Abstract
To develop a novel acute graft-versus-host disease (GVHD) Risk Score, we examined the GVHD clinical stage and grade of 1723 patients at the onset of treatment with systemic steroids. Using clinical grouping, descriptive statistics and recursive partitioning, we identified poorly responsive, high-risk (HR) acute GVHD by the number of involved organs and severity of GVHD at onset. The overall response [(complete response/partial response (CR/PR)] rate 28 days after initiation of steroid therapy for acute GVHD was lower in the 269 patients with HR-GVHD than in the 1454 patients with standard risk (SR)-GVHD [44% (95% CI 38–50%) vs. 68% (95% CI 66–70%), p<0.001. Patients with HR-GVHD were less likely to respond at day 28 [odds ratio (OR), 0.3, 95% CI 0.2–0.4, p<0.001], and had higher risks of mortality [relative risk (RR) 2.1, 95% CI 1.7–2.6, P<0.001] and transplant-related mortality (RR 2.5, 95% CI 2.0–3.2%, p<0.001) compared to patients with SR-GVHD. This refined definition of acute GVHD risk is a better predictor of response, survival and transplant-related mortality than other published acute GVHD risk scores. Patients with HR-GVHD are candidates for studies investigating new treatment approaches. Likewise, patients with SR-GVHD are candidates for studies investigating less toxic therapy.
Introduction
Acute graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality after hematopoietic cell transplantation (HCT). Corticosteroids are the standard initial therapy but are effective in only approximately half of the cases.1,2 Methods to identify patients who are unlikely to respond to this conventional initial therapy and who warrant alternative, more effective initial therapy are needed. Earlier analyses have defined high-risk acute GVHD at time of onset by their clinical stage and grade2–5 and more recently by serum biomarkers.6–8
The Minnesota group recently defined high-risk acute GVHD by a novel acute GVHD risk score.9 Initial high-risk (HR) acute GVHD was defined as either skin stage 4; lower gastrointestinal (GI) stage 3–4 or liver stage 3–4; or skin stage 3+ and either lower GI 2–4 or liver stage 2–4 GVHD. Patients with this HR-GVHD were less likely to respond to steroid therapy and had a two-fold increased risk of treatment related (non-relapse) mortality (TRM) compared to patients with standard-risk (SR)-GVHD. In order to validate this new GVHD risk score, we examined a larger, heterogeneous group of patients who received steroids as initial systemic therapy for acute GVHD. A database of 1723 patients was created from 5 cohorts; the previously reported patients from the University of Minnesota (n=864)9, Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Study 0302 (n=155)10, BMT CTN Study 0802 (n=213)11, Hôpital Saint Louis, Paris (n=184) and the University of Michigan (n=307). Patients from Minnesota or Michigan enrolled in the BMT CTN trials (n=33) were counted only once. The distribution of initial GVHD organ staging combinations and subsequent response to therapy was analyzed to predict responses and define HR and SR GVHD.
Patients and Methods
Study Design
Between 1990 and 2007, 1723 allogeneic HCT patients developed grade I–IV acute GVHD and were treated with prednisone 2mg/kg/day or 60 mg/m2 PO (or methylprednisolone 48 mg/m2 IV) as initial therapy and are included in this analysis. All HCT and data collection protocols were reviewed and approved by the Institutional Review Board at the respective HCT centers. De-identified data were compiled for analysis.
Patient and Transplant Characteristics
Patient demographics including year of transplant, recipient age, gender, cytomegalovirus (CMV) serostatus, and underlying diagnosis are shown in Table 1. Median patient age was 40 years (range, 0.2–76) and 24% were <20 years of age. Standard-risk disease was defined as acute leukemia in first or second complete remission, chronic myelogenous leukemia (CML) in first chronic phase, or myelodysplastic syndrome (MDS) without excess blasts. All other diseases were considered high risk.
Table 1.
Patient and Transplant Characteristics
| Factors | N (%) |
|---|---|
| Total | 1723 |
| Year of Transplant | |
| 1990–1995 | 289 (17%) |
| 1996–2000 | 292 (17%) |
| 2001–2005 | 409 (24%) |
| 2006–2007 | 733 (43%) |
| Age | |
| <20 years | 421 (24%) |
| 21–40 years | 459 (27%) |
| 41+ years | 843 (49%) |
| Median (range) | 40 (0.2–76) |
| Gender | |
| Male | 1067 (62%) |
| Female | 656 (38%) |
| Disease | |
| Acute Leukemia | 741 (43%) |
| CML | 217 (13%) |
| CLL/Other Leukemia | 73 (4%) |
| MDS/MPN | 194 (11%) |
| HL/NHL | 236 (14%) |
| Other Malignancies | 69 (4%) |
| SAA/FA | 81 (5%) |
| Immune Deficiency/Hemoglobinopathy | 28 (2%) |
| Inborn Error of Metabolism | 28 (2%) |
| Disease Risk* | |
| Standard | 816 (47%) |
| High | 907 (53%) |
| Donor Type | |
| Matched Sibling BM/PBSC | 598 (35%) |
| Mismatched Related Donor BM/PBSC | 73 (4%) |
| Matched URD BM/PBSC | 626 (36%) |
| Mismatched URD BM/PBSC | 164 (10%) |
| Single or Double UCB | 262 (15%) |
| Conditioning | |
| Myeloablative | 1273 (74%) |
| Reduced Intensity | 450 (26%) |
CML = chronic myelogenous leukemia; CLL = chronic lymphocytic leukemia; MDS = myelodysplastic syndrome; MPN = myeloproliferative neoplasm; HL = Hodgkin lymphoma; NHL = Non-Hodgkin lymphoma; SAA = severe aplastic anemia; FA = Fanconi anemia. BM = bone marrow; PBSC = peripheral blood stem cells; URD = unrelated donor; UCB = umbilical cord blood
Standard risk = acute leukemia in CR1 or CR2, CML in first chronic phase, MDS without excess blasts or non-malignant diseases. High risk = all others.
Transplant characteristics including donor type and preparative therapy are shown in Table 1. Graft sources included HLA-identical sibling bone marrow (BM) or peripheral blood stem cells (PBSC; n = 598), HLA-mismatched related donor BM or PBSC, (n = 73), HLA-matched unrelated donor (URD) BM or PBSC (n = 626), HLA-mismatched URD BM or PBSC (n=164), single or double umbilical cord blood (UCB) (n=262). The majority (74%) of patients received myeloablative conditioning regimens.
GVHD Therapy and Measurement of Response to Prednisone
All patients received prednisone at approximately 2 mg/kg or 60 mg/m2/day PO (or methylprednisolone IV equivalent) as initial therapy, while 353 (20%) received additional agents for initial treatment. Patients enrolled in the BMT CTN 0302 trial received steroids and one of 4 additional agents (etanercept, mycophenolate mofetil (MMF), denileukin diftitox or pentostatin)10. Those enrolled in the BMT CTN 0802 trial received steroids with MMF or placebo11. The median time from HCT to initiation of steroid therapy was 30 days (range 2–178; interquartile range 21–43). All patients had at least 3 months follow-up from GVHD treatment (median 4.9 years, range 0.3–17.7).
Response was determined comparing the initial acute GVHD stage and grade in each organ to the best recorded stage and grade at day 28 (±7 days) after prednisone treatment was started. Complete response (CR) was defined as the complete resolution of acute GVHD manifestations in all organs, without need for secondary GVHD therapy. Partial response (PR) was defined as improvement in GVHD stage in all initially affected organs, without resolution in all organs, worsening in any other GVHD target organs or need for secondary GVHD therapy. No response (NR) was defined as the same severity of GVHD in any organ or death, or the addition of secondary GVHD therapy before day 28. Patients who experienced a flare of acute GVHD before day 28 and required therapy with increased steroids or additional GVHD therapy were also considered to have no response. Progression was defined as worsening GVHD in at least 1 organ with or without improvement in any other organ.
Statistical Analysis
Simple proportions with 95% confidence intervals were used to estimate the rate of overall response defined as CR+PR or CR alone. Survival after GVHD treatment was analyzed by Kaplan-Meier estimates.12 Non-relapse, treatment-related mortality (TRM) was analyzed using cumulative incidence estimates, treating relapse of the underlying HCT diagnosis as a competing risk.13 Outcomes were evaluated with the log-rank test for trend or the simple log-rank test when comparing two categories.
Logistic regression was used to examine the independent association of factors with CR+PR and CR alone at day 28 after onset of GVHD. Cox regression was used to assess the independent association of the high and low-risk categories with the probability of 6 month overall survival,14 and Fine and Gray proportional hazards regression was used to assess the independent association of the categories with risks of TRM and chronic GVHD.15 Clinical groupings, probabilities within organ staging categories and recursive partitioning was used to identify the optimal cut-point among initial acute GVHD stage groupings for response at day 28.16 The Net Reclassification Index (NRI) defined as the net proportion of events assigned to a higher risk category plus the net proportion of nonevents assigned to a lower risk category, was used to assess and quantify the net improvement in risk prediction by our refined acute GVHD risk determination as compared to our original GVHD risk score17 or other GVHD grading schemes2,9,18,19
Results
The initial GVHD stage in each organ is shown in Table 2. Initial GVHD organ involvement was skin only (n = 910; 53%), upper and/or lower GI only (n = 346; 20%), liver only (n = 23; 1%) or multi-organ (n = 556; 25%). Overall response (CR + PR) at day 28 was observed in 1111 of 1723 patients (64%, 95% confidence interval (CI) 62–66%). CR was observed in 843 (49%, 95% CI 47–51) patients and PR in 268 (15%, 95% CI 13–17%). For the entire cohort of 1723 patients, survival at 6 months after initiation of steroid therapy was 68% (95% CI 66–70%), and TRM at 6 months was 26% (95% CI 23–28%).
Table 2.
GVHD Organ Stage and Clinical Grade in 1723 patients at Onset of Treatment
| Organ Stage | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Skin | 400 (23%) | 262 (15%) | 409 (24%) | 639 (37%) | 13 (1%) |
| Liver | 1579 (92%) | 57 (3%) | 52 (3%) | 25 (1%) | 10 (1%) |
| Lower GI | 1197 (69%) | 262 (15%) | 122 (7%) | 122 (7%) | 20 (1%) |
| Upper GI | 1263 (73%) | 460 (27) |
| Initial CIBMTR Grade
|
|
|||
|---|---|---|---|---|
| Initial MN Grade
|
A | B | C | D |
| I | 146 | 280 | 0 | 0 |
| II | 0 | 383 | 570 | 0 |
| III | 0 | 121 | 183 | 7 |
| IV | 0 | 0 | 0 | 33 |
Initial GVHD grades by the Minnesota (MN)2,9 and Center for International Blood and Marrow Transplant Research (CIBMTR) grading systems19 at the initiation of steroid therapy are shown in Table 2. The day 28 CR/PR rate was 67% among patients with CIBMTR grades A–B GVHD and 61% among those with grades C–D GVHD (p = 0.008). The day 28 CR/PR rate was 68% among patients with MN grades I–II GVHD and 50% among those with grades III–IV GVHD (p<0.001).
MN Acute GVHD Risk Score
According to a novel, previously published MN score that combined the MN & CIBMTR grading systems,9 146 (8%) patients were classified as IA, 280 (16%) IB, 383 (22%) IIB, 570 (33%) IIC, 121 (7%) IIIB, 183 (11%) IIIC, 7 (<1%) IIID and 33 (2%) IVD GVHD. This classification was used to categorize standard-risk (SR) and high-risk (HR) groups. The day 28 CR/PR rate was 67% among 1500 patients with SR-GVHD (initial grade IA–IIIB) and 46% among 223 patients with HR-GVHD (initial grade IIIC–IVD) (p<0.001). In multivariate analysis, factors associated with CR/PR at day 28 after starting steroid treatment included GVHD risk group, donor type and organ involvement, but not patient age. Additionally, factors associated with a higher likelihood of survival and lower TRM at 6 months included GVHD risk group, younger patient age, disease risk, donor type and earlier onset of acute GVHD.
GVHD Organ Stage and Outcomes
Recognizing this heterogeneity in GVHD grading, particularly within clinical grades II and III, we then examined the details of initial GVHD organ stage combinations to determine whether stage groupings would better identify the patients at highest risk. We studied the 1723 patients and divided them into 67 categories by organ stage and thus extent of GVHD involvement at onset (Supplemental Table 1). We collapsed these categories into 17 larger categories clustered as clinically similar cohorts with comparable CR/PR at day 28, and evaluated these new GVHD staging categories for CR/PR, survival and TRM (Table 3). We found a clear demarcation between categories according to the CR/PR rate at day 28 and risk of 6-month mortality and TRM, thus dividing the cohort into SR- and HR-GVHD groups.
Table 3.
GVHD Organ Staging Categories
| GVHD Categories Grouped by Day 28 CR/PR | N | Day 28 CR/PR | Odds Ratio of day 28 CR/PR (95% CI) | P Value | Relative Risk of Mortality (95% CI) | P Value | Relative Risk of TRM (95% CI) | P Value | GVHD Risk** |
|---|---|---|---|---|---|---|---|---|---|
| 1. Stage 1–3 Skin only* | 901 | 68% | 1.0 | 1.0 | 1.0 | SR | |||
| 2. UGI only | 115 | 78% | 1.6 (1.0–2.6) | 0.04 | 0.6 (0.4–0.9) | 0.02 | 0.5 (0.3–0.9) | 0.03 | SR |
| 3. Stage 1–2 Lower GI only | 100 | 73% | 1.3 (0.8–2.1) | 0.29 | 1.2 (0.8–1.7) | 0.41 | 1.6 (1.0–2.3) | 0.04 | SR |
| 4. Stage 1–3 Skin + UGI | 90 | 69% | 1.0 (0.6–1.6) | 0.89 | 0.9 (0.6–1.4) | 0.64 | 0.9 (0.6–1.5) | 0.71 | SR |
| 5. Stage 1–3 Skin + Stage 1 Lower GI | 71 | 61% | 0.7 (0.4–1.2) | 0.16 | 1.1 (0.7–1.8) | 0.60 | 1.3 (0.8–2.1) | 0.35 | SR |
| 6. Stage 1 Lower GI + Upper GI | 64 | 64% | 0.8 (0.5–1.4) | 0.42 | 1.2 (0.7–1.9) | 0.54 | 1.4 (0.9–2.4) | 0.18 | SR |
| 7. Stage 1–3 Skin + Stage 1–4 Liver | 51 | 71% | 1.1 (0.6–2.1) | 0.71 | 1.3 (0.8–2.0) | 0.30 | 1.4 (0.9–2.3) | 0.17 | SR |
| 8. Stage 1–3 Skin + Stage 1 Lower GI + Upper GI | 62 | 61% | 0.6 (0.4–0.9) | 0.12 | 0.6 (0.3–1.1) | 0.11 | 0.6 (0.3–1.3) | 0.18 | SR |
|
| |||||||||
| 9. Stage 1–2 Lower GI + Stage 1–3 Liver | 12 | 50% | 0.4 (0.1–1.4) | 0.15 | 2.9 (1.4–6.3) | 0.005 | 3.2 (1.5–7.0) | 0.003 | HR |
| 10. Stage 1–3 Skin + (Stage 1–2 Lower GI or Upper GI) + Stage 1–3 Liver | 23 | 35% | 0.2 (0.1–0.6) | 0.001 | 3.0 (1.7–5.1) | <0.001 | 3.2 (1.8–5.6) | <0.001 | HR |
| 11. Stage 3 Lower GI only | 65 | 55% | 0.5 (0.3–0.9) | 0.02 | 2.1 (1.4–3.1) | 0.003 | 2.7 (1.8–4.2) | <0.001 | HR |
| 12. Stage 1–3 Skin + Stage 2 Lower GI | 54 | 52% | 0.5 (0.3–0.9) | 0.01 | 1.5 (1.0–2.3) | 0.08 | 1.6 (1.0–2.6) | 0.06 | HR |
| 13. Stage 3–4 Lower GI + (Stage 1–3 Skin or Liver Stage 1–4) | 55 | 36% | 0.2 (0.1–0.4) | <0.001 | 2.5 (1.7–3.6) | <0.001 | 3.0 (2.0–4.5) | <0.001 | HR |
| 14. Stage 1–4 Liver alone | 25 | 48% | 0.3 (0.2–0.8) | 0.01 | 1.0 (0.5–2.3) | 0.96 | 1.7 (0.8–3.8) | 0.19 | HR |
| 15. Stage 1–3 Skin + Stage 3–4 Lower GI + Stage 1–4 Liver | 13 | 8% | 0.1 (0.01–0.3) | 0.002 | 4.3 (2.3–8.2) | <0.001 | 7.4 (3.6–15.2) | <0.001 | HR |
| 16. Stage 4 Skin only | 13 | 38% | 0.3 (0.1–0.8) | 0.02 | 0.8 (0.2–3.1) | 0.71 | 2.2 (0.7–6.9) | 0.16 | HR |
| 17. Stage 4 Lower GI only | 22 | 22% | 0.1 (0.03–0.7) | 0.02 | 3.5 (1.5–7.9) | 0.003 | 3.1 (1.2–8.1) | 0.02 | HR |
reference group; regression analysis includes adjustment for donor type [HLA-matched sibling vs. other related vs. umbilical cord blood vs. HLA-matched unrelated donor (URD) vs. HLA-mismatched URD], days to acute GVHD (< 28 days vs. ≥ 28 days), center/dataset (Minnesota vs. BMTCTN 0302 vs. BMTCTN 0802 vs. Paris vs. Michigan), age (<21 vs. 21–40 vs. 41+).
SR = standard risk; HR = high risk (categories assigned by recursive partitioning for all 3 endpoints)
Refined Acute GVHD Risk Score
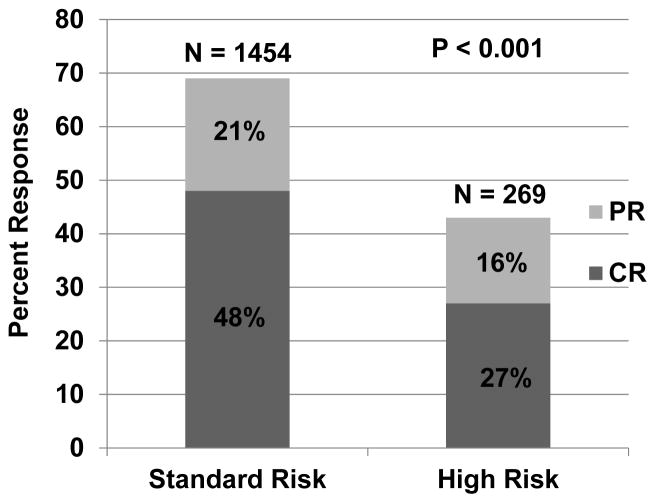
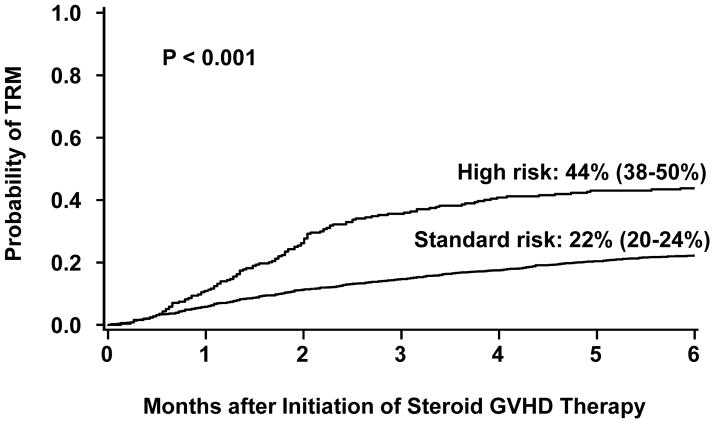
This refined definition of SR-GVHD includes single organ involvement (either stage 1–3 skin or stage 1–2 GI) or two organ involvement (either stage 1–3 skin plus stage 1 GI; or stage 1–3 skin plus stage 1–4 liver). All other patients are considered high risk. Table 4 describes the stage and organ involvement (and subgroup frequency) by risk group for the patients in this analysis. The day 28 CR/PR rate was lower in the 269 patients with HR-GVHD than in the 1454 patients with SR-GVHD [44% (95% CI, 38–50%) vs. 68% (95% CI, 66–70%) CR/PR, p<0.001, Figure 1]. At day 28, the CR rates in HR- and SR-GVHD cohorts were 27% (95% CI 22–33%) and 48% (95% CI 45–51%); PR rates were 16% (95% CI 12–21%) and 21% (95% CI 19–23%). Additionally, the 6-month incidence of TRM was twice as high in patients with HR-GVHD as in those with SR-GVHD [44% (95% CI 38–50%) vs. 22% (95% CI 20–24%), p<0.001, Figure 2]. Finally, survival at 6 months after the onset of steroid treatment was lower in patients with HR-GVHD than in those with SR-GVHD [52% (95% CI 46–58%) vs 71% (95% CI 69–73%), p<0.001].
Table 4.
GVHD Risk Definition by Organ Stage at Onset
| GVHD Risk Score | One Organ (n) | Two Organs (n) | Three Organs (n) |
|---|---|---|---|
| Standard Risk (N=1454, 84%) | Stage 1–3 Skin (901) | Stage 1–3 Skin plus Stage 1 GI (223)† | -- |
| Stage 1–2 GI (279)** | Stage 1–3 Skin plus Stage 1–4 Liver (51) | -- | |
|
| |||
| High Risk* (N=269, 16%) | Stage 4 Skin (13) | Stage 1–3 Skin plus Stage 2 GI (54) | Stage 1–3 Skin plus Stage 1–2 GI plus Stage 1–3 Liver (23) |
| Stage 3–4 GI (74)†† | Stage 1–2 Lower GI plus Stage 1–3 Liver (12) | Stage 1–3 Skin plus Stage 3–4 GI plus Stage 1–4 Liver (13) | |
| Stage 1–4 Liver (25)*** | Stage 3–4 GI plus Stage 1–3 Skin (45) Stage 3–4 GI plus Stage 1–4 Liver (10) |
||
UGI plus Lower GI considered as single organ disease
For high risk disease, the degree of organ involvement is the minimum necessary to be deemed high risk. Patients with higher stage of GVHD than observed in the high-risk group should also be considered high risk.
- UGI alone (n=115)
- Stage 1–2 Lower GI alone (100)
- UGI and Stage 1 Lower GI (64)
- Stage 3 Lower GI alone (65)
- Stage 4 Lower GI alone (9)
- Stage 1 Liver alone (7)
- Stage 2 Liver alone (10)
- Stage 3 Liver alone (5)
- Stage 4 Liver alone (3)
- Stage 1–3 Skin plus UGI (90)
- Stage 1–3 Skin plus Stage 1 Lower GI (71)
- Stage 1–3 Skin plus UGI and Stage 1 Lower GI (62)
Figure 1.
Day 28 response by risk group. CR = complete response. PR = partial response.
Figure 2.
Cumulative incidence (+/− 95% CI) of transplant related mortality at 6 months after onset of steroid therapy according to risk group.
In multiple regression analysis adjusted for clinically significant variables in addition to the risk group, the probability of CR/PR at day 28 in patients with HR-GVHD was lower than in those with SR-GVHD [Odds ratio (OR) 0.3, 95% CI 0.2–0.4, p<0.001, Table 5]. Donor type was the only other factor associated with response. Patients who received a graft from an HLA-matched (OR 0.7, 95% CI 0.6–0.9, p=0.01) or mismatched URD (OR 0.3, 95% CI 0.2–0.5 p<0.001) were less likely to respond than those who received either a related donor or UCB graft. The number of days from HCT to GVHD onset, patient age, original center/study cohort and other factors showed no statistically significantly association with response.
Table 5.
Factors Associated with Day 28 CR/PR, 6 Month Mortality and TRM: Multivariate Analysis
| Factors | N | Odds Ratio of day 28 CR/PR (95% CI) | P | Relative Risk of Mortality (95% CI) | P | Relative Risk of TRM (95% CI) | P |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| <20* | 421 | 1.0 | 1.0 | 1.0 | |||
| 21–40** | 459 | 0.8 (0.6–1.1) | 0.10 | 1.3 (1.0–1.8) | 0.03 | 1.6 (1.2–2.1) | 0.004 |
| 41+ | 843 | 0.8 (0.6–1.1) | 0.15 | 1.7 (1.3–2.3) | <0.001 | 2.4 (1.8–3.3) | <0.001 |
| Disease Risk† | |||||||
| Standard* | 812 | 1.0 | 1.0 | 1.0 | |||
| High | 717 | 1.1 (0.9–1.4) | 0.45 | 1.2 (1.0–1.5) | 0.05 | 1.1 (0.9–1.3) | 0.52 |
| Non–malignant | 191 | 0.9 (0.6–1.3) | 0.47 | 1.06 (0.8–1.5) | 0.74 | 1.8 (1.3–2.5) | 0.001 |
| Conditioning Regimen | |||||||
| Myeloablative* | 1273 | 1.0 | 1.0 | 1.0 | |||
| Reduced Intensity | 450 | 1.2 (0.9–1.6) | 0.24 | 1.0 (0.8–1.2) | 0.78 | 0.9 (0.6–1.1) | 0.25 |
| Donor Type | |||||||
| Matched Sibling* | 598 | 1.0 | 1.0 | 1.0 | |||
| Other related | 73 | 0.9 (0.5–1.5) | 0.62 | 1.5 (1.0–2.2) | 0.07 | 1.6 (1.0–2.6) | 0.42 |
| Matched URD | 626 | 0.7 (0.6–0.9) | 0.01 | 1.5 (1.2–1.8) | <0.001 | 1.6 (1.2–2.0) | <0.001 |
| Mismatched URD | 164 | 0.3 (0.2–0.5) | <0.001 | 2.2 (1.6–2.9) | <0.001 | 2.4 (1.7–3.2) | <0.001 |
| UCB | 262 | 0.9 (0.7–1.3) | 0.45 | 1.1 (0.8–1.4) | 0.67 | 1.0 (0.7–1.4) | 0.81 |
| Days from HCT to Initial Steroid Rx | |||||||
| <28 days* | 717 | 1.0 | 1.0 | 1.0 | |||
| ≥28 days | 1006 | 1.2 (1.0–1.5) | 0.06 | 0.8 (0.7–0.9) | 0.006 | 0.8 (0.7–1.0) | 0.02 |
| GVHD Risk†† | |||||||
| Standard Risk* | 1454 | 1.0 | 1.0 | 1.0 | |||
| High Risk | 269 | 0.3 (0.2–0.4) | <0.001 | 2.1 (1.7–2.6) | <0.001 | 2.5 (2.0–3.2) | <0.001 |
reference group
comparison of 21–40 vs. 41+: for CR/PR, P=0.27; for mortality, P=0.02; for TRM, P<0.001.
Standard-risk indicates acute leukemia in CR1 or CR2, CML in first chronic phase, MDS without excess blasts, or non-malignant disease. High risk indicates all others.
As defined in Table 3 with Standard Risk = single organ involvement (stage 1–3 skin or stage 1–2 GI) or two organ involvement (stage 1–3 skin plus stage 1 GI; or stage 1–3 skin plus stage 1–4 liver). All other patients are High Risk.
In multivariate analysis, factors associated with CR/PR were also associated with 6-month survival and TRM. These included patient age, disease risk, donor type, time to onset of acute GVHD and the new, refined GVHD risk group. Patients with HR-GVHD had a 2-fold increase in risk of mortality (RR, 2.1, 95% CI, 1.7–2.6, p<0.001) and a 2.5 increased risk of TRM (RR 2.5, 95% CI, 2.0–3.2, p<0.001) compared to patients with SR-GVHD. Risks of mortality and TRM were also significantly higher in older patients, recipients of HLA-matched or mismatched URD grafts and in those with onset of GVHD within 28 days after HCT (Table 5).
The refined GVHD risk score more effectively redistributes patients based on their predicted outcomes compared to the original GVHD Risk score, the CIBMTR or the MN grading systems (Table 6). Seventy-six of 1500 (5%) SR patients in the original scoring system, 72 of 930 (8%) patients with CIBMTR grades A–B and 14 (1%) of the 1379 patients with MN I–II GVHD were reclassified as HR according to this refined scoring system. More strikingly, 30 of 223 (13%) HR patients from the original scoring system, 596 of 793 (75%) patients with CIBMTR grades C–D and 89 of 344 (26%) of MN grade III–IV were reclassified as SR according to this refined scoring system.
Table 6.
GVHD Risk Classification by Different Scoring Systems
| Original Acute GVHD Risk Score9 | CIBMTR Grading20 | MN Grading18,19 | N (%) | ||||
|---|---|---|---|---|---|---|---|
| Refined Acute GVHD Risk Score | Standard Risk | High Risk | Standard Risk (A–B) | High Risk (C–D) | Standard Risk (I–II) | High Risk (III–IV) | |
| Standard Risk | 1424 | 30 | 858 | 596 | 1365 | 89 | 1454 (84%) |
| High Risk | 76 | 193 | 72 | 197 | 14 | 255 | 269 (16%) |
| Total | 1500 (87%) | 223 (13%) | 930 (54%) | 793 (46%) | 1379 (80%) | 344 (20%) | |
As shown in Table 7, the refined GVHD risk score better demarcates day 28 CR/PR (SR 68% vs. HR 44%, p <0.001) compared to our initial GVHD risk score (SR 67% vs. HR 46%, p<0.001), CIBMTR grading system (A–B 67% vs. C–D 61%, p=0.008), or the MN GVHD grading system (I–II 68% vs. III–IV 50%, p <0.001). Similarly, our new refined risk classification better predicts 6-month survival (SR 71% vs HR 53%, p <0.001) compared to our original GVHD risk score (SR 70% vs. HR 54%, p<0.001), the CIBMTR grading system (A–B 71% vs. C–D 65%, p = 0.008) or the MN grading system (grade I–II 71% vs. grade III–IV 56%, p <0.001). TRM at 6 months was similar using this refined risk score (SR 22% vs. HR 43%, p <0.001) and the original GVHD risk score (SR 23% vs. HR 42%, p<0.001), but is better delineated than with the CIBMTR grading system (A–B 23% vs. C–D 29%, p = 0.006) or the MN grading system (grade I–II 22% vs. grade III–IV 40%, p <0.001). As measured by the net reclassification index (NRI), our refined definition of GVHD improves both the true-positive and false-positive rates among our study population. The net percentage of patients more appropriately reclassified both in the positive and negative direction (e.g. response and no response) is 5% (p<0.001) for response and 4% for 6-month survival (p<0.001) compared to our original definition of GVHD risk. Importantly, the NRI is 6% (p<0·001) for response, 4% for 6-month survival (p=0·002) and 5% for 6-month TRM (p<0·001) compared to the MN grading system and is a better discriminant of outcome for all endpoints than the NRI of our original GVHD risk score and MN grading system.
Table 7.
Outcomes Based on Published GVHD Grading Systems
| GVHD Grading System | N | CR/PR at day 28 | P | 6 month survival | P | 6 month TRM | P | ||
|---|---|---|---|---|---|---|---|---|---|
| CIBMTR | |||||||||
| A–B | 930 | 626 (67%) | 660 (71%) | 213 (23%) | |||||
| C–D | 793 | 485 (61%) | 0·008 | 515 (65%) | 0·008 | 228 (29%) | 0·006 | ||
| MN | |||||||||
| I–II | 1379 | 938 (68%) | 984 (71%) | 304 (22%) | |||||
| III–IV | 344 | 173 (50%) | <0·001 | 192 (56%) | <0·001 | 137 (40%) | <0·001 | ||
| Original GVHD Risk Score | |||||||||
| Standard risk | 1500 | 1008 (67%) | 1055 (70%) | 347 (23%) | |||||
| High risk | 223 | 103 (46%) | <0·001 | 121 (54%) | <0·001 | 94 (42%) | <0·001 | ||
| Refined GVHD Risk Score | |||||||||
| Standard risk | 1454 | 993 (68%) | 1039 (71%) | 326 (22%) | |||||
| High risk | 269 | 118 (44%) | <0·001 | 137 (53%) | <0·001 | 115 (43%) | <0·001 | ||
| Number changed from SR-to HR-GVHD | Number changed from HR-to SR-GVHD | % Reclassified | % Reclassified | % Reclassified | |||||
| NRI* (Refined vs. Original) | 76 | 30 | 5% | <0·001 | 4% | <0·001 | 0% | 0·21 | |
| NRI* (Refined vs. MN) | 14 | 89 | 6% | <0·001 | 4% | 0·002 | 5% | <0·001 | |
| NRI* (Refined vs. CIBMTR) | 72 | 596 | 19% | <0.001 | 7% | 0.001 | 14% | <0.001 | |
| NRI* (Original vs. MN) | 0 | 121 | 1% | 0·74 | 2% | 0·02 | 4% | 0·001 |
Net reclassification Index (NRI) defined as the net % of patients more appropriately reclassified both in positive and negative direction (e.g. response and no response).17 The p-value is comparing against the null hypothesis that there is no net benefit.
Discussion
In our previous analysis of GVHD scoring systems, we observed that the heterogeneity of the CIBMTR grades B and C (which include MN grades I–III) contributed to weak predictive utility for GVHD therapy outcomes.9 We have, therefore, derived a combined scoring system, which better identified HR-GVHD patients who need more intensive initial therapy than prednisone alone.9 In order to develop this refined GVHD risk score, we included independent data from 2 prospective BMT CTN trials as well as from 2 other large HCT centers (Hôpital Saint Louis, Paris, and the University of Michigan). The refined definition of SR and HR groups based upon initial GVHD stage serves as a better predictor of response and survival than either the previous risk score which was based upon initial GVHD grade9 or the MN or CIBMTR grading systems. Patients in the HR group were much less likely to respond to initial steroid therapy and had at least 2-fold increased risk of mortality and TRM than those in the SR group. Outcomes are poor for patients with HR-GVHD treated with steroids alone, and these patients should be considered for enrollment in clinical trials testing new approaches for treatment of acute GVHD. Conversely, those with SR-GVHD should be considered for studies using therapeutic approaches designed to limit risks of treatment toxicity or risks of chronic GVHD.
Our refined definition of GVHD risk is confounded by a few small subsets of patients. Twenty-five (1%) patients with stage 1–4 liver disease alone were deemed HR-GVHD, as their outcomes were universally poor. However, 51 (3%) patients with stage 1–3 skin plus stage 1–4 liver had favorable outcomes and were scored SR-GVHD. Although this discrepancy cannot be fully explained, patients with liver only disease consistently had poor outcomes and should still be considered as having high risk while raising questions about the validity of their GVHD diagnoses compared to non-recognized alternative hepatic syndromes complicating the diagnosis of liver only GVHD. Ten patients (<0.5%) did not easily fit into any category as shown in the supplemental figure and were included within a clinically similar category. Additionally, not all potential organ stage combinations and permutations were observed in our cohort of 1723 patients. Given the poor outcomes of patients in the HR-GVHD category, future patients presenting with higher stage involvement than those observed should also be considered high risk.
We chose 2 GVHD risk groups because when we examined CR/PR at day 28, as well as mortality and TRM, 2 groups became apparent; a large group consisting of 84% of the patients with similar outcomes and a smaller group comprised of 16% of the patients with far worse outcomes. Within the large SR-GVHD group, there was no definable staging cohort with better outcomes. For instance the CR/PR rate of the 426 patients with stage 1–2 skin only GVHD (69%) was the same as the 475 patients with stage 3 skin only GVHD (67%). Therefore, although one might consider 3 groups to be more practical (i.e. low, standard and high risk), the data showed that there were 2 GVHD risk groups not 3 or more.
We also chose to use the entire cohort for analysis rather than divide into a training and validation set. Even in this largest data set ever analyzed for GVHD staging and grading characteristics, some of the identified groups are small. Subdivision of the population further would have either missed these cohorts entirely or limited our ability to classify them correctly. Future, independent analyses will thus better be able to confirm or refine the utility of this new classification.
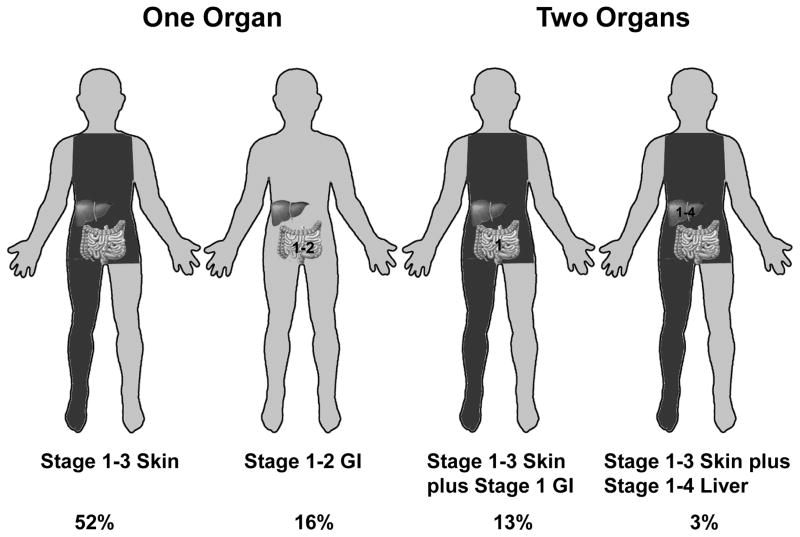
It is simple to define SR-GVHD in our cohort (Table 4; Figure 3). These 84% patients were defined by single organ involvement (stage 1–3 skin or stage 1–2 GI) or two organ involvement (stage 1–3 skin plus stage 1 GI; or stage 1–3 skin plus stage 1–4 liver). Since our definition of HR-GVHD includes 9 distinct subgroups, we more simply define HR-GVHD as those who are not SR. These remaining 16% of patients had HR-GVHD characterized as having either severe single-organ involvement or complex multi-organ GVHD. We have designed a free web-based program to easily determine the GVHD risk group for a given patient using our refined risk score available at: http://z.umn.edu/MNAcuteGVHDRiskScore.
Figure 3.
Organ stages defining high-risk acute GVHD with % involved of total cohort of 1723 patients.
As more than 25,000 people worldwide develop acute GVHD annually, our new scoring system would reclassify 20,750 cases (83%) if CIBMTR grading used and 6750 (27%) if MN grading system used, allowing for more appropriate therapy upfront. Biomarkers have been recently studied as a means to identify HR-GVHD patients but their prognostic capability varies and no validated biomarker test is currently available for real time decision making.6,7,8,21 By contrast, this new revised clinical scoring system is available to clinicians at the bedside in real time. A future prospective study to examine this GVHD score along with informative biomarkers in the same GVHD population could help further refine the prognostic capability of each and determine whether a combination of clinical score and biomarker levels could even better identify HR-GVHD.
Supplementary Material
Supplemental Table 1: Initial GVHD Organ Staging Categories
High risk (HR)-GVHD patients less likely to respond to steroids vs standard risk(SR).
Patients with HR-GVHD have higher risks of mortality and TRM vs SR-GVHD patients.
Refined GVHD risk score better predicts outcomes vs published GVHD risk scores.
Patients with HR-GVHD are candidates for novel treatment approaches.
Patients with SR-GVHD are candidates for studies investigating less toxic therapy.
Acknowledgments
The authors thank the nurses, nurse coordinators, and physicians who cared for these patients and their families. In addition, we gratefully acknowledge the research nurses at all sites whose dedicated efforts facilitated the prospective collection of the GVHD data. We also thank the patients and their families for participating in the clinical research trials.
Footnotes
Authorship
M.L.M. and D.J.W. were responsible for the study concept and design. T.E.D. was responsible for the statistical design and analysis. M.L.M., M.R. A.C.H., A.A., V.T.H., J,B-M, J.L.M.F., R.J., M.A., B.R.B., S.G.H., D.J., M.P., G.S., J.H.A., J.E.L., AND D.J.W were responsible for patient accrual, and care. M.L.M. drafted the manuscript. All authors reviewed, edited and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Financial Disclosure: This study was supported in part by the National Institutes of Health, National Cancer Institute grant 2P01CA065493 and the National Institutes of Health, Blood and Marrow Transplant Clinical Trials Network grant BMT CTN U10HL069290.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–30. [PubMed] [Google Scholar]
- 2.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–94. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 3.Martino R, Romero P, Subira M, et al. Comparison of the classic Glucksberg criteria and the IBMTR Severity Index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. International Bone Marrow Transplant Registry Bone Marrow Transplant. 1999;24:283–7. doi: 10.1038/sj.bmt.1701899. [DOI] [PubMed] [Google Scholar]
- 4.Weisdorf DJ, Hurd D, Carter S, et al. Prospective grading of graft-versus-host disease after unrelated donor marrow transplantation: a grading algorithm versus blinded expert panel review. Biol Blood Marrow Transplant. 2003;9:512–8. doi: 10.1016/s1083-8791(03)00162-9. [DOI] [PubMed] [Google Scholar]
- 5.Cahn JY, Klein JP, Lee SJ, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106:1495–500. doi: 10.1182/blood-2004-11-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine JE, Logan BR, Wu J, et al. Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: a Blood and Marrow Transplant Clinical Trials Network study. Blood. 2012;119:3854–60. doi: 10.1182/blood-2012-01-403063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vander Lugt MT, Braun TM, Hanash S, et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N Engl J Med. 2013;369:529–39. doi: 10.1056/NEJMoa1213299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine JE, Harris AC, Taylor A, Braun TM, Magenau J, Ferrara JLM. A biomarker-based grading system at onset of GVHD predicts TRM better than the modified Glucksberg Grading System. Blood. 2013;122:145. [Google Scholar]
- 9.MacMillan ML, Defor TE, Weisdorf DJ. What predicts high risk acute graft-versus-host disease (GVHD) at onset?: identification of those at highest risk by a novel acute GVHD risk score. Br J Haematol. 2012;157:732–41. doi: 10.1111/j.1365-2141.2012.09114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–7. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolanos-Meade J, Logan BR, Alousi AM, et al. A Multi-Center, Randomized, Double Blind, Phase III Clinical Trial Comparing Steroids/Placebo Vs. Steroids/Mycophenolate Mofetil As Initial Therapy for Acute Graft-Versus-Host Disease. Blood and Marrow Transplant Clinical Trials Network Study 0802; BMT Tandem Meetings; Salt Lake City, Utah. 2013. [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–81. [Google Scholar]
- 13.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Statistics in Medicine. 1997;16:901–10. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Cox DR. Regression models and life tables. Journal of the Royal Stastistical Society. 1972:187–220. [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 16.Breiman L, Freidman J, Olshen R, Stone C. Classification and Regression Trees. Belmont, CA: Wadsworth and Brooks; 1984. [Google Scholar]
- 17.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 19.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–64. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 20.Weisdorf DJ, Snover DC, Haake R, et al. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood. 1990;76:624–9. [PubMed] [Google Scholar]
- 21.Paczesny S. Discovery and validation of graft-versus-host disease biomarkers. Blood. 2013;121:585–94. doi: 10.1182/blood-2012-08-355990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Initial GVHD Organ Staging Categories