Abstract
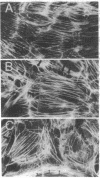
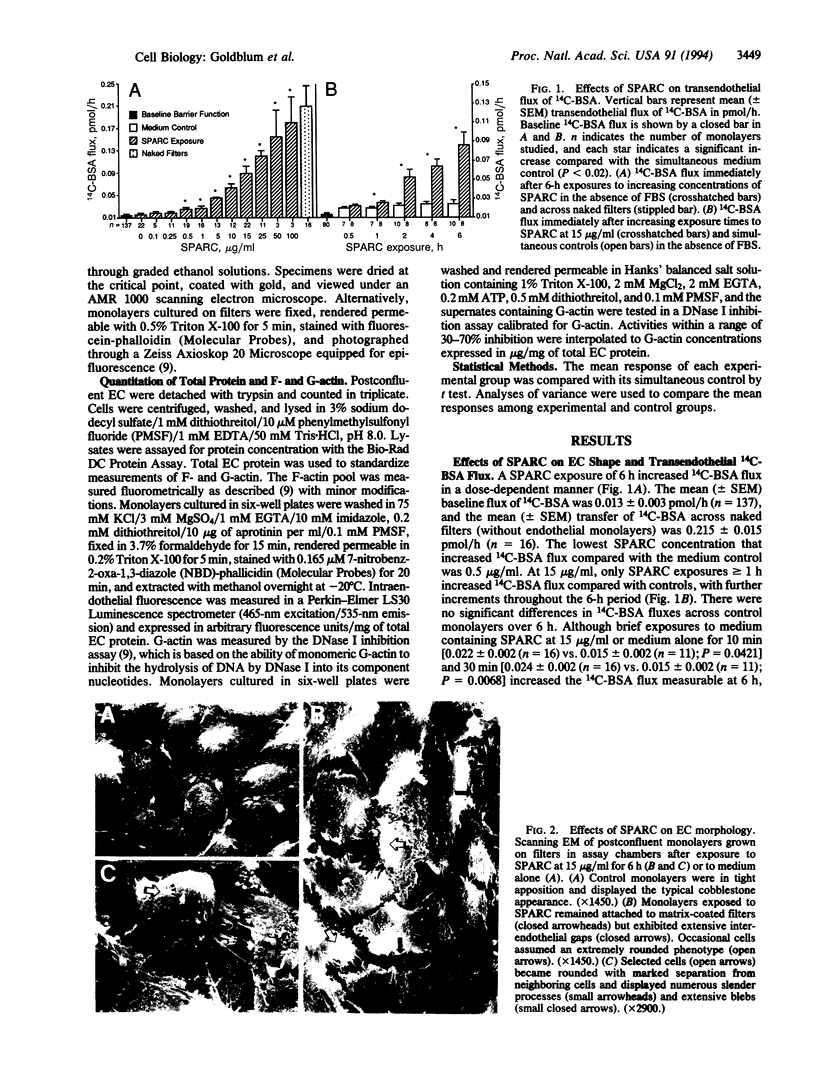
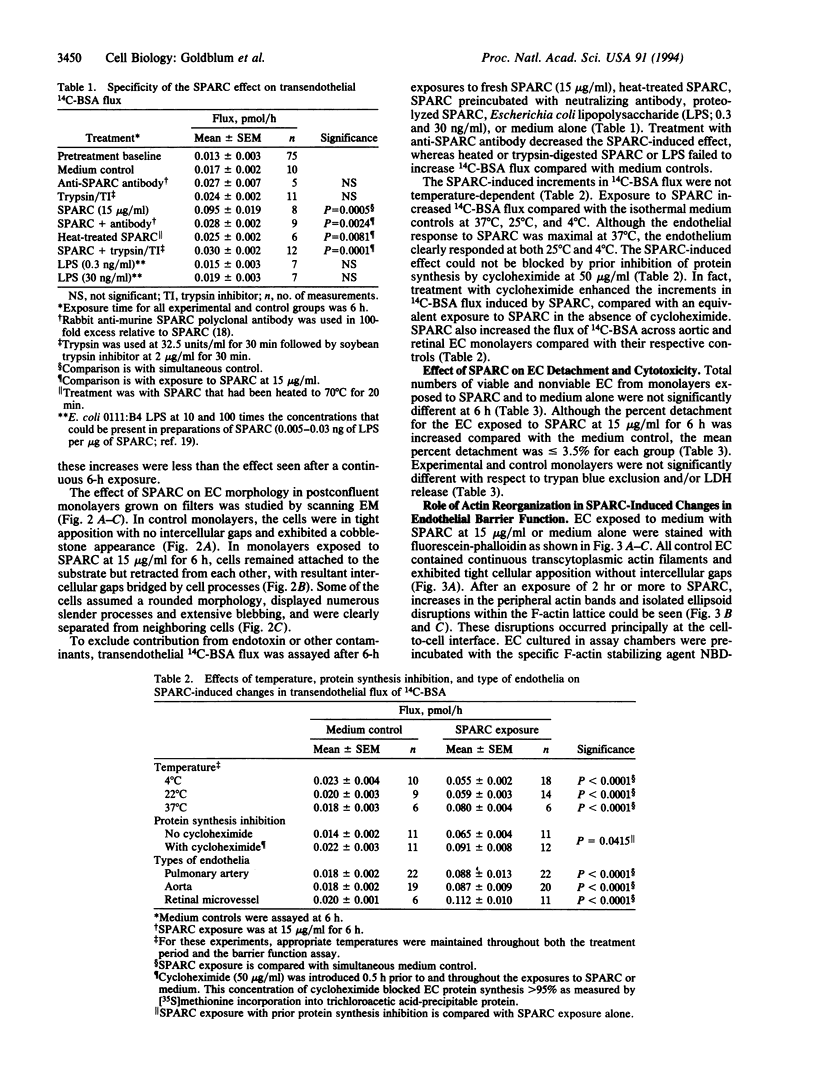
SPARC (secreted protein acidic and rich in cysteine) can be selectively expressed by the endothelium in response to certain types of injury and induces rounding in adherent endothelial cells in vitro. To determine whether SPARC might influence endothelial permeability, we studied the effect of exogenous SPARC on the movement of 14C-labeled bovine serum albumin across postconfluent bovine pulmonary artery endothelial cells. SPARC increased (P < 0.02) transendothelial albumin flux in a dose-dependent manner at concentrations > or = 0.5 microgram/ml. At a fixed dose (15 micrograms/ml), exposure times > or = 1 h augmented (P < 0.005) albumin flux by 1.3- to 3.6-fold; this increase was blocked by anti-SPARC antibodies but not by inhibition of protein synthesis. Barrier dysfunction was not associated with loss of cell viability. Monolayers exposed to SPARC exhibited a rounded morphology and intercellular gaps. Prior stabilization of F-actin with phallicidin protected against the changes in barrier function (P = 0.0001) that were otherwise induced by SPARC. Bovine aortic and retinal microvascular endothelia also responded to SPARC. We propose that SPARC regulates endothelial barrier function through F-actin-dependent changes in cell shape, coincident with the appearance of intercellular gaps, that provide a paracellular pathway for extravasation of macromolecules.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Sampson P. M., Haselton F. R., McNiff J. M., Mueller S. N., Williams S. K., Fishman A. P., Levine E. M. Permeability characteristics of cultured endothelial cell monolayers. J Appl Physiol (1985) 1988 Jan;64(1):308–322. doi: 10.1152/jappl.1988.64.1.308. [DOI] [PubMed] [Google Scholar]
- Alexander J. S., Hechtman H. B., Shepro D. Phalloidin enhances endothelial barrier function and reduces inflammatory permeability in vitro. Microvasc Res. 1988 May;35(3):308–315. doi: 10.1016/0026-2862(88)90085-4. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis. 1986 May;133(5):913–927. [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Camussi G., Aglietta M., Braquet P., Bosia A., Pescarmona G., Sanavio F., D'Urso N., Marchisio P. C. Human endothelial cells are target for platelet-activating factor. I. Platelet-activating factor induces changes in cytoskeleton structures. J Immunol. 1987 Oct 1;139(7):2439–2446. [PubMed] [Google Scholar]
- Goldblum S. E., Ding X., Campbell-Washington J. TNF-alpha induces endothelial cell F-actin depolymerization, new actin synthesis, and barrier dysfunction. Am J Physiol. 1993 Apr;264(4 Pt 1):C894–C905. doi: 10.1152/ajpcell.1993.264.4.C894. [DOI] [PubMed] [Google Scholar]
- Goldblum S. E., Sun W. L. Tumor necrosis factor-alpha augments pulmonary arterial transendothelial albumin flux in vitro. Am J Physiol. 1990 Feb;258(2 Pt 1):L57–L67. doi: 10.1152/ajplung.1990.258.2.L57. [DOI] [PubMed] [Google Scholar]
- Hasselaar P., Loskutoff D. J., Sawdey M., Sage E. H. SPARC induces the expression of type 1 plasminogen activator inhibitor in cultured bovine aortic endothelial cells. J Biol Chem. 1991 Jul 15;266(20):13178–13184. [PubMed] [Google Scholar]
- Kelm R. J., Jr, Mann K. G. Human platelet osteonectin: release, surface expression, and partial characterization. Blood. 1990 Mar 1;75(5):1105–1113. [PubMed] [Google Scholar]
- Lane T. F., Sage E. H. Functional mapping of SPARC: peptides from two distinct Ca+(+)-binding sites modulate cell shape. J Cell Biol. 1990 Dec;111(6 Pt 2):3065–3076. doi: 10.1083/jcb.111.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterra J., Goldstein G. W. Astroglial-induced in vitro angiogenesis: requirements for RNA and protein synthesis. J Neurochem. 1991 Oct;57(4):1231–1239. doi: 10.1111/j.1471-4159.1991.tb08284.x. [DOI] [PubMed] [Google Scholar]
- Mason I. J., Murphy D., Münke M., Francke U., Elliott R. W., Hogan B. L. Developmental and transformation-sensitive expression of the Sparc gene on mouse chromosome 11. EMBO J. 1986 Aug;5(8):1831–1837. doi: 10.1002/j.1460-2075.1986.tb04434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B. O., Ryan U. S., Brigham K. L. Direct effects of E coli endotoxin on structure and permeability of pulmonary endothelial monolayers and the endothelial layer of intimal explants. Am J Pathol. 1986 Jan;122(1):140–151. [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Lightner V. A., Aukhil I., Yan Y. Z., Erickson H. P., Hök M. Focal adhesion integrity is downregulated by the alternatively spliced domain of human tenascin. J Cell Biol. 1991 Nov;115(4):1127–1136. doi: 10.1083/jcb.115.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P. G., Lum H., Malik A. B., Tsan M. F. Phallacidin prevents thrombin-induced increases in endothelial permeability to albumin. Am J Physiol. 1989 Sep;257(3 Pt 1):C562–C567. doi: 10.1152/ajpcell.1989.257.3.C562. [DOI] [PubMed] [Google Scholar]
- Pober J. S. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium. Physiology and pathology. Am J Pathol. 1988 Dec;133(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- Rinaldo J. E., Rogers R. M. Adult respiratory-distress syndrome: changing concepts of lung injury and repair. N Engl J Med. 1982 Apr 15;306(15):900–909. doi: 10.1056/NEJM198204153061504. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986 Dec;103(6 Pt 1):2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage E. H., Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991 Aug 15;266(23):14831–14834. [PubMed] [Google Scholar]
- Sage H., Johnson C., Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem. 1984 Mar 25;259(6):3993–4007. [PubMed] [Google Scholar]
- Sage H., Tupper J., Bramson R. Endothelial cell injury in vitro is associated with increased secretion of an Mr 43,000 glycoprotein ligand. J Cell Physiol. 1986 Jun;127(3):373–387. doi: 10.1002/jcp.1041270305. [DOI] [PubMed] [Google Scholar]
- Sage H., Vernon R. B., Decker J., Funk S., Iruela-Arispe M. L. Distribution of the calcium-binding protein SPARC in tissues of embryonic and adult mice. J Histochem Cytochem. 1989 Jun;37(6):819–829. doi: 10.1177/37.6.2723400. [DOI] [PubMed] [Google Scholar]
- Sage H., Vernon R. B., Funk S. E., Everitt E. A., Angello J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J Cell Biol. 1989 Jul;109(1):341–356. doi: 10.1083/jcb.109.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittler H. J., Wilke A., Gress T., Suttorp N., Drenckhahn D. Role of actin and myosin in the control of paracellular permeability in pig, rat and human vascular endothelium. J Physiol. 1990 Dec;431:379–401. doi: 10.1113/jphysiol.1990.sp018335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Siflinger-Birnboim A., Del Vecchio P. J., Cooper J. A., Blumenstock F. A., Shepard J. M., Malik A. B. Molecular sieving characteristics of the cultured endothelial monolayer. J Cell Physiol. 1987 Jul;132(1):111–117. doi: 10.1002/jcp.1041320115. [DOI] [PubMed] [Google Scholar]
- Stenner D. D., Tracy R. P., Riggs B. L., Mann K. G. Human platelets contain and secrete osteonectin, a major protein of mineralized bone. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6892–6896. doi: 10.1073/pnas.83.18.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttorp N., Polley M., Seybold J., Schnittler H., Seeger W., Grimminger F., Aktories K. Adenosine diphosphate-ribosylation of G-actin by botulinum C2 toxin increases endothelial permeability in vitro. J Clin Invest. 1991 May;87(5):1575–1584. doi: 10.1172/JCI115171. [DOI] [PMC free article] [PubMed] [Google Scholar]