Abstract
Objective
Enhanced HIV prevention interventions, such as pre-exposure prophylaxis for high-risk individuals, require substantial investments. We sought to estimate the medical cost saved by averting one HIV infection in the United States.
Methods
We estimated lifetime medical costs in persons with and without HIV to determine the cost saved by preventing one HIV infection. We used a computer simulation model of HIV disease and treatment (CEPAC) to project CD4 cell count, antiretroviral treatment status, and mortality after HIV infection. Annual medical cost estimates for HIV-infected persons, adjusted for age, sex, race/ethnicity, and transmission risk group, were from the HIV Research Network (range $1,854–$4,545/month) and for HIV-uninfected persons were from the Medical Expenditure Panel Survey (range $73–$628/month). Results are reported as lifetime medical costs from the US health system perspective discounted at 3% (2012 US dollars).
Results
The estimated discounted lifetime cost for persons who become HIV infected at age 35 is $326,500 (60% for antiretroviral medications, 15% for other medications, 25% non-drug costs). For individuals who remain uninfected but at high risk for infection, the discounted lifetime cost estimate is $96,700. The medical cost saved by avoiding one HIV infection is $229,800. The cost saved would reach $338,400 if all HIV-infected individuals presented early and remained in care. Cost savings are higher taking into account secondary infections avoided and lower if HIV infections are temporarily delayed rather than permanently avoided.
Conclusions
The economic value of HIV prevention in the US is substantial given the high cost of HIV disease treatment.
Keywords: HIV, AIDS, health care cost, prevention, computer modeling
INTRODUCTION
Since early in the HIV epidemic in the United States, researchers have sought to provide decision makers with the best available information about the costs of HIV care and costs avoided by HIV prevention to support fiscal planning, program evaluation, resource allocation, and public health policy [1–6]. We previously examined the lifetime cost of providing guideline-concordant care to HIV-infected adults in the US in 2004. We estimated that the discounted lifetime cost from time of infection was $303,100 ($361,400 in 2012 US dollars) [7]. This finding has been widely used as a benchmark for evaluating HIV prevention interventions [8, 9]. Recently, the Centers for Disease Control and Prevention (CDC) recommended prescribing pre-exposure prophylaxis (PrEP) in high-risk populations, a potentially highly effective but costly prevention intervention [10]. Updated cost and cost savings estimates are needed that reflect the reality of current HIV care in the US [11], including current treatments and treatment initiation practices [12], costs for disease management of chronic HIV disease and other chronic diseases [13], and inconsistent access and adherence to effective treatment [14].
Our objective was to project the lifetime medical cost avoided by averting one HIV infection in the United States, taking into account the medical costs that would have been incurred in the absence of HIV infection, considering costs of medications for conditions other than HIV, and incorporating realistic assumptions about lack of continuity of HIV care compared to optimal care delivery.
METHODS
Analytic Overview
We estimated the lifetime medical cost savings from averting one HIV infection. The analysis was conducted from the US health care system perspective [15]. We first estimated mean lifetime medical costs accrued by HIV-infected persons beginning at the time of HIV infection. We then subtracted the mean estimated lifetime costs accrued by demographically similar individuals who remain HIV uninfected. The estimation period began at age 35, which we estimated to be the mean age at infection in the US [15]. We also conducted analyses in which the estimation period began at age 25 and age 55.
For HIV-infected persons, we projected mortality and immune function (CD4 count distribution) using the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model, a widely-published state-transition model of HIV disease [7, 16, 17]. Annual medical costs were estimated using data from the HIV Research Network (HIVRN) [18]. These costs were stratified based on age, sex, race/ethnicity, HIV transmission risk factors (men who have sex with men (MSM), injection drug user (IDU), heterosexual sex), and CD4 count.
We estimated non-HIV mortality from race/ethnicity and sex-specific life tables from CDC [19]. We adjusted these estimates to reflect differences in mortality observed among HIV-uninfected MSM, IDU, and heterosexual individuals at risk of becoming HIV infected [20] (see Appendix). We used age, race/ethnicity, and sex-specific costs from the 2009 Medical Expenditure Panel Survey (MEPS) to estimate annual medical costs of HIV-uninfected individuals [21].
We also considered the case where HIV infection is delayed – but not permanently averted – for 5 years. In this case we compared the discounted lifetime cost for individuals who become HIV-infected at age 35 to the discounted lifetime costs for individuals who are HIV-uninfected at age 35 and then become infected at age 40.
We addressed uncertainty about the medical costs by deriving cost distributions from 1,000 random draws based on the standard errors around the predicted costs provided by a generalized linear model for HIV-infected individuals and by using the MEPS standard errors for HIV-uninfected individuals. The resulting empiric standard errors (standard deviations of the distribution of mean values) are presented in parentheses after mean estimates.
Results are reported as projected lifetime medical costs in 2012 US dollars. Life expectancy results are reported undiscounted, and cost results are reported both undiscounted and discounted to present value at a 3% annual rate [22]. Discounted costs are highlighted throughout because they represent economic costs that take into account time preferences of individuals and society and the opportunity cost of funds [22].
Computer Simulation Model
We used the CEPAC model of HIV natural history and treatment to project annual mortality and immune function status (represented by CD4 count [17]) over time in a hypothetical cohort of newly HIV-infected individuals. In our analysis, patients enter the model at the time of HIV infection and follow natural disease progression until antiretroviral therapy (ART) initiation. Patients’ HIV RNA level as well as current CD4 count determine their CD4 count decline. Patients in the model may die due to acute opportunistic infections, chronic HIV-related causes, or non-HIV-related causes. Risks for opportunistic infections and mortality depend on CD4 count. Non-HIV-related mortality is dependent on age, sex, race/ethnicity, and HIV transmission risk factor (MSM, IDU, heterosexual sex) [23].
ART is initiated after simulated HIV-infected individuals enter HIV care [12], and prophylaxis against opportunistic infections is initiated using the CD4 threshold criteria specified in US guidelines [24]. Once patients initiate ART, they have a probability of achieving virologic suppression and consequent CD4 count gain, with the greatest CD4 count gain during the first two months on therapy [25]. Increasing CD4 counts reduces the likelihood of developing opportunistic infections and HIV-related mortality. After achieving viral suppression, patients are subject to a monthly probability of virologic failure and a monthly probability of loss to follow-up while on ART. Probabilities of achieving virologic suppression, virologic failure, and loss to follow-up vary depending on ART adherence (see Appendix).
Medical Services Utilization and Annual Cost Estimation
We used data from the HIVRN, a consortium of hospital- and community-based HIV care sites in the US, to calculate medical costs of HIV-infected individuals [18, 26, 27]. HIVRN sites annually abstract specified data elements from patients’ medical records and assemble them into a uniform database. Of the 12 HIVRN sites that treat adult patients, 7 collect comprehensive prescription medication data. Analyses were limited to adult patients (>18 years) at these 7 sites who were in HIV primary care, defined by having at least 1 visit to the primary HIV care provider and a CD4 count drawn between January 1, 2009 and December 31, 2009.
Each site provided data on several cost components: inpatient hospital days for any reason, outpatient primary care visits to the HIV clinic, emergency department (ED) visits for any reason, CD4 and viral load laboratory tests, and HIV-related and HIV-unrelated prescription medications recorded in the HIV clinic charts. We counted the total numbers of outpatient visits to the HIV primary care provider, inpatient days, ED visits, CD4 tests, and HIV RNA tests for each patient within strata defined by median CD4 in 2009 (<= 50, 51–200, 201–350, 351–500, >500/μL). For each type of service, we estimated costs by multiplying utilization data by an appropriate unit cost estimate (see Appendix). Medical record data provided detailed information and start and stop dates on all prescribed medications. For each patient, we calculated the number of months that each medication was prescribed, which we multiplied by an estimated monthly cost based on the discounted Red Book average wholesale price for that medication [27, 28]. Total annual expenditures were the sum of estimated costs for each cost component.
To take into account variations in medical costs by patient demographics and immune status, we estimated a multivariable Poisson regression model that included sex, race/ethnicity, HIV risk group, age, CD4 count stratum, any ART use, and indicators for HIVRN provider site. For each combination of independent variables, we calculated mean predicted costs based on this model, averaging predictions across sites. The predicted annual costs were adjusted to 2012 US dollars using the medical care component of the Consumer Price Index [29] (Table 1) and served as inputs for the subsequent analysis.
Table 1.
Model inputs
| Variable | Base Case | Reference |
|---|---|---|
| Cohort characteristics at time of HIV infection | ||
| CD4 cell count, mean cells/μl (SD) | 751 (267) | [45] |
| Age, years | 35 | [19] |
| HIV RNA distribution post-acute infection (%) | [46] | |
| >100,000 copies/ml | 25 | |
| 30,001–100,000 copies/ml | 42 | |
| 10,001–30,000 copies/ml | 21 | |
| <10,000 copies/ml | 12 | |
| Baseline ART adherence, % | derived from [47, 48] | |
| Adherence < 50% | 3.1 | |
| 50% ≤ Adherence < 95% | 50.7 | |
| Adherence ≥ 95% | 46.2 | |
| First-line ART efficacy | ||
| HIV RNA suppressed at 6 months, % | 83.5 | [49] |
| Loss to follow-up and return to care, per 100 person years* | ||
| Loss to follow-up rate on ART | 7.5 | HIVRN data |
| Return to care rate | 16.9 | HIVRN data |
| Treatment initiation | ||
| CD4 count at ART initiation (mean cells/μl) | 342† | [30, 31] |
| Unit Costs | ||
| % discount from AWP applied to non-generic drugs | 23 | [50] |
| Inpatient visit (2012 US dollars) | 2,228 | See Appendix |
| Outpatient visit (2012 US dollars) | 123 | See Appendix |
| Emergency department visit (2012 US dollars) | 723 | See Appendix |
| CD4 test (2012 US dollars) | 72 | [51] |
| HIV RNA test (2012 US dollars) | 131 | [51] |
| Estimated mean annual costs (SE, 2012 US dollars)‡ | ||
| Age and disease stage | ||
| 18–29 years | ||
| HIV-uninfected individuals | 1,468 (168) | MEPS§ |
| HIV-infected individuals (CD4 cells) | HIVRN | |
| <= 50/μl | 35,272 (2,426) | |
| 51–200/μl | 26,934 (1,420) | |
| 201–350/μl | 22,891 (1,131) | |
| 351–500/μl | 20,881 (1,013) | |
| >500/μl | 19,682 (940) | |
| 30–39 years | ||
| HIV-uninfected individuals | 1,998 (381) | MEPS§ |
| HIV-infected individuals (CD4 cells) | HIVRN | |
| <= 50/μl | 40,633 (2,416) | |
| 51–200/μl | 31,028 (1,214) | |
| 201–350/μl | 26,371 (920) | |
| 351–500/μl | 24,055 (815) | |
| >500/μl | 22,674 (736) | |
| 40–49 years | ||
| HIV-uninfected individuals | 3,347 (488) | MEPS§ |
| HIV-infected individuals (CD4 cells) | HIVRN | |
| <= 50/μl | 44,745 (2,490) | |
| 51–200/μl | 34,167 (1,118) | |
| 201–350/μl | 29,039 (759) | |
| 351–500/μl | 26,489 (660) | |
| >500/μl | 24,968 (565) | |
| 50+ years | ||
| HIV-uninfected individuals | 6,622 (499) | MEPS§ |
| HIV-infected individuals (CD4 cells) | HIVRN | |
| <= 50/μl | 48,217 (2,863) | |
| 51–200/μl | 36,818 (1,327) | |
| 201–350/μl | 31,292 (880) | |
| 351–500/μl | 28,545 (790) | |
| >500/μl | 26,905 (675) | |
Loss to follow-up is defined as an interruption in care of at least 12 months
Range 261–480 depending on sex, race/ethnicity, and transmission category (see Appendix Table 1)
Estimated mean monthly costs summarized in this table are weighted by transmission risk group (see Appendix Table 2)
Medical Expenditure Panel Survey (MEPS) data are assigned to transmission risk groups based on sex and race/ethnicity for each risk group at each age
SD: standard deviation; ART: antiretroviral therapy; HIVRN: HIV Research Network; AWP: average wholesale price; MEPS: Medical Expenditure Panel Survey; SE: standard error
To calculate annual medical costs of HIV-uninfected individuals, we used data from the MEPS, a nationally representative survey of households in the US [21]. Using the online MEPS query tool [21], we calculated mean annual medical care expenditures from the MEPS household survey in 2009 stratified by sex, race/ethnicity, and age. We did not include costs of physical therapy, occupational therapy, chiropractic care, optometry, and home health care, because these costs were not included in the HIVRN cost estimates for HIV-infected individuals. We then adjusted these costs to 2012 dollars (Table 1).
Base case analysis
We conducted analyses separately for 15 subpopulations: 5 transmission risk groups (MSM, male IDU, male heterosexual, female IDU, female heterosexual) stratified by race/ethnicity (White, Black, Hispanic). CD4 at entry into care was specified separately for each population [30, 31]. We assumed ART was initiated for all individuals at care entry and retention was consistent with HIVRN sites’ experience. Overall costs were estimated by an average of model results for the 15 subpopulations, weighted in proportion to each subpopulation’s contribution to HIV incidence in the US between 2006 and 2009 [15]. This approach necessitated using only one age at time of infection for each analysis. For HIV-infected individuals, we used the CEPAC model to project the proportion of individuals alive in each year after HIV infection, stratified by CD4 category and whether the individuals were or were not in care and were or were not on ART. Probabilities of achieving virologic suppression, subsequent virologic failure, and loss to follow-up depend on model inputs for ART adherence (Table 1). We then applied the relevant estimated annual costs from the HIVRN. We assigned to individuals out of care (pre-ART or lost to follow-up) the cost of care for HIV-infected individuals not on ART with the same demographic characteristics and CD4 count.
For HIV-uninfected individuals, we used the adjusted life tables to project annual mortality [19, 20], assuming they remained HIV uninfected. Costs for each year were then applied using the relevant MEPS annual cost estimates for individuals remaining alive. Discounted annual costs were summed across years from age 35 until death. We were limited to choosing one initial age for each analysis by the overall method used to estimate costs, which requires a weighted average of model results for 15 subpopulations.
Sensitivity analyses
We conducted a sensitivity analysis that assumed an optimal care scenario in which all HIV-infected individuals were identified and linked to care at a CD4 count of 500/μl, initiated ART immediately, and were never lost from care. We also varied costs to reflect the possibility that the HIV-uninfected individuals would be more frequent users of medical services in this optimal care scenario even if they remained HIV uninfected. To consider the potential impact of the availability of generic antiretroviral medication regimens in the future, we conducted a sensitivity analysis in which we assumed the costs for 85% of individuals on first-line regimens would be reduced by 40% [32]. We also varied costs for HIV-infected individuals based on the 95% confidence interval around predicted costs for each subpopulation.
Additional sensitivity analyses included: varying the CEPAC model inputs for ART adherence (e.g. proportion achieving ≥ 95% adherence); assuming non-HIV mortality is that of individuals of the same sex, age, and race/ethnicity in the US population without further risk adjustment; and repeating the base case analyses assuming ages at infection of 25 and 55.
To incorporate the effect of secondary HIV transmission, we applied per person probabilities of sexual transmission during acute infection, post-acute unaware, and post-acute aware periods [33, 34] to HIV-infected patients alive in each year since infection. For each subpopulation, we assumed that each secondary transmission averted would accrue the same discounted incremental cost as the primary transmission starting at the time of secondary infection. The savings were discounted to present value and the sum of these additional cost savings was added to the base case cost savings. We assumed that if a secondary transmission did not occur in a given year, the HIV infection would not be permanently avoided due to ongoing risk behavior in the population, but would be delayed for five years before the infection eventually occurred.
RESULTS
Base case
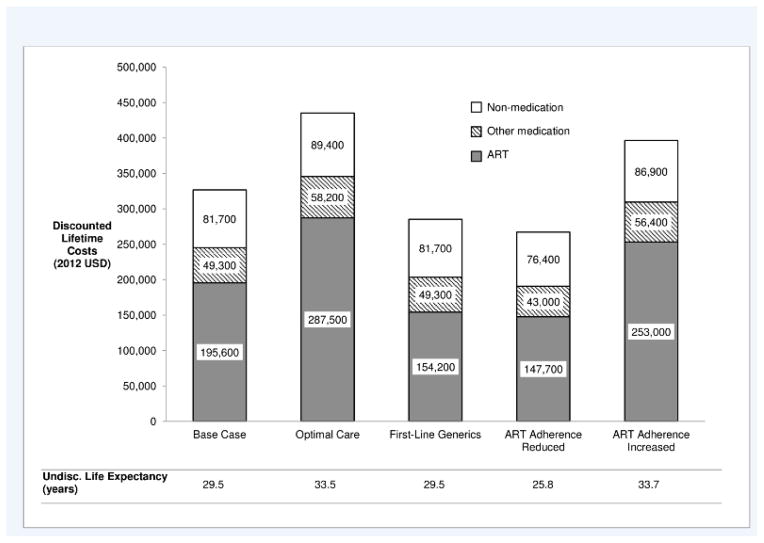
Based on current data on time of entry into HIV care [31], estimated life expectancy for persons who became HIV infected in 2012 at age 35 [15] is 29.5 years and discounted mean lifetime medical cost is $326,500 (standard error $2,100). Of the total cost, 60% is for antiretroviral medications, 15% is for chronic disease medications and for opportunistic infection prophylaxis and treatment medications, and 25% is for non-medication costs (Figure 1). Undiscounted mean lifetime costs are $597,300 ($4,200). After entry into care, which was estimated using the CEPAC model to occur on average 5.1 years after infection, individuals spend 17% of total life years with CD4 <500/μl and 83% with CD4 ≥500/μl. For individuals who remain uninfected, estimated life expectancy is 38.1 years, discounted lifetime cost is $96,700 ($2,700), and undiscounted lifetime cost is $197,100 ($5,400). Therefore the estimated discounted medical cost saved by avoiding an HIV infection, based on current linkage to and retention in HIV care, is $229,800 ($3,400) ($326,500 minus $96,700, Table 2).
Figure 1. Lifetime discounted cost from time of HIV infection by cost category (2012 US dollars).
Other medication = opportunistic infection prophylaxis and treatment medication and other chronic disease medication; ART = antiretroviral therapy; Optimal Care = entry to care at CD4 <500/μL, no interruption in care
Table 2.
Medical cost saved from preventing an HIV infection in the United States, excluding secondary transmission effects (2012 US dollars)
| Mean per Person Lifetime Medical Cost (SE) | Lifetime Cost Saved (SE)* | |||
|---|---|---|---|---|
|
| ||||
| Discounted | Undiscounted | Discounted | Undiscounted | |
| Cost for HIV-uninfected individual | 96,700 (2,700) | 197,100 (5,400) | N/A | N/A |
| Cost for HIV-infected individual | ||||
| Base case (current entry to and retention in HIV care) | 326,500 (2,100) | 597,300 (4,200) | 229,800 (3,400) | 400,200 (6,900) |
| Entry to care at CD4 <500/μL, no interruption in care, no change in cost for HIV-uninfected individual (“Optimal Care”) | 435,200 (2,700) | 798,300 (5,800) | 338,400 (3,600) | 601,200 (7,800) |
| Entry to care at CD4 <500/μL, no interruption in care, increased cost for HIV-uninfected individual | 435,200 (2,700) | 798,300 (5,800) | 326,100 (3,600)† | 576,100 (7,900)† |
| First-line generic ART | 285,200 (2,000) | 525,700 (4,200) | 188,400 (3,300) | 328,600 (6,900) |
| ART adherence reduced‡ | 267,100 (1,600) | 470,900 (3,300) | 170,300 (3,100) | 273,800 (6,300) |
| ART adherence increased‡ | 396,300 (2,600) | 749,100 (5,900) | 299,600 (3,700) | 552,100 (8,000) |
| Low cost of medical care for HIV-infected individuals estimate | 300,700 (1,900) | 553,100 (4,400) | 203,900 (2,600) | 356,100 (7,000) |
| High cost of medical care for HIV-infected individuals estimate | 352,400 (2,000) | 641,500 (4,400) | 255,600 (3,200) | 444,400 (7,000) |
Lifetime cost saved is equal to the difference between the mean person lifetime cost for HIV-infected and HIV-uninfected individuals; however, these numbers do not sum exactly due to rounding.
Increased lifetime cost for HIV-uninfected individual for entry to care at CD <500/μL, Discounted: 106,800; Undiscounted: 221,200.
The proportions of individuals with baseline ART adherence of <50%, 50–94%, and ≥95% were adjusted so that initial suppression on first-line ART at 6 months was 75% (ART adherence reduced) or 90% (ART adherence increased).
SE: standard error; ART: antiretroviral therapy.
Across the 15 subpopulations, life expectancies vary from 13.9 to 34.2 years for HIV-infected and 15.5 to 45.7 years for HIV-uninfected persons. The time from infection to diagnosis varies from 3.2 to 5.7 years; and discounted medical cost saved by avoiding an HIV infection, based on current linkage to and retention in HIV care, varies from $121,900 ($5,400) to $283,800 ($7,800) (Appendix Table 3).
Results of sensitivity analyses
For individuals who receive optimal care, estimated life expectancy is 33.5 years, discounted lifetime cost is $435,200 ($2,700), antiretroviral medications costs are 47% higher than the base case, and other medication and non-medication costs are 13% higher than the base case (Figure 1). Estimated undiscounted lifetime cost is $798,300 ($5,800). The discounted medical cost saved by avoiding an HIV infection, assuming this optimal HIV care, is estimated at $338,400 ($3,600). If costs for HIV-uninfected individuals are also increased by 13%, the discounted medical cost saving for the optimal care scenario is estimated at $326,100 ($3,600).
Additional sensitivity analyses indicate that the discounted cost saving is $188,400 ($3,300), 13% lower than the base case, when assuming first-line antiretroviral treatment with generic medication regimens. Results are also sensitive to model assumptions about ART adherence as well as medical costs at the highest and lowest estimates for HIV-infected individuals (Table 2). Assuming HIV-infected individuals out of care incurred only hospitalization costs yielded results similar to using the lowest medical cost estimates (results not shown). Discounted cost savings increase by 7% to $245,600 ($3,300) when non-HIV mortality is based on life tables without adjustment by risk group.
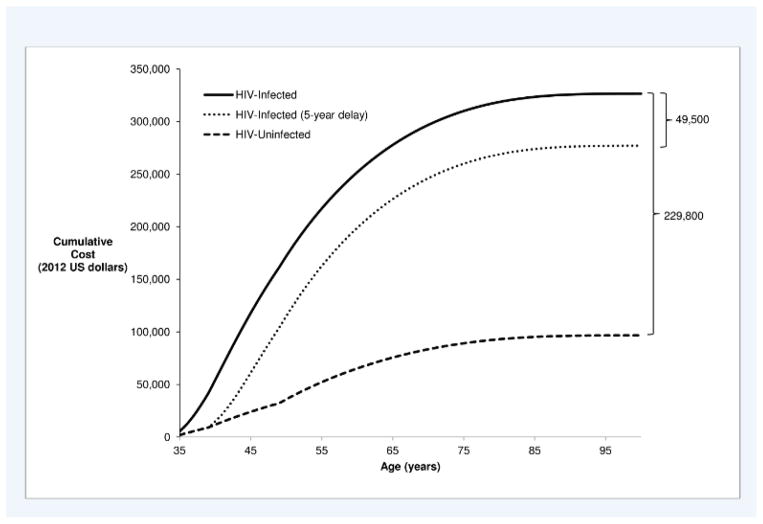
Discounted savings vary substantially depending on whether primary HIV infections are temporarily delayed rather than permanently avoided as assumed in the base case. For example, discounted lifetime medical cost savings are $49,500 ($2,900) if a primary infection is delayed for 5 years (Figure 2 – difference between solid and dotted lines) versus $229,800 ($3,400) if the infection is avoided (Figure 2 – difference between solid and dashed lines). Discounted savings also vary depending on age at HIV infection, ranging from $248,700 ($3,000) discounted lifetime medical savings if an infection is permanently avoided in a 25 year-old individual to $146,300 ($4,100) if an infection is permanently avoided in a 55 year-old individual. The impact of assuming the HIV infection is temporarily delayed rather than permanently avoided also varies by age of infection (Appendix Figures 1 and 2).
Figure 2.
Cumulative discounted lifetime costs from time of infection at age 35 (2012 US dollars)
Impact of Secondary Transmission
In sensitivity analyses that included secondary transmission, the average HIV-infected person is expected to transmit HIV to 0.97 HIV-uninfected persons over his or her lifetime. The discounted medical cost saved by avoiding an HIV infection and delaying secondary transmission by 5 years is $270,300 ($229,800 plus $40,500).
DISCUSSION
Effective treatment has increased life expectancy after HIV infection, and deaths from non-AIDS-related causes now exceed deaths from AIDS for those with HIV in the US [35]. Medical costs of treating HIV-infected individuals as they age now include costs of both HIV-related and HIV-unrelated medical care. We estimated the medical cost saved by averting one HIV infection in the United States, taking into account the costs that would have been incurred by similar at-risk individuals in the absence of HIV infection. We project discounted medical cost savings of $229,800 by permanently averting one HIV infection based on current care patterns in the US and $49,500 if one HIV infection is delayed by 5 years. Our analysis shows that as HIV care becomes more effective, the cost avoided by averting one HIV infection also increases. Improved care is cost-effective by accepted standards in the US, it is not cost-saving [36]. The added years of life, however, result in additional costs for treatment that would not have occurred in the absence of an infection.
Our projections of lifetime medical costs for HIV-infected individuals of $326,500 in the base case and $435,200 in the optimal care case are comparable to recent model-based estimates of lifetime costs for individuals in the US entering care with CD4 201–350/μl ($332,300 in 2012 US dollars) and >500/μl ($443,000) respectively [37], and costs from entry into care (not shown) are consistent with previous estimates of these costs in France using the CEPAC model [38]. Our projection of medical cost savings of $229,800 is substantially lower than the previous estimate of $303,100 in 2004 US dollars ($361,400 in 2012 US dollars) [7] for several reasons. First, we now account for medical costs that would have been incurred in the absence of an HIV infection. Second, our previous analysis did not adjust mortality for risk group characteristics that lower average life expectancy [23], thereby reducing costs, nor did they adjust costs for health service utilization by different risk groups. Our life expectancy estimates are lower than two other recent model-based analyses in the United States and the United Kingdom [37, 39], likely reflecting the race/ethnicity and risk-category mortality effects in our model. Our results are consistent with these models, however, in projecting substantial life expectancy losses associated both with becoming HIV infected and with delayed initiation of treatment after infection.
Consistent with other analyses [7, 37], we found that ART medications represent the largest component of cost for HIV-infected individuals. We found that non-HIV chronic care medications represent a substantial component of cost as well, emphasizing the significant cost of managing non-HIV comorbidities in an aging HIV-infected population [13, 40]. These comorbidities are frequently managed by HIV primary care providers [41]. Our results are somewhat sensitive to assumptions about future use of generic HIV drugs in the US. This points to the potential importance of future availability of generic drugs in lowering the cost of HIV care, depending on regimens selected and adherence [32].
Our analysis also indicates that the value of HIV primary prevention may be greater when the effects of preventing secondary transmission to HIV-uninfected partners are taken into account, which would increase the value of interventions targeting individuals at high risk of transmitting to multiple partners. The magnitude of this impact is greater the longer individuals remain uninfected after avoiding a secondary transmission. The current relatively stable HIV incidence trends in the US [15] suggest these uninfected partners are at high risk for eventual HIV infection. If the probabilities of secondary transmission we used already take into account this additional risk, the value of primary prevention would be even higher.
Our analysis is subject to several limitations. By focusing on health system costs, our analysis does not reflect quality-of-life gains and broader societal benefits of avoiding HIV infections. The unit costs we applied to determine lifetime medical costs for HIV-infected individuals represent costs to the health care system. In contrast, the costs applied to non-HIV-infected individuals were from the MEPS survey that collected data on medical expenditures, which may be different from costs to the health care system. Although we removed out-of-pocket costs and costs reported in MEPS for services not included in HIVRN, which could lead to a slight underestimate of costs for HIV-infected individuals, costs for HIV-uninfected individuals may be overstated nevertheless. In contrast, costs for HIV-infected individuals may be overstated because, in the base case, we assume that their costs prior to diagnosis and when lost to follow-up are similar to those of HIV-infected individuals with the same CD4 count who are in care but not receiving ART.
Model-based analyses use available data to project future mortality and costs. To address this limitation, we conducted several sensitivity analyses to consider the impact of potential improvements in ART efficacy and reductions in the cost of ART, which are the principal drivers of life expectancy and total cost for HIV-infected individuals. We did not explicitly model the impact of hepatitis C infection and treatment; treatment options for HCV are evolving rapidly, and future HCV treatment efficacy, costs, and acceptance in HIV co-infected populations are uncertain [42]. Our lifetime cost projections include HIV-related medical costs and some HIV-unrelated medical costs, but we also did not separately model end-of-life care costs or other specific comorbidities when deriving these estimates [43]; explicitly modeling comorbidities could result in shorter forecasted life expectancies but higher costs while alive. Our health services utilization data from HIVRN include data from individuals across a broad range of demographics, ensuring representation of costs associated with comorbidities and aging. Nevertheless data collected in 2009 may not reflect current services utilization. There were some medical services that HIVRN did not capture because patients received services from providers outside the HIVRN or from HIVRN providers who were not designated as the primary HIV provider. The HIVRN data also did not include diagnostic tests and procedures other than CD4 and HIV RNA tests. The estimated savings associated with prevention of HIV disease would be higher if these costs were included.
The costs to society of an HIV infection extend well beyond the medical domain, including social services, housing, patient time, lost productivity, and physical and emotional distress to patients and their families. Estimates of medical costs averted represent a lower bound on the societal value of averting HIV infections. Nevertheless, the potential medical cost savings from avoiding or even delaying HIV infections in the US are substantial, given the high cost of HIV treatment. Combination prevention interventions, including targeted PrEP, that fundamentally address transmission and reduce HIV incidence have the potential to permanently prevent HIV infections [44]. With greater success in effectively treating HIV as a chronic disease, the economic value of HIV prevention in the US increases.
Supplementary Material
Acknowledgments
This work was funded by the National Institute for Allergy and Infectious Diseases (R37 AI42006; P30 AI094189); the Agency for Healthcare Research and Quality (HHSA290201100007C); and the Health Resources and Services Administration (HHSH250201200008C).
References
- 1.Hay JW, Osmond DH, Jacobson MA. Projecting the medical costs of AIDS and ARC in the United States. J Acquir Immune Defic Syndr. 1988;1:466–485. [PubMed] [Google Scholar]
- 2.Hellinger FJ. The lifetime cost of treating a person with HIV. JAMA. 1993;270:474–478. [PubMed] [Google Scholar]
- 3.Pinkerton SD, Holtgrave DR. Lifetime costs of HIV/AIDS medical care. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:380–382. doi: 10.1097/00042560-199704010-00012. [DOI] [PubMed] [Google Scholar]
- 4.Gebo KA, Chaisson RE, Folkemer JG, et al. Costs of HIV medical care in the era of highly active antiretroviral therapy. AIDS. 1999;13:963–969. doi: 10.1097/00002030-199905280-00013. [DOI] [PubMed] [Google Scholar]
- 5.Hellinger FJ, Fleishman JA. Estimating the national cost of treating people with HIV disease: Patient, payer, and provider data. J Acquir Immune Defic Syndr. 2000;24:182–188. doi: 10.1097/00126334-200006010-00016. [DOI] [PubMed] [Google Scholar]
- 6.Bozzette SA, Joyce G, McCaffrey DF, et al. Expenditures for the care of HIV-infected patients in the era of highly active antiretroviral therapy. N Engl J Med. 2001;344:817–823. doi: 10.1056/NEJM200103153441107. [DOI] [PubMed] [Google Scholar]
- 7.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 8.Farnham PG, Holtgrave DR, Sansom SL, et al. Medical costs averted by HIV prevention efforts in the United States, 1991–2006. J Acquir Immune Defic Syndr. 2010;54:565–567. doi: 10.1097/QAI.0b013e3181e461b2. [DOI] [PubMed] [Google Scholar]
- 9.Lasry A, Sansom SL, Hicks KA, et al. Allocating HIV prevention funds in the United States: Recommendations from an optimization model. PLoS One. 2012;7:e37545. doi: 10.1371/journal.pone.0037545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States - 2014 clinical practice guideline. Center for Disease Control and Prevention; [Accessed May 6, 2014]. Available at: http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf. [Google Scholar]
- 11.Schackman BR. The value of HIV screening in the United States in the era of effective treatment. Med Decis Making. 2013;33:457–459. doi: 10.1177/0272989X13486978. [DOI] [PubMed] [Google Scholar]
- 12.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2013. [Accessed May 6, 2014]. Available at: http://www.aidsinfo.nih.gov/contentfiles/adultandadolescentGL.pdf. [Google Scholar]
- 13.Gebo KA. Epidemiology of HIV and response to antiretroviral therapy in the middle aged and elderly. Aging Health. 2008;4:615–627. doi: 10.2217/1745509X.4.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleishman JA, Yehia BR, Moore RD, et al. Disparities in receipt of antiretroviral therapy among HIV-infected adults (2002–2008) Med Care. 2012;50:419–427. doi: 10.1097/MLR.0b013e31824e3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–831. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 17.Cost-effectiveness of preventing AIDS complications. Massachusetts General Hospital; [Accessed December 18, 2013]. Available at: http://web2.research.partners.org/cepac/mainpage.html. [Google Scholar]
- 18.HIV Research Network. Hospital and outpatient health services utilization among HIV-infected patients in care in 1999. J Acquir Immune Defic Syndr. 2002;30:21–26. doi: 10.1097/00126334-200205010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Arias E. United States life tables, 2008. Centers for Disease Control and Prevention; 2012. [Accessed May 26, 2014]. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_03.pdf. [PubMed] [Google Scholar]
- 20.Seage GR, 3rd, Holte SE, Metzger D, et al. Are US populations appropriate for trials of human immunodeficiency virus vaccine? The HIVNET Vaccine Preparedness Study. Am J Epidemiol. 2001;153:619–627. doi: 10.1093/aje/153.7.619. [DOI] [PubMed] [Google Scholar]
- 21.Agency for Healthcare Research and Quality. [Accessed January 23, 2014];Medical Expenditure Panel Survey. 2009 Available at: http://meps.ahrq.gov/mepsweb/about_meps/survey_back.jsp.
- 22.Gold MR. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 23.Losina E, Schackman BR, Sadownik SN, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the United States: Impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009;49:1570–1578. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan JE, Benson C, Holmes KH, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. [PubMed] [Google Scholar]
- 25.Pozniak AL, Gallant JE, DeJesus E, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: Virologic, immunologic, and morphologic changes - a 96-week analysis. J Acquir Immune Defic Syndr. 2006;43:535–540. doi: 10.1097/01.qai.0000245886.51262.67. [DOI] [PubMed] [Google Scholar]
- 26.Fleishman JA, Gebo KA, Reilly ED, et al. Hospital and outpatient health services utilization among HIV-infected adults in care 2000–2002. Med Care. 2005;43:III40–52. doi: 10.1097/01.mlr.0000175621.65005.c6. [DOI] [PubMed] [Google Scholar]
- 27.Gebo KA, Fleishman JA, Conviser R, et al. Contemporary costs of HIV healthcare in the HAART era. AIDS. 2010;24:2705–2715. doi: 10.1097/QAD.0b013e32833f3c14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Red Book. Montvale, NJ: Thompson PDR; 2009. [Google Scholar]
- 29.United States Department of Labor. [Accessed June 23, 2014];Measuring price change for medical care in the CPI. 2010 Available at: http://www.bls.gov/cpi/cpifact4.htm.
- 30.Althoff KN. Personal communication to K. Gebo requesting NA-ACCORD data for CD4 count at presentation to care stratified by race, sex, and risk group (2007 data) 2012 [Google Scholar]
- 31.Althoff KN, Gange SJ, Klein MB, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010;50:1512–1520. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walensky RP, Sax PE, Nakamura YM, et al. Economic savings versus health losses: The cost-effectiveness of generic antiretroviral therapy in the United States. Ann Intern Med. 2013;158:84–92. doi: 10.7326/0003-4819-158-2-201301150-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhu VS, Hutchinson AB, Farnham PG, et al. Sexually acquired HIV infections in the United States due to acute-phase HIV transmission: An update. AIDS. 2009;23:1792–1794. doi: 10.1097/QAD.0b013e32832e7d04. [DOI] [PubMed] [Google Scholar]
- 34.Pinkerton SD. How many sexually-acquired HIV infections in the USA are due to acute-phase HIV transmission? AIDS. 2007;21:1625–1629. doi: 10.1097/QAD.0b013e32826fb6a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 36.Gopalappa C, Farnham PG, Hutchinson AB, et al. Cost effectiveness of the National HIV/AIDS Strategy goal of increasing linkage to care for HIV-infected persons. J Acquir Immune Defic Syndr. 2012;61:99–105. doi: 10.1097/QAI.0b013e31825bd862. [DOI] [PubMed] [Google Scholar]
- 37.Farnham PG, Holtgrave DR, Gopalappa C, et al. Lifetime costs and quality-adjusted life years saved from HIV prevention in the test and treat era. J Acquir Immune Defic Syndr. 2013;64:e15–18. doi: 10.1097/QAI.0b013e3182a5c8d4. [DOI] [PubMed] [Google Scholar]
- 38.Sloan CE, Champenois K, Choisy P, et al. Newer drugs and earlier treatment: Impact on lifetime cost of care for HIV-infected adults. AIDS. 2012;26:45–56. doi: 10.1097/QAD.0b013e32834dce6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa F, Lodwick RK, Smith CJ, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS. 2012;26:335–343. doi: 10.1097/QAD.0b013e32834dcec9. [DOI] [PubMed] [Google Scholar]
- 40.High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: State of knowledge and areas of critical need for research. J Acquir Immune Defic Syndr. 2012;60 (Suppl 1):S1–18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu C, Umanski G, Blank A, et al. Comorbidity-related treatment outcomes among HIV-infected adults in the Bronx, NY. J Urban Health. 2011;88:507–516. doi: 10.1007/s11524-010-9540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linas BP, Barter DM, Leff JA, et al. The cost-effectiveness of improved hepatitis C virus therapies in HIV/hepatitis C virus coinfected patients. AIDS. 2014;28:365–376. doi: 10.1097/QAD.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, aging, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 44.Celum C, Baeten JM, Hughes JP, et al. Integrated strategies for combination HIV prevention: Principles and examples for men who have sex with men in the Americas and heterosexual African populations. J Acquir Immune Defic Syndr. 2013;63 (Suppl 2):S213–220. doi: 10.1097/QAI.0b013e3182986f3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Multicenter AIDS Cohort Study (MACS) Public Dataset: Release P04. Springfield, VA: National Technical Information Service; 1995. [Google Scholar]
- 46.Samet JH, Freedberg KA, Savetsky JB, et al. Understanding delay to medical care for HIV infection: The long-term non-presenter. AIDS. 2001;15:77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- 47.Hirsch JD, Gonzales M, Rosenquist A, et al. Antiretroviral therapy adherence, medication use, and health care costs during 3 years of a community pharmacy medication therapy management program for Medi-Cal beneficiaries with HIV/AIDS. J Manag Care Pharm. 2011;17:213–223. doi: 10.18553/jmcp.2011.17.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris BL, Scott CA, Wilkin TJ, et al. Cost-effectiveness of adding an agent that improves immune responses to initial antiretroviral therapy (ART) in HIV-infected patients: Guidance for drug development. HIV Clin Trials. 2012;13:1–10. doi: 10.1310/hct1301-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: A randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379:2439–2448. doi: 10.1016/S0140-6736(12)60917-9. [DOI] [PubMed] [Google Scholar]
- 50.Department of Health and Human Services: Office of Inspector General. [Accessed March 26, 2014];AIDS drug assistance program cost containment strategies. 2000 Available at: http://oig.hhs.gov/oei/reports/oei-05-99-00610.pdf.
- 51.Centers for Medicare and Medicaid Services. [Accessed June 21, 2014];Physician fee schedule search. 2009 Available at: http://www.cms.hhs.gov/PFSlookup/02_PFSSearch.asp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.