Abstract
Background
Ethiopia, a high tuberculosis (TB) burden country, reports one of the highest incidence rates of extra-pulmonary TB dominated by cervical lymphadenitis (TBLN). Infection with Mycobacterium bovis has previously been excluded as the main reason for the high rate of extrapulmonary TB in Ethiopia.
Methods
Here we examined demographic and clinical characteristics of 953 pulmonary (PTB) and 1198 TBLN patients visiting 11 health facilities in distinct geographic areas of Ethiopia. Clinical characteristics were also correlated with genotypes of the causative agent, Mycobacterium tuberculosis.
Results
No major patient or bacterial strain factor could be identified as being responsible for the high rate of TBLN, and there was no association with HIV infection. However, analysis of the demographic data of involved patients showed that having regular and direct contact with live animals was more associated with TBLN than with PTB, although no M. bovis was isolated from patients with TBLN. Among PTB patients, those infected with Lineage 4 reported “contact with other TB patient” more often than patients infected with Lineage 3 did (OR = 1.6, CI 95% 1.0-2.7; p = 0.064). High fever, in contrast to low and moderate fever, was significantly associated with Lineage 4 (OR = 2.3; p = 0.024). On the other hand, TBLN cases infected with Lineage 4 tended to get milder symptoms overall for the constitutional symptoms than those infected with Lineage 3.
Conclusions
The study suggests a complex role for multiple interacting factors in the epidemiology of extrapulmonary TB in Ethiopia, including factors that can only be derived from population-based studies, which may prove to be significant for TB control in Ethiopia.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-015-0846-7) contains supplementary material, which is available to authorized users.
Keywords: Mycobacterium, Tuberculosis, Bovis, Pulmonary, Extrapulmonary, Lymphadenitis, Zoonotic, Ethiopia
Background
There were approximately 8.6 million new cases and 1.3 million deaths due to tuberculosis (TB) in 2012 [1]. Although pulmonary TB is the most common manifestation, an estimated one million people (~15%) develop extrapulmonary TB, of which TB lymphadenitis in the cervical lymph nodes (TBLN) is the most frequent form [2,3]. Failure to diagnose and treat TBLN can lead to serious health consequences such as disseminated TB [2].
The majority of all human TB cases are caused by Mycobacterium tuberculosis but the closely-related Mycobacterium bovis, the causative agent of TB in cattle and a range of domestic and wild animals, can also cause disease in humans. Zoonotic transmission can occur through the aerosol route during close contact with animals leading to pulmonary disease [4], but M. bovis is primarily transmitted through consumption of contaminated milk and is often associated with TBLN [5].
Ethiopia, with a population of over 90 million people, is among the countries with the highest TB burdens in the world, with an incidence rate of 247 per 100,000 in 2012 [1]. Moreover, Ethiopia has reported a higher than average incidence of extrapulmonary TB since records started in the 1990s. What are the risk factors that can explain this high rate of extrapulmonary TB in Ethiopia? More than 80% of all extrapulmonary cases involve cervical TB lymphadenitis that currently accounts for around 33% of all incident TB cases in the country, with a prevalence that roughly increases from 20% to 45% along a south to north geographic axis (data from Ethiopian Federal Ministry of Health; Figure 1). In parallel, Ethiopia is home to the largest livestock population in Africa with ~52 million cattle [6], and more than 80% of the labour force works in the agricultural sector. As several investigations have shown that bovine TB is endemic in Ethiopian cattle [7,8] and reaches high prevalence in regions with intensive husbandry systems [9-11], it would seem plausible that zoonotic transmission of M. bovis would have an influence on the prevalence of TBLN in the country. However, in a large nation-wide molecular epidemiology study [12] we explored the public health risk for zoonotic TB in Ethiopia and concluded that the overall role of M. bovis as a causative agent of TB in humans was approximately only 1%. This led us to conclude that the high incidence rate of extrapulmonary TB reported in Ethiopia is likely due to other factors.
Figure 1.

Mapping of collection sites and extrapulmonary TB in Ethiopia. Information received from the Federal Ministry of Health (FMoH), Ethiopia.
In our molecular epidemiological study [12], nearly 1,000 mycobacterial strains were isolated from over 2,000 patients nationwide (Figure 1) who were diagnosed with either TB lymphadenitis or pulmonary TB. In parallel to identifying the pathogen population structure, demographic and clinical data were collected from the same patients. Here we report the results of our analyses of these demographic and clinical data in an attempt to elucidate the factors that influence rates of extrapulmonary TB in Ethiopia and to inform public health control measures generally.
Methods
Selection of study sites
The location of the study sites is shown in Figure 1 with Gondar, Woldiya, Ghimbi, Fiche, and Butajira in the Ethiopian highlands where subsistence farming dominates, while Negelle, Filtu, and Jinka (“NFJ”; referred to as one site in this paper) are located in the southern parts of the country where people are mainly pastoralists or agro-pastoralists. Fine needle aspirates (FNA) and sputum samples, for diagnosis of TBLN and pulmonary TB respectively, were collected from patients attending hospitals in Gondar, Woldiya, Ghimbi, Butajira and Negelle. In addition, sputum samples were collected in hospitals at Fiche, Jinka and Filtu, as well as health centres at three suburban sites of Addis Ababa where residents are mainly engaged in subsistence farming or in intensified dairy farming (Holeta, Sululta, and Chancho).
Collection of specimens and demographic and clinical information
All consecutive and consenting patients with pulmonary TB or TBLN presenting at hospitals or health centres located in the study sites were recruited. Patients with disseminated TB (e.g. evidence of combined lung and lymph node disease) were excluded from the study. Demographic and clinical information (including cardinal symptoms and their duration: fever, night sweat, weight loss, poor appetite, weakness, cough, and blood in sputum as well as information on nodes such as number, size, and consistency) was collected from each patient through an interview with a clinician using the same questionnaire in all health centres. Sputum samples were collected from newly diagnosed pulmonary TB patients confirmed by the health facility laboratory to have acid-fast bacilli (AFB) in the sputum. The Ethiopian national algorithm (2008; Additional file 1: Figure S1) was used for recruitment of TB lymphadenitis patients, and patients diagnosed with TBLN had an opportunity to be serologically tested for HIV (no information on HIV status for pulmonary TB cases was recorded in this study). FNA material was collected aseptically from enlarged cervical lymph nodes with a 21-gauge needle attached to a 10 ml syringe and smeared onto glass slides for routine cytology. The remaining material in the syringe was rinsed into a tube with 1 ml PBS solution and used for culture. Sputum and FNA samples were stored at 4°C at the field sites and during transportation to the TB laboratory of the Armauer Hansen Research Institute (AHRI) in Addis Ababa where they were further processed within five days after sampling. For remote collection sites (NFJ), samples were commonly stored at the field site at −20°C until transport to AHRI. Informed consent was obtained from all patients enrolled in the study. All participating health facilities and regional health bureaus provided support letters and ethical approval was obtained from the AHRI and the All Africa Leprosy, Tuberculosis and Rehabilitation Training Centre (ALERT) Ethics Review Committee, regional health bureau ethics committees and the Ethiopian National Research Ethical Review Committee.
Cytology and HIV testing
FNA smears were air dried on glass slides (at the collection site) and brought to AHRI for Ziehl-Neelsen and Wright-staining (BDH chemicals Ltd, England). Cytological examination was performed by an experienced pathologist for evidence of pathology indicative of tuberculosis and the results reported to the respective health facilities for appropriate patient management. HIV testing was done according to national guidelines at the health facility where the patient was treated for TB and test procedures followed manufacturer’s instructions. Up to three different tests were used: KHB rapid HIV; Stat-Pak HIV; and Uni-gold HIV [13].
Culture and molecular typing
The procedures used for isolation and typing of the causative agents from the recruited patients as well as the molecular epidemiology outcomes have been published elsewhere [7,12]. In brief, FNA and sputum samples collected from patients in this study were processed and cultured at 37°C on three different media, including two Löwenstein-Jensen (LJ) media (supplemented with either glycerol or pyruvate) and a modified Middlebrook 7H11 medium optimised for the culture of M. bovis [12,14]. Slants with no growth at week 8 were considered negative. Bacterial colonies from culture-positive samples were Ziehl-Neelsen stained according to a standardized protocol to identify AFB. Cultures positive for AFB were prepared as 20% glycerol stocks and stored at −80°C. In parallel, heat-inactivated AFB positive samples were investigated by multiplex PCR for Large Sequence Polymorphism (LSP; e.g. RD4 and RD9) regions and by lineage-specific single-nucleotide polymorphism (SNP) analysis [7,15]. Isolates genetically typed as belonging to the M. tuberculosis complex were spoligotyped as previously described [16].
Statistical analysis
All data collected from patients and the laboratory were double-entered into a Microsoft Access database and the entry errors corrected with EpiInfo 3.5.1 (data compare utility). Questionnaire and laboratory data were linked by a unique identification code. Analyses of data were done in STATA/IC 10 for Windows (StataCorp, College Station, TX). Logistic regression models were used for univariate analyses of crude odds ratios (OR) of demographic and clinical data and models were also adjusted to age group (≤20 years, >20 to ≤45 years, >45 years), sex and site (seven sites) to generate ORs adjusted to expected confounders ORs of demographic and potential risk factors were further compared to those obtained in the full model with all variables. We created binary outcome variables for Lineages 3, 4 and 7 (the respective lineage vs. all other lineages) to test associations with explanatory variables such as pulmonary TB and TBLN. The statistical significance threshold was set at 0.05, p ≤ 0.05.
Results
Study population and Sample collected
During a period of five years from 2006–2010, we screened 2151 patients with untreated clinically diagnosed pulmonary TB and TB lymphadenitis (TBLN) in seven sites distributed across Ethiopia (Figure 1). Patients attending clinics in Gondar and Woldiya were predominantly Amhara, while the majority of people in Ghimbi, Fiche and the suburban clinics of Addis Ababa were Oromo. The population in Butajira was mainly Gurage, and clinics in the south (Negelle/Filtu/Jinka) included people of Oromo, Somali, Amhara, and Ari ethnic groups.
Clinics at Fiche and Addis Ababa did not use fine needle aspirates (FNA) for diagnosis of TBLN and therefore only sputum from pulmonary TB cases was collected from these sites. The yield of AFB positive cultures differed between sites, likely reflecting logistic issues associated with sample transport (Table 1).
Table 1.
Patient numbers enrolled and AFB culture-positivity per collection site
| Collection site | Pulmonary TB | TB lymphadenitis | ||
|---|---|---|---|---|
| Patients | Culture-positive | Patients | Culture-positive | |
| Gondar | 120 | 103 (86%) | 156 | 46 (29%) |
| Woldiya | 36 | 30 (83%) | 333 | 160 (48%) |
| Ghimbi | 68 | 65 (96%) | 264 | 99 (38%) |
| Fiche | 223 | 203 (91%) | 0 | - |
| Addis Ababa | 122 | 76 (62%) | 0 | - |
| Butajira | 101 | 99 (98%) | 360 | 138 (38%) |
| Negelle/Filtu/Jinka | 283 | 180 (64%) | 85 | 13 (15%) |
| Total | 953 | 756 (79%) | 1198 | 456 (38%) |
Bacterial culture and clinical features
Acid-fast bacilli were isolated from 1212 of the 2151 patients (Table 1). As expected, AFB positive cultures were obtained more frequently from the 953 patients with smear-positive pulmonary disease than from the 1198 patients with suspected TBLN, with an average yield between the collection sites of 79% and 38% culture positivity, respectively. In comparison, Ziehl-Neelsen staining of 302 FNA samples demonstrated only 10% smear-positivity, reflecting challenges in diagnosis of TBLN by different methods. However, the frequency of positive TBLN cultures was higher in FNA samples that showed cytological evidence of tuberculosis (220 positive cultures from 469 samples; 47%) than those with negative cytology (23 positive cultures from 131 samples; 18%).
Our analysis of clinical features of patients diagnosed with TBLN and pulmonary TB with regard to culture outcome can be found in Additional file 2: Table S2 and Additional file 3: Table S3A. Constitutional symptoms (i.e. fever, night sweat, weight loss, poor appetite, and weakness) were more frequent in patients where a positive culture could be confirmed, reaching statistical significance for “night sweat” and “weakness” for patients of both disease outcomes compared to those negative on culture. For patients diagnosed with TBLN, significant differences were also noticed in culture positivity related to perceived increased rate and duration of neck swelling; culture positivity was higher in “fast” swelling as compared to “slow” swelling of the cervical lymph nodes (38% versus 54%) (Additional file 3: Table S3), and with regard to the duration, TBLN patients seeking health care within one month of the appearance of swelling were significantly more likely to be culture-positive than those who were examined only one year after appearance. Culture-positivity also tended to increase in proportion to node size; 103/288 (36%) of samples from lymph nodes less than 1 cm yielded positive cultures, in comparison to 58/137 (42%) of samples from lymph nodes greater than 4 cm [adjusted OR (95% CI) 2.3 (0.9-5.8)]. The median duration of swelling recorded for all recruited TBLN patients was 12 weeks and the average size of swollen lymph nodes was approximately 3 cm. For additional results on clinical features, see Additional file 2: Table S2 and Additional file 3: Table S3.
Patients diagnosed with TBLN at the clinics had an opportunity to be serologically tested for HIV. We obtained 395 records for HIV status, of which 14 (3.6%) were HIV positive. Five of these 14 HIV positive patients were confirmed with TBLN by positive mycobacterial culture. No HIV data were collected from pulmonary TB patients.
Demographic information
In an attempt to elucidate risk factors associated with disease type, we then compared 626 patients with pulmonary TB and 328 with TBLN for their demographic characteristics (Table 2). Having regular and direct contact with live animals, was a significant risk factor for TBLN when compared to pulmonary TB. In addition, an association was observed between education and TBLN, with patients having a degree from secondary school or higher education being at lower risk of developing TBLN in comparison to pulmonary TB (Table 2). We also analysed our data within each study site to investigate if there was any association between ethnic group and disease outcome. This could not be analysed on a national level as sites and ethnic groups were highly correlated and affected by sampling bias. No associations between ethnic groups and TBLN or pulmonary TB were found (data not shown).
Table 2.
Demographic characteristic of 954 culture-positive patients with identified strain
| PTB | TBLN | Crude OR | Adjusted OR | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | 95% CI | 95% CI | ||
| Age a | ≤20 years | 128 | 51.6 | 120 | 48.4 | 1 | 1 |
| >20 - ≤45 years | 355 | 70.6 | 148 | 29.4 | 0.4 (0.3-06.)*** | 0.5 (0.4-0.8)** | |
| >45 years | 87 | 80.6 | 21 | 19.4 | 0.3 (0.2-0.4)*** | 0.3 (0.2-0.6)*** | |
| Sex b | Male | 331 | 70.6 | 138 | 29.4 | ||
| Female | 246 | 59.6 | 167 | 40.4 | 1.6 (1.2-2.2)*** | 1.6 (1.1-2.3)* | |
| Education | Illiterate | 311 | 67.2 | 152 | 32.8 | 1 | 1 |
| Primary | 158 | 58.5 | 112 | 41.5 | 1.5 (1.1-2.0)* | 0.7 (0.4-1.1) | |
| Secondary | 86 | 71.7 | 34 | 28.3 | 0.8 (0.5-1.3) | 0.3 (0.2-0.6)*** | |
| Higher degree | 22 | 81.5 | 5 | 18.5 | 0.5 (0.2-1.3) | 0.2 (0.1-0.7)** | |
| Previous TB contact | No | 331 | 59.8 | 223 | 40.2 | ||
| Yes | 154 | 66.7 | 77 | 33.3 | 0.7 (0.5-1.0) | 0.8 (0.5-1.3) | |
| Intake of raw milk | No | 221 | 66.4 | 112 | 33.6 | ||
| Yes | 353 | 64.6 | 193 | 35.4 | 1.1 (0.8-1.4) | 1.9 (1.2-2.8)** | |
| Living with animals in same household | No | 289 | 70.2 | 123 | 29.8 | ||
| Yes | 273 | 60.7 | 177 | 39.3 | 1.5 (1.1-2.0)** | 1.7 (1.1-2.6)* | |
| Regular and direct contact with live animals | No | 214 | 72.0 | 83 | 28.0 | ||
| Yes | 327 | 64.9 | 177 | 35.1 | 1.4 (1.0-1.9)* | 3.0 (1.9-4.5)*** | |
| BCG vaccination | No | 329 | 82.5 | 70 | 17.5 | ||
| Yes | 89 | 77.4 | 26 | 22.6 | 1.4 (0.8-2.3) | 1.6 (0.8-3.3) |
aadjusted for sex and site alone; badjusted for age and site alone; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
The role of M. tuberculosis lineages in clinical presentation
The M. tuberculosis complex strains that caused TB in the 954 patients have been previously reported by Firdessa et al. [12]. A summary of the lineage distribution of these strains is shown in Figure 2, stratified by TBLN and pulmonary TB cases, respectively. Here we have made use of this information to seek correlations between demographic/clinical features and lineages of M. tuberculosis. Demographic and clinical data from the 328 patients with TBLN and the 626 cases with pulmonary TB were therefore stratified by M. tuberculosis lineage. Due to low statistical power for lineages with limited isolates, only Lineage 3 and Lineage 4 were compared individually to all other lineages (Additional file 2: Table S2A and Additional file 3: Table S3A). Analysis of demographic factors among pulmonary TB patients showed a borderline statistical difference between TB contact and M. tuberculosis lineage causing the disease; patients infected with Lineage 4 reported “contact with other TB patient” more often than patients infected with Lineage 3 did (OR = 1.6, CI 95% 1.0-2.7; p = 0.064). The analogous comparison in TBLN patients showed no significant difference. Moreover, pulmonary TB patients infected with Lineage 4 strains – in contrast to Lineage 3 – tended to have a more severe manifestation (OR > 1) for a majority of the constitutional symptoms (Additional file 2: Table S2A and Additional file 3: Table S3A). Fever was the most distinct symptom in this regard and it was further enhanced when data on type of fever was included (data not shown); high fever, in contrast to low and moderate fever, was significantly associated with Lineage 4 (OR = 2.3; p = 0.024). Corresponding data collected from the TBLN patients generated a similar but reverse result: TBLN cases infected with Lineage 4 tended to get milder symptoms overall for the constitutional symptoms than those infected with Lineage 3.
Figure 2.
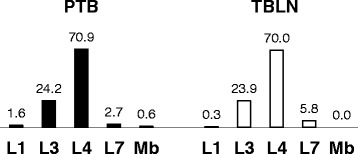
Representative lineages (in percentage) of M. tuberculosis complex strains collected from TB lymphadenitis and pulmonary TB patients in Ethiopia (data taken from Firdessa et al. [ 10 ]). Mb, M. bovis) PTB = pulmonary TB, TBLN = tuberculous lymphadenitis.
Discussion
This study identified no major patient or bacterial strain factor that alone could be responsible for the high rate of TBLN in Ethiopia. In addition, no association was found with HIV infection.
The incidence of extrapulmonary TB, of which the vast majority is TBLN, among TB patients in Ethiopia has steadily increased since the 1990s, reaching an average of around 33% across the country. Iwnetu et al. [17] questioned if this increase was simply due to over-diagnosis, with their study concluding that up to 15% of all TBLN cases could be wrongly diagnosed; however, this clearly does not completely explain the high incidence of extrapulmonary TB. We recently tested the hypothesis that TBLN in Ethiopia would be associated with zoonotic transmission of M. bovis from cattle [12]. Molecular identification of the causative agents showed that the contribution of M. bovis to human TB was less than 1%. Even more unexpected was the fact that no TB lymphadenitis patient was diagnosed with M. bovis infection, but instead, the four human cases identified with M. bovis infection all had pulmonary TB. This suggests that other risk factors need to be considered to explain the high incidence of extrapulmonary TB in Ethiopia [18,19]. To search for additional risk factors, our extensive health centre-based survey was also designed to overlap geographically with areas previously examined for bovine TB in cattle [7,10,20,21] and to include sites distributed across the documented south–north gradient (20-45%) of extrapulmonary TB (Figure 1). Based on further analysis of our epidemiological study presented here and elsewhere [7,12], we suggest a set of possible factors that may influence the high incidence of extrapulmonary TB as well as the lower than expected burden of M. bovis infections in humans.
Zoonotic TB – M. bovis
The low incidence of human infection by M. bovis may simply reflect a relatively low prevalence of bovine TB. In the 1930s and 40s, it was estimated that 30-40% of cattle in England and Wales had TB, with zoonotic transmission suggested to account for around 5-6% of the human TB burden [22,23]. In comparison, prevalence rates of 1-10% for bovine TB in Ethiopian zebu cattle grazing in pasture are relatively low [7,21] and may therefore have a lower impact on the overall contribution to human TB in Ethiopia.
The low rate of isolation of M. bovis in humans recorded by us [12] can be compared to other reports that used genotyping of isolates for definitive characterisation. Recent studies identified 3.5% of human TB in Madagascar as caused by M. bovis [24], but found no M. bovis in Côte d’Ivoire [25], in Brazil [26], or in Chad [27] despite the fact that these countries are endemic for bovine TB in cattle [28-32]. Higher rates were found in Mexico, with separate studies identifying 6% and 28% of human mycobacterial isolates as M. bovis [33,34]. Taken together, these findings suggest a low overall rate of zoonotic transmission of M. bovis, particularly in Africa, but do not exclude this as a public health concern in selected areas. Of particular note in the context of the present study is that we did not sample TBLN cases in urban areas where there are high rates of bovine TB associated with intensified dairy farming [9,11,35]. At a global level, systematic surveys in additional geographic regions will be important in making a realistic assessment of the human health risk posed by zoonotic transmission of M. bovis. The high frequency of M. bovis isolated from Mexican patients may be linked to the low overall TB incidence rate (17/100,000), and it can be anticipated that the relative contribution of zoonotic transmission will increase as control programmes for humans progress towards TB elimination, particularly when programmes against bovine TB in cattle are lagging behind [36].
Accurate speciation of the causative agent in individuals with TB is not trivial and demands time and resources. Although newly developed PCR-based techniques can identify specific mycobacteria directly from infected human specimens [37], isolation of the bacteria by culturing - prior to further characterisation – is considered as the diagnostic gold standard due to significantly higher sensitivity and specificity. We do not think that our low rates of M. bovis isolation from humans were simply due to sample processing techniques that selected against M. bovis; we routinely cultured M. bovis from cattle samples from the same sites using the same protocols. The traditional method to distinguish between species within the M. tuberculosis complex has primarily been biochemical testing based on phenotypic differences such as nitrate reduction [38] and pyrazinamide resistance [39]. However, the reliability of phenotype-based assays to accurately distinguish between M. tuberculosis and M. bovis has been contested [40,41]. Therefore, reservations regarding conclusions of earlier studies based solely on biochemical tests or cultural characteristics are justified. The currently well-established genotyping methods (e.g. LSP and SNP typing), as used in this study, should be a requirement for definitive differentiation of species from the M. tuberculosis complex.
Zoonotic TB – M. tuberculosis
From our initial hypothesis that the high prevalence of extrapulmonary TB may reflect zoonotic transmission, we anticipated that TBLN patients might have close contact with farm animals and/or consume raw milk. This was in fact the case. Our demographic analysis identified contact with livestock as a risk factor for TBLN when compared to pulmonary TB. Given the large cattle population in Ethiopia (~50 million) and that humans and cattle live in close proximity, together with reports that M. tuberculosis has been isolated both from cattle [7,42,43] and milk samples [44], there would appear to be ample opportunity for direct involvement of livestock in the transmission cycle of M. tuberculosis. In our study of bovine TB in Ethiopia based on visual inspection of carcasses and culture of suspect lesions, we obtained 8 M. tuberculosis isolates from 32,800 animals [7], corresponding to a “culture-proven” prevalence of 24 cases per 100,000. In comparison, the prevalence of smear-positive TB in humans is 74 cases per 100,000 in Ethiopia [1]. In addition, the comparative intradermal skin test that is used to assess bovine TB prevalence may overestimate true prevalence of M. bovis infection as the test cannot distinguish between cattle infected with M. bovis or M. tuberculosis. In fact, a recent Ethiopian study isolated both M. bovis and M. tuberculosis at similar rates from skin-test reactor cattle in small-holder farms owned by households with a TB index case [43]. Taken together, even if the extent of cattle infected with M. tuberculosis is unknown it should be considered as a potential risk factor for human transmission, with for example ingestion of contaminated milk being one possible source of infection. The contribution of zoonotic transmission of M. tuberculosis to the high rate of extrapulmonary TB remains to be clarified. As epidemiological data from Ethiopia suggest a higher prevalence of M. bovis in cattle as compared to M. tuberculosis, one would expect that the rate of zoonotic transmission of M. bovis would be higher than that for M. tuberculosis. However, several unanswered questions remain to be solved in this human-cattle-human transmission cycle, including transmission routes and rates as well as differences in pathogenicity between M. bovis and M. tuberculosis in cattle and humans.
Lineage and disease presentation
Having ruled out M. bovis as a cause for the high incidence of TBLN, we can ask whether particular features of the circulating M. tuberculosis strains might play a role. An association of strains belonging to M. tuberculosis Lineage 4 with pulmonary TB as opposed to extrapulmonary disease in the form of TB meningitis has been described in Vietnam [45]. Similarly, Lari et al. [46] have shown an association between Lineage 3 and extrapulmonary TB. Across Ethiopia as a whole, we did not observe any association between M. tuberculosis lineage and disease presentation (Figure 2). Increased representation of Lineage 4 in contrast to Lineage 3 in TBLN (data not shown) was however observed in the north of the country but it only reached statistical significance in Gondar (Table 3). Lineage differences were also identified by comparison of disease severity indicators; infection with Lineage 4 was weakly associated with increased severity in pulmonary TB patients, and with decreased severity in TBLN. While these results are consistent with the hypothesis that genetic diversity of M. tuberculosis is associated with phenotypic diversity linked to disease presentation [47-49], these differences do not provide an obvious explanation for the high overall rate, or for the North–south gradient, of extrapulmonary TB in Ethiopia.
Table 3.
Representation of M. tuberculosis Lineage 4 among TB lymphadenitis and pulmonary TB cases from respective collection sites
| PTB | TBLN | Crude OR | ||||
|---|---|---|---|---|---|---|
| N | % | n | % | |||
| Gondar | 60 | 84.5 | 11 | 15.5 | ||
| 32 | 60.4 | 21 | 39.6 | 3.6 (1.5-8.3)** | 0.003 | |
| Woldiya | 14 | 20.0 | 56 | 80.0 | ||
| 9 | 14.3 | 54 | 85.7 | 1.5 (0.6-3.8) | 0.4 | |
| Ghimbi | 7 | 38.9 | 11 | 61.1 | ||
| 40 | 40.8 | 58 | 59.2 | 0.9 (0.3-2.6) | 0.9 | |
| Butajira | 10 | 34.5 | 19 | 65.5 | ||
| 60 | 39.7 | 91 | 60.3 | 0.8 (0.3-1.8) | 0.6 | |
| Negelle/Filtu/Jinka | 38 | 95.0 | 2 | 5.0 | ||
| 123 | 96.1 | 5 | 3.9 | 0.8 (0.1-4.1) | 0.8 |
**p ≤ 0.01 PTB = pulmonary tuberculosis; TBLN = tuberculous lymphadenitis; OR = odds ratio.
Adjusted OR; adjusted for site (n = 7), age category (3) and sex (binary). L3 = Lineage 3 (CAS); L4 = Lineage 4 (Euro-American); L7 = Lineage 7 (new).
There is evidence of distinct geographic structuring of M. tuberculosis populations in Ethiopia. Representation of Lineage 3 was up to four times higher in the north as compared to the other sites. A recent study in neighbouring Sudan also reported a high frequency of Lineage 3, with SIT 25 (the predominant Lineage 3 spoligotype in Ethiopia) accounting for almost half of the M. tuberculosis isolates [50]. In contrast, Lineage 1, which has been reported at high frequency in Somalia (~50% (129/256); SITVIT2), was isolated only from sites in southern Ethiopia. In comparison to Ethiopia, Sudan (25%) and Somalia (20%) reported lower case notifications of extrapulmonary TB in 2012 [1]. Based on these reports from Sudan and current data from Ethiopia, we cannot propose a correlation between a high rate of extrapulmonary TB and infection with Lineage 3 (subtype SIT 25) strains; this is also shown by our observation that Lineage 4 (rather than Lineage 3) is associated with TBLN in Gondar. Similarly, the two major subtypes of Lineage 4 (Spoligotypes SIT 149 and SIT 53) were relatively well distributed among the collection sites in Ethiopia and no strong clustering towards the north (e.g. Gondar) was observed. Thus, no obvious relationship between subtype and disease manifestation was found, but such a correlation cannot be excluded. We conclude that while M. tuberculosis genotype may have some effect on disease presentation, it cannot account for the overall elevation in the prevalence of extrapulmonary TB in Ethiopia.
Other possible factors driving disease presentation
Co-infection with HIV is strongly associated with increased rates of extrapulmonary TB [51-53]. While the rate of HIV-TB co-infected patients in Ethiopia (10.2% of all incident cases) is higher than in neighbouring Sudan and Somalia (7.5% and 3.6% respectively), it is much lower than that in Kenya (39%), which has an incident rate of extrapulmonary TB (18%) approximately half of that reported for Ethiopia [1]. Working within national control programme guidelines in our present study, we obtained HIV infection data for 399 patients with TBLN; the rate of HIV-positivity (3.6%) was below the national average reported for all TB cases. Though limited number of patients were compared, we conclude that HIV co-infection does not explain the high rate of extrapulmonary TB, confirming observations of several previous studies in Ethiopia [17,54].
Data from the Ethiopian FMoH (2008) and analysed by us (data not shown) suggests that the total incidence of both TB and TBLN are positively associated with the proportion of urban population in Ethiopian regions. This likely reflects an urban population with easier access to health services and TB diagnostics than people living in more rural regions, and thus, may also influence the proportion of TBLN cases among all TB cases. Specifically, seeking health care may be particularly low for people with TBLN among rural populations with long distances from a health centre, as has been suggested for pastoralists living in the Afar region [55,56].
The Ethiopian human population is characterised by a high level of genetic diversity [57,58] and, although not studied here, host genetics may well have an influence on disease presentation [59]. In general, the incidence of extrapulmonary TB in North African countries is higher than that in Sub-Saharan Africa [1]. Assuming a human genetic effect, the high rates in Ethiopia may reflect the known admixture of northern and southern African human ancestry, and/or perhaps particular host and pathogen genotype combinations. Future analyses of disease outcomes as a function of different host-pathogen combinations may provide insights into the pathogenesis of TB and complement conventional genome-wide association studies [60,61].
A limitation of this study is that it is based on clinical cases visiting health facilities that enrolled smear positive pulmonary and cervical lymphadenitis TB cases. There was no prospective follow up to determine clinical outcome. Smear negative pulmonary and non-cervical extrapulmonary TB cases have not been included. Pulmonary TB cases were not tested for HIV. An epidemiological investigation with active case detection would have avoided the possible bias due to differential health seeking-behaviour and access to care. Nevertheless, our study provides very valuable insight into the epidemiology of TB in Ethiopia and will inform control in this and other high burden TB countries.
Conclusions
Why Ethiopia stands out as a country with a high burden of extrapulmonary TB is enigmatic. Zoonotic transmission of M. bovis infection has been excluded as a major factor in TBLN, as was infection with HIV. Instead, the answer is likely to be more complex, and here we have presented several factors that could play significant roles, including zoonotic transmission of M. tuberculosis and genetic features of the pathogen and/or the host population. The study suggests a complex role for multiple interacting factors in the epidemiology of extrapulmonary TB in Ethiopia, including factors that can only be derived from population-based studies, which may prove to be significant for TB control in Ethiopia. We conclude that elucidation of the epidemiology of TBLN will contribute to a better understanding of the factors that maintain TB as a whole, rather than just pulmonary TB alone, and hence to improved disease control.
Acknowledgments
This study was sponsored by the Wellcome Trust, United Kingdom (grant number 075833/A/04/Z), under their “Animal health in the developing world” initiative. We thank the staffs in hospitals and health centres of the participating towns/regions in Ethiopia as well as staff at AHRI, especially Wondu Wagaye, Girma Beyene, Yusuf Sani, and Fikerte Mulatu for their invaluable contributions to this study. Information on TB prevalence rates used in Figure 1 was kindly provided by the Ethiopian Federal Ministry of Health.
Abbreviations
- TB
Tuberculosis
- TBLN
TB lymphadenitis in the cervical lymph node
- FNA
Fine needle aspirate
- AFB
Acid fast bacilli
Additional files
Algorithms for recruitment of extrapulmonary TB (A) and pulmonary TB (B) patients according to the Ethiopian national TB and Leprosy control programme.
Factors and clinical features of Pulmonary TB with regard to culture outcome (A) and M. tuberculosis lineage (B).
Factors and clinical features of TB lymphadenitis with regard to culture outcome (A) and M. tuberculosis lineage (B).
Footnotes
Stefan Berg, Esther Schelling, Elena Hailu and Rebuma Firdessa contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Study design was performed by AA, SVG, GH, JZ, ES, AM, RT, RF, BG, GA and StB. Data collection (e.g. sampling, mycobacterial culturing, molecular typing, histology) was completed by AM, EG, RF, EH, GE, JH, MH, RT, ShB, TK, YD, WM and StB, while data analyses were performed by LY, StB, ES, RT, EH, , RT, AA, SVG, and SG. Manuscript was mainly written by StB, ES, AA, SVG, and SG. All authors have read and agreed for publication of the manuscript.
Contributor Information
Stefan Berg, Email: stefan.berg@apha.gsi.gov.uk.
Esther Schelling, Email: esther.schelling@unibas.ch.
Elena Hailu, Email: elenahailu@yahoo.com.
Rebuma Firdessa, Email: rebumab@yahoo.com.
Balako Gumi, Email: balako.gumi@yahoo.com.
Girume Erenso, Email: girum1825@yahoo.com.
Endalamaw Gadisa, gadisa.endalamaw@googlemail.com.
Araya Mengistu, Email: armen.kassa@gmail.com.
Meseret Habtamu, Email: mekonnen.meseret@gmail.com.
Jemal Hussein, Email: jemaldr@yahoo.com.
Teklu Kiros, Email: kmengie@yahoo.com.
Shiferaw Bekele, Email: shiferawbekele92@yahoo.com.
Wondale Mekonnen, Email: hevewondale@yahoo.com.
Yohannes Derese, Email: yohannesderese@yahoo.com.
Jakob Zinsstag, Email: jakob.zinsstag@unibas.ch.
Gobena Ameni, Email: gobenachimdi2009@yahoo.co.uk.
Sebastien Gagneux, Email: sebastien.gagneux@unibas.ch.
Brian D Robertson, Email: b.robertson@imperial.ac.uk.
Rea Tschopp, Email: rea.tschopp@unibas.ch.
Glyn Hewinson, Email: glyn.hewinson@apha.gsi.gov.uk.
Lawrence Yamuah, Email: yamuahlk@ahrialert.org.
Stephen V Gordon, Email: stephen.gordon@ucd.ie.
Abraham Aseffa, Email: aseffaa@gmail.com.
References
- 1.Global tuberculosis report 2013. World Health Organisation. www.who.int/tb/data
- 2.Black TC, Chapman JS, German JL. Tuberculosis of superficial lymph nodes. Diseases of the Chest. 1956;30(3):326–337. doi: 10.1378/chest.30.3.326. [DOI] [PubMed] [Google Scholar]
- 3.Fontanilla J-M, Barnes A, von Reyn F. Current diagnosis and management of peripheral tuberculous lymphadenitis. Clin Infect Dis. 2011;53:555–562. doi: 10.1093/cid/cir454. [DOI] [PubMed] [Google Scholar]
- 4.Sunder S, Lanotte P, Godreuil S, Martin C, Boschiroli M, Besnier JM. Human-to-human transmission of tuberculosis caused by mycobacterium bovis in immunocompetent patients. J Clin Microbiol. 2009;47(4):1249–1251. doi: 10.1128/JCM.02042-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grange JM. Mycobacterium bovis infection in human beings. Tuberculosis (Edinb) 2001;81(1–2):71–77. doi: 10.1054/tube.2000.0263. [DOI] [PubMed] [Google Scholar]
- 6.CSA. Agricultural Sample Survey 2010/11. In., vol. 2: Central Statistical Agency of Ethiopia; 2011. http://www.csa.gov.et/newcsaweb/…/surveys/Agricultural_sample_survey/Agri. Viewed 27 Feb 2015
- 7.Berg S, Firdessa R, Habtamu M, Gadisa E, Mengistu A, Yamuah L, et al. The burden of mycobacterial disease in ethiopian cattle: implications for public health. PLoS One. 2009;4(4):e5068. doi: 10.1371/journal.pone.0005068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shitaye JETW, Pavlik I. Bovine tuberculosis infection in animal and human populations in Ethiopia: a review. Veterinarni Medicina. 2007;52(8):317–332. [Google Scholar]
- 9.Elias K, Hussein D, Asseged B, Wondwossen T, Gebeyehu M. Status of bovine tuberculosis in Addis Ababa dairy farms. Rev Sci Tech. 2008;27(3):915–923. doi: 10.20506/rst.27.3.1850. [DOI] [PubMed] [Google Scholar]
- 10.Ameni G, Aseffa A, Engers H, Young D, Gordon S, Hewinson G, et al. High prevalence and increased severity of pathology of bovine tuberculosis in Holsteins compared to zebu breeds under field cattle husbandry in central Ethiopia. Clin Vaccine Immunol. 2007;14(10):1356–1361. doi: 10.1128/CVI.00205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Firdessa R, Tschopp R, Wubete A, Sombo M, Hailu E, Erenso G, et al. High prevalence of bovine tuberculosis in dairy cattle in central ethiopia: implications for the dairy industry and public health. PLoS One. 2012;7(12):e52851. doi: 10.1371/journal.pone.0052851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firdessa R, Berg S, Hailu E, Schelling E, Gumi B, Erenso G, et al. Mycobacterial lineages causing pulmonary and extrapulmonary tuberculosis. Ethiopia. Emerg Infect Dis. 2013;19(3):460–463. doi: 10.3201/eid1903.120256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FMoH . National guideline for Lab, HIV testing in blood safely, surveillance, VCT and ARV use. Addis Ababa, Ethiopia: Federal Ministry of Health; 2003. [Google Scholar]
- 14.Gallagher J, Horwill DM. A selective oleic acid albumin agar medium for the cultivation of Mycobacterium bovis. Journal of Hygiene. 1977;79(1):155–160. doi: 10.1017/S0022172400052943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hershberg R, Lipatov M, Small PM, Sheffer H, Niemann S, Homolka S, et al. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 2008;6(12):e311. doi: 10.1371/journal.pbio.0060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35(4):907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwnetu R, van den Hombergh J, Woldeamanuel Y, Asfaw M, Gebrekirstos C, Negussie Y, et al. Is tuberculous lymphadenitis over-diagnosed in Ethiopia? Comparative performance of diagnostic tests for mycobacterial lymphadenitis in a high-burden country. Scand J Infect Dis. 2009;41(6–7):462–468. doi: 10.1080/00365540902897697. [DOI] [PubMed] [Google Scholar]
- 18.Lin JN, Lai CH, Chen YH, Lee SS, Tsai SS, Huang CK, et al. Risk factors for extra-pulmonary tuberculosis compared to pulmonary tuberculosis. Int J Tuberc Lung Dis. 2009;13(5):620–625. [PubMed] [Google Scholar]
- 19.Yang Z, Kong Y, Wilson F, Foxman B, Fowler AH, Marrs CF, et al. Identification of risk factors for extrapulmonary tuberculosis. Clin Infect Dis. 2004;38(2):199–205. doi: 10.1086/380644. [DOI] [PubMed] [Google Scholar]
- 20.Tschopp R, Schelling E, Hattendorf J, Young D, Aseffa A, Zinsstag J. Repeated cross-sectional skin testing for bovine tuberculosis in cattle kept in a traditional husbandry system in Ethiopia. Vet Rec. 2010;167(7):250–256. doi: 10.1136/vr.c3381. [DOI] [PubMed] [Google Scholar]
- 21.Gumi B, Schelling E, Firdessa R, Aseffa A, Tschopp R, Yamuah L, et al. Prevalence of bovine tuberculosis in pastoral cattle herds in the Oromia region, southern Ethiopia. Trop Anim Health Prod. 2011;43(6):1081–1087. doi: 10.1007/s11250-010-9777-x. [DOI] [PubMed] [Google Scholar]
- 22.de la Rua-Domenech R. Human mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis (Edinb) 2006;86(2):77–109. doi: 10.1016/j.tube.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Hardie RM, Watson JM. Mycobacterium bovis in England and Wales: past, present and future. Epidemiol Infect. 1992;109(1):23–33. [PMC free article] [PubMed] [Google Scholar]
- 24.Rakotosamimanana N, Raharimanga V, Andriamandimby SF, Soares JL, Doherty TM, Ratsitorahina M, et al. Variation in gamma interferon responses to different infecting strains of Mycobacterium tuberculosis in acid-fast bacillus smear-positive patients and household contacts in Antananarivo, Madagascar. Clin Vaccine Immunol. 2010;17(7):1094–1103. doi: 10.1128/CVI.00049-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouassa T, Borroni E, Loukou GY, Faye-Kette H, Kouakou J, et al. High prevalence of shared international type 53 among Mycobacterium tuberculosis complex strains in retreated patients from Côte d’Ivoire. PLoS ONE. 2012;7(9):e45363. doi: 10.1371/journal.pone.0045363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocha A, Elias AR, Sobral LF, Soares DF, Santos AC, Marsico AG, et al. Genotyping did not evidence any contribution of Mycobacterium bovis to human tuberculosis in Brazil. Tuberculosis (Edinb) 2011;91(1):14–21. doi: 10.1016/j.tube.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Diguimbaye C, Hilty M, Ngandolo R, Mahamat HH, Pfyffer GE, Baggi F, et al. Molecular characterization and drug resistance testing of Mycobacterium tuberculosis isolates from Chad. J Clin Microbiol. 2006;44(4):1575–1577. doi: 10.1128/JCM.44.4.1575-1577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quirin R, Rasolofo V, Andriambololona R, Ramboasolo A, Rasolonavalona T, Raharisolo C, et al. Validity of intradermal tuberculin testing for the screening of bovine tuberculosis in Madagascar. Onderstepoort J Vet Res. 2001;68(3):231–238. [PubMed] [Google Scholar]
- 29.Nassar AF, Miyashiro S, Oliveira CG, Pacheco WA, Ogata RA. Isolation and identification of bovine tuberculosis in a Brazilian herd (São Paulo) Mem Inst Oswaldo Cruz. 2007;102(5):639–642. doi: 10.1590/S0074-02762007005000073. [DOI] [PubMed] [Google Scholar]
- 30.Franco MM, Paes AC, Ribeiro MG, de Figueiredo Pantoja JC, Santos AC, Miyata M, et al. Occurrence of mycobacteria in bovine milk samples from both individual and collective bulk tanks at farms and informal markets in the southeast region of Sao Paulo, Brazil. BMC Vet Res. 2013;24(9):85. doi: 10.1186/1746-6148-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller B, Dürr S, Alonso S, Hattendorf J, Laisse CJ, Parsons SD, et al. Zoonotic Mycobacterium bovis-induced tuberculosis in humans. Emerg Infect Dis. 2013;19(6):899–908. doi: 10.3201/eid1906.120543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diguimbaye-Djaibe C, Hilty M, Ngandolo R, Mahamat HH, Pfyffer GE, Baggi F, et al. Mycobacterium bovis isolates from tuberculous lesions in Chadian zebu carcasses. Emerg Infect Dis. 2006;12(5):769–771. doi: 10.3201/eid1205.050691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milian-Suazo F, Perez-Guerrero L, Arriaga-Diaz C, Escartin-Chavez M. Molecular epidemiology of human cases of tuberculosis by Mycobacterium bovis in Mexico. Prev Vet Med. 2010;97(1):37–44. doi: 10.1016/j.prevetmed.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Portillo-Gomez L, Sosa-Iglesias EG. Molecular identification of Mycobacterium bovis and the importance of zoonotic tuberculosis in Mexican patients. Int J Tuberc Lung Dis. 2011;15(10):1409–1414. doi: 10.5588/ijtld.10.0608. [DOI] [PubMed] [Google Scholar]
- 35.Tsegaye A, Aseffa A, Mache A, Mengistu Y, Berg S, Ameni G. Conventional and Molecular Epidemiology of bovine tuberculosis in dairy farms in Addis Ababa City, the capital of Ethiopia. J Appl Res Vet Med. 2010;8(2):143–151. [Google Scholar]
- 36.World Health Organization. 2011. The Global Plan to Stop TB 2011-2015. Stop TB Partnership. http://www.stoptb.org/global/plan/plan1115.asp Last viewed 27 February 2015
- 37.Teo J, Jureen R, Chiang D, Chan D, Lin R. Comparison of two nucleic acid amplification assays, the Xpert MTB/RIF assay and the amplified Mycobacterium tuberculosis direct assay, for detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. J Clin Microbiol. 2011;49(10):3659–3662. doi: 10.1128/JCM.00211-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Virtanen S. A study of nitrate reduction by mycobacteria. The use of the nitrate reduction test in the identification of mycobacteria. Acta tuberculosea Scandinavica Supplementum. 1960;48:1–119. [PubMed] [Google Scholar]
- 39.Wayne LG. Simple pyrazinamidase and urease tests for routine identification of mycobacteria. Am Rev Respir Dis. 1974;109(1):147–151. doi: 10.1164/arrd.1974.109.1.147. [DOI] [PubMed] [Google Scholar]
- 40.Witebsky FG, Kruczak-Filipov P. Identification of mycobacteria by conventional methods. Clin Lab Med. 1996;16(3):569–601. [PubMed] [Google Scholar]
- 41.Kubica T, Agzamova R, Wright A, Rakishev G, Rusch-Gerdes S, Niemann S. Mycobacterium bovis isolates with M. tuberculosis specific characteristics. Emerg Infect Dis. 2006;12(5):763–765. doi: 10.3201/eid1205.050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ameni G, Vordermeier M, Firdessa R, Aseffa A, Hewinson G, Gordon SV, et al. Mycobacterium tuberculosis infection in grazing cattle in central Ethiopia. Vet J. 2011;188(3):359–361. doi: 10.1016/j.tvjl.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ameni G, Tadesse K, Hailu E, Deresse Y, Medhin G, Aseffa A, et al. Transmission of Mycobacterium tuberculosis between farmers and cattle in central Ethiopia. PLoS One. 2013;8(10):e76891. doi: 10.1371/journal.pone.0076891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srivastava K, Chauhan DS, Gupta P, Singh HB, Sharma VD, Yadav VS, et al. Isolation of Mycobacterium bovis & M. tuberculosis from cattle of some farms in north India--possible relevance in human health. Indian J Med Res. 2008;128(1):26–31. [PubMed] [Google Scholar]
- 45.Caws M, Thwaites G, Dunstan S, Hawn TR, Lan NT, Thuong NT, et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 2008;4(3):e1000034. doi: 10.1371/journal.ppat.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lari N, Rindi L, Cristofani R, Rastogi N, Tortoli E, Garzelli C. Association of Mycobacterium tuberculosis complex isolates of BOVIS and Central Asian (CAS) genotypic lineages with extrapulmonary disease. Clin Microbiol Infect. 2009;15(6):538–543. doi: 10.1111/j.1469-0691.2009.02712.x. [DOI] [PubMed] [Google Scholar]
- 47.Tsenova L, Ellison E, Harbacheuski R, Moreira AL, Kurepina N, Reed MB, et al. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J Infect Dis. 2005;192(1):98–106. doi: 10.1086/430614. [DOI] [PubMed] [Google Scholar]
- 48.Reed MB, Gagneux S, Deriemer K, Small PM, Barry CE., 3rd The W-Beijing lineage of Mycobacterium tuberculosis overproduces triglycerides and has the DosR dormancy regulon constitutively upregulated. J Bacteriol. 2007;189(7):2583–2589. doi: 10.1128/JB.01670-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coscolla M, Gagneux S. Does M. tuberculosis genomic diversity explain disease diversity? Drug Discovery Today: Disease Mechanisms. 2010;7(1):e43–e59. doi: 10.1016/j.ddmec.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharaf Eldin GS, Fadl-Elmula I, Ali MS, Ali AB, Salih AL, Mallard K, et al. Tuberculosis in Sudan: a study of Mycobacterium tuberculosis strain genotype and susceptibility to anti-tuberculosis drugs. BMC Infect Dis. 2011;11:219. doi: 10.1186/1471-2334-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bem C. Human immunodeficiency virus-positive tuberculous lymphadenitis in Central Africa: clinical presentation of 157 cases. Int J Tuberc Lung Dis. 1997;1(3):215–219. [PubMed] [Google Scholar]
- 52.Bem C, Patil PS, Bharucha H, Namaambo K, Luo N. Importance of human immunodeficiency virus-associated lymphadenopathy and tuberculous lymphadenitis in patients undergoing lymph node biopsy in Zambia. Br J Surgery. 1996;83(1):75–78. doi: 10.1002/bjs.1800830124. [DOI] [PubMed] [Google Scholar]
- 53.Harries AD, Dye C. Tuberculosis. Ann Trop Med Parasitol. 2006;100(5–6):415–431. doi: 10.1179/136485906X91477. [DOI] [PubMed] [Google Scholar]
- 54.Yassin MA, Olobo JO, Kidane D, Negesse Y, Shimeles E, Tadesse A, et al. Diagnosis of tuberculous lymphadenitis in Butajira, rural Ethiopia. Scand J Infect Dis. 2003;35(4):240–243. doi: 10.1080/00365540310004027. [DOI] [PubMed] [Google Scholar]
- 55.Belay M, Bjune G, Ameni G, Abebe F. Diagnostic and treatment delay among Tuberculosis patients in Afar Region, Ethiopia: a cross-sectional study. BMC Public Health. 2012;12:369. doi: 10.1186/1471-2458-12-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JDH. The social determinants of tuberculosis: from evidence to action. Am J Public Health. 2011;101(4):654–662. doi: 10.2105/AJPH.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kivisild T, Reidla M, Metspalu E, Rosa A, Brehm A, Pennarun E, et al. Ethiopian mitochondrial DNA heritage: tracking gene flow across and around the gate of tears. Am J Hum Genet. 2004;75(5):752–770. doi: 10.1086/425161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lovell A, Moreau C, Yotova V, Xiao F, Bourgeois S, Gehl D, et al. Ethiopia: between Sub-Saharan Africa and western Eurasia. Ann Hum Genet. 2005;69(Pt 3):275–287. doi: 10.1046/J.1469-1809.2005.00152.x. [DOI] [PubMed] [Google Scholar]
- 59.Pareek M, Evans J, Innes J, Smith G, Hingley-Wilson S, Lougheed KE, et al. Ethnicity and mycobacterial lineage as determinants of tuberculosis disease phenotype. Thorax. 2013;68(3):221–229. doi: 10.1136/thoraxjnl-2012-201824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oki NO, Motsinger-Reif AA, Antas PR, Levy S, Holland SM, Sterling TR. Novel human genetic variants associated with extrapulmonary tuberculosis: a pilot genome wide association study. BMC Res Notes. 2011;4(1):28. doi: 10.1186/1756-0500-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thye T, Vannberg FO, Wong SH, Owusu-Dabo E, Osei I, Gyapong J, et al. Genome-wide association analyses identifies a susceptibility locus for tuberculosis on chromosome 18q11.2. Nature Genet. 2010;42(9):739–741. doi: 10.1038/ng.639. [DOI] [PMC free article] [PubMed] [Google Scholar]


