Abstract
Background
No single-nucleotide polymorphisms (SNPs) specific for aggressive prostate cancer have been identified in genome-wide association studies (GWAS).
Objective
To test if SNPs associated with other traits may also affect the risk of aggressive prostate cancer.
Design, setting, and participants
SNPs implicated in any phenotype other than prostate cancer (p ≤ 10−7) were identified through the catalog of published GWAS and tested in 2891 aggressive prostate cancer cases and 4592 controls from the Breast and Prostate Cancer Cohort Consortium (BPC3). The 40 most significant SNPs were followed up in 4872 aggressive prostate cancer cases and 24 534 controls from the Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL) consortium.
Outcome measurements and statistical analysis
Odds ratios (ORs) and 95% confidence intervals (CIs) for aggressive prostate cancer were estimated.
Results and limitations
A total of 4666 SNPs were evaluated by the BPC3. Two signals were seen in regions already reported for prostate cancer risk. rs7014346 at 8q24.21 was marginally associated with aggressive prostate cancer in the BPC3 trial (p = 1.6 × 10-6), whereas after meta-analysis by PRACTICAL the summary OR was 1.21 (95%CI 1.16–1.27; p = 3.22 × 10−18). rs9900242 at 17q24.3 was also marginally associated with aggressive disease in the meta-analysis (OR 0.90, 95% CI 0.86–0.94; p = 2.5 × 10−6). Neither of these SNPs remained statistically significant when conditioning on correlated known prostate cancer SNPs. The meta-analysis by BPC3 and PRACTICAL identified a third promising signal, marked by rs16844874 at 2q34, independent of known prostate cancer loci (OR 1.12,95% CI 1.06–1.19; p = 4.67 × 10−5); it has been shown that SNPs correlated with this signal affect glycine concentrations. The main limitation is the heterogeneity in the definition of aggressive prostate cancer between BPC3 and PRACTICAL.
Conclusions
We did not identify new SNPs for aggressive prostate cancer. However, rs16844874 may provide preliminary genetic evidence on the role of the glycine pathway in prostate cancer etiology.
Patient summary
We evaluated whether genetic variants associated with several traits are linked to the risk of aggressive prostate cancer. No new such variants were identified.
Keywords: Aggressive prostate cancer, Genome-wide association study, Pleiotropy, Single-nucleotide polymorphism, Glycine
1. Introduction
Prostate cancer is a clinically heterogeneous disease entity ranging from microscopic, well-differentiated indolent tumors to aggressive disease; the latter comprises 10–20% of all tumors and can lead to considerable morbidity and mortality [1]. This clinical heterogeneity may reflect underlying heterogeneity in disease etiology and has implications for screening, treatment, and prognosis. Many genetic risk factors have been robustly associated with the disease through genome-wide association studies (GWAS). Currently, almost 100 loci, explaining ∼30% of the genetic variance of the disease, have been discovered and replicated through GWAS and replication studies [2].
Recent GWAS have identified SNPs possibly associated with aggressive prostate cancer [3], but none of these SNPs show specificity only for the aggressive phenotype [2]. The paucity of loci uniquely associated with aggressive disease may be because of low study power arising from insufficient sample sizes [3], heterogeneity and misclassification of the definitions of disease aggressiveness, and a high proportion of false-negative findings in current GWAS [4,5], especially with regard to low-frequency variants and variants conferring a small increase in risk [6,7]. Protection against the large number of false-positive findings has led to adoption of strict thresholds of statistical significance (eg, p < 5 × 10−8), which in turn increase the number of false-negative results that may be noteworthy but are not explored further in replication efforts [8].
One approach to discover additional noteworthy loci is to evaluate SNPs that have been robustly associated with other human traits through GWAS and large-scale meta-analyses thereof [9,10]. This genome-wide pleiotropy scan approach has previously yielded novel associations for risk of pancreatic adenocarcinoma [11] and endometrial [12] and colorectal cancer [13]. The hypothesized pleiotropic effects (when a genetic locus is associated with multiple phenotypes or phenotypic traits) [14] are especially meaningful in cancer. For example, the TERT locus at 5p15.33 has been associated with more than ten different conditions, including bladder [15], prostate, [16] and other cancers [17], as well as nonmalignant [18] diseases.
Therefore, we aimed to identify new loci associated with risk of aggressive prostate cancer by estimating the associations for loci previously associated with other complex traits in GWAS.
2. Materials and methods
2.1. Study design and populations
We applied a two-stage design. In the first stage, data from the GWAS on aggressive prostate cancer from the Breast and Prostate Cancer Cohort Consortium (BPC3) were used to examine whether previously GWAS-identified SNPs associated with other traits were also associated with the risk of aggressive prostate cancer. In the second stage, replication of the 40 most significant SNPs from the first stage was performed using data from the Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL) consortium, and the results for the two consortia were combined through meta-analysis.
For the first stage, we used data from 2891 cases with aggressive prostate cancer and 4592 matched controls on age, ethnicity, and in some cohorts, the region of recruitment, who have been previously genotyped in the seven large and well-established cohorts of BPC3 (Supplementary text) [19]. All subjects included in the current analysis were of European ancestry. Aggressive prostate cancer was defined as having either high histologic grade (Gleason score ≥8) or extraprostatic extension (stage C/D). The specimens used to determine the Gleason grading included surgical specimens from radical prostatectomy or autopsy as well as from diagnostic biopsy (either needle biopsy or transurethral resection of the prostate). When multiple Gleason scores were available, we used the surgical value.
The study population, genotyping methods, and quality control criteria applied in the PRACTICAL consortium at the replication stage have been described in detail elsewhere [3,20]. In brief, the total sample size consisted of 23 631 prostate cancer cases, 4872 (21%) of whom had aggressive prostate cancer, and 24 534 disease-free control subjects. All individuals were of European ancestry. Aggressive disease was defined as Gleason score ≥8, prostate-specific antigen (PSA) >100 ng/ml, a disease stage of distant (outside the pelvis), or death from prostate cancer.
2.2. Selection of SNPs
We used the Catalog of Published Genome-Wide Association Studies hosted by the National Human Genome Research Institute [9] as of June 26,2013 to select eligible SNPs. The catalog is a regularly updated online database that lists genetic associations from published GWAS that have p ≤ 10−5. We included only SNPs that have previously been associated with any complex disease or trait at p = 10−7 or lower, excluding those associated with prostate cancer. Empirical evidence suggests that the widely adopted level of genome-wide significance at 5 × 10−8 is strict and that associations with p ≤ 10−7 are likely to represent true signals [21]. We included only associations that pertained to SNPs and excluded copy number and structural variants. Haplotypes of SNPs were broken down to the individual SNPs whenever possible. We also excluded associations for which the corresponding p values were not reported in the catalog and could not be estimated using the data reported in the original GWAS publications. In addition, we excluded catalog entries that could not be mapped to an rs-numbered SNP after reviewing the original publications.
For SNPs that had not been directly genotyped in BPC3, we used the SNP Annotation and Proxy (SNAP) tool [22] to identify proxies in high linkage disequilibrium (LD; ie, r2 > 0.9).
2.3. Statistical analysis
In the BPC3 GWAS, the association between each SNP and aggressive prostate cancer was examined using logistic regression to estimate the per-allele odds ratio (OR) and the corresponding 95% confidence interval (CI) after adjusting for the second principal component of genetic covariance, as explained previously [19]. For SNPs that were present in four or more BPC3 cohorts, fixed-effects meta-analysis was used to estimate the summary per-allele OR.
In the PRACTICAL GWAS, per-allele ORs and corresponding 95% CIs were estimated using logistic regression after adjusting for principal components, as explained in detail previously [20]. Fixed-effects meta-analysis was used to combine the effect estimates from the individual studies. To clarify whether SNPs reaching significance thresholds in regions previously associated with prostate cancer are independent signals, we performed conditional analyses.
The top 40 SNPs from BPC3 with the lowest p values were followed up for replication in PRACTICAL. Results from the two consortia were combined using random-effects meta-analysis, which is more powerful than fixed-effect meta-analysis when the aim is to replicate an association [10]. Between-study heterogeneity was evaluated using Cochran's Q statistic and was quantified with the I2 metric [23]. We evaluated the statistical significance of the pleiotropy scan results at the genome-wide level using p = 10−7 [21].
In a secondary analysis, we performed a cross-phenotype association analysis in the replication stage. To determine whether any SNPs from the discovery stage are specific for the risk of aggressive prostate cancer, we examined their association with overall prostate cancer in the BPC3, PRACTICAL, and combined data sets.
3. Results
As of June 26,2013, the GWAS catalog listed a total of 13 613 associations, of which 4438 pertained to SNPs eligible for our analyses (Supplementary Fig. 1). As described in the Supplementary text, these corresponded to a total of 5003 SNPs that were tested in BPC3, of which 337 SNPs were duplicates, resulting in a final set of 4666 SNPs.
3.1. Association of SNPs with aggressive prostate cancer
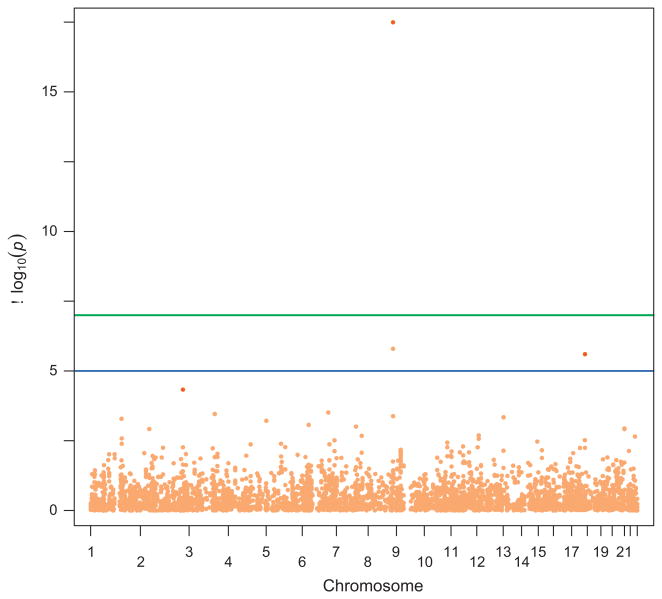
A total of 265 SNPs either directly genotyped (140 catalog SNPs and 87 proxy SNPs) or imputed (34 HapMap-imputed SNPs and four SNPs imputed from 1000 Genomes) had nominally significant (p < 0.05) associations with aggressive prostate cancer in BPC3, but none of them reached the genome-wide significance level of p = 10−7 (Fig. 1 and Supplementary Table 1). Table 1 shows the 40 SNPs with the lowest p values in BPC3 that were followed up in PRACTICAL. After meta-analysis with PRACTICAL, ten SNPs were nominally significant (p < 0.05), and five (rs7014346, rs10505477, rs10069690, rs2315008, and rs4809330) also reached genome-wide significance at p ≤ 1 × 10−7 (Table 1).
Figure 1.
Manhattan plot for the 4666 single-nucleotide polymorphisms (SNPs) evaluated in the genome-wide pleiotropy scan. Breast and Prostate Cancer Cohort Consortium (BPC3) results are shown in Light Orange and the meta-analysis results for the three strongest SNPs are shown in orange.
Table 1. Top 40 SNPs in BPC3 and associations with aggressive prostate cancer in BPC3, PRACTICAL, and random-effects meta-analysis.
| SNP | Alleles | Region | Trait in GWAS catalog | BPC3 | PRACTICAL | Meta-analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | phet | I2 (%) | ||||
| rs7014346 | A vs G | 8q24.21 | Colorectal cancer | 1.19 (1.11–1.28) | 1.60 × 10−6 | 1.22 (1.16–1.29) | 3.49 × 10−13 | 1.21 (1.16–1.27) | 3.22 × 10−18 | 0.64 | 0 |
| rs6569648 | C vs T | 6q23.1 | Height | 0.86 (0.79–0.93) | 3.08 × 10−4 | 0.97 (0.91–1.04) | 4.16 × 10−1 | 0.92 (0.81–1.04) | 0.175 | 0.02 | 82.8 |
| rs2687720a | A vs G | 3q21.3 | Menarche (age at onset) | 1.15 (1.07–1.24) | 3.48 × 10−4 | 1.09 (1.03–1.16) | 4.02 × 10−3 | 1.11 (1.06–1.17) | 6.74 × 10−5 | 0.26 | 19.8 |
| rs10505477 | C vs T | 8q24.21 | Colorectal cancer | 0.85 (0.77–0.93) | 4.15 × 10−4 | 0.79 (0.75–0.83) | 7.72 × 10−20 | 0.81 (0.76–0.86) | 3.56 × 10−11 | 0.21 | 35.4 |
| rs9318086 | A vs G | 13q12.12 | Myopia | 1.13 (1.06–1.22) | 4.53 × 10−4 | 1.01 (0.96–1.06) | 7.38 × 10−1 | 1.07 (0.95–1.20) | 2.65 × 10−1 | <0.01 | 85.2 |
| rs753955 | T vs C | 13q12.12 | Lung cancer | 1.14 (1.06–1.22) | 4.60 × 10−4 | 1.03 (0.98–1.09) | 2.74 × 10−1 | 1.08 (0.98–1.19) | 1.18 × 10−1 | 0.03 | 77.9 |
| rs3180018 | A vs G | 1q22 | Crohn disease | 1.16 (1.07–1.25) | 5.15 × 10−4 | 0.99 (0.93–1.05) | 7.47 × 10−1 | 1.07 (0.92–1.24) | 4.02 × 10−1 | <0.01 | 88.7 |
| rs10069690 | C vs T | 5p15.33 | Breast cancer | 1.21 (1.08–1.35) | 6.10 × 10−4 | 1.20 (1.13–1.28) | 3.45 × 10−9 | 1.21 (1.14–1.27) | 8.82 × 10−12 | 0.97 | 0 |
| rs6910071 | G vs A | 6p21.32 | Rheumatoid arthritis | 1.16 (1.06–1.27) | 8.53 × 10−4 | 1.02 (0.96–1.09) | 5.67 × 10−1 | 1.08 (0.95–1.23) | 2.16 × 10−1 | 0.02 | 81.8 |
| rs7788750a | T vs C | 7q22.1 | Ulcerative colitis | 1.36 (1.13–1.63) | 9.83 × 10−4 | 0.88 (0.76–1.00) | 5.39 × 10−2 | 1.09 (0.71–1.67) | 7.10 × 10−1 | <0.01 | 93.1 |
| rs2315008 | T vs G | 20q13.33 | Inflammatory bowel disease | 0.88 (0.82–0.95) | 1.15 × 10−3 | 0.86 (0.81–0.91) | 1.45 × 10−7 | 0.87 (0.83–0.91) | 7.43 × 10−10 | 0.57 | 0 |
| rs6756629 | A vs G | 2p21 | Total and LDL cholesterol | 1.26 (1.09–1.44) | 1.20 × 10−3 | 0.96 (0.86–1.07) | 4.49 × 10−1 | 1.09 (0.84–1.42) | 5.07 × 10−1 | <0.01 | 89 |
| rs4809330 | A vs G | 20q13.33 | Crohn disease | 0.88 (0.82–0.95) | 1.21 × 10−3 | 0.86 (0.81–0.91) | 1.77 × 10−7 | 0.87 (0.83–0.91) | 9.50 × 10−10 | 0.57 | 0 |
| rs3764021 | A vs G | 12p13.31 | Type 1 diabetes | 0.89 (0.82–0.95) | 2.04 × 10−3 | 1.02 (0.97–1.07) | 4.91 × 10−1 | 0.95 (0.83–1.09) | 4.91 × 10−1 | <0.01 | 88.3 |
| rs10229583 | A vs G | 7q32.1 | Type 2 diabetes | 1.14 (1.05–1.23) | 2.10 × 10−3 | 1.02 (0.96–1.08) | 5.45 × 10−1 | 1.07 (0.96–1.19) | 1.99 × 10−1 | 0.04 | 77.4 |
| rs968451 | T vs G | 22q13.1 | Primary biliary cirrhosis | 1.15 (1.05–1.26) | 2.24 × 10−3 | 1.02 (0.95–1.09) | 5.82 × 10−1 | 1.08 (0.96–1.21) | 2.10 × 10−1 | 0.04 | 77.5 |
| rs12563627a | T vs C | 1q22 | Inflammatory bowel disease | 1.12 (1.04–1.21) | 2.61 × 10−3 | 1.02 (0.96–1.08) | 5.20 × 10−1 | 1.07 (0.97–1.17) | 1.91 × 10−1 | 0.04 | 75.9 |
| rs10466829 | G vs A | 12p13.31 | Multiple sclerosis | 1.11 (1.04–1.20) | 2.69 × 10−3 | 0.99 (0.94–1.04) | 6.52 × 10−1 | 1.05 (0.93–1.18) | 4.50 × 10−1 | <0.01 | 85.7 |
| rs8079702 | G vs A | 17q24.3 | Primary tooth development (number of teeth) | 1.11 (1.04–1.20) | 3.02 × 10−3 | 0.96 (0.91–1.01) | 1.40 × 10−1 | 1.03 (0.89–1.19) | 6.69 × 10−1 | <0.01 | 90.6 |
| rs2048327 | G vs A | 6q25.3 | Coronary heart disease | 1.12 (1.04–1.20) | 3.06 × 10−3 | 1.05 (1.00–1.11) | 5.69 × 10−2 | 1.08 (1.02–1.14) | 6.00 × 10−3 | 0.22 | 33.4 |
| rs4905994a | T vs C | 14q32.2 | Platelet counts | 1.12 (1.04–1.21) | 3.37×10−3 | 0.96 (0.91–1.02) | 1.83×10−1 | 1.04 (0.89–1.20) | 6.42 × 10−1 | <0.01 | 89.9 |
| rs7901695 | C vs T | 10q25.2 | Type 2 diabetes | 0.89 (0.83–0.96) | 3.67 × 10−3 | 1.00 (0.94–1.06) | 9.89 × 10−1 | 0.95 (0.85–1.06) | 3.39 × 10−1 | 0.02 | 81.4 |
| rs3846662 | C vs T | 5q13.3 | Total and LDL cholesterol | 0.90 (0.84–0.97) | 4.04 × 10−3 | 1.04 (0.98–1.10) | 1.71 × 10−1 | 0.97 (0.85–1.11) | 6.58 × 10−1 | <0.01 | 89.7 |
| rs625658a | A vs C | 1q22 | Inflammatory bowel disease | 1.12 (1.04–1.20) | 4.05 × 10−3 | 1.02 (0.97–1.08) | 4.87 × 10−1 | 1.06 (0.97–1.16) | 1.73 × 10−1 | 0.06 | 72.1 |
| rs6569992 | A vs G | 6q23.3 | Red blood cell traits | 0.88 (0.80–0.96) | 4.24 × 10−3 | 0.93 (0.87–1.00) | 5.68 × 10−2 | 0.91 (0.86–0.96) | 1.00 × 10−3 | 0.32 | 100 |
| rs10033464 | T vs G | 4q25 | Atrial fibrillation | 1.19 (1.06–1.33) | 4.25 × 10−3 | 0.95 (0.87–1.04) | 2.69 × 10−1 | 1.06 (0.85–1.31) | 6.07 × 10−1 | <0.01 | 88.5 |
| rs1562990 | C vs A | 11q12.2 | Alzheimer disease | 1.11 (1.03–1.19) | 5.05 × 10−3 | 1.02 (0.96–1.07) | 5.35 × 10−1 | 1.06 (0.97–1.15) | 1.86 × 10−1 | 0.06 | 71.4 |
| rs4869272a | C vs T | 5q15 | Fasting glucose-related traits | 1.11 (1.03–1.20) | 5.33 × 10−3 | 1.01 (0.95–1.08) | 7.49 × 10−1 | 1.06 (0.96–1.17) | 2.34 × 10−1 | 0.06 | 71.7 |
| rs7903146 | T vs C | 10q25.2 | Type 2 diabetes; Fasting glucose-related traits; Fasting insulin-related traits; Proinsulin levels; HbA1c levels | 0.89 (0.83–0.97) | 5.40 × 10−3 | 1.00 (0.94–1.06) | 9.64 × 10−1 | 0.95 (0.85–1.06) | 3.33 × 10−1 | 0.03 | 79.3 |
| rs2970818 | T vs A | 12p13.32 | Phosphorus levels | 0.83 (0.73–0.95) | 5.42 × 10−3 | 0.98 (0.89–1.07) | 6.26 × 10−1 | 0.91 (0.77–1.06) | 2.31 × 10−1 | 0.05 | 75.1 |
| rs16844874a | C vs T | 2q34 | Glycine | 1.16 (1.05–1.29) | 5.44 × 10−3 | 1.11 (1.04–1.18) | 2.18 × 10−3 | 1.12 (1.06–1.19) | 4.67 × 10−5 | 0.46 | 0 |
| rs4374383 | A vs G | 2q13 | Hepatitis C–induced liver fibrosis | 1.11 (1.03–1.19) | 5.63 × 10−3 | 1.00 (0.93–1.07) | 9.20 × 10−1 | 1.05 (0.95–1.17) | 3.56 × 10−1 | 0.04 | 76.7 |
| rs9900242a | A vs G | 17q24.3 | Pulmonary function in smokers | 0.90 (0.84–0.97) | 5.67 × 10−3 | 0.90 (0.85–0.95) | 1.39 × 10−4 | 0.90 (0.86–0.94) | 2.50 × 10−6 | 0.94 | 0 |
| rs9303521 | T vs G | 17q21.31 | Bone mineral density | 0.90 (0.84–0.97) | 5.42 × 10−3 | 0.99 (0.94–1.04) | 6.26 × 10−1 | 0.95 (0.87–1.04) | 2.35 × 10−1 | 0.05 | 74.1 |
| rsl0804533a | T vs C | 3ql3.32 | Menarche (age at onset) | 0.91 (0.85–0.97) | 5.93 × 10−3 | 1.04 (0.98–1.09) | 1.93 × 10−1 | 0.97 (0.85–1.11) | 6.57 × 10−1 | <0.01 | 88.7 |
| rsl0757274 | A vs G | 9p21.3 | Coronary heart disease | 0.91 (0.85–0.97) | 6.64 × 10−3 | 1.03 (0.98–1.09) | 2.31 × 10−1 | 0.97 (0.85–1.10) | 6.39 × 10−1 | <0.01 | 88 |
| rsl 6948098 | A vs G | 15ql5.3 | Serum albumin level | 0.79 (0.66–0.94) | 5.42 × 10−3 | 0.94 (0.81–1.08) | 6.26 × 10−1 | 0.87 (0.73–1.03) | 9.80 × 10−2 | 0.13 | 56.8 |
| rs944797 | CvsT | 9p21.3 | Coronary heart disease | 1.10(1.03–1.18) | 7.01 × 10−3 | 0.97 (0.92–1.03) | 2.89 × 10−1 | 1.03 (0.91–1.17) | 6.11 × 10−1 | <0.01 | 87.2 |
| rsl0757278 | G vs A | 9p21.3 | Myocardial infarction | 1.11 (1.03–1.19) | 5.42 × 10−3 | 0.98 (0.93–1.03) | 6.26 × 10−1 | 1.04(0.92–1.17) | 5.60 × 10−1 | <0.01 | 86.2 |
| rs9510787 | G vs A | 13ql2.12 | Nasopharyngeal carcinoma | 1.11 (1.03–1.21) | 7.18 × 10−3 | 1.00(0.94–1.07) | 9.82 × 10−1 | 1.05 (0.95–1.17) | 3.47 × 10−1 | 0.04 | 77.5 |
BPC3 = Breast and Prostate Cancer Cohort Consortium; HbAlc = glycated hemoglobin; LDL = low-density lipoprotein; OR = odds ratio; CI = confidence interval; PRACTICAL = Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome; SNP = single-nucleotide polymorphism; GWAS = genome-wide association studies; phet = p value for Cochran's Q statistic for between-study heterogeneity.
Logistic regression was applied in BPC3 and PRACTICAL. Meta-analysis was performed under a random-effects model. All ORs are per-copy of the first allele shown in the Alleles column.
These SNPs are correlated with GWAS catalog-indexed SNPs not found in BPC3 per se as follows: rs9900242 and rsl 1654749 (r2 = 0.93, D′ = 1); rs2687720 and rs2687729 (r2 = 1, D′ = l), rsl0804533 and rs6438424 (r2 = 1, D′ = 1), rs7788750 and rs7809799 (r2 = 1, D′ = 1), rsl2563627 and rs670523 (r2 = 0.97, D′ = 1), rs4905994 and rs7149242 (r2 = 0.96, D′ = 1), rs625658 and rs670523 (r2 = 1, D′ = 1), rsl6844874 and rs2216405 (r2 = 0.94, D′ = 1), rs4869272 and rsl3179048 (r2 = 1, D′ = 1).
Of the 40 SNPs, rs7014346 was the strongest SNP associated with aggressive prostate cancer in BPC3. The OR per copy for the A allele was 1.19 in BPC3 (95% CI 1.11–1.28; p = 1.6 × 10−6), whereas after in silico replication it was 1.22 in PRACTICAL (95% CI 1.16-1.29; p = 3.49 × 10−13). The summary combined OR was 1.21 (95% CI 1.16–1.27; p = 3.22 × 10−18) with no evidence of heterogeneity between BPC3 and PRACTICAL (I2 = 0%, p = 0.64). This SNP, located at 8q24.21, has previously been associated with colorectal cancer [24]. This SNP is correlated (r2 = 0.44, D′ = 1) with a previously established prostate cancer SNP (rs6983267) [19,25,26], so we performed a conditional analysis to explore whether it represents a novel signal. After conditioning on rs6983267, rs7014346 was no longer associated with aggressive prostate cancer (OR 1.06, 95% CI 0.99-1.13; p = 0.07).
Another four SNPs (rs10505477, rs10069690, rs2315008, and rs4809330) were associated with aggressive prostate cancer at p < 10−7 in the meta-analysis. However, for all of these SNPs there was at least one previously established prostate cancer SNP (rs6983267, rs2242652, or rs6062509) in high LD (r2 > 0.80, D′ = 1).
Another promising SNP, rs9900242 at 17q24.3, did not reach genome-wide significance in PRACTICAL (OR per copy of the A allele 0.90, 95% CI 0.85–0.95; p = 1.38 × 10−5) or BPC3 (OR 0.90, 95% CI 0.84–0.97; p = 5.7 × 10−3). However, the meta-analysis yielded a lower p value of 2.5 × 10−6 (OR 0.90, 95% CI 0.86–0.94) with no evidence of heterogeneity (I2 = 0%, p = 0.94). This SNP is a proxy (r2 = 0.93, D′ = 1) for the catalog-indexed rs11654749 at the KCNJ2-SOX9 locus, which has been implicated in an interaction between smoking and pulmonary function [27], and is also partially correlated (r2 = 0.29, D′ = 0.68) with a previously reported genome-wide significant prostate cancer SNP (rs1859962) in that region [19,28,29]. To clarify whether the effect of rs9900242 is independent of rs1859962, we performed a conditional analysis in which rs9900242 was no longer associated with aggressive prostate cancer (OR 0.98,95% CI 0.93–1.04; p = 0.61).
Similarly, another SNP (rs16844874) that did not reach statistical significance in BPC3 (OR per copy of the C allele 1.16, 95% CI 1.05–1.29; p = 5.44 × 10−3) or in PRACTICAL (OR 1.12, 95% CI 1.04–1.18; p = 2.18 × 10−3) reached a lower significance level after meta-analysis with a per-allele OR of 1.12 (95% CI 1.06–1.19; p = 4.67 × 10−5)and no evidence of heterogeneity (I2 = 0%, p = 0.46). rs16844874 is independent of previously reported prostate cancer SNPs (r2 < 0.001 according to the SNAP tool for correlation with all known prostate cancer SNPs) and is a proxy for rs2216405 (r2 = 0.94, D′ = 1) in CPS1 that has been previously shown to increase the serum concentrations of glycine and other metabolites [30]. Sarcosine is a glycine derivative recently shown to be associated with prostate cancer [31], so we examined the association between rs16844874 and rs2216405 and circulating sarcosine concentrations in one of the BPC3 studies with available data (Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, PLCO). Neither rs16844874 (p = 0.72) nor rs2216405 (p = 0.74) was associated with the log-transformed concentrations of sarcosine normalized for alanine levels (log[sarcosine/alanine]) in linear regression models of 990 prostate cancer cases and 821 control subjects after adjustment for case-control status, age, smoking, and diabetes (Supplementary Table 2). Results were identical when we examined this association only in the controls (data not shown).
3.2. Association of SNPs with overall prostate cancer
Of the 40 SNPs with the strongest associations with aggressive prostate cancer in BPC3 that were tested for cross-phenotype associations with overall prostate cancer in BPC3 and PRACTICAL (Supplementary Table 3), eight reached p < 10−7 in the meta-analysis (Table 2). Seven of these SNPs (rs7014346, rs2687720, rs10505477, rs10069690, rs2315008, rs4809330, and rs2048327) are in high LD (r2 > 0.40, D′ > 0.95) with known prostate cancer SNPs (rs6983267, rs10934853, rs2242652, rs6062509, or rs9364554). The summary per-allele OR for the remaining SNP, rs9900242, was 0.90 per copy of the A allele (95% CI 0.87–0.92; p = 7.47 × 10−17) with no evidence of heterogeneity (I2 = 0%, p = 0.83). However, after conditioning on the moderately correlated known prostate cancer SNP rs1859962 (r2 = 0.29, D′ = 0.68), rs9900242 was not associated with overall prostate cancer (OR 0.99, 95% CI 0.96–1.03; p = 0.70). We found no significant association between rs16844874 and overall prostate cancer risk (summary OR 1.08, 95% CI 0.97–1.21; p = 0.17; Supplementary Table 3), but this association was not significantly different from the summary result for aggressive disease (p heterogeneity 0.57).
Table 2. BPC3 SNPs associated with overall prostate cancer at PRACTICAL and meta-analysis.
| SNP | Alleles | BPC3 | PRACTICAL | Meta-analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | phet | I2 (%) | ||
| rs7014346 | A vs G | 1.19 (1.11–1.28) | 1.60 × 10−6 | 1.20 (1.16–1.23) | 3.21 × 10−34 | 1.20 (1.17–1.23) | 3.33 × 10−43 | 0.97 | 0 |
| rs2687720 | A vs G | 1.15 (1.07–1.24) | 3.48 × 10−4 | 1.11 (1.08–1.15) | 9.43 × 10−13 | 1.12 (1.09–1.15) | 1.92 × 10−15 | 0.44 | 0 |
| rs10505477 | C vs T | 0.85 (0.77–0.93) | 4.15 × 10−4 | 0.80 (0.78–0.83) | 5.15 × 10−45 | 0.81 (0.78–0.83) | 5.26 × 10−42 | 0.30 | 6.70 |
| rs10069690 | C vs T | 1.21 (1.08–1.35) | 6.10 × 10−4 | 1.15 (1.12–1.19) | 1.61 × 10−19 | 1.16 (1.12–1.19) | 3.14 × 10−22 | 0.43 | 0 |
| rs2315008 | T vs G | 0.88 (0.82–0.95) | 1.15 × 10−3 | 0.90 (0.87–0.93) | 4.31 × 10−13 | 0.90 (0.87–0.92) | 2.25 × 10−15 | 0.63 | 0 |
| rs4809330 | A vs G | 0.88 (0.82–0.95) | 1.21 × 10−3 | 0.90 (0.87–0.93) | 6.20 × 10−13 | 0.90 (0.87–0.92) | 3.39 × 10−15 | 0.63 | 0 |
| rs2048327 | G vs A | 1.12 (1.04–1.20) | 3.06 × 10−3 | 1.10 (1.07–1.13) | 5.67 × 10−12 | 1.10 (1.08–1.13) | 6.89 × 10−14 | 0.74 | 0 |
| rs9900242 | A vs G | 0.90 (0.84–0.97) | 5.67 × 10−3 | 0.90 (0.87–0.92) | 3.55 × 10−15 | 0.90 (0.87–0.92) | 7.47 × 10−17 | 0.83 | 0 |
BPC3 = Breast and Prostate Cancer Cohort Consortium; OR = odds ratio; PRACTICAL = Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome; SNP = single-nucleotide polymorphism; phet = p value for Cochran's Q statistic for between-study heterogeneity.
Logistic regression was applied in BPC3 and PRACTICAL Meta-analysis was performed under a random-effects model. All ORs are per-copy of the first allele shown in the Alleles column.
4. Discussion
Using a genome-wide pleiotropy scan, we tested more than 4600 SNPs previously associated with various complex traits for association with aggressive prostate cancer in approximately 7800 men of Caucasian ancestry and replicated the strongest SNPs in an independent sample of more than 30 000 men. No new associations were identified.
Pleiotropic effects of GWAS-discovered SNPs have been identified in the past and different types of pleiotropy have been described [14]. Biological pleiotropy and pleiotropy mediated through an intermediate phenotype can be particularly useful when documented, because they may offer new insights into biological functions that are common among apparently unrelated phenotypes, increasing current knowledge about disease pathophysiology. They may also provide functional explanations about associations that have been observed in epidemiological studies [14].
In addition, an SNP in LD with several SNPs, each associated with a different phenotype, may cause spurious pleiotropy [14]. The strongest SNP identified in our study, rs7014346 at 8q24.21, has previously been associated with colorectal cancer risk [24]. This SNP had an OR of 1.19, conferring a small to modest increase in disease risk. This is not uncommon in GWAS, for which the majority of the SNPs discovered have relatively small effect sizes [32]. Such SNPs explain a small percentage of total heritability [33,34], implying that additional factors such as gene-environment interactions and rare variants [7] could explain some of the missing heritability [33]. This is the second SNP in this region for which pleiotropic effects have been discovered; rs6983267 is associated with colorectal and prostate cancer [26,35]. Although the two SNPs are correlated and do not confer independent risks, as documented in a conditional analysis in the current study, rs7014346 has not been reported to be associated with other phenotypes besides colorectal cancer such as rs6983267 has [36]. It is likely that rs7014346 has been a false-negative result so far because of strict genome-wide significance thresholds, which may not always take LD patterns into account [21]; however we cannot exclude the fact it may not have been reported because of its correlation with known prostate cancer SNPs. The 8q24 region contains a large gene desert with SNPs showing pleiotropic effects for various phenotypes including different cancers [37] and cardiovascular and cerebrovascular disease [38]. This region harbors the MYC proto-oncogene, which seems to have long-range interactions with 8q24 loci that act as enhancers regulating the expression of this gene [39]. Similarly, rs9900242 did not confer independent risk for aggressive prostate cancer in our study from the previously known rs1859962. Detailed fine-mapping of the 8q24 and 17q24.3 regions along with appropriate epidemiological approaches [38] can provide additional evidence on the biological mechanisms underlying the respective phenotypes.
Of interest, rs16844874 was nominally significantly associated with aggressive prostate cancer in our study but did not reach genome-wide significance. Because associations may reach genome-wide significance levels when additional data are combined [8,21], this SNP could potentially constitute a new signal, but it should be followed up in future studies. This SNP is not in LD with known prostate cancer loci, but is highly correlated with SNPs previously implicated in glycine metabolism. Glycine is one of the 20 amino acids that form human proteins, and one of its derivatives, sarcosine, has been implicated in the progression of prostate cancer [40]. Although evidence from functional analyses [41] is inconclusive, a recent prospective epidemiological study showed that elevated serum concentrations of sarcosine were associated with an increased risk of prostate cancer, especially the nonaggressive type [31]; however, a second larger study reported an inverse association with circulating concentrations of glycine and sarcosine [42]. Nevertheless, rs16844874 was not associated with circulating sarcosine concentrations in a subset of our study.
Our study has some limitations that should be acknowledged. First, BPC3 and PRACTICAL used slightly different definitions of aggressive prostate cancer as a result of differences in classifications used by pathologists by study and country and over time. These differences may contribute to heterogeneity of outcomes and disease misclassification, as some patients diagnosed with aggressive prostate cancer may actually have nonaggressive disease. They can also increase the noise around a true signal, making detection of SNPs specific for aggressive disease challenging. Second, our analysis pertained exclusively to SNPs included in the GWAS catalog, which lists SNPs with p < 10−5 in published reports, and thus we cannot exclude the possibility that some additional SNPs with weaker associations could also represent false-negative findings, although this possibility seems less likely. Third, the total sample size for BPC3 might have insufficient statistical power to detect SNPs with small effect sizes. Nevertheless, for the replication stage, we used data from the PRACTICAL Consortium, which is the largest sample with GWAS data on prostate cancer to date. Finally, we applied a strict significance threshold of p = 10−7 instead of adjusting the p value for the 4666 tests performed in the discovery stage. This protects our results from false-positive findings. Other approaches such as the false-discovery rate are expected to give similar results with the family-wise error rate (eg, Bonferroni correction) [43].
5. Conclusions
Our genome-wide pleiotropy scan for aggressive prostate cancer that interrogated all known SNPs pertaining to complex traits did not identify any new SNPs. Although rs16844874 did not reach genome-wide significance levels, it may warrant follow-up because of its high correlation with SNPs involved in metabolic pathways potentially implicated in prostate cancer. Given the lack of loci specifically associated with aggressive disease, future study designs should focus on identifying SNPs specific for this outcome, which is more clinically relevant. GWAS with larger sample sizes and denser genotyping platforms, as well as sequencing studies, could reveal clinically useful genetic associations. There is evidence that integration of such associations in prognostic studies and clinical translational research may improve the efficacy of targeted prostate cancer screening programs, risk stratification, and treatment [2,44,45].
Supplementary Material
Acknowledgments
The BPC3 consortium was supported by the United States National Cancer Institute (NCI) of the National Institutes of Health (NIH) (U01-CA98233-07 to D.J. Hunter, U01-CA98710-06 to M.J. Thun, U01-CA98216-06 to E. Riboli and R. Kaaks, U01-CA98758-07 to B.E. Henderson, and the Intramural Research Program of the NIH/NCI to the Division of Cancer Epidemiology and Genetics). K.K. Tsilidis was supported by the Hellenic Republic, General Secretary of Research and Technology, Aristeia II funding program (grant no. 4320) for this work. Acknowledgment and funding details for the PRACTICAL Consortium are provided in the Supplementary text.
Funding/Support and role of the sponsor: Supported by US National Cancer Institute, Hellenic Republic (General Secretary of Research and Technology), Canadian Institutes of Health Research, European Commission's Seventh Framework Programme, and Cancer Research UK. The sponsors played no role in the study.
Footnotes
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/ j.eururo.2014.09.020.
Author contributions: Konstantinos K. Tsilidis had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Panagiotou, Travis, Campa, Berndt, Lindstrom, Kraft, Schumacher, Siddiq, Papatheodorou, Stanford, Chanock, Wacholder, Key, Tsilidis.
Acquisition of data: Berndt, Kraft, Virtamo, Gapstur, Riboli, Kaaks, Boeing, Khaw, Krogh, Bueno-de-Mesquita, Overvad, Barricarte Gurrea, Tricho-poulos, Giovannucci, Le Marchand, Henderson, Stampfer, Gaziano, Hunter, Albanes, Hoover, The PRACTICAL Consortium, Chanock, Key.
Analysis and interpretation of data: Panagiotou, Travis, Campa, Berndt, Lindstrom, Kraft, Schumacher, Siddiq, Papatheodorou, Stanford, Chanock, Wacholder, Key, Tsilidis.
Drafting of the manuscript: Panagiotou, Tsilidis.
Critical revision of the manuscript for important intellectual content: Panagiotou, Travis, Campa, Berndt, Lindstrom, Kraft, Schumacher, Siddiq, Papatheodorou, Stanford, Virtamo, Gapstur, Stevens, Diver, Riboli, Kaaks, Boeing, Khaw, Krogh, Bueno-de-Mesquita, Overvad, Barricarte Gurrea, Trichopoulos, Giovannucci, Haiman, Le Marchand, Henderson, Stampfer, Gaziano, Hunter, Weinstein, Albanes, Koutros, Hoover, Yeager, the PRACTICAL Consortium, Chanock, Wacholder, Key, Tsilidis.
Statistical analysis: Panagiotou, Tsilidis.
Obtaining funding: Henderson, Hunter, Riboli, Kaaks, the PRACTICAL Consortium.
Administrative, technical, or material support: None.
Supervision: None.
Other (specify): None.
Financial disclosures: Konstantinos K. Tsilidis certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Eeles R, Goh C, Castro E, et al. The genetic epidemiology of prostate cancer and its clinical implications. Nat Rev Urol. 2014;11:18–31. doi: 10.1038/nrurol.2013.266. [DOI] [PubMed] [Google Scholar]
- 3.Al Olama AA, Kote-Jarai Z, Schumacher FR, et al. A meta-analysis of genome-wide association studies to identify prostate cancer susceptibility loci associated with aggressive and non-aggressive disease. Hum Mol Genet. 2013;22:408–15. doi: 10.1093/hmg/dds425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ioannidis JP, Tarone R, McLaughlin JK. The false-positive to false-negative ratio in epidemiologic studies. Epidemiology. 2011;22:450–6. doi: 10.1097/EDE.0b013e31821b506e. [DOI] [PubMed] [Google Scholar]
- 5.Wacholder S. On standards of evidence. Epidemiology. 2011;22:464–6. doi: 10.1097/EDE.0b013e31821d127d. [DOI] [PubMed] [Google Scholar]
- 6.Vineis P, Brennan P, Canzian F, et al. Expectations and challenges stemming from genome-wide association studies. Mutagenesis. 2008;23:439–44. doi: 10.1093/mutage/gen042. [DOI] [PubMed] [Google Scholar]
- 7.Panagiotou OA, Evangelou E, Ioannidis JP. Genome-wide significant associations for variants with minor allele frequency of 5% or less— an overview: a HuGE review. Am J Epidemiol. 2010;172:869–89. doi: 10.1093/aje/kwq234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazzeroni LC, Lu Y, Belitskaya-Levy I. P-values in genomics: apparent precision masks high uncertainty. Mol Psychiatry. doi: 10.1038/mp.2013.184. In press. http://dx.doi.org/10.1038/mp.2013.184. [DOI] [PMC free article] [PubMed]
- 9.Hindorff LA, Sethupathy P, Junkins HA, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106:9362–7. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panagiotou OA, Willer CJ, Hirschhorn JN, Ioannidis JP. The power of meta-analysis in genome-wide association studies. Annu Rev Genomics Hum Genet. 2013;14:441–65. doi: 10.1146/annurev-genom-091212-153520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce BL, Ahsan H. Genome-wide “pleiotropy scan” identifies HNF1A region as a novel pancreatic cancer susceptibility locus. Cancer Res. 2011;71:4352–8. doi: 10.1158/0008-5472.CAN-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Setiawan VW, Schumacher F, Prescott J, et al. Cross-cancer pleio-tropic analysis of endometrial cancer: PAGE and E2C2 consortia. Carcinogenesis. 2014;35:2068–73. doi: 10.1093/carcin/bgu107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng I, Kocarnik JM, Dumitrescu L, et al. Pleiotropic effects of genetic risk variants for other cancers on colorectal cancer risk: PAGE, GECCO and CCFR consortia. Gut. 2014;63:800–7. doi: 10.1136/gutjnl-2013-305189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solovieff N, Cotsapas C, Lee PH, Purcell SM, Smoller JW. Pleiotropy in complex traits: challenges and strategies. Nat Rev Genet. 2013;14:483–95. doi: 10.1038/nrg3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figueroa JD, Ye Y, Siddiq A, et al. Genome-wide association study identifies multiple loci associated with bladder cancer risk. Hum Mol Genet. 2014;23:1387–98. doi: 10.1093/hmg/ddt519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kote-Jarai Z, Al Olama AA, Giles GG, et al. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat Genet. 2011;43:785–91. doi: 10.1038/ng.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mocellin S, Verdi D, Pooley KA, et al. Telomerase reverse transcriptase locus polymorphisms and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2012;104:840–54. doi: 10.1093/jnci/djs222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fingerlin TE, Murphy E, Zhang W, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45:613–20. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schumacher FR, Berndt SI, Siddiq A, et al. Genome-wide association study identifies new prostate cancer susceptibility loci. Hum Mol Genet. 2011;20:3867–75. doi: 10.1093/hmg/ddr295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eeles RA, Al Olama AA, Benlloch S, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom geno-typing array. Nat Genet. 2013;45:385–91. doi: 10.1038/ng.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panagiotou OA, Ioannidis JP. for the Genome-Wide Significance Project. What should the genome-wide significance threshold be?. Empirical replication of borderline genetic associations. Int J Epidemiol. 2012;41:273–86. doi: 10.1093/ije/dyr178. [DOI] [PubMed] [Google Scholar]
- 22.Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O'Donnell CJ, de Bakker PI. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–9. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenesa A, Farrington SM, Prendergast JG, et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on 11q23 and replicates risk loci at 8q24 and 18q21. Nat Genet. 2008;40:631–7. doi: 10.1038/ng.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eeles RA, Kote-Jarai Z, Giles GG, et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet. 2008;40:316–21. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 26.Thomas G, Jacobs KB, Yeager M, et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40:310–5. doi: 10.1038/ng.91. [DOI] [PubMed] [Google Scholar]
- 27.Hancock DB, Artigas MS, Gharib SA, et al. Genome-wide joint meta-analysis of SNP and SNP-by-smoking interaction identifies novel loci for pulmonary function. PLoS Genet. 2012;8:e1003098. doi: 10.1371/journal.pgen.1003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eeles RA, Kote-Jarai Z, Al Olama AA, et al. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet. 2009;41:1116–21. doi: 10.1038/ng.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gudmundsson J, Sulem P, Steinthorsdottir V, et al. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39:977–83. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 30.Suhre K, Shin SY, Petersen AK, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;477:54–60. doi: 10.1038/nature10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koutros S, Meyer TE, Fox SD, et al. Prospective evaluation of serum sarcosine and risk of prostate cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Carcinogenesis. 2013;34:2281–5. doi: 10.1093/carcin/bgt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manolio TA, Brooks LD, Collins FS. A HapMap harvest of insights into the genetics of common disease. J Clin Invest. 2008;118:1590–605. doi: 10.1172/JCI34772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JH, Wacholder S, Gail MH, et al. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet. 2010;42:570–5. doi: 10.1038/ng.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomlinson I, Webb E, Carvajal-Carmona L, et al. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat Genet. 2007;39:984–8. doi: 10.1038/ng2085. [DOI] [PubMed] [Google Scholar]
- 36.Guarrera S, Ricceri F, Polidoro S, et al. Association between total number of deaths, diabetes mellitus, incident cancers, and haplo-types in chromosomal region 8q24 in a prospective study. Am J Epidemiol. 2012;175:479–87. doi: 10.1093/aje/kwr430. [DOI] [PubMed] [Google Scholar]
- 37.Chung CC, Chanock SJ. Current status of genome-wide association studies in cancer. Hum Genet. 2011;130:59–78. doi: 10.1007/s00439-011-1030-9. [DOI] [PubMed] [Google Scholar]
- 38.Wacholder S, Yeager M, Liao LM. Invited commentary: more surprises from a gene desert. Am J Epidemiol. 2012;175:488–91. doi: 10.1093/aje/kwr429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grisanzio C, Freedman ML. Chromosome 8q24-associated cancers and MYC. Genes Cancer. 2010;1:555–9. doi: 10.1177/1947601910381380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sreekumar A, Poisson LM, Rajendiran TM, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–4. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 41.Pavlou M, Diamandis EP. The search for new prostate cancer biomarkers continues. Clin Chem. 2009;55:1277–9. doi: 10.1373/clinchem.2009.126870. [DOI] [PubMed] [Google Scholar]
- 42.de Vogel S, Ulvik A, Meyer K, et al. Sarcosine and other metabolites along the choline oxidation pathway in relation to prostate cancer— a large nested case-control study within the JANUS cohort in Norway. Int J Cancer. 2014;134:197–206. doi: 10.1002/ijc.28347. [DOI] [PubMed] [Google Scholar]
- 43.Ziegler A, Konig IR, Thompson JR. Biostatistical aspects of genome-wide association studies. Biometr J. 2008;50:8–28. doi: 10.1002/bimj.200710398. [DOI] [PubMed] [Google Scholar]
- 44.Pashayan N, Duffy SW, Chowdhury S, et al. Polygenic susceptibility to prostate and breast cancer: implications for personalised screening. Br J Cancer. 2011;104:1656–63. doi: 10.1038/bjc.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khoury MJ, Janssens AC, Ransohoff DF. How can polygenic inheritance be used in population screening for common diseases? Genet Med. 2013;15:437–43. doi: 10.1038/gim.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.