Abstract
Purpose
In patients with malignant pleural mesothelioma (MPM) who are unable to undergo a pneumonectomy, it is difficult to deliver tumoricidal doses of radiation to the pleura without significant toxicity. We have implemented a technique of using intensity-modulated radiotherapy (IMRT) to treat these patients, and we hereby report the feasibility and toxicity of this approach.
Methods and Materials
Between 2005 and 2010, 36 patients with MPM and two intact lungs (i.e. no prior pneumonectomy) were treated with pleural IMRT to the hemithorax (median dose: 46.8 Gy, range: 41.4–50.4 Gy) at Memorial Sloan-Kettering Cancer Center.
Results
Patient characteristics were: right sided (56%), histology (epithelial – 78%, sarcomatoid – 6%, mixed – 17%), stage (I – 6%, II – 28%, III – 33%, IV – 33%). Thirty-two patients (89%) received induction chemotherapy (mostly cisplatin and pemetrexed). 56% underwent pleurectomy/decortication (P/D) prior to IMRT and 44% did not undergo resection. Of 36 patients evaluable for acute toxicity, 7 (20%) had grade 3 or worse pneumonitis (including one death) and 2 had grade 3 fatigue. In 30 patients assessable for late toxicity, 5 had continuing grade 3 pneumonitis. For patients treated with surgery, the 1-year and 2-year survival rates were 75%, 53%, and the median survival was 26 months. For patients who did not have surgical resection, the 1-year and 2-year survival rates were 69%, 28%, and the median survival was 17 months.
Conclusions
Treating the intact lung with pleural IMRT in patients with MPM is a safe and feasible treatment option with an acceptable rate of pneumonitis. Additionally, the survival rates were encouraging in this retrospective series, particularly for the patients who underwent P/D. We have initiated a phase II trial of induction chemotherapy with pemetrexed and cisplatin +/− pleurectomy/decortication followed by pleural IMRT in order to prospectively evaluate toxicity and survival.
Keywords: Malignant pleural mesothelioma, IMRT, pneumonitis
Introduction
Malignant pleural mesothelioma (MPM) is difficult to treat due to its locally aggressive behavior. Controversy surrounds the optimal treatment for early stage mesothelioma; however studies using multimodality therapy have shown promising local control and survival. Since the most common site of treatment failure for MPM is the ipsilateral hemithorax, optimizing local control provides the best chance for long-term survival.
Two types of surgery are performed for mesothelioma: extrapleural pneumonectomy (EPP) and pleurectomy/decortication (P/D). EPP involves en bloc resection of the entire pleura, lung, diaphragm, and ipsilateral half of the pericardium. P/D involves resection of all gross tumor without removing the lung. Neither P/D nor EPP alone offers adequate long-term local control or survival rates. After EPP, adjuvant hemithoracic radiation to the chest cavity has been shown to improve local control and survival, but distant failure remains a problem. A number of studies have investigated whether more advanced RT techniques, such as intensity modulated radiation therapy (IMRT), lead to better local control than standard RT after EPP. However, IMRT can be associated with increased toxicity, including fatal pneumonitis, if radiation exposure to the remaining lung is not carefully restricted.1–3
More recently, enthusiasm for EPP has waned, particularly since this surgery is associated with increased morbidity and mortality relative to P/D. A report compiling data on 663 patients with MPM managed surgically suggested that those who underwent EPP had an inferior survival compared to patients who had P/D.4 Although this analysis was subject to significant retrospective biases, it has had a major influence on the practice of many thoracic surgeons. However, with P/D local control remains the primary issue and radiation is more challenging to administer due to the risk of pneumonitis in the intact lung. Our prior institutional experience with standard pleural radiation was not promising.5 The difference in the effectiveness of radiation therapy in these situations can be partially explained by the fact that the complete removal of the ipsilateral lung with EPP allows higher doses of radiation to be delivered. However, implementation of an IMRT technique could enable higher doses to be administered even with an intact lung, and thereby may improve efficacy. This paper reports on our experience using a novel technique to treat the pleura with IMRT.
Materials and Methods
Thirty-six patients with biopsy proven MPM were treated with IMRT to the hemithorax without pneumonectomy at MSKCC between 2005 and 2010. Eleven of these patients were enrolled on an MSKCC Institutional Review Board approved feasibility protocol. The others were treated after the feasibility of the technique had been established. Their records were reviewed with an IRB waiver. All patients were not candidates for EPP due to impaired pulmonary function or extent of tumor and instead underwent either P/D, pleurectomy only, or no surgery. Specific patient characteristics are presented in Table 1.
Table 1.
Patient Characteristics
| N (%) | |
|---|---|
|
| |
| Age (years) | |
| Median | 67 |
| Range | 42–82 |
|
| |
| Gender | |
| Male | 29 (81) |
| Female | 7 (19) |
|
| |
| Histological subtype | |
| Epithelial | 28 (78) |
| Sarcomatoid | 2 (6) |
| Mixed | 6 (17) |
|
| |
| Surgery | |
| P/D or P | 20 (56) |
| Nonoperative | 16 (44) |
|
| |
| Stage | |
| I | 2 (6) |
| II | 10 (28) |
| III | 12 (33) |
| IV | 12 (33) |
|
| |
| Laterality | |
| Right | 20 (56) |
| Left | 16 (44) |
|
| |
| Chemotherapy | |
| Yes | 32 (89) |
| No | 4 (11) |
Abbreviations: P/D= pleurectomy/decortication, P= pleurectomy. Stage is based on the International Mesothelioma Interest Group Staging System. Data is presented as number of patients (N), with percentages in parentheses.
All patients were immobilized in a supine position with their arms raised in a customized alpha-cradle mold (Alpha Cradle Molds, Akron, OH) prior to CT simulation. All patients received a planning CT scan (Model PQ 5000 AcQSim, Marconi/Philips Medical Systems, Cleveland, OH or GE Discovery/ST PET-CT simulator). Based on this study, an initial PTV (PTV-CT) was defined as a rind composed of the pleura (if remaining) and the chest wall of the entire hemithorax. The PTV-CT typically began superiorly at the thoracic inlet and continued inferiorly until the insertion of the diaphragm into approximately the T12 or L1 vertebral body. Any visible gross disease was also included in the PTV-CT with a margin of approximately 8 mm. The typical thickness of the PTV-CT rind was 14–16 mm.
PET-CT scans were used for treatment planning. In each case, the patient was required to fast for at least 6 hours and then received an intravenous administration of 370 mCi of F-18 labeled FDG. Following this, the patients rested for approximately one hour before obtaining the PET scan. For patients simulated on the AcQSim, the PET scan was acquired several days after simulation, using a GE Discovery/LS in the Nuclear Medicine Department and in-house image registration software was used to register the planning CT with the PET study and to display the CT and PET images side by side. The patients simulated on the Discovery/ST PET-CT simulator received their PET study immediately after the planning scan and the vendor’s software was used to register and display the studies. The initial PTV was modified to include any areas of increased FDG uptake and sometimes to exclude areas of very low uptake, thus forming the PTV_PET. To assess respiratory motion, most patients simulated received a respiratory correlated cine-CT scan immediately following the planning CT and PET scans. If necessary, the PTV was further modified to account for estimated respiratory motion.
Treatment planning was performed with the MSKCC treatment-planning system, which has been described previously.6–10 The optimization algorithm9 minimizes a quadratic objective function which has terms to control target homogeneity, maximum and minimum doses and normal organ maximum dose, dose-volume combinations and mean dose (mean dose below 20–21 Gy is a useful lung constraint). Tissue inhomogeneity corrections were applied to all dose calculations.8 Treatments were delivered with 6 MV photons utilizing the sliding window IMRT method on Varian linear accelerators (600C, 2100C, or 2100EX depending on machine availability) with dynamic multi-leaf collimator (DMLC).10 Beam directions consisted of 6–8 angles, approximately equi-spaced over a 200° to 240° sector encompassing the ipsilateral lung; Figure 1 is an example of a typical isodose distribution. Specific directions were manually chosen by the planner to satisfy the clinical dosimetric objectives for the tumor and surrounding normal structures. Since a requirement of Varian DMLC delivery is that the separation of the most extended and most retracted leaves on the same leaf carriage be less than 14.5–15 cm (depending on MLC model), fields wider than this limit had to be “split” into two separate deliveries. The planners generally attempted to keep dose to the contralateral lung low, although formal dosimetric criteria were not applied to this structure. The planning goal was to deliver the prescription dose to at least 95% of the PTV while meeting normal tissue constraints described below. All patients were treated with conventional fractionation (1.8 Gy) with no planned treatment breaks. The MU’s per beam ranged from 80–200.
Figure 1. Isodense beam distribution.
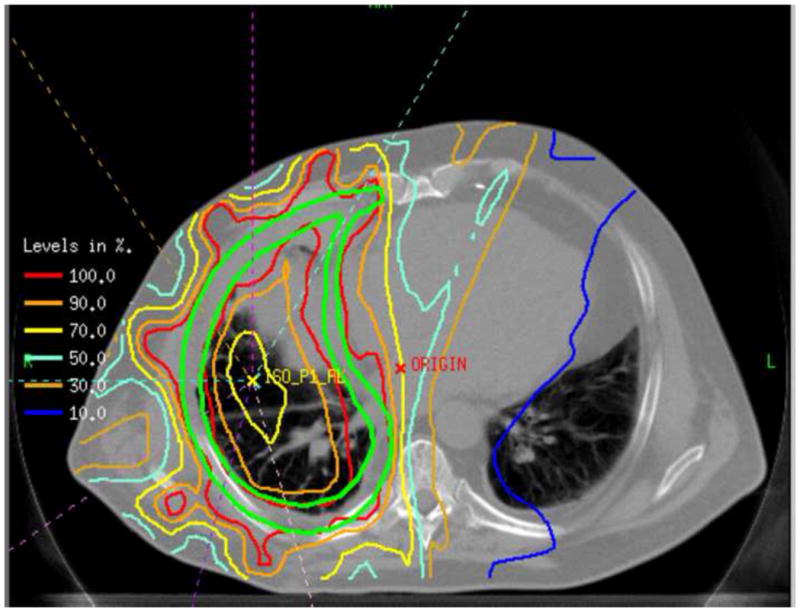
Depicted above is an example of a typical isodose distribution using 8 angles equispaced over 200° to 240° sector encompassing the ipsilateral lung. The area within the green lines is the target volume.
The prescription dose goal for this group of patients was 50.4 Gy but was reduced if normal tissue dose constraints (usually lung) could not be met. The spinal cord and total lung were dose-limiting tissues. For the lung, we required that the NTCP not exceed 25%11 as calculated with the Lyman model12 using the dose volume histogram (DVH) reduction scheme of Kutcher and Burman and the model parameters of Burman et al. for severe radiation pneumonitis.13,14 For this model, NTCP of 25% corresponds to an approximate mean lung dose of 20 Gy. The maximum spinal cord dose was kept below 50 Gy. A volumetric dose display was used to detect hot spots exceeding 110–115% of prescription outside the PTV which were reduced or eliminated by re-optimizing with maximum dose constraints applied to dummy structures surrounding hot-spots. In general, these are challenging plans requiring several repeat optimizations (average 20–25 for our dosimetrists).
Acute toxicities and late treatment-related complications were evaluated during routine on-treatment visits and follow-up visits one month after completion of treatment and then every 3–4 months. Side effects were graded based on the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Acute reactions included those experienced during treatment or within the first 4 months after the end of treatment and were graded according to the most severe reaction. Any complications that began or persisted after 4 months were considered to be late reactions. Overall survival was estimated using Kaplan-Meier method, starting from the time of diagnosis until death or last available follow-up, whichever came first. In addition, time to local control failure (defined as disease progression within the radiation field) and time to distant recurrence (defined as disease occurrence or progression outside of the radiation field) were estimated using as time origin the end of IMRT treatment, with patients followed until local/distant failure or last available follow-up. Group comparisons were performed using the log-rank test, with the level of significance α = 0.05.
Results
The median radiation dose was 4680 cGy (range, 4140–5040 cGy). Acute treatment-related toxicity frequencies are presented in Table 2. Patients were able to complete treatment with medical support and without the need for treatment breaks. No patients required intravenous hydration during treatment. One patient required the placement of a PEG tube prior to the initiation of the radiation treatment due to poor nutritional status and was able to maintain an adequate nutritional status throughout treatment. Of 36 patients evaluable for acute toxicity, 2 (6%) experienced grade 3 fatigue, one of whom had pre-existing grade 2 fatigue prior to treatment. No other severe non-pulmonary toxicities were observed.
Table 2.
Acute Toxicity
| Grade (N=36) | ||||||
|---|---|---|---|---|---|---|
| Acute Toxicity | 0 | 1 | 2 | 3 | 4 | 5 |
| Arrhythmia | 36 | 0 | 0 | 0 | 0 | 0 |
| Dermatitis | 20 | 16 | 0 | 0 | 0 | 0 |
| Dyspnea | 4 | 20 | 5 | 5 | 1 | 1 |
| Esophagitis | 17 | 15 | 4 | 0 | 0 | 0 |
| Fatigue | 5 | 18 | 11 | 2 | 0 | 0 |
| Nausea | 21 | 7 | 8 | 0 | 0 | 0 |
| Pericarditis/cardiac | 36 | 0 | 0 | 0 | 0 | 0 |
| Pneumonitis | 24 | 5 | 0 | 5 | 1 | 1 |
| Vomiting | 33 | 3 | 0 | 0 | 0 | 0 |
Seven patients (20%) experienced severe pneumonitis. All received steroids but, as some were managed at outside hospitals, we do not know the doses or length of treatment for everyone. Five patients had grade 3 pneumonitis. Three of these 5 patients underwent resection and 3 of the 5 also received chemotherapy prior to radiation treatment. Figure 2 is an example of the grade 3 pneumonitis typically experienced by these patients. These images are from a 65 year old man who initially presented with stage III left-sided disease and received three cycles of pemetrexed and cisplatin before he underwent P/D. He received 45 Gy IMRT to the left pleura. Within 3 months of completing his radiation therapy, he experienced significant shortness of breath that required oxygen supplementation. He received a course of steroids for about 4 months which allowed his oxygen to be weaned. At the time of this manuscript, he remains off steroids, off oxygen, and without evidence of recurrence 29 months after completing radiation therapy.
Figure 2. Typical imaging of radiation pneumonitis after IMRT.
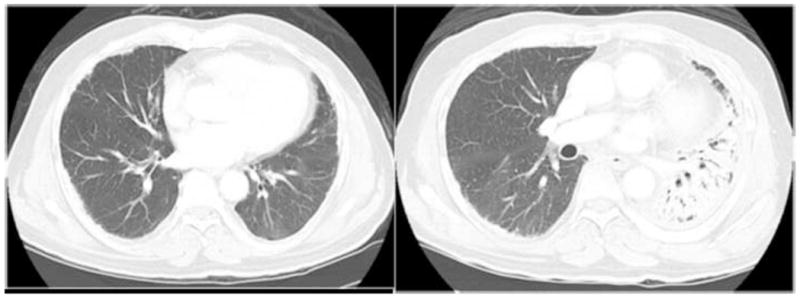
Left: prior to any therapy; Right: at the time of this patient’s most severe pneumonitissymptoms. Despite complete recovery, the imaging findings persist.
One patient, an 82 year old woman, who presented with a solitary paraspinal lesion, required mechanical ventilation due to acute pneumonitis after radiation therapy. She was the oldest patient in this cohort and had poor baseline lung function with an FEV1 38% of predicted that precluded surgical intervention for her mesothelioma. After treatment with steroids, she was successfully extubated.
There was one death possibly attributable to acute pneumonitis in an 81 year old man, the second oldest in the cohort, who had grade 2 fatigue and a poor performance status to his treatment. Additionally, he had progression of disease after chemotherapy as well as after surgery, reflecting the aggressiveness of his disease. He had moderate impairment of his baseline lung function prior to treatment with an FEV1 61% of predicted and DLCO 50% of predicted. His death was likely due to a combination of progression of disease and worsening fatigue exacerbated by radiation therapy.
In 30 patients assessable for late toxicity, 5 had continuing grade 3 pneumonitis. None of these patients required prolonged oxygen supplementation and all were eventually tapered off steroids. No new late pulmonary toxicities appeared in patients who did not have acute toxicities. The six patients not assessable for late toxicity died within 4 months of completing IMRT.
With a median followup of 18 months from diagnosis (range, 8–80 months), the median overall survival was 18 months (95% CI: 15–33 months). Survival at 1 year and 2 years was 72% and 42% respectively. For patients who underwent P/D in addition to IMRT, median survival was 26 months from the time of diagnosis (Figure 3). Survival at 1 year and 2 years was 75% and 53% respectively. 16 patients did not undergo resection: 11 (69%) due to unresectable disease and 5 (31%) due to poor lung function. Among these patients, median survival following diagnosis was 17 months (Figure 4). Survival at 1 year and 2 years was 69% and 28% respectively.
Figure 3.
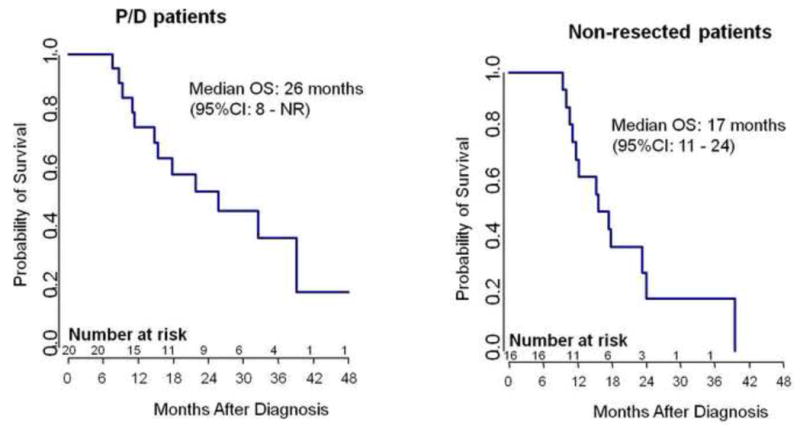
A -- P/D Patient Survival
Kaplan-Meier survival curve for the patients who underwent resection of their MPM by P/D.
B -- Non-resected Patient Survival
Kaplan-Meier survival curve for the patients who did not undergo resection of their MPM.
Patterns of failure were different in surgical and non-surgical patients. Among those who underwent P/D, the probability of experiencing local and distant failure during the first 12 months post-treatment was 48% and 10% respectively. In contrast, among non-resected patients, the probability of experiencing local and distant failure during the first 12 months post-treatment was 63% and 31% respectively. However, among non-resected patients, the median time to local recurrence after completion of IMRT was 4 months, suggesting the possibility of benefit of IMRT even in the absence of surgical intervention.
Discussion
This report demonstrates that pleural IMRT in patients with MPM and an intact ipsilateral lung is a feasible treatment option with an acceptable toxicity profile. Historically, our institution has favored EPP followed by adjuvant hemithorax radiation for the treatment of MPM. In a phase II trial, there was a median survival of 17 months using this treatment paradigm.15 Radiation was well tolerated since the removal of the ipsilateral lung during EPP minimized pleural toxicity. Median overall survival in stage I/II patients was 34 months and 10 months in stage III/IV patients. Additionally, local failure rates were low: 4% (2) locoregional failure only; 9% (5) locoregional and distant failure; 55% (30) distant failure only. Notably, the few local recurrences that did occur were at the margins of the radiation field. Several subsequent studies have incorporated this hemithoracic radiation technique in a multimodality approach after induction chemotherapy and EPP. The median survival for patients treated on these trials has ranged from 17–20 months.
Despite these promising results, many patients are unable to tolerate an EPP either because of the extent of their disease or other co-morbid medical conditions. Additionally, while the decision between EPP and P/D remains controversial, a multi-institutional retrospective analysis reported improved survival among patients receiving P/D compared to those receiving EPP.4 Consequently, at many institutions, fewer EPPs are being performed which has increased the population of patients seeking radiation treatment for MPM with two intact lungs.
Limited data are available on pleural radiation in patients with two intact lungs. At our center, in a series of 123 patients treated with P/D followed by standard radiation therapy with a median dose of 42.5 Gy, with or without brachytherapy, the median overall survival was 13.5 months and the 2-year survival was 23%.5 Local failure rates were high: 33% (40) local failure only; 24% (29) local and distant failure; 11% (14) distant failure only. Toxicity was also significant with 2 deaths from toxicity within one month of treatment and 35 patients with grade 3 or 4 toxicity. Another series of 35 patients treated with radical pleurectomy, chemotherapy, and radiation, reported a median overall survival of 30 months and 1-, 2-, and 3-year survival of 69%, 50%, and 31% respectively.16
Since conventional radiation techniques in this patient population have been unable to provide adequate local control without significant toxicity, more advanced treatment delivery techniques such as IMRT were investigated. In other disease sites with complex target volumes, IMRT improved dose distribution and decreased normal tissue toxicity when compared to conventional techniques. Previous reports have described IMRT use after EPP and local control and survival have been promising. In one series of 63 patients from MD Anderson Cancer Center treated with IMRT after EPP, median survival was 14 months and there was a 5% incidence of local recurrence.17 However, there have also been reports of severe pulmonary toxicity including fatal pneumonitis ranging from 8–46% at 4 different institutions.2,3,18 One small series described 11 patients with unresectable MPM who were treated with up to 50 Gy of IMRT.19 This therapy was tolerated quite well with only 4 patients experiencing grade 2 skin reactions. No pneumonitis or late toxicity was reported. The 2-year overall survival was 36% with a median follow up of 23 months.
Based on these encouraging results and the need to identify a safe, feasible, and efficacious radiation technique for MPM patients with 2 intact lungs, we have reported our institutional experience with IMRT in this setting. There was a 20% rate of acute grade 3 or worse toxicity, including one possible treatment related death in an older patient with preexisting frailty. Even this possible 3% rate of grade 5 pneumonitis is favorable when compared to the risks associated with other treatment paradigms involving other major surgical procedures. All but one of the patients with pneumonitis recovered with steroids. Late severe toxicity included 5 cases of grade 3 pneumonitis, all of whom had experienced acute severe pneumonitis.
Finally, survival in our cohort was encouraging. Among patients who underwent pleural IMRT after P/D, median overall survival was 26 months with a median follow-up of 28 months from the time of diagnosis. These rates compare favorably with those reported in the multimodality studies described above. Survival was also substantially increased among patients who did not undergo resection with a median overall survival of 17 months. After chemotherapy, all patients eventually progress and secondary treatment options are limited. In the phase III Vogelzang trial of pemetrexed/cisplatin which enrolled patients with unresectable MPM, the median time to progression was 6 months and the median survival was 12 months. Thus, IMRT after other treatment modalities appears to improve progression free and overall survival in these patients. Therefore, we believe this modest risk of toxicity to be acceptable. Also, as we gain more experience with this technique and employ better patient selection for this therapy, the 20% rate of severe toxicity will likely decrease: since the only two cases of grade 4 and 5 pneumonitis occurred in patients with poor baseline lung function, restricting pleural IMRT use in those with severe pre-existing compromised lung function or significantly compromised performance status may help eliminate grade 4–5 pneumonitis. Consequently, we are currently enrolling patients in a phase II trial of induction chemotherapy, possible P/D, and pleural IMRT to better examine the safety and efficacy of IMRT in the multi-modality treatment paradigm.
Table 3.
Late Toxicity
| Grade (N=30) | ||||||
|---|---|---|---|---|---|---|
| Late Toxicity | 0 | 1 | 2 | 3 | 4 | 5 |
| Esophagitis | 30 | 0 | 0 | 0 | 0 | 0 |
| Pulmonary | 9 | 12 | 4 | 5 | 0 | 0 |
Footnotes
Conflicts of Interest Notification
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahamad A, Stevens CW, Smythe WR, et al. Intensity-modulated radiation therapy: a novel approach to the management of malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2003;55:768–75. doi: 10.1016/s0360-3016(02)04151-2. [DOI] [PubMed] [Google Scholar]
- 2.Allen AM, Czerminska M, Janne PA, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys. 2006;65:640–5. doi: 10.1016/j.ijrobp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Rice DC, Smythe WR, Liao Z, et al. Dose-dependent pulmonary toxicity after postoperative intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2007;69:350–7. doi: 10.1016/j.ijrobp.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135:620–6. 6 e1–3. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 5.Gupta V, Mychalczak B, Krug L, et al. Hemithoracic radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2005;63:1045–52. doi: 10.1016/j.ijrobp.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 6.Burman C, Chui CS, Kutcher G, et al. Planning, delivery, and quality assurance of intensity-modulated radiotherapy using dynamic multileaf collimator: a strategy for large-scale implementation for the treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1997;39:863–73. doi: 10.1016/s0360-3016(97)00458-6. [DOI] [PubMed] [Google Scholar]
- 7.Chui CS, LoSasso T, Spirou S. Dose calculation for photon beams with intensity modulation generated by dynamic jaw or multileaf collimations. Med Phys. 1994;21:1237–44. doi: 10.1118/1.597206. [DOI] [PubMed] [Google Scholar]
- 8.Mohan R, Barest G, Brewster LJ, et al. A comprehensive three-dimensional radiation treatment planning system. Int J Radiat Oncol Biol Phys. 1988;15:481–95. doi: 10.1016/s0360-3016(98)90033-5. [DOI] [PubMed] [Google Scholar]
- 9.Spirou SV, Chui CS. A gradient inverse planning algorithm with dose-volume constraints. Med Phys. 1998;25:321–33. doi: 10.1118/1.598202. [DOI] [PubMed] [Google Scholar]
- 10.Spirou S, Chui C. Generation of arbitrary intensity profiles by dynamic jaws or multileaf collimators. Med Phys. 1994;21:1031–47. doi: 10.1118/1.597345. [DOI] [PubMed] [Google Scholar]
- 11.Rosenzweig KE, Mychalczak B, Fuks Z, et al. Final report of the 70.2-Gy and 75. 6-Gy dose levels of a phase I dose escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable non-small cell lung cancer. Cancer J. 2000;6:82–7. [PubMed] [Google Scholar]
- 12.Lyman J. Complication probability as assessed from dose-volume histograms. Radiat Res. 1985;104:13–9. [PubMed] [Google Scholar]
- 13.Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991;21:123–35. doi: 10.1016/0360-3016(91)90172-z. [DOI] [PubMed] [Google Scholar]
- 14.Kutcher GJ, Burman C, Brewster L, Goitein M, Mohan R. Histogram reduction method for calculating complication probabilities for three-dimensional treatment planning evaluations. Int J Radiat Oncol Biol Phys. 1991;21:137–46. doi: 10.1016/0360-3016(91)90173-2. [DOI] [PubMed] [Google Scholar]
- 15.Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2001;122:788–95. doi: 10.1067/mtc.2001.116560. [DOI] [PubMed] [Google Scholar]
- 16.Bolukbas S, Manegold C, Eberlein M, Bergmann T, Fisseler-Eckhoff A, Schirren J. Survival after trimodality therapy for malignant pleural mesothelioma: Radical Pleurectomy, chemotherapy with Cisplatin/Permetrexed and radiotherapy. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Rice DC, Stevens CW, Correa AM, et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2007;84:1685–92. doi: 10.1016/j.athoracsur.2007.04.076. discussion 92–3. [DOI] [PubMed] [Google Scholar]
- 18.Miles EF, Larrier NA, Kelsey CR, et al. Intensity-modulated radiotherapy for resected mesothelioma: the Duke experience. Int J Radiat Oncol Biol Phys. 2008;71:1143–50. doi: 10.1016/j.ijrobp.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Munter MW, Thieke C, Nikoghosyan A, Nill S, Debus J. Inverse planned stereotactic intensity modulated radiotherapy (IMRT) in the palliative treatment of malignant mesothelioma of the pleura: the Heidelberg experience. Lung Cancer. 2005;49 (Suppl 1):S83–6. doi: 10.1016/j.lungcan.2005.03.031. [DOI] [PubMed] [Google Scholar]


