Abstract
H7N9 influenza A first caused human infections in early 2013 in China. Virus genetics, histories of patient exposures to poultry, and previous experimental studies suggest the source of the virus is a domestic avian species, such as chickens. In order to better understand the ecology of this H7N9 in chickens, we evaluated the infectious dose and pathogenesis of A/Anhui/1/2013 H7N9 in two common breeds of chickens, White Leghorns (table-egg layers) and White Plymouth Rocks (meat chickens). No morbidity or mortality were observed with doses of 106 or 108 EID50 per bird when administered by the upper-respiratory route, and the mean infectious dose (106 EID50) was higher than expected, suggesting that the virus is poorly adapted to chickens. Virus was shed at higher titers and spread to the kidneys in chickens inoculated by the intravenous route. Challenge experiments with three other human-origin H7N9 viruses showed a similar pattern of virus replication.
Keywords: Avian influenza virus, H7N9 influenza, chicken disease, influenza pathogenesis
Introduction
A novel H7N9 influenza A virus emerged in humans in Eastern China in February of 2013. Cases were often severe and the case fatality rate is approximately 32%. The viral genome was rapidly characterized and was revealed to contain a novel constellation of genes from avian influenza viruses from several Eurasian sub-lineages most of which had been previously detected in China (Kageyama et al., 2013; Van Ranst and Lemey, 2013; Xiong et al., 2013; Zhang et al., 2013a). An avian reservoir was immediately suspected as a likely origin of human infections because of the avian-origin influenza virus genes and because recent contact with poultry was documented with numerous human cases (Lee et al., 2013; Murhekar et al., 2013).
Isolates were obtained from the environment of live poultry markets (LPMs) and directly from avian species, including chickens, ducks and pigeons, during the surveillance that was implemented soon after the human cases began (Shi et al., 2013; Wang et al., 2013; Yu et al., 2014). Among poultry, chickens are considered to be a highly likely reservoir species for the H7N9 influenza virus because of the structure of the poultry markets in the affected areas and a previous experimental study of infectivity and transmission supported chickens and Japanese quail as likely reservoirs, but not pigeons, domestic ducks, or domestic geese (Pantin-Jackwood et al., 2014). Since Japanese quail are only available in the LPMs sporadically and typically only in low numbers, chickens were suspected as being a primary exposure source for human cases (FAO, 2014; Hamilton and Swayne, 2013)
The viral genomes of the chicken isolates were genetically similar to the human isolates, although some reassortment has occurred, so that the level of identity varies slightly between genes and isolates (Zhang et al., 2013a). Based on the hemagglutinin (HA) proteolytic cleavage site, the primary virulence marker of avian influenza virus (AIV) for gallinaceous poultry, the virus would be low pathogenic (LP) in chickens. In the absence of clinical disease, identifying infected birds is more difficult and people are less likely to handle poultry that appear healthy with adequate sanitation measures, which can make control of the virus in poultry more difficult. The regulatory LP classification was soon confirmed by in vivo testing by several reference laboratories using the World Organization for Animal Health (OIE) procedure (OIE, 2012) which primarily evaluates mortality with a non-standardized dose administered by the intravenous (IV) route.
The 2013 H7N9 LPAIV lineage is unusual for an influenza A virus that genetically belongs to avian lineages because of its ability to infect and cause disease in humans. The paucity of pathogenesis or transmission data makes it difficult to understanding the role of chickens in the maintenance and transmission of H7N9 to humans. In order to better characterize the 2013 H7N9 LPAIV in chickens, comprehensive pathogenesis studies, and infectious dose studies were conducted.
Materials and Methods
Virus
Egg passage 2 of the A/Anhui/1/2013 H7N9 influenza A strain was obtained from the US, Centers for Disease Control and original material was provided by Chinese Centers for Diseases Control through World Health Organization Influenza Network. A single additional virus passage was completed in embryonating chickens eggs (ECE) using standard methods (Senne, 2008). Egg passage 3 was titrated in ECE with standard methods (Senne, 2008) and was used as inoculum for chicken studies or as antigen for hemagglutination inhibition (HI) assays.
Six additional isolates were evaluated for transmission among chickens (Table 1). Two were sequence variants of A/Anhui/1/2013 that were isolated from chickens from earlier studies (Pantin-Jackwood et al., 2014) and included: 1) a variant had a leucine at position 217 of the HA (A/Anhui/1/2013 L217) which is the same as the parental virus and that is associated with human adaption and 2) a variant with glutamine at position 217 (A/Anhui/1/2013 Q217) , which is the amino acid typically found in avian H7 viruses. Finally, 3 additional H7N9 human isolates: 3) A/Hong Kong/5942/2013; 4) A/Hong Kong/734/2014; 5) A/Hong Kong/2212982/2014; and 6) an H9N2 human isolate, A/Hong Kong/308/2014 with internal proteins that are all >99% similar to the H7N9 isolates in amino acid sequence.
Table 1.
Infection, shed titers and seroconversion of chickens exposed to variants of A/Anhui/1/2013 H7N9 and H9N2 viruses isolated form humans. Viruses recovered from A/Anhui/1/2013 variants (L217 or Q217) were confirmed to have the same sequence as the inoculum.
| Isolate | Dose per bird (log10) | Direct inoculates | Contact exposed chickens | ||||
|---|---|---|---|---|---|---|---|
| Number infected/total chickens | Oral shed titer (mean of 2 & 4 DPI) | Number antibody positive/total chickensc | Number infected/total chickens | Oral shed titer (mean of 2 & 5 DPI) | Number antibody positive/total chickens | ||
| A/Anhui/1/2013 L217 | 4 | 3/5a | 2.4b | 3/5 | 0/3 | NDd | 0/3 |
| 6 | 5/5 | 3.5 | 4/5 | 1/3c | 3.4 | 2/3 | |
| 7.6 | 5/5 | 3.8 | 5/5 | 3/3 | 2.0 | 0/3 | |
| A/Anhui/1/2013 Q217 | 4 | 1/5 | 2.0 | 2/5 | 0/3 | ND | 1/3 |
| 6 | 5/5 | 3.2 | 5/5 | 0/3 | ND | 1/3 | |
| 7.6 | 5/5 | 3.1 | 5/5 | 1/3 | 3.2 | 0/3 | |
| A/Hong Kong/5942/13 (H7N9) | 4 | 1/5 | 2.4 | 1/5 | 0/3 | ND | 0/3 |
| 6 | 5/5 | 2.5 | 5/5 | 0/3 | ND | 0/3 | |
| 7.5 | 5/5 | 3.0 | 5/5 | 0/3 | ND | 1/3 | |
| A/Hong Kong/734/14 (H7N9) | 4 | 5/5 | 2.4 | 5/5 | 0/3 | ND | 1/3 |
| 6 | 5/5 | 2.8 | 5/5 | 0/3 | ND | 2/3 | |
| 7.5 | 5/5 | 3.7 | 5/5 | 3/3 | 2.0 | 2/3 | |
| A/Hong Kong/2212982/14 (H7N9) | 4 | 0/5 | ND | 2/5 | 0/3 | ND | 0/3 |
| 6 | 4/5 | 3.6 | 5/5 | 0/3 | ND | 1/3 | |
| 7.5 | 5/5 | 3.3 | 5/5 | 0/3 | ND | 0/3 | |
| A/Hong Kong/308/2014 (H9N2) | 4 | 5/5 | 5.0 | 5/5 | 3/3 | 4.8 | NTe |
| 6 | 5/5 | 5.8 | 5/5 | 3/3 | 4.7 | NT | |
A chicken was considered virus positive if RNA was detected on either day 2 or day 4 post-challenge.
Virus titers were determined by quantitative real-time RT-PCR
Antibody was detected by homologous hemagglutination inhibition assay.
ND=No virus detected
NT=Not tested
All studies were conducted in accordance with procedures approved by the SEPRL institutional biosafety committee.
Chickens and animal care
Four week-old specific pathogen free (SPF) White Leghorn (WL) (table-egg layer type) and 4 and 10 week-old SPF White Plymouth Rock (WR) (meat type) chickens were obtained from SEPRL in-house flocks. Each bird was individually tagged and housed in HEPA ventilated modified Horsfall isolators with ad libitum access to feed and water. Chickens were observed daily for clinical signs and mortality. All studies were conducted in accordance with procedures approved by the SEPRL institutional animal care and use committee in BSL-3E certified facilities.
Mean infectious dose (MID), transmission and pathogenesis
Five chickens were inoculated with each dose of a 10-fold dilution series of 101 through 108 EID50/bird in 0.1ml of A/Anhui/1/2013 H7N9 by the intrachoanal (ICh) route (simulates natural upper respiratory exposure as the virus is administered into the middle nasal cavity through the choanal cleft and is very accurate because it is easy to ensure that each bird receives the full dose). Two extra WL and 8 extra WR chickens were inoculated with the dose of 106 EID50/bird for the pathogenesis studies. The extra chickens were housed separately to maintain the same ratio of directly inoculated chickens (n=5) to contact exposure chickens (n=3) among the MID dose groups. The contact exposure chickens were non-inoculated hatch-mates that were added to each dose group at 2 DPI. Oropharyngeal (OP) swabs were collected 2, 4 and 7 days post inoculation (DPI) from direct inoculates and from the contact transmission birds at 2 and 5 DPI. The birds were observed daily for clinical signs and mortality. Serum was collected from all surviving chickens at 14 DPI.
Challenge studies with the A/Anhui/1/2013 sequence variants and the additional H7N9 and H9N2 human isolates were identical, however the doses were adjusted higher to: 104, 106, and 107.5 EID50/bird based on the results with the original A/Anhui/1/2013 experiment.
To evaluate gross and microscopic lesions 3 WR chickens and 2 WL chickens from the 106 EID50/bird dose ICh inoculated groups were euthanized and necropsied at 3 DPI (two non-infected hatch-mates were also necropsied to provide a set of age-matched control tissue samples). A full set of tissue samples were collected for microscopic evaluation: lungs (multiple transverse sections, including secondary and tertiary bronchi), bursa, kidney, adrenal gland, thymus, thyroid, brain, liver, heart, ventriculus, pancreas, intestine, spleen, gonads, trachea (several sections anterior to the syrinx), beak (nasal cavity with middle and posterior regions) and thigh muscle. Tissues were fixed in 10% neutral buffered formalin solution, sectioned, and stained with hematoxylin-and-eosin. Duplicate sections were stained by IHC methods (Driskell et al., 2010; Pantin-Jackwood, 2008) to visualize influenza viral antigen distribution in individual tissues.
High challenge dose pathogenesis study with intrachoanal inoculation
A second set of pathogenesis studies was conducted with a high challenge dose of 108 EID50 per bird administered by the ICh route in a volume of 0.1 ml. Sixteen, 4-week-old WL and ten, 10-week-old WR chickens were ICh-inoculated with A/Anhui/1/2013 H7N9. Five chickens served as age-matched, sham-inoculated controls. OP and cloacal (CL) swabs were collected at days 2, 4, 6, 8 and 10 DPI from all chickens. Titers of virus in OP and CL swabs were determined by quantitative real-time RT-PCR (qRRT-PCR).
Two to 4 chickens from each group were euthanized and necropsied at 3 and 6 DPI. Two non-infected controls per age group were also necropsied to serve as age-matched controls. Gross lesions were recorded and lung, spleen, kidney, brain, and muscle tissues were collected separately from each bird and were examined for virus replication by virus isolation in ECE. A full set of tissue samples was collected from the same birds for microscopic evaluation as described above. Serum was collected from all surviving chickens 10 DPI to evaluate antibody titers by HI assay.
Intravenous versus intrachoanal route of inoculation at high challenge dose
To compare the effect of route of inoculation on pathogenesis, eighteen, 4-week-old WL were separated into 3 groups of 6 birds and each was inoculated by either the ICh or intravenous (IV) route with a dose of 108 EID50 in 0.1ml per bird, or sham inoculated with sterile PBS by the each of the same routes and volume. OP and CL swabs were collected at 2 and 4 DPI to evaluate virus shed by virus isolation. Two birds from each group were euthanized and necropsied at 5 DPI (2 non-infected hatch-mates were also necropsied to provide a set of age-matched control tissue samples) and tissues were collected for microscopic evaluation and to evaluate virus replication as described above. Necropsy was conducted at 5 DPI instead of 3 DPI based on previous studies with LPAIV in chickens with IV inoculation which showed that a later time was optimal to observe lesions in visceral organs (Slemons et al., 1990; Slemons and Swayne, 1995; Swayne and Slemons, 1994).
Quantitative real-time RT-PCR and RNA extraction
RNA was extracted from OP swabs and CL swabs by adding 750[μ541]l Trizol LS (Invitrogen, Inc., Carlsbad, CA) to 250[μ541]l swab material. The sample material-Trizol LS mixture was mixed by vortexing and incubated at room temperature for 5 min., then 200[μ541]l of chloroform was added. The material was mixed again by vortexing, incubated at room temperature a minimum of 10 min. and centrifuged for 15 min. at 12,000Xg. The RNA extraction was completed by binding and eluting the RNA from the aqueous phase using the MagMAX 96 AI/ND Viral RNA isolation kit (Ambion, Inc. Austin, TX) in accordance with kit instructions using the KingFisher magnetic particle processor (Thermo Scientific, Waltham, MA) (Das et al., 2009).
Quantitative RRT-PCR which targets the influenza M gene (Spackman et al., 2002) was performed using the 7500 FAST Real-time PCR System, (Applied Biosystems, Foster City, CA) and the AgPath-ID OneStep RT-PCR kit (Ambion, Inc.) (Spackman, 2014). The standard curve for virus quantification was established with RNA extracted from dilutions of the same titrated stock of A/Anhui/1/2013 H7N9 that was used to inoculate the chickens and was run in duplicate. The cut-off for qRRT-PCR detection was calculated to be 102EID50 per ml.
Hemagglutination inhibition assay
Sera were adsorbed for 30min. at room temperature with 1% chicken red blood cells to remove non-specific agglutinins. HI assay was performed in accordance with standard procedures (Pedersen, 2008). Briefly, 2-fold serial dilutions of 25[g541]l of serum were made in 25[μ541]l of phosphate buffered saline. Diluted sera were incubated for 30 min. at room temperature with 4HAU/25[μ541]l of antigen, then 50[μ541]l of 0.5% chicken red blood cells were added. The test was evaluated after 30 min. of incubation at room temperature. Titers were calculated as the reciprocal of the last HI positive serum dilution and samples with HI titers of 4 or below were considered negative.
Virus isolation
Virus detection from OP and CL swabs and tissues collected on day 2 and 4 post-inoculation from the route of inoculation study was performed as described previously (Tumpey et al., 2004). Briefly, swabs were collected into 2 ml of brain-heart infusion broth with antibiotics (1,000 units/mL of penicillin G, 200 [μ541]g/ml of gentamicin sulfate, and 4 [μ541]g/ml of amphotericin B; Sigma Chemical Company, St. Louis, MO) from each bird and 0.2 ml was injected in 9- to 11-day-old embryonating SPF chicken eggs. Inoculated eggs were incubated at 37°C for 72 to 96 hours and allantoic fluid was harvested and screened for the presence of AI virus by hemagglutination assay following standard procedures (Killian, 2008; Senne, 2008). Virus titers are reported as log10 EID50/ml and the threshold of detection was 100.9 EID50/ml.
Statistical methods
Virus titers were tested for statistical significance among treatment groups with one-way ANOVA within a DPI. If normality failed then one-way ANOVA on ranks, Dunn's method was used (SigmaPlot 12.0, Systat Software, Richmond, CA). The proportion of chickens positive for virus shed between treatment groups was tested by Fisher's Exact test for statistical significance. A p value of ≤ 0.05 was considered to be significant for all statistical tests.
Results
Mean infectious dose and pathogenesis
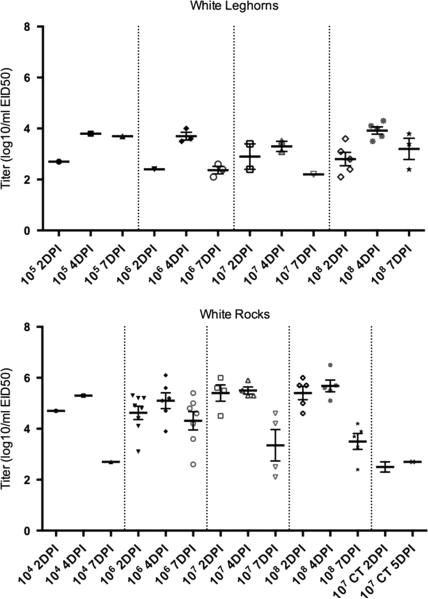
The 50% infectious dose of A/Anhui/1/2013 H7N9 for WR and WL breed chickens was determined based on oral virus shed at 2 DPI. Titers from 4 and 7 days were used to evaluate shed patterns, but because of possible secondary spread within a group of birds, they were not used to determine infectious dose. A dose of 106 EID50 (mid-dose) resulted in 50% infection for both chicken breeds based virus shed at 2 DPI (Table 2). A 100% infection rate was only achieved at the highest dose of 108 EID50 per bird. The chickens (of both breeds) that seroconverted were always the same birds that shed virus (Table 1) (excluding two WR chickens which shed virus at 2 DPI but were euthanized and necropsied at 3 DPI so there was no sera available for HI assay). Virus detectable by qRRT-PCR was shed by the OP route at 2, 4 and 7 DPI by the directly inoculated WR starting at the dose of 104 EID50 per bird through a dose of 108 EID50 per bird, and starting at a dose of 10 EID50 per bird through dose of 108 EID50 per bird by the directly inoculated WL (Figure 1).
Table 2.
Number of directly inoculated chickens positive for virus shed at 2 DPI and antibody 14 DPI by dose of A/Anhui/1/2013 H7N9. Virus shed was detected by quantitative real-time RT-PCR and antibody was detected by hemagglutination inhibition assay with homologous antigen.
| Dose per bird (EID50) | White Leghorns | White Plymouth Rocks | ||
|---|---|---|---|---|
| rRT-PCR | Antibody | rRT-PCR | Antibody | |
| 101 | 0/5a | 0/5 (0)b | 0/5 | 0/5 (0) |
| 102 | 0/5 | 0/5 (0) | 0/5 | 0/5 (0) |
| 103 | 0/5 | 0/5 (0) | 0/5 | 0/5 (0) |
| 104 | 0/5 | 0/5 (0) | 1/5 | 1/5 (64) |
| 105 | 1/5 | 1/5 (64) | 0/5 | 0/5 (0) |
| 106 | 3/5 | 3/5 (81) | 7/10 | 7/10 (18) |
| 107 | 2/5 | 2/5 (45) | 4/5 | 4/5 (38) |
| 108 | 5/5 | 5/5 (37) | 5/5 | 5/5 (28) |
Number positive/total
Number positive/total (geometric mean titer)
Figure 1.
Oral virus titers for White Plymouth Rock (WR) and White Leghorn (WL) chickens inoculated by the intrachoanal route with A/Anhui/1/2013 H7N9 by dose (50% egg infectious doses per bird) and day post inoculation as determined by quantitative real-time RT-PCR. Groups which did not shed virus are not shown (WL: 101 through 104 EID50 per bird not shown, WR: 101 through 103, and 105 EID50 per bird not shown). The thicker bar represents the mean and the error bars represent standard error. Each symbol represents an individual bird within the treatment group. The number of positive WL was insufficient for statistical camparison of titers to WR. CT= contact transmission, all other groups were directly exposed. The cut-off for rRT-PCR sensitivity was determined to be 102EID50/ml (equal to 100EID50/RT-PCR reaction).
Only two contact transmission birds in the WR group housed with the group that received 107EID50/bird could be confirmed to have been infected. One of these contact exposed chickens was positive for OP virus shed at 2 and 5 DPI and the other was only positive at 2 DPI (Figure 1). Both were negative for antibody by HI assay; all contact exposure birds from both experiments were negative for antibody.
Pathogenesis with ICh inoculation with a dose of 106 EID50 per bird
No clinical signs, gross lesions or mortality were observed in any of the chickens. At the exposure dose of 106 EID50 per bird WL and WR shed sufficient virus by the OP route at 2, 4 and 7 DPI as detected by qRRT-PCR (Figure 1). There was a trend (i.e. not statistically significant) toward higher titers to be shed by the WR (mean titers 4.6, 5.1 and 4.3 log10 EID50 at 2, 4, 7 and DPI respectively) versus the WL (mean titers 2.4, 3.6 and 2.4 log10 EID50 at 2, 4, 7 and DPI respectively). The number of positive WL was insufficient for statistical analysis of titers. The number of birds shedding was not significantly different between the WL and WR at any time (Table 3).
Table 3.
Number of chickens positive for influenza A virus in oropharyngeal (OP) and cloacal (CL) swabs by rRT-PCR or virus isolation by day post inoculation (DPI) and number of chickens positive by hemagglutination inhibiton assay for antibody to A/Anhui/1/2103 H7N9 at 10 or 14 (DPI). There were no significant differences between the number of white leghorns and white rocks shedding except in the 108EID50/bird dose group inoculated intrachoanally at 10DPI by the oral route (*).
| Dose | Route of Inoculation |
Breed | 2 DPI | 4 DPI | 6 DPI | 8 DPI | 10 DPI | Serology | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OP | CL | OP | CL | OP | CL | OP | CL | OP | CL | # Pos/ Total (% pos) |
GMTc | |||
| 106EID50/bird | Intrachoanal | White Leghorns | 1/5 (20)a | Not done | 3/5 (60) | Not done | 3/5 (60)b | Not done | Not done | Not done | Not done | Not done | 3/5 (60) | 40 |
| Intrachoanal | White Plymouth Rocks | 8/13 (61.5) | Not done | 6/10 (60) | Not done | 7/10 (70)b | Not done | Not done | Not done | Not done | Not done | 7/10 (70) | 18 | |
| 108EID50/bird | Intrachoanal | White Leghorns | 14/14 (100) | 2/14 (12.5) | 12/12 (100) | 2/12 (16.7) | 11/12 (91.6) | 3/12 (25) | 4/9 (44.4) | 2/9 (22.2) | 5/9* (55.5) | 2/9 (22.2) | 9/9 (100) | 102 |
| Intrachoanal | White Plymouth Rocks | 10/10 (100) | 1/10 (10) | 8/8 (100) | 3/8 (37.5) | 8/8 (100) | 2/8 (25) | 2/6 (33.3) | 1/6 (16.7) | 0/6* (0) | 0/6 (0) | 6/6 (100) | 66 | |
| 108EID50/bird | Intrachoanal | White Leghorns | 6/6 (100) | 2/6 (33.3) | 6/6 (100) | 6/6 (100) | Not done | Not done | Not done | Not done | Not done | Not done | 4/4 (100) | 256 |
| Intravenous | White Leghorns | 6/6 (100) | 6/6 (100) | 6/6 (100) | 6/6 (100) | Not done | Not done | Not done | Not done | Not done | Not done | 4/4 (100) | 724 | |
Number positive/total (percent).
Samples collected 7DPI.
GMT=Geometric mean titer
Because 100% infection was only observed at the dose of 108 EID50 per bird and observations of mortality in concurrent vaccination studies (manuscript in preparation) a second higher dose study with 108 EID50 per bird was conducted. Also both the IV and ICh routes were used to simulate the OIE standard test which uses the IV inoculation route and natural infection, respectively. Since the titer of the virus preparation utilized was 109 EID50 per ml, a 1:10 dilution as called for in the OIE procedure would have resulted in a dose of 108 EID50 per bird, therefore this would replicate the OIE test.
High dose pathogenesis study with intrachoanal inoculation
No clinical signs, lesions or mortality were observed in any of the pathogenesis studies with ICh virus inoculation regardless of chicken breed at a dose of 108 EID50 per bird.
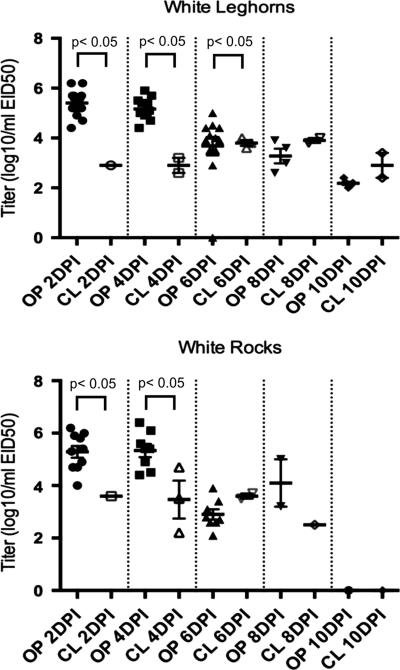
In chickens inoculated by the ICh route with the high virus dose (108 EID50 per bird), virus was detectable from 100% of OP swabs from both breeds taken at 2, 4 and 6 DPI (Table 2), with birds shedding an average of 104-6.2EID50/ml on days 2 and 4 DPI (Figure 2). By 8 DPI less than half of the birds were shedding virus through the OP route (Table 3) and at 10 DPI the number of birds shedding was significantly higher in the WL versus the WR (no virus was detected). Virus was detected in CL swabs in fewer birds at each time point than OP swabs and mean virus titers were lower at each time point (Figure 2). Mean OP shed titers were significantly higher than CL titers from the WL at 2, 4 and 6 DPI and from WR at 2 and 4 DPI. There was no significant difference in the number of WL versus WR shedding detectable levels of virus at any time. At 10 DPI, all chickens inoculated with 108 EID50 of A/Anhui/1/2013 by the ICh route had detectable antibody titers against the virus (Table 2).
Figure 2.
Titers of virus in oropharyngeal (OP) and cloacal (CL) swabs from White Plymouth Rock and White Leghorn chickens inoculated by the intrachoanal route with 108 EID50 of A/Anhui/1/2013 H7N9 per chicken as determined by quantitative real-time RT-PCR. There were no positive samples from White Rocks at 10 DPI (days post inoculation). The thicker bar represents the mean and the error bars represent standard error. Each symbol represents an individual bird within the treatment group. The cutoff for rRT-PCR sensitivity was determined to be 102EID50/ml (equal to 100EID50/RT-PCR reaction).
The titers of virus shed orally and number of chickens shedding virus was compared between the high dose and mid-dose groups at 2 and 4 DPI. The number of both WL and WR chickens shedding at 2 DPI was significantly higher in the high dose groups at 2 DPI, but not at 4 DPI. The titers were significantly higher from the high dose group at 2 DPI for both WL and WR, and at 4 DPI from WL.
Only mild gross lesions were observed at necropsy in chickens inoculated by the ICh route regardless of breed. Sinusitis was the primary lesion observed. Microscopic lesions were consistent with LPAIV infection; lesions were generally confined to the upper respiratory tract. Moderate to severe catarrhal and/or lymphocytic rhinitis and sinusitis, with mucocellular exudates containing sloughed epithelial cells, submucosal edema, and glandular hyperplasia was present. Mild degenerative changes of the overlying epithelium, and mild lymphocytic infiltration in the submucosa, and mild edema were observed in the trachea. In the lungs, mild congestion, mild interstitial inflammation with mixed mononuclear cells, and mild catarrhal bronchitis was observed. Lesions in the gastrointestinal tract consisted of mild to moderate proliferation of gut-associated lymphoid tissues. Mild focal lymphocytic infiltrations in the liver were also present. In one bird, lymphocytic infiltrations were also found in the heart. Other organs lacked significant histopathologic lesions. No lesions were found in age-matched controls.
Selected tissues were tested for virus. At 3 DPI, virus was detected by qRRT-PCR in the lung of two birds (102.3 and 103.4 EID50/g), in the kidney of two birds (102 and 104.5 EID50/g), in muscle tissue of three birds (101.9-3.9 EID50/g), and in the brain and spleen of all four birds examined (102-3.2 and 103.5-3.9 EID50/g respectively). At 6 DPI, virus was detected in the lung of one bird (102.5 EID50/g) and in the spleen of all four birds (102-2.6 EID50/g) examined.
Immunohistochemistry on tissues from ICh exposed chickens
Using immunohistochemistry viral staining in tissues from all of the chickens examined from the ICh inoculated groups (mid- and high dose) was commonly present in epithelial cells and macrophage in the nasal cavity and adjacent glands (Figure 3). Minimal staining was present in tubule epithelium in the kidney. Rare viral antigen staining was observed in epithelial cells and infiltrating inflammatory cells in the intestine, in resident phagocytes in the spleen and liver, and in microglial cells in the brain.
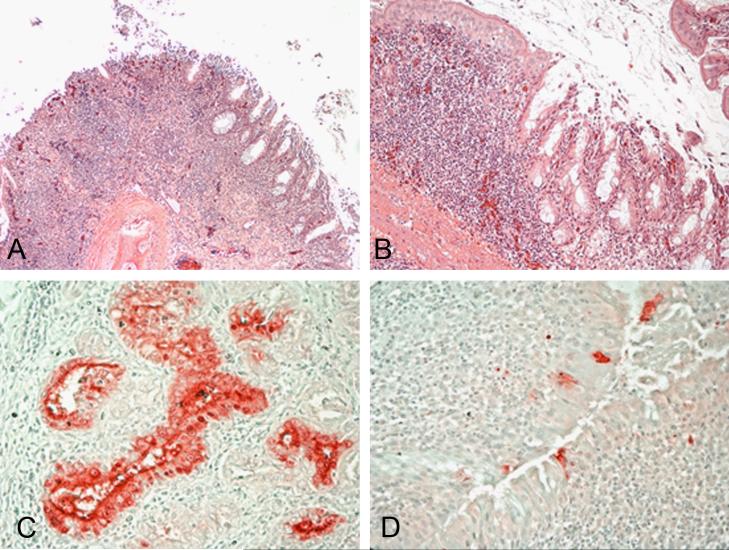
Figure 3.
Histopathology and immunohistochemical staining for avian influenza virus antigen in tissues of chickens infected by the ICh route with the A/Anhui/1/2013 H7N9 virus, 3 and 6 DPI. Photomicrographs, magnification 200-400X; virus staining in red. Nasal turbinates. Severe necrotizing rhinitis with submucosal congestion and edema, glandular hyperplasia, and lymphoplasmacytic infiltration (A and B). Demonstration of viral antigen in the epithelial cells of nasal glands (C) and nasal epithelium of the turbinates (D).
Intravenous versus intrachoanal route of inoculation
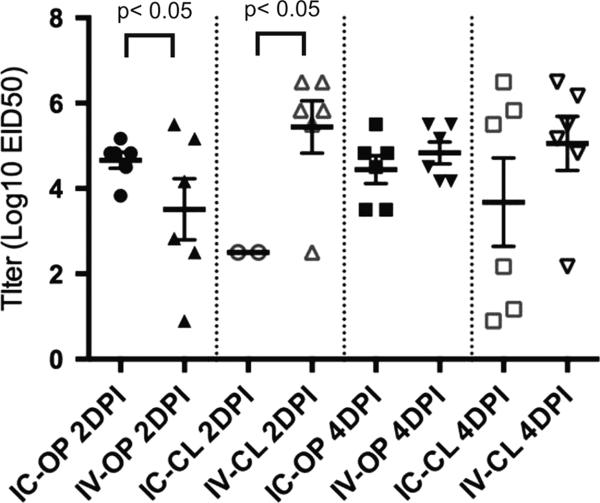
No mortality or clinical signs were observed in chickens inoculated with 108 EID50 per bird by either the ICh or IV routes or in sham inoculates. Oral and CL virus shed was evaluated at 2 and 4 DPI (Figure 4). The only significant difference between the IV and ICh inoculated chickens in either mean titers or the number of birds shedding was at 2DPI where the mean titers were significantly lower from the ICh as compared to the IV inoculated chickens (the difference in the number of chickens shedding was not significant).
Figure 4.
Oropharyngeal (OP) and cloacal (CL) viral shed titers at 2 and 4 days post inoculation (DPI) for White Leghorn chickens inoculated by the intrachoanal (ICh) or intravenous (IV) route with 108 EID50 of A/Anhui/1/2013 H7N9 per chicken as determined by virus isolation. The thicker bar represents the mean and the error bars represent standard error. Each symbol represents an individual bird within the treatment group.
Gross lesions observed at 5DPI in chickens inoculated by the IV route included necrotic areas in the kidney and petechial hemorrhages on the liver. Virus isolation was attempted with kidney tissue from each of the two birds necropsied from each treatment (sham, ICh inoculation, and IV inoculation). No virus was isolated from the kidneys from the 2 sham inoculates or the 2 ICh inoculates. Kidney tissue from both of the IV inoculates was positive for virus and both had a titer of 106.5EID50/g.
Microscopic lesions and viral antigen staining observed by immunohistochemistry in the tissues from the ICh-inoculated birds were similar to what was seen in the other studies described above. In the tissues from the birds that received the virus by the IV route, severe lymphocytic tubule-interstitial nephritis with necrosis of tubule epithelium, edema and hemorrhage was observed (Figure 5). Mild to moderate phagocyte ellipsoid-associated proliferation was present in the spleen (Figure 5) as well as mild necrosis of hepatocytes with sinusoidal histiocytosis in the liver. Viral antigen was present in necrotic tubule epithelium, infiltrating mononuclear cells, and debris in the kidney and in resident phagocytic cells in the spleen, associated with the microscopic lesions observed. No virus staining was found in any other tissue.
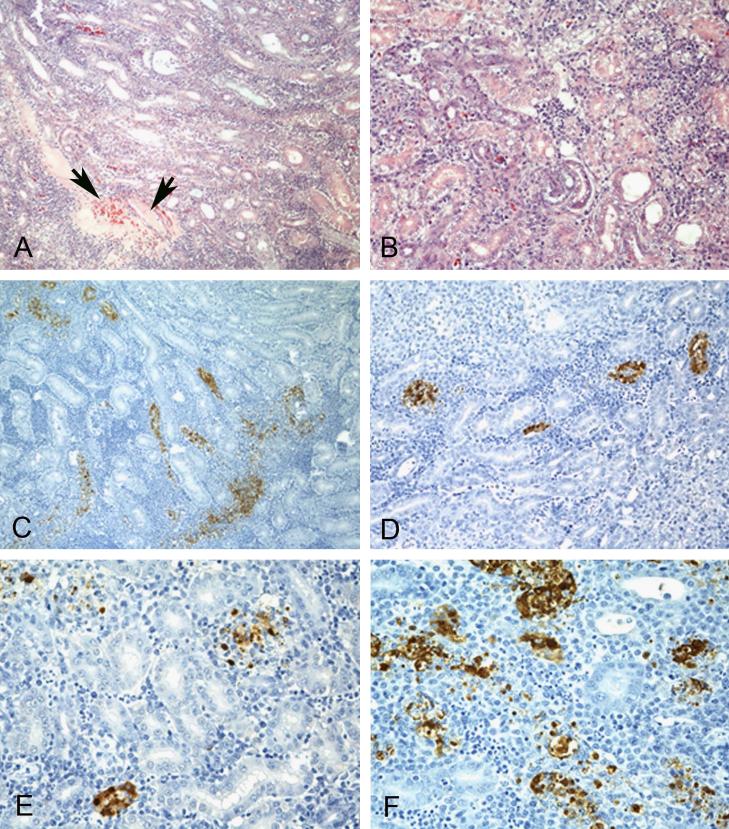
Figure 5.
Histopathology and immunohistochemical staining for avian influenza virus antigen in tissues of chickens intravenously infected with the A/Anhui/1/2013 H7N9 virus, 3 DPI. Photomicrographs, magnification 100-400X; virus staining in brown. A-F. Kidney. Severe lymphocytic tubulointerstitial nephritis with necrosis of tubular epithelium and tubular cast (arrows)(A and B). Demonstration of viral antigen in tubule epithelium, infiltrating mononuclear cells, and debri (C-F).
Transmission of other H7N9 isolates and an H9N2 human isolate in chickens
Chickens were exposed to three additional human isolates of H7N9 at doses of 104, 106, and 107.5 EID50 per bird by the ICh route to evaluate viral replication and transmission. All three viruses infected 100% of the chickens at 106 and 107.5 EID50 per bird, but transmission by contact to non-inoculated cage-mates was limited to none or 1 of 3 birds with 2 isolates, and 2 of 3 with the third isolate (Table 1). Antibody titers detected by HI assay correlated closely to viral RNA detection by qRRT-PCR (i.e. the same birds were positive with both assays). In contrast, chickens exposed to the H9N2 human isolate were 100% infected and transmitted to 100% of the non-inoculated cage-mates when exposed at doses of 104 and 106, EID50 per bird (Table 3). Also, the amount of virus in swabs was much lower from the contact transmission group as compared to the directly inoculated chickens. Additionally, the amount of virus detected in swabs from contact exposed birds was much lower than from the directly inoculated chickens.
Transmission of A/Ahui/1/2013 sequence variants
Two isolates of A/Anhui/1/2013 from chickens from an earlier challenge study with the A/Anhui/1/2013 virus (Pantin-Jackwood et al., 2014) which had variant HA sequences: leucine or glutamine at site 217 (H7 numbering). The change at residue 217 was the most prominent variation observed in the A/Anhui/1/2013 HA after passage in chickens or quail and this position is known to be important in sialic acid binding; leucine is correlated to human isolates and glutamine associated with avian isolates. Therefore, an isolate with each of these residues were directly compared. The isolate with Q217 infected 100% of the chickens at 106 and 107.5 EID50 per bird, but transmission by contact to non-inoculated cage-mates was limited to 1 of 3 birds (Table 3). The isolate with L217 transmitted to all 3 un-inoculated cage-mates at the same doses.
Discussion
The mean infectious dose of A/Anhui/1/2013 H7N9 was 106EID50 in both WL and WR chickens. This dose seems higher than one would expect if this lineage was well adapted to chickens. In a previous study the mean infectious dose for 10 LPAIV isolates in WL chickens varied from <102.9 EID50 to greater than 107.5 EID50 per bird (Swayne and Slemons, 2008). In that study the isolates which had a history of circulating in chickens had mean infectious doses of <102.9 EID50 per bird versus doses above 104.2EID50 per bird with isolates from waterfowl, ratites and shore birds (Swayne and Slemons, 2008). The 2013 H7N9 lineage has been isolated from numerous and diverse avian species, which makes it difficult to speculate to what species this lineage is best adapted. Surveillance data from LPMs shows that the H7N9 lineage is detected in chickens at the LPMs at higher rates than from other species (Chen et al., 2014). However the number of chickens tested was much higher than any other species, so there may be some sample bias since the prevalence, even in chickens was very low (2.1%) (Chen et al., 2014). Since the LPM's are comprised of numerous species mixed together and in Chen's study the highest rates of virus detection were in environmental samples, the chickens may be infected at the markets (which is supported by the lack of detection at farms) (Chen et al., 2014).
Several reports on the pathogenesis of A/Anhui/1/2013 in gallinaceous and other avian species have been reported. In older WL chickens a dose of 106EID50 per bird by the ICh route achieved 100% infection (Pantin-Jackwood et al., 2014) versus 60% (WL) and 70% (WR) here. In that study the chickens were 59 week-old hens that were in-lay, so the difference may be age related and be affected by the physiological stress of egg laying which could cause the hens to be more susceptible to infection with a lower dose of virus (Pantin-Jackwood et al., 2014). That examined infection of numerous species including quail, pigeons and Pekin ducks with doses of 102, 104 and 106 EID50 per bird and only quail could be infected 100% at 102 EID50 versus 106 for 50% infection in Pekin ducks and >106 for pigeons (Pantin-Jackwood et al., 2014). This shows that a relatively high 50% bird infectious dose is needed to infect pigeons and Pekin ducks. Other studies in younger chickens with a single dose have also shown similar results. Ku et al. observed poor replication and transmission at a dose of 109.6EID50/bird (Ku et al., 2014). Kalthoff et al. were able to achieve 100% infection of young chickens at 106EID50/bird with a small group of 4 chickens (Kalthoff et al., 2014). Similarly, Zhang et al. were able to achieve a high proportion of infection in chickens using a chicken and a pigeon origin isolate at a dose of 106EID50/bird (Zhang et al., 2013b). The varying data suggest that chicken adaptation may vary among isolates.
Importantly laboratory studies often do not replicate the real world situation. In the field chickens are physiologically stressed, nutrition may not be adequate, other infectious agents, including the immunosuppressive viruses of chickens could affect susceptibility to infection. The difference between the lab and field is not a complete explanation because the H9N2 and other viruses have much lower infectious doses for chickens under the same conditions (Swayne and Slemons, 2008; Tumpey et al., 2004).
Consideration must be given that the A/Anhui/1/2013 is a human isolate, and there may be some adaptation of the virus to humans which potentially limits its replication in poultry. However, different lines of evidence don't support this theory. First the deduced protein sequences of all viral proteins of the A/Anhui/1/2013 have 100% identity with numerous chicken isolates based on sequence from 35 chicken isolates in public databases (Eurosurveillance editorial, 2013; Van Ranst and Lemey, 2013; Xiong et al., 2013). Two mutations in the Anhui strain, Q217L in the hemagglutinin and the PB2 E627K mutation, are associated with mammalian adaptation of avian influenza viruses (Subbarao et al., 1993). However, some poultry H7N9 isolates had both human associated mutations, which makes it unlikely that these mutations are responsible for the poor replication in chickens. Furthermore, numerous isolates of H5N1 HPAIV from chickens with the PB2 E627K mutation have been reported (Kayali et al., 2011; Li et al., 2010; Wasilenko et al., 2011), which suggests that influenza with this sequence is able to sufficiently transmit among chickens in a natural setting. Secondly, three additional H7N9 viruses from humans were compared at several different doses, and a similar pattern of virus replication and transmission and shedding were observed. Although, all three viruses had leucine in the hemagglutinin at 217 and lysine in the PB2 at 627, it provides evidence that the Anhui strain is not an outlier in its ability to replicate in chickens. Finally, the two variants of residue 217, either Q or L, in A/Anhui/1/2013 behaved similarly to the parental strain, which appeared to be a mixed population with both variants. Based on the current information in public data bases most chicken viruses have Q and most human viruses have L, but neither is exclusive to a species.
One of the primary reasons that chickens were suspected in the epidemiology of H7N9 was that the virus appeared to be a reassortant virus which contained the 6 internal gene segments derived from the chicken adapted H9N2 lineage that is endemic in the region. The expectation was this would allow efficient virus replication in poultry. As a positive control, a human H9N2 from this lineage (>99% similarity in internal proteins) was included in the study and transmitted more efficiently than the H7N9 viruses, indicating that the HA and NA are important for transmission among chickens.
Importantly, although WL and WR derived commercial chicken lines are widespread in China, the most common chicken breed in the live poultry markets in China is a native, local breed, the “yellow chicken”. Therefore a breed difference in host susceptibility cannot be ruled out for the ecology of the 2013 H7N9 lineage in the live poultry markets where humans were being exposed. Other factors including: stress, infections with other agents, and immunosuppression, can affect the susceptibility of chickens to the H7N9 virus in field conditions. Additional infectious dose work with H7N9 isolates from different species and breeds of poultry are needed to elucidate the species adaptation of the lineage from genetic and biological perspectives.
As expected, the pathogenesis of A/Anhui/1/2013 is clinically consistent with LPAIV in chickens (Morales et al., 2009; OIE, 2012; Pillai et al., 2010; Spackman et al., 2010; Swayne et al., 2008) regardless of breed or dose. Virus shed did differ based on challenge dose, where more chickens shed higher titers after a higher challenge dose earlier post exposure. This same dose effect has been reported with porcine reproductive and respiratory syndrome virus and foot and mouth disease virus in swine (Howey et al., 2009; Yoon et al., 1999) and demonstrates how challenge dose in laboratory studies can affect the course of infection. Also, virus was detected in internal organs at the high challenge dose, but not the lower dose.
Route of inoculation did have an effect on pathogenesis. Since a human or mammalian influenza isolate had not been evaluated for differences in pathogenesis in chickens based on these two common experimental routes of exposure, we evaluated A/Anhui/1/2013 by both the ICh and IV routes of infection. Unlike ICh inoculation, gross lesions, microscopic lesions and high viral titers was observed in the kidneys of chickens inoculated by the IV route. Intravenous inoculation of chickens has previously been shown to alter the pathogenesis of LPAIV by allowing direct exposure beyond the respiratory and enteric tracts, particularly to the kidneys (Condobery and Slemons, 1992; Slemons et al., 1990; Slemons and Swayne, 1995; Swayne et al., 1994; Swayne and Slemons, 1994). Thus infection and damage in tubular epithelial cells of the kidney may result in low level mortality, which could make an isolate appear more pathogenic than it is by more natural respiratory exposure route. We have observed kidney infection and nephrosis in vaccination studies in several chickens that died following ICh exposure to a high dose (108EID50 per bird) of the Anhui H7N9 virus (manuscript in preparation). Importantly, a high rate of mortality from renal infection could result in incorrectly classifying an LPAIV as HPAIV, which is important because IV inoculation is the standard route of inoculation for the OIE pathogenicity test (OIE, 2012). This has been reported with an H10 (Wood et al., 1996). In addition to the kidney lesions, virus shed titers by the CL route were increased early after exposure (2 DPI) as compared to the respiratory route. These data reinforce that IV inoculation will alter the apparent tissue tropism and pathogenesis of LPAIV in chickens versus a respiratory route of inoculation.
Conclusions
Consistent with other reports, no mortality or morbidity was observed in chickens regardless of breed, dose or route of inoculation. The mean infectious dose of A/Anhui/1/2013 in WL and WR chickens was 106 EID50 per chicken and 100% infection required a dose of 108EID50 per bird. Similar results were observed with variants of A/Anhui/1/2013 and other H7N9 isolate. However, an H9N2 isolate with similar internal gene segments, did have a lower mean infectious dose indicating that the H7 and/or N9 are important for chicken susceptibility. More importantly, for chickens to serve as a primary reservoir for the H7N9 lineage one would expect for the infectious dose to be lower and more inline with the infectious doses of other AIV isolates that are adapted to chickens. Therefore the role of chickens in the ecology of the H7N9 lineage, at least early on (as the virus has continued to circulate it may have changed), may not have been efficient.
Research highlights.
The 2013 H7N9 avian influenza virus was evaluated in two common breeds of chickens.
No morbidity or mortality were observed with doses up to108 EID50 per bird.
The mean infectious dose for chickens was 106 EID50.
The pathogenesis was more invasive when inoculated by the intravenous route.
Acknowledgments
The authors gratefully acknowledge, Scott Lee, Diane Smith, Aniko Zsak, Mar Costa-Hurtado, Eric Shepherd, Hai Jun Jiang, Suzanne DeBlois, Mari Rodriguez, and Kira Moresco for technical assistance with this work. This research was supported by US Department of Agriculture, ARS CRIS Project 6612-32000-063-00D and with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under IAA No. AAI12004-001-00001. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen Z, Li K, Luo L, Lu E, Yuan J, Liu H, Lu J, Di B, Xiao X, Yang Z. Detection of avian influenza A(H7N9) virus from live poultry markets in Guangzhou, China: a surveillance report. PloS one. 2014;9:e107266. doi: 10.1371/journal.pone.0107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condobery PK, Slemons RD. Biological properties of waterfowl-origin type A influenza viruses in chickens. Avian Dis. 1992;36:17–23. [PubMed] [Google Scholar]
- Das A, Spackman E, Pantin-Jackwood MJ, Suarez DL. Removal of real-time reverse transcription polymerase chain reaction (RT-PCR) inhibitors associated with cloacal swab samples and tissues for improved diagnosis of Avian influenza virus by RT-PCR. J Vet Diagn Invest. 2009;21:771–778. doi: 10.1177/104063870902100603. [DOI] [PubMed] [Google Scholar]
- Driskell EA, Jones CA, Stallknecht DE, Howerth EW, Tompkins SM. Avian influenza virus isolates from wild birds replicate and cause disease in a mouse model of infection. Virology. 2010;399:280–289. doi: 10.1016/j.virol.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Eurosurveillance editorial, t. Novel influenza A(H7N9) virus linked to human disease in China, April 2013. Euro surveillance : bulletin europeen sur les maladies transmissibles = European communicable disease bulletin 18. 2013 [Google Scholar]
- FAO Addressing avian influenza A(H7N9) Qualitative risk assessment update. 2014 http://www.fao.org/docrep/019/i3631e/i3631e.pdf.
- Hamilton K, Swayne DE. World Organisation for Animal Health (OIE) visit to the People’s Republic of China to investigate influenza A (H7N9) infections in poultry. 2013 http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/RD_China_H7N9_June2013.pdf.
- Howey R, Quan M, Savill NJ, Matthews L, Alexandersen S, Woolhouse M. Effect of the initial dose of foot-and-mouth disease virus on the early viral dynamics within pigs. Journal of the Royal Society, Interface / the Royal Society. 2009;6:835–847. doi: 10.1098/rsif.2008.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama T, Fujisaki S, Takashita E, Xu H, Yamada S, Uchida Y, Neumann G, Saito T, Kawaoka Y, Tashiro M. Genetic analysis of novel avian A(H7N9) influenza viruses isolated from patients in China, February to April 2013. Euro Surveill. 2013;18 [PMC free article] [PubMed] [Google Scholar]
- Kalthoff D, Bogs J, Grund C, Tauscher K, Teifke JP, Starick E, Harder T, Beer M. Avian influenza H7N9/13 and H7N7/13: a comparative virulence study in chickens, pigeons, and ferrets. Journal of virology. 2014;88:9153–9165. doi: 10.1128/JVI.01241-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayali G, Webby RJ, Ducatez MF, El Shesheny RA, Kandeil AM, Govorkova EA, Mostafa A, Ali MA. The epidemiological and molecular aspects of influenza H5N1 viruses at the human-animal interface in Egypt. PLoS One. 2011;6:e17730. doi: 10.1371/journal.pone.0017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian ML. Hemagglutination assay for the avian influenza virus. Methods Mol Biol. 2008;436:47–52. doi: 10.1007/978-1-59745-279-3_7. [DOI] [PubMed] [Google Scholar]
- Ku KB, Park EH, Yum J, Kim HM, Kang YM, Kim JC, Kim JA, Kim HS, Seo SH. Transmissibility of novel H7N9 and H9N2 avian influenza viruses between chickens and ferrets. Virology. 2014;450-451:316–323. doi: 10.1016/j.virol.2013.12.022. [DOI] [PubMed] [Google Scholar]
- Lee SS, Wong NS, Leung CC. Exposure to avian influenza H7N9 in farms and wet markets. Lancet. 2013;381:1815. doi: 10.1016/S0140-6736(13)60949-6. [DOI] [PubMed] [Google Scholar]
- Li Y, Shi J, Zhong G, Deng G, Tian G, Ge J, Zeng X, Song J, Zhao D, Liu L, Jiang Y, Guan Y, Bu Z, Chen H. Continued evolution of H5N1 influenza viruses in wild birds, domestic poultry, and humans in China from 2004 to 2009. J Virol. 2010;84:8389–8397. doi: 10.1128/JVI.00413-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AC, Jr., Hilt DA, Williams SM, Pantin-Jackwood MJ, Suarez DL, Spackman E, Stallknecht DE, Jackwood MW. Biologic characterization of H4, H6, and H9 type low pathogenicity avian influenza viruses from wild birds in chickens and turkeys. Avian Dis. 2009;53:552–562. doi: 10.1637/8877-041509-Reg.1. [DOI] [PubMed] [Google Scholar]
- Murhekar M, Arima Y, Horby P, Vandemaele KA, Vong S, Zijian F, Lee CK, Li A, World Health Organization Regional Office for the Western Pacific Event Management, T. Avian influenza A(H7N9) and the closure of live bird markets. Western Pacific surveillance and response journal : WPSAR. 2013;4:4–7. doi: 10.5365/WPSAR.2013.4.2.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE Avian Influenza. 2012 http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.03.04_AI.pdf.
- Pantin-Jackwood M, Miller P, Spackman E, Swayne DE, Susta L, Costa-Hurtado M, Suarez DL. Role of poultry in spread of novel H7N9 influenza virus in China. Journal of virology. 2014 doi: 10.1128/JVI.03689-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin-Jackwood MJ. Immunohistochemical staining for the detection of the avian influenza virus in tissues. Methods Mol Biol. 2008;436:77–83. doi: 10.1007/978-1-59745-279-3_10. [DOI] [PubMed] [Google Scholar]
- Pedersen JC. Hemagglutination-inhibition test for avian influenza virus subtype identification and the detection and quantitation of serum antibodies to the avian influenza virus. Methods Mol Biol. 2008;436:53–66. doi: 10.1007/978-1-59745-279-3_8. [DOI] [PubMed] [Google Scholar]
- Pillai SP, Pantin-Jackwood M, Suarez DL, Saif YM, Lee CW. Pathobiological characterization of low-pathogenicity H5 avian influenza viruses of diverse origins in chickens, ducks and turkeys. Arch Virol. 2010;155:1439–1451. doi: 10.1007/s00705-010-0727-8. [DOI] [PubMed] [Google Scholar]
- Senne D. Virus Propagation in Embryonating Eggs. In: Dufour-Zavala L, editor. A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens. 5th ed. American Association of Avian Pathologists; Jacksonville, FL: 2008. pp. 204–208. [Google Scholar]
- Shi J, Deng G, Liu P, Zhou J, Guan L, Li W, Li X, Guo J, Wang G, Fan J, Wang J, Li Y, Jiang Y, Liu L, Tian G, Li C, Chen H. Isolation and characterization of H7N9 viruses from live poultry markets — Implication of the source of current H7N9 infection in humans. Chin. Sci. Bull. 2013;58:1857–1863. [Google Scholar]
- Slemons RD, Locke LN, Sheerar MG, Duncan RM, Hinshaw VS, Easterday BC. Kidney lesions associated with mortality in chickens inoculated with waterfowl influenza viruses. Avian Dis. 1990;34:120–128. [PubMed] [Google Scholar]
- Slemons RD, Swayne DE. Tissue tropism and replicative properties of waterfowl-origin influenza viruses in chickens. Avian Dis. 1995;39:521–527. [PubMed] [Google Scholar]
- Spackman E. Avian influenza virus detection and quantitation by real-time RT-PCR. Methods Mol Biol. 2014;1161:105–118. doi: 10.1007/978-1-4939-0758-8_10. [DOI] [PubMed] [Google Scholar]
- Spackman E, Gelb J, Jr., Preskenis LA, Ladman BS, Pope CR, Pantin-Jackwood MJ, McKinley ET. The pathogenesis of low pathogenicity H7 avian influenza viruses in chickens, ducks and turkeys. Virol J. 2010;7:331. doi: 10.1186/1743-422X-7-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao EK, London W, Murphy BR. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J Virol. 1993;67:1761–1764. doi: 10.1128/jvi.67.4.1761-1764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne DE, Radin MJ, Hoepf TM, Slemons RD. Acute renal failure as the cause of death in chickens following intravenous inoculation with avian influenza virus A/chicken/Alabama/7395/75 (H4N8). Avian Dis. 1994;38:151–157. [PubMed] [Google Scholar]
- Swayne DE, Senne D, Suarez DL. Avian Influenza. In: Dufour-Zavala L, Swayne DE, Glisson J, Pearson J, Reed W, Jackwood M, Woolcock P, editors. Isolation and Identification of Avian Pathogens. 5th ed. American Association of Avian Pathologists; Jacksonville, FL: 2008. pp. 128–134. [Google Scholar]
- Swayne DE, Slemons RD. Comparative pathology of a chicken-origin and two duck-origin influenza virus isolates in chickens: the effect of route of inoculation. Vet Pathol. 1994;31:237–245. doi: 10.1177/030098589403100211. [DOI] [PubMed] [Google Scholar]
- Swayne DE, Slemons RD. Using mean infectious dose of high-and low-pathogenicity avian influenza viruses originating from wild duck and poultry as one measure of infectivity and adaptation to poultry. Avian Dis. 2008;52:455–460. doi: 10.1637/8229-012508-Reg.1. [DOI] [PubMed] [Google Scholar]
- Tumpey TM, Kapczynski DR, Swayne DE. Comparative susceptibility of chickens and turkeys to avian influenza A H7N2 virus infection and protective efficacy of a commercial avian influenza H7N2 virus vaccine. Avian Dis. 2004;48:167–176. doi: 10.1637/7103. [DOI] [PubMed] [Google Scholar]
- Van Ranst M, Lemey P. Genesis of avian-origin H7N9 influenza A viruses. Lancet. 2013;381:1883–1885. doi: 10.1016/S0140-6736(13)60959-9. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang J, Su W, Gao S, Luo J, Zhang M, Xie L, Liu S, Liu X, Chen Y, Jia Y, Zhang H, Ding H, He H. Relationship Between Domestic and Wild Birds in Live Poultry Market and a Novel Human H7N9 Virus in China. The Journal of infectious diseases. 2013 doi: 10.1093/infdis/jit478. [DOI] [PubMed] [Google Scholar]
- Wasilenko JL, Arafa AM, Selim AA, Hassan MK, Aly MM, Ali A, Nassif S, Elebiary E, Balish A, Klimov A, Suarez DL, Swayne DE, Pantin-Jackwood MJ. Pathogenicity of two Egyptian H5N1 highly pathogenic avian influenza viruses in domestic ducks. Arch Virol. 2011;156:37–51. doi: 10.1007/s00705-010-0813-y. [DOI] [PubMed] [Google Scholar]
- Wood GW, Banks J, Strong I, Parsons G, Alexander DJ. An avian influenza virus of H10 subtype that is highly pathogenic for chickens, but lacks multiple basic amino acids at the haemagglutinin cleavage site. Avian Pathol. 1996;25:799–806. doi: 10.1080/03079459608419182. [DOI] [PubMed] [Google Scholar]
- Xiong C, Zhang Z, Jiang Q, Chen Y. Evolutionary characteristics of A/Hangzhou/1/2013 and source of avian influenza virus H7N9 subtype in China. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57:622–624. doi: 10.1093/cid/cit294. [DOI] [PubMed] [Google Scholar]
- Yoon KJ, Zimmerman JJ, Chang CC, Cancel-Tirado S, Harmon KM, McGinley MJ. Effect of challenge dose and route on porcine reproductive and respiratory syndrome virus (PRRSV) infection in young swine. Veterinary research. 1999;30:629–638. [PubMed] [Google Scholar]
- Yu X, Jin T, Cui Y, Pu X, Li J, Xu J, Liu G, Jia H, Liu D, Song S, Yu Y, Xie L, Huang R, Ding H, Kou Y, Zhou Y, Wang Y, Xu X, Yin Y, Wang J, Guo C, Yang X, Hu L, Wu X, Wang H, Liu J, Zhao G, Zhou J, Pan J, Gao GF, Yang R, Wang J. Coexistence of influenza H7N9 and H9N2 in poultry linked to human H7N9 infection and their genome characteristics. Journal of virology. 2014 doi: 10.1128/JVI.02059-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang Z, Weng Z. Rapid reassortment of internal genes in avian influenza A(H7N9) virus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013a;57:1059–1061. doi: 10.1093/cid/cit414. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shi J, Deng G, Guo J, Zeng X, He X, Kong H, Gu C, Li X, Liu J, Wang G, Chen Y, Liu L, Liang L, Li Y, Fan J, Wang J, Li W, Guan L, Li Q, Yang H, Chen P, Jiang L, Guan Y, Xin X, Jiang Y, Tian G, Wang X, Qiao C, Li C, Bu Z, Chen H. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science. 2013b;341:410–414. doi: 10.1126/science.1240532. [DOI] [PubMed] [Google Scholar]