Abstract
STUDY QUESTION
Is first trimester phthalate exposure associated with anogenital distance (AGD), a biomarker of prenatal androgen exposure, in newborns?
SUMMARY ANSWER
Concentrations of diethylhexyl phthalate (DEHP) metabolites in first trimester maternal urine samples are inversely associated with AGD in male, but not female, newborns.
WHAT IS KNOWN ALREADY
AGD is a sexually dimorphic measure reflecting prenatal androgen exposure. Prenatal phthalate exposure has been associated with shorter male AGD in multiple animal studies. Prior human studies, which have been limited by small sample size and imprecise timing of exposure and/or outcome, have reported conflicting results.
STUDY DESIGN, SIZE, DURATION
The Infant Development and the Environment Study (TIDES) is a prospective cohort study of pregnant women recruited in prenatal clinics in San Francisco, CA, Minneapolis, MN, Rochester, NY and Seattle, WA in 2010–2012. Participants delivered 787 infants; 753 with complete data are included in this analysis.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Any woman over 18 years old who was able to read and write English (or Spanish in CA), who was <13 weeks pregnant, whose pregnancy was not medically threatened and who planned to deliver in a study hospital was eligible to participate. Analyses include all infants whose mothers provided a first trimester urine sample and who were examined at or shortly after birth. Specific gravity (SpG) adjusted concentrations of phthalate metabolites in first trimester urine samples were examined in relation to genital measurements. In boys (N = 366), we obtained two measures of anogenital distance (AGD) (anoscrotal distance, or AGDAS and anopenile distance, AGDAP) as well as penile width (PW). In girls (N = 373), we measured anofourchette distance (AGDAF) and anoclitoral distance (AGDAC). We used multivariable regression models that adjusted for the infant's age at exam, gestational age, weight-for-length Z-score, time of day of urine collection, maternal age and study center.
MAIN RESULTS AND THE ROLE OF CHANCE
Three metabolites of DEHP were significantly and inversely associated with both measures of boys' AGD. Associations (β, 95% confidence interval (CI)) between AGDAS and (log10) SpG-adjusted phthalate concentrations were: −1.12 (−2.16, −0.07) for mono-2-ethylhexyl phthalate (MEHP), −1.43, (−2.49, −0.38) for mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and −1.28 (−2.29, −0.27) for mono-2-ethyl-5-hydroxyhexyl (MEHHP). Associations were of similar magnitude for AGDAP. Associations were weaker and not statistically significant for PW. No other phthalate metabolites were associated with any genital measurement in boys. No phthalate metabolites were associated with either AGD measure in girls.
LIMITATIONS, REASONS FOR CAUTION
Exposure assessment was based on a single first trimester urine sample, which may have introduced exposure misclassification. In addition, significant between-center differences suggest that this measurement is difficult to standardize.
WIDER IMPLICATIONS OF THE FINDINGS
Our findings are consistent with multiple rodent studies and most human studies which were far smaller. The data we report here suggest that even at current low levels, environmental exposure to DEHP can adversely affect male genital development resulting in reproductive tract changes that may impact reproductive health later in life. These findings have important implications for public policy since most pregnant women are exposed to this ubiquitous chemical.
STUDY FUNDING/COMPETING INTEREST(S)
Funding for TIDES was provided by the following grants from the National Institute of Environmental Health Sciences: R01ES016863-04 and R01 ES016863-02S4. The authors report no conflict of interest.
Keywords: anogenital distance, phthalates, prenatal development, environmental chemicals, phthalate syndrome
Introduction
Phthalates comprise a class of environmentally pervasive industrial chemicals, several of which are potent anti-androgens (Gray et al., 2000). According to the Centers for Disease Control (CDC), nearly 100% of the US population exhibits measurable levels of one or more of these chemicals (CDC, 2013). Phthalates comprise a large class of ubiquitous chemicals that have been measured in residential indoor environments in both indoor air and house dust (Rudel et al., 2003), as well as in foods, milk and drinking water. High molecular weight phthalates, such as diethylhexyl phthalate (DEHP), are primarily used as plasticizers in the manufacture of polyvinyl chloride (PVC) which is used extensively in various consumer products, flooring, wall coverings, food contact applications and medical devices (ATSDR, 2002). Lower molecular weight phthalates, including dibutyl phthalate (DBP) are used as solvents and plasticizers for cellulose acetate in making lacquers, varnishes, coatings and personal-care products (e.g. perfumes, lotions and cosmetics) (ATSDR, 2001).
Phthalates have recently been subjected to increased scrutiny because of a growing body of evidence implicating them in a spectrum of male reproductive disorders. In rats, prenatal exposure to several phthalates, particularly, DBP, DEHP and butyl benzyl phthalate (BBzP), elicits a syndrome of genital dysmorphology in males, including shortened AGD, which has been termed the ‘phthalate syndrome’ (Foster, 2005). This syndrome includes incomplete testicular descent, smaller testis weight and penile size, alterations to the vas deferens and epididymis, and most notably, shortened anogenital distance (AGD) (Foster et al., 2000). AGD, the distance between the anus and the genitals, is frequently 50–100% longer in males than females of many species, including humans. It is a sensitive marker of prenatal disruption of male genital tract development, particularly by anti-androgenic exposures during the critical prenatal window (Macleod et al., 2010). For that reason, AGD (as well as testicular descent, and weight and structure of the external genitalia) is included in the United State Environmental Protection Agency (USEPA) guidelines for evaluation of chemicals for reproductive toxicity (USEPA, 1996).
Several recent studies suggest that short male AGD may have reproductive implications for humans as well as rodents. In males, children with hypospadias and cryptorchidism have significantly shorter AGD than controls (Hsieh et al., 2008, 2012; Thankamony et al., 2013). In male adults, shorter AGD predicts poorer semen quality (Eisenberg et al., 2011; Mendiola et al., 2011) and reduced testosterone and testicular volume (Eisenberg et al., 2012). Among men seen in an infertility clinic, AGD was longer in fathers than in infertile men (Eisenberg et al., 2011). In females, a longer AGD has been associated with conditions linked to increased androgenization such as congenital adrenal hyperplasia (Callegari et al., 1987) and multifollicular ovaries (Mendiola et al., 2012). AGD may therefore be an important biomarker of reproductive health in humans, as it is in rodents.
In our prior Study for Future Families (SFF), we found that in males (N = 105), shorter AGD as well as smaller penile width (PW) and incomplete testicular descent in infancy were associated with prenatal exposure to DEHP metabolites (Swan, 2008). Two additional studies (N = 111 and N = 73) reported results consistent with these (Suzuki et al., 2012; Bustamante-Montes et al., 2013), while one study (N = 196) found similar, but not statistically significant associations with DEHP metabolite (Bornehag et al., 2014) and a fourth (N = 33) (Huang et al., 2008) did not find an association. Therefore, whether DEHP and other phthalates can cause a ‘phthalate syndrome’ in human males remains uncertain. No animal study has reported altered AGD in females exposed to phthalates. In the SFF, AGD in girls (N = 102) was not associated with any phthalate metabolite. In the only other human study to examine female AGD in relation to prenatal phthalate exposure, girls' AGD index (AGD/weight) at birth (N = 33) was inversely associated with higher concentrations of monobutyl phthalate (MBP) in amniotic fluid (Huang et al., 2008).
Given the ubiquity of phthalate exposure and the growing evidence that altered AGD may be associated with impaired reproductive development, a definitive study examining these associations was needed. The Infant Development and the Environment Study (TIDES) is far more powerful and precise than any that has looked at this question to date, with a sample size (N = 753) exceeding all published studies on this question combined, carefully timed collection of urine samples during the first trimester, the critical window for genital development (Welsh et al., 2008), and precise genital measurements at birth. In addition, population levels of many phthalate metabolites (and DEHP in particular) have been decreasing (Zota et al., 2014) and prior studies of phthalates and AGD examined associations with higher exposure. Therefore, whether phthalates at current lower exposure levels can alter fetal androgen exposure and perhaps produce a human analogue of the phthalate syndrome seen in rodents was an open question motivating this study. Based on the animal literature and prior human studies, the objective of this study was to test the a priori hypotheses that phthalate metabolite concentrations will be inversely associated with male (but not female) AGD, and that those associations will be strongest for DEHP, a potent reproductive toxicant (Gray et al., 2006) and the phthalate most clearly linked to genital changes in male infants.
Methods
Study population
TIDES is an ongoing prospective cohort study designed to examine prenatal phthalate exposure in relation to infant genital morphology. Pregnant women were recruited between August 2010 and August 2012 at university-based prenatal clinics in San Francisco, CA (University of California, San Francisco, UCSF), Rochester, NY (University of Rochester Medical Center, URMC), Minneapolis, MN (University of Minnesota, UMN) and Seattle, WA (University of Washington/Seattle Children's Hospital, UW/SCH). Any woman over 18 years old who was able to read and write English (or Spanish at the CA center), who was <13 weeks pregnant, whose pregnancy was not medically threatened, and who planned to deliver in a study hospital was eligible to participate. Participants provided urine samples and completed a questionnaire in each trimester, and gave a serum sample in the first trimester. The institutional review board (IRB) at all participating institutions approved TIDES prior to study implementation and all subjects provided signed informed consent before starting any study activities. IRB approval was also obtained at the Icahn School of Medicine at Mount Sinai, which served as the TIDES Coordinating Center after 2011. Here we report on all TIDES women who provided a first trimester urine sample and gave birth to a singleton infant whose AGD was measured at or shortly after birth.
Maternal urinary phthalate metabolite concentrations
Urine samples were collected in phthalate-free polypropylene cups and all collection and storage materials were shown to be phthalate-free. Specific gravity (SpG) was measured within 30 min using a hand-held refractometer (National Instrument Company, Inc., USA) which was calibrated with deionized water before each measurement. Ambient temperature at SpG determination was noted and adjustment was made if required. Samples were stored at −80°C before shipment at the end of prenatal sample collection (up to 2 years). All samples from mothers of boys were analyzed at the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention (CDC) using an analytical approach that involves the enzymatic deconjugation of the metabolites from their glucuronidated form, automated online solid-phase extraction, separation with high performance liquid chromatography and detection by isotope-dilution tandem mass spectrometry (Silva et al., 2007). Samples from mothers of girls were analyzed in two laboratories. Most samples (N = 288) were measured at the Environmental Health Laboratory at the University of Washington (UW). At this laboratory glucuronidated phthalate monoesters underwent enzymatic deconjugation, followed by online solid-phase extraction (SPE) coupled with reversed high performance liquid chromatography-electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS) to quantify the simple monoesters in urine (Calafat, 2010). A quarter of the samples from mothers of girls (N = 95) were measured at the CDC using the method described above. In both laboratories, procedure blanks were run with each batch of samples and isotopically labeled internal standards were used along with conjugated internal standards to increase precision and accuracy of the measurements. Travel blanks, collected at each study site at the beginning, middle and end of the sample collection period were analyzed with participants' samples. Limits of detection (LOD) were somewhat lower at the CDC lab than at the UW lab. For statistical analyses, values below the LOD were assigned the value LOD divided by the square root of 2, as has been recommended when the data are not highly skewed (Hornung and Reed, 1990).
While our a priori hypothesis focused on four DEHP metabolites, we also examined associations between our study end-points and seven other phthalate metabolites (listed in Table I). We also examined associations between genital measurements and the molar sum of the four measured DEHP metabolites (Wolff et al., 2008): ΣDEHP = (MEHP*(1/278)) + (MEHHP*(1/294)) + (MEOHP*(1/292)) + (MECPP*(1/308)) nmol/ml.
Table I.
Concentration (ng/ml) of phthalate metabolites in first trimester urine samples in TIDES women (NHANES1 values in italics).
| Phthalate | Metabolite | Geometric mean (95% CI) | Percentiles |
Limit of detection (LOD) |
%> LOD | Sample size | |||
|---|---|---|---|---|---|---|---|---|---|
| 50th | 75th | 90th | CDC1 | UW2 | |||||
| Diethylhexyl (DEHP) | Mono-2-ethylhexyl (MEHP) | 1.93 (1.76, 2.11) | 2.00 | 4.70 | 9.20 | 0.5 | 1.0 | 66.4 | 753 |
| 1.39 (1.21, 1.60) | 1.30 | 3.00 | 6.49 | ||||||
| Mono-2-ethyl-5-oxohexyl (MEOHP) | 4.22 (3.84, 4.63) | 4.40 | 9.90 | 19.9 | 0.2 | 1.0 | 96.9 | 753 | |
| 7.09 (6.17, 8.14) | 7.35 | 15.2 | 27.1 | ||||||
| Mono-2-ethyl-5-hydroxyhexyl (MEHHP) | 6.04 (5.49, 6.64) | 6.10 | 14.0 | 29.7 | 0.2 | 0.2 or 1.0 | 97.2 | 753 | |
| 11.0 (9.58, 12.8) | 11.6 | 22.5 | 71.0 | ||||||
| Mono-2-ethyl-5-carboxypentyl (MECPP) | 8.12 (7.42, 8.89) | 8.60 | 18.00 | 37.6 | 0.2 | 0.2 or 1.0 | 97.6 | 753 | |
| 18.5 (16.2, 21.0) | 18.8 | 34.7 | 79.2 | ||||||
| ΣDEHP4 | 71.7 (65.6, 78.3) | 75.0 | 165.0 | 333 | NA | NA | NA | 753 | |
| Diethyl (DEP) | Mono-ethyl (MEP) | 28.4 (25.3, 31.9) | 26.0 | 81.0 | 213 | 0.6 | 1.0 | 99.1 | 753 |
| 67.8 (60.3, 76.4) | 59.6 | 164 | 548 | ||||||
| Butylbenzyl (BBzP) | Mono-benzyl (MBzP) | 3.31 (2.98. 3.68) | 3.10 | 9.00 | 23.0 | 0.3 | 1.0 | 87.4 | 753 |
| 6.04 (5.38. 6.77) | 6.36 | 15.3 | 29.3 | ||||||
| Dibutyl (DBP) | Mono-n-butyl (MnBP) | 6.36 (5.77, 7.00) | 7.00 | 16.6 | 30.4 | 0.4 | 1.0 or 2.0 | 92.4 | 753 |
| 14.7 (13.1, 16.5) | 16.4 | 32.7 | 57.7 | ||||||
| Di-isobutyl (DIBP) | Mono-isobutyl (MiBP) | 3.97 (3.60, 4.38) | 4.40 | 10.6 | 21.7 | 0.2 | 0.2 | 96.9 | 753 |
| 7.50 (6.68, 8.43) | 8.07 | 16.4 | 29.1 | ||||||
| Di-n-octyl (DnOP) | Mono-3-carboxy-propyl (MCPP) | 1.91 (1.72, 2.13) | 1.60 | 5.00 | 14.6 | 0.2 | 1.0 | 75.2 | 753 |
| 2.72 (2.29, 3.22) | 2.79 | 5.78 | 12.6 | ||||||
| Di-isodecyl (DIDP) | Mono-carboxy-isononyl (MCNP) | 2.03 (1.80, 2.29) | 2.10 | 4.80 | 12.1 | 0.2 | NA | 95.9 | 4643 |
| 2.66 (2.32, 3.04) | 2.60 | 5.21 | 11.6 | ||||||
| Di-isononyl (DINP) | Mono-carboxy-isooctyl (MCOP) | 14.5 (12.5, 16.8) | 13.2 | 45.2 | 121 | 0.2 | NA | 100 | 4643 |
| 11.4 (9.17, 14.1) | 10.6 | 29.6 | 70.4 | ||||||
Centers for Disease Control (CDC) Fourth Report Updated, unadjusted values for females 2009–2010.
Environmental Health Laboratory at the University of Washington.
MCNP and MCOP not measured in UW lab.
ΣDEHP = (MEHP*(1/278)) + (MEHHP*(1/294)) + (MEOHP*(1/292)) + (MECPP*(1/308)) × 1000 (nmol/L).
Anogenital distance
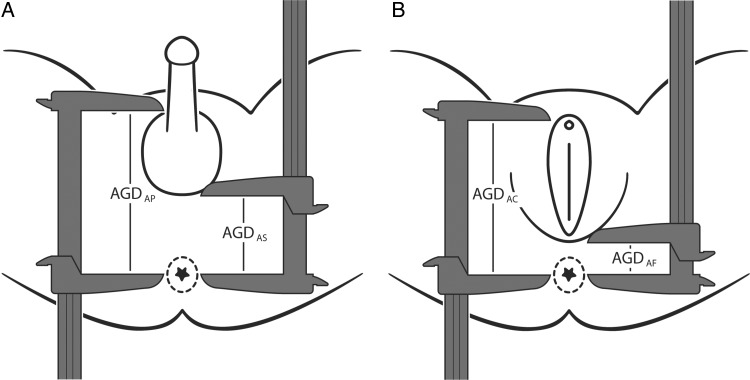
Following methods described elsewhere (Sathyanarayana et al.), which were similar to methods used in our previous study (Swan et al., 2005; Swan, 2008), two measures of AGD and one measure of penile width (PW) were obtained on all male infants and two measures of AGD were obtained on all females. All measurements were made with the infant lying on his/her back with the buttocks at the edge of a flat surface. An assistant held the legs in frog leg position at a 60–90° angle from the torso at the hip. All measurements were made using dial calipers (SPI Model 31-415-3, Style B). All AGD measurements are distances from the center of the anus to a genital landmark. In males the genital landmarks are (i) the anterior base of the penis where the penile tissue meets the pubic bone (AGDAP) and (ii) the base of the scrotum where the skin changes from rugated to smooth (AGDAS) (Fig. 1B). In females, the genital landmarks are (i) the anterior tip of the clitoral hood (AGDAC) and (ii) the base of the posterior fourchette where skin folds fuse (AGDAF) (Fig. 1A). PW was measured at the base of the penis.
Figure 1.
Landmarks for two measurements of anogenital distance. In females (A) AGDAC (shown as AGD) is measured from the center of the anus to the anterior tip of the clitoral hood and AGDAF (shown as AFD) is measured from the center of the anus to the base of the posterior fourchette where skin folds fuse. In males (B) AGDAP (shown as AGD) is measured from the center of the anus to the cephalad insertion of the penis and AGDAS (shown as ASD) is measured from the center of the anus to the posterior base (first fold) of the scrotum.
Statistical methods
Phthalate metabolite concentrations were log10 transformed to normalize distributions. SpG was used to adjust for urine dilution using the following formula:
where Pc is the SpG-corrected phthalate concentration (ng/ml), P is the observed phthalate concentration (ng/ml), 1.014 is the mean SpG for all TIDES samples and SpG is the specific gravity of the individual urine sample (Boeniger et al., 1993).
A rigorous quality control program was implemented, which is described in detail elsewhere (Sathyanarayana et al.). Briefly, all measurements were made in triplicate and averaged. Approximately every 10th infant was measured a second time by an independent examiner. These data were used to calculate inter and intra-observer intra-class correlation coefficients (ICCs) and coefficients of variations (CVs). Between-center differences were examined and controlled in several ways. We included center terms in all multivariable models examining AGD, both those that included phthalate concentration and those that examined predictors of AGD without controlling for phthalate concentration. We examined homogeneity of variance for all AGD variables across centers using Levene's test (Levene, 1960). We also ran models that included interaction terms to examine the possible modification of AGD-phthalate associations by center.
We calculated univariate descriptive statistics (means, standard deviation, median, percentiles and frequencies, as appropriate) for all genital end-points, phthalate metabolites and covariates. Before conducting multivariable linear regression analyses, we examined a number of covariates potentially predictive of AGD and PW including infant's age at exam, gestational age at birth, and clinical center. As described in more detail elsewhere (Sathyanarayana et al.), we used weight-for-length Z-scores (ZWL) calculated from World Health Organization (WHO) standard curves (WHO, 2009) to control for infant body size. These Z-scores provide the best predictor of genital measurements among the measures of size we considered (including weight, weight-for- age and length-for-age Z-scores) and are independent of both age and phthalate metabolite concentration. Maternal race/ethnicity, education and smoking were also considered, but did not alter effect estimates by >10% and were not included in final models. Final multivariable models adjusted for ZWL, clinical center, gestational age at birth, age at birth exam, specific gravity, time of day of urine collection and maternal age. Regression assumptions were examined using residual plots and outliers (defined as data points with standardized residuals exceeding 3 standard deviations) were removed. We used these models to estimate the change in genital measurements (ΔAGD) expected with an increase of phthalate metabolite concentration from the 10th to the 90th percentiles.
We used a commonly accepted method of imputation to deal with metabolite values less than the limit of detection (LOD): substitution by LOD/ (Sqrt2) (Hornung and Reed, 1990). However, several authors have reported that this method can introduce bias, usually towards the null (Helsel, 2005; Chen et al., 2011; Hewett and Ganser, 2007). Therefore when modeling associations between DEHP metabolites and boys' AGD, we compared the usual method of imputation to the deletion method (e.g. deleting all values below the LOD).
All analyses were conducted using SAS software, Version 9.3 (Cary, NC, USA).
Results
TIDES enrolled 969 women of whom 894 completed a questionnaire and gave a urine sample in the first trimester. Of these 758 women gave birth to an infant (371 boys and 387 girls) who received a birth exam at a median age of 1 day (25th and 75th percentiles, 1 and 2 days, respectively). First trimester phthalate metabolite concentrations were available for 370 boys and 383 girls who received a birth exam. Four boys were then dropped from the final analytic data set; two because their data were outliers, one because birth length was not measured, and one because of missing maternal age. Seven girls missing weight-for-length Z-score due to missing length and three girls missing maternal age were also dropped from final analyses. In addition, AGDAP was not measured for one boy, leaving 366 boys for analysis of AGDAS and PW and 365 boys for analysis of AGDAP. AGDAC was not measured for one girl leaving 373 girls for analysis of AGDAF and 372 for AGDAC.
Mothers' mean age was 31.1 years, mean pre-pregnancy BMI was 26.2 kg/m2 and 83% were married or living as married (Table II). Nearly three-quarters of our participants were college graduates and only 43 women (5.8%) reported smoking. Three of our four clinical centers were quite similar demographically but URMC participants included more Hispanic and non-Caucasian women (56%), unmarried women (46%) and fewer college graduates (31%) than the other centers. Among the singleton infants included in our study, median gestational age at delivery was 39 weeks, with 7.9% of infants (N = 67) born preterm (<37 weeks gestation). Most exams were performed in the first or second post-natal day in the mother's hospital room, although 118 (15.6%) infants had a birth exam >7 days post-delivery due to out-of-hospital birth, discharge before examination could be scheduled, or medical complications making an exam immediately after birth contraindicated. The median ZWL was similar in boys and girls and slightly negative (−0.35, −0.33 in boys and girls, respectively) reflecting the minor weight loss that infants typically experience during the first days of life. All genital measurements were normally distributed. In boys, mean (SD) AGDAS, AGDAP and PW was 24.7(4.6) mm, 49.7 (5.9) mm and 10.8 (1.3) mm, respectively. In girls, mean (SD) AGDAF and AGDAC was 16.0 (3.2) and 36.6 (3.9) respectively. Thus, the male to female ratios for the shorter and longer AGD measures were 1.5 and 1.4, respectively.
Table II.
The Infant Development and the Environment Study: characteristics of mothers and infants.
| Mothers (N = 753) | Categorical variables | N | (%) | ||
| Center | |||||
| San Francisco, CA | 187 | (24.83) | |||
| Minneapolis, MN | 202 | (26.83) | |||
| Rochester, NY | 212 | (28.15) | |||
| Seattle, WA | 152 | (20.19) | |||
| Race/Ethnicity | |||||
| White/Not Hispanic | 500 | (66.40) | |||
| Other | 253 | (33.60) | |||
| Smoking | |||||
| Yes | 43 | (5.81) | |||
| No | 697 | (94.19) | |||
| Education | |||||
| Less than college | 191 | (25.64) | |||
| Graduated college | 554 | (74.36) | |||
| Marital status | |||||
| Married/ Living as married | 622 | (83.16) | |||
| Separated/divorced/single | 126 | (16.84) | |||
| Continuous variables | Mean (SD) | Percentiles |
|||
| 25th | 50th | 75th | |||
| All Infants | Age | 31.09 (5.52) | 27.37 | 31.52 | 35.03 |
| Pre-pregnancy BMI (kg/m2) | 26.23 (6.29) | 21.81 | 24.55 | 29.21 | |
| Weight at exam (kg) | 3.37 (0.71) | 2.96 | 3.30 | 3.66 | |
| Gestational age (weeks) | 39.31 (1.79) | 38.71 | 39.57 | 40.57 | |
| Age at exam (days) | 6.44 (15.55) | 1 | 1 | 2 | |
| Weight-for-length z-score (ZWL) | −0.39 (1.26) | −1.09 | −0.34 | 0.36 | |
| Boys (N = 366) | AGDAS (mm) | 24.73 (4.55) | 21.73 | 24.33 | 27.17 |
| AGDAP (mm) | 49.66 (5.91) | 45.6 | 49.03 | 53.23 | |
| Penile width (mm) | 10.81 (1.32) | 9.93 | 10.78 | 11.5 | |
| Girls (N = 373) | AGDAF (mm) | 16.02 (3.22) | 13.9 | 15.73 | 18.20 |
| AGDAC (mm) | 36.60 (3.89) | 34.07 | 36.78 | 39.17 | |
First trimester urine samples were assayed for 11 phthalate metabolites (Table I). Percent detectable (values exceeding the limit of detection or LOD) ranged from 66.4% for mono-2-ethylhexyl phthalate (MEHP) to 100% for mono-carboxy-isooctyl phthalate (MCOP). No other phthalate metabolites measured in T1 collection were associated with the concentrations of all DEHP metabolites. Hour of urine collection was associated with the concentrations of all DEHP metabolites. For example, for the sum of the DEHP metabolites (ΣDEHP), the regression coefficient (CI, P) for hour of urine collection was 0.03, [CI (0.016, 0.459), P = 0.0001].
All genital measurements were significantly correlated. In boys, the Pearson correlation between AGDAP and AGDAS was 0.65 (P < 0.0001), and between AGD measurements and PW, it was 0.33 and 0.23 (P < 0.0001), for AGDAP and AGDAS, respectively. For girls, the correlation between the two AGD measurements was 0.47 (P < 0.0001). All genital measurements increased with age and weight, which were highly correlated (R = 0.61, P < 0.0001). Infant weight was also inversely related to prenatal DEHP metabolite concentration (Zhang et al., 2009). For example, the correlation between weight and log10 MEHP concentration was −0.10 (P = 0.046). Therefore, we adjusted for body size using the infant's weight-for-length Z-scores (ZWL) (Multicentre Growth Reference Study Group, 2009) which is uncorrelated with age (R = 0.02, P = 0.64) or prenatal phthalate concentration (e.g. R = 0.001, P = 0.985, for log10 MEHP concentration). Models predicting our genital measurements, independent of phthalate exposure, have been described previously (Sathyanarayana et al.). Briefly, in addition to age at exam, which was associated with all genital measurements, gestational age, maternal age, center and ZWL were associated with most outcomes.
Six examiners measured most infants (70.7%) with a total of 20 examiners across all sites and study years. Intra-examiner ICCs ranged from 0.89 for PW, to 0.92 for AGDAF and AGDAC and 0.94 and 0.95 for AGDAF and AGDAS. Inter-examiner ICCs were somewhat higher for boys' AGD (0.82 and 0.84 for AGDAP and AGDAS) than for girls' AGD (0.73 and 0.79). In models run to identify predictors of outcomes measures independent of phthalate exposure, there were no significant between-center differences for AGDAS and AGDAC. For AGDAP, measurements at three centers were somewhat shorter than those in UMN (average 3% of mean). Between-center differences were greatest for AGDAF (average 9.8% of mean) and somewhat less for PW (average 6% of mean).
In multivariable regression analyses adjusting for infant's age at exam, gestational age at birth, ZWL, time of day of urine collection, specific gravity, maternal age and study center, the strongest associations between male AGD measurements and phthalate metabolite concentrations were seen for MEHP, MEOHP and MEHHP (Table III). The associations between AGDAS and (log10) phthalate concentrations for male infants were: (β = −1.12, 95% confidence interval (CI) = (−2.16, −0.07), P = 0.036) for MEHP; (β = −1.43, CI = (−2.49, −0.38), P = 0.008) for MEOHP, (β = −1.28, CI (−2.29, −0.27), P = 0.013) for MEHHP and (β = −1.26, CI (−2.40, −0.13), P = 0.029) for ΣDEHP. Associations were slightly stronger for AGDAP. For PW, the strongest relationship was seen between PW and MECPP (β = −0.32, CI (−0.66, 0.02), P = 0.065). The estimated percent change in boys' AGD measurements (ΔAGD) expected for an increase in metabolite concentration of MEHP, MEHHP and MEOHP from the 10th to the 90th percentiles, all other covariates remaining unchanged, is contained in Table IV. These ranged from 2.23% for the expected change in AGDAP with increasing MEHP concentration to 5.05% for the expected change in AGDAS with increasing in MEOHP concentration from the 10th to the 90th percentile.
Table III.
Measures of association between first trimester urinary phthalate metabolite concentrations and genital measurements in newborn boys1,2 in multivariable models.
| Boys | AGDAS |
AGDAP |
PW |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P-value | β | 95% CI | P-value | β | 95% CI | P-value | |
| MEHP | −1.12 | (−2.16, −0.07) | 0.036 | −1.21 | (−2.43, −0.01) | 0.051 | −0.26 | (−0.57, 0.06) | 0.107 |
| MEOHP | −1.43 | (−2.49, −0.38) | 0.008 | −1.60 | (−2.84, −0.36) | 0.011 | −0.26 | (−0.58, 0.06) | 0.113 |
| MEHHP | −1.28 | (−2.29, −0.27) | 0.013 | −1.47 | (−2.65, −0.29) | 0.015 | −0.24 | (−0.54, 0.09) | 0.128 |
| MECPP | −0.97 | (−2.12, 0.18) | 0.097 | −0.93 | (−2.27, 0.41) | 0.171 | −0.32 | (−0.66, 0.02) | 0.065 |
| ΣDEHP | −1.26 | (−2.40, −0.13) | 0.029 | −1.35 | (−2.67, −0.02) | 0.046 | −0.31 | (−0.64, 0.04) | 0.078 |
| MEP | −0.32 | (−1.04, 0.39) | 0.374 | −0.03 | (−0.87, 0.82) | 0.953 | −0.12 | (−0.33, 0.10) | 0.275 |
| MBzP | 0.16 | (−0.81, 1.12) | 0.750 | 0.38 | (−0.64, 1.50) | 0.507 | 0.001 | (−0.29, 0.29) | 0.994 |
| MBP | −0.91 | (−2.02, 0.20) | 0.108 | −0.78 | (−2.08, 0.51) | 0.235 | −0.03 | (−0.36, 0.31) | 0.877 |
| MiBP | −0.49 | (−1.69, 0.72) | 0.427 | −0.49 | (−1.89, 0.91) | 0.490 | −0.07 | (−0.43, 0.29) | 0.699 |
| MCPP | 0.18 | (−0.60, 0.95) | 0.654 | 0.35 | (−0.56, 1.26) | 0.447 | −0.10 | (−0.33, 0.12) | 0.413 |
| MCNP | 0.31 | (−0.71, 1.33) | 0.551 | 0.14 | (−1.06, 1.34) | 0.816 | 0.10 | (−0.21, 0.40) | 0.537 |
| MCOP | 0.39 | (−0.39, 1.16) | 0.326 | −0.02 | (−0.93, 0.88) | 0.958 | −0.05 | (−0.28, 0.18) | 0.681 |
Adjusted for age at exam, weight-for-length Z-score, gestational age at birth, center and time of day of urine collection and maternal age.
Phthalate metabolite concentrations <LOD set to LOD/(√2).
Table IV.
ΔAGD: Decrease in boys' AGD with increase in metabolite concentration1 from 10th to 90th percentiles.2
| Percentile (ng/ml) |
ΔAGD |
|||
|---|---|---|---|---|
| 10th | 90th | AGDAS | AGDAP | |
| MEHP | 0.73 | 5.80 | 4.09% | 2.23% |
| MEHHP | 2.32 | 20.02 | 4.87% | 2.81% |
| MEOHP | 1.68 | 12.37 | 5.05% | 2.84% |
Log10 (SG-adjusted) phthalate metabolite concentration.
Average of center-specific estimates from models adjusted for age at exam, weight-for-length Z-score, gestational age at birth, time of day of urine collection and maternal age.
Associations between AGD measures and most covariates differed somewhat by study center in stratified analyses. For example, gestational age was more strongly associated with all genital measures at UW/SCH, while body size (weight-for-age Z score) was more strongly associated with genital outcomes at UCSF. Associations with phthalate metabolite concentration also varied by center, as did estimates of ΔAGD. Therefore we ran models examining modification of AGD-phthalate associations by center. Across all centers, AGD measures and DEHP metabolites, these models yielded 24 estimates of center-phthalate interactions, of which only one was statistically significant. Thus, we see little evidence of center-phthalate interactions between boys' AGD measurement and DEHP metabolite concentration.
No other phthalate metabolites measured in first trimester (T1) urine samples were associated with any genital measurements in male infants (Table III), nor were there any associations between either measure of girls' AGD and any of the phthalate metabolites measured (Table V).
Table V.
Measures of association between first trimester urinary phthalate metabolite concentrations and genital measurements in newborn girls1,2 in multivariable models.
| Girls | AGDAF |
AGDAC |
||||
|---|---|---|---|---|---|---|
| β | 95% CI | P-value | β | 95% CI | P-value | |
| MEHP | 0.03 | (−0.72, 0.77) | 0.929 | −0.16 | (−1.09, 0.77) | 0.739 |
| MEOHP | 0.29 | (−0.51, 1.10) | 0.473 | 0.00 | (−1.01, 1.02) | 0.997 |
| MEHHP | 0.22 | (−0.56, 1.00) | 0.578 | −0.29 | (−1.28, 0.69) | 0.560 |
| MECPP | 0.23 | (−0.55, 1.01) | 0.566 | −0.42 | (−1.40, 0.57) | 0.407 |
| ΣDEHP | 0.25 | (−0.59, 1.09) | 0.558 | −0.33 | (−1.39, 0.73) | 0.542 |
| MEP | 0.04 | (−0.48, 0.55) | 0.893 | 0.22 | (−0.43, 0.86) | 0.507 |
| MBzP | 0.42 | (−0.27, 1.12) | 0.232 | 0.26 | (−0.62, 1.14) | 0.567 |
| MBP | 0.66 | (−0.19, 1.50) | 0.128 | 0.04 | (−1.62, 1.11) | 0.948 |
| MiBP | −0.25 | (−1.06, 0.55) | 0.533 | −0.55 | (−1.56, 0.47) | 0.288 |
| MCPP | −0.10 | (−0.64, 0.43) | 0.708 | −0.44 | (−1.11, 0.24) | 0.202 |
| MCNP | 0.21 | (−0.89, 1.30) | 0.710 | 0.08 | (−1.36, 1.53) | 0.909 |
| MCOP | 0.23 | (−1.09, 1.54) | 0.733 | 0.09 | (−1.65, 1.83) | 0.920 |
Adjusted for age at exam, weight-for-length Z-score, gestational age at birth, center and time of day of urine collection and maternal age.
Phthalate metabolite concentrations <LOD set to LOD/(√2).
We conducted a sensitivity analysis comparing two methods of handling values below the LOD. As described above, for the primary analyses we imputed values below the LOD by replacing them with LOD/(sqrt2). An alternative method is to delete all values below the LOD (deletion method). We compared results using these two methods for all metabolites for which some concentrations were below the LOD. Almost all associations were somewhat stronger (higher beta and smaller P-value) when the deletion method was used than when imputation was used (data available upon request).
While all urine samples from mothers of boys were measured at a single lab (CDC), samples from mothers of girls were measured in two labs (CDC and UW). Therefore we compared metabolite concentrations in 10 samples from mothers of girls at the CDC and UW laboratories. Median concentrations differed for some metabolites, some significantly. However ΣDEHP was similar in the two labs: median 39.5 and 43.2 ng/ml in UW and CDC, respectively (P = 0.617).
Discussion
The Infant Development and the Environment Study (TIDES) was designed to test the a priori hypothesis that boys' AGD and PW at birth are significantly and negatively associated with first trimester phthalate metabolite concentrations, particularly with the metabolites of the potent anti-androgen, DEHP. Our current data support that hypothesis and are consistent with much of the available literature, though not all. They also support the absence of associations between first trimester phthalate exposure to other phthalate metabolites and boys' AGD, or between any phthalate metabolite and girls' AGD.
TIDES, which includes 737 infants (365 boys and 372 girls), has a sample size that exceeds that in all prior human studies of AGD and prenatal phthalate exposure combined (N = 652). These prior studies include the US Study for Future Families (SFF) with 207 infants (105 boys and 102 girls) (Swan, 2008), a Taiwanese study including 33 boys and 32 girls (Huang, 2008), a Mexican study of 111 boys (Bustamante-Montes et al., 2013), a Japanese study of 73 boys (Suzuki, 2011) and a recent Swedish study of 196 boys (Bornehag et al., 2014). TIDES protocols were designed to minimize exposure and outcome misclassification, thus reducing unexplained variability and increasing statistical power. Urine samples were collected during the first trimester (mean gestational age 11 weeks), corresponding to the male programming window for genital development (Welsh et al., 2008). Most prior studies that examined AGD and prenatal phthalate exposure collected samples in late pregnancy, except one other study that collected samples in the first trimester (Bornehag et al., 2014) and one which collected samples in the second trimester (Huang et al., 2008). In TIDES, all birth exam measurements (including weight and length, which were also measured with standardized equipment at the TIDES exam) were collected according to rigorous protocols and timed to minimize variability. For example, the exam was performed at least 4 h after birth to minimize genital swelling that might contribute to measurement variation, and in most cases, before the infant was discharged from the birth hospital. All other studies measured AGD either at the time of delivery or much later in childhood. We developed detailed measurement and training protocols and maintained strict quality control of all measurements, resulting in high inter- and intra-rater reliability. In addition, our statistical models were selected to minimize residual and collinearity. To this end, we controlled for body size using weight-for-length Z-scores rather than weight, because infant weight is strongly correlated both with age and prenatal phthalate exposure (Zhang et al., 2009). Analytic methods for measuring phthalate metabolite concentration were more sensitive than those available for earlier studies due to improvements in assay technology. Because of these strengths, our study provides the most definitive data on this question to date.
Concentrations of many phthalate metabolites have declined in the USA over the past 10 years due to decreased use of these chemicals in industrial processes and consumer products, with many substitutions, such as DEHP being replaced by di-isononyl phthalates (DINP) (Zota et al., 2014). In TIDES, concentrations of DEHP metabolites were ∼50% lower than in our previous pregnancy cohort study (SFF), which had enrolled participants between 2000 and 2002. Median metabolite concentrations in our population were also somewhat lower than those reported in the 2009–2010 cycle of the National Health and Nutrition Examination Survey (NHANES) (CDC, 2013). For example, median MEOHP and MEHHP were 4.0 and 5.8 (ng/ml) in the TIDES population compared with 7.4 and 11.6 (ng/ml), respectively, in NHANES (Table I). Whether these differences reflect further decreases in DEHP exposure or differences between pregnant and non-pregnant women is uncertain, since phthalate metabolite concentrations were measured in only 28 pregnant women in the 2009–2010 cycle of NHANES. However, despite lower exposure in TIDES, the associations between DEHP and AGD were consistent with those in SFF, suggesting that these chemicals may have developmental effects on the male reproductive system even at very low levels.
In the SFF, the first study to examine AGD in relation to phthalate exposure, we found AGD and PW to be inversely associated with DEHP metabolites (Swan, 2008). Beta coefficients in models predicting AGDAP in relation to (log10) concentration of MEHP, MEOHP and MEHHP were −3.50, −4.98 and −5.13 in our prior study (SFF), compared with −1.43, −1.71 and −1.49 in TIDES, with similar P-values in the two studies. These differences in beta coefficients may reflect the 50% lower concentration of DEHP metabolites in TIDES relative to the SFF. Differences may also be influenced by the 40% longer AGD in SFF boys whose AGD was measured at (median) 12.8 months of age, rather than at birth in the current study.
Results from two smaller studies were consistent with our findings (Suzuki et al., 2012; Bustamante-Montes et al., 2013), while one was not (Huang et al., 2008). A recent study reported significant inverse associations between male AGD and two of three DINP metabolites, as well as the sum of DINP metabolites, in 196 Swedish infants at 21 weeks of age (Bornehag et al., 2014). In TIDES we measured only one DINP metabolite (MCOP), which was not associated with boys' AGD. In the Swedish study this metabolite was associated with a modest decrease in boy's AGD. In addition, DEHP metabolites were less strongly (and not significantly) associated with shorter boys' AGD in the Swedish study than in TIDES even though metabolites concentrations were similar in the two populations. We have no explanation for these between-study differences, which suggest the need for further replication.
We did not expect to see associations between these anti-androgens and female AGD, which is lengthened by prenatal testosterone exposure, but unchanged by anti-androgen exposure in rodent models (Hotchkiss et al., 2007). Only one prior study has examined prenatal phthalate exposure and female AGD in humans (Huang et al., 2008). That study reported a significant inverse association between MBP concentration in amniotic fluid at amniocentesis and the anogenital index (AGD divided by weight) in 31 females at birth. Few rodent studies have examined prenatal exposure to DEHP or DBP in relation to female AGD. One such study (Grande et al., 2007) found a significant delay in the age at vaginal opening associated with moderate DEHP exposure prenatally but no change in AGD. A recent rodent study suggested that early prenatal phthalate exposure to high doses of DBP may result in a ‘female phthalate syndrome’ with features similar to Mayer–Rokitansky–Kuster–Hauser (MRKH) syndrome in women, a condition characterized by uterine and vaginal canal aplasia (Hannas et al., 2013). However, these were not end-points that we could examine in TIDES, nor were they included in any other human study of prenatal phthalate exposure.
Because concentrations of most phthalate metabolites were low in this population, we cannot rule out associations between higher phthalate exposure and female AGD, or between male AGD and phthalates that were not associated in this study, including metabolites of DBP (Fisher, 2004) and DINP (Boberg et al., 2011), compounds that have been shown to reduce AGD and cause features of the ‘phthalate syndrome’ following high dose exposure in rats. In fact, a recent Swedish study found inverse associations between early pregnancy exposure to DINP at environmental levels and male AGD at 21 weeks (Bornehag et al., 2014).
Potential limitations of our study include an exposure assessment based on a single first trimester sample, which may have resulted in exposure misclassification. Our population was relatively homogeneous, and largely Caucasian, limiting our ability to examine race/ethnicity in relation to exposure and outcome. The use of two different laboratories for phthalate analysis, with slightly different analytic methods and limits of detection may have increased variability in metabolite concentrations. However, since all samples from mothers of boys were measured at the CDC laboratory, these differences did not affect associations between measurements in boys and phthalate metabolite concentration.
In addition, though we controlled for center in multivariable models, between-center differences suggest that this measurement is difficult to standardize. In a prior TIDES publication, we examined predictors of AGD measurements, including center (Sathyanarayana et al.). For two measures (AGDAS and AGDAC), none of the center differences were significant, while for AGDAP and AGDAF, between-center differences were 1–2 mm, with no consistent pattern seen across centers. While we adjusted for center in our multivariable regression models, we also considered possible modification of associations by center. To address this question we ran our final multivariable models including terms for the interaction of center and (SpG-adjusted log10) phthalate metabolite concentration. Only one of the 24 interaction terms was statistically significant. We also examined homogeneity of variance of each AGD measurements across centers using Levene's test (Levene, 1960) which showed no heterogeneity of variance (P-values were 0.256, 0.247, 0.143 and 0.372 for AGDAS AGDAP, AGDAF and AGDAC, respectively).
We have identified associations between male genital end-points at birth and measures of first trimester DEHP exposure. In the future it will be important to examine potential downstream clinical sequelae of these findings in later childhood and adulthood. Animal studies suggest that shorter AGD at birth is permanent and results in significant lifelong consequences. In rodent studies, shorter male AGD predicts altered function in puberty and adulthood, including decreased sperm production (Foster, 2006) and fertility (Akingbemi et al., 2004; Barlow et al., 2004), as well as testicular tumors (Akingbemi et al., 2004). Recent studies report reproductive correlates of shorter male AGD in humans as well, suggesting potentially important clinical implications of shorter male AGD in infants. These include shorter AGD among infants with cryptorchidism and/or hypospadias (Hsieh et al., 2008, 2012; Jain and Singal, 2013) and significant associations between shorter male AGD and reduced semen quality (Eisenberg et al., 2011; Mendiola et al., 2011), infertility (Eisenberg et al., 2011), lower testosterone and smaller testicular volume (Eisenberg et al., 2012) in adult men. Smaller AGD was also seen in prostate cancer cases, compared with healthy controls (Castano-Vinyals et al., 2012).
In summary, we report inverse associations between first trimester exposure to DEHP and AGD in newborn boys, with no associations in girls. These findings are consistent with an extensive rodent literature and most human studies. The data we report here suggest that even at current low levels, environmental exposure to DEHP may affect male genital development resulting in reproductive tract changes with potentially significant health consequences for these boys as they mature. These findings have important implications for public policy since virtually all pregnant women and infants are exposed to this ubiquitous chemical.
Authors' roles
S.H.S. contributed to the conception and design of the study, the acquisition of data and the analysis/interpretation of data. She was also responsible for drafting and revising the manuscript. S.S., E.S.B., S.J., and J.B.R. contributed to conception and design of the study, data acquisition, analysis and interpretation of data and manuscript revisions. F.L. was responsible for data management and contributed to analysis. R.H.N.N. contributed to data interpretation and manuscript revision. All authors reviewed and approved the final manuscript. S.H.S. is the guarantor and takes full responsibility for the work as a whole.
Funding
Funding for TIDES was provided by the following grants from the National Institute of Environmental Health Sciences: R01ES016863-04 and R01 ES016863-02S4.
Conflict of interest
All authors declare they have no potential conflicts of interest including any relevant financial interests, activities, relationships and affiliations.
Acknowledgements
We wish to acknowledge the contributions of the TIDES Study Team: Coordinating Center: Fan Liu, Erica Scher; UCSF: Marina Stasenko, Erin Ayash, Melissa Schirmer, Jason Farrell, Mari-Paule Thiet, Laurence Baskin; UMN: Heather L. Gray Chelsea Georgesen, Brooke J. Rody, Carrie A. Terrell, Kapilmeet Kaur; URMC: Erin Brantley, Heather Fiore, Lynda Kochman, Lauren Parlett, Jessica Marino, William Hulbert, Robert Mevorach, Eva Pressman; UW/SCH: Kristy Ivicek, Bobbie Salveson, Garry Alcedo and the families who participated in the study. We thank Antonia Calafat (Center for Disease Control) for urinary phthalate metabolite analyses, Dr Sally Thurston (URMC) for statistical advice, the TIDES families for their participation and the residents at URMC and UCSF who assisted in birth exams.
References
- Akingbemi BT, Ge R, Klinefelter GR, Zirkin BR, Hardy MP. Phthalate-induced Leydig cell hyperplasia is associated with multiple endocrine disturbances. Proc Natl Acad Sci USA. 2004;101:775–780. doi: 10.1073/pnas.0305977101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for di-n-butyl Phthalate. Atlanta, GA: Agency for Toxic Substances and Disease Registry, Division of Toxicology; 2001. Anonymous (ed) (2002) [PubMed] [Google Scholar]
- Barlow NJ, McIntyre BS, Foster PM. Male reproductive tract lesions at 6, 12, and 18 months of age following in utero exposure to di(n-butyl) phthalate. Toxicol Pathol. 2004;32:79–90. doi: 10.1080/01926230490265894. [DOI] [PubMed] [Google Scholar]
- Boberg J, Christiansen S, Axelstad M, Kledal TS, Vinggaard AM, Dalgaard M, Nellemann C, Hass U. Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod Toxicol. 2011;31:200–209. doi: 10.1016/j.reprotox.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54:615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Carlstedt F, Jonsson BA, Lindh CH, Jensen TK, Bodin A, Jonsson C, Janson S, Swan SH. Prenatal phthalate exposures and anogenital distance in Swedish boys. Environ Health Perspect. 2014;123:101–107. doi: 10.1289/ehp.1408163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante-Montes L, Hernandez-Valero M, Flores-Pimentel D, Garcia-Fabila M, Amaya-Chavez A, Barr D, Borja-Aburto V. Prenatal exposure to phthalates is associated with decreased anogenital distance and penile size in male newborns. J Dev Orig Health Dis. 2013;4:300–306. doi: 10.1017/S2040174413000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM. Phthalate Metabolites Method 6306.03. Atlanta, GA: Centers for Disease Control and Prevention; 2010. [Google Scholar]
- Callegari C, Everett S, Ross M, Brasel JA. Anogenital ratio: measure of fetal virilization in premature and full-term newborn infants. J Pediatr. 1987;111:240–243. doi: 10.1016/s0022-3476(87)80075-6. [DOI] [PubMed] [Google Scholar]
- Castano-Vinyals G, Carrasco E, Lorente JA, Sabate Y, Cirac-Claveras J, Pollan M, Kogevinas M. Anogenital distance and the risk of prostate cancer. BJU Int. 2012;110:E707–E710. doi: 10.1111/j.1464-410X.2012.11516.x. [DOI] [PubMed] [Google Scholar]
- CDC. Atlanta, GA: 2013. Fourth National Report on Human Exposure to Environmental Chemicals: Updated Tables. [Google Scholar]
- Chen H, Quandt SA, Grzywacz JG, Arcury TA. A distribution-based multiple imputation method for handling bivariate pesticide data with values below the limit of detection. Environ Health Perspect. 2011;119:351–356. doi: 10.1289/ehp.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Hsieh MH, Walters RC, Krasnow R, Lipshultz LI. The relationship between anogenital distance, fatherhood, and fertility in adult men. PLoS One. 2011;6:e18973. doi: 10.1371/journal.pone.0018973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Jensen TK, Walters RC, Skakkebaek NE, Lipshultz LI. The relationship between anogenital distance and reproductive hormone levels in adult men. J Urol. 2012;187:594–598. doi: 10.1016/j.juro.2011.10.041. [DOI] [PubMed] [Google Scholar]
- Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- Foster PMD. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2005;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Foster PMD, Mylchreest E, Gaido K, Sar M. Effects of phthalate esters on the developing reproductive tract of male rats, ed. Anonymous (Proceedings of the RH Workshop, Hormones and endocrine disrupters in food and water: possible impact on human health; 2000 May 27–30) 2000;37.
- Grande SW, Andrade AJ, Talsness CE, Grote K, Golombiewski A, Sterner-Kock A, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): reproductive effects on adult female offspring rats. Toxicology. 2007;229:114–122. doi: 10.1016/j.tox.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Gray LEJ, Ostby J, Furr J, Price M, Veeramachaneni DNR, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Gray LE, Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, Howdeshell K, Ankley GT, Guillette L. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int J Androl. 2006;29:96–104. doi: 10.1111/j.1365-2605.2005.00636.x. discussion 05-8-96-04; discussion 05-8. [DOI] [PubMed] [Google Scholar]
- Hannas BR, Howdeshell KL, Furr J, Gray LE., Jr In utero phthalate effects in the female rat: a model for MRKH syndrome. Toxicol Lett. 2013;223:315–321. doi: 10.1016/j.toxlet.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsel DR. Nondetections and Data Analysis: Statistics for Censored Environmental Data. Hoboken, NJ: John Wiley and Sons; 2005. [Google Scholar]
- Hewett P, Ganser GH. A comparison of several methods for analyzing censored data. Ann Occup Hyg. 2007;51:611–632. doi: 10.1093/annhyg/mem045. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Hotchkiss AK, Lambright CS, Ostby JS, Parks-Saldutti L, Vandenbergh JG, Gray LE., Jr Prenatal testosterone exposure permanently masculinizes anogenital distance, nipple development, and reproductive tract morphology in female Sprague-Dawley rats. Toxicol Sci. 2007;96:335–345. doi: 10.1093/toxsci/kfm002. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, Breyer BN, Eisenberg ML, Baskin LS. Associations among hypospadias, cryptorchidism, anogenital distance, and endocrine disruption. Curr Urol Rep. 2008;9:137–142. doi: 10.1007/s11934-008-0025-0. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, Eisenberg ML, Hittelman AB, Wilson JM, Tasian GE, Baskin LS. Caucasian male infants and boys with hypospadias exhibit reduced anogenital distance. Hum Reprod. 2012;27:1577–1580. doi: 10.1093/humrep/des087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Chou YY, Lin SJ, Lee CC. Association between prenatal exposure to phthalates and the health of newborns. Environ Int. 2008;35:14–20. doi: 10.1016/j.envint.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Jain VG, Singal AK. Shorter anogenital distance correlates with undescended testis: a detailed genital anthropometric analysis in human newborns. Hum Reprod. 2013;28:2343–2349. doi: 10.1093/humrep/det286. [DOI] [PubMed] [Google Scholar]
- Levene H. In: Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling. Olkin I, editor. Stanford, CA: Stanford University Press; 1960. pp. 278–292. [Google Scholar]
- Macleod DJ, Sharpe RM, Welsh M, Fisken M, Scott HM, Hutchison GR, Drake AJ, van den Driesche S. Androgen action in the masculinization programming window and development of male reproductive organs. Int J Androl. 2010;33:279–287. doi: 10.1111/j.1365-2605.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Stahlhut RW, Jorgensen N, Liu F, Swan SH. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect. 2011;119:958–963. doi: 10.1289/ehp.1103421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Roca M, Minguez-Alarcon L, Mira-Escolano MP, Lopez-Espin JJ, Barrett ES, Swan SH, Torres-Cantero AM. Anogenital distance is related to ovarian follicular number in young Spanish women: a cross-sectional study. Environ Health. 2012;11:90. doi: 10.1186/1476-069X-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multicentre Growth Reference Study Group, W. WHO Child Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference: Methods and Development. Geneva: WHO; 2009. [Google Scholar]
- Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol. 2003;37:4543–4553. doi: 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Grady R, Redmon B, Ivicek K, Barrett E, Janssen S, Swan SH TIDES Study Team. doi: 10.1016/j.jpurol.2014.11.018. Anogenital distance and penile width measurements in The Infant Development and the Environment Study (TIDES): methods and predictors, Pediatric Urology. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860:106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int J Androl. 2012;35:236–244. doi: 10.1111/j.1365-2605.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thankamony A, Lek N, Carroll D, Williams M, Dunger DB, Acerini CL, Ong KK, Hughes IA. Anogenital distance and penile length in infants with hypospadias or cryptorchidism: comparison with normative data. Environ Health Perspect. 2013;122:207–211. doi: 10.1289/ehp.1307178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA. Guidelines for Reproductive Toxicity Risk Assessment (61: Federal Register) 1996. pp. 56274–56322.
- Welsh M, Saunders PTK, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008;118:1479–1490. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. WHO Child Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference. WHO Press; 2009. [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lin L, Cao Y, Chen B, Zheng L, Ge RS. Phthalate levels and low birth weight: a nested case-control study of Chinese newborns. J Pediatr. 2009;155:500–504. doi: 10.1016/j.jpeds.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Calafat AH, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect. 2014;122:235–241. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]