Abstract
Objectives
The aim of this study was to assess the effect of Sodium Nitroprusside-enhanced Cardiopulmonary Resuscitation (SNPeCPR) on heat exchange during surface cooling. We hypothesized that SNPeCPR would decrease the time required to reach brain temperature < 35 °C compared to Active Compression-Decompression plus Impedance Threshold Device (ACD-ITD) CPR alone, in the setting of intra-CPR cooling. We further hypothesized that the addition of epinephrine during SNPeCPR would mitigate heat exchange.
Design
Prospective randomized animal investigation.
Setting
Preclinical animal laboratory.
Subjects
Female farm pigs (n = 28)
Interventions
After 10 minutes of untreated VF, animals were randomized to 3 different protocols: SNPeCPR (n = 8), SNPeCPR plus epinephrine (SNPeCPR+EPI, n = 10), and ACD-ITD alone (Control, n = 10). All animals received surface cooling at the initiation of CPR. SNPeCPR included ACD-ITD plus abdominal binding and 2 mg of SNP at 1, 4 and 8 minutes of CPR. No epinephrine was used during CPR in the SNPeCPR group. Control and SNPeCPR+EPI groups received 0.5 mg of epinephrine at minute 4.5 and 9 of CPR. Defibrillation occurred after 10 minutes of CPR. After ROSC, an Arctic Sun® was applied at maximum cooling on all animals. The primary endpoint was the time required to reach brain temperature < 35 °C beginning from the time of CPR initiation. Data are presented as mean ± SEM.
Results
The time required to reach a brain temperature of 35°C was decreased with SNPeCPR vs. Control or SNPeCPR+EPI (24 ± 6 min, 63 ± 8 min, and 50 ± 9 min, respectively, p = 0.005). Carotid blood flow was higher during CPR in the SNPeCPR group (83 ± 15 ml/min versus 26 ± 7 and 35 ± 5 in the Control and SNPeCPR+EPI group, respectively, p=0.001).
Conclusion
This study demonstrates that SNPeCPR facilitates intra-CPR hypothermia. The addition of epinephrine to SNPeCPR during CPR reduced its improvement in heat exchange.
Keywords: Therapeutic hypothermia, intra arrest cooling, Sodium Nitroprusside, Cardiac arrest, Cardiopulmonary resuscitation, active compression-decompression, impedance threshold device, left ventricular function, neurologic function
Introduction
Mild therapeutic hypothermia has been shown to improve survival and neurologic outcome for comatose survivors after cardiac arrest with the recommendation for initiation of cooling as soon as possible after return of spontaneous circulation (ROSC) (1-3).
Initiation of therapeutic hypothermia (TH) during cardiopulmonary resuscitation (CPR), termed intra-CPR TH, has been shown to improve survival and neurologic outcomes in animal models (4-7). In out-of-hospital cardiac arrest, cold saline infusion immediately after ROSC is the most common method of early hypothermia induction but should be distinguished from intra-CPR TH (8, 9). Despite it's effectiveness, cooling with cold saline has not been associated with improvement in clinical outcomes and has even shown an increased rate of re-arrest and pulmonary edema when given at the time of ROSC (8).
Sodium nitroprusside (SNP) is a potent vasodilator which, when used during CPR, promotes forward blood flow by decreasing arterial resistance. The combination of SNP with a mechanical CPR platform [active compression-decompression (ACD) CPR combined with an inspiratory impedance threshold device (ITD) plus abdominal binding] is referred to as SNPeCPR. SNPeCPR has been shown to optimize hemodynamics during CPR and divert blood flow to the brain and heart with improved 24h survival, neurologic function, left ventricular function, and vital organ blood flow after prolonged cardiac arrest in pigs (10-12).
Intra-CPR TH is more effective when the method of CPR provides higher blood flow thereby exchanging heat more efficiently (13). The brain temperature decreased more quickly when intra-CPR intravenous cold saline was combined with ACD-ITD CPR rather than standard CPR (13). Furthermore, the use of epinephrine during CPR induces profound vasoconstriction and may therefore decrease external heat exchange.
The aim of this study was to assess the effect of SNPeCPR on heat exchange with external cooling and without cold saline infusion. We hypothesized that SNPeCPR would achieve target brain temperature faster when compared with ACD-ITD CPR alone. We further hypothesized that use of epinephrine during resuscitation would impair the rate of cooling achieved with external cooling.
Methods
This study was approved by the Institutional Animal Care Committee of the Minneapolis Medical Research Foundation of Hennepin County Medical Center. All animal care was compliant with the National Research Council's 1996 Guidelines for the Care and Use of Laboratory Animals. All studies were performed by a qualified and experienced research team in Yorkshire female farm bred pigs weighing 39 ± 0.3 kg. A certified and licensed veterinarian assured the protocols were performed in accordance with the National Research Council's Guidelines.
Preparatory Phase
The surgical preparation, anesthesia, data monitoring, and recording procedures used in this study have been previously described (14, 15). Under aseptic surgical conditions, we used initial sedation with intramuscular ketamine (10 mL of 100 mg/mL, Ketaset, Fort Dodge Animal Health, Fort Dodge, Iowa) followed by inhaled isoflurane at a dose of 0.8–1.2%. Pigs were intubated with a size 7.0 endotracheal tube. Central aortic blood pressure was recorded continuously with a catheter (Mikro-Tip Transducer, Millar Instruments, Houston, Texas) placed in the descending thoracic aorta. A second Millar catheter was inserted in the right atrium via the right external jugular vein. An ultrasound flow probe (Transonic 420 series multichannel, Transonic Systems, Ithaca, New York) was surgically placed on the left common carotid artery to quantify carotid blood flow (ml/min). After creating a burr hole, a Millar catheter was inserted into the parietal lobe to measure intracranial pressure (ICP). The left femoral artery, left femoral vein, and right external jugular vein were cannulated using an ultrasound guided modified Seldinger percutaneous technique. Three temperature probes were inserted into the following tissues: a) parietal lobe to measure brain temperature, b) inferior vena cava via the right femoral vein to measure central blood temperature and c) subcutaneous space to measure skin temperature. The animal's central blood temperature was maintained at 37 ± 0.2 before induction of VF, with a warming blanket (Bair Hugger, Augustine Medical, Eden Prairie, Minnesota). All animals received an intravenous heparin bolus (100 units/kg). Animals were fasted overnight and received up to 1000 ml of normal saline solution over an hour period prior to the induction of ventricular fibrillation (VF) in order to maintain the mean right atrial pressure between 3-5 mmHg. The animals were then ventilated with room air, using a volume-control ventilator (Narcomed, Telford, Pennsylvania), with a tidal volume of 10 mL/kg and a respiratory rate adjusted to continually maintain a PaCO2 of 40 mmHg and PaO2 of 80 mmHg (blood oxygen saturation > 95%), as measured from arterial blood (Gem 3000, Instrumentation Laboratory). Surface electrocardiographic tracings were continuously recorded. Coronary perfusion pressure (CPP) was calculated from the difference between aortic pressure during the decompression phase of CPR and the right atrial pressure. Cerebral perfusion pressure (CePP) was calculated from the difference between mean arterial pressure and mean ICP. Ultrasound derived carotid blood flow velocity was reported in ml/sec. Return of spontaneous circulation (ROSC) was defined using the Utstein guidelines for uniform reporting in animal research (16).
Time to target brain temperature was measured from the initiation of CPR. All data were recorded with a digital recording system (BIOPAC MP 150, BIOPAC Systems, Inc., CA, USA). End tidal CO2 (ETCO2), tidal volume, minute ventilation, and blood oxygen saturation were continuously measured with a respiratory monitor (Cosmo Plus, Novametrix Medical Systems, Wallingford, Connecticut).
Arterial blood gases were obtained at baseline, 5, 60 and 120 minutes after ROSC.
Experimental protocol
After the surgical preparation was complete, the animal was stabilized with oxygen saturation on room air greater than 95%, and ETCO2 was between 35 and 42 mmHg for 5 minutes. Ventricular fibrillation was then induced by delivering direct intracardiac current via a temporary pacing wire. The ventilator was disconnected from the endotracheal tube. ACD-CPR was performed with a pneumatically driven automatic piston device (Pneumatic Compression Controller, Ambu International, Glostrup, Denmark) as previously described (17). During ACD-CPR, uninterrupted chest compressions were performed at a rate of 100 compressions/min, with a 50% duty cycle and a compression depth of 25% of the anteroposterior chest diameter. After each compression the chest was actively pulled upwards with suction cup attached to the skin with a decompression force of approximately 20 lbs.
Asynchronous positive pressure ventilations were delivered with room air (FiO2 of 0.21) using the volume-control ventilator. The tidal volume was maintained at approximately 10 mL/kg and the respiratory rate was 10 breaths/min.
Protocol
The primary endpoint was the time required to reach brain temperature < 35 °C beginning from the time of CPR initiation.
Following 10 minutes of untreated ventricular fibrillation, 28 animals were randomized to 3 groups: SNPeCPR (n = 8), ACD-ITD alone (Control, n = 10), and SNPeCPR plus epinephrine (SNPeCPR+EPI, n = 10) (Figure 1). ACD-ITD CPR was provided in all groups for 10 minutes. External cooling using ice bags was initiated immediately after the beginning of CPR. In the SNPeCPR group, a bolus of 2 mg of SNP was administered intravenously at 1, 4 and 8 minutes of CPR. Epinephrine was administered intravenously as a 0.5 mg (15 μg/kg) bolus at 4.5 and 9 minutes after CPR was started in the Control and SNPeCPR+EPI groups. After 10 minutes of CPR, three biphasic defibrillation shocks with 275-J (Lifepak 15, Physio-Control, Inc, Redmond, WA, USA) were delivered. If ROSC was not achieved after 3 defibrillation shocks, an additional bolus of 0.5mg of epinephrine was administered in all groups as well as 25 mg of amiodarone. If ROSC was not achieved, a defibrillation shock was delivered every 2 minutes as well as epinephrine bolus every 4 minutes thereafter until ROSC was achieved or for up to the point in time when a total of 15 minutes of CPR had been performed.
Figure 1.
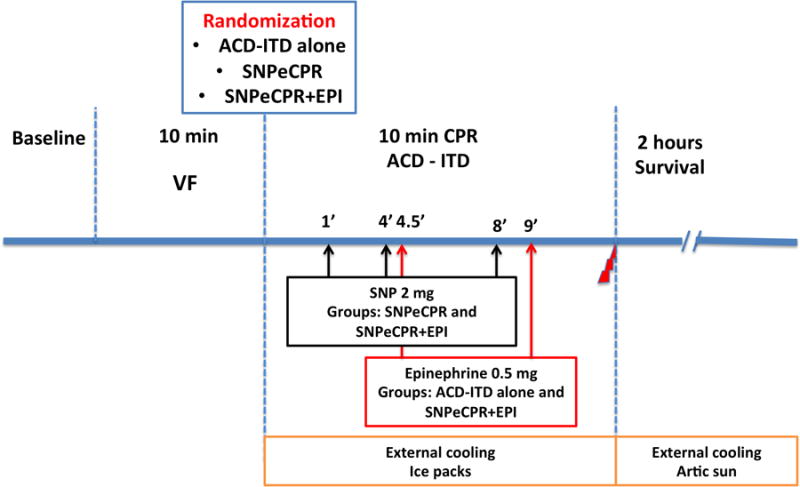
Study protocol.
VF = Ventricular Fibrillation, CPR = Cardiopulmonary Resuscitation, SNPeCPR = Sodium Nitroprusside-enhanced Cardiopulmonary Resuscitation; ACD = Active Compression-Decompression, ITD = Impedance Threshold Device, Epi = Epinephrine
Post-ROSC Care
After ROSC was achieved, the ITD was removed and the animals were allowed to recover under general anesthesia with isoflurane. Supplemental oxygen was added only if arterial saturation was lower than 90% with a SaO2 target between 90-94%.
Initial external cooling was performed with an Artic Sun device (Medivance, Inc., Louiseville, CO, USA) at maximum cooling rate. After reaching a brain temperature under 34°C therapeutic hypothermia was sustained between 33 and 34 °C until the end of the 2-hour survival period. Animals that had a stable post-ROSC rhythm but were hypotensive (mean arterial pressure < 50 mm Hg) received increments of 0.1–0.2 mg intravenous epinephrine every 5 minutes until mean arterial pressure rose above 50 mm Hg. If pH was lower than 7.2, 50–100 mEq of NaHCO3 was given intravenously. This was repeated as needed for significant acidosis. Resuscitated animals were observed for a 2 hours period.
Echocardiographic Evaluation of Left Ventricular Function
A transthoracic echocardiogram (Philips Sonos 5500 Ultrasound, Amsterdam, Netherlands with an Agilent Technology S3 probe, Santa Clara, CA, USA) was obtained on all survivors 2 hours post ROSC.
Images were obtained from the right parasternal window, which provides similar views as the long and short parasternal windows in humans (18). Ejection fraction was assessed using Simpson's method of volumetric analysis by an independent clinical echocardiographer blinded to the treatments (19). Before echocardiographic evaluation, any inotropic support was stopped for at least 20 minutes and, if needed, was restarted immediately after the echocardiographic evaluation.
Statistical analysis
Data are expressed as mean ± SEM. ANOVA or Kruskal-Wallis non-parametric test and post hoc analysis were used to determine statistical significance of differences of continuous variables between groups and pairwise comparison of subgroups. The Chi-square or Fisher exact tests were used for comparison of proportions. All statistical tests were two-sided, and a P value of less than 0.05 was required to reject the null hypothesis. Statistical analysis was performed using SPSS 21 (IBM Corporation, New York, USA).
Results
There were no significant differences between treatment groups at baseline (Tables 1). One animal in each study group failed to complete the 2-hour post-ROSC recovery period (one control animal in each of the ACD-ITD and SNPeCPR+EPI groups failed to achieve ROSC while one SNPeCPR animal required euthanasia 20 min after ROSC due to severe hemodynamic instability). There were no differences between groups in time to ROSC or number of shocks needed (time to ROSC: 11.2 ± 0.7 min, 10.4 ± 0.2 min, and 10.4 ± 0.2 min; number of shocks: 2.6 ± 0.8, 1.8 ± 0.4, and 2.1 ± 0.7 for SNPeCPR, ACD-ITD, and SNPeCPR+EPI, respectively; p = 0.19 and p = 0.66). There was no difference in the total dose of epinephrine needed to achieve ROSC in groups treated with epinephrine (1.3 ± 0.3 for ACD-ITD vs. 1.2 ± 0.15 for SNPeCPR+EPI).
Table 1. Hemodynamic Evaluation.
| CPR | ROSC | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3min | 5min | 8min | 10min | 30 min | 60 min | ||
| SBP | ACD-ITD | 100 ± 2 | 86 ± 7 | 108 ± 9 | 95 ± 7 | 108 ± 11 | 97 ± 4 | 97 ± 4 |
| SNPeCPR+EPI | 102 ± 6 | 71 ± 5 | 84 ± 8 | 85 ± 8 | 99 ± 11 | 99 ± 6 | 101 ± 3 | |
| SNPeCPR | 111 ± 7 | 78 ± 6 | 89 ± 7 | 90 ± 6 | 89 ± 7 | 87 ± 7 | 83 ± 9 | |
|
| ||||||||
| DBP | ACD-ITD | 65 ± 2 | 27 ± 2 | 40 ± 4 | 34 ± 4 | 42 ± 6 | 63 ± 5 | 62 ± 4 |
| SNPeCPR+EPI | 67 ± 5 | 23 ± 4 | 28 ± 2§ | 30 ± 3 | 40 ± 5 | 60 ± 6 | 61 ± 3 | |
| SNPeCPR | 77 ± 5 | 24 ± 2 | 23 ± 3* | 27 ± 2 | 27 ± 1 | 56 ± 4 | 54 ± 7 | |
|
| ||||||||
| RA | ACD-ITD | 2 ± 1 | 3 ± 1 | 4 ± 1 | 4 ± 1 | 4 ± 1 | 5 ± 1 | 4 ± 1 |
| SNPeCPR+EPI | 1 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 1 | 4 ± 1 | 4 ± 1 | 4 ± 1 | |
| SNPeCPR | 0 ± 1 | 3 ± 1 | 3 ± 1 | 4 ± 1 | 3 ± 1 | 6 ± 1 | 3 ± 1 | |
|
| ||||||||
| CBF | ACD-ITD | 263 ± 23 | 36 ± 5 | 31 ± 7 | 42 ± 5 | 26 ± 7 | 119 ± 31 | 184 ± 33 |
| SNPeCPR+EPI | 247 ± 39 | 74 ± 8§ | 46 ± 6 | 61 ± 7 | 35 ± 5 | 161 ± 32 | 157 ± 24 | |
| SNPeCPR | 338 ± 43 | 90 ± 15* | 103 ± 18*, # | 90 ± 15* | 83 ± 15*, # | 76 ± 12 | 102 ± 20 | |
|
| ||||||||
| ICP | ACD-ITD | 23 ± 1 | 30 ± 2 | 34 ± 3 | 28 ± 2 | 29 ± 2 | 14 ± 1 | 16 ± 2 |
| SNPeCPR+EPI | 21 ± 2 | 31 ± 3 | 31 ± 3 | 36 ± 3 | 31 ± 4 | 15 ± 2 | 15 ± 2 | |
| SNPeCPR | 20 ± 2 | 32 ± 2 | 34 ± 3 | 29 ± 2 | 34 ± 2 | 14 ± 1 | 14 ± 1 | |
|
| ||||||||
| CPP | ACD-ITD | 63 ± 2 | 25 ± 2 | 37 ± 3 | 30 ± 4 | 38 ± 5 | 58 ± 5 | 59 ± 5 |
| SNPeCPR+EPI | 66 ± 5 | 21 ± 4 | 25 ± 2 | 27 ± 3§ | 35 ± 5 | 56 ± 6 | 57 ± 3 | |
| SNPeCPR | 77 ± 5 | 20 ± 2 | 20 ± 3 | 23 ± 3* | 24 ± 2 | 50 ± 5 | 50 ± 7 | |
|
| ||||||||
| CePP | ACD-ITD | 59 ± 2 | 17 ± 4 | 29 ± 7 | 23 ± 6 | 30 ± 8 | 54 ±10 | 51 ±9 |
| SNPeCPR+EPI | 53 ± 3 | 13 ± 4 | 21 ± 3 | 17 ± 4 | 26 ± 5 | 54 ± 3 | 60 ± 3 | |
| SNPeCPR | 72 ± 6# | 14 ± 3 | 15 ± 3 | 14 ± 3 | 15 ± 5 | 56 ± 6 | 54 ± 8 | |
|
| ||||||||
| ETCO2 | ACD-ITD | 41 ± 1 | 38 ± 4 | 37 ± 3 | 28 ± 2 | 27 ± 2 | 44 ± 1 | 40 ± 1 |
| SNPeCPR+EPI | 40 ± 1 | 41 ± 2 | 39 ± 2 | 36 ± 3§ | 31 ± 2 | 45 ± 1 | 41 ± 1 | |
| SNPeCPR | 39 ± 1 | 32 ± 3 | 28 ± 2# | 29 ± 2 | 25 ± 4 | 36 ± 3*, # | 36 ± 3 | |
SBP = Systolic Blood Pressure, DBP = Diastolic Blood Pressure, RA = Diastolic Right Atrial Pressure, CBF = Carotid Blood Flow, ICP = Intracranial Pressure, CPP = Coronary Perfusion Pressure, CePP = Cerebral Perfusion Pressure. ACD-ITD = Active Compression Decompression with Impedance Threshold Device, SNPeCPR = Sodium Nitroprusside enhanced CPR and EPI = Epinephrine. Values are shown as mean ± SEM. Pressures are presented in mm Hg and flow in ml/min.
denotes p < 0.05 comparing SNPeCPR and ACD-ITD alone.
denotes p < 0.05 comparing SNPeCPR and SNPeCPR+EPI.
denotes p < 0.05 comparing SNPeCPR+EPI and ACD-ITD alone.
Temperatures during CPR
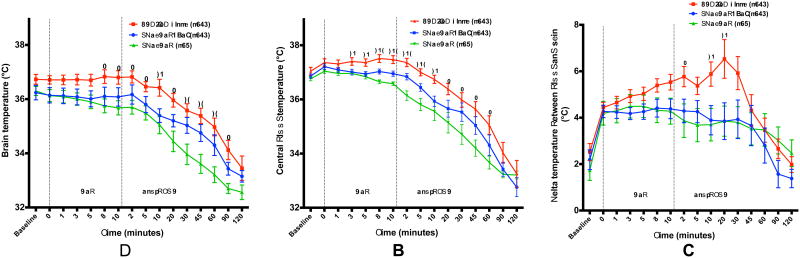
Time to reach target brain temperature of <35°C was accelerated with SNPeCPR compared to Control or SNPeCPR+EPI (24 ± 6 min, 63 ± 8 min, and 50 ± 9 min, respectively, p = 0.005, Figure 2). The same improvement was seen with the time to reach brain temperature of 34°C (46 ± 9 min, 91 ± 8 min and 74 ± 9 min, respectively, p = 0.006). Blood temperature was significantly reduced in the SNPeCPR group compared to the ACD-ITD group at an earlier time point compared to the brain temperature (Figure 2).
Figure 2.
A - Brain temperature, B - Central blood temperature and C – Temperature delta between blood and skin in the 3 groups. CPR = Cardiopulmonary Resuscitation, ROSC = Return of Spontaneous Circulation, ACD-ITD alone = Active Compression Decompression with Impedance Threshold Device, SNPeCPR = Sodium Nitroprusside enhanced CPR, EPI = Epinephrine. (*) denotes p < 0.05 comparing SNPeCPR and ACD-ITD alone. (#) denotes p < 0.05 comparing SNPeCPR and SNPeCPR+EPI. (§) denotes p < 0.05 comparing SNPeCPR+EPI and ACD-ITD alone.
A temperature gradient developed between skin and blood. After 10 minutes of CPR, the difference in temperature between blood and skin was greater in the ACD-ITD group though the difference had not reached statistical significant when compared to the other treatment groups (5.5 ± 0.3°C vs. 4.4 ± 0.4 and 4.3± 0.6 for ACD-ITD, SNPeCPR+EPI, and SNPeCPR, p = 0.08). Once ROSC was achieved, the temperature difference between blood and skin was significantly greater in the ACD-ITD group (5.8 ± 0.4°C vs. 4.4 ± 0.4 and 3.9 ± 0.7 for ACD-ITD, SNPeCPR+EPI, and SNPeCPR, respectively, p = 0.048) (Figure 3).
CPR Hemodynamics
Carotid artery blood flow (CBF) was higher in the SNPeCPR group compared to ACD-ITD throughout CPR (Table 1). There were no differences in CPP, CePP, CBF, or blood pressure once ROSC was achieved.
Arterial Blood gas
Acidosis was significantly improved in the SNPeCPR group 5 minutes after ROSC compared to ACD-ITD and SNPeCPR+EPI animals (7.26 ± 0.03, 7.19 ± 0.02, 7.16 ± 0.02, respectively, p = 0.048). There was no other significant difference in blood gas analysis between groups (Table 2).
Table 2. Arterial blood gas analysis.
| Baseline | ROSC | ||||
|---|---|---|---|---|---|
| 5 min | 60 min | 2h | |||
| pH | ACD-ITD | 7.46 ± 0.02 | 7.19 ± 0.02 | 7.23 ± 0.03 | 7.25 ± 0.03 |
| SNPeCPR+EPI | 7.44 ± 0.01 | 7.16 ± 0.02 | 7.25 ± 0.03 | 7.28 ± 0.02 | |
| SNPeCPR | 7.44 ± 0.02 | 7.26 ± 0.03* | 7.18 ± 0.05 | 7.19 ± 0.07 | |
| PCO2 | ACD-ITD | 47 ± 3 | 73 ± 7 | 51 ± 4 | 55 ± 3 |
| SNPeCPR+EPI | 44 ± 1 | 68 ± 5 | 56 ± 3 | 55 ± 2 | |
| SNPeCPR | 44 ± 1 | 59 ± 5 | 47 ± 2 | 47 ± 3 | |
| PO2 | ACD-ITD | 90 ± 2 | 63 ± 7 | 113 ± 10 | 108 ± 6 |
| SNPeCPR+EPI | 82 ± 6 | 108 ± 31 | 101 ± 9 | 108 ± 10 | |
| SNPeCPR | 91 ± 3 | 135 ± 36 | 112 ± 14 | 91 ± 7 | |
| HCO3- | ACD-ITD | 30 ± 1 | 25 ± 1 | 21 ± 1 | 24 ± 1 |
| SNPeCPR+EPI | 30 ± 1 | 26 ± 1 | 24 ± 1 | 26 ± 1 | |
| SNPeCPR | 30 ± 1 | 26 ± 1 | 21 ± 2 | 25 ± 2 | |
| BE | ACD-ITD | 6 ± 1 | -4 ± 1 | -6 ± 1 | -3 ± 2 |
| SNPeCPR+EPI | 6 ± 1 | -3 ± 1 | -4 ± 1 | -1 ± 2 | |
| SNPeCPR | 6 ± 1 | -1 ± 2 | -6 ± 2 | -3 ± 3 | |
BE = Base Excess, HCO3- = Bicarbonate, SNPeCPR = Sodium Nitroprusside-enhanced CPR, ACD-ITD = Active Compression-Decompression - Impedance Threshold Device. Values are shown as mean ± SEM. All pressures are in mm Hg.
denotes p < 0.05 compared to ACD-ITD.
Left ventricular Function
There was no difference in the left ventricular ejection fraction between the groups at 2 h after ROSC (53 ± 3%, 54 ± 3%, and 53 ± 3% for SNPeCPR, ACD-ITD and SNPeCPR+EPI, respectively, p = 0.91)
Discussion
This study demonstrated for the first time that SNPeCPR greatly increases the brain temperature cooling rate. The target temperature of 35 °C was reached in 24 minutes after ROSC in the SNPeCPR animals.
Hypothermia induced by surface cooling leads to intense vasoconstriction and shivering (20). Blood is diverted from the cold skin to the deeper layers of the body, thus insulating the core blood flow from the surface cooling agent. If vasoconstriction and shivering are diminished the body should follow Newton's law of cooling which states that the rate of cooling of a body which is hotter than its surroundings is proportional to its surface area and the difference in temperature between the body and its surroundings (21). Epinephrine enhances the natural vasoconstriction further reducing heat exchange. Alternatively, using vasodilators such as Chlorpromazine, or intravenous alcohol to induce hypothermia was described a few decades ago for cardiac and neurosurgery (22). SNP by its potent vasodilator effect reduced the temperature difference between skin and central blood temperature and thus enhanced the heat exchange.
The combination of ice-cold saline, improved hemodynamics during CPR, and ACD-ITD induced cerebral hypothermia more rapidly after ROSC compared to standard CPR in a similar model of cardiac arrest and CPR (13). SNPeCPR improved vital organ perfusion pressure, carotid blood flow, and 24h survival with good neurologic outcome in similar studies (10-12, 23). The increase of cerebral blood flow provided by SNPeCPR in combination with improved heat exchange due to the peripheral vasodilation explains the faster cooling rate observed.
Intra-CPR cooling has been associated with better outcomes in animal models. Several experimental studies suggested that intra-CPR cooling could be important to limit reperfusion injury (4, 5, 24-26). In a previous study using a porcine model of cardiac arrest, intra-CPR cooling significantly reduced myocardial infarct size. Avoidance of intra-CPR volume loading further improved outcomes (27). In this study, infusion of cold saline during CPR was associated with a decrease in coronary perfusion pressure when compared to external cooling after ROSC (27). In humans with out-of-hospital cardiac arrest, cold saline infusion after ROSC was associated with increased re-arrest and pulmonary edema (8). Our results suggest that inducing therapeutic hypothermia quickly during CPR without the use of cold saline is feasible and safe.
It is also important to note that SNP administration during ACD-ITD CPR did not decrease central aortic pressure, coronary perfusion pressure, or cerebral perfusion pressure during prolonged CPR. Conversely, carotid blood flow was significantly higher during CPR in the SNPeCPR group compared to ACD-ITD CPR alone.
Limitations
Our study has several limitations. First, although the study was prospective and animals were randomized to the method of CPR after induction of VF, the study could not be blinded. However, compression rate, force, and cooling method were closely monitored and controlled. Second, the ice-bags used during CPR for external cooling did not cover the entire pig skin surface area, therefore cooling rate could have been further improved with a more complete surface of exchange. Third, abdominal binding was performed only in the SNPeCPR and SNPeCPR+EPI groups. Abdominal binding reduces the blood circulation in the lower part of the body, and thus could have also reduced the heat exchange in the SNPeCPR groups. Finally, we did not assess the clinical benefits of this treatment strategy. Further studies are planned to assess the effect of this strategy on neurologically intact survival.
Conclusion
SNPeCPR significantly accelerates intra-CPR heat exchange with surface cooling when compared to ACD+ITD CPR. SNPeCPR coupled with intra-CPR surface cooling reduced the time to reach target brain temperature more than 60% compared with ACD-ITD CPR. The addition of epinephrine to SNPeCPR attenuates its effect on heat exchange.
Acknowledgments
source of funding: This study was funded by an NIH grant: R01 HL108926
Guillaume Debaty received a grant from the Region Rhône-Alpes (France) and from the Société Française de Medecine d'Urgence (SFMU) for a post-doctoral fellowship.
Dr. Yannopoulos is employed by the University of Minnesota and received support for article research from the National Institutes of Health (NIH). Dr. Yannopoulos and his institution received grant support from NIH grants for research and a Medtronic Foundation grant for resuscitation. His institution received grant support (NIH grant: R01HL108926). Dr. Debaty received grant support from the French Society of Emergency Medicine and Region Rhone-Alpes (France) and received support for article research from the NIH. His institution received grant support from the NIH: R01 HL108926. Dr. Matsuura received support for article research from the NIH. His institution received grant support and support for travel from the NIH. Dr. Rees is employed by the University of Minnesota and received support for article research from the NIH. Her institution received grant support from the NIH. Dr. McKnite received support for article research from the NIH. His institution received grant support from the NIH. Dr. Lick is employed by Advanced Circulatory Systems, Inc.
Footnotes
Conflict of interest: The authors do not have any conflict of interest.
Copyright form disclosures: The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Nolan JP, Morley PT, Vanden Hoek TL, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108:118–121. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 2.Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.Nozari A, Safar P, Stezoski SW, et al. Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation. 2006;113:2690–2696. doi: 10.1161/CIRCULATIONAHA.106.613349. [DOI] [PubMed] [Google Scholar]
- 5.Abella BS, Zhao D, Alvarado J, et al. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109:2786–2791. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- 6.Riter HG, Brooks LA, Pretorius AM, et al. Intra-arrest hypothermia: both cold liquid ventilation with perfluorocarbons and cold intravenous saline rapidly achieve hypothermia, but only cold liquid ventilation improves resumption of spontaneous circulation. Resuscitation. 2009;80:561–566. doi: 10.1016/j.resuscitation.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staffey KS, Dendi R, Brooks LA, et al. Liquid ventilation with perfluorocarbons facilitates resumption of spontaneous circulation in a swine cardiac arrest model. Resuscitation. 2008;78:77–84. doi: 10.1016/j.resuscitation.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim F, Nichol G, Maynard C, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA. 2014;311:45–52. doi: 10.1001/jama.2013.282173. [DOI] [PubMed] [Google Scholar]
- 9.Bernard SA, Smith K, Cameron P, et al. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation. 2010;122:737–742. doi: 10.1161/CIRCULATIONAHA.109.906859. [DOI] [PubMed] [Google Scholar]
- 10.Schultz J, Segal N, Kolbeck J, et al. Sodium nitroprusside enhanced cardiopulmonary resuscitation (SNPeCPR) improves vital organ perfusion pressures and carotid blood flow in a porcine model of cardiac arrest. Resuscitation. 2012;83:374–377. doi: 10.1016/j.resuscitation.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz JC, Segal N, Caldwell E, et al. Sodium nitroprusside-enhanced cardiopulmonary resuscitation improves resuscitation rates after prolonged untreated cardiac arrest in two porcine models. Crit Care Med. 2011;39:2705–2710. doi: 10.1097/CCM.0b013e31822668ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultz J, Segal N, Kolbeck J, et al. Sodium nitroprusside enhanced cardiopulmonary resuscitation prevents post-resuscitation left ventricular dysfunction and improves 24-hour survival and neurological function in a porcine model of prolonged untreated ventricular fibrillation. Resuscitation. 2011;82(Suppl 2):S35–40. doi: 10.1016/S0300-9572(11)70149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivasan V, Nadkarni VM, Yannopoulos D, et al. Rapid induction of cerebral hypothermia is enhanced with active compression-decompression plus inspiratory impedance threshold device cardiopulmonary resusitation in a porcine model of cardiac arrest. J Am Coll Cardiol. 2006;47:835–841. doi: 10.1016/j.jacc.2005.09.062. [DOI] [PubMed] [Google Scholar]
- 14.Yannopoulos D, Segal N, McKnite S, et al. Controlled pauses at the initiation of sodium nitroprusside-enhanced cardiopulmonary resuscitation facilitate neurological and cardiac recovery after 15 mins of untreated ventricular fibrillation. Crit Care Med. 2012;40:1562–1569. doi: 10.1097/CCM.0b013e31823e9f78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lurie KG, Yannopoulos D, McKnite SH, et al. Comparison of a 10-breaths-per-minute versus a 2-breaths-per-minute strategy during cardiopulmonary resuscitation in a porcine model of cardiac arrest. Respir Care. 2008;53:862–870. [PubMed] [Google Scholar]
- 16.Idris AH, Becker LB, Ornato JP, et al. Utstein-style guidelines for uniform reporting of laboratory CPR research. A statement for healthcare professionals from a Task Force of the American Heart Association, the American College of Emergency Physicians, the American College of Cardiology, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Institute of Critical Care Medicine, the Safar Center for Resuscitation Research, and the Society for Academic Emergency Medicine. Resuscitation. 1996;33:69–84. doi: 10.1016/s0300-9572(96)01055-6. [DOI] [PubMed] [Google Scholar]
- 17.Shultz JJ, Coffeen P, Sweeney M, et al. Evaluation of standard and active compression-decompression CPR in an acute human model of ventricular fibrillation. Circulation. 1994;89:684–693. doi: 10.1161/01.cir.89.2.684. [DOI] [PubMed] [Google Scholar]
- 18.Marino BS, Yannopoulos D, Sigurdsson G, et al. Spontaneous breathing through an inspiratory impedance threshold device augments cardiac index and stroke volume index in a pediatric porcine model of hemorrhagic hypovolemia. Crit Care Med. 2004;32:S398–405. doi: 10.1097/01.ccm.0000139950.39972.68. [DOI] [PubMed] [Google Scholar]
- 19.Quinones MA, Waggoner AD, Reduto LA, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–753. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 20.Dundee JW, King R. Clinical aspects of induced hypothermia; methods of production and indications for its use. Br J Anaesth. 1959;31:106–133. doi: 10.1093/bja/31.3.106. [DOI] [PubMed] [Google Scholar]
- 21.Dhruva AJ, Javeri PM, Parulkar GB, et al. Mechanism of temperature fall during hypothermia by surface cooling. An experimental and clinical study. Anesth Analg. 1963;42:306–315. [PubMed] [Google Scholar]
- 22.Dundee JW, Clarke RS. Pharmacology of Hypothermia. Int Anesthesiol Clin. 1964;2:857–872. doi: 10.1097/00004311-196408000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Yannopoulos D, Matsuura T, Schultz J, et al. Sodium nitroprusside enhanced cardiopulmonary resuscitation improves survival with good neurological function in a porcine model of prolonged cardiac arrest. Crit Care Med. 2011;39:1269–1274. doi: 10.1097/CCM.0b013e31820ed8a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation. 2008;77:242–249. doi: 10.1016/j.resuscitation.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Barbut D, Tsai MS, et al. Intra-arrest selective brain cooling improves success of resuscitation in a porcine model of prolonged cardiac arrest. Resuscitation. 2010;81:617–621. doi: 10.1016/j.resuscitation.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Scolletta S, Taccone FS, Nordberg P, et al. Intra-arrest hypothermia during cardiac arrest: a systematic review. Crit Care. 2012;16:R41. doi: 10.1186/cc11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yannopoulos D, Zviman M, Castro V, et al. Intra-cardiopulmonary resuscitation hypothermia with and without volume loading in an ischemic model of cardiac arrest. Circulation. 2009;120:1426–1435. doi: 10.1161/CIRCULATIONAHA.109.848424. [DOI] [PubMed] [Google Scholar]