Abstract
Background
Pneumocystis pneumonia (PCP) may develop as a clinical manifestation of nosocomial pneumonia by means of either reactivation of resident P. jirovecii or de novo infection. However, there have been no studies describing the clinical characteristics of hospital-onset PCP.
Methods
A retrospective review of medical records was performed to identify episodes of hospital-onset PCP in a tertiary care centre in Korea between May 2007 and January 2013. We investigated whether human-to-human contact during hospitalisation contributed to PCP development by molecular analysis of the genes encoding mitochondrial large ribosomal subunit (mtLSU) rRNA and dihydropteroate synthase (DHPS) and a review of hospitalisation history.
Results
During the study period, 129 patients (130 episodes) were diagnosed with PCP. Of these, respiratory specimens from 94 patients during 95 PCP episodes were available for analysis. Sixteen episodes (16.8%) were categorised as hospital-onset PCP. There was a trend toward a higher proportion of haematological malignancy (43.8% [7/16] vs. 20.3% [16/79]; P = 0.058) in patients with hospital-onset PCP compared to patients with community-onset PCP. mtLSU genotype 1 was the most common, occurring in 41 (43.2%) patients. There were four possible cases of nosocomial transmission. Mutation in DHPS was not observed in any PCP episode.
Conclusions
PCP can be one of the causes of nosocomial pneumonia, although the mode of acquisition and transmission of P. jirovecii remains uncertain. mtLSU genotype 1 is the predominant P. jirovecii strain in Korea.
Keywords: Pneumocystis pneumonia, Large ribosomal subunit of mitochondrial rRNA, Transmission, Epidemiology
Background
Pneumocystis pneumonia (PCP) is a major opportunistic infection in immunocompromised patients principally acquired and transmitted via an airborne route [1]. It has been proposed previously that Pneumocystis jirovecii infects the host during childhood, becomes part of the resident microbial flora and remains latent for extended periods, reactivating when the host becomes immunocompromised. This theory is based on the observation that most humans become seropositive for P. jirovecii early in life [2], and that the colonisation of P. jirovecii in animal models persists over long periods of time [3]. By contrast, other researchers suggest that P. jirovecii infects transiently and that active transmission is possible. This idea is supported by early clearance of P. jirovecii from the lungs of adult animals [4] and detection of mutations in dihydropteroate synthase (DHPS) in human immunodeficiency virus (HIV)-infected patients without prior sulfamethoxazole prophylaxis [5]. Recent reports of clusters or outbreaks of a single genetic strain of P. jirovecii also suggest de novo infection by human-to-human transmission [6-10].
Based on these previous observations, PCP may develop as a clinical manifestation of nosocomial pneumonia by means of either reactivation of resident P. jirovecii or de novo infection [11]. Damiani et al. provided additional data supporting P. jirovecii exhalation by infected patients, showing that a full match of P. jirovecii genotypes was found for 4 (57.1%) pairs of pulmonary and room air samples [7]. Therefore, de novo infection by human-to-human transmission may be a cause of some of PCP development during hospitalisation. Thus, it is necessary to apply measures preventing airborne transmission of P. jirovecii in hospitals.
In this study, we retrospectively identified the episodes of hospital-onset PCP. We performed a molecular analysis of the genes encoding mitochondrial large ribosomal subunit (mtLSU) rRNA and DHPS and a review of hospitalisation history of patients with PCP to investigate whether human-to-human contact during hospitalisation contributed to PCP development.
Methods
Ethics statement
This work was approved by the Research Ethics Committee at Asan Medical Centre, Seoul, Republic of Korea. Informed consent was waived by the Institutional Review Board of Asan Medical Center since this work was a retrospective study without intervention and did not involve extra clinical specimens.
Study design and patients
This study was performed at Asan Medical Center, a 2,700-bed tertiary care teaching hospital, from May 1, 2007 to January 31, 2013. The study included patients with compatible symptoms and radiological findings confirmed as PCP by a direct immunofluorescence assay (Light Diagnostics™ Pneumocystis carinii DFA Kit, Millipore, Billerica, MA, USA) using respiratory specimens. We excluded patients whose respiratory specimens were not available for molecular analysis. Respiratory specimens were not collected between September 1, 2009 and September 31, 2011 because the principal investigator was on sabbatical leave. During a retrospective medical review, we identified episodes of hospital-onset PCP which was defined as pneumonia arising more than 5 days after admission when no signs and symptoms compatible with PCP were documented at the time of admission. The 5 day-cutoff for diagnosing hospital-onset PCP is same as that used in previous studies of late-onset ventilator-associated pneumonia [12]. Other patients were considered to have community-onset PCP.
Data collection
The patients’ medical records were reviewed retrospectively, and clinical information was collected, including demographics, underlying diseases and conditions, hospitalisation history, reason for admission, signs and symptoms of PCP, radiological findings, history of prior prophylaxis against PCP, laboratory findings (neutrophil and lymphocyte counts in bronchoalveolar lavage [BAL] fluid, absolute neutrophil and lymphocyte counts, lactate dehydrogenase, and C-reactive protein), initial severity of PCP, treatment regimens and response, need for mechanical ventilation and 30-day mortality from the time of initial PCP diagnosis. Using this information, we compared the clinical characteristics of patients with hospital-onset and community-onset PCP. A possible case of nosocomial transmission was defined as a patient with a history of admission to the same ward in which another patient infected with a genetically identical strain of P. jirovecii, determined by mtLSU and DHPS sequencing, had stayed.
Genotyping
The majority of specimens (n = 92) were acquired through from BAL obtained using a fibre-optic bronchoscope and standard techniques. Three specimens obtained by endotracheal aspiration. We performed a retrospective molecular analysis of the P. jirovecii mtLSU and DHPS loci. BAL specimens (350 μl) were treated with proteinase K and DNA was extracted using QIAamp DNA Stool Mini Kits (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The single-copy DHPS gene was amplified by nested PCR using the primers F1 and B45 (first round) and AHUM and BN (second round) in all positive samples, as described previously [13,14]. mtLSU rRNA gene was amplified by nested PCR using the primers pAZ102-E and pAZ102-H (first round) and pAZ102-X and pAZ102-Y (second round) in all samples, as described previously [2,15]. Amplicons were purified using a Power Gel Extraction kit (TaKaRa Bio Inc., Shiga, Japan) and directly sequenced on an ABI Prism 3130xl genetic analyser (Applied Biosystems, Foster City, CA, USA) using a BigDye Terminator v. 3.1 cycle sequencing kit (Applied Biosystems). DHPS genotypes were as follows: genotype 1, 165A and 171C (resulting in Thr and Pro); genotype 2, 165G and 171C (resulting in Ala and Pro); genotype 3, 165A and 171T (resulting in Thr and Ser); and genotype 4, 165G and 171T (resulting in Ala and Ser). mtLSU genotypes were as follows: genotype 1 = 85C/248C, 2 = 85A/248C, 3 = 85T/248C and 4 = 85C/248T [16].
Statistical analyses
Chi-square or Fisher’s exact test was used to compare categorical variables, and either Student’s t-test or the Mann–Whitney U-test was used to compare continuous variables, as appropriate. Data were analysed using SPSS for Windows, version 15.0 (SPSS Inc., Chicago, IL, USA). A value of P <0.05 was taken to indicate statistical significance.
Results
Identification of hospital-onset PCP
During the study period, a total of 130 PCP episodes were documented in 129 patients. There were 95 episodes between May 2007 and August 2009, 54 during the interruption period, and 35 between October 2011 and January 2013. Of these, adequate specimens for the study were available for 94 patients experiencing 95 episodes: 70 (73.7%; out of 95 episodes) obtained between May 2007 and August 2009, and 25 (71.4%; out of 35 episodes) obtained between October 2011 and January 2013. In one patient with recurrent episodes, PCP developed in both June 2008 and April 2009. Sixteen patients were categorised as having hospital-onset PCP (Table 1). At the time of admission, none of the patients had signs or symptoms compatible with PCP, and all were admitted as a result of problems not associated with the respiratory system. The median number of days from admission to diagnosis of PCP was 38 (range, 18–63 days).
Table 1.
Characteristics of patients with hospital-onset and community-onset PCP
| Characteristics | Hospital-onset PCP * | Community-onset PCP | P | Characteristics | Hospital-onset PCP * | Community-onset PCP | P |
|---|---|---|---|---|---|---|---|
| (n = 16) | (n = 79) | (n = 16) | (n = 79) | ||||
| Gender, male | 9 (56.3) | 51 (64.6) | 0.58 | Interstitial lung disease | 0 (0.0) | 8 (10.1) | 0.34 |
| Age, median years (IQR) | 42 (27–59) | 52 (39 to 63) | 0.13 | Connective tissue disease | 1 (6.3) | 4 (5.1) | 1.00 |
| Genotype | Others† | 2 (12.5) | 5 (6.3) | 0.34 | |||
| 1 | 8 (46.7) | 33 (41.7) | 0.58 | History of priory exposure to SMX more than 3 months | 2 (12.5) | 23 (29.1) | 0.22 |
| 2 | 2 (12.5) | 3 (3.8) | 0.20 | Days of BAL from admission, median (IQR) | 38 (18 to 63) | 2 (1 to 5) | <0.001 |
| 3 | 2 (12.5) | 19 (24.1) | 0.51 | Days of BAL from last chemotherapy, median (IQR)‡ | 17 (9 to 31) | 19 (15 to 30) | 0.44 |
| 4 | 0 (0.0) | 1 (1.3) | 1.00 | BAL neutrophil, median cells/mm3 (IQR) | 11 (0 to 80) | 19 (7 to 62) | 0.43 |
| Mixed | 4 (25.0) | 23 (29.1) | 1.00 | BAL lymphocyte, median cells/mm3 (IQR) | 50 (10 to 177) | 48 (12 to 145) | 0.84 |
| 1 and 2 | 1 (6.3) | 12 (15.2) | 0.69 | ANC, median cells/mm3 (IQR) | 5053 (883 to 8863) | 5968 (3818 to 9007) | 0.18 |
| 1 and 3 | 2 (12.5) | 9 (11.4) | 1.00 | ALC, median cells/mm3 (IQR) | 416 (130 to 1000) | 648 (331 to 1108) | 0.11 |
| 2 and 3 | 1 (6.3) | 2 (2.5) | 0.43 | LDH, median IU/L (IQR) | 364 (240 to 632) | 448 (330 to 620) | 0.35 |
| Underlying conditions | CRP, median mg/dL (IQR) | 10.2 (4.5 to 14.2) | 9.0 (4.0 to 20.3) | 0.43 | |||
| HIV infection | 0 (0.0) | 13 (16.5) | 0.12 | Initial severity at diagnosis§ | |||
| Transplantation | Severe | 11 (68.8) | 64 (81.0) | 0.32 | |||
| HSCT | 3 (18.8) | 6 (7.6) | 0.17 | Treatment | |||
| SOT | 3 (18.8) | 19 (23.1) | 0.76 | TMP/SMX usage as initial treatment | 14 (93.8) | 79 (100) | 0.17 |
| Malignancy | Treatment failure to initial regimen¶ | 6 (37.5) | 23 (29.1) | 0.57 | |||
| Solid tumour | 0 (0.0) | 8 (10.1) | 0.34 | Mechanical ventilation | 11 (68.8) | 42 (53.2) | 0.28 |
| Haematologic | 7 (43.8) | 16 (20.3) | 0.06 | 30-day mortality | 7 (43.8) | 18 (22.8) | 0.12 |
Data are numbers (%) of patients, unless otherwise indicated.
*Hospital-onset PCP was defined as pneumonia arising more than 5 days after admission when no signs and symptoms compatible with PCP were documented at the time of admission. Other patients were considered to have community-onset PCP.
†Autoimmune haemolytic anaemia and Steven-Johnson’s syndrome in patients with hospital-onset PCP. Henoch-Schönlein purpura, severe combined immunodeficiency, idiopathic thrombocytopenic purpura, ulcerative colitis, and unspecified glomerulonephritis in patients with community-onset PCP.
‡It was checked only in patients with a haematologic malignancy on chemotherapy.
§Severe PCP was defined as partial arterial oxygen pressure <60 mmHg while breathing room air or an alveolar-arterial oxygen difference ≥45.
¶Treatment failure was defined as one of the following situations: (1) progressive clinical deterioration as demonstrated by the inability to maintain a stable partial pressure of arterial oxygen despite an increase in the fraction of inspired oxygen, or (2) progressive deterioration of vital signs with a requirement for an increased fraction of inspired oxygen after 7 days of therapy.
ALC, absolute lymphocyte count; ANC, absolute neutrophil count; BAL, bronchoalveolar lavage; CRP, C-reactive protein; HSCT, haematopoietic stem cell transplantation; IQR, interquartile range; LDH, lactate dehydrogenase; PCP, Pneumocystis jirovecii pneumonia; SOT, solid organ transplantation; TMP/SMX, trimethoprim/sulfamethoxazole.
Clinical characteristics of patients with hospital-onset community-onset PCP
Malignancy and transplantation were common underlying diseases and conditions. All patients with connective tissue disease or interstitial lung disease were on immunosuppression. Trimethoprim/sulfamethoxazole was chosen as the initial regimen in almost all patients. Among these patients, 30.5% (29/95) failed to respond to the initial therapy. The 30-day all-cause mortality rate was 26.3% (25/95). A comparison of the clinical characteristics of patients with hospital-onset and community-onset PCP demonstrated no statistically significant differences (Table 1) except a trend toward a higher proportion of haematological malignancy as an underlying disease (hospital-onset PCP, 43.8% [7/16] vs. community-onset, 20.3% [16/79]; P = 0.058) and a higher 30-day mortality in patients with PCP developing during hospitalisation (hospital-onset PCP, 43.8% [7/16] vs. community-onset, 22.8% [18/79]; P = 0.12). To adjust for a possible confounding effect on outcome, we performed a subgroup analysis of patients with haematological malignancy. There was no statistically significant difference between the groups, possibly due to the small number of patients (hospital-onset PCP, 3/7 [42.9%] vs. community-onset, 2/16 [12.5%]; P = 0.14).
Possible nosocomial transmission of P. jirovecii
We identified four possible cases of nosocomial transmission (Figure 1). Of these, three patients with hospital-onset PCP with mtLSU genotype 1 strains (patients 49, 58, and 66) stayed in ward 74 during a similar period, although they did not share the same room (Figure 2). It was unclear who might be an index patient among them. Patient 65, who developed hospital-onset PCP with a mixed mtLSU genotype 1, 2 , stayed in ward 174, where patient 56 who had a strain with the same mixed genotype was hospitalised. Patients 56 and 65 also did not share the same room (Figure 2).
Figure 1.
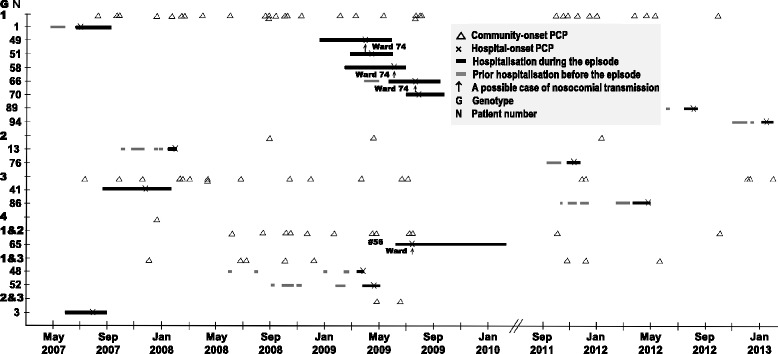
A transmission map for patients with Pneumocystis pneumonia (PCP) based on mitochondrial large ribosomal subunit ( mtLSU ) rRNA genotypes. Genotypes were determined by direct sequencing of nucleotides 85 and 248: genotype 1 = 85C/248C, 2 = 85A/248C, 3 = 85T/248C, and 4 = 85C/248T. Patients 49, 58, and 66 with genotype 1 stayed in ward 74 during a similar period, although they did not share the same room. Patient 65, who developed hospital-onset PCP with a mixed mtLSU genotype 1, 2 , stayed in ward 174, in which patient 56 who had a strain with the same mixed genotype was hospitalised. Patients 56 and 65 also did not share the same room
Figure 2.
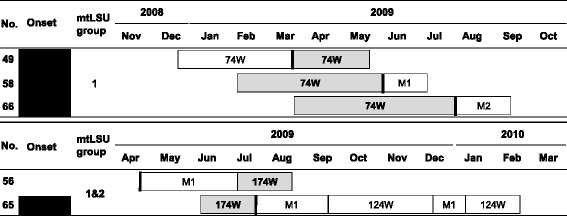
A transmission map for possible cases of nosocomial transmission of Pneumocystis jirovecii. Genotypes of the mitochondrial large ribosomal subunit (mtLSU) were determined by direct sequencing of nucleotides 85 and 248: genotype 1 = 85C/248C, 2 = 85A/248C, 3 = 85T/248C, and 4 = 85C/248T. A dark grey box indicates the period of hospitalisation during the PCP episode. The thick vertical line is the time when hospital-onset PCP was diagnosed. Patients 49, 58, and 66 stayed in ward 74 during a similar period. Patients 56 and 65 stayed in ward 174 during the same period. No., case number; Hospital-onset PCP marked by a black box; M1, the first medical intensive care unit; M2, second medical intensive care unit; W, ward
Genotype distribution of P. jirovecii
Genotype results of strains from all episodes, analysed by mtLSU and DHPS sequencing, are summarised in Table 2. mtLSU genotype 1 was the most common, occurring in 41 (43.2%) cases, followed by type 3 in 21 (22.1%) cases. Only one episode with genotype 4 was identified. Strains with mixed genotypes were found in 27 episodes (28.4%). In the patient with recurrent episodes, a mixture of genotypes 2 and 3 was identified in both episodes. DHPS mutation was not observed in any episode. Nine of thirteen episodes in HIV-infected patients were with genotype 1, which was a higher proportion than in non-HIV-infected patients (HIV, 69.2% [9/13] vs. non-HIV, 37.8% [31/82]; P = 0.04).
Table 2.
Variant mtLSU and dihydropteroate synthetase ( DHPS ) genotypes in patients with P. jirovecii pneumonia
| Genotype (n = 95) | mtLSU | DHPS | ||
|---|---|---|---|---|
| Nucleotide position/identity | No. (%) | Nucleotide position/identity | No. (%) | |
| 1 | 85C/248C | 41 (43.2) | 165 (55)/A (Thr); 171 (57)/C (Pro) | 95 (100) |
| 2 | 85A/248C | 5 (5.2) | 165 (55)/G (Ala); 171 (57)/C (Pro) | 0 (0.0) |
| 3 | 85T/248C | 21 (22.1) | 165 (55)/A (Thr); 171 (57)/T (Ser) | 0 (0.0) |
| 4 | 85C/248T | 1 (1.0) | 165 (55)/G (Ala); 171 (57)/T (Ser) | 0 (0.0) |
| Mixed (total) | 27 (28.1) | |||
| 1 and 2 | 13 (13.5) | |||
| 1 and 3 | 11 (11.5) | |||
| 2 and 3 | 3 (3.1) | |||
Discussion
In the present study, a significant proportion of PCP cases developed as a clinical manifestation of nosocomial pneumonia. We found a trend toward a higher proportion of haematological malignancy in patients with hospital-onset PCP, which can be explained by the long hospitalisation period during chemotherapy. This finding indicates that PCP should be considered in the differential diagnosis of nosocomial pneumonia and that the list of differential diagnosises was heavily dependent on the immune status and clinical characteristics. mtLSU genotype 1 was the most common P. jirovecii strain in Korea. We did not identify an association of any particular genetic strain with hospital-onset PCP. Thus, the impact of genotype on the occurrence of hospital-onset PCP and clinical outcomes should be further investigated.
Several studies have suggested that PCP results from de novo infection by nosocomial transmission, rather than from reactivation of latent infection. Clustering or outbreak strains with the same genotypes determined by various molecular methods suggest that P. jirovecii can be acquired by human-to-human transmission [6-10,17-19]. Additionally, recent studies suggest that the hospital environment and patients colonised with P. jirovecii can be a source of transmission [7,20]. Unlike these previous studies investigating whether nosocomial transmission contributed to PCP development during outbreak or clusters, the present study investigated the possibility of nosocomial transmission during the period without outbreak or clusters. Our findings also suggest human-to-human transmission to be a possible mode of P. jirovecii acquisition in hospitalised patients. Based on these results, patients with PCP may need to be isolated to prevent human-to-human transmission, and universal surveillance for PCP colonisation may be necessary before admission to wards containing immunocompromised patients. However, these results should be interpreted with caution before they are applied to a strategy for PCP prevention. First, in the present study, most of the patients with hospital-onset PCP had no history of possible nosocomial transmission. This suggests that the de novo acquisition of P. jirovecii, at least, may rarely occur during hospitalisation. Second, no DHPS mutations were found despite a prior history of exposure to sulfamethoxazole in many patients. In a previous study, DHPS mutations were not detected in patients in whom PCP developed prior to the widespread use of sulfamethoxazole to treat and prevent the disease [5]. This implies that patients with hospital-onset PCP may be infected from reservoir populations in the community that have not been exposed to sulfamethoxazole. Additionally, the genotype distributions were not different between patients with hospital-onset and community-onset PCP developing. Finally, owing to the long incubation period of P. jirovecii [21], it was impossible to strictly differentiate between de novo acquisition and reactivation. Therefore, the mode of acquisition and transmission of P. jirovecii causing pneumonia during hospitalisation could not be determined in the present study.
The genetic epidemiological features identified in this study determined that the mtLSU genotype 1 was the predominant P. jirovecii strain, there was a high prevalence of mixed-genotype strains, and DHPS mutations were absent. In previous studies in Spain and Japan, genotype 1 was also the most common, occurring in 30% and 49% of patients, respectively, which is comparable to the present study [16,22]. By contrast, genotypes 2 and 3 were reported to be the most prevalent strains in India [23] and Tunisia [24], respectively. In addition, a significant prevalence of mutations in DHPS (20–37%) was reported in several countries [16,23-25], while only one DHPS mutant was found among 52 strains in Japan [22]. These variable genetic epidemiological findings may be due to geographical differences. We found that genotype 1 occurred more frequently in HIV-infected patients compared to non-HIV-infected patients, which is similar to the report by Montes-Cano et al. [16].
The 30-day all-cause mortality rate was 26.3%. This high rate seems to be associated with the high proportion of patients with a non-haematologic malignancy and interstitial lung disease [26]. The rate of treatment failure in the initial therapy was similar to that in our previous study [27]. Our group has already published a number of articles on various clinical issues related to PCP, including the efficacy of salvage therapy regimens and the role of adjunctive corticosteroids [27,28]. Therefore, clinical issues were not extensively reviewed in the present study.
The present study had several limitations. First, because the incubation period of P. jirovecii is quite broad (range, 7–188 days) [21], 5 days may not be an appropriate cut-off for differentiating between cases of hospital-acquired and community-acquired PCP. Therefore, we have applied the terms ‘hospital-onset’ and ‘community-onset’ rather than ‘hospital-acquired’ and ‘community-acquired’ PCP. Second, a significant number of patients with PCP (36.9%, 35/130) were not included in the molecular analysis due to a lack of suitable specimens. Third, DHPS and mtLSU genotyping may not be optimal for epidemiological investigations. To get better discriminatory power, other molecular methods such as multilocus sequence typing should be done [29]. Fourth, other sources of nosocomial transmission, such as patient-to-patient transmission in other hospital departments and the acquisition of P. jirovecii from other hospital environments, were not investigated. Moreover, we did not search for P. jirovecii-infected individuals without definite signs and symptoms, who can act as problematic reservoirs. Finally, generalisations cannot be made from this study, as it was conducted in a single centre. Different conclusions may result from studies of other populations.
Conclusions
In conclusion, some episodes of PCP develop as nosocomial pneumonia. PCP should be considered as one of the causes of nosocomial pneumonia in immunocompromised patients, such as those with haematological malignancy. Molecular analysis of mtLSU rRNA indicated that a strain of genotype 1 was predominant in Korea. Further investigation should be performed to identify the mode of acquisition and transmission of P. jirovecii causing pneumonia during hospitalisation.
Financial support
This study was supported by a grant (2011–0915) from the Asan Institute for Life Sciences, Seoul, Korea.
Abbreviations
- PCP
Pneumocystis pneumonia
- mtLSU
mitochondrial large ribosomal subunit
- DHPS
Dihydropteroate synthase
- BAL
Bronchoalveolar lavage
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TK, SOL, HS conceived and designed the experiments. MNK, HS performed the in vitro experiments. TK, HS analysed data and wrote the paper. HLH, JYL, SHK, SHC, YSK, JHW collected data and contributed to editing the manuscript. All authors read and approved the final manuscript.
Contributor Information
Tark Kim, Email: ktocc2@naver.com.
Sang-Oh Lee, Email: soleemd@amc.seoul.kr.
Hyo-Lim Hong, Email: popear01@naver.com.
Ju Young Lee, Email: mizigun@hanmail.net.
Sung-Han Kim, Email: shkimmd@amc.seoul.kr.
Sang-Ho Choi, Email: sangho@amc.seoul.kr.
Mi-Na Kim, Email: mnkim@amc.seoul.kr.
Yang Soo Kim, Email: yskim@amc.seoul.kr.
Jun Hee Woo, Email: junheewoo@amc.seoul.kr.
Heungsup Sung, Email: sung@amc.seoul.kr.
References
- 1.Hughes WT. Natural mode of acquisition for de novo infection with Pneumocystis carinii. J Infect Dis. 1982;145(6):842–8. doi: 10.1093/infdis/145.6.842. [DOI] [PubMed] [Google Scholar]
- 2.Vargas SL, Hughes WT, Santolaya ME, Ulloa AV, Ponce CA, Cabrera CE, et al. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin Infect Dis. 2001;32(6):855–61. doi: 10.1086/319340. [DOI] [PubMed] [Google Scholar]
- 3.Frenkel JK, Good JT, Shultz JA. Latent Pneumocystis infection of rats, relapse, and chemotherapy. Lab Invest. 1966;15(10):1559–77. [PubMed] [Google Scholar]
- 4.Garvy BA, Harmsen AG. Susceptibility to Pneumocystis carinii infection: host responses of neonatal mice from immune or naive mothers and of immune or naive adults. Infect Immun. 1996;64(10):3987–92. doi: 10.1128/iai.64.10.3987-3992.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang L, Beard CB, Creasman J, Levy D, Duchin JS, Lee S, et al. Sulfa or sulfone prophylaxis and geographic region predict mutations in the Pneumocystis carinii dihydropteroate synthase gene. J Infect Dis. 2000;182(4):1192–8. doi: 10.1086/315824. [DOI] [PubMed] [Google Scholar]
- 6.Gianella S, Haeberli L, Joos B, Ledergerber B, Wuthrich RP, Weber R, et al. Molecular evidence of interhuman transmission in an outbreak of Pneumocystis jirovecii pneumonia among renal transplant recipients. Transpl Infect Dis. 2010;12(1):1–10. doi: 10.1111/j.1399-3062.2009.00447.x. [DOI] [PubMed] [Google Scholar]
- 7.Damiani C, Choukri F, Le Gal S, Menotti J, Sarfati C, Nevez G, et al. Possible nosocomial transmission of Pneumocystis jirovecii. Emerg Infect Dis. 2012;18(5):877–8. doi: 10.3201/eid1805.111432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sassi M, Ripamonti C, Mueller NJ, Yazaki H, Kutty G, Ma L, et al. Outbreaks of Pneumocystis pneumonia in 2 renal transplant centers linked to a single strain of Pneumocystis: implications for transmission and virulence. Clin Infect Dis. 2012;54(10):1437–44. doi: 10.1093/cid/cis217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmoldt S, Schuhegger R, Wendler T, Huber I, Sollner H, Hogardt M, et al. Molecular evidence of nosocomial Pneumocystis jirovecii transmission among 16 patients after kidney transplantation. J Clin Microbiol. 2008;46(3):966–71. doi: 10.1128/JCM.02016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas S, Vivancos R, Corless C, Wood G, Beeching NJ, Beadsworth MB. Increasing frequency of Pneumocystis jirovecii pneumonia in renal transplant recipients in the United Kingdom: clonal variability, clusters, and geographic location. Clin Infect Dis. 2011;53(3):307–8. doi: 10.1093/cid/cir329. [DOI] [PubMed] [Google Scholar]
- 11.Nevez G, Chabe M, Rabodonirina M, Virmaux M, Dei-Cas E, Hauser PM, et al. Nosocomial Pneumocystis jirovecii infections. Parasite. 2008;15(3):359–65. doi: 10.1051/parasite/2008153359. [DOI] [PubMed] [Google Scholar]
- 12.Kollef MH, Silver P, Murphy DM, Trovillion E. The effect of late-onset ventilator-associated pneumonia in determining patient mortality. Chest. 1995;108(6):1655–62. doi: 10.1378/chest.108.6.1655. [DOI] [PubMed] [Google Scholar]
- 13.Beard CB, Carter JL, Keely SP, Huang L, Pieniazek NJ, Moura IN, et al. Genetic variation in Pneumocystis carinii isolates from different geographic regions: implications for transmission. Emerg Infect Dis. 2000;6(3):265–72. doi: 10.3201/eid0603.000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lane BR, Ast JC, Hossler PA, Mindell DP, Bartlett MS, Smith JW, et al. Dihydropteroate synthase polymorphisms in Pneumocystis carinii. J Infect Dis. 1997;175(2):482–5. doi: 10.1093/infdis/175.2.482. [DOI] [PubMed] [Google Scholar]
- 15.Wakefield AE, Pixley FJ, Banerji S, Sinclair K, Miller RF, Moxon ER, et al. Amplification of mitochondrial ribosomal RNA sequences from Pneumocystis carinii DNA of rat and human origin. Mol Biochem Parasitol. 1990;43(1):69–76. doi: 10.1016/0166-6851(90)90131-5. [DOI] [PubMed] [Google Scholar]
- 16.Montes-Cano MA, de la Horra C, Martin-Juan J, Varela JM, Torronteras R, Respaldiza N, et al. Pneumocystis jiroveci genotypes in the Spanish population. Clin Infect Dis. 2004;39(1):123–8. doi: 10.1086/421778. [DOI] [PubMed] [Google Scholar]
- 17.Hocker B, Wendt C, Nahimana A, Tonshoff B, Hauser PM. Molecular evidence of Pneumocystis transmission in pediatric transplant unit. Emerg 343. Infect Dis. 2005;11(2):330–2. doi: 10.3201/eid1102.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsson M, Eriksson BM, Elvin K, Strandberg M, Wahlgren M. Genotypes of clustered cases of Pneumocystis carinii pneumonia. Scand J Infect Dis. 2001;33(4):285–9. doi: 10.1080/003655401300077324. [DOI] [PubMed] [Google Scholar]
- 19.Rabodonirina M, Vanhems P, Couray-Targe S, Gillibert RP, Ganne C, Nizard N, et al. Molecular evidence of interhuman transmission of Pneumocystis pneumonia among renal transplant recipients hospitalized with HIV-infected patients. Emerg Infect Dis. 2004;10(10):1766–73. doi: 10.3201/eid1010.040453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Boer MG, Bruijnesteijn van Coppenraet LE, Gaasbeek A, Berger SP, Gelinck LB, van Houwelingen HC, et al. An outbreak of Pneumocystis jiroveci pneumonia with 1 predominant genotype among renal transplant recipients: interhuman transmission or a common environmental source? Clin Infect Dis. 2007;44(9):1143–9. doi: 10.1086/513198. [DOI] [PubMed] [Google Scholar]
- 21.Yazaki H, Goto N, Uchida K, Kobayashi T, Gatanaga H, Oka S. Outbreak of Pneumocystis jiroveci pneumonia in renal transplant recipients: P. jiroveci is contagious to the susceptible host. Transplantation. 2009;88(3):380–5. doi: 10.1097/TP.0b013e3181aed389. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura Y, Shindo Y, Iinuma Y, Yamamoto M, Shirano M, Matsushima A, et al. Clinical characteristics of Pneumocystis pneumonia in non-HIV patients and prognostic factors including microbiological genotypes. BMC Infect Dis. 2011;11:76. doi: 10.1186/1471-2334-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta R, Mirdha BR, Guleria R, Agarwal SK, Samantaray JC, Kumar L, et al. Genotypic variation of Pneumocystis jirovecii isolates in India based on sequence diversity at mitochondrial large subunit rRNA. Int J Med Microbiol. 2011;301(3):267–72. doi: 10.1016/j.ijmm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Jarboui MA, Mseddi F, Sellami H, Sellami A, Makni F, Ayadi A. Genetic diversity of Pneumocystis jirovecii strains based on sequence variation of different DNA region. Med Mycol. 2013;51(6):561–7. doi: 10.3109/13693786.2012.744879. [DOI] [PubMed] [Google Scholar]
- 25.Rabodonirina M, Vaillant L, Taffe P, Nahimana A, Gillibert RP, Vanhems P, et al. Pneumocystis jirovecii genotype associated with increased death rate of HIV-infected patients with pneumonia. Emerg Infect Dis. 2013;19(1):21–8. doi: 10.3201/eid1901.120140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim T, Moon SM, Sung H, Kim MN, Kim SH, Choi SH, et al. Outcomes of non-HIV-infected patients with Pneumocystis pneumonia and concomitant pulmonary cytomegalovirus infection. Scand J Infect Dis. 2012;44(9):670–7. doi: 10.3109/00365548.2011.652665. [DOI] [PubMed] [Google Scholar]
- 27.Kim T, Kim SH, Park KH, Cho OH, Sung H, Kim MN, et al. Clindamycin-primaquine versus pentamidine for the second-line treatment of pneumocystis pneumonia. J Infect Chemother. 2009;15(5):343–6. doi: 10.1007/s10156-009-0710-Z. [DOI] [PubMed] [Google Scholar]
- 28.Moon SM, Kim T, Sung H, Kim MN, Kim SH, Choi SH, et al. Outcomes of moderate-to-severe Pneumocystis pneumonia treated with adjunctive steroid in non-HIV-infected patients. Antimicrob Agents Chemother. 2011;55(10):4613–8. doi: 10.1128/AAC.00669-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maitte C, Leterrier M, Le Pape P, Miegeville M, Morio F. Multilocus sequence typing of Pneumocystis jirovecii from clinical samples: how many and which loci should be used? J Clin Microbiol. 2013;51(9):2843–9. doi: 10.1128/JCM.01073-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


