Abstract
Background
Dysphagia is a major stroke complication but lacks effective therapy that can facilitate the course of recovery. Non-invasive brain-stimulation with and without peripheral sensorimotor activities may be an attractive treatment option for swallowing recovery but has not been systematically investigated in the stroke population.
Objective
To describe the rationale and methodology for the first prospective, single-center, double-blinded trial of anodal versus sham transcranial direct current stimulation (tDCS) used in combination with swallowing exercises in patients with dysphagia from an acute ischemic stroke. The aim of this study is to gather safety data on cumulative sessions of tDCS in acute-subacute phases of stroke, obtain information about effects of this intervention on important physiological and clinically relevant swallowing parameters, and examine possible dose effects.
Methods
99 consecutive patients with dysphagia from an acute unilateral hemispheric infarction with a Penetration and Aspiration Scale (PAS) score ≥ 4 and without other confounding reasons for dysphagia will be enrolled at a single tertiary care center. Subjects will be randomized to either a high or low dose tDCS or a sham group and will undergo 10 sessions over 5 consecutive days concomitantly with effortful swallowing maneuvers. The main efficacy measures are a change in the PAS score before and after treatment; the main safety measures are mortality, seizures, neurological, motor and swallowing deterioration.
Conclusion
The knowledge gained from this study will help plan a larger confirmatory trial for treating stroke related dysphagia and advance our understanding of important covariates influencing swallowing recovery and response to the proposed intervention.
Keywords: dysphagia, acute stroke, recovery, rehabilitation, tDCS, treatment
Introduction
Dysphagia occurs frequently after a stroke and often leads to serious complications such as pneumonia or death.[1–6] Despite its common occurrence treatment options for stroke related dysphagia remains inadequate. The usual clinical practice revolves around implementing compensatory measures such as dietary modifications, head and neck maneuvers to minimize aspiration, and supervision/assistance during food intake.[7] Patients with more severe dysphagia usually receive nutritional supplementation via nasogastric or percutaneous gastrostomy tubes till their swallowing improves spontaneously, if at all. The efficacy of these techniques in preventing complications of dysphagia has not been systematically investigated in adequately powered clinical trials though they are accepted as standard of care at most stroke centers. On the other hand, implementation of an intervention that improves swallowing in the early aftermath of a stroke may improve patient outcomes and decrease costs of care. To date, attention has mainly focused on medications, exercise therapy, and different stimulation techniques though their efficacy in stroke patients remains unproven.[8, 9] A recent metaanalysis largely derived from small studies reported some improvement in dysphagia through behavioral interventions and acupuncture, but no effect was found for peripheral or cortical stimulation techniques.[10]
Non-invasive brain stimulation techniques, such as transcranial direct current stimulation (tDCS) or transcranial magnetic stimulation (TMS) are attractive options for facilitating recovery of swallowing after a stroke. Recovery of swallowing in patients with hemispheric strokes has been demonstrated to occur via compensatory reorganization of the unaffected cerebral hemisphere.[11, 12] The reorganization of the swallowing cortex shows predictable patterns of expansion of the pharyngeal representation in an anterolateral direction, irrespective of lesion site or laterality, making it a potential target for neuromodulating therapies such as tDCS or TMS.[11] Both, tDCS and TMS are non-invasive brain stimulation techniques but with markedly different characteristics. TMS uses magnetic fields to stimulate brain neurons via an electromagnetic coil held against the skull through which electromagnetic pulses are administered; these pulses are capable of eliciting neuronal action potentials. On the other hand, tDCS uses a constant, low current delivered directly to the area of interest in the brain via small electrodes on the scalp, which either increases (anodal stimulation) or decreases (cathodal stimulation) the neuronal excitability in the targeted brain region, thereby, increasing or decreasing the likelihood of neuronal firing. [13] The short lived effects of tDCS are mediated by changes in membrane potential while the longer lasting effects occur through synaptic mechanisms similar to induction of neuroplasticity.[14] tDCS can be combined with physical or behavioral therapies to enhance its effects. In addition, tDCS has a sham mode which makes it possible to examine its effect in a blinded trial paradigm.
tDCS has been shown to improve motor functions in chronic stroke patients [15–20] though its effect on improved dysphagia has not been systematically investigated. Furthermore, data about its safety in the acute stroke phase remains sparse. Jefferson and colleagues have recently shown that anodal tDCS can modulate neuronal excitability of the swallowing motor cortex, and make it more amenable to plastic changes.[16] In a previous pilot study we demonstrated that anodal tDCS application to the unaffected swallowing cortex combined with swallowing exercises was safe and feasible in the acute-subacute stroke phases and showed promise in improving dysphagia.[21] In light of prior investigations which have demonstrated that the bihemispheric innervation of brain stem swallowing centers allow the healthy hemisphere to possess sufficient drive to effect recovery of dysphagia[11], we hypothesize that with hemispheric lesions where the brainstem and peripheral structures are intact but the upper circuitry of the swallowing apparatus are dysfunctional, a cortical stimulation technique applied to the healthy swallowing cortex can be effective in restoring swallowing functions.
Trial Design and Methods
Overview
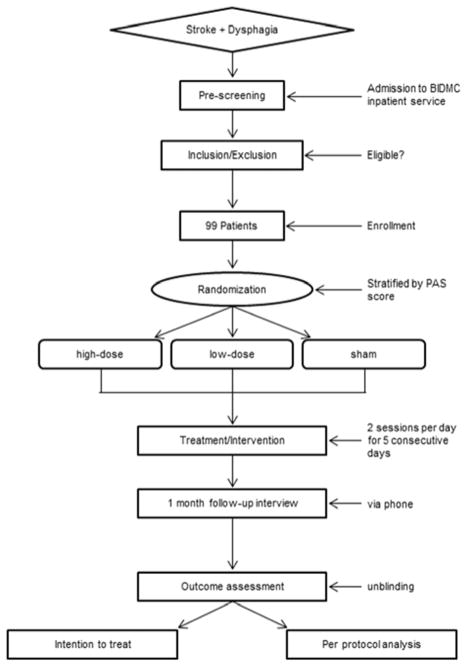
The Fostering Eating After Stroke with tDCS (FEASt) trial is an NIH funded, single-center, phase I/II randomized double-blind trial that will test the safety of tDCS in the acute-subacute phases of stroke recovery and obtain preliminary data on its efficacy in improving swallowing functions in dysphagic stroke patients by combining it concomitantly with swallowing exercises - an approach not previously employed in dysphagia treatment. Subjects will be randomized to one of three intervention arms – a low dose tDCS arm, a high dose tDCS arm, or a sham group. All patient enrollments will occur at Beth Israel Deaconess Medical Center (BIDMC) while the evaluation of videofluoroscopic swallowing studies (VFSS) will be done at Boston University Medical Center (BUMC). A Data Coordination Center at the Boston University School of Public Health (BUSPH) will systematically collect and store all study data. A Data Safety Monitoring Board (DSMB) will review the progress of the study. Figure 1 provides a flowchart of the study overview.
Figure 1.
Trial Design Flowchart
This trial has been approved by the local institutional review boards at BIDMC and BUSPH. It has been registered with ClinicalTrials.gov (# NCT01919112).
Aims of the study
The primary aim of the FEASt study is to assess the safety of the proposed intervention, obtain preliminary data about its efficacy and explore any dose effects.
For assessing efficacy, the following outcomes will be collected and analyzed:
Changes in Penetration and Aspiration Scale (PAS) scores between the 2 tDCS and sham groups
Effects of different doses of anodal tDCS versus sham stimulation on several physiological measures of swallowing derived from VFSS
Durability of any observed effects of tDCS on dietary status as determined by changes in Functional Oral Intake Scale (FOIS) score at study onset and 1 month.
Safety of the proposed intervention will be analyzed by comparing the anticipated and observed incidence of the major adverse events, i.e. seizures, stroke specific mortality, neurological, motor and swallowing deterioration as measured by changes in NIH Stroke Scale (NIHSS), FOIS and PAS scores, respectively.
The secondary aim is to investigate the impact of subject-specific predictors (namely, baseline NIHSS score, dysarthria, corticobulbar tract (CBT)-lesion load and intubation) of dysphagia recovery on the outcome of the proposed intervention by examining differences in the effect size of this intervention across different strata of subject specific-predictors of dysphagia recovery.
Participants
We will prospectively enroll 99 stroke patients with dysphagia due to an acute unilateral hemispheric infarction into the study.
Inclusion/Exclusion Criteria
The detailed inclusion and exclusion criteria are outlined in table 1. Study candidates with a unilateral hemispheric cortical or subcortical infarction as documented by imaging, between 21 and 90 years of age and who are between 25 hours (day 2) to 144 hours (day 6) after stroke onset will be invited to participate if they have moderate to severe dysphagia (PAS score ≥ 4) on a standardized VFSS and do not have other conditions which may independently cause dysphagia.
Table 1.
Inclusion/Exclusion Criteria
| INCLUSION CRITERIA |
| 21 – 90 years of age |
| Between 25 hours (day 2) to 144 (day 6) hours since stroke onset |
| Unilateral hemispheric (cortical or subcortical) infarction documented by imaging |
| Moderate to severe dysphagia with a PAS score ≥ 4 |
| EXCLUSION CRITERIA |
| Prior history of swallowing difficulties |
| Any other condition that may independently cause dysphagia |
| NIHSS score at enrollment > 25 |
| Drowsiness or marked cognitive impairment that interferes with participation in swallowing maneuvers |
| Severe language comprehension difficulties, requiring only proxy consent for participation |
| Intubation ≥ 4 days |
| Ongoing use of the following CNS-active medications that can interfere with the effect of tDCS-carbamazepine, pheyntoin, valproic acid and dextromethorphan |
| Severe COPD (oxygen dependent) |
| Advanced CHF |
| Any other medical condition that in the opinion of the investigator significantly shortens life expectancy |
| Significant hemorrhagic transformation [defined as dense hematoma >30% of the infarcted area with substantial space-occupying effect or as any hemorrhagic lesion outside the infarcted area] on brain imaging studies prior to enrollment |
| Unable to undergo an MRI due to claustrophobia or presence of electrically, magnetically or mechanically activated implant (including cardiac pacemaker), intracerebral vascular clips or any other electrically sensitive support system, metal in any part of the body, including metallic injury to eye, or pregnancy |
| Likely candidates for hemicraniectomy, carotid surgery or stenting |
Presence of a potential tDCS risk factor:
|
Outcome Measures
Primary Outcome
The trial will use changes in the PAS scores as primary outcome measure to assess efficacy. PAS is a validated 8 point ordinal scale that quantifies penetration and aspiration events observed during VFSS.[22] A cut off PAS score ≥ 4 has been adopted for enrollment as minor degrees of penetration can be seen in normal individuals.
Secondary Outcomes
The secondary outcomes will assess the impact of the intervention on changes in swallowing physiology (a, b & c) and (d) diet.
Pharyngeal Constriction Ratio (PCR) is a measure of the pharyngeal area visible in the lateral radiograph view at the point when a bolus is held in the oral cavity divided by the pharyngeal area at the point of maximum pharyngeal constriction during the swallow.[23]
Hyoid, Laryngeal, and Pharyngeal excursion (HLPE) and Pharyngoesophageal (PES) opening will measure the actual excursion of these structures and landmarks from their resting point to maximal excursion.[24]
Pharyngeal Delay Time (PDT) will provide a temporal measure of the briskness of the swallow onset. All the 3 measures (a, b, c) will be collected before the first and after the final session of tDCS/sham.
The Functional Oral Intake Scale (FOIS) will be used as a dietary outcome measure. The FOIS has been tested and validated in stroke population and demonstrated to be sensitive to changes in swallowing functions in acute stroke patients.[25] It will be collected at four time points: 1) prior to initiating tDCS/sham stimulation (same day); 2) after the 5th session of tDCS/sham stimulation; 3) after the last session of tDCS/sham stimulation; and 4) by telephone at 1 month after the last stimulation session using a questionnaire.
Safety Outcomes
The adverse events - seizures, deterioration in global neurological, motor and swallowing functions, and stroke specific mortality during the period of active stimulation will serve as major safety outcome measures. The study DSMB have adopted thresholds for stopping the trial if the incidence of these events exceeds their “natural” probability based on historical data from published literature on acute stroke patients.
The incidence of any seizure during the 5 days of active stimulation will be analyzed.
Neurological Deterioration will be defined as a ≥ 4-point increase in the total NIHSS score or ≥ 2 points increase in the motor sub-item of the NIHSS score on the same limb between each consecutive day during active or sham stimulation.
Swallowing deterioration will be assessed by measuring changes in the FOIS score after the 5th session. To minimize radiation exposure VFSS will not be routinely used for this interim assessment. However, subjects with ≥3 point decrease in the FOIS score will undergo an interim VFSS to document changes in PAS scores. For the purposes of this trial, swallowing deterioration has been defined as an increase in PAS score by ≥ 2 points compared to baseline.
For stroke specific mortality, deaths due to direct consequences of brain injury such as brain edema or seizure will count, but not deaths from recurrent ischemic events, hemorrhage, pneumonia, cardiac events, and infections.
Non serious events (headaches, skin erythema, fatigue and visual perceptual changes) will be tabulated to assess patient tolerability. Patient Health Questionnaire-9 (PHQ-9)[26] will be used for tracking changes in subject’s mood over the course of the study based on recent reports suggesting an effect of depression.[27] The PHQ-9 will be administered prior to the first session and after the last session of stimulation, as well as at 1 month.
Pre-Screening and Enrollment
All potential study candidates will be pre-screened using the online medical records as well as the patient charts. Patients who fail the bedside swallow evaluation will undergo a standardized VFSS conducted by the Speech and Language Pathologists (SLPs) at BIDMC. Patients who have a PAS score ≥ 4 will be enrolled in the study if they fulfill all other study criteria and provide consent.
Swallowing evaluation
All participants will undergo 3 bedside swallowing evaluations and 2 standardized VFSS to quantify dysphagia severity. The first bedside swallow evaluation will be performed before the first stimulation session, the second will be done after the 5th session and the final evaluation will be done after the final session. The VFSS will be done prior to the first and after the final stimulation session. It will use a total of 3 bolus types and will assess 5 successful swallows. The order of the bolus presentation will not be randomized but given in an incremental way due to the uncertainty about patient’s swallowing status and for safety: 5 ml nectar thick liquid followed by 5 ml pudding, followed by 5 ml thin liquid, followed by 10 ml thin liquid and lastly by 30 ml thin liquid.
If a patient cannot take a bolus because of prior failures, a score of 8 will be automatically assigned to that bolus. The arithmetic mean of the PAS score will be computed for each patient, based on the number of swallows on lateral view. The PCR, PDT, and HLPE will be measured on each successful swallow. Inter-rater reliability of study analyses will be conducted for 30% of the studies, selected randomly. The SLPs will incorporate the information from bedside and VFSS to assign the first (prior to stimulation) and final FOIS score (after stimulation) though the intermediate FOIS score (after 5th session) will only be based on the bedside swallow evaluation.
Randomization
The DCC will institute computer based randomization to randomize subjects to the two interventions or sham groups by stratifying them according to their baseline PAS score (4–6 vs. 7–8).
Intervention
The experimental interventions will be performed over 5 consecutive days for each subject, twice daily (total of 10 sessions). Each session will be of 20 minutes duration and involve anodal tDCS or sham carried simultaneously with swallowing exercises. The subject and all investigators except those involved in programming the device will be blinded to the group allocation; the investigators programming the device will not be involved in any patient evaluation nor delivering the intervention. Subjects will be unable to subjectively distinguish between tDCS versus sham stimulation.[28]
tDCS
TDCS will be delivered through a battery-driven, constant current stimulator (NeuroConn-DC Stimulator Plus) with the following electrode dimensions: anode (active electrode) will be 3 × 5 cm and the reference electrode will be 5 × 7 cm. For real tDCS an anodal current of 2 mA will be delivered for 20 minutes continuously. For sham simulation, the electrodes will be set in exactly the same way as for real tDCS, the current however will only be ramped up for 8 seconds to 2 mA and after 40s gradually decreased to produce a sensation of transient tingling, which is indistinguishable from active stimulation.[28] The programmers of the device will verify after each session that the device delivered the appropriate stimulation and record their findings.
The high dose group will receive active stimulation during all sessions (i.e. 10 sessions with 2 mA tDCS) whereas the low dose tDCS group will receive active stimulation alternating with sham (i.e. 5 sessions of 2 mA) over 5 days. The sham group will receive sham during all sessions.
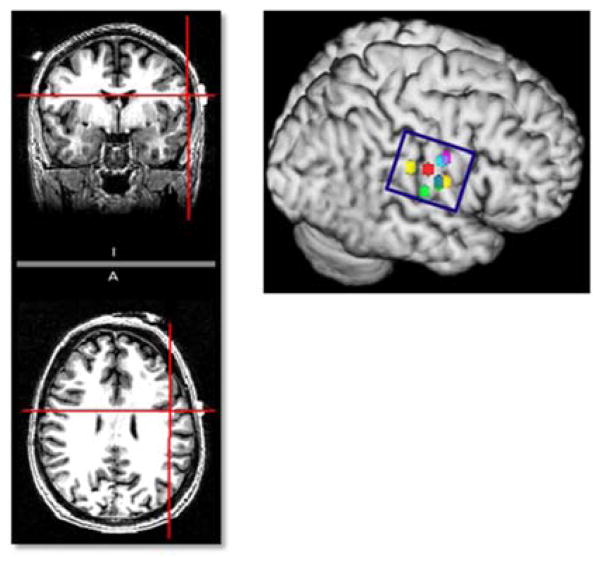
We will use the international 10–20 EEG electrode system to guide our electrode montage.[29] The anode will be placed mid-distance between C3/T3 [left] or C4/T4 [right] over the unaffected hemisphere and the reference electrode over the contralateral supraorbital region. We have previously verified this location using MRI markers on high resolution anatomical brain MRI scans and fMRI scans in healthy patients during swallowing tasks (see figure 2). This montage has been developed with the aim of stimulating the inferolateral regions of the primary sensorimotor and premotor cortex of the unaffected hemisphere, based on studies which consistently point to the importance of the inferolateral primary sensorimotor cortex in regulation of swallowing[30–32] as well as information from TMS research that show a pattern of anterolateral expansion of the pharyngeal cortex in the unaffected hemisphere in patients with successful swallowing recovery.[11]
Figure 2. Confirmation of electrode position with imaging.
Co-registration of anode midposition between C3/T3 using 10–20 EEG systems with a T1-weighted dataset demonstrates it to be centered over the caudal end of the left primary motor cortex in a healthy volunteer. The position and dimensions of the anode depicted by the blue circle appears to completely cover the regions of maximal activation, depicted by colored dots, seen during swallowing tasks on fMRI across a group of healthy elderly volunteers.
Swallowing Exercises
All tDCS or sham sessions will be simultaneously combined with swallowing exercises. The exercises will provide the necessary sensory and motor activation of the swallowing cortex and augment the effect of cortical stimulation. We will use the effortful swallow maneuver [33] which produces greater cortical activation as compared to dry swallows.[34]
Patients will be given a lemon flavored lollipop, ice chips, a cold spoon, and/or water spritzer to stimulate saliva production during these sessions. Occurrence of a swallow response will assessed by palpating the excursion of the thyroid cartilage coupled with sound recordings from a laryngeal microphone taped externally to the patient’s throat. Effortful swallows will be alternated with ‘regular’ swallows over the session until the patient has attempted 40 effortful swallows. A total swallowing tally will be kept for each session.
Training and Standardization
All SLPs involved in performing VFSS have undergone comprehensive training on standardizing the VFSS procedure, which includes uniform methods for bolus presentation, patient positioning, issuing instructions to the patients and obtaining images. They have also been trained to standardize the instructions and recording of the effortful swallowing maneuvers. The performance of all SLPs involved with bedside swallowing exercises will be reviewed randomly and rated to assess their competence in performing and recording the swallowing efforts. SLPs who score 75% or less on the rating scale will need to undergo further training before they can resume working with study subjects. The investigators involved in clinical assessments using NIHSS scores have undergone video training offered by the NINDS and have been certified. The investigators responsible for programming the tDCS device have been trained by representative from the NeuroConn device company on all aspects of device programming, recording and trouble shooting.
Data acquisition for secondary Aim
The secondary aim constitutes investigating the impact of subject-specific predictors of dysphagia recovery (baseline NIHSS score, dysarthria, CBT-lesion load and intubation)[35] on the outcome of our proposed intervention. We will use our trial cohort to extract relevant variables needed for this study arm.
Acquisition and Analysis of MRI Data
To obtain reliable estimates of foci of activation with swallowing tasks and construct coticobulbar tracts, functional MRI and high resolution Diffusion Tensor Imaging (DTI) will be performed in 8 age matched healthy volunteers. The CBT-lesion load will be computed by overlapping the fiber tracts with the lesion maps manually drawn on the MRI scans of the stroke patients enrolled in the trial arm.
Statistical Considerations
Sample Size and Power Calculations
The trial will enroll and randomize 99 subjects; 33 subjects for each of the two active and the sham treatment groups. With the above sample size, after 40% attrition, it is estimated that a difference of 1.0 and 1.15 standard deviations between the active and sham treatment in the mean primary outcome measure of the study can be detected with a type I error rate of 2.5% and power 80% and 90%, respectively. In our pilot study the estimated difference was 1.35 with an approximate standard deviation for the differences in the two groups of 1.2, with a standardized difference of 1.1 standard deviations. Thus we expect to have >87% power to detect the expected differences in our study.
Statistical Analyses
We will conduct Intent-to-Treat (ITT) and Per-Protocol (PP) analyses.
Analytic plan Primary Aim
For the primary analysis – an intent-to-treat approach of all randomized subjects will be used. A linear model will be fitted to the data of the primary outcome variable PAS using PROC MIXED in SAS. The outcome is a change in mean PAS score and treatment will be included as a categorical variable. Additionally baseline PAS and other variables that are identified as confounders will be included as covariates. Adjusted means in the two tDCS groups will be compared to those in the sham group. To control for multiple testing, the Hochberg-Benjamini procedure will be employed, which will result in a positive study result if both null hypotheses are rejected at the 0.05 level or one is rejected at the 0.025 level. Similar analyses will be performed on the FOIS, PCR, HLPE, and PDT outcomes. To assess the durability of the intervention effects, a repeated measures analysis will be used. The outcome will be FOIS score at the onset of the trial and at 1 month. Treatment time and their interactions will be included as categorical variables. Additionally other variables that are identified as confounders will be included as covariates. Adjusted means at the onset of trial and 1 month in the two tDCS groups will be compared to those in the sham group at each time point. Safety analyses will be run on all subjects receiving at least one round of intervention. The incidence rates of adverse events will be described as a whole and by treatment group.
Analytic plan for Secondary Aim
To assess the effect size of the proposed intervention across subject-specific predictors, subgroup analyses will be conducted. For such analyses, regression models will be employed that include intervention, covariate and covariate by treatment interactions as predictors and PAS scores, PCR, PDT, HLPE as outcomes, to examine the modifying effect of the covariates on the intervention. If a significant interaction is detected, analyses of major efficacy endpoints (PAS scores, PCR, PDT, HLPE) will be performed within each group.
Missing Data
We anticipate a 35% – 40% attrition rate in this study, which includes drop-out, spontaneous recovery and discharge, based on review of our hospital records over the past 5 years. Missing data will be handled by employing multiple imputation methods.[36]
Interim Monitoring
Once in progress, the study will be monitored by the DSMB, an independent group that will periodically review the results in order to assess safety. No interim efficacy assessment is planned.
DISCUSSION
Despite the importance of stroke related dysphagia there is little data at present to guide treatment. Several interventions have been examined in small studies and some have provided optimistic results about efficacy.[8–10, 37, 38] However, thus far none have been scrutinized in a larger clinical trial and most published studies have had significant methodological limitations including issues pertaining to power, standardization of the intervention, assessment and procedures, as well as blinding. The FEASt trial is a phase I/II randomized controlled double blind trial designed to assess the safety of concomitantly using anodal tDCS with swallowing exercises in an acute-subacute stroke population with dysphagia. It is expected to provide preliminary efficacy data and serve as a prelude to a larger multicenter, confirmatory trial.
This trial is expected to make several unique contributions to dysphagia and stroke research. It will advance our knowledge and experience in trial conduct in this patient population. Stroke patients with dysphagia often have more disabling strokes with associated functional deficits which can hamper patient participation. The challenges of systematically performing an investigational intervention requiring active subject participation in this patient population are different from trials where patients are passive recipients of treatments like medications or surgery. Experience gained from the conduct of this study will have potential implications for investigating other therapies (behavioral, physical or occupational) in the acute stroke phase. The limitations of active subject participation may also hinder acquisition of detailed, standardized swallowing assessments in these patients. We have, therefore, adapted and standardized our assessment based on the experiences from our pilot study. The protocols for this trial will allow us to test the feasibility of our methods in a larger patient cohort and provide an opportunity to optimize it further. The methods used for swallowing assessment, blinding the raters and exercise protocols can serve as a template for future studies on dysphagia in acute stroke patients.
The experimental intervention proposed here is unique and has not been systematically investigated. It will provide rich data about the safety and tolerability of tDCS in an acute stroke population as most prior studies on tDCS have been conducted in chronic stroke patients or healthy volunteers. Furthermore, information about adverse events in prior studies have been largely volunteered by investigators and not collected as an a priori study requirement, leaving questions about its safety in the acute stroke phase. This trial will employ rigorous double-blinded predefined methods for recording and reporting all potential side effects and clarify issues pertaining to safety of this intervention. Another unique aspect of this trial is the use of low and high dose tDCS, which will provide evidence for any potential dose effects on both safety and efficacy. Previous studies have shown strong additive effects of multiple sessions of tDCS administered over consecutive days.[19–21, 33, 39] The FEASt trial will use a novel design where all subjects undergo a more intense twice daily stimulation/sham session to maximize treatment exposure. Exceptional methods will be applied to not only blinding the subject and the investigator assessing outcomes but also the investigator who delivers the stimulation, to eliminate any inadvertent biases.
The neuroimaging data from the FEASt trial will likely improve understanding of the neuroanatomical correlates of dysphagia and swallowing recovery. Previous neuroimaging studies have implicated several neuroanatomical sites in producing dysphagia[30, 32, 40–43] but suffered from methodological limitations such as a failure to account for differences in lesion size or the potential effects of other confounders. Consequently, analysis of lesion topography or lesion size, in isolation, has been insufficient to explain the great variability in swallowing functions or potential for recovery. While techniques such as TMS and fMRI have improved our understanding about stroke related dysphagia they offer limited quantitative information on how damage to the cortical projections influence severity of dysphagia or potential for recovery. In this study we will use the previously developed variable lesion-load which combines lesion size and location and quantitatively relates this variable to behavioral measures in a multivariate analysis. This method has recently been applied in a motor as well as aphasia study[44, 45], though has never been attempted in a dysphagic stroke population. If proven reliable, it has the potential to be automated in the future and has the potential to improve future study designs and refine study inclusion criteria by identifying candidates likely to respond to treatment.
A barrier in optimal planning of clinical studies has been a current lack of large scale epidemiological investigations on stroke related dysphagia. This trial will generate a rich data collected prospectively using standardized protocols and methods and help bridge important gaps in epidemiological knowledge about swallowing recovery. The information gained from this analysis can aid in planning other studies in stroke related dysphagia.
In summary, the FEASt trial is expected to advance our knowledge on stroke related dysphagia. This research will gather additional safety data on cumulative sessions of tDCS in acute-subacute phases of stroke, obtain information about effects of this intervention on important physiological and clinically relevant swallowing parameters, examine possible dose effects, and identify candidates who are more likely to benefit from this intervention. The experience gained from this study will guide planning of future confirmatory trials that use relevant clinical outcomes to assess potential benefits of this intervention and utilize important subject-specific parameters to refine study inclusion criteria and aid in severity adjusted analysis.
Acknowledgments
Research reported in this publication was supported by the National Institute On Deafness And Other Communication Disorders of the National Institutes of Health under Award Number R01DC012584. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Members of the FEASt team (Beth Israel Deaconess Medical Center, Boston, MA –Sandeep Kumar, MD; Sarah Marchina, PhD; Gottfried Schlaug, MD, PhD; David E. Searls, MD; Cynthia Wagner, MS CCC-SLP; Amanda Warren, MS CCC-SLP; Brooke Littleton, MS CCC-SLP; Rebecca Baars, MS CCC-SLP; Boston University Medical Center, Boston, MA – Susan Langmore, PhD CCC-SLP; Jessica M. Pisegna, MS; Gintas Krisciunas, MPH MA; Boston University School of Public Health, Boston, MA – Christine E. Chaisson, MPH; Alice B. Bisbee, MPH; Joseph Palmisano; Joseph Massaro, PhD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. British medical journal. 1987;295:411–4. doi: 10.1136/bmj.295.6595.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe C. Deaths from stroke in younger people. Bmj. 1993;307:1020–1. doi: 10.1136/bmj.307.6911.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odderson IR. Acute and subacute stroke rehabilitation. Archives of physical medicine and rehabilitation. 1995;76:889–90. doi: 10.1016/s0003-9993(95)80562-1. [DOI] [PubMed] [Google Scholar]
- 4.Smithard DG, O’Neill PA, Parks C, Morris J. Complications and outcome after acute stroke. Does dysphagia matter? Stroke; a journal of cerebral circulation. 1996;27:1200–4. doi: 10.1161/01.str.27.7.1200. [DOI] [PubMed] [Google Scholar]
- 5.Mann G, Hankey GJ, Cameron D. Swallowing disorders following acute stroke: prevalence and diagnostic accuracy. Cerebrovascular diseases. 2000;10:380–6. doi: 10.1159/000016094. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Hamdy S. Dysphagia in stroke patients. Postgraduate medical journal. 2006;82:383–91. doi: 10.1136/pgmj.2005.043281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kind A, Anderson P, Hind J, Robbins J, Smith M. Omission of dysphagia therapies in hospital discharge communications. Dysphagia. 2011;26:49–61. doi: 10.1007/s00455-009-9266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bath PM, Bath FJ, Smithard DG. Interventions for dysphagia in acute stroke. The Cochrane database of systematic reviews. 2000:CD000323. doi: 10.1002/14651858.CD000323. [DOI] [PubMed] [Google Scholar]
- 9.Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomised controlled trials. Age and ageing. 2008;37:258–64. doi: 10.1093/ageing/afn064. [DOI] [PubMed] [Google Scholar]
- 10.Geeganage C, Beavan J, Ellender S, Bath PM. Interventions for dysphagia and nutritional support in acute and subacute stroke. The Cochrane database of systematic reviews. 2012;10:CD000323. doi: 10.1002/14651858.CD000323.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Hamdy S, Aziz Q, Rothwell JC, Power M, Singh KD, Nicholson DA, et al. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115:1104–12. doi: 10.1016/s0016-5085(98)70081-2. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Luo C, Yu B, Yan B, Gong Q, He C, et al. Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: a preliminary study. Journal of neurology, neurosurgery, and psychiatry. 2009;80:1320–9. doi: 10.1136/jnnp.2009.176214. [DOI] [PubMed] [Google Scholar]
- 13.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain stimulation. 2008;1:206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Archives of neurology. 2008;65:1571–6. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baird AE, Benfield A, Schlaug G, Siewert B, Lovblad KO, Edelman RR, et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Annals of neurology. 1997;41:581–9. doi: 10.1002/ana.410410506. [DOI] [PubMed] [Google Scholar]
- 16.Jefferson S, Mistry S, Singh S, Rothwell J, Hamdy S. Characterizing the application of transcranial direct current stimulation in human pharyngeal motor cortex. American journal of physiology Gastrointestinal and liver physiology. 2009;297:G1035–40. doi: 10.1152/ajpgi.00294.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64:872–5. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- 18.Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain : a journal of neurology. 2005;128:490–9. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 19.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restorative neurology and neuroscience. 2007;25:123–9. [PubMed] [Google Scholar]
- 20.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75:2176–84. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, Wagner CW, Frayne C, Zhu L, Selim M, Feng W, et al. Noninvasive brain stimulation may improve stroke-related dysphagia: a pilot study. Stroke; a journal of cerebral circulation. 2011;42:1035–40. doi: 10.1161/STROKEAHA.110.602128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 23.Leonard RJ, Kendall KA, McKenzie S, Goncalves MI, Walker A. Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15:146–52. doi: 10.1007/s004550010017. [DOI] [PubMed] [Google Scholar]
- 24.Leonard R, Belafsky PC, Rees CJ. Relationship between fluoroscopic and manometric measures of pharyngeal constriction: the pharyngeal constriction ratio. The Annals of otology, rhinology, and laryngology. 2006;115:897–901. doi: 10.1177/000348940611501207. [DOI] [PubMed] [Google Scholar]
- 25.Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Archives of physical medicine and rehabilitation. 2005;86:1516–20. doi: 10.1016/j.apmr.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 26.Turner A, Hambridge J, White J, Carter G, Clover K, Nelson L, et al. Depression screening in stroke: a comparison of alternative measures with the structured diagnostic interview for the diagnostic and statistical manual of mental disorders, fourth edition (major depressive episode) as criterion standard. Stroke; a journal of cerebral circulation. 2012;43:1000–5. doi: 10.1161/STROKEAHA.111.643296. [DOI] [PubMed] [Google Scholar]
- 27.Kalu UG, Sexton CE, Loo CK, Ebmeier KP. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychological medicine. 2012;42:1791–800. doi: 10.1017/S0033291711003059. [DOI] [PubMed] [Google Scholar]
- 28.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2006;117:845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Steinmetz H, Furst G, Meyer BU. Craniocerebral topography within the international 10–20 system. Electroencephalography and clinical neurophysiology. 1989;72:499–506. doi: 10.1016/0013-4694(89)90227-7. [DOI] [PubMed] [Google Scholar]
- 30.Hamdy S, Aziz Q, Rothwell JC, Singh KD, Barlow J, Hughes DG, et al. The cortical topography of human swallowing musculature in health and disease. Nature medicine. 1996;2:1217–24. doi: 10.1038/nm1196-1217. [DOI] [PubMed] [Google Scholar]
- 31.Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2003;114:2226–44. doi: 10.1016/s1388-2457(03)00237-2. [DOI] [PubMed] [Google Scholar]
- 32.Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, et al. Cortical activation during human volitional swallowing: an event-related fMRI study. The American journal of physiology. 1999;277:G219–25. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- 33.Lazarus C, Logemann JA, Song CW, Rademaker AW, Kahrilas PJ. Effects of voluntary maneuvers on tongue base function for swallowing. Folia phoniatrica et logopaedica : official organ of the International Association of Logopedics and Phoniatrics. 2002;54:171–6. doi: 10.1159/000063192. [DOI] [PubMed] [Google Scholar]
- 34.Peck KK, Branski RC, Lazarus C, Cody V, Kraus D, Haupage S, et al. Cortical activation during swallowing rehabilitation maneuvers: a functional MRI study of healthy controls. The Laryngoscope. 2010;120:2153–9. doi: 10.1002/lary.21125. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S, Doughty C, Doros G, Selim M, Lahoti S, Gokhale S, et al. Recovery of swallowing after dysphagic stroke: an analysis of prognostic factors. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2014;23:56–62. doi: 10.1016/j.jstrokecerebrovasdis.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Statistics in medicine. 1991;10:585–98. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 37.Khedr EM, Abo-Elfetoh N, Rothwell JC. Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta neurologica Scandinavica. 2009;119:155–61. doi: 10.1111/j.1600-0404.2008.01093.x. [DOI] [PubMed] [Google Scholar]
- 38.Bulow M, Speyer R, Baijens L, Woisard V, Ekberg O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia. 2008;23:302–9. doi: 10.1007/s00455-007-9145-9. [DOI] [PubMed] [Google Scholar]
- 39.Froc DJ, Chapman CA, Trepel C, Racine RJ. Long-term depression and depotentiation in the sensorimotor cortex of the freely moving rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:438–45. doi: 10.1523/JNEUROSCI.20-01-00438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniels SK, Foundas AL. Lesion localization in acute stroke patients with risk of aspiration. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 1999;9:91–8. doi: 10.1111/jon19999291. [DOI] [PubMed] [Google Scholar]
- 41.Broadley S, Croser D, Cottrell J, Creevy M, Teo E, Yiu D, et al. Predictors of prolonged dysphagia following acute stroke. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2003;10:300–5. doi: 10.1016/s0967-5868(03)00022-5. [DOI] [PubMed] [Google Scholar]
- 42.Flowers HL, Skoretz SA, Streiner DL, Silver FL, Martino R. MRI-based neuroanatomical predictors of dysphagia after acute ischemic stroke: a systematic review and meta-analysis. Cerebrovascular diseases. 2011;32:1–10. doi: 10.1159/000324940. [DOI] [PubMed] [Google Scholar]
- 43.Mosier KM, Liu WC, Maldjian JA, Shah R, Modi B. Lateralization of cortical function in swallowing: a functional MR imaging study. AJNR American journal of neuroradiology. 1999;20:1520–6. [PMC free article] [PubMed] [Google Scholar]
- 44.Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, Schlaug G. Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke; a journal of cerebral circulation. 2011;42:2251–6. doi: 10.1161/STROKEAHA.110.606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke; a journal of cerebral circulation. 2010;41:910–5. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]