Abstract
Purpose
Renal cell carcinoma (RCC) most commonly metastasizes to the lung. Preoperative indeterminate pulmonary nodules (IPNs) occur in half of patients with localized RCC; however clinical significance remains poorly defined. We aimed to determine whether IPNs, their size, or number, are associated with RCC outcomes.
Materials and Methods
Data of 1,102 patients with RCC who had chest computed tomography within 6 months before nephrectomy from 2002-2012 were reviewed. Patients with metastatic disease at presentation, benign tumors, pulmonary nodules >2cm, or concurrent pulmonary disease were excluded, leaving 748 for analysis. Study outcomes included lung metastases, any distant metastases, or death from RCC. Cox proportional hazards models were used to assess whether presence of IPNs, nodule size, or number were associated with outcomes. Models were evaluated by comparing discrimination using Harrell's c-index.
Results
IPNs were present in 382/748 patients (51%). Median follow-up was 4.1 years (IQR, 2.2–6.1). Presence of IPNs was not associated with distant metastases or death from kidney cancer; however, compared to sub-centimeter IPNs, nodules >1cm were associated with metastatic disease after adjusting for tumor histology, stage, and size (HR=2.48; 95% CI, 1.08–5.68; p=0.031). C-indices for outcomes increased slightly after adding size of nodules to a predictive model adjusted for tumor characteristics.
Conclusions
In the current study, no evidence suggested IPNs <1cm were associated with RCC progression, although large nodules significantly predicted metastatic disease. Patients with sub-centimeter IPNs will unlikely benefit from extensive postoperative chest imaging surveillance, which should be reserved for patients with nodules >1cm.
Keywords: Chest CT scan, metastatic disease, prognosis, pulmonary nodules, carcinoma, renal cell
Introduction
RCC accounts for 4% of newly diagnosed malignancies in adults, and 3% of cancer-related deaths in males.1 Metastatic RCC is present in 20–30% of patients with newly diagnosed disease. Furthermore, 20–30% of patients undergoing nephrectomy for clinically localized disease will develop metastases during follow-up.2 The most common metastatic site for RCC is the lung, which is involved in 45% of patients with metastatic disease.3 The EAU guidelines recommend that initial radiologic evaluation of RCC should include chest CT, which may detect metastatic disease in the lung at an early stage, except in low-risk patients, in which chest x-ray may be sufficient.4,5
IPNs are defined as round opacities of unknown nature that are <3cm at maximal diameter.6,7 In patients diagnosed with cancer, non-calcified pulmonary nodules may represent metastatic disease in 19% of cases.8 Studies in colorectal and breast cancers suggest that most IPNs will not progress to metastatic disease; therefore, their presence should not delay curative treatment, nor trigger additional diagnostic tests.9,10 Despite the high rate of RCC metastasizing to the lung, the presence of IPNs on preoperative chest imaging do not influence current postoperative follow-up protocols.11,12 In the only study to evaluate the prognostic significance of IPNs in patients with RCC, the presence of IPNs was shown to be significantly associated with worse DFS on multivariate analysis. However, the study was limited by a relatively small sample size and did not evaluate the value of adding the presence of IPNs to a prognostic model with known predictors of outcome.13
We aimed to assess whether the presence of IPNs, nodule size, or nodule number were associated with the development of lung metastases, any distant metastases, or death from kidney cancer in a group of non-metastatic, surgically-treated patients with RCC. We also evaluated the value of IPN status in determining RCC prognosis.
Materials and Methods
After obtaining institutional review board approval, we queried our prospectively maintained nephrectomy database and identified 1,102 patients with renal cortical tumors who underwent nephrectomy at Memorial Sloan Kettering Cancer Center from 2002 through 2012 and had a chest CT within 6 months before surgery. Patients were excluded if they had metastatic disease at presentation (n=165), a benign renal tumor (n=91), pulmonary nodules >2cm (n=2), or concurrent pulmonary disease (n=96), which left 748 patients for analysis. To better characterize the study cohort, characteristics of the study cohort were compared to those of a similar patient cohort who underwent nephrectomy at our institution during the same years and did not have a chest CT performed prior to surgery (n=1,662).
Patient characteristics including age, gender, body mass index, presentation status (incidental, local, or systemic), and smoking status (never, former, or current) were collected. IPNs were defined according to recommendations by the Fleischner Society as round, moderately well-marginated opacities of unknown nature, ≤3cm at maximal diameter.6 Since all but two of the lung nodules identified were ≤2cm in diameter, nodules >2cm were excluded. The presence of IPNs, number of IPNs (solitary or multiple), and the size of the largest nodule were noted based on the imaging report. A pre-specified threshold of 1cm was used to define pulmonary nodules as large (>1cm) or small (≤1cm). Nephrectomy specimens were reviewed by genitourinary pathologists. Pathologic features included tumor maximal diameter, histologic subtype, and stage according to the 2010 AJCC staging system.
Patients with IPNs were followed with chest and abdominal CT every 3–6 months for 2 years and annually thereafter. Patients without IPNs were followed with annual chest x-ray. Similar to previous reports, a lung nodule was considered malignant in the presence of a documented radiographic increase in size or number of lesions, or a histologic diagnosis of cancer.14 Treatment of metastatic disease included further monitoring, systemic therapy and/or lung resection based on patient and disease characteristics. When available, follow-up chest imaging of patients who presented with IPNs and developed lung metastasis were reviewed by a radiologist to evaluate whether the metastatic lesion originated from an IPN.
Univariate Cox proportional hazards models were used to examine the association between the presence of IPNs, nodule size, or nodule number, and the risk of lung metastases, any distant metastases, or death from kidney cancer in all patients. To assess whether the presence of IPNs is a significant independent predictor of RCC outcomes in all patients (n=748), two multivariable Cox proportional hazard models were created; one included number of nodules and the other included size of nodules. Both models were adjusted for previously known predictors of outcome including AJCC stage (stage I/II or stage III/IV), tumor size (cm), and histology (clear cell or non-clear cell). Due to the correlation between nodule number and nodule size, we could not include both in the same multivariable model for all patients.
Additionally, we created two multivariable Cox models to assess whether size and number of nodules were independent predictors of oncologic outcome among patients with IPNs (n=382). One model assessed whether both nodule size and number were associated with any distant metastases when adjusting for AJCC stage, tumor size, and histology. The other assessed whether nodule size was associated with risk of lung metastases, adjusting for the same tumor characteristics. Number of nodules was excluded from the model for lung metastases due to a limited number of events.
After testing the statistical significance of nodule size and number in predicting outcomes, we evaluated whether adding size or number of IPNs to a base model, adjusted for tumor stage, size, and histology, increased its ability to discriminate between outcomes. We used Harrell's c-index to estimate discrimination. All c-indices were corrected for optimism using 10-fold cross validation. All analyses were performed using Stata 12 (StataCorp, College Station, TX).
Results
Of 748 patients, 382 (51%) had IPNs on preoperative imaging. Patient characteristics are reported by pulmonary nodule status in Table 1. Patients with IPNs were older than patients without pulmonary nodules (p=0.0004); however, no other significant differences were found between the two groups.
Table 1.
Patient characteristics. Data are presented as frequency (percent) or median (interquartile range).
| All Patients (N=748) | No IPNs (N=366) | IPNs (N=382) | p value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 484 (65%) | 239 (65%) | 245 (64%) | 0.7 |
| Female | 264 (35%) | 127 (35%) | 137 (36%) | |
| Age at surgery | 61 (52, 70) | 60 (50, 67) | 62 (53, 72) | 0.0004 |
| Body mass index | 28.8 (25.9, 32.9) | 29.2 (26.0, 33.4) | 28.6 (25.8, 32.5) | 0.12 |
| Presentation | ||||
| Incidental | 562 (75%) | 272 (74%) | 290 (76%) | 0.8 |
| Local | 157 (21%) | 78 (21%) | 79 (21%) | |
| Systemic | 29 (4%) | 16 (5%) | 13 (3%) | |
| Number of nodules | ||||
| None | 366 (49%) | 366 (100%) | - | - |
| Single | 127 (17%) | - | 127 (33%) | |
| Multiple | 255 (34%) | - | 255 (67%) | |
| Size of nodules | - | |||
| None | 366 (49%) | 366 (100%) | - | - |
| ≤ 1cm | 353 (47%) | - | 353 (92%) | |
| > 1cm | 29 (4%) | - | 29 (8%) | |
| Tumor size (cm) | 4.4 (2.8, 7.5) | 4.3 (2.5, 7.8) | 4.5 (2.9, 7.2) | 0.8 |
| Tumor histology | ||||
| Clear cell | 521 (70%) | 259 (71%) | 262 (69%) | 0.5 |
| Non-clear cell | 227 (30%) | 107 (29%) | 120 (31%) | |
| AJCC stage | ||||
| Stage I | 432 (58%) | 212 (58%) | 220 (58%) | 0.9 |
| Stage II | 76 (10%) | 40 (11%) | 36 (9%) | |
| Stage III | 235 (31 %) | 112 (31%) | 123 (32%) | |
| Stage IV | 5 (1%) | 2 (1%) | 3 (1%) |
Abbreviations: IPN = indeterminate pulmonary nodules, AJCC = American Joint Committee on Cancer
Patients who underwent preoperative chest CT had larger tumors (median size of 4.4cm, IQR 2.8–7.5 vs. 3.3cm IQR 2.2–4.9, p <0.0001), lower rate of AJCC stage I–II disease (67% vs. 81%, p <0.0001), and more commonly had clear cell histology (70% vs. 64%, p=0.003), compared to patients who did not undergo preoperative chest CT. On univariate analysis, patients who underwent chest CT had worse outcomes than those who did not, consistent with the finding that patients with less favorable tumor characteristics were selected to undergo chest CT prior to surgery. However, in patients with AJCC stage I disease, no significant differences in outcomes were found between imaged and non-imaged patients (Figure 1).
Figure 1.
Kaplan-Meier estimates of (A) lung metastases-free survival, (B) any metastases-free survival and (C) disease-specific survival, for AJCC stage I patients, stratified by those who underwent chest CT and those who did not.
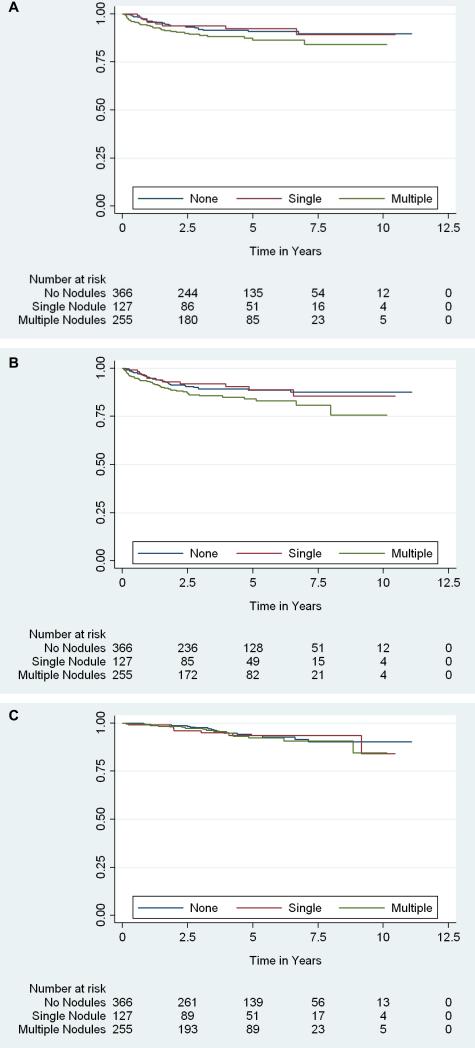
Median follow-up time was 4.1 years (IQR 2.2–6.1) for patients without metastases. Kaplan-Meier curves of lung metastases-free, any metastases-free and disease-specific survival are shown in Figures 2 and 3. At 5 years, 27% of patients with 1–2cm IPNs had developed lung metastases compared to 10% and 9% of patients with sub-centimeter or no nodules, respectively. Thirty-seven of the 64 patients (58%) who developed lung metastases within 5 years of surgery had preoperative IPNs, 33 of which had follow-up chest CT available for review. Metastatic lung lesions in 25/33 patients (76%) originated from IPNs identified preoperatively, while in 24% of the patients, IPNs did not progress to metastatic lesions.
Figure 2.
Kaplan-Meier estimates of (A) lung metastases-free survival, (B) any metastases-free survival and (C) disease-specific survival, stratified by indeterminate pulmonary nodule size.
Figure 3.
Kaplan-Meier estimates of (A) lung metastases-free survival, (B) any metastases-free survival and (C) disease-specific survival, stratified by indeterminate pulmonary nodule number.
Presence of IPNs was not found to be associated with lung metastases (p=0.2), any distant metastases (p=0.2), or death from kidney cancer (p=0.6) on univariate analysis. However, analysis by nodule size showed that having pulmonary nodules >1cm was significantly associated with lung metastases (HR = 3.97; 95%CI, 1.74–9.10; p=0.001), any distant metastases (HR=3.27; 95% CI, 1.45–7.38; p=0.004), and death from kidney cancer (HR=3.39; 95% CI, 1.14–10.06; p=0.028), when compared to having no nodules. Having pulmonary nodules ≤1cm was not significantly associated with any of the outcomes when compared to having no nodules (Table 2). After adjusting for tumor histology, AJCC stage and size, having pulmonary nodules >1cm remained significantly associated with lung metastases (HR=4.53; 95% CI, 1.95–10.52; p=0.0004) and any distant metastases (HR=3.77; 95% CI, 1.66–8.57; p=0.002), when compared to having no nodules. Having multiple nodules was associated with any distant metastases (p=0.034). On multivariable analysis of all patients, neither the presence of >1cm nodules, nor the presence of multiple pulmonary nodules were significantly associated with death from kidney cancer when compared to patients with no nodules (p=0.11 and p=0.7, respectively, Table 3).
Table 2.
Univariate Cox models for lung metastases, any distant metastases, and death from kidney cancer, N = 748. For size and number of indeterminate pulmonary nodules, having no nodules is the reference category.
| Lung Metastases | Any Distant Metastases | Death from Kidney Cancer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value |
| Pulmonary nodules present | 1.33 | 0.82-2.16 | 0.2 | 1.36 | 0.89-2.10 | 0.2 | 1.17 | 0.63-2.18 | 0.6 |
| Nodule size | |||||||||
| ≤ 1 cm | 1.16 | 0.70-1.93 | 0.6 | 1.25 | 0.80-1.95 | 0.3 | 1.02 | 0.53-1.97 | 0.9 |
| > 1 cm | 3.97 | 1.74-9.10 | 0.001 | 3.27 | 1.45-7.38 | 0.004 | 3.39 | 1.14-10.06 | 0.028 |
| Number of nodules | |||||||||
| Single | 0.92 | 0.44-1.96 | 0.8 | 0.98 | 0.51-1.88 | 0.9 | 1.10 | 0.46-2.63 | 0.8 |
| Multiple | 1.53 | 0.92-2.57 | 0.10 | 1.56 | 0.98-2.47 | 0.058 | 1.21 | 0.61-2.40 | 0.6 |
| AJCC stage | |||||||||
| I-II | Ref. | - | - | Ref. | - | - | Ref. | - | - |
| II-IV | 7.60 | 4.38-13.18 | <0.0001 | 7.75 | 4.77-12.61 | <0.0001 | 7.81 | 3.82-15.99 | <0.0001 |
| Tumor histology | |||||||||
| Non-clear cell | Ref. | - | - | Ref. | - | - | Ref. | - | - |
| Clear cell | 2.16 | 1.16-4.03 | 0.016 | 1.65 | 0.99-2.74 | 0.056 | 1.23 | 0.61-2.46 | 0.6 |
| Tumor size (per cm) | 1.15 | 1.11-1.19 | <0.0001 | 1.14 | 1.11-1.18 | <0.0001 | 1.14 | 1.10-1.19 | <0.0001 |
Abbreviations: HR = hazard ratio, CI = confidence interval, AJCC = American Joint Committee on Cancer
Table 3.
Multivariable Cox models for size and number of indeterminate pulmonary nodules for all patients. Each model was adjusted for American Joint Committee on Cancer stage, histology, and tumor size, N = 748.
| Lung Metastases | Any Distant Metastases | Death from Kidney Cancer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value |
| Size of nodules | |||||||||
| No nodules | Ref. | - | - | Ref. | - | - | Ref. | - | - |
| ≤ 1 cm | 1.26 | 0.76-2.10 | 0.4 | 1.33 | 0.85-2.08 | 0.2 | 1.08 | 0.56-2.09 | 0.8 |
| > 1 cm | 4.53 | 1.95-10.52 | 0.0004 | 3.77 | 1.66-8.57 | 0.002 | 2.45 | 0.81-7.37 | 0.11 |
| AJCC stage | |||||||||
| Stages 1 and 2 | Ref. | - | - | Ref. | - | - | Ref. | - | - |
| Stages 3 and 4 | 2.67 | 1.90-3.76 | <0.0001 | 2.80 | 2.08-3.78 | <0.0001 | 3.17 | 2.01-5.00 | <0.0001 |
| Histology | |||||||||
| Non-clear cell | Ref. | - | - | Ref. | - | - | Ref. | - | - |
| Clear cell | 3.13 | 1.57-6.22 | 0.001 | 2.05 | 1.19-3.53 | 0.01 | 1.37 | 0.67-2.82 | 0.4 |
| Tumor size (cm) | 1.14 | 1.08-1.20 | <0.0001 | 1.12 | 1.07-1.17 | <0.0001 | 1.09 | 1.02-1.17 | 0.012 |
| Number of nodules | |||||||||
| No nodules | Ref. | - | - | Ref. | - | - | Ref. | - | - |
| Single | 1.08 | 0.51-2.29 | 0.8 | 1.06 | 0.55-2.05 | 0.9 | 1.30 | 0.54-3.14 | 0.6 |
| Multiple | 1.61 | 0.96-2.71 | 0.072 | 1.65 | 1.04-2.62 | 0.034 | 1.16 | 0.58-2.32 | 0.7 |
| AJCC stage | |||||||||
| Stages 1 and 2 | Ref. | - | - | Ref. | - | - | Ref. | - | - |
| Stages 3 and 4 | 2.67 | 1.90-3.73 | <0.0001 | 2.79 | 2.07-3.75 | <0.0001 | 3.28 | 2.06-5.21 | <0.0001 |
| Histology | |||||||||
| Non-clear cell | Ref. | - | - | Ref. | - | - | Ref. | - | - |
| Clear cell | 2.87 | 1.45-5.69 | 0.002 | 1.99 | 1.15-3.45 | 0.013 | 1.34 | 0.65-2.76 | 0.4 |
| Tumor size (cm) | 1.14 | 1.08-1.20 | <0.0001 | 1.12 | 1.07-1.17 | <0.0001 | 1.09 | 1.02-1.17 | 0.011 |
Abbreviations: HR = hazard ratio, CI = confidence interva , AJCC = American Joint Committee on Cancer
When both the size and number of IPNs were included in the multivariable model for patients with preoperative IPNs, nodule size remained a significant predictor of any distant metastases (HR=2.48; 95% CI, 1.08–5.68; p=0.031), while the number of nodules was no longer a significant predictor of any distant metastases (p=0.3; Table 4). A similar trend was observed when including both nodule size and nodule number in a model predicting lung metastases among patients with pulmonary nodules. However, due to a limited number of events, we excluded nodule number from our final model for lung metastases, since nodule size, tumor size, AJCC stage, and histology were all significant predictors (Table 4).
Table 4.
Multivariable Cox models for predictors of any distant metastases and lung metastases in patients with indeterminate pulmonary nodules (n=382).
| Any Distant Metastases | Lung Metastases | |||||
|---|---|---|---|---|---|---|
| Predictors | HR | 95% CI | p value | HR | 95% CI | p value |
| Size of nodules | ||||||
| ≤ 1 cm | Ref. | - | - | Ref. | - | - |
| > 1 cm | 2.48 | 1.08-5.68 | 0.031 | 3.62 | 1.58-8.32 | 0.002 |
| Number of nodules | ||||||
| Single | Ref. | - | - | |||
| Multiple | 1.45 | 0.74-2.82 | 0.3 | |||
| AJCC Stage | ||||||
| Stages 1 and 2 | Ref. | - | - | Ref. | - | - |
| Stages 3 and 4 | 2.62 | 1.82-3.78 | <0.0001 | 2.49 | 1.63-3.80 | <0.0001 |
| Histology | ||||||
| Non-clear cell | Ref. | - | - | Ref. | - | - |
| Clear cell | 2.56 | 1.22-5.35 | 0.013 | 4.27 | 1.62-11.22 | 0.003 |
| Tumor size (cm) | 1.13 | 1.07-1.20 | <0.0001 | 1.16 | 1.09-1.24 | <0.0001 |
Abbreviations: HR = hazard ratio, CI = confidence interva , AJCC = American Joint Committee on Cancer
When comparing c-indices between the base model for all patients (adjusted for tumor stage, size, and histology) to the base model including nodule size or number, the results were consistent with those from the multivariable Cox models, which demonstrated that size of IPNs was a stronger predictor for all outcomes than the number of nodules. For all three outcomes, adding number of nodules to the base model did not increase the c-index (Table 5). Adding IPN size to the base model slightly increased the c-index for lung metastases and any distant metastases from 0.831 to 0.839 and 0.821 to 0.828, respectively, but it did not improve the c-index for death from disease. Similarly, among patients with pulmonary nodules, adding the size of pulmonary nodules to the base model increased the c-index of the base model for the outcomes of lung metastases and any distant metastases (Table 5). The value of this model for death from kidney cancer among patients with IPNs was not assessed due to a limited number of events.
Table 5.
C-indices for the base model, the base model plus number of nodules, and the base model plus size of nodules for all patients (N=748) and for patients with indeterminate pulmonary nodules (N=382). The base model is a multivariable Cox model adjusted for American Joint Committee on Cancer stage, histology, and tumor size. All c-indices are corrected by cross validation.
| Lung Metastases | Any Distant Metastases | Death from Kidney Cancer | |
|---|---|---|---|
| For all patients (N=748) | |||
| Base model | 0.831 | 0.821 | 0.799 |
| Base model + size of nodules | 0.839 | 0.828 | 0.799 |
| Base model + number of nodules | 0.830 | 0.819 | 0.794 |
| For patients with indeterminate pulmonary nodules (N=382) | |||
| Base model | 0.833 | 0.816 | -* |
| Base model + size of nodules | 0.850 | 0.823 | -* |
| Base model + number of nodules | 0.830 | 0.813 | -* |
No comparison was done for death from kidney cancer among patients with indeterminate pulmonary nodules due to a limited number of events.
Discussion
In the current study, IPNs were a common occurrence in patients with clinically localized RCC, apparent in 51% of patients. Contrary to previous findings, we did not find an association between the presence of IPNs and development of lung metastases, any distant metastases, or death from RCC. While our analysis including nodule size showed that the presence of IPNs >1cm was significantly associated with lung metastasis and any distant metastasis, the clinical significance of this finding for the whole cohort was limited.
Pulmonary nodules in patients diagnosed with extra-pulmonary cancer may represent scarring, metastatic disease, or primary lung tumors.8 In a study utilizing thick-section chest CT in a group of patients with extra-pulmonary cancers and non-calcified pulmonary nodules or masses, 42% of lung lesions were malignant, of which 44% represented metastatic spread of the primary cancer.8 In a recent report, utilizing thin-section chest CT, one or more non-calcified pulmonary nodules were detected in 75% of patients with extra-pulmonary malignancies. In this series, while 80% of all nodules were benign, 85% of nodules >1cm were malignant, and nodule size was predictive of malignancy (p <0.0001).14 Xu et al. identified IPNs on preoperative chest CT in 52% of non-metastatic patients with RCC,13 which was similar to the 51% identified in our cohort. Despite their frequent occurrence, the prognostic value of IPNs and their role in determining the appropriate postoperative follow-up are not well defined.
In determining the significance of IPNs in predicting RCC outcome, Xu et al. evaluated a cohort of 240 patients who underwent either chest X-ray or CT prior to surgery. DFS was associated with the presence of IPNs on multivariate analysis adjusted for tumor stage and grade (HR=1.90; 95% CI, 1.04–3.46; p=0.0362). Among patients with IPNs, nodule size was associated with DFS (HR=4.16; 95% CI, 1.25– 13.82; p=0.0201) on univariate analysis, however this lost significance on multivariate analysis. The number of nodules was not associated with survival. In addition, overall survival was not associated with the presence of IPNs.13 Xu et al. reported similar findings in the subgroup of 170 patients who underwent preoperative chest-CT.13 In the current study, the presence of IPNs was not associated with lung metastases, any distant metastases, or disease-specific survival. However, nodule size >1cm predicted progression to metastatic disease on multivariable analysis.
Current guidelines for chest imaging follow-up of patients undergoing surgery for clinically localized renal masses are based on disease risk as defined by pathologic tumor stage. According to the American Urologic Association, patients with low-risk disease should undergo chest x-ray yearly for 3 years and as clinically indicated thereafter. In patients with high-risk disease, a baseline chest CT should be obtained within 3–6 months following the operation, after which chest imaging (CT or x-ray) should be performed every 6 months for at least 3 years and annually thereafter to year 5.11 The National Comprehensive Cancer Network guidelines for kidney cancer recommend performing a chest CT at 4–6 months after surgery and as indicated thereafter.12 Neither guideline incorporates the finding of IPNs in the decision for the appropriate follow-up. The Fleischner Society recommends obtaining follow-up CT scans at 6–12 months intervals for up to 2 years for incidentally detected IPNs based on nodule size and the risk of developing lung cancer, but specific recommendations in the presence of extra-pulmonary malignancies are not indicated.7 Based on the association found between IPNs and worse DFS, Xu et al. suggested that baseline imaging should be performed followed by vigilant surveillance in all patients with preoperative IPNs.13 While the current study did not directly evaluate the validity of follow-up imaging protocols, our findings suggest that the presence of sub-centimeter IPNs should not change regular follow-up protocols. Moreover, chest CT may not be required for patients with AJCC stage I disease, as it does not alter outcomes. However, as the rate of metastatic disease to the lung is significantly higher in patients with IPNs >1cm, frequent follow-up chest imaging may enable earlier identification of disease progression in these patients. Further studies are required to evaluate whether different follow-up protocols may enable early identification of progression and affect outcome, and whether chest X-ray may be sufficient for detecting clinically significant pulmonary nodules. In addition, the use of advanced imaging modalities, including 18F-fluoro-2-deoxyglucose positron emission tomography, may assist in detecting the true nature of IPNs and help identify suspicious nodules that may be targeted for tissue sampling.15,16
Limitations of the study include its retrospective nature and the absence of standardized preoperative imaging parameters, which may affect the prevalence of IPNs. In addition, adjuvant therapies were not standardized during the years of the study, possibly affecting survival. Furthermore, the postoperative imaging follow-up protocol was not standardized. In this setting, patients without IPNs are less likely to undergo frequent chest imaging during follow-up, delaying the identification of disease progression. Nevertheless, the finding that sub-centimeter IPNs were not associated with worse cancer-related outcomes when compared to patients without IPNs would most likely be unchanged in the presence of a more frequent follow-up for patients without IPNs.
Conclusions
In the current study, IPNs were identified in half of the patients undergoing chest CT before surgery for localized RCC, most of which were ≤1cm in size. While the presence of sub-centimeter nodules was not indicative of disease progression, patients with IPNs >1cm had significantly higher rates of metastatic disease.
The presence of IPNs ≤1cm should not delay curative treatment nor promote a more vigilant follow-up protocol, thus avoiding unnecessary imaging. However, patients with IPNs >1cm may benefit from frequent follow-up imaging aimed at earlier identification of metastatic disease.
Acknowledgments
Funding
Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers and the Hanson Family Renal Cancer Research Fund.
Abbreviations and Acronyms
- RCC
renal cell carcinoma
- EAU
European Association of Urology
- CT
computed tomography
- IPNs
indeterminate pulmonary nodules
- DFS
disease-free survival
- AJCC
American Joint Committee on Cancer
- IQR
interquartile range
- HR
hazard ratio
- CI
confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors have nothing to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B. The role of metastasectomy in renal cell carcinoma in the era of targeted therapy. Curr Urol Rep. 2013;14:19. doi: 10.1007/s11934-012-0293-6. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973. doi: 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 5.Griffin N, Gore ME, Sohaib SA. Imaging in metastatic renal cell carcinoma. AJR Am J Roentgenol. 2007;189:360. doi: 10.2214/AJR.07.2077. [DOI] [PubMed] [Google Scholar]
- 6.Austin JH, Muller NL, Friedman PJ, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology. 1996;200:327. doi: 10.1148/radiology.200.2.8685321. [DOI] [PubMed] [Google Scholar]
- 7.MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237:395. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 8.Khokhar S, Vickers A, Moore MS, et al. Significance of non-calcified pulmonary nodules in patients with extrapulmonary cancers. Thorax. 2006;61:331. doi: 10.1136/thx.2005.051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordholm-Carstensen A, Wille-Jorgensen PA, Jorgensen LN, et al. Indeterminate Pulmonary Nodules at Colorectal Cancer Staging: A Systematic Review of Predictive Parameters for Malignancy. Ann Surg Oncol. 2013;20:4022. doi: 10.1245/s10434-013-3062-y. [DOI] [PubMed] [Google Scholar]
- 10.Lee B, Lim A, Lalvani A, et al. The clinical significance of radiologically detected silent pulmonary nodules in early breast cancer. Ann Oncol. 2008;19:2001. doi: 10.1093/annonc/mdn421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donat SM, Diaz M, Bishoff JT, et al. Follow-up for Clinically Localized Renal Neoplasms: AUA Guideline. J Urol. 2013;190:407. doi: 10.1016/j.juro.2013.04.121. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Agarwal N, Beard C, et al. NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw. 2009;7:618. doi: 10.6004/jnccn.2009.0043. [DOI] [PubMed] [Google Scholar]
- 13.Xu R, Horick N, McGovern FJ, et al. Prognostic significance of indeterminate lung nodules in renal cell carcinoma. Urol Oncol. 2014;32:355. doi: 10.1016/j.urolonc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Hanamiya M, Aoki T, Yamashita Y, et al. Frequency and significance of pulmonary nodules on thin-section CT in patients with extrapulmonary malignant neoplasms. Eur J Radiol. 2012;81:152. doi: 10.1016/j.ejrad.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Chang CH, Shiau YC, Shen YY, et al. Differentiating solitary pulmonary metastases in patients with renal cell carcinomas by 18F-fluoro-2-deoxyglucose positron emission tomography--a preliminary report. Urol Int. 2003;71:306. doi: 10.1159/000072683. [DOI] [PubMed] [Google Scholar]
- 16.Manohar K, Bhattacharya A, Vyas S, et al. Metastatic lung nodule diagnosed on 18F-FDG PET/CT-guided fine needle aspiration in a patient with renal cell carcinoma. Clin Nucl Med. 2013;38:e38. doi: 10.1097/RLU.0b013e3182485323. [DOI] [PubMed] [Google Scholar]