Abstract
Background
Hospital-acquired infections are a major cause of morbidity and mortality in acute ischemic stroke patients. While prior scoring systems have been developed to predict pneumonia in ischemic stroke patients, these scores were not designed to predict other infections. We sought to develop a simple scoring system for any hospital-acquired infection.
Methods
Patients admitted to our stroke center (07/08-06/12) were retrospectively assessed. Patients were excluded if they had an in-hospital stroke, unknown time from symptom onset, or delay from symptom onset to hospital arrival >48 hours. Infections were diagnosed via clinical, laboratory, and imaging modalities using standard definitions. A scoring system was created to predict infections based on baseline patient characteristics.
Results
Of 568 patients, 84 (14.8%) developed an infection during their stays. Patients who developed infection were older (73 vs. 64, p<0.0001), more frequently diabetic (43.9% vs. 29.1%, p=0.0077), and had more severe strokes on admission (National Institutes of Health Stroke Scale 12 vs. 5, p<0.0001). Ranging from 0-7, the overall infection score consists of age ≥ 70 (1 point), history of diabetes (1 point), and National Institutes of Health Stroke Scale (0-4 conferred 0 points, 5-15 3 points, >15 5 points). Patients with an infection score of ≥4 were at 5 times greater odds of developing an infection (OR 5.67, 95% CI 3.28-9.81, p<0.0001).
Conclusion
In our sample, clinical, laboratory, and imaging information available at admission identified patients at risk for infections during their acute hospitalizations. If validated in other populations, this score could assist providers in predicting infections after ischemic stroke.
Keywords: infection, acute ischemic stroke, outcome, risk factors, modeling
Introduction
Hospital-acquired infections (HAI) are a major cause of morbidity and mortality among patients admitted with an ischemic stroke.[1] The National Healthcare Safety Network defines a HAI, or nosocomial infection, as a “localized or systemic condition that is preventable and results from an adverse reaction to the presence of an infectious agent with no evidence that the infection was present or incubating at the time of admission to the acute care setting”.[2] Post-ischemic stroke infections are particularly problematic because they increase the risk of death and disability after discharge through fever, immobilization of the patient, and end-organ damage resulting from shock.[3][4] Furthermore, infections are known to complicate ischemic stroke recovery by increasing hospital costs and by prolonging hospitalization.[5][6][7]
While patient-related factors such as stroke severity and age are indicators of outcome following acute ischemic stroke (AIS), HAIs may also play a long-term role.[8][9][10] Prior research has shown that infections present on admission (POA) are not associated with poor outcomes after ischemic stroke, whereas HAIs are a major contributor to poor functional outcomes.[11] Urinary tract infection (UTI), pneumonia (PNA), and bacteremia are among the most common types of HAI, presenting a barrier to long term recovery in this population.[6][12] Together, these three infection types comprise nearly half of all HAIs.13]
Early identification of risk factors for infection during hospitalization for AIS is important, considering effective management may prevent the development of a HAI and subsequently improve long-term outcomes, although this remains controversial.[14][15][16] Prior research has shown that infections occur more commonly in the acute phase following ischemic stroke.[17] Several investigators have identified risk factors for hospital-acquired PNA after stroke and prediction models have been generated,[18][19] but to date, no model has been generated to assess the risk of any nosocomial infection. The purpose of this study was to develop a simple risk prediction model useful in a broad spectrum of infection subtypes in patients hospitalized for AIS.
Methods
Study Population
A retrospective analysis of previously collected data of all patients with AIS who presented to our single academic institution between July 2008 and June 2012 was performed using previously described methods.[20] The registry includes demographic variables, baseline clinical, laboratory, medication, and imaging variables, as well as inpatient clinical, laboratory, medication, and imaging variables on all patients admitted with a stroke. Patients were excluded if they were last seen normal >48 hours prior to admission, had an unknown time of last seen normal, were transferred to our center from an outside hospital, experienced an in-hospital stroke, or had an infection present on admission (defined as an infection diagnosed within the first 24 hours of admission).
Definition of Outcomes
All-cause HAIs were defined as any type of bacterial, fungal, or viral infection. Infection types not included in the detailed scoring mechanisms (e.g. cellulitis, pseudomembranous colitis, meningitis, ventriculitis) were diagnosed clinically or via laboratory/imaging findings. Subsequent analyses were performed to assess predictors of UTI, PNA, and bacteremia. UTIs were defined as >100,000 colony forming units per millimeter of urine in a patient with signs and symptoms. Urinalysis, which is ordered for all patients on admission as part of a standardized order set, distinguishes HAI from bacteriuria present on admission. PNA was defined as an infiltrate on chest radiography with appropriate clinical correlates, but is considered present on admission (and therefore excluded) if identified on baseline chest radiography, which is also part of a standardized admission order set.[21] Bacteremia was defined as >100,000 colony forming units in at least two venous blood samples (excluding contaminants). We considered coagulase-negative staphylococci, diphtheroids, Micrococcus spp., Bacillus spp., and Viridans group streptococci as contaminants if these bacteria did not grow out of all available blood culture vials from a given date and time (e.g. if only one out of two blood culture vials speciated the organism).
Statistical Analysis
We compared admission variables of interest between patients who contracted a HAI and those who did not contract a HAI. Pearson Chi-Square (or Fisher's exact test where appropriate) was used to compare proportions. The Wilcoxon Rank Sum test was used to compare medians of continuous data. A prediction score for HAIs was created by dividing the patient sample into a random sample of 55% of the dataset (build group). The remaining 45% constituted the test group. Once the score was tested in the test group, the score was tested in the entire dataset. Logistic regression models were used to assess the association between admission variables and the outcome of interest, HAI. Every variable collected at the time of admission as part of the registry was tested in a univariable logistic regression model to assess whether it was an independent predictor of HAIs. Independent predictors of HAIs (e.g. age, history of diabetes) with p-values ≤0.2 were considered for the final score as score variables and were evaluated at different values and dichotomizations by calculating the sensitivity and specificity of each binary exposure. Further testing on the categorized variable through crude logistic regression models to identify cutoff points was conducted. Each continuous variable was evaluated using receiver operator characteristics (ROC) curves. Spearman's correlation and ROC curves were used to evaluate the final score. The points assigned to the variables in the score were determined using the beta coefficients from the final adjusted logistic regression model for predicting all-cause infections. This process was repeated to create a prediction score for UTIs, PNA and bacteremia. Logistic regression was then used to assess what prediction score cut off was most predictive of each outcome of interest. As this was an exploratory analysis, no adjustments were made for multiple comparisons.[22] An alpha of 0.05 was used as the level of significance.
Results
Baseline Characteristics
Of the 568 patients included in this study, 84 (14.8%) were found to have a HAI. Of these patients, 56 (66.7%) developed a UTI, 28 (33.3%) developed PNA, and 20 (23.8%) developed bacteremia. These infection groups were not mutually exclusive as 20 patients (23.8%) in our cohort with HAI experienced more than one HAI during admission. In the multivariable models, an age of greater than or equal to 70 years old on admission was a significant independent predictor of HAI (OR 2.49, 95%CI 1.55-4.00, p=0.0002). History of diabetes was also a significant independent predictor of HAI (OR 1.91, 95%CI 1.18-3.09, p=0.0084). We categorized baseline NIHSS into three categories (NIHSS 0-7, 8-14, >14) as reported in a prior prognostic study,[23] which was also found to be significantly higher in patients with HAIs than patients without HAIs (OR=2.10, 95% CI 1.60-2.77, p<0.0001).
HAI Prediction Score
In the HAI model, history of diabetes met the <0.2 univariable p-value cut off. Glucose on admission was not included in the final prediction model because of colinearity with history of diabetes. History of diabetes was selected over admission glucose due to better sensitivity and specificity in predicting HAIs. The cutoff age was determined by testing different cut-points in the final multivariable model to assess which threshold was more predictive in the total model. NIHSS on admission categories were determined using previously established categories.[23] The final prediction score for HAIs is shown in Table 2. The HAI Prediction Score ranged from 0 to 7 and produced an area under the curve (AUC) of 0.7311. Using the entire cohort, 76.8% of patients with a HAI Prediction Score greater than or equal to 4 developed a HAI. Figure 2 illustrates the distribution of the HAI Prediction Score among patients without HAI compared to patients who developed a HAI. Additionally, the odds of a patient with a HAI Prediction Score greater than or equal to 4 of developing a HAI was nearly 6 times the odds of patients with HAI Prediction Scores of 0-3 of developing a HAI (OR 5.67 95% CI 3.28-9.81, p<0.0001).
Table 2. Characteristics of the Overall HAI score, the UTI prediction score, the pneumonia prediction score and the bacteremia prediction score.
| Score | Variables Included | ROC Characteristics |
|---|---|---|
| HAI Prediction Score (Range 0-7) | AUC 0.731 | |
| Age ≥ 70= 1 point History of Diabetes= 1 point Baseline NIHSS 4-15= 3 points Baseline NIHSS >15 = 5points |
||
| UTI Infection Score (Range 0-7) | AUC 0.687 | |
| Age ≥ 70= 1 point History of Diabetes= 1 point Baseline NIHSS 4-15= 3 points Baseline NIHSS >15 = 5points |
||
| Pneumonia Infection Score (Range 0-7) | AUC 0.786 | |
| Age ≥ 70= 1 point History of Diabetes= 1 point Baseline NIHSS 4-15= 3 points Baseline NIHSS >15 = 5points |
||
| Bacteremia Infection Score (Range 0-8) | AUC 0.689 | |
| Age ≥ 70= 1 point History of Diabetes= 1 point Baseline NIHSS 4-15= 3 points Baseline NIHSS >15 = 5points History of atrial fibrillation= 1 point |
Figure 2. Distribution of HAI prediction Score.

UTI Prediction Score
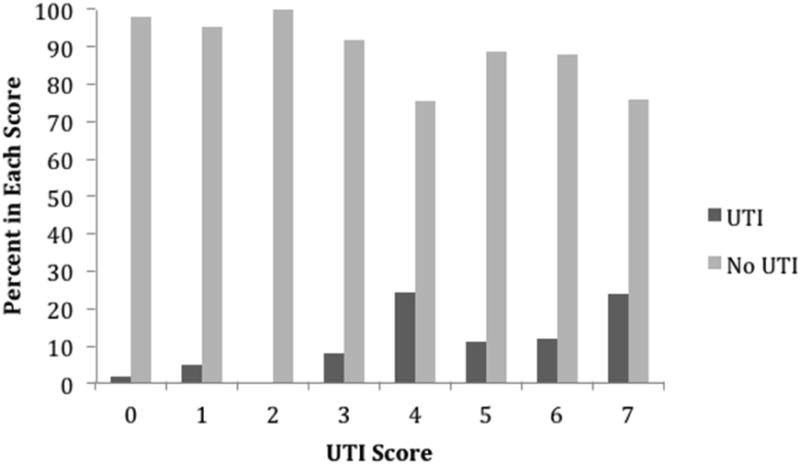
In the UTI model, history of diabetes met the <0.2 univariable p-value cut off. The previously described method for creating a HAI prediction score was applied to patients with UTI.[23] The final prediction score for UTI is shown in Table 2. The UTI Prediction Score ranged from 0 to 7 and produced an AUC of 0.6867 (Figure 1). Figure 3 illustrates the distribution of the UTI Prediction Score among patients without nosocomial UTI compared to patients who developed a nosocomial UTI. The odds of a patient with a UTI Prediction Score greater than or equal to 4 of developing a nosocomial UTI was nearly 5 times the odds of patients with UTI Prediction Scores of 0-3 of developing a nosocomial UTI (OR 4.84 95% CI 2.53-9.28, p<0.0001).
Figure 1. Receiver Operating Characteristic Curve for the HAI Predictor Score, the UTI Prediction Score, the Pneumonia Prediction score and the bacteremia prediction score.

Figure 3. Distribution of UTI Prediction Score.
PNA Prediction Score
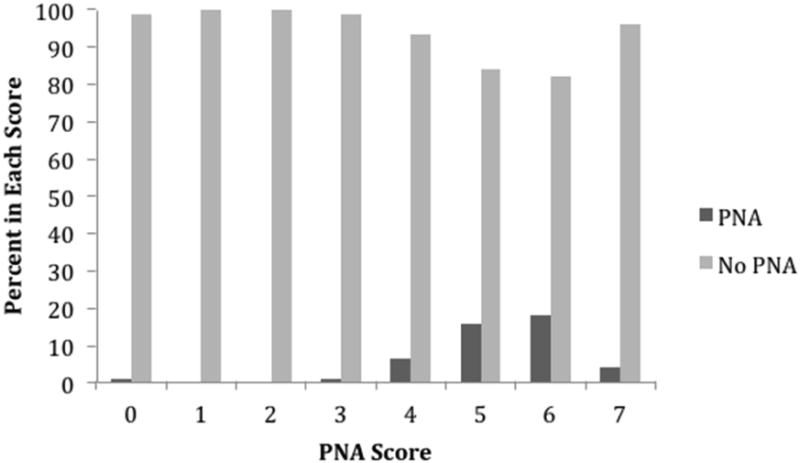
In the PNA model, history of diabetes met the <0.2 univariable p-value cut off. The previously described method for creating HAI and UTI prediction scores was applied to patients with PNA.[23] The final prediction score for PNA is shown in Table 2. The PNA Prediction Score ranged from 0 to 7 and produced an AUC of 0.7862 (Figure 1). Figure 4 illustrates the distribution of the PNA Prediction Score among patients without nosocomial PNA compared to patients who developed a nosocomial PNA. The odds of a patient with a PNA Prediction Score greater than or equal to 5 of developing nosocomial PNA was 7 times the odds of patients with PNA Prediction Scores of 0-4 of developing nosocomial PNA (OR 7.26 95% CI 3.12-16.9, p<0.0001).
Figure 4. Distribution of PNA Prediction Score.
Bacteremia Prediction Score
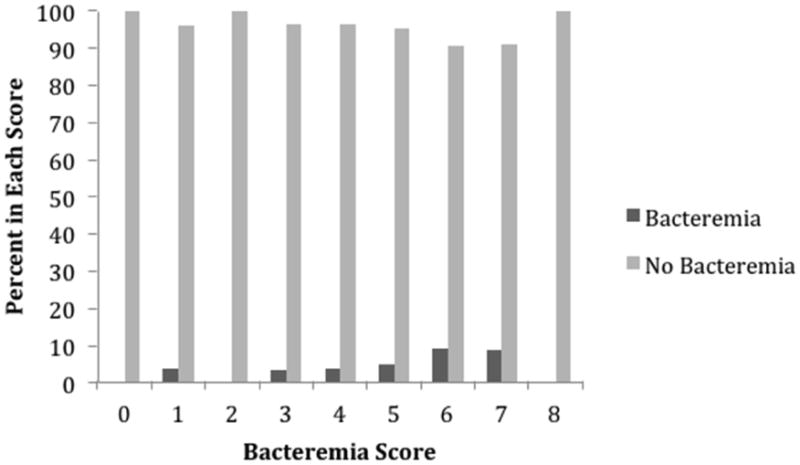
In the HAI model, history of diabetes and history of atrial fibrillation met the <0.2 univariable p-value cut off. The previously described methods for creating HAI, UTI, and PNA scores was applied to patients with bacteremia.[23] The final prediction score for bacteremia is shown in Table 1. The Bacteremia Prediction Score ranged from 0 to 8 and produced an AUC of 0.6891 (Figure 1). Figure 5 illustrates the distribution of the Bacteremia Prediction Score among patients without nosocomial bacteremia compared to patients who developed nosocomial bacteremia. The odds of a patient with a Bacteremia Prediction Score greater than or equal to 6 of developing bacteremia was 3 times the odds of patients with Bacteremia Prediction Scores of 0-5 of developing bacteremia (OR 3.34 95% CI 1.27-8.75, p=0.0143).
Table 1.
Baseline characteristics of patients with and without a hospital inquired infection (HAI) during stroke service admission.
| No HAI (N=484) | HAI (N=84) | p-value | |
|---|---|---|---|
| Age, median y (range) | 64 (24-102) | 73 (19-95) | <0.0001 |
| Gender, No. female (%) | 211 (45.9%) | 39 (46.4%) | 0.9247 |
| Black Race, (%) | 309 (67.3%) | 57 (67.9%) | 0.9231 |
| Past Medical History, No. (%) | |||
| Coronary Artery Disease | 77 (16.8%) | 22 (26.2%) | 0.1043 |
| Diabetes | 132 (29.1%) | 36 (43.9%) | 0.0077 |
| Hypertension | 346 (75.9%) | 66 (78.6%) | 0.5936 |
| Dyslipidemia | 209 (45.7%) | 31 (36.9%) | 0.2571 |
| Atrial Fibrillation | 50 (11.0%) | 14 (16.7%) | 0.1436 |
| Congestive Heart Failure | 27 (13.0%) | 5 (17.2%) | 0.5363 |
| Stroke | 185 (40.2%) | 29 (34.5%) | 0.3260 |
| Baseline NIHSS, median (IQR) | 5 (0-31) | 12 (1-33) | <0.0001 |
| Simple TOAST | 0.1736 | ||
| Cardioembolic | 116 (25.2%) | 31 (36.9%) | |
| Large vessel disease | 97 (21.1%) | 21 (25%) | |
| Small vessel disease | 88 (19.1%) | 11 (13.1%) | |
| Cryptogenic | 114 (24.8%) | 15 (17.9%) | |
| >1 Cause | 12 (2.6%) | 1 (1.2%) | |
| Other | 33 (7.2%) | 5 (5.9%) | |
| Baseline Laboratory values | |||
| Serum Glucose, median mg/dl (IQR) | 115 (58-569) | 133 (78-481) | 0.0427 |
| Serum Leukocyte, median per mL (IQR) | 8 (0.2-18) | 8 (2.4-26.9) | 0.2024 |
| Hematocrit, median % (IQR) | 39.7(23-55.3) | 39.3 (24.7-48.6) | 0.5203 |
| Serum Platelet count, median per mL (IQR) | 222 (54-751) | 210 (10-750) | 0.1732 |
| IV tPA, No. (%) | 186 (40.3%) | 37 (44.1%) | 0.5159 |
Abbreviations: IQR, interquartile range; Baseline NIHSS, National Institutes of Stroke Scale score at admission; TOAST, Trial of Org 10172 in Acute Stroke Treatment; IV tPA, intravenous tissue plasminogen activator.
Figure 5. Distribution of Bacteremia Prediction Score.
Discussion
In this current study, we derived a simple risk score for HAI during post-stroke hospitalization using clinical and demographic variables available on admission. Additionally, we developed individual risk scores for the three main types of HAI: UTI, PNA, and bacteremia. These models predict the potential risk of a HAI using information available at the time of hospital presentation, regardless of subsequent in-hospital management. These simple scores can identify patients at risk for a HAI, and have the potential to subsequently guide in-hospital care to mitigate HAIs and their effects on outcomes.
In our cohort, we found that 1 in 7 patients experienced a HAI during their stroke admission. Our results are consistent with previous studies in showing that HAIs occur in older patients with higher NIHSS at baseline and in patients with diabetes.[18][24][25][26] Our data is also consistent with previous reports where the prevalence of HAI, while lower in our sample than other reports of AIS (14% vs. 16-42%),[27][28] remains higher than the general prevalence of HAI reported in larger cohort studies of non-stroke patients (16-42% vs. 6-9%).[27][29][30]
Predictive scores have been developed in prior research for PNA. The A2DS2 score, a 10-point scoring system that included age, history of atrial fibrillation, dysphagia, sex, and stroke severity as calculated by NIHSS, was used to determine the risk of PNA following ischemic stroke.[31] Several other studies assessed risk factors and created scores for nosocomial post-stroke PNA.[18][24][32][33] While these previous models are adequate for predicting post-stroke PNA, our model incorporates multiple HAI subtypes. Further, these studies included lab values in their algorithms, while our score requires no more than patient history and short clinical assessments.[34][35][36] Our study demonstrates that a score using only variables available at the time of admission is feasible. Through our model, we were able to identify patients who were at 6-fold higher odds of developing a HAI after an ischemic stroke. This score may eventually prove valuable in an effort to prevent HAIs; however, this remains unknown at this time.
A prior meta-analysis investigated the effects of prophylactic antibiotic use in acute ischemic stroke patients. Unfortunately, the clinical trials used in the meta-analysis were not designed or powered to investigate the use of prophylactic antibiotics to reduce post stroke infections.[16] Further research is needed in the form of prospective studies to evaluate the efficacy of certain safety precautions or prophylactic antibiotic use in AIS patients in preventing HAIs and improving outcome.
Our study is limited by its retrospective nature and relatively small sample size. Our sample is derived from a large, urban area seeking services at a single academic center. Furthermore, a weakness in the development of any prediction score is the difficulty inherent to widespread implementation, which could be mitigated by the use of centralized freeware sources. Due to the high prevalence of UTI, PNA, and bacteremia within HAIs, we focused our prediction models on these three subtypes only. Confirmation of additional infection subtypes is technically challenging (e.g., rhinosinusitis, lower extremity cellulitis) or infrequent (e.g., meningitis/encephalitis), which thereby limits the broad range of infections we could include in our modeling. Because of the relative rarity in these infection subtypes, we do not believe their inclusion would significantly affect the results presented here. Additional validation is needed to examine the generalizability of our HAI prediction score.
Despite its limitations, our study is unique in that we were able to develop sensitive and specific prediction scores for all-cause HAI, as well as specific types of HAIs in AIS patients. If validated, our scoring system has the potential to identify AIS patients at higher odds of developing a HAI. This could then be used for future prospective studies designed to evaluate safety precautions and treatment methods to reduce HAIs and improve patient outcome. Future work may incorporate blood-based immune and stress markers to further improve upon our simple HAI scoring system.
Acknowledgments
Sources of Funding: Dr. Boehme is supported by NINDS NIH T32 NS007153-31. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIH. This study was conducted at Tulane Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elmsley HC, Hopkins SJ. Acute ischaemic stroke and infection: Recent and emerging concepts. Lancet Neurol. 2008;7(4):341–353. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- 2.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases--a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66:301–311. doi: 10.1093/gerona/glq208. [DOI] [PubMed] [Google Scholar]
- 4.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: A manifestation of brain-induced immunodepression. Stroke. 2007;38:1097–1103. doi: 10.1161/01.STR.0000258346.68966.9d. [DOI] [PubMed] [Google Scholar]
- 5.Ingeman A, Andersen G, Hundborg HH, et al. In-hospital medical complications, length of stay, and mortality among stroke unit patients. Stroke. 2011;42(11):3214–3218. doi: 10.1161/STROKEAHA.110.610881. [DOI] [PubMed] [Google Scholar]
- 6.Spratt N, Wang Y, Levi C, et al. A prospective study of predictors of prolonged hospital stay and disability after stroke. J Clin Neurosci. 2003;10(6):665–669. doi: 10.1016/j.jocn.2002.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Raut M, Schein J, Mody S, et al. Estimating the economic impact of a half-day reduction in length of hospital stay among patients with community-acquired pneumonia in the US. Curr Med Res Opin. 2009;25(9):2151–2157. doi: 10.1185/03007990903102743. [DOI] [PubMed] [Google Scholar]
- 8.Koennecke HC, Belz W, Berfelde D, et al. Factors influencing in-hospital mortality and morbidity in patients treated on a stroke unit. Neurology. 2011;77(10):965–972. doi: 10.1212/WNL.0b013e31822dc795. [DOI] [PubMed] [Google Scholar]
- 9.Weimar C, Mieck T, Buchthal J, et al. Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol. 2005;62(3):393–397. doi: 10.1001/archneur.62.3.393. [DOI] [PubMed] [Google Scholar]
- 10.Siegler JE, Boehme AK, Kumar AD, et al. Identification of modifiable and nonmodifiable risk factors for neurologic deterioration after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22(7):e207–213. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boehme AK, Kumar AD, Dorsey AM, et al. Infections present on admission compared with hospital-acquired infections in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. 2013;22(8):e582–589. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weimar C, Roth MP, Zillessen G, et al. Complications following acute ischemic stroke. Eur Neurol. 2002;48:133–140. doi: 10.1159/000065512. [DOI] [PubMed] [Google Scholar]
- 13.Magill SS, Edwards JR, Bamberg W, et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lampl Y, Boaz M, Gilad R, et al. Minocycline treatment in acute stroke: An open-label, evaluator-blinded study. Neurology. 2007;69(14):1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- 15.Westendorp WF, Vermeij JD, Vermeij F, et al. Antibiotic therapy for preventing infections in patients with acute stroke. Cochrane Database Syst Rev. 2012;1:CD008530. doi: 10.1002/14651858.CD008530.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Meisel C, Prass K, Braun J, et al. Preventive antibacterial treatment improves the general medical and neurological outcome in a mouse model of stroke. Stroke. 2004;35(1):2–6. doi: 10.1161/01.STR.0000109041.89959.4C. [DOI] [PubMed] [Google Scholar]
- 17.Langhorne P, Stott DJ, Robertson L, et al. Medical complications after stroke: a multicenter study. Stroke. 2000;31(6):1223–1229. doi: 10.1161/01.str.31.6.1223. [DOI] [PubMed] [Google Scholar]
- 18.Ji R, Shen H, Pan Y, et al. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke. 2013;44(5):1303–1309. doi: 10.1161/STROKEAHA.111.000598. [DOI] [PubMed] [Google Scholar]
- 19.Kwon HM, Jeong SW, Lee SH, et al. The pneumonia score: A simple grading scale for prediction of pneumonia after acute stroke. Am J Infect Control. 2006;34(2):64–68. doi: 10.1016/j.ajic.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Siegler JE, Boehme AK, Dorsey AM, et al. A comprehensive stroke center patient registry: Advantages, limitations, and lessons learned. Med Stud Res J. 2013;1:21–29. doi: 10.15404/msrj.002.002.spring/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones EM, Albright KC, Fossati-Bellani M, et al. Emergency department shift change is associated with pneumonia in patients with acute ischemic stroke. Stroke. 2011;42:3226–3230. doi: 10.1161/STROKEAHA.110.613026. [DOI] [PubMed] [Google Scholar]
- 22.Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol. 2001;54(4):343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 23.Saver JL, Yafeh B. Confirmation of tPA treatment effect by baseline severity-adjusted end point reanalysis of the NINDS-tPA stroke trials. Stroke. 2007;38(2):414–416. doi: 10.1161/01.STR.0000254580.39297.3c. [DOI] [PubMed] [Google Scholar]
- 24.Sellars C, Bowie L, Bagg J, et al. Risk factors for chest infection in acute stroke: A prospective cohort study. Stroke. 2007;38(8):2284–2291. doi: 10.1161/STROKEAHA.106.478156. [DOI] [PubMed] [Google Scholar]
- 25.Hilker R, Poetter C, Findeisen N, et al. Nosocomial pneumonia after acute stroke: Implications for neurological intensive care medicine. Stroke. 2003;34(4):975–981. doi: 10.1161/01.STR.0000063373.70993.CD. [DOI] [PubMed] [Google Scholar]
- 26.Westendorp WF, Nederkoorn PJ, Vermeij JD, et al. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wartenberg KE, Stoll A, Funk A, et al. Infection after acute ischemic stroke: Risk factors, biomarkers, and outcome. Stroke Res Treat. 2011;14:830614. doi: 10.4061/2011/830614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meisel C, Schwab JM, Prass K, et al. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6(10):775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- 29.Andersen BM, Ringertz SH, Gullord TP, et al. A three-year survey of nosocomial and community-acquired infections, antibiotic treatment and re-hospitalization in a Norwegian health region. J Hosp Infect. 2000;44(3):214–223. doi: 10.1053/jhin.1999.0677. [DOI] [PubMed] [Google Scholar]
- 30.Emmerson AM, Enstone JE, Griffin M, et al. The second national prevalence survey of infection in hospitals--overview of the results. J Hosp Infect. 1996;32(3):175–190. doi: 10.1016/s0195-6701(96)90144-9. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann S, Malzahn U, Harms H, et al. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke. 2012;43(10):2617–2623. doi: 10.1161/STROKEAHA.112.653055. [DOI] [PubMed] [Google Scholar]
- 32.Harms H, Grittner U, Dröge H, et al. Predicting post-stroke pneumonia: The PANTHERIS score. Acta Neurol Scand. 2013;128(3):178–184. doi: 10.1111/ane.12095. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Wang F, Zhang Y, et al. Risk factors for developing pneumonia in patients with diabetes mellitus following acute ischaemic stroke. J Int Med Res. 2012;40(5):1860–1865. doi: 10.1177/030006051204000524. [DOI] [PubMed] [Google Scholar]
- 34.Chumbler NR, Williams LS, Wells CK, et al. Derivation and validation of a clinical system for predicting pneumonia in acute stroke. Neuroepidemiology. 2010;34(4):193–199. doi: 10.1159/000289350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian J, Payabvash S, Kemmling A, et al. Variable selection and prediction using a nested, matched case-control study: Application to hospital acquired pneumonia in stroke patients. Biometrics. 2014;70(1):153–163. doi: 10.1111/biom.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniels SK, Brailey K, Priestly DH, et al. Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79(1):14–19. doi: 10.1016/s0003-9993(98)90200-3. [DOI] [PubMed] [Google Scholar]


