Abstract
Dry eye (DE) disease is commonly associated with ocular surface inflammation, an unstable tear film and symptoms of irritation. However, little is known about the role of central neural mechanisms in DE. This study used a model for persistent aqueous tear deficiency, exorbital gland removal, to assess the effects of mustard oil, a TRPA1 agonist, on eyeblink and eyewipe behavior and Fos-like immunoreactivity (Fos-LI) in the trigeminal brainstem of male rats. Spontaneous tear secretion was reduced by about 50% and spontaneous eyeblinks were increased more than 100% in DE rats compared to sham rats. Mustard oil (0.02–0.2%) caused dose-related increases in eyeblink and forelimb eyewipe behavior in DE and sham rats. Exorbital gland removal alone was sufficient to increase Fos-LI at the ventrolateral pole of trigeminal interpolaris/caudalis (Vi/Vc) transition region, but not at more caudal regions of the trigeminal brainstem. Under barbiturate anesthesia ocular surface application of mustard oil (2–20%) produced Fos-LI in the Vi/Vc transition, in the mid-portions of Vc and in the trigeminal caudalis/upper cervical spinal cord (Vc/C1) region that was significantly greater in DE rats than in sham controls. Mustard oil caused an increase in Fos-LI ipsilaterally in superficial laminae at the mid-Vc and Vc/C1 regions in a dose-dependent manner. Smaller, but significant, increases in Fos-LI also were seen in the contralateral Vc/C1 region in DE rats. TRPA1 protein levels in trigeminal ganglia from DE rats ipsilateral and contralateral to gland removal were similar.
Persistent tear reduction enhanced the behavioral and trigeminal brainstem neural responses to ocular surface stimulation by mustard oil. These results suggested that TRPA1 mechanisms play a significant role in the sensitization of ocular-responsive trigeminal brainstem neurons in this model for tear deficient DE.
Keywords: Dry eye, central sensitization, eyeblink behavior, c-Fos, TRPA1
1. Introduction
Dry eye (DE) is a multifactorial disease characterized by an unstable tear film and symptoms of ocular discomfort (DEWS Report Classification 2007). Symptoms of ocular dryness, irritation, and itch as well as visual disturbances, have a negative impact on quality of life and are the main reasons DE patients seek medical attention (Begley et al. 2002; Pflugfelder 2011; Uchino and Schaumberg 2013). Although nearly all forms of DE are accompanied by elevated proinflammatory biomarkers in tears and other peripheral signs of tear dysfunction (Calonge et al. 2010; Lambiase et al. 2011; Bron et al. 2014; Stern et al. 2013), treatments directed at reducing ocular inflammation often fail to adequately manage symptoms of irritation in moderate to severe DE (Asbell and Spiegel 2010). This suggests that factors other than peripheral nerve activity contribute to the symptoms of discomfort in DE.
Tear film integrity depends on a reflex circuit termed the lacrimal functional unit (LFU) that consists of trigeminal sensory nerves in the eye, integrative mechanisms in the brain, and autonomic efferent nerves that drive lacrimal gland secretion (Stern et al. 1998; 2004). Although dysfunction of any element within the circuit may result in DE-like conditions, the vast majority of studies on LFU activity and DE have focused on peripheral mechanisms and far fewer on brain pathways. All tissues of the eye are supplied by sensory branches of the trigeminal nerve that terminate centrally in two main regions of the lower trigeminal brainstem complex, the trigeminal interpolaris/caudalis (Vi/Vc) transition and the Vc/upper cervical cord (Vc/C1) region (Marfurt 1981; Marfurt and del Toro 1987; Marfurt and Echtenkamp 1988; Panneton et al. 2010). Previously, we reported that only neurons at the Vi/Vc transition responded to changes in moisture status of the ocular surface (Hirata et al. 2004), whereas only neurons at the Vc/C1 region became sensitized after systemic (Bereiter et al. 2005) or ocular surface inflammation (Tashiro et al. 2010). This suggested that neurons in each region serve different aspects of ocular function. The present study used exorbital gland removal to determine if Transient Receptor Potential Ankyrin (TRPA1) contributes to altered behavior and sensitization of trigeminal brainstem neurons during conditions of reduced tear secretion. TRPA1 is an excitatory ion channel that is gated by wide variety of chemical irritants (Bautista et al. 2006) and is expressed in ~35% of trigeminal ganglion neurons (Jordt et al. 2004; Kobayashi et al. 2005; Kim et al. 2010). TRPA1 is reportedly upregulated by proinflammatory molecules found in tears of DE patients (Diogenes et al. 2007; Malsch et al. 2014). TRPA1 contributes to behavioral hypersensitivity in a variety of persistent inflammatory pain models (Bautista et al. 2006; Dai et al. 2007; McNamara et al. 2007; Malin et al. 2011; Lennertz et al. 2012). TRPA1 activation also has been linked to chronic itch (Wilson et al. 2013), a common symptom in DE patients (Begley et al. 2002), and enhanced tearing and blinking in an animal model of allergic keratoconjunctivitis (Acosta et al. 2013).
2. Experimental procedures
2.1. Animal preparation
The animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Minnesota (USA) and conformed to the established guidelines set by The National Institutes of Health guide for the care the use of laboratory animals (PHS Law 99–158, revised 2002). All efforts were made to minimize the number of animals used for these experiments and to minimize their suffering. Animals were housed in pairs with free access to food and water. Cages remained in climate and light controlled protected units (25 ± 2 °C, 12:12-h light/dark cycle with light on at 7:00AM) for at least 5 days before experiments.
Male rats (200–320 g, Sprague-Dawley, Harlan, Indianapolis, IN) were anesthetized with isoflurane (3.0–5.0 %) and a small incision was made in skin approximately 5 mm in front of and inferior to the left tragus to expose and excise the left exorbital gland. Sham rats received the same surgical procedure for exposure, but without gland removal. Rats survived 7 days or 14 days after gland removal, while sham rats survived 14 days after surgery.
2.2. Tear volume measurement
Spontaneous tear volume was measured under pentobarbital sodium (50 mg/kg) anesthesia by the increase in wet length of phenol red thread (ZONE-QUICK, Menicon INC., San Mateo, CA; Hamano et al., 1983). The thread was placed at the inferior-lateral edge of the cornea/conjunctiva interface immediately after removing excess tears with a fine filter paper. Spontaneous tear volume was sampled over 2 min in all animals prior to subsequent ocular surface stimulation. For comparison, tear volume also was measured in naïve controls (n = 6). The data were assessed by analysis of variance (ANOVA) corrected for repeated measures. Significant treatment effects were further assessed by Newman-Keuls test after ANOVA. The data were presented as mean ± standard error of mean (SEM) and significance level set at p<0.05.
2.3. Eyeblink and eyewipe behavior
Rats (n = 5) were placed in a Plexiglas chamber and allowed to habituate for 1h prior to testing. Test solutions (0.02% and 0.20% mustard oil (MO), 20μl) were applied to the ipsilateral eye by micropipette in ascending order of concentration at 30 min intervals. Eyewipe behavior was observed directly and defined as purposeful wiping of the periorbital face by the forelimb, adapted from the method of Shimada and LaMotte (2008). Eyeblinks included partial and full lid closures. Spontaneous and evoked eyewiping and eyeblinks were counted over 5 min prior to exorbital gland removal and on days 7 and 14 after exorbital gland removal. In pilot studies eyeblink responses to repeated application 0.2% MO did not change when presented at 30 min intervals. Rats used for behavioral testing were separate from those used for Fos immunohistochemistry.
2.4.1. Experimental design – Fos immunohistochemistry
On the day of the experiment, rats (n = 53) were anesthetized with pentobarbital sodium (50 mg/kg, i.p.) and tear volume measured as noted above. Body temperature was maintained at 37°C with a heating blanket. Adequate depth of anesthesia was confirmed by loss of the hind limb withdrawal reflex. The effect of ocular instillation of vehicle, 2% MO or 20% MO (allyl isothiocyanate, 10 μl, Sigma-Aldrich, St. Louis, MO, dissolved in mineral oil, Bereiter 2001) on Fos-like immunoreactivity (Fos-LI) was assessed in sham, 7 day DE (7d DE) and 14 day DE (14d DE) rats. Chemicals were applied and remained on the ocular surface throughout the 2 h survival period. For sham rats, the number of animals per treatment was: vehicle = 5, 2% MO = 4 and 20% MO = 5. For 7d DE rats, the number of rats per treatment was: vehicle = 5, 2% MO = 5 and 20% MO = 5. For 14d DE rats, the number of rats per treatment was: vehicle = 6, 2% MO = 4 and 20% MO = 5. Additional rats served as stimulus controls and were anesthetized for 2 h, but received no ocular surface stimulation (sham NS = 3; 7d DE NS = 3; 14d DE NS = 3). Fluorescein staining was performed in a small group of rats at 14 days after gland removal to assess ocular surface epithelial barrier function (n = 3). Fluorescein (2%, 10 μl, Sigma, St. Louis, MO) was applied to the ocular surface and after 2 min flushed with artificial tears. The ocular surface was examined through a halogen light source and Wratten yellow 12 filter. Fluorescein staining was negligible in all animals.
2.4.2. Fos Immunohistochemistry
At 2 h after ocular stimulation animals were given a bolus injection of pentobarbital sodium (70 mg/kg, i.p.) and perfused through the heart with heparinized saline followed by 250 ml cold fixative (4% paraformaldehyde, 0.1 M phosphate, pH 7.4). The lower brainstem /upper cervical spinal cord segment was removed and post-fixed for 24 h. Transverse sections (40 μm) were cut on a vibratome and collected in cold 0.01 M phosphate-buffered saline (PBS). Sections were incubated sequentially in 5% normal donkey serum and 0.3% Triton X-100 (60 min), affinity-purified rabbit polyclonal anti-Fos antibody (Ab-5, Calbiochem, La Jolla, CA; 1:5000, 40 h at 4 °C), biotinylated donkey anti-rabbit antibody (Millipore, Billerica, MA; 1:300, 90 min) and avidin-biotin-peroxidase complex (Vector, Burlingame, CA; 60 min). Fos-LI was visualized by incubation in a nickel-cobalt diaminobenzidine solution activated by 0.01% hydrogen peroxide. After rinsing in PBS, sections were mounted on slides, air-dried and coverslipped with mounting medium (Thermo Scientific, Waltham, MA). Under bright-field illumination, Fos-positive neuronal nuclei appeared as homogenous, gray-black elements with a well-defined border. Specific staining was abolished by omission of primary antiserum.
2.4.3. Data analysis
Brainstem sections were categorized according to rostrocaudal level at 500 μm intervals from 2 mm rostral to 6.5 mm caudal to the obex. The obex is a surface landmark defined by the caudal end of the fourth ventricle approximately 500 μm rostral to the most caudal tip of trigeminal subnucleus interpolaris (Yoshida et al., 1991). Seventy to 90 sections per animal were counted at 40 × magnification without prior knowledge of treatment. To facilitate statistical comparisons across multiple treatments, the average number of Fos-LI neurons per section was compared for the following brainstem regions (see Fig. 5): Vi/Vc transition (+0.5 to −1.5 mm); middle portion of Vc (mid-Vc, −2 to −4 mm); and the Vc/C1 region (−4.5 – −6.5 mm). At the mid-Vc and Vc/C1 regions separate cells counts were made for superficial (I – II) and deeper laminae (III – V) using established cytological landmarks (Molander et al., 1989). Mean cell counts per rat were compared by two-way analysis of variance (ANOVA). Individual comparisons were made by the Newman-Keuls test after significant main effects by ANOVA. Data were expressed in the text and figures as mean ± standard error of mean (SEM). Significance levels were defined at p<0.05.
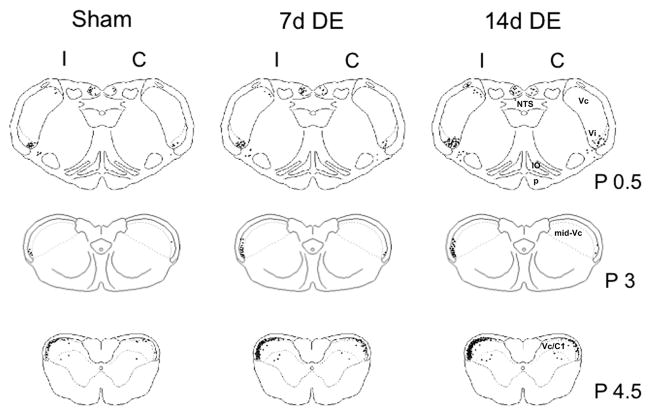
Figure 5.
Camera lucida examples of Fos-LI produced at the level of the Vi/Vc transition, midVc and Vc/C1 regions after 20% MO. One dot = 1 Fos-positive neuronal nucleus. Numbers to the right of the outlines indicate distance (in mm) from obex. Abbreviations (right side): IO, inferior olive; NTS, nucleus tractus solitarus; p, pyramidal tract. See list of abbreviations for further descriptions.
2.5. Immunoblot for TRPA1 of trigeminal ganglion
Rats were anesthetized with pentobarbital sodium (50 mg/kg, i.p.) and perfused through the heart with heparinized saline followed by 250 ml cold fixative (4% paraformaldehyde, 0.1 M phosphate, pH 7.4). Trigeminal ganglia from 14d DE rats (n = 4), ipsilateral and contralateral to exorbital gland removal, were homogenized with ceramic beads in buffer of tris-buffered saline, pH 7.4, 1% Triton X-100, 10mM ethylene glycol tetraacetic acid (EGTA), 10mM ethylene diamine tetraacetic acid (EDTA), and protease inhibitor cocktail (Complete Mini, Roche, Indianapolis, IN). Protein concentrations were determined after centrifugation (15,000×G, 20 min, 4°C) by BCA Protein Assay Kit (Pierce, Rockford, IL). Protein (40 μg) was separated bySDS-PAGE in 7.5% Tris-HCl gels (Bio-Rad, Berkeley, CA) and transferred onto 0.45 μM nitrocellulose membrane (Bio-Rad), blocked for 1 h in Odyssey blocking buffer (Li-Cor, Lincoln, NE), and incubated with primary rabbit polyclonal anti-TRPA1 antibody (Thermo Scientific; 1:1000) in TBS with Triton X-100 (TBST) overnight at 4°C. Mouse monoclonal anti-β-tubulin antibody (Millipore, Billerica, MA; 1:1000) was simultaneously included for normalization. After TBST wash, the following secondary antibodies were used at 1:15,000 dilution: anti-rabbit IgG IRDye 680 and anti-mouse IgG IRDye 800CW (Li-Cor, 60 min). Protein bands were visualized on a Li-Cor Odyssey Infrared scanner and quantified with Odyssey 2.1 analysis software.
3. Results
3.1. Spontaneous tear volume
Spontaneous tear volume was reduced significantly (F6,41 = 14.1, p<0.001) in the ipsilateral eye of 7d DE (n = 6) and 14d DE rats (n = 5) compared to the contralateral eye of DE rats and compared to sham (n = 10) or naïve controls (n = 6) (Fig 1). However, tear volume was similar for 7d and 14d DE rats.
Figure 1.
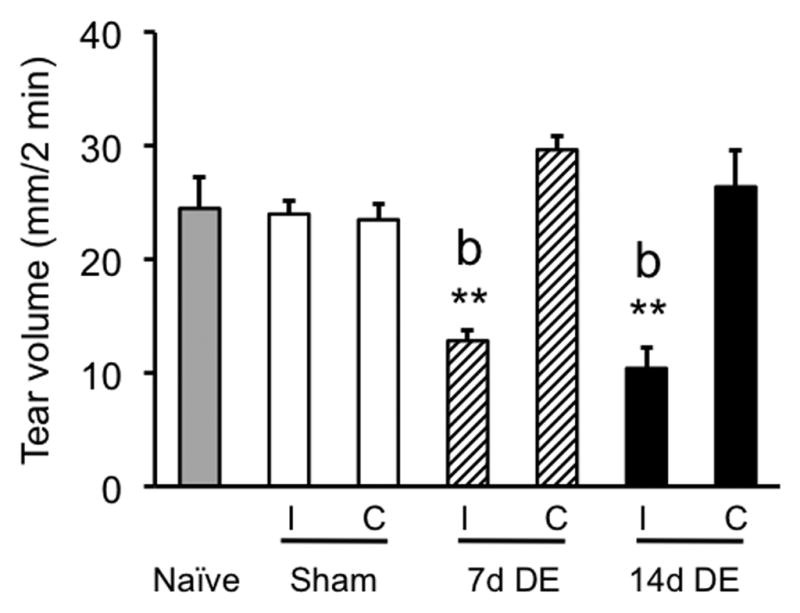
Spontaneous tear volume was reduced ipsilateral (I), but not contralateral (C) to exorbital gland removal at 7 days and 14 days after surgery. Tear volume was measured over 2 min under barbiturate anesthesia. Sample sizes (rats per group) = naïve (6), sham (10), 7d DE (6), and 14d DE (5). **p<0.01 versus contralateral eye; b = p<0.01 versus other groups.
3.2. Eyeblink and eyewipe behavior
Spontaneous eyeblink behavior was increased significantly in 14d DE rats compared to sham controls (24 ± 4.8 versus 9.1 ± 2.5 blinks per 5 min; F1,6 = 8.5, p < 0.05, n = 4). Ocular instillation of 0.2% MO caused a marked increase in eyeblinks compared to 0.02% MO in all groups (F1,12 = 44.2, p<0.001) (Fig 2, upper panel). Post hoc analysis revealed a further enhancement in eyeblinks to 0.2% MO of 14d DE rats compared to other groups and compared to spontaneous blink rates (F2,22 = 4.9, p<0.025). Forelimb eyewipe behavior (Fig 2, lower panel) was increased after 0.2% MO stimulation in sham and DE groups compared to the response to 0.02% MO (F1,12 = 26.4, p<0.001).
Figure 2.
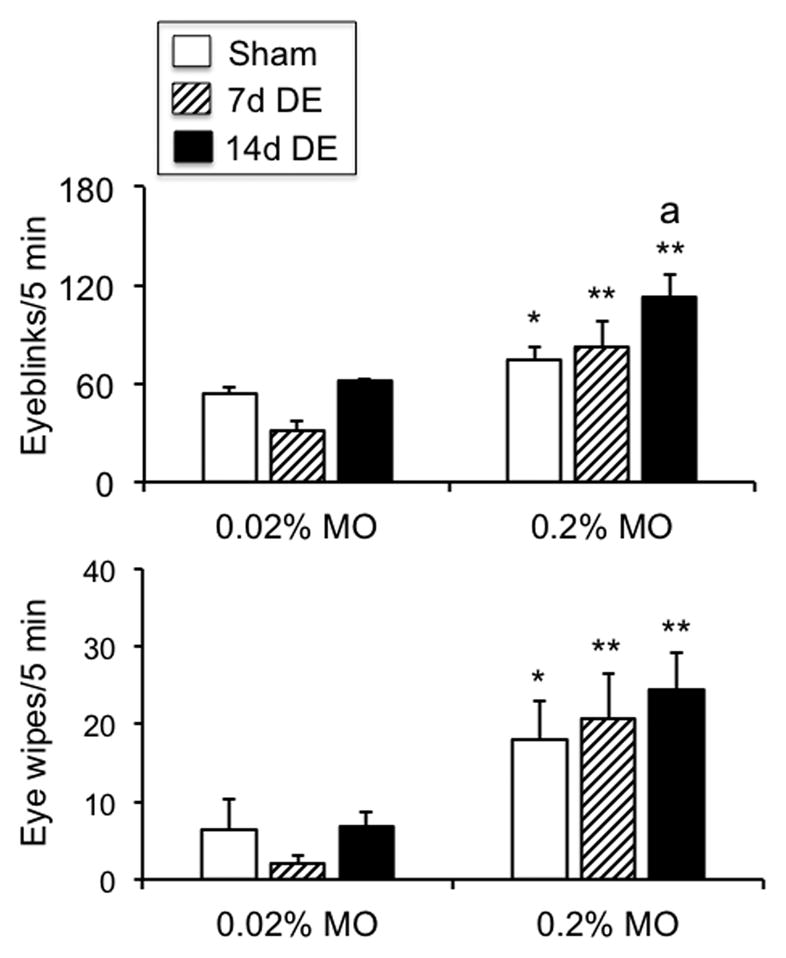
Eyeblink (upper panel) and forelimb eyewipe behavior (lower panel) was evoked by low doses of MO in all groups. Sample sizes (rats per group) = sham (5), 7d DE (5), and 14d DE (5). *p<0.05, **p<0.01 versus response to 0.02% MO; a = p<0.05 versus sham.
3.3. Fos Immunohistochemistry
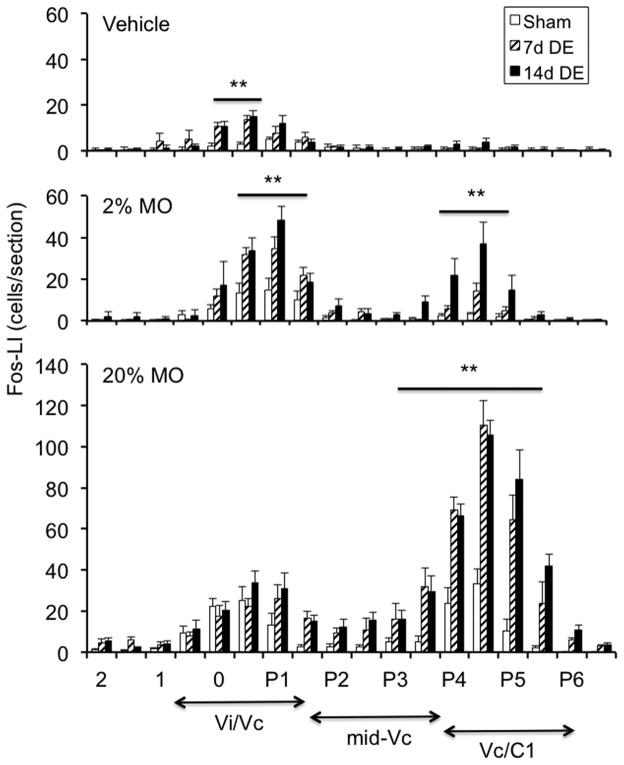
Persistent tear reduction alone was sufficient to increase Fos-LI at the Vi/Vc transition ipsilateral to exorbital gland removal (Fig 3, F17,204 = 9.8, p<0.001) with smaller, and insignificant, increases seen contralateral to gland removal in sham, 7d DE and 14d DE rats (data not shown). Note that gland removal alone did not cause increases in Fos-LI in more caudal regions of Vc in the absence of ocular surface stimulation, in agreement with earlier results after acute drying of the ocular surface (Hirata et al. 2004). As seen in Fig 4 (upper panel), vehicle application to the ipsilateral eye produced a single peak of Fos-LI at the Vi/Vc transition in 7d and 14d DE rats that was significantly greater than in sham rats (F5,26 = 11.1, p<0.001). Smaller increases in Fos-LI also occurred at the contralateral Vi/Vc transition after vehicle application in 7d and 14d DE groups (data not shown). Following 2% MO (Fig 4, middle panel) sham and 7d DE rats displayed a single peak of Fos-LI at the ipsilateral Vi/Vc transition, while in 14d DE rats Fos-LI was also significantly increased at the Vc/C1 region (F5,20 = 35.2, p<0.001). After 20% MO (Fig 4, lower panel) Fos-LI was increased at the ipsilateral Vi/Vc transition and at the Vc/C1 region in sham, 7d DE and 14d DE groups (F5,24 = 31.4, p<0.001) with caudal increases predominating.
Figure 3.
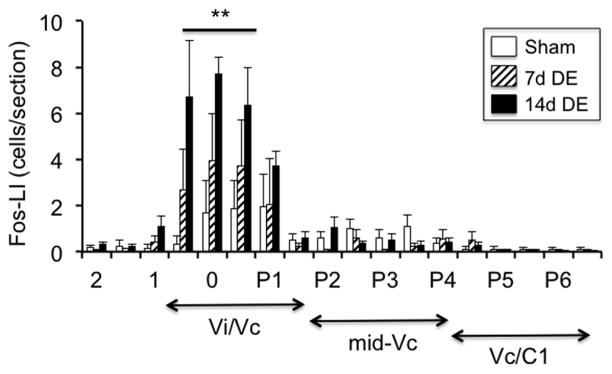
Tear reduction alone was sufficient to increase Fos-LI at the Vi/Vc transition in 7d DE and 14d DE rats ipsilateral to exorbital gland removal. Sample size = 3 rats per group. **p<0.01 versus sham group. Numbers on the x-axis refer to the distance from obex (mm). The approximate rostrocaudal boundaries of trigeminal subnucleus regions and upper cervical segments are indicated below.
Figure 4.
Rostrocaudal distribution of Fos-LI within the trigeminal brainstem complex of sham, 7d DE and 14d DE rats. Upper panel: vehicle application produced Fos-LI only at the Vi/Vc transition. Middle panel: 2% MO produced a bimodal pattern of Fos-LI at the Vi/Vc transition and at the Vc/C1 region in 14d DE rats, while Fos-LI in sham and 7d DE rats was mainly at the Vi/Vc transition. Lower panel: 20% MO increased Fos-LI at the Vi/Vc transition and at the Vc/C1 region in all groups. Numbers on the x-axis refer to the distance from obex (mm). The approximate rostrocaudal boundaries of trigeminal subnucleus regions and upper cervical segments are indicated below. Sample sizes (rats per group) = sham (4–5), 7d DE (4–5), and 14d DE (4–6). **p<0.01 versus sham.
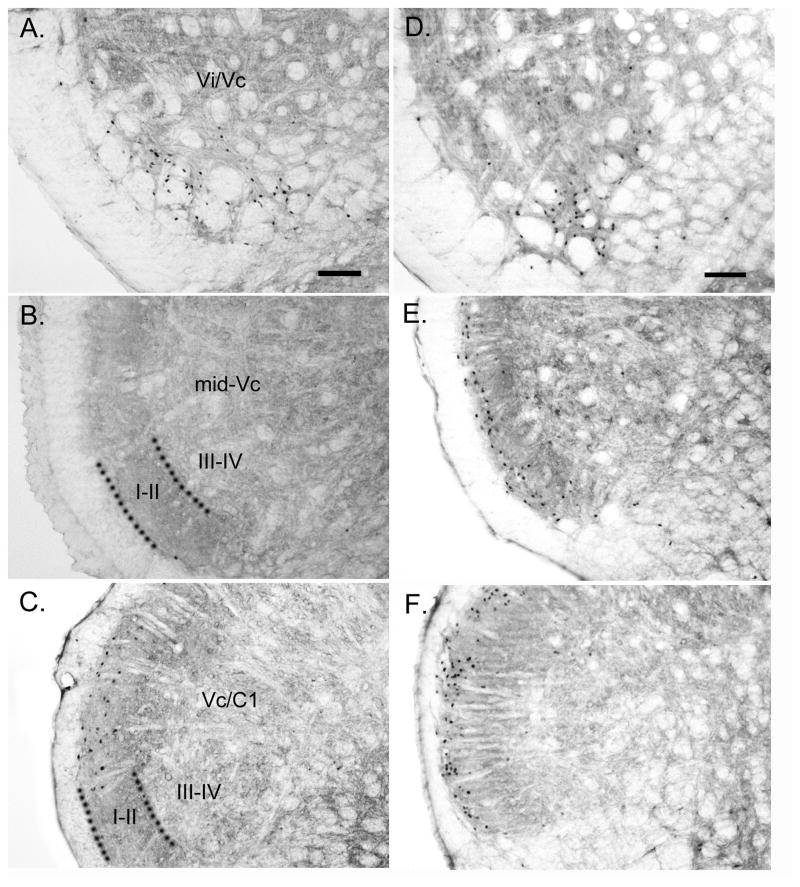
Representative examples of Fos-LI produced at the level of the Vi/Vc transition, mid-Vc and Vc/C1 regions after 20% MO were drawn by camera lucida and mapped onto a series of rat brainstem outlines (Fig 5). As seen in Fig 5 (upper panels) Fos-LI was produced mainly at the ventrolateral pole of the Vi/Vc transition with scattered cells in the dorsomedial pole of Vi/Vc transition and in the dorsal column nuclei. Fos-LI also was produced inconsistently in the nucleus tractus solitarius and inferior olive in sham, 7d DE and 14d DE groups (not shown). At the level of the mid-Vc (Fig 5, middle panels) Fos-LI was restricted to the superficial laminae ipsilateral to the MO stimulus. At the level of the Vc/C1 region (Fig 5, lower panels) Fos-LI also was produced mainly in superficial laminae; however, unlike at the mid-Vc, Fos-positive neurons also were seen contralateral to the applied MO stimulus. Figure 6 presents examples of Fos staining for sham (Figs 6A, B and C; Vi/Vc transition, mid-Vc and Vc/C1 region, respectively) and 14d DE rats (Figs 6D, E, and F; Vi/Vc transition, mid-Vc and Vc/C1 region, respectively) following instillation of 20% MO into the ipsilateral eye.
Figure 6.
Micrograph examples of Fos staining in sham and 14d DE rats after 20% MO applied to the ipsilateral ocular surface. A, B and C = Vi/Vc transition, mid-Vc and Vc/C1 region, respectively, in sham rats. D, E and F = Vi/Vc transition, mid-Vc and Vc/C1 region, respectively, in 14d DE rats. Calibration bar in (A) and (F) = 100 μm. Dotted lines in (B) and (C) indicate border between laminae II and III. Abbreviations (left side): I–II, superficial laminae; III–IV, deeper laminae. See list of abbreviations for further descriptions.
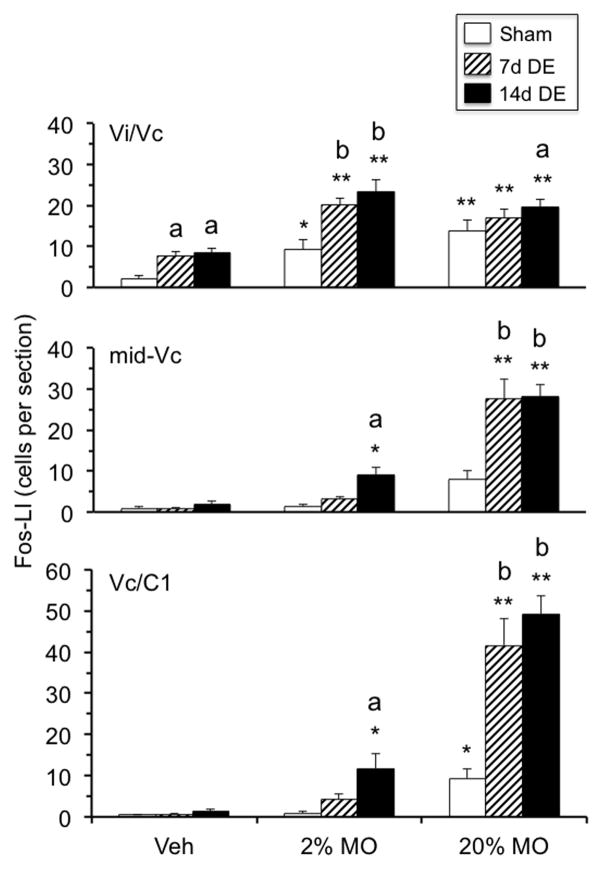
To more clearly assess Fos-LI responses across animal treatments (sham, 7d DE and 14d DE) and ocular stimuli (vehicle, 2% MO and 20% MO), the data were analyzed separately for the Vi/Vc/transition, mid-Vc and Vc/C1 regions, regions identified from the rostrocaudal distributions in Figs 4 and Fig 5 and based on our previous studies indicating a close association of these brainstem regions with ocular sensory processing (Meng et al. 1997; Hirata et al. 2004). At the Vi/Vc transition (Fig 7, upper panel) 2% and 20% MO produced a significant increase in Fos-LI ipsilateral to the stimulus in sham, 7d DE and 14d DE rats compared to the contralateral side (F1,35 = 244, p<0.001, contralateral data not shown). Further analysis revealed no significant group differences for Fos-LI produced at the contralateral Vi/Vc transition regardless of the applied stimulus (data not shown). Individual comparisons revealed that the Fos-LI responses to vehicle and 2% MO in 7d DE and 14d DE rats were greater than the response in sham rats (F8,35 = 9.7, p<0.001). However, the Fos-LI responses at the Vi/Vc transition in 7d DE and 14d DE rats were similar regardless of the applied stimulus. At the mid-Vc region (Fig 7, middle panel) highly significant (F8,35 = 20.5, p<0.001) treatment main effects were seen as Fos-LI increased in a dose-related manner in 14d DE rats and was greater than sham controls after 2% (p<0.01) and 20% MO (p<0.001). The Fos-LI response at mid-Vc in 7d DE rats was significantly different from sham rats only after 20% MO stimulation (p<0.001). Note that Fos-LI did not increase significantly at the mid-Vc in sham rats after 2% or 20% MO (see examples in Figs 6B and 6F). At the Vc/C1 region (Fig 7, lower panel) highly significant (F8,35 = 39.4, p<0.001) treatment main effects were seen and were similar to those seen at mid-Vc. That is, Fos-LI displayed a MO dose-related increase, whereas sham and 7d DE rats displayed significant increases only after 20% MO. Fos-LI also was produced at the Vc/C1 region contralateral to 20% MO in 14d DE rats and was significantly greater in 7d DE and 14d DE rats compared to sham rats (F8,63 = 10.2, p<0.001, data not shown). Fos-LI produced in deep laminae at the mid-Vc and Vc/C1 regions of 7d DE and 14d DE rats revealed numerical, but non-significant, increases after 20% MO (data not shown).
Figure 7.
Summary of treatment effects on Fos-LI produced ipsilateral to exorbital gland removal in sham, 7d DE and 14d DE rats. Upper panel: Vi/Vc transition, Fos-LI increased after vehicle, 2% MO and 20% MO in 7d DE and 14d DE rats. Note that 2% and 20% MO produced similar responses in all groups. Middle panel: midVc, Fos-LI increased after MO in a dose-related manner in DE rats, whereas sham rats displayed no significant increase. Lower panel: Vc/C1 region, Fos-LI increased after MO in a dose-related manner in all groups. Note that at midVc and Vc/C1 regions only 14d DE rats displayed significant increases in Fos-LI after 2% MO. Sample sizes (rats per group) = sham (4–5), 7d DE (4–5), and 14d DE (4–6). *p<0.05, **p<0.01 versus vehicle, a = p<0.05, b = p<0.01 versus sham group.
3.4. TRPA1 protein levels in trigeminal ganglion
TRPA1 protein levels from trigeminal ganglia (n = 4) of 14d DE rats, ipsilateral and contralateral to exorbital gland removal, were similar and averaged 1.94 ± 0.2 and 2.3 ± 0.6 relative intensity units, respectively. These results suggested that TRPA1 protein levels of peripheral sensory neurons were not altered in this model for aqueous tear deficient DE.
4. Discussion
These results indicated that persistent tear reduction enhanced the behavioral and trigeminal brainstem responses to TRPA1 receptor activation. Exorbital gland removal alone was sufficient to increase Fos-LI at the Vi/Vc transition region, whereas Fos-LI produced after ocular instillation of MO was significantly greater at the Vi/Vc transition, mid-Vc and Vc/C1 regions in 7d DE and 14d DE rats compared to sham controls. Significant increases in Fos-LI at the mid-Vc and Vc/C1 regions in 14d DE rats occurred at a lower MO concentration than in sham rats and occurred bilaterally. Spontaneous eyeblink and MO-evoked eyeblink and forelimb eyewipe behavior were increased in 14d DE rats compared to sham rats.
It is understood that many forms of DE can be classified as chronic inflammatory conditions (DEWS Report 2007; Calonge et al. 2010; Stern et al. 2013). Accordingly, current management strategies are designed to reduce the peripheral signs of ocular inflammation (Behrens et al. 2006; Alves et al. 2013). Although current treatments are effective in reducing ocular inflammation, such treatments often are not adequate to manage the symptoms of irritation in moderate to severe forms of DE (Asbell and Siegel 2010). In animal models of other chronic inflammatory pain conditions such as arthritis (Schaible et al. 2009) and visceral pain (Vergnolle 2008, Bradesi 2010) persistent exposure to proinflammatory agents, even at low levels, leads to sensitization of spinal cord neurons. Although a persistent elevation of proinflammatory cytokines and neuropeptides reported in tears of DE patients might be expected to sensitize second-order ocular neurons in trigeminal brainstem, this has never been tested directly in an animal model for DE.
A unique feature of the trigeminal somatosensory system, unlike spinal systems, is that craniofacial structures are represented somatotopically at multiple levels of the trigeminal brainstem complex (Bereiter et al. 2009). In all species examined thus far, trigeminal primary afferent fibers that innervate the eye terminate centrally at the Vi/Vc transition and at the Vc/C1 region (Marfurt 1981; Marfurt and del Toro 1987; Marfurt and Echtenkamp 1988; Panneton et al. 2010). The functional significance of this dual representation is not certain. However, based on encoding properties, responses to analgesics and ascending projection targets in naïve rats, we (Meng et al. 1997; Hirata et al. 1999; Hirata et al. 2000; Hirata et al. 2004) and others (Pelligrini et al. 1995) have proposed that neurons at each region serve different aspects of ocular function. Previously we reported that only ocular neurons at the Vc/C1 region, and not those at the Vi/Vc transition, displayed evidence consistent with central sensitization in models for uveitis (Bereiter et al. 2005) or photokeratitis (Tashiro et al. 2010; Chang et al. 2010). By contrast, in the present study the Fos-LI response to 2% and 20% MO was significantly greater at the Vi/Vc transition, and in the mid-Vc and Vc/C1 regions in DE rats compared to sham controls. Furthermore, the increased Fos-LI response to 2% MO in 14d DE rats compared to sham controls suggested that persistent tear reduction lowered the threshold for activation and increased the spread of neural excitation for ocular-responsive neurons within the caudal trigeminal brainstem in a TRPA1-dependent manner. At high concentrations of MO (20%) the Fos-LI response at the Vi/Vc transition was similar to that seen after 2% MO and was not different between DE and sham rats. By contrast, Fos-LI produced at the mid-Vc and Vc/C1 regions was clearly dose-dependent and after 20% MO was markedly greater in DE rats than sham rats. Interestingly, exorbital gland removal alone was sufficient to produce Fos-LI at the Vi/Vc transition consistent with results seen after acute drying of the ocular surface (Hirata et al. 2004). This suggested that the reduced spontaneous tear volume in our DE model (~50% reduction) was sufficient to excite ongoing activity of moisture-sensitive corneal afferent fibers that would be expected to increase during ocular surface dryness (Meng and Kurose 2013). These data support previous studies indicating that ocular stimulus intensity coding is a more pronounced feature of Vc/C1 neurons compared to Vi/Vc transition neurons (see Bereiter et al. 2000), whereas the Vi/Vc transition may be more involved in homeostatic reflex responses such as lacrimation and eyeblink (Hirata et al. 2004; Rahman et al. 2014) and maintenance of a stable tear film.
The classification and diagnosis of DE is based largely on peripheral signs of tear film instability and symptoms of discomfort (DEWS Report 2007; Sullivan 2014). Although an elevated level of proinflammatory molecules in tear samples is a consistent finding in DE (Calonge et al. 2010; Pflugfelder 2011; Stern et al. 2013), the relationship between inflammatory molecules in tears and the central integration of ocular sensory input that underlies the symptoms in DE is poorly defined. In the present study, behavioral and neural responses were evoked by mustard oil, a selective TRPA1 agonist, applied to the ocular surface. It was not possible to use the same concentration of MO for behavior measurements in awake animals and for Fos-LI counts in anesthetized rats. In pilot studies concentrations of MO greater than 0.2% caused pronounced eyelid closure and rats did not blink. Also, MO-evoked eyeblink and forelimb wiping behavior likely would have produced a Fos-LI response that may have confounded the interpretation of Fos-LI produced only by ocular surface stimulation. By contrast, the Fos-LI response in trigeminal brainstem to 0.2% MO would be expected to be low, since in an earlier study we found that injection of 0.2% MO or vehicle into the temporomandibular joint of barbiturate-anesthetized males rats produced similar responses (Bereiter 2001). TRPA1 is a member of the TRP family of non-selective cationic channels and is gated by a wide variety of proinflammatory agents (Bandell et al. 2004; Bautista et al. 2006). Although TRPA1 has been shown to be upregulated in other persistent inflammatory conditions (Malin et al. 2011; Lennertz et al. 2012), the fact that trigeminal ganglia protein levels were similar ipsilateral and contralateral to exorbital gland removal suggested that this DE model did not cause severe inflammation. This does not, however, exclude the possibility that peripheral TRPA1 may contribute to the maintenance of ocular irritation in DE, since elevated levels of proinflammatory agents such as nerve growth factor (Lambiase et al. 2011) and IL-6 in tears of DE patients (Lam et al. 2009; Na et al. 2012) could enhance TRPA1 functionality in neurons without necessarily increasing receptor expression (Diogenes et al. 2007; Malin et al. 2011; Malsch et al. 2014).
The present results were consistent with a central mechanism to account for the effects of persistent tear reduction on TRPA1-induced behavioral and central neural responses to ocular surface stimulation. However, despite no difference in trigeminal ganglion protein levels of TRPA1 ipsilateral and contralateral to gland removal, these results cannot exclude a role for peripheral mechanisms as a driving force for longer-term changes in the brain during persistent inflammation (Woolf and Costigan 1999; Baron et al. 2013). Dryness and irritation are common symptoms in DE (Begley et al. 2002). Considerable evidence suggests that TRPM8-positive trigeminal afferent neurons are responsible for a sense of dryness and provide the afferent signal for spontaneous tearing (Parra et al. 2010; Robbins et al. 2012). Although we did not specifically test for possible TRPM8 involvement, TRPM8- and TRPA1-positive neurons likely represent separate populations of trigeminal ganglion cells (Kobayashi et al. 2005). Several lines of evidence suggest that the afferent signals that drive the sensation of irritation versus those necessary to maintain a stable film represent input from different classes of nerve fibers. For example, sensitization of polymodal corneal afferent neurons after acute exposure to inflammatory agents did not affect the response to hyperosmolar tears (Parra et al. 2014), while topical application of selective TRPA1 antagonists did not influence trigeminal ganglion responses to drying the cornea or to hyperosmolar tears (Hirata and Oshinsky 2012). In preliminary studies, we found that ocular instillation of menthol, a TRPM8 agonist, did not produce significant increases in Fos-LI in the rat trigeminal brainstem nuclear complex.
Conclusions
The present results indicated that persistent reduction in tears significantly enhanced the responsiveness of neurons to ocular surface stimulation by MO. Enhanced responsiveness was seen in the form of a reduced threshold concentration of MO needed to evoke significant increases in Fos-LI in the trigeminal brainstem and in the spread of the Fos-LI response to a greater number of trigeminal brainstem regions. These data support the hypothesis that TRPA1-related mechanisms play a key role in mediating the effects of persistent tear deficiency on central neuron excitability and symptoms of irritation in DE.
Highlights.
Exorbital gland removal reduced tear volume by ~50%.
Enhanced eyeblink responses to 0.2% MO in 14 day dry eye rats.
Enhanced Fos-like immunoreactive neurons in caudal trigeminal brainstem of dry eye rats.
No change in TRPA1 protein in trigeminal ganglion of dry eye rats.
Acknowledgments
This work was supported by a grant from NIH (EY021447).
Abbreviations
- DE
dry eye
- Fos-LI
Fos-like immunoreactivity
- mid-Vc
middle portion of trigeminal subnucleus caudalis
- MO
mustard oil
- TRPA1
transient receptor potential ankyrin
- TRPM8
transient receptor potential melanostatin
- Vi/Vc
trigeminal subnucleus interpolaris/caudalis
- Vc/C1
trigeminal subnucleus caudalis/upper cervical region
Footnotes
The authors have no financial or other relationships to report that might lead to conflict interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- Acosta MC, Luna C, Quirce S, Belmonte C, Gallar J. Changes in sensory activity of ocular surface sensory nerves during allergic keratoconjunctivitis. Pain. 2013;154:2335–2352. doi: 10.1016/j.pain.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Alves M, Fonseca EC, Alves MF, Malki LT, Arruda GV, Reinach PS, Rocha EM. Dry eye disease treatment: a systematic review of published trials and a critical appraisal of therapeutic strategies. Ocul Surf. 2013;11:181–192. doi: 10.1016/j.jtos.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Asbell PA, Spiegel S. Ophthalmologist perceptions regarding treatment of moderate-to-severe dry eye: results of a physician survey. Eye Contact Lens. 2010;36:33–38. doi: 10.1097/ICL.0b013e3181c739ad. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Baron R, Hans G, Dickenson A. Peripheral input and its importance for central sensitization. Ann Neurol. 2013 doi: 10.1002/ana.24017. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Begley CG, Caffery B, Chalmers RL, Mitchell GL. Use of the dry eye questionnaire to measure symptoms of ocular irritation in patients with aqueous tear deficient dry eye. Cornea. 2002;21:664–670. doi: 10.1097/00003226-200210000-00007. [DOI] [PubMed] [Google Scholar]
- Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, Dua HS, Hom M, Karpecki PM, Laibson PR, Lemp MA, Meisler DM, Del Castillo JM, O’Brien TP, Pflugfelder SC, Rolando M, Schein OD, Seitz B, Tseng SC, van Setten G, Wilson SE, Yiu SC. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25:900–907. doi: 10.1097/01.ico.0000214802.40313.fa. [DOI] [PubMed] [Google Scholar]
- Bereiter DA. Sex differences in brainstem neural activation after injury to the TMJ region. Cells Tissues Organs. 2001;169:226–237. doi: 10.1159/000047886. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Hargreaves KM, Hu JW. Trigeminal mechanisms of nociception: peripheral and brainstem organization. In: Basbaum A, Bushnell MC, editors. Science of Pain. Vol. 5. New York: Elsevier; 2009. pp. 435–460. [Google Scholar]
- Bereiter DA, Hirata H, Hu JW. Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain. 2000;88:221–224. doi: 10.1016/S0304-3959(00)00434-6. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Okamoto K, Tashiro A, Hirata H. Endotoxin-induced uveitis causes long-term changes in trigeminal subnucleus caudalis neurons. J Neurophysiol. 2005;94:3815–3825. doi: 10.1152/jn.00616.2005. [DOI] [PubMed] [Google Scholar]
- Bradesi S. Role of spinal cord glia in the central processing of peripheral pain perception. Neurogastroenterol Motil. 2010;22:499–511. doi: 10.1111/j.1365-2982.2010.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron AJ, Tomlinson A, Foulks GN, Pepose JS, Baudouin C, Geerling G, Nichols KK, Lemp MA. Rethinking Dry Eye Disease: A Perspective on Clinical Implications. Ocul Surf. 2014;12:S1–S31. doi: 10.1016/j.jtos.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Calonge M, Enriquez-de-Salamanca A, Diebold Y, Gonzalez-Garcia MJ, Reinoso R, Herreras JM, Corell A. Dry eye disease as an inflammatory disorder. Ocul Immunol Inflamm. 2010;18:244–253. doi: 10.3109/09273941003721926. [DOI] [PubMed] [Google Scholar]
- Chang Z, Okamoto K, Tashiro A, Bereiter DA. UV irradiation of the eye and Fos-positive neurons induced in trigeminal brainstem after intravitreal or ocular surface TRPV1 activation. Neuroscience. 2010;170:678–685. doi: 10.1016/j.neuroscience.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogenes A, Akopian AN, Hargreaves KM. NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res. 2007;86:550–555. doi: 10.1177/154405910708600612. [DOI] [PubMed] [Google Scholar]
- Hamano H, Hori M, Hamano T, Mitsunaga S, Maeshima J, Kojima S, Kawabe H, Hamano T. A new method for measuring tears. CLAO J. 1983;9:281–289. [PubMed] [Google Scholar]
- Hirata H, Hu JW, Bereiter DA. Responses of medullary dorsal horn neurons to corneal stimulation by CO2 pulses in the rat. J Neurophysiol. 1999;82:2092–2107. doi: 10.1152/jn.1999.82.5.2092. [DOI] [PubMed] [Google Scholar]
- Hirata H, Okamoto K, Tashiro A, Bereiter DA. A novel class of neurons at the trigeminal subnucleus interpolaris/caudalis transition region monitors ocular surface fluid status and modulates tear production. J Neurosci. 2004;24:4224–4232. doi: 10.1523/JNEUROSCI.0381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Oshinsky ML. Ocular dryness excites two classes of corneal afferent neurons implicated in basal tearing in rats: involvement of transient receptor potential channels. J Neurophysiol. 2012;107:1199–1209. doi: 10.1152/jn.00657.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Takeshita S, Hu JW, Bereiter DA. Cornea–responsive medullary dorsal horn neurons: modulation by local opioid agonists and projections to thalamus and brainstem. J Neurophysiol. 2000;84:1050–1061. doi: 10.1152/jn.2000.84.2.1050. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibers through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Kim YS, Son JY, Kim TH, Paik SK, Dai Y, Noguchi K, Ahn DK, Bae YC. Expression of transient receptor potential ankyrin 1 (TRPA1) in the rat trigeminal sensory afferents and spinal dorsal horn. J Comp Neurol. 2010;518:687–698. doi: 10.1002/cne.22238. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147:198–205. e191. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Micera A, Sacchetti M, Cortes M, Mantelli F, Bonini S. Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol. 2011;129:981–986. doi: 10.1001/archophthalmol.2011.200. [DOI] [PubMed] [Google Scholar]
- Lennertz RC, Kossyreva EA, Smith AK, Stucky CL. TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PLoS One. 2012;7:e43597. doi: 10.1371/journal.pone.0043597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin S, Molliver D, Christianson JA, Schwartz ES, Cornuet P, Albers KM, Davis BM. TRPV1 and TRPA1 function and modulation are target tissue dependent. J Neurosci. 2011;31:10516–10528. doi: 10.1523/JNEUROSCI.2992-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malsch P, Andratsch M, Vogl C, Link AS, Alzheimer C, Brierley SM, Hughes PA, Kress M. Deletion of Interleukin-6 Signal Transducer gp130 in Small Sensory Neurons Attenuates Mechanonociception and Down-Regulates TRPA1 Expression. J Neurosci. 2014;34:9845–9856. doi: 10.1523/JNEUROSCI.5161-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF. The central projections of trigeminal primary afferent neurons in the cat as determined by the transganglionic transport of horseradish peroxidase. J Comp Neurol. 1981;203:785–798. doi: 10.1002/cne.902030414. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Del Toro DR. Corneal sensory pathway in the rat: a horseradish peroxidase tracing study. J Comp Neurol. 1987;261:450–459. doi: 10.1002/cne.902610309. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Echtenkamp SF. Central projections and trigeminal ganglion location of corneal afferent neurons in the monkey, macaca fascicularis. J Comp Neurol. 1988;272:370–382. doi: 10.1002/cne.902720307. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Benetti AP, Bereiter DA. Encoding of corneal input in two distinct regions of the spinal trigeminal nucleus in the rat: cutaneous receptive field properties, responses to thermal and chemical stimulation, modulation by diffuse noxious inhibitory controls, and projections to the parabrachial area. J Neurophysiol. 1997;77:43–56. doi: 10.1152/jn.1997.77.1.43. [DOI] [PubMed] [Google Scholar]
- Meng ID, Kurose M. The role of corneal afferent neurons in regulating tears under normal and dry eye conditions. Exp Eye Res. 2013;117:79–87. doi: 10.1016/j.exer.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander C, Xu Q, Rivero-Melian C, Grant G. Cytoarchitectonic organization of the spinal cord in the rat: II. The cervical and upper thoracic cord. J Comp Neurol. 1989;289:375–385. doi: 10.1002/cne.902890303. [DOI] [PubMed] [Google Scholar]
- Na KS, Mok JW, Kim JY, Rho CR, Joo CK. Correlations between tear cytokines, chemokines, and soluble receptors and clinical severity of dry eye disease. Invest Ophthalmol Vis Sci. 2012;53:5443–5450. doi: 10.1167/iovs.11-9417. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Hsu H, Gan Q. Distinct central representations for sensory fibers innervating either the conjunctiva or cornea of the rat. Exp Eye Res. 2010;90:388–396. doi: 10.1016/j.exer.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra A, Gonzalez-Gonzalez O, Gallar J, Belmonte C. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. Pain. 2014;155:1481–1491. doi: 10.1016/j.pain.2014.04.025. [DOI] [PubMed] [Google Scholar]
- Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, Gallar J, Dhaka A, Viana F, Belmonte C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010;16:1396–1399. doi: 10.1038/nm.2264. [DOI] [PubMed] [Google Scholar]
- Pellegrini JJ, Horn AK, Evinger C. The trigeminally evoked blink reflex. I. Neuronal circuits. Exp Brain Res. 1995;107:166–180. doi: 10.1007/BF00230039. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC. Tear dysfunction and the cornea: LXVIII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2011;152:900–909. e901. doi: 10.1016/j.ajo.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Okamoto K, Thompson R, Bereiter DA. Trigeminal pathways for hypertonic saline- and light-evoked corneal reflexes. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A, Kurose M, Winterson BJ, Meng ID. Menthol Activation of Corneal Cool Cells Induces TRPM8-Mediated Lacrimation but Not Nociceptive Responses in Rodents. Invest Ophthalmol Vis Sci. 2012;53:7034–7042. doi: 10.1167/iovs.12-10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible HG, Richter F, Ebersberger A, Boettger MK, Vanegas H, Natura G, Vazquez E, Segond von Banchet G. Joint pain. Exp Brain Res. 2009;196:153–162. doi: 10.1007/s00221-009-1782-9. [DOI] [PubMed] [Google Scholar]
- Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78:409–416. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32:19–41. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan B. Challenges in using signs and symptoms to evaluate new biomarkers of dry eye disease. Ocul Surf. 2014;12:2–9. doi: 10.1016/j.jtos.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Chang Z, Bereiter DA. Behavioral and neurophysiological correlates of nociception in an animal model of photokeratitis. Neuroscience. 2010;169:455–462. doi: 10.1016/j.neuroscience.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino M, Schaumberg DA. Dry Eye Disease: Impact on Quality of Life and Vision. Curr Ophthalmol Rep. 2013;1:51–57. doi: 10.1007/s40135-013-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N. Postinflammatory visceral sensitivity and pain mechanisms. Neurogastroenterol Motil. 2008;20(Suppl 1):73–80. doi: 10.1111/j.1365-2982.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA, Bautista DM. The Ion Channel TRPA1 Is Required for Chronic Itch. J Neurosci. 2013;33:9283–9294. doi: 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Dostrovsky JO, Sessle BJ, Chiang CY. Trigeminal projections to the nucleus submedius of the thalamus in the rat. J Comp Neurol. 1991;307:609–625. doi: 10.1002/cne.903070408. [DOI] [PubMed] [Google Scholar]