Abstract
Most research on neurodevelopmental disorders has focused on their abnormalities. However, what remains intact may also be important. Increasing evidence suggests that declarative memory, a critical learning and memory system in the brain, remains largely functional in a number of neurodevelopmental disorders. Because declarative memory remains functional, and because this system can learn and retain numerous types of information, functions, and tasks, it should be able to play compensatory roles for multiple types of impairments across the disorders. Here, we examine this hypothesis for specific language impairment, dyslexia, autism spectrum disorder, Tourette syndrome, and obsessive-compulsive disorder. We lay out specific predictions for the hypothesis and review existing behavioral, electrophysiological, and neuroimaging evidence. Overall, the evidence suggests that declarative memory indeed plays compensatory roles for a range of impairments across all five disorders. Finally, we discuss diagnostic, therapeutic and other implications.
Keywords: declarative memory, compensation, developmental disorders, neurodevelopmental disorders, specific language impairment, SLI, language disorder, dyslexia, specific learning disorder, autism, autism spectrum disorder, ASD, Tourette syndrome, obsessive-compulsive disorder, OCD, attention deficit hyperactivity disorder, ADHD, developmental coordination disorder, DCD, Parkinson's disease, PD, sex differences, underdiagnosis, hippocampus, medial temporal lobe, MTL, striatum, basal ganglia, procedural memory, explicit, explicit memory, habit reversal training, cognitive behavioral therapy, CBT, functional magnetic resonance imaging, fMRI, positron emission tomography, PET, neuroimaging, electrophysiology, event-related potentials, ERPs
1. Introduction
Not surprisingly, most research on neurodevelopmental disorders has focused on the behavioral and neurobiological abnormalities that characterize them, and on the underlying causes of these abnormalities. However, what remains normal may be as important as what is abnormal. It has long been known that relatively intact circuitry can play compensatory roles in brain disorders – even subsequent to adult-onset lesions, where less plasticity is generally expected than in children. For example, the right hemisphere may compensate for left hemisphere damage (Basso et al., 1989), and spared sensory systems can be employed in the face of sensory deficits, such as Braille reading by the blind.
It has previously been suggested that declarative memory, an important learning and memory system in the brain, may compensate for certain deficits in some neurodevelopmental disorders. In particular, it has been proposed that this memory system can at least partially take over certain functions that normally rely heavily on other systems. Such compensation has been posited for grammatical deficits in specific language impairment (Ullman and Pierpont, 2005), procedural memory impairments in obsessive-compulsive disorder (Rauch et al., 1997), and deficits of theory of mind and of implicit learning in autism spectrum disorder (Frith, 2004; Klinger and Dawson, 2001; Klinger et al., 2007). However, this evidence has not been synthesized or systematically reviewed, within let alone across disorders. Nor has declarative memory-based compensation been examined more broadly, including in related neurodevelopmental disorders and for a broader range of deficits. Thus the nature and extent of compensation by the declarative memory system remains unclear.
Here we present an in-depth examination of the declarative memory compensation hypothesis, which posits that declarative memory should play compensatory roles for multiple impairments across disorders, as long as the system remains largely functional. Specifically, we examine whether and how declarative memory plays compensatory roles across a range of deficits (beyond grammar and procedural memory) in several neurodevelopmental disorders that are often comorbid with each other (Bradshaw, 2001; Gillberg, 2010; Goorhuis-Brouwer and Wijnberg-Williams, 1996; Pauc, 2005; Pennington and Bishop, 2009): specific language impairment (SLI; i.e., developmental language disorder), dyslexia, autism spectrum disorder (ASD), Tourette syndrome, and obsessive-compulsive disorder (OCD). Because declarative memory is powerful and flexible in that it can learn and retain multiple types of information, functions and tasks (Eichenbaum, 2012; Squire and Wixted, 2011; Ullman, In Press-b), it should be able to support the learning and use of many types of compensatory strategies. Importantly, because this memory system is quite well understood in both humans and non-human animals, elucidating its compensatory roles in neurodevelopmental disorders has the potential to be highly informative, and could lead to therapeutic and diagnostic advances as well as to an increased understanding of the disorders themselves.
The main goals of this paper are to present the declarative memory compensation hypothesis, to lay out clear testable predictions, to review and synthesize existing evidence for these predictions for each disorder, to elucidate gaps and weaknesses that can guide future studies, and to outline potential impacts for basic and translational research.
Below, we first provide a brief review of declarative memory. Next, we show that learning and retention in declarative memory remain largely intact in the disorders examined here, allowing the memory system to play compensatory roles across them. We then outline the main predictions of the compensation hypothesis, and examine the evidence for each of these predictions for each disorder. Finally, we discuss therapeutic, diagnostic, and other implications.
2. Declarative Memory: What Is It?
Traditionally, the declarative memory system has referred to the brain system that underlies the learning and storage of explicit knowledge, that is, knowledge that is available to conscious awareness. This includes explicit knowledge both about facts (semantic knowledge; e.g., that the capital of Swaziland is Mbabane) and about events (episodic knowledge; e.g., that you ate spicy tender but crunchy calamari at a Thai restaurant last night). However, accumulating evidence suggests that this brain system underlies much more, and that it can learn implicit as well as explicit knowledge, for a wide range of information, functions, and tasks, across cognitive domains and sensory modalities (Cabeza and Moscovitch, 2013; Eichenbaum, 2012; Eichenbaum et al., 2012; Henke, 2010; Squire and Wixted, 2011; Ullman, 2004, In Press-b). Thus the system seems very well suited to compensate for a wide variety of impairments across disorders. Moreover, the system is quite well understood at many levels – including its behavioral, computational, neuroanatomical, electrophysiological, cellular, biochemical, and genetic correlates – providing a rich foundation for generating testable predictions regarding its compensation in various disorders.
So what constitutes this brain system? (For reviews, see Cabeza and Moscovitch, 2013; Eichenbaum, 2012; Eichenbaum et al., 2012; Henke, 2010; Squire and Wixted, 2011; Ullman, 2004, In Press-b) Perhaps most importantly, learning and consolidation in the system crucially depend on the hippocampus and other medial temporal lobe (MTL) structures. Within the MTL, the hippocampus appears to underlie the rapid linking (binding, associating) of different bits of knowledge or experience, across a wide range of domains and modalities, including what may be characterized as knowledge of “what” (facts, meanings), “where” (landmarks), and “when” (when an event occurred). The ability of the hippocampus to bind complex relational associations, including contextual and temporal information, may explain why it seems to be critical for episodic knowledge (Brown et al., 2010; Eichenbaum et al., 2012; Squire and Wixted, 2011). Other MTL structures, in particular the entorhinal, perirhinal, and parahippocampal cortices, also play important roles in declarative memory. For example, it has been suggested that perirhinal cortex underlies the familiarity of newly learned information, while the hippocampus subserves its explicit recollection (Brown and Aggleton, 2001; Brown et al., 2010) (but see Wixted and Squire, 2011). Additionally, perirhinal cortex may support memories of single items, and might be particularly important (perhaps in addition to the hippocampus) for aspects of semantic and other non-episodic (e.g., lexical) knowledge. Structures connected to the MTL, including the fornix and various diencephalic structures, also support declarative memory (Squire and Wixted, 2011).
Although the MTL and these connected structures play key roles in learning and consolidation, ultimately most long-term knowledge learned in this system seems to rely mainly on neocortical regions, especially but not only in the temporal lobes (Eichenbaum, 2012; Eichenbaum et al., 2012; Squire and Wixted, 2011; Ullman, 2004). Nevertheless, some evidence suggests that the MTL may continue to underlie long-term memories, particularly for autobiographical (episodic) knowledge (Winocur and Moscovitch, 2011), though this claim has been disputed (Squire and Wixted, 2011).
Within neocortex, different regions appear to underlie different types of knowledge (Martin, 2007; Martin and Chao, 2001; Squire and Wixted, 2011). For example, knowledge of faces involves portions of the fusiform gyrus (the so-called fusiform face area), whereas written words depend more on the “visual word form area” (also in the fusiform gyrus). Additionally, higher-level concepts may rely on more anterior temporal regions (Barense et al., 2012; Bussey and Saksida, 2007; Martin and Chao, 2001). Finally, other brain structures interact with the core learning and storage functions of declarative memory. For example, certain frontal regions (e.g., Brodmann's areas 45/47) seem to be involved in the encoding of information that is learned in declarative memory and the subsequent recall of this knowledge (Fletcher et al., 2003; Hofer et al., 2007; Ullman, 2004). And a posterior parietal region may underlie aspects of encoding and retrieval (Uncapher and Wagner, 2009; Wagner et al., 2005).
The molecular and genetic bases of declarative memory have also been well studied, in both humans and animals (Green and Dunbar, 2012; Ullman, 2004). For example, various genes, including those for brain-derived neurotrophic factor (BDNF) and apolipoprotein E (APOE), seem to play roles in declarative memory and hippocampal function (Green and Dunbar, 2012; Pezawas et al., 2004), as does the neurotransmitter acetylcholine (Freo et al., 2002; Packard, 1998) and the hormone estrogen (Phillips and Sherwin, 1992; Sherwin, 1988). For example, higher levels of estrogen are associated with better declarative memory (Maki and Resnick, 2000; Sherwin, 1998).
The functional characteristics of declarative memory are also quite well understood (Eichenbaum, 2012; Henke, 2010; Squire and Wixted, 2011; Ullman, 2004, In Press-b). As mentioned above, this memory system can support a wide range of information, tasks, and functions across domains and modalities. It can acquire not only semantic and episodic knowledge in the restricted sense of meaning and event knowledge, but also words (lexical knowledge), images, scripts, instructions, and much more (Eichenbaum, 2012; Squire and Wixted, 2011; Ullman, 2004, In Press-b). For example, this system, and perhaps the hippocampus in particular, is involved in such diverse functions as inhibitory learning (learning to suppress a prepotent response; Chan et al., 2001) and aspects of social learning and memory (likely due the system's role integrating the complex information necessary for this function; Hitti and Siegelbaum, 2014; Kogan et al., 2000). More generally, the system may be specialized for learning and representing arbitrary pieces of information, as well as linking them together. Unlike other learning and memory systems, such as basal ganglia-based procedural memory or amygdala-based fear conditioning, this system can rapidly learn many types of knowledge, even from a single exposure of a stimulus, although additional exposures strengthen memories. The acquired knowledge can be either explicit or implicit (Chun, 2000; Henke, 2010; Schendan et al., 2003). However, declarative memory appears to be the only long-term memory system that underlies explicit knowledge; thus, any knowledge that is explicit was likely learned in this memory system. Once learned, information in declarative memory can be generalized and used flexibly across different contexts.
Finally, multiple factors have been found to affect learning and retention in declarative memory, including subject-related variables such as age, sex, sleep, and stress; learning context-related variables such as deep vs. shallow encoding and spaced vs. massed presentation; and item-level variables such as imageability (Delaney and Knowles, 2005; Marshall and Born, 2007; Prado and Ullman, 2009; Ullman, 2005a; Ullman et al., 2008; Wolf, 2009).
3. The Status of Declarative Memory in Neurodevelopmental Disorders
Here we examine the functionality of declarative memory in each of the five neurodevelopmental disorders: specific language impairment, dyslexia, autism spectrum disorder, Tourette syndrome, and obsessive-compulsive disorder. As we will see, evidence suggests that individuals with these disorders generally learn and retain knowledge in declarative memory at least adequately if not normally, and in some cases even show superior performance as compared to typically developing individuals. Moreover, individuals with the disorders appear to be able to learn multiple types of knowledge in declarative memory, suggesting that they can also learn multiple types of compensatory strategies. Note that most such strategies likely rely primarily on various types of non-episodic knowledge (since most compensatory strategies do not require remembering the context in which they were learned), and such knowledge seems to be particularly spared in the disorders. Thus, declarative memory appears to show sufficient functionality across the disorders to underlie the learning and retention of a wide range of compensatory strategies.
Where weaknesses have been reported in tests of declarative memory, they are generally observed where the tests also depend on functions that are compromised in the disorders, such as phonological processing, working memory, or free recall (interestingly, these functions tend to rely at least in part on frontal, basal ganglia or cerebellar circuits, which often show abnormalities in the disorders; Amaral et al., 2008; Eckert et al., 2005; Hoekstra et al., 2004; Pernet et al., 2009; Rauch et al., 2007; Ullman and Pierpont, 2005; Ullman et al., Under Review). Indeed, tests of declarative memory that minimize or avoid such functions generally show spared performance. Importantly, these compromised functions do not seem to prevent the eventual learning and retention of substantial knowledge, especially non-episodic knowledge. For example, long-term memories of common knowledge, such as lexical and semantic knowledge, do not show particular impairments, perhaps because learners were exposed to the relevant information many times, as well as in multiple contexts that do not always heavily involve the full range of compromised functions (e.g., that minimize the need for working memory). Thus, any such weaknesses are not likely to preclude the learning of compensatory strategies by declarative memory.
3.1. Specific Language Impairment (SLI)
Specific language impairment is a neurodevelopmental disorder of language that is not attributable to hearing or other sensory impairments, motor dysfunction, environmental deprivation, emotional problems, or another medical or neurological condition, and is not better explained by intellectual disability (American Psychiatric Association, 2013; Leonard, 1998, 2014). It is referred to as “language disorder” in the DSM-5 (American Psychiatric Association, 2013). Grammatical aspects of language, in particular syntax, morphology, and phonology, are especially affected in SLI. Learning in declarative memory appears to remain largely unaffected in individuals with SLI, particularly for non-verbal material, but possibly even in the verbal domain once working memory and language impairments have been controlled for (Lum and Conti-Ramsden, 2013) (Dewey and Wall, 1997; Lum et al., 2012; Lum et al., Under Review; Ullman and Pierpont, 2005). For example, in one study children with SLI showed no impairments in tests that probed the learning of visual information, and normal learning of verbal information after working memory and language deficits were statistically controlled for; in contrast, the same subjects were impaired at working memory and procedural memory, and these impairments were found even after controlling for other deficits (Lum et al., 2012). In another study of children with SLI, impairments at learning verbal information were found only in those children with co-occuring working memory deficits (Lum et al., Under Review). Interestingly, two recent studies found that children with SLI may actually consolidate information in declarative memory better than typically developing children (Lukacs et al., In Preparation; Lum et al., In Preparation). Importantly, long-term common knowledge also seems to remain relatively normal in SLI, especially after controlling for working memory and language deficits (Ullman and Pierpont, 2005). For reviews of declarative memory in SLI, see Ullman and Pierpont (2005), Lum et al (2012), and Lum and Conti-Ramsden (2013).
3.2. Dyslexia
Dyslexia is a neurodevelopmental disorder in which reading is impaired below expectation given the individual's age, where the impairment is not better accounted for by intellectual disability, sensory impairments, other mental or neurological disorders, psychosocial adversity, or inadequate educational instruction (American Psychiatric Association, 2013; Lyon et al., 2003). It is categorized as a specific learning disorder in the DSM-5 (American Psychiatric Association, 2013). Learning non-verbal visual information in declarative memory appears to remain normal in dyslexia (Jorm, 1983; Kibby, 2009; Kibby and Cohen, 2008). Learning verbal material also seem to be unimpaired, especially when difficulties in encoding, which are likely due to phonological and working memory deficits, are accounted for (Felton et al., 1987; Jorm, 1983; Kibby, 2009; Kibby and Cohen, 2008; Kramer et al., 2000). For example, in a list-learning task (in which subjects are asked to remember a list of words), children with dyslexia may learn fewer words during the encoding phase, but have no difficulty in subsequently remembering the words they have learned (Kibby, 2009; Kramer et al., 2000). Lexical knowledge also remains largely unaffected in the disorder, as evidenced by studies of receptive vocabulary (e.g., which present words to subjects and ask them to pick the correct meaning) (Richardson et al., 2004; Swan and Goswami, 1997). When deficits are found in tasks that probe lexical or other long-term common knowledge, such as in object naming tests (subjects see a picture of an object, and have to name it), the problems often seem to reflect phonological or recall deficits rather than memory impairments per se (Jorm, 1983; Swan and Goswami, 1997). Adults with dyslexia have also been found to show unimpaired performance at a spatial contextual learning task, which probes implicit aspects of declarative memory (Bennett et al., 2008; Howard et al., 2006). In fact, in one study subjects showed a trend towards superior contextual learning as compared to typically developing controls, but were impaired on a different task that relies on procedural learning (Howard et al., 2006). In a similar contrast between the two memory systems, children with dyslexia showed deficits at a sequence learning task that involves procedural learning, but not at a sequence learning task with explicit instruction that depends on declarative memory (Vicari et al., 2003). Finally, in a recent study of incidental encoding and subsequent recognition of objects (which minimized working memory, phonological processing, and recall) (Hedenius et al., 2013), children with dyslexia showed better learning and retention than age-matched typically developing children. This result, as well as the trend of superior performance at contextual learning (Howard et al., 2006), remains to be explained, though it may be due to the “seesaw effect” – that is, the enhancement of declarative memory as a result of abnormalities in the frontal/basal-ganglia circuits underlying procedural memory (Ullman, 2004).
3.3. Autism Spectrum Disorder (ASD)
Autism spectrum disorder, here also referred to as autism, is characterized by impairments of social interaction and communication, and by restricted repetitive and stereotyped patterns of behavior, interests, and activities (American Psychiatric Association, 2013). The status of declarative memory in ASD appears to be more complex than in SLI and dyslexia.
In high functioning individuals with autism, learning in declarative memory seems to remain largely intact, as tested by both recognition and cued recall tests (e.g., in which subjects are given a phonological cue to help them recall a word) (Boucher and Mayes, 2011, 2012; Boucher et al., 2012; Walenski et al., 2006). Indeed, recognition has been found to be spared even when the underlying neural processes appear to be atypical (Massand et al., 2013). Spatial contextual learning (implicit learning in declarative memory; see above) also seems to be unimpaired (Barnes et al., 2008). Importantly, so does long-term non-episodic (lexical and semantic) knowledge (Walenski et al., 2008; Walenski et al., 2006). In contrast, tests of free recall (uncued recall; e.g., in which subjects are asked to recall a list of words that they saw previously) have yielded inconsistent results across studies of high functioning autism (Boucher and Mayes, 2011; Boucher et al., 2012). Person- and emotion-related memory as well as memory of personally-experienced events are also sometimes impaired, possibly as a result of the broader social and emotional deficits in autism (Boucher and Mayes, 2011, 2012; Boucher et al., 2012; Walenski et al., 2008), and the dependence of memory for personally-experienced events (episodic knowledge) on frontal structures, which are compromised in the disorder (Ben Shalom, 2003). Overall, individuals with high functioning autism may be best described as having intact memory for facts, percepts, and associations, with mild episodic memory impairments and a greater vulnerability for person- and emotion-related memories (Boucher and Mayes, 2012).
Declarative memory may be more impaired in low-functioning autism (Boucher and Mayes, 2011; Boucher et al., 2008, 2012), which might help explain the more severe deficits found in this form of the disorder (see section 5.2) – though note that even in low functioning individuals, cued recall and paired associate learning (e.g., in which subjects learn pairs of words) have been found to be intact (Boucher and Mayes, 2011; Boucher et al., 2012).
Finally, enhanced (Walenski et al., 2008) or even savant-like memory has often been reported in individuals with autism (Boucher and Mayes, 2011) – for example, drawing complex scenes from memory (Howlin et al., 2009). Such memory advantages could be due to various factors, including the seesaw effect (Ullman, 2004; Walenski et al., 2008; Walenski et al., 2006), enhanced perceptual functioning and pattern recognition (Mottron et al., 2013; Toichi and Kamio, 2002), and an obsessive focus on particular informational domains (Boucher and Mayes, 2011). Indeed, such obsessions might help explain why some individuals with ASD who have excellent memory for particular topics still do not seem to compensate effectively for social and other deficits.
3.4. Tourette Syndrome
Tourette syndrome is a neurodevelopmental disorder that manifests itself with multiple motor tics and at least one vocal tic, where these are not explained by medications or another medical condition (American Psychiatric Association, 2013). Although few studies have investigated declarative memory in Tourette syndrome, the evidence suggests that it remains largely unimpaired (for a review, see Walenski et al., 2007). Learning new information in declarative memory, including verbal and non-verbal list learning (Brookshire et al., 1994; Channon et al., 2003) and remembering the locations of objects (Alexander and Peterson, 2004; Marsh et al., 2004), appears quite typical, as does long-term lexical and semantic knowledge (Channon et al., 2003; Scheurholz et al., 1996). And whereas the implicit learning of procedural knowledge in the “weather prediction” task was found to be impaired in children and adults with Tourette syndrome, typical performance was observed in a test of explicit knowledge in the same subjects (Marsh et al., 2004). In contrast, as we have seen for other neurodevelopmental disorders, free recall is often compromised in Tourette syndrome (Channon et al., 2003; Sutherland et al., 1982).
3.5. Obsessive-Compulsive Disorder (OCD)
Obsessive-compulsive disorder, which is closely related to Tourette syndrome, is marked by obsessions (repeated and persistent thoughts, impulses, or images that the individual attempts to suppress) and/or compulsions (repetitive and excessive behaviors due to an obsession, or performed in order to follow a rigid set of rules), where these cause distress and/or interfere with appropriate functioning (American Psychiatric Association, 2013). OCD, like Tourette syndrome, seems to leave declarative memory essentially unimpaired. Learning verbal information is generally unaffected (Olley et al., 2007; Roth et al., 2004), while difficulties learning in the visual domain have been attributed to executive deficits (Deckersbach et al., 2004; Olley et al., 2007; Savage et al., 2000). Lexical knowledge also appears to be typical in OCD, as revealed by tasks probing receptive vocabulary (Deckersbach et al., 2000) and lexical decision (in which subjects have to decide which items are real words and which are made-up words) (Unoki et al., 1999).
3.6. Summary
Overall, the data suggest that individuals with these five neurodevelopmental disorders are generally able to learn and in particular retain information in declarative memory. Thus, although impairments in interacting functions such as working memory or phonological processing may slow the learning of information in declarative memory, the information can nonetheless be largely acquired and retained, suggesting that compensatory strategies can also be learned and retained in this system.
4. A Compensatory Role for Declarative Memory?
To date, relatively few studies have been specifically designed to test the hypothesis that declarative memory supports compensation in neurodevelopmental disorders. Nevertheless, an increasing number of studies provide evidence that directly or indirectly tests the hypothesis in the five disorders examined here, thus allowing it to be evaluated in these disorders. In this section we first lay out the three major predictions derived from the hypothesis. These predictions are expected to hold across symptoms and disorders. We then examine a wide range of relevant evidence, and identify existing gaps in the literature, thus providing a roadmap for future research (also see Table 1). Relevant studies were identified via online searches (e.g., using PubMed, Google Scholar), our own database of articles, references in identified articles as well as articles that cite them, and queries to researchers in the field.
Table 1.
Predictions and evidence for compensation by declarative memory in neurodevelopmental disorders.
| Predictions | Evidence | ||||
|---|---|---|---|---|---|
|
| |||||
| SLI | Dyslexia | Autism Spectrum Disorder | Tourette syndrome | OCD | |
| Increased reliance (from either spontaneous learning or therapy) on strategies thought to depend on declarative memory |
|
|
|
Habit reversal therapy, which involves the explicit control of tics, is effective in TS (Frank and Cavanna, 2013; Himle et al., 2006) | Habit reversal therapy, as well as other cognitive behavioral therapies, are effective in OCD (March, 1995; Watson and Rees, 2008) |
|
| |||||
| Increased reliance on the neural system underlying declarative memory | Electrophysiological evidence: N400 ERP components found for grammatical processing (Fonteneau and van der Lely, 2008; Neville et al., 1993; Ullman and Pierpont, 2005) |
|
fMRI evidence: Increased activation in medial temporal lobe structures during some social processing tasks (Dichter et al., 2012; Vaidya et al., 2011) | TBD | fMRI and PET evidence: Increased activation in MTL structures (and decreased activation in the basal ganglia) during procedural memory and executive tasks (Rauch et al., 1997; Rauch et al., 2007; Rauch et al., 2001; Roth et al., 2003; van den Heuvel et al., 2005) |
|
| |||||
| Indicators of better declarative memory are associated with better compensation | Better grammatical abilities in individuals with better declarative memory (Lum et al., 2012) | Better reading ability in individuals with better declarative memory and larger hippocampi/MTL structures (Hedenius et al., 2013; Krafnick et al., 2011) | TBD |
|
OCD symptoms better in individuals with larger hippocampi/MTL structures (Carmona et al., 2007; Peterson et al., 2007; van den Heuvel et al., 2009) |
Note. SLI: specific language impairment. TS: Tourette syndrome. OCD: obsessive-compulsive disorder. TBD: To be determined; we are not aware of any relevant studies. (f)MRI: (functional) magnetic resonance imaging. PET: positron emission tomography. ERP: event-related potential. MTL: medial temporal lobe.
The three main predictions are as follows. First, individuals with the disorders should at least partly compensate for their impairments by using cognitive and other behavioral strategies that depend on declarative memory. (Note that this does not preclude either an additional reliance on strategies that do not involve declarative memory, or improvements in the compromised systems themselves, for example due to developmental changes, experience, or therapy.) Thus, individuals with the disorders should rely more than typically developing individuals on declarative memory-based strategies. At least two types of compensatory strategies could be learned in this system. First, the system could at least partially take over certain functions that normally rely heavily on other systems, such as grammar or sequence learning. Second, the system could learn strategies that allow individuals to inhibit or control inappropriate behaviors, such as tics or obsessions. Compensatory strategies could be learned explicitly or implicitly, and either spontaneously or through behavioral therapies. Indeed, given the proposed importance of declarative memory, we expect that existing therapies tend to take advantage of declarative memory even if they were not specifically designed to do so.
Second, an increased behavioral dependence on declarative memory should be reflected in an increased reliance on the neural underpinnings of this system. Thus, imaging and electrophysiological studies should indicate greater involvement of the neural substrates of declarative memory in individuals with the disorders as compared to typically developing controls. This pattern should be found in particular when probing impairments that can be compensated for by this system. Note that activation in the MTL should be observed primarily in studies in which compensatory strategies are still being learned or were recently learned rather than in studies examining long-established strategies, since the latter are less likely to still rely on MTL structures (see section 2). In addition, therapeutic interventions may be expected to lead to an increased reliance on declarative memory brain structures, especially (but not only) if they were designed to take advantage of this system. We emphasize that an increased dependence on declarative memory in no way precludes an additional reliance on other (non-declarative) brain systems; that is, one type of compensation does not necessarily exclude others.
Third, better declarative memory should result in better compensation. Correlations should be observed, such that individuals or groups with evidence of superior declarative memory should show superior compensation, and thus fewer or less severe symptoms. Note that even though declarative memory seems to remain relatively spared in the disorders, its functionality should still show between-subject variability. Such variability could be due to the sort of variation in declarative memory functioning that is also found in typically developing individuals (Ullman, 2001, 2004, 2005a; Ullman et al., 2008), or to a degree of dysfunction of declarative memory in some individuals (e.g., from the same pathological processes that result in other abnormalities in the disorders; see section 5.2), or even to the enhancement of this memory system (e.g., from the seesaw effect).
4.1. Specific Language Impairment
Previous studies of SLI provide some supporting evidence for all three predictions. First, behavioral evidence suggests that individuals with SLI use declarative memory in at least two ways to compensate for their grammatical deficits: chunking and explicit rules. Typically developing individuals generally compute complex forms such as “the cat” or “walked” with highly-automatized grammatical composition (e.g., the + cat, walk + -ed), a process that appears to depend on procedural memory (Ullman, 2004, In Press-b). In contrast, individuals with SLI often memorize complex forms as (structured or unstructured) wholes, that is as chunks, which appear to be stored in declarative memory (Oetting and Horohov, 1997; Ullman and Gopnik, 1999; Ullman and Pierpont, 2005). For example, children with SLI disproportionately use high-frequency phrases (i.e., those that are more often encountered in the language) (Thordardottir and Ellis Weismer, 2002), which are particularly likely to be memorized. And evidence from experimental approaches such as the examination of “frequency effects”, which can distinguish whether complex forms are chunked or are composed from their parts, suggests that children and adults with SLI tend to depend more than typically developing controls on chunking (Oetting and Horohov, 1997; Ullman and Pierpont, 2005; van der Lely and Ullman, 2001). Individuals with SLI also learn explicit grammar rules, that is, rules that appear to be learned and applied with conscious awareness (Ullman and Pierpont, 2005). For example, one child with developmental language impairment reported that “at school, learn it at school. In the past tense put -e-d on it. If it's today it's -i-n-g. Like swimming: ‘I went swimming today’ and ‘Yesterday I swammed’” (Ullman and Gopnik, 1999). As indicated above, since declarative memory appears to be the only long-term memory system underlying explicit knowledge, any such knowledge is likely to have been learned in this memory system.
Second, evidence suggests that individuals with SLI rely more than typically developing individuals on the neural system underlying declarative memory. Specifically, electrophysiological evidence from the recording of event-related potentials (ERPs) implicates declarative memory in a compensatory role for grammar (Fonteneau and van der Lely, 2008; Neville et al., 1993; Ullman and Pierpont, 2005). For example, one study (Fonteneau and van der Lely, 2008) showed that in typically developing children and adults syntactic anomalies elicited a left anterior negativity, a pattern often found in typical individuals, whereas in matched participants with SLI these anomalies elicited an N400, an ERP component that has been linked to lexical/semantic processing and declarative memory, perhaps perirhinal cortex in particular (Kutas and Federmeier, 2011; McCarthy et al., 1995; Mormann et al., 2005; Ullman, 2001, In Press-b); see Figure 1. In contrast, both groups showed similar N400s for lexical anomalies, indicating, as expected, that an increased dependence on declarative memory in SLI does not occur for functions that already depend on this system in typically developing individuals. Additional electrophysiological studies of grammar in SLI seem warranted. Note that previous functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies of SLI have, to our knowledge, ignored both syntax and morphology, focusing instead on lexical, phonological, and executive tasks (Ullman and Pierpont, 2005; Ullman et al., Under Review). Thus, previous studies using these techniques have not yet tested whether individuals with SLI rely on declarative memory brain structures for the compensation of these grammatical functions.
Figure 1.
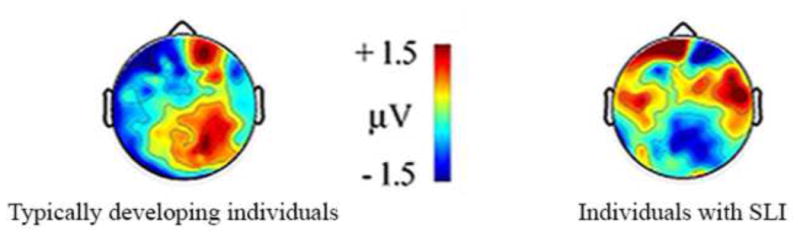
Electrophysiological evidence from children and adults with specific language impairment (SLI). In an event-related potential (ERP) study (Fonteneau and van der Lely, 2008), syntactic anomalies elicited a left anterior negativity in typically developing children and adults. In contrast, in children and adults with SLI the anomalies elicited an N400, which has been linked to lexical/semantic processing and declarative memory (Kutas and Federmeier, 2011; Ullman, 2001). This suggests that, unlike typically developing individuals, individuals with SLI may rely on lexical/semantic processing and declarative memory to compensate for impairments at syntactic processing. Figure adapted from Fonteneau and van der Lely (2008).
Third, correlational evidence suggests that individuals with SLI who demonstrate better declarative memory also show better grammatical performance. Grammatical abilities have been found to correlate with declarative memory learning abilities in children with SLI, but not with learning in procedural memory or working memory measures in the same children (Lum et al., 2012). In contrast, in matched typically developing children, grammar correlated with procedural memory, but not with declarative memory or working memory. As expected, lexical abilities correlated with declarative memory in both groups (and not with either procedural memory or working memory measures). Overall, this suggests that whereas typically developing children depend importantly on procedural memory for grammar, children with SLI use declarative memory to help them compensate for their grammatical deficits.
4.2. Dyslexia
Previous studies also provide evidence for all three predictions in dyslexia, though again further research is needed. First, behavioral evidence suggests that individuals with dyslexia rely disproportionately on declarative memory for reading, using at least three strategies: chunking, a reliance on semantics, and the use of explicit rules. As in SLI, chunking, in particular whole word memorization, seems to be important in dyslexia. Whereas typically developing individuals rely on both phonological decoding and chunking for reading, individuals with dyslexia appear to have deficits with phonological decoding, with relatively spared chunking, leading to a disproportionate reliance on the latter. Thus, they have particular problems reading made-up words (which cannot have been previously memorized as whole words), but are less impaired at reading real words (which could have been memorized), especially those that occur that occur with high frequency in the language (which are especially likely to have been memorized) (Bruck, 1990; Rack et al., 1992; Shaywitz et al., 2003; Snowling et al., 1994). This is analogous to the pattern found in SLI for complex forms. Reading by individuals with dyslexia also relies heavily on semantic knowledge, which is learned in declarative memory. For example, one study found that children with dyslexia improved their reading of single words when these were supported by a strong semantic context, whereas such semantic facilitation appeared to be weaker in typically developing children (Nation and Snowling, 1998). The authors suggest that the children with dyslexia used semantic context to compensate for poor decoding skills. Such semantic facilitation effects have also been found in adults with dyslexia (Ben-Dror et al., 1991). Moreover, studies of adult dyslexics suggest that improvements in reading during childhood and adolescence are due not to improvements in phonological processing but rather to an increased reliance on semantic knowledge (Chiarello et al., 2006; Shaywitz et al., 2003). Finally, explicit rules also appear to play an important role in reading in dyslexia. Therapeutic programs designed to teach reading in dyslexia often focus on learning explicit phonological rules (Alexander and Slinger-Constant, 2004; Ehri and Nunes, 2001). Moreover, such programs may lead to more significant reading gains than therapies that do not depend on explicit rules (Alexander and Slinger-Constant, 2004; Ehri and Nunes, 2001).
Second, behavioral interventions in dyslexia can lead not only to reading improvements, but also to concomittant changes in the hippocampus and other MTL structures that underlie declarative memory, in both functional and structural imaging studies. At least three fMRI studies of children or adults with dyslexia have found that various interventions can lead to both improved reading and increased activation in the hippocampus and other MTL structures (as well as other brain regions) in phonological processing and pseudoword reading tasks, suggesting an increased dependence on these structures (Eden et al., 2004; Gebauer et al., 2012; Temple et al., 2003). It has been argued that this pattern may be due to compensation by hippocampal-based memory (Temple et al., 2003) – though for this and other findings, it is important to keep in mind that inference of function from a given activation pattern is often uncertain (for a discussion of the issue of inference in neuroimaging studies, see Poldrack, 2006). To our knowledge, other studies have not reported MTL activation increases following intervention (Barquero et al., 2014), although such negative findings might be due to factors such as small subject numbers (Temple et al., 2000) or a much briefer intervention (Aylward et al., 2003). In a structural MRI study of children with dyslexia, intensive training (not specifically designed to emphasize explicit memory) led to better reading skills as well as to increased gray matter volumes in the hippocampi bilaterally (Krafnick et al., 2011); see Figure 2. Although unfortunately declarative memory performance was not assessed in this study (or in the fMRI studies described above), larger hippocampal volumes have often been associated with better declarative memory (Protopopescu et al., 2008; Schofield et al., 2009). Thus, intensive behavioral intervention in dyslexia may lead not only to better reading, but also to improvements in declarative memory, presumably because this system was relied upon during training. Indeed, training paradigms that clearly involve memorization in declarative memory have been found to lead to larger hippocampal volumes (Draganski et al., 2006; Woollett and Maguire, 2011), underscoring the plausibility of this outcome. We are aware of no other studies examining the effects of intervention on gray matter volumes in dyslexia. Finally, electrophysiological evidence also suggests a compensatory role for declarative memory in the disorder. As in SLI, individuals with dyslexia may show grammatical impairments (Cantiani et al., 2012; Cantiani et al., 2013). And similarly to SLI, morphosyntactic violations elicit N400s (which are linked to lexical/semantic processing and declarative memory; see above) in adults and children with dyslexia, but not in matched typically developing individuals (Cantiani et al., 2012; Cantiani et al., 2013).
Figure 2.
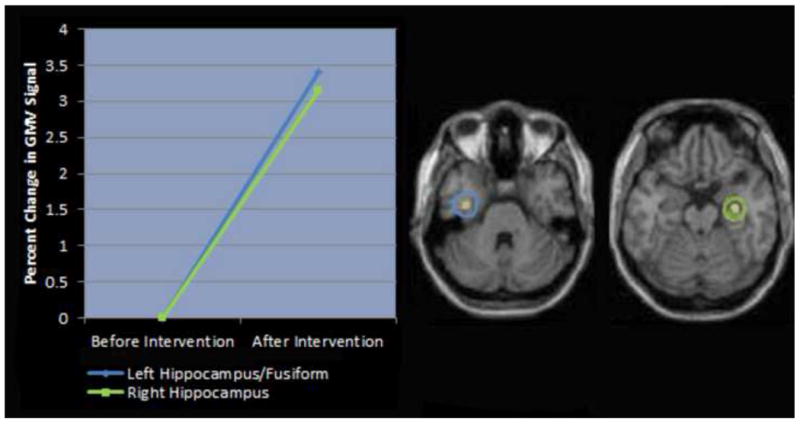
Structural imaging evidence from children with dyslexia. In a structural magnetic resonance imaging (MRI) study (Krafnick et al., 2011), gray matter volume (GMV) was examined in children with dyslexia before and after a behavioral intervention. Following the intervention, there was a significant increase not only of reading abilities, but also of gray matter volumes in the left hippocampus/fusiform and the right hippocampus (both shown here), as well as the left precuneus and right cerebellum (not shown). Since larger hippocampal volumes have independently been shown to correlate with better declarative memory (Protopopescu et al., 2008; Schofield et al., 2009), the findings suggest that intensive behavioral intervention in dyslexia can lead to improved declarative memory as well as improved reading. Declarative memory performance was not directly assessed in this study. Figure adapted from Krafnick et al. (2011).
Third, evidence suggests that better declarative memory in dyslexia may correlate with better reading. First of all, the structural MRI study discussed above (Krafnick et al., 2011) found that subsequent to training, children with dyslexia showed not only improved reading but also larger hippocampi, which are linked to superior declarative memory. Thus, within subjects, an indication of improved declarative memory is associated with improved reading in dyslexia. Further studies seem warranted to test whether positive reading outcomes from such interventions may be explained at least in part by improvements of declarative memory. More direct evidence comes from a recent behavioral study, which found that performance at learning and retention in a recognition memory task was positively associated with reading performance in children with dyslexia (Hedenius et al., 2013). In contrast, no such association was observed in typically developing children in the same study. The results suggest that better declarative memory might in fact lead to better reading, but only in dyslexia, consistent with this memory system playing a compensatory role for reading in the disorder. We are not aware of other studies that examine (separately in dyslexic and typically developing individuals) correlations between measures of reading and direct or indirect measures of declarative memory.
4.3. Autism Spectrum Disorder
In ASD (also referred to here as autism), research supports the first prediction (an increased dependence on behavioral strategies that rely on declarative memory), and has begun to shed light on the second prediction (an increased reliance on the brain structures underlying declarative memory). We are not aware of any research on individuals with autism pertaining to the third (correlational) prediction.
Behavioral evidence indicates that declarative memory plays an important role in compensating for a range of language, pragmatic, and social problems in the disorder. First, many individuals with autism use formulaic speech (memorized, i.e., chunked, phrases and sentences), which can compensate for both linguistic and social deficits (Dobbinson et al., 2003; Tager-Flusberg and Calkins, 1990; Walenski et al., 2006). Second, they often explicitly memorize and apply rules, scripts, and event schemas to compensate for social deficits (Bishop and Norbury, 2002; Eales, 1993; Landa, 2000; Portman, 2006). For example, they may memorize entire scripts for common social situations and scenarios, a strategy that is often evident in the sometimes overly formal tone of their language (Eales, 1993; Portman, 2006). Third, whereas individuals with autism can learn to perform specific tasks of theory of mind, this learning does not appear to apply more broadly (Klinger et al., 2007); it has been suggested that these individuals learn explicit rules for the specific tasks rather than implicitly learning underlying principles (Klinger et al., 2007). Similarly, it has been suggested that the automatic implicit ability to attribute mental states to others is dysfunctional in ASD, but at least individuals with Asperger syndrome (American Psychiatric Association, 2000) can compensate by learning an explicit theory of mind (Frith, 2004; Senju et al., 2009). Fourth, whereas typically developing individuals can implicitly learn fuzzy mental categories (where some category members are more prototypical than others), a skill that may depend on procedural memory (Knowlton and Squire, 1993), individuals with ASD appear to show impairments at this skill, and instead attempt to rely on a memorized set of explicit rules for category membership (e.g., a dog has to bark, have two ears, four legs, and a tail) (Klinger and Dawson, 2001). Fifth, behavioral therapeutic approaches for autism often target explicit rules, scripts, and schemas (Charlop-Christy and Kelso, 2003; Ganz et al., 2008; Krantz and McClannahan, 1993, 1998). For example, “Social Stories”, a type of cognitive behavioral therapy, is a popular and often effective treatment in which individuals with ASD are provided with sentences and pictures that explicitly guide them through specific types of social situations, such as how to behave at a birthday party or how to get friends to look at a toy (Crozier and Tincani, 2007; Ivey et al., 2004; Thiemann and Goldstein, 2001). More generally, Social Stories, as well as and other types of cognitive behavioral therapy (often referred to simply as CBT, these are goal-oriented therapies that rely heavily on explicit knowledge), have been shown to improve social skills as well as associated symptoms like anxiety in children with autism (Crozier and Tincani, 2007; Ivey et al., 2004; Lang et al., 2010; Thiemann and Goldstein, 2001).
The second (neural) prediction is supported by at least some functional neuroimaging research, though further, more targeted, studies of ASD are clearly needed. One recent fMRI study examined the neural basis of social motivation deficits in autism with a reward anticipation task (Dichter et al., 2012). Adults with ASD showed decreased activation in the nucleus accumbens (part of the basal ganglia) and increased activation in the hippocampus while performing the task, as compared to typically developing controls, even though there were no behavioral differences between the groups. The authors suggest this pattern may reflect an MTL memory-based compensatory mechanism in the ASD subjects. In an fMRI study of children with ASD, increased activation in the parahippocampal gyrus was observed during the processing of social stimuli (gaze processing) in more as compared to less severe cases of ASD (Vaidya et al., 2011). Activation was not compared between the more severe cases of ASD and typically developing controls. The authors suggest that the results may reflect MTL-based associative learning of the task, and that this compensation was relied on more in individuals with more severe underlying impairments. Overall, these studies suggest that children and adults with autism may indeed rely on declarative memory and its neural substrates to compensate for disparate tasks and functions. However, it is important to emphasize that many functional imaging studies of ASD have not reported increased activation in medial temporal or other regions associated with declarative memory. Further studies directly testing the declarative memory compensation hypothesis in ASD seem warranted, in particular to investigate under what conditions (e.g., which types of subjects, which types of tasks) such compensation might occur.
4.4. Tourette Syndrome
In Tourette syndrome, therapeutic evidence provides some support for the first (behavioral) prediction. Habit reversal training, a type of cognitive behavioral therapy in which the individual increases conscious awareness of a tic and learns to explicitly perform a competing response, can successfully suppress tics in the disorder (Frank and Cavanna, 2013; Himle et al., 2006). Indeed, it is the most widely used behavioral therapy for Tourette syndrome, and has been shown to be highly effective (Frank and Cavanna, 2013; Himle et al., 2006). Although various explanatory accounts for habit reversal training have been proposed (Himle et al., 2006), its dependence on learned explicit responses indicates that it is based at least in part on declarative memory. Note that any later automatization of these responses does not preclude an initial dependence on explicit knowledge in declarative memory, nor even on later implicit declarative memory processes. Other cognitive behavioral therapies may also be effective in Tourette syndrome (Frank and Cavanna, 2013). Finally, note that the apparent role of declarative memory in inhibitory learning (i.e., learning to suppress a response) (Chan et al., 2001) underscores the plausibility of declarative memory playing an inhibitory role in suppressing tics in Tourette syndrome. Additional studies directly testing the behavioral prediction in the disorder seem warranted.
Although we are aware of no non-correlational evidence that is relevant to the second (neural) prediction, neuroimaging evidence supports the third (correlational) prediction for Tourette syndrome. Specifically, larger hippocampal and other medial temporal lobe volumes are associated with lower tic severity in both children and adults with the disorder (Peterson et al., 2007; Plessen et al., 2009; Worbe et al., 2010). Since larger hippocampal volumes are linked to better declarative memory (Protopopescu et al., 2008; Schofield et al., 2009), this suggests that tic suppression may depend at least in part on this memory system. Indeed, in one study, hippocampal volumes correlated negatively with tic severity as well as with co-occurring OCD symptoms (Peterson et al., 2007). The authors suggest a compensatory role for the hippocampus in attenuating or controlling symptoms of both disorders (Peterson et al., 2007).
4.5. Obsessive-Compulsive Disorder
In OCD, the evidence, which is somewhat similar to that for Tourette syndrome, provides some support for all three predictions. First, just as in Tourette syndrome, habit reversal training and other cognitive behavioral therapies have been shown to be effective (March, 1995; Watson and Rees, 2008). Indeed, cognitive behavioral therapies are among the most widely used treatments for OCD, and may have larger treatement effects than pharmacological approaches (Watson and Rees, 2008). Moreover, they have been recommended as the first-line treatment, at least for pediatric OCD and for adolescents with OCD who have mild to moderate symptoms (Watson and Rees, 2008). Additional research is needed to examine the behavioral prediction for OCD, including the possibility (analogous to Tourette syndrome) that declarative memory may be involved in inhibitory learning in the suppression of obsessions and compulsions.
Second, a number of functional neuroimaging studies directly support the neural prediction. PET and fMRI studies of procedural learning, using both serial reaction time tasks (which probe sequence learning) and weather prediction tasks (which probe probabilistic category/rule learning), have found that while adults with OCD show equivalent learning as compared to typically developing controls, they show altered patterns of brain activation. In particular, they show less activation in basal ganglia circuits, which are normally recruited for these procedural learning tasks, but more in declarative memory brain substrates (hippocampal and parahippocampal regions) (Rauch et al., 1997; Rauch et al., 2007; Rauch et al., 2001; Roth et al., 2003); see Figure 3. This suggests that individuals with OCD can, at least in some circumstances, fully compensate with declarative memory for tasks that normally depend on procedural memory. Since primary OCD symptoms might be at least partly explained by dysfunctional procedural memory (Rauch and Savage, 2000; Rauch et al., 2007), these findings suggest the possibility that such compensation may also play a more general role in alleviating the symptoms of OCD. Another study of adults with OCD examined the Tower of London task (van den Heuvel et al., 2005), which probes executive functioning and planning, domains that have been found to be impaired in OCD (Greisberg and McKay, 2003). The study found less activation in frontal and basal ganglia structures in the subjects with OCD than in typically developing controls, but more and “presumably compensatory” activation of parahippocampal cortex, among other regions (van den Heuvel et al., 2005). Performance at the task was worse in the subjects with OCD than in controls, suggesting that, unlike in the tasks designed to probe procedural learning, declarative memory may only partially compensate for certain executive and planning deficits in the disorder. Overall, the functional imaging evidence suggests that adults with OCD can use declarative memory to either fully or partially compensate for at least certain frontal/basal-ganglia based tasks.
Figure 3.
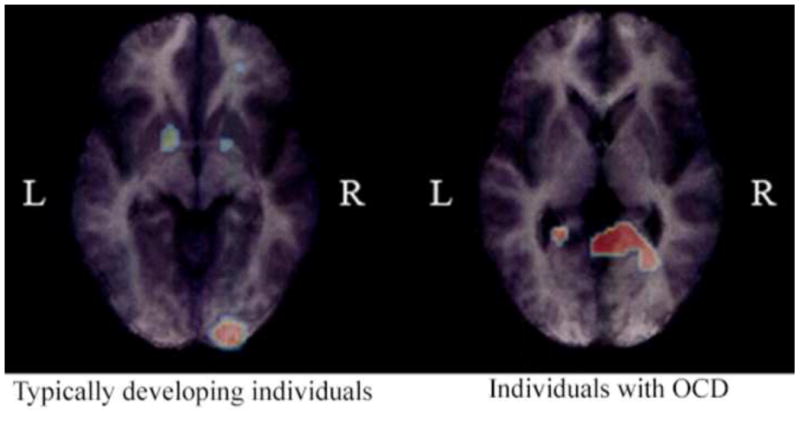
Functional imaging evidence from adults with obsessive-compulsive disorder (OCD). Typically developing adults and adults with OCD were given a procedural learning task (the serial reaction time task) during positron emission tomography scanning (Rauch et al., 1997). The two groups showed no behavioral differences on the task. However, their functional neuroanatomy differed. Whereas the typically developing group showed bilateral basal ganglia (and other) activation, the OCD group instead activated medial temporal lobe structures, including the hippocampus bilaterally. This suggests that OCD individuals may use declarative memory to fully compensate for at least some tasks that normally depend on procedural memory. L = left side; R = right side. Figure adapted from Rauch et al. (1997).
Third, correlational evidence for declarative memory-based compensation is found in OCD, similarly to Tourette syndrome. Specifically, OCD symptoms such as obsessive thoughts and actions have been found to correlate negatively with hippocampal and other medial temporal lobe volumes (Carmona et al., 2007; Peterson et al., 2007; van den Heuvel et al., 2009). Note however that one study (Kwon et al., 2003) found a positive correlation between OCD symptoms and glucose metabolism (as measured by PET) in the hippocampus. Further studies examining such correlations seem warranted.
5. Discussion
In summary, accumulating evidence suggests the following. First, across the five neurodevelopmental disorders examined here (SLI, dyslexia, ASD, Tourette syndrome, and OCD), declarative memory generally remains sufficiently intact to learn and retain the sort of knowledge that could be used for many compensatory strategies. Second, this memory system does indeed underlie compensation for wide range of impairments across the disorders, supporting the declarative memory compensation hypothesis.
5.1. The Nature of Declarative Memory Compensation
Declarative memory can apparently compensate for deficits in at least two ways. First, it can to some extent actually carry out various functions that are comprised in the disorders, such as grammar in SLI and dyslexia, and sequence learning in OCD. That is, it can compensate by acting as a redundant (backup) mechanism for the systems that generally support these functions but are compromised in the disorders. In some cases individuals with neurodevelopmental disorders seem to rely on declarative memory-based strategies that are also used, though to a lesser extent, by typically developing individuals: for example, chunking complex forms in SLI, whole word reading in dyslexia, and perhaps even formulaic speech in autism (Ullman, 2005a; Ullman et al., 2008). Other strategies appear to be qualitatively different from those used by typically developing individuals, such as social scripts in autism. Evidence from populations other than those with developmental disorders underscores the plausibility of a redundant role (Schlaggar and McCandliss, 2007) for declarative memory (Ullman, 2004, 2005b). For example, the memory system has been found to support grammar both in adult second language learners (Ullman, 2001, 2005a, In Press-a) and individuals with aphasia (see section 5.3).
Second, declarative memory seems to support the learning and use of strategies that allow individuals to inhibit, control, or otherwise minimize inappropriate behaviors. Notably, such strategies may help individuals with Tourette syndrome or OCD control their tics, obsessions, and compulsions. For certain strategies, such as the use of social scripts by individuals with ASD, declarative memory can be thought of both as playing a redundant functional role and as controlling inappropriate behaviors. Finally, given the flexibility of declarative memory, it seems likely that other types of compensation by this system will also be found.
Evidently, declarative memory does not fully compensate for all impairments in the disorders. The compensation hypothesis leads to a number of predictions regarding compensatory success, which should vary as a function of several factors. The degree to which compensation is successful should increase with the functionality of declarative memory – in particular those aspects of declarative memory that are involved in the relevant compensatory strategies (e.g., verbal or non-verbal). Compensatory success should decrease, however, with the severity of the underlying problem. Additionally, some deficits are more likely to be amenable to effective compensation than others, and some strategies should lead to better compensation. For example, whereas declarative memory may compensate for local grammatical relations via chunking, this strategy is less likely to succeed for long-distance dependencies (e.g., “John walks” is easier to memorize as a chunk than where John and walks are separated by additional words or phrases) (Ullman, 2005a). It remains to be seen whether even the best compensatory strategies (e.g., perhaps chunking for local grammatical relations) are as efficient as the processes generally used in typically developing individuals. Finally, impairments that cannot be easily compensated for with declarative memory should be particularly characteristic of the disorders – for example, long-distance dependencies in SLI, or reading novel words in dyslexia. Similarly, to the extent to which declarative memory-based strategies in Tourette syndrome or OCD rely on executive control to implement memorized rules, any executive impairments in individuals with these disorders should limit the effectiveness of such strategies.
5.2. Implications for Therapy, Diagnosis and Basic Research
The declarative memory compensation hypothesis has significant implications for therapy, diagnosis and basic research. First, it suggests that therapies should target not only the disordered behaviors and neurobiology, but also declarative memory and its neurobiological substrates. Such an approach is promising not just because of the power and flexibility of this memory system, but also because the system is quite well understood, leading to a number of specific therapeutic predictions and recommendations, including the following. Behavioral therapies that depend on declarative memory (including cognitive behavioral therapies or any other treatments that rely heavily on explicit knowledge) could be further enhanced by incorporating approaches that have been shown to improve learning and retention in this memory system, such as deep vs. shallow encoding, spaced vs. massed presentation, use of imageable items, sleep, exercise, and stress avoidance (Delaney and Knowles, 2005; Erickson et al., 2011; Marshall and Born, 2007; Prado and Ullman, 2009; Wolf, 2009). For example, given that sleep may promote consolidation in declarative memory (Marshall and Born, 2007), sufficient sleep may be important for the development of compensatory strategies in this system. (Note that sleep may also enhance learning in procedural memory; more generally, as discussed in section 4, we emphasize that compensation by declarative memory should not preclude either compensation by other means, or improvements in any systems that are abnormal in the disorders, such as procedural memory.) Pharmacological agents that have been found to enhance declarative memory, such as acetylcholinesterase inhibitors, methylphenidate, or memantine (Dommett et al., 2008; Repantis et al., 2010), may also be useful, whether alone or in combination with behavioral therapies. Therapies that depend on declarative memory should be designed to minimize the involvement of other functions that are compromised in the disorders, such as phonological processing and working memory, since their involvement would impede such therapies. Therapies that rely on declarative memory may be especially effective in groups or individuals with better declarative memory, allowing for more individualized therapeutic approaches – for example, females as compared to males (Ullman et al., 2008), individuals with particular genotypes (see below), or those who show direct evidence of enhanced declarative memory (see section 2). In contrast, those with worse declarative memory should benefit from behavioral or pharmacological approaches (e.g., using spaced presentation, or memantine) that may improve their learning in the memory system, which in turn should lead to better compensation with this system. Finally, an understanding of declarative memory-based compensation may also allow the development of therapies that prevent such compensation (e.g., by focusing on long-distance dependencies, or relying more on working memory if it is dysfunctional), with the goal of increasing reliance on the compromised system(s) rather than encouraging compensation. Consistent with the principles of constraint-induced therapy (Cherney et al., 2008b; Pulvermuller et al., 2001; Taub et al., 1999), such an approach could potentially augment functionality, though in this case by targeting and improving the underlying neurocognitive mechanisms (e.g., procedural memory) rather than the surface behavior or function (e.g., grammar or movement). This constraint-induced targeting of mechanisms or systems rather than behaviors may constitute a useful extension of traditional constraint-induced therapeutic approaches.
Second, the hypothesis has various diagnostic implications. Perhaps most importantly, even reasonably effective compensation by declarative memory may delay or even preclude diagnosis, especially where declarative memory is strong and the underlying impairments are not too severe. Thus declarative memory-based compensation may be responsible for a fair degree of underdiagnosis. Our understanding of declarative memory allows this problem to be at least partially addressed. For example, diagnostic instruments should target symptoms and deficits that cannot easily be compensated by declarative memory (e.g., long-distance dependencies in SLI), and hence would be revealed even in individuals with superior compensation. Note that such individuals should still benefit from diagnosis, since even their sub-clinical symptoms may be detrimental, and may be ameliorated with therapy. Investigation of such individuals may also lead to a better understanding of how the disorders should be defined. Population based studies (i.e., that examine the broader population rather than targeting already-identified or at-risk individuals) should reveal the prevalence of such underdiagnosis. Importantly, since it is precisely those individuals who have escaped diagnsosis who may be compensating most effectively, such studies should also help elucidate the nature and limits of declarative memory-based compensation.
The prediction that compensation by declarative memory should lead to underdiagnosis has various further implications. First of all, it might help to explain one of the major conundrums regarding all of the neurodevelopmental disorders examined here: why they appear to be more prevalent in males than females (Geller, 2006; Rutter et al., 2003; Scharf et al., 2012). Because females seem to have better declarative memory, even as young children (Ullman et al., 2008), females with the disorders should tend to compensate more successfully than males. As a consequence, females on the whole should show fewer (obvious) impairments than males, and so should be identified and diagnosed less often, even if males and females are in fact afflicted with underlying neurobiological abnormalities at the same rate (Ullman et al., 2008). This line of reasoning leads to testable predictions, including the following. First, the generally observed male-to-female ratio should be higher than the actual sex ratio of the true population with each disorder, as identified by the underlying pathology. Second, for a given level of underlying pathology, females should show fewer or less severe symptoms than males, even to the point of escaping diagnosis. However, at different levels of pathology, different degrees of female compensation and thus different sex ratios should be observed. At mild to moderate pathological levels females could compensate effectively, even to the point of avoiding diagnosis, thus leading to an apparently higher male prevalence. In contrast, at severe levels the sex ratio should tend to even out, since it should be difficult for any individuals (even females with their better declarative memory) to compensate sufficiently to avoid diagnosis. Finally, in the sample of those who are actually diagnosed with the disorder, females should tend to have worse underlying pathology than males, since those with similar pathology to males should be less likely to be diagnosed, biasing the sample of diagnosed females towards those with worse pathology. We emphasize that that this novel account based on compensation by declarative memory is not mutually exclusive with other accounts for the observed sex ratios in these disorders (Baron-Cohen et al., 2005; Hartung and Widiger, 1998; Hawke et al., 2009; Holden, 2005; Levy et al., 2011; Raz et al., 1994; Sawada and Shimohama, 2000), and more than one account may explain the pattern. Future studies directly examining the testable predictions of the sex difference compensation hypothesis proposed here should reveal its explanatory power.
Generalizing the same point beyond sex differences, any individual or group with better declarative memory should compensate for their impairments more effectively, and thus should also be at increased risk for underdiagnosis. For example, individuals with the disorders who have genotypes that confer declarative memory advantages should, like females, compensate better and thus be diagnosed less frequently. Some evidence suggests that val/val homozygotes of the val66met single nucleotide polymorphism of the BDNF gene may have declarative memory advantages as compared to met carriers, and possibly likewise for APOE non-E4 carriers as compared to E4 carriers , though the findings are not consistent (Green and Dunbar, 2012; Molendijk et al., 2012). If these or other genotypes are shown to be reliably associated with better declarative memory, they may be “protective” for the disorders, simply (or at least in part) because they allow for greater compensation.
Such variability in compensation due to variability in declarative memory functionality may be found not only between subjects, but also within subjects, though it is not clear whether individuals showing such intra-subject variability would be diagnosed at a lower rate. For example, since evidence suggests that declarative memory improves at higher estrogen levels (Maki and Resnick, 2000; Sherwin, 1998), women with the disorders may be expected to show superior compensation and thus fewer symptoms at high-estrogen points of their menstrual cycle. Some evidence is consistent with this prediction. Tic severity in women with Tourette syndrome has been found to improve at high-estrogen points of the menstrual cycle as compared to low-estrogen points (Sandyk et al., 1987; Schwabe and Konkol, 1992). Similarly, OCD symptoms such as obsessive thoughts and actions seem to decrease at high-estrogen points of the menstrual cycle (Vulink et al., 2006). However, at least one study has not found this pattern, in Tourette syndrome (Kompoliti et al., 2001). Additionally, it is important to point out that such correlations may instead or additionally be explained by other mechanisms, such the action of estrogen on dopamine (Becker, 1990; Jacobs and D'Esposito, 2011), a neurotransmitter that plays a role in both Tourette syndrome and OCD (Buse et al., 2013; Denys et al., 2004). Further research seems warranted to examine whether higher estrogen levels are indeed reliably associated with fewer symptoms in these and other disorders, and what mechanisms underlie such patterns.
Finally, the declarative memory compensation hypothesis should elucidate our understanding of the neurodevelopmental disorders themselves. Some or perhaps much of their behavioral profiles might be due not to the underlying dysfunctions, but to the roles and characteristics of declarative memory. Well-studied examples may include the inappropriate use of memorized formulas by individuals with autism, and the often formal tone of their language. Additionally, improvements during the course of childhood may be explained at least in part by increasing declarative memory-based compensation (Chiarello et al., 2006; Shaywitz et al., 2003). Such increasing compensation over time could be due to strategies gradually being learned as well as to maturational changes in declarative memory, since evidence suggests that learning in this system improves during childhood (Ullman, 2005a). Findings that individuals with the disorders may compensate successfully for procedural memory deficits as adults (Rauch et al., 1997; Rauch et al., 2007; Rauch et al., 2001; Roth et al., 2003) but apparently less so as children (Barnes et al., 2010; Howard et al., 2006; Lum et al., 2012; Marsh et al., 2004; Vicari et al., 2003) may in part be explained by such changes over time. Finally, the most striking and persistent anomalies in the disorders might be not just those functions that are most impaired, but also those that cannot easily be compensated for.
An interesting question is why declarative memory seems to be relatively spared in so many neurodevelopmental disorders. One possibility is that since declarative memory functionality develops quite late, with abilities peaking in late adolescence and young adulthood (Ullman, 2005a, In Press-b), the early onset of dysfunction in developmental disorders might tend to leave declarative memory relatively unaffected. Another possibility is that many individuals who might otherwise be diagnosed with one of the disorders discussed here are not given such a diagnosis due to accompanying impairments of declarative memory (which could be caused by the same pathological processes that result in other abnormalities). In particular, if such impairments lead to dense amnesia, such that they significantly affect learning non-episodic (as well as episodic) knowledge (c.f., Vargha-Khadem et al., 1997), this may lead to lower IQ and more generally low functionality, thus precluding such diagnoses (Nicolson and Fawcett, 2007; Ullman and Pierpont, 2005). In other words, many individuals may have the same underlying problems as are found in these disorders, but are not given such a diagnosis due to accompanying declarative memory (and perhaps other) impairments. Thus, it might be the case that these disorders are under-diagnosed not only because of highly successful compensation due to intact declarative memory (see above), but also because of significantly compromised declarative memory that leads to other difficulties, perhaps preventing an appropriate diagnosis of the disorder. Note that in the case of autism, individuals with low IQ are still diagnosed with the disorder, and indeed such low functioning individuals may have impairments of declarative memory (see section 3.3).
5.3. Other Disorders
The declarative memory compensation hypothesis may extend beyond the five disorders examined here. Neurodevelopmental disorders that are comorbid with some of these disorders, that show similar neuroanatomical profiles (e.g., frontal, basal ganglia, or cerebellar anomalies) and relatively spared declarative memory, and that have been found to have a higher prevalence in males than females, are particularly likely candidates. Both attention deficit hyperactivity disorder (ADHD) (Froehlich et al., 2007; Krain and Castellanos, 2006) and developmental coordination disorder (DCD) (Barnhart et al., 2003; Zwicker et al., 2009) fit the bill. Note that declarative memory can underlie even some aspects of motor function (Keisler and Shadmehr, 2010), and thus could play a compensatory role in motor disorders such as DCD. Indeed, some evidence hints at declarative memory compensation in both disorders. Analogous to both Tourette syndrome and OCD, symptom severity in ADHD has been found to correlate negatively with hippocampal volumes (Peterson et al., 2007; Plessen et al., 2006), findings that have been attributed to a hippocampal-based compensatory response (Peterson et al., 2007; Plessen et al., 2006). Also as with Tourette syndrome and OCD, females with ADHD seem to have less severe symptoms at higher than at lower estrogen points in the menstrual cycle (Quinn, 2005; Stevenson and Williams, 2000). Although less research has been carried out on DCD, one recent fMRI study of motor function found increased MTL (parahippocampal) activation in children with DCD as compared to typically developing peers, despite equivalent performance (Zwicker et al., 2010), suggesting the possibility of declarative memory-based compensation of motor function in this disorder. Further investigation of the compensation hypothesis in these disorders seemed desirable.
Compensation by declarative memory may also be found in some adult-onset disorders. Likely disorders are those with somewhat similar cognitive and/or anatomical profiles to the neurodevelopmental disorders examined here. First of all, a number of studies suggest that declarative memory may underlie compensation and recovery in aphasia, including in agrammatic aphasia (which is associated with the broader designations of Broca's and non-fluent aphasias; Tesak and Code, 2008). Just as in SLI, agrammatic aphasics have been found to chunk complex linguistic forms (Drury and Ullman, 2002). As in some of the neurodevelopmental disorders examined here, behavioral therapies based on explicit knowledge or strategies are commonly used for the treatment of aphasias. For example, the computerized AphasiaScripts™ program, which involves the repetitive practice of individualized scripts, promotes the memorization of phrases and sentences to use in everyday situtations. Initial studies suggest that the program seems to be effective in Broca's and other aphasics: patients improve on the scripts, and in fact more generally improve their scores on standardized aphasia tests (Cherney, 2012; Cherney and Halper, 2008; Cherney et al., 2008a). Pharmacological evidence is also consistent with declarative memory-based compensation: both cholinesterase inhibitors and memantine (both which can enhance declarative memory; see section 5.2) have also been shown to enhance recovery in individuals with Broca's and other aphasias (Berthier et al., 2009; Berthier et al., 2011). Additionally, electrophysiological evidence is suggestive: similar to SLI, agrammatic aphasics have been found to elicit an N400 in response to syntactic anomalies (Hagoort et al., 2003). And recent neuroimaging evidence suggests that the hippocampus may be important in aphasia recovery. One study found that the success of Broca's aphasics at relearning words in a training paradigm correlated positively with activation in the hippocampus (and other structures) (Menke et al., 2009). Another study found that Broca's and global aphasics' success at relearning words in the same training paradigm correlated positively with the integrity of the hippocampus, but not with overall lesion size or global brain integrity (Meinzer et al., 2010). These studies reveal the importance of the hippocampus in aphasia recovery, specifically at relearning real words; it remains to be seen whether the same patterns are observed for learning complex forms, as may be expected if aphasics can rely on declarative memory for grammatical compensation. Finally, some evidence suggests the possibility of an intriguing sex difference in aphasia recovery, with women showing better recovery than men in some measures of language function (Basso et al., 1982; Pizzamiglio et al., 1985), although this pattern has not been reliably observed (Basso, 1992; Pedersen et al., 1995). Examination of this possible sex difference from the perspective of the compensation hypothesis may clarify these inconsistencies in aphasia recovery. More generally, the compensatory role of declarative memory in aphasia seems to warrant further study.
Cognitive and perhaps even motor deficits in Parkinson's disease (PD) may also be compensatory targets, especially in earlier stages of the disorder when declarative memory tends to be relatively spared. Indeed, some evidence suggests declarative memory-based compensation in PD. Analogously to findings with OCD (see section 4.5; van den Heuvel et al., 2005), in a Tower of London task patients with mild PD showed decreased basal ganglia activation, but increased activation in the hippocampus, as compared to normal controls; additionally, these PD patients showed normal performance on the task, suggesting that compensation was successful (Dagher et al., 2001). Another study found a similar pattern in mild to moderate PD patients (Beauchamp et al., 2008). In yet another study, mild PD patients learned the weather prediction task while showing the same pattern of decreased basal ganglia activation and increased hippocampal activation (Moody et al., 2004). In each of these studies, the authors suggest compensation by the declarative memory system (Beauchamp et al., 2008; Dagher et al., 2001; Moody et al., 2004). Other lines of evidence also suggests that PD patients may depend more on declarative memory, not only for the weather prediction task (Shohamy et al., 2004), but also for sequence learning (Carbon et al., 2010; Gobel et al., 2013) and grammar (Johari et al., In Preparation; Ullman and Estabrooke, 2004). And, analogous to OCD and Tourette syndrome, motor symptom severity in pre-menopausal women with Parkinson's disease has been found to worsen during low estrogen points of the menstrual cycle as compared to high estrogen points (Quinn and Marsden, 1986). Interestingly, as in the neurodevelopmental disorders discussed above, PD appears to be more prevalent in males than females (Elbaz et al., 2002; Gillies et al., 2014). It is possible that this sex difference might also be partially explained by better female compensation – though as discussed above, we emphasize that the sex difference may instead or additionally be explained by other accounts (Gillies et al., 2014). Finally, an intriguing possibility that might warrant investigation is whether the delayed onset of PD symptoms relative to neuronal degeneration might be due in part to compensation (Gillies et al., 2014) by declarative memory (perhaps especially of non-motor symptoms, which might be easier to compensate for by this system). Further studies examining declarative memory-based compensation in Parkinson's disease (including for motor symptoms) seem warranted.
In contrast, we would not expect declarative memory-based compensation in disorders in which this memory system is not functional. It is thus not predicted in disorders such as anterograde amnesia or Alzheimer's disease, at least to the extent that learning non-episodic knowledge is impaired. Therefore it is not the case that declarative memory-based compensation is always expected. Rather, it should only be found where this memory system remains functional, and thus can play a compensatory role.
5.4. Gaps in the Literature and Future Research
We have identified a number of gaps and weaknesses in previous research relevant to the declarative memory compensation hypothesis. Indeed, as can be seen in Table 1, not all predictions have been examined in all five neurodevelopmental disorders, or by all methodologies. For example, previous research has investigated such compensation less in Tourette syndrome than in SLI. Even less research relevant to the compensation hypothesis has examined other disorders, such as ADHD and developmental coordination disorder. The hypothesis also warrants further investigation in agrammatic and other aphasias, as well as Parkinson's disease. Given the flexibility and power of declarative memory and its resulting ability to compensate for a wide range of impairments, the compensation hypothesis should also be considered in multiple developmental, psychiatric, and neurological disorders disorders not discussed here (e.g., schizophrenia, Huntington's disease, traumatic brain injury, alexia, and dysgraphia). Declarative memory based compensation might also take place in normal aging (Rieckmann and Backman, 2009; Rieckmann et al., 2010).
These gaps both limit our current understanding of the nature of declarative memory-based compensation, and provide a useful roadmap for guiding future research. Additionally, our synthesis and review of the literature has raised questions that can be targeted by further studies. For example, it remains to be seen whether the free recall difficulties often found in the disorders might limit compensation by hindering the recall of compensatory knowledge, or whether perhaps the context in which strategies are learned might cue and thus facilitate their retrieval. Similarly, additional studies may elucidate whether the problems that individuals with ASD have in learning person- and emotion-related memories might contribute to difficulties in learning social strategies. And as we have seen, the therapeutic, diagnostic, and basic research implications generated by the compensation hypothesis have raised numerous questions, and generate testable predictions. These predictions should be directly examined in future studies, not only for the five disorders focused on here, but also for additional disorders in which declarative memory may also play compensatory roles.
6. Conclusion
In this paper we presented and examined the declarative memory compensation hypothesis. The hypothesis posits that declarative memory should play compensatory roles for a range of impairments across disorders, as long as the system remains functional. We focused on five neurodevelopmental disorders: SLI, dyslexia, ASD, Tourette syndrome, and OCD.
First, we presented evidence that declarative memory generally remains sufficiently functional across these disorders to allow it to play compensatory roles. To our knowledge, this is the first review of the status of declarative memory across these disorders. Its apparent functionality in the disorders may be of interest and have implications beyond the compensation hypothesis.
We next laid out the major predictions for the compensation hypothesis, and reviewed a wide range of evidence for all five disorders. Behavioral evidence showing how individuals compensate using declarative memory (for example, by relying on chunking, semantics, or explicit rules and strategies) is complemented by electrophysiological and neuroimaging evidence implicating the brain system underlying declarative memory. Additionally, correlational evidence from both behavioral and neuroimaging studies suggests that better declarative memory is associated with fewer impairments, consistent with better compensation by the system. Overall, the evidence supports the hypothesis that declarative memory can at least partially compensate for a range of impairments across the disorders. Future studies should further elucidate the nature and limits of this compensation.
The compensation hypothesis has therapeutic, diagnostic, and other implications. It suggests specific behavioral and pharmacological treatment approaches for the disorders, based on our independent understanding of declarative memory. It predicts that compensation may lead to underdiagnosis, and proposes ways in which such underdiagnosis may be avoided. Underdiagnosis due to compensation may also help explain the apparently greater prevalence of the disorders in males than females: female advantages at declarative memory should lead to better compensation, potentially resulting in higher rates of underdiagnosis. The hypothesis may also account for aspects of the behavioral profiles of the disorders, which may be explained not just by the underlying dysfunctions, but also by the roles and characteristics of declarative memory in its compensatory role. Finally, the compensation hypothesis may extend to additional developmental as well as adult-onset disorders, including ADHD, developmental coordination disorder, aphasia, and Parkinson's disease.
In conclusion, the declarative memory compensation hypothesis may contribute significantly to our understanding of a number of disorders, with potentially important impacts for basic and translational research.
Highlights.
Declarative memory compensates for multiple deficits across disorders
The disorders include specific language impairment, dyslexia, autism spectrum disorder, Tourette syndrome, and OCD
Compensation by declarative memory has therapeutic, diagnostic, and basic research implications
Acknowledgments
Support for this project was provided to MTU by the National Institute of Health under RO1 HD049347, the Simons Foundation, and the Mabel H. Flory Trust.
References
- Alexander AW, Slinger-Constant AM. Current status of treatments for dyslexia: critical review. J Child Neurol. 2004;19:744–758. doi: 10.1177/08830738040190100401. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Peterson BS. Testing the prenatal hormone hypothesis of tic-related disorders: Gender identity and gender role behavior. Dev Psychopathol. 2004;16:407–420. doi: 10.1017/s095457940404458x. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association; Washington, D.C.: 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. American Psychiatric Association; Washington, D.C.: 2013. [Google Scholar]
- Aylward EH, Richards TL, Berninger VW, Nagy WE, Field KM, Grimme AC, Richards AL, Thomson JB, Cramer SC. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61:212–219. doi: 10.1212/01.wnl.0000068363.05974.64. [DOI] [PubMed] [Google Scholar]
- Barense MD, Groen IIA, Lee ACH, Yeung LK, Brady SM, Gregori M, Kapur N, Bussey TJ, Saksida LM, Henson RNA. Intact Memory for Irrelevant Information Impairs Perception in Amnesia. Neuron. 2012;75:157–167. doi: 10.1016/j.neuron.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KA, Howard JHJ, Howard DV, Gilotty L, Kenworthy L, Gaillard WD, Vaidya CJ. Intact implicit learning of spatial context and temporal sequences in childhood autism spectrum disorder. Neuropsychology. 2008;22:563–570. doi: 10.1037/0894-4105.22.5.563. [DOI] [PubMed] [Google Scholar]
- Barnes KA, Howard JHJ, Howard DV, Kenealy L, Vaidya CJ. Two forms of implicit learning in childhood ADHD. Developmental Neuropsychology. 2010;35:494–505. doi: 10.1080/87565641.2010.494750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart RC, Davenport MJ, Epps SB, Nordquist VM. Developmental coordination disorder. Phys Ther. 2003;83:722–731. [PubMed] [Google Scholar]
- Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310:819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- Barquero LA, Davis N, Cutting LE. Neuroimaging of reading intervention: A systematic review and activation likelihood estimate meta-analysis. PLoS ONE. 2014;9:e83668. doi: 10.1371/journal.pone.0083668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso A. Prognostic factors in aphasia. Aphasiology. 1992;6:337–348. [Google Scholar]
- Basso A, Capitani E, Moraschini S. Sex differences in recovery from aphasia. Cortex. 1982;18:469–475. doi: 10.1016/s0010-9452(82)80044-0. [DOI] [PubMed] [Google Scholar]
- Basso A, Gardelli M, Grassi MP, Mariotti M. The role of the right hemisphere in recovery from aphasia. Two case studies Cortex. 1989;25:555–566. doi: 10.1016/s0010-9452(89)80017-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Dagher A, Panisset M, Doyon J. Neural substrates of cognitive skill learning in Parkinson's disease. Brain Cogn. 2008;68:134–143. doi: 10.1016/j.bandc.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: Sex differences in dopamine release. Synapse. 1990;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Ben-Dror I, Pollatsek A, Scarpati S. Word identification in isolation and in context by college dyslexic students. Brain Lang. 1991;40:471–490. doi: 10.1016/0093-934x(91)90144-p. [DOI] [PubMed] [Google Scholar]
- Ben Shalom D. Memory in autism: review and synthesis. Cortex. 2003;39:1129–1138. doi: 10.1016/s0010-9452(08)70881-5. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Romano JC, Howard J, James H, Howard DV. Two forms of implicit learning in young adults with dyslexia. Ann N Y Acad Sci. 2008;1145:184–198. doi: 10.1196/annals.1416.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthier ML, Green C, Lara JP, Higueras C, Barbancho MA, Davila G, Pulvermuller F. Memantine and constraint-induced aphasia therapy in chronic poststroke aphasia. Ann Neurol. 2009;65:577–585. doi: 10.1002/ana.21597. [DOI] [PubMed] [Google Scholar]
- Berthier ML, Pulvermuller F, Davila G, Casares NG, Gutierrez A. Drug therapy of post-stroke aphasia: a review of current evidence. Neuropsychol Rev. 2011;21:302–317. doi: 10.1007/s11065-011-9177-7. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Norbury CFN. Exploring the borderlands of autistic disorder and specific language impairment: A study using standardised diagnostic instruments. J Child Psychol Psyc. 2002;43:917–929. doi: 10.1111/1469-7610.00114. [DOI] [PubMed] [Google Scholar]
- Boucher J, Mayes A. Memory in ASD. In: Fein D, editor. The neuropsychology of autism. Oxford University Press; 2011. [Google Scholar]
- Boucher J, Mayes A. Memory in ASD: Have we been barking up the wrong tree? Autism. 2012:1–9. doi: 10.1177/1362361311417738. [DOI] [PubMed] [Google Scholar]
- Boucher J, Mayes A, Bigham S. Memory, language and intellectual ability in low-functioning autism. In: Boucher J, Bowler DM, editors. Memory in Autism. Cambridge University Press; 2008. pp. 268–290. [Google Scholar]
- Boucher J, Mayes A, Bigham S. Memory in autistic spectrum disorder. Psychol Bull. 2012;138:458–496. doi: 10.1037/a0026869. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL. Developmental Disorders of the Frontostriatal System. Psychology Press; Hove, East Sussex, Great Britain: 2001. [Google Scholar]
- Brookshire B, Butler I, Ewing-Cobbs L, Fletcher J. Neuropsychological characteristics of children with Tourette syndrome: evidence for a nonverbal learning disability? J Clin Exp Neuropsychol. 1994;16:289–302. doi: 10.1080/01688639408402639. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Review Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brown MW, Warburton EC, Aggleton JP. Recognition memory: Material, processes, and substrates. Hippocampus. 2010;20:1228–1244. doi: 10.1002/hipo.20858. [DOI] [PubMed] [Google Scholar]
- Bruck M. Word-Recognition Skills of Adults With Childhood Diagnoses of Dyslexia. Dev Psychol. 1990;26:439–454. [Google Scholar]
- Buse J, Schoenefeld K, Munchau A, Roessner V. Neuromodulation in Tourette syndrome: Dopamine and beyond. Neurosci Biobehav Rev. 2013;37:1069–1084. doi: 10.1016/j.neubiorev.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Memory, perception, and the ventral visual-perirhinal-hippocampal stream: Thinking outside of the boxes. Hippocampus. 2007;17:898–908. doi: 10.1002/hipo.20320. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Moscovitch M. Memory Systems, Processing Modes, and Components: Functional Neuroimaging Evidence. Perspect Psychol Sci. 2013;8:49–55. doi: 10.1177/1745691612469033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantiani C, Guasti MT, Perego P, Lorusso ML. Impaired inflectional morphology in children with Developmental Dyslexia: Converging evidence from behavioral and electrophysiological measures. In: Cantiani AK, Chung EY, Kimball AE, editors. Proceedings of the 36th annual Boston University Conference on Language Development. Cascadilla Press; Somerville, MA: 2012. pp. 114–125. [Google Scholar]
- Cantiani C, Lorusso ML, Perego P, Molteni M, Guasti MT. Event-related potentials reveal anomalous morphosyntactic processing in developmental dyslexia. Appl Psycholinguist. 2013;34:1135–1162. [Google Scholar]
- Carbon M, Reetz K, Ghilardi MF, Dhawan V, Eidelberg D. Early Parkinson's disease: Longitudinal changes in brain activity during sequence learning. Neurobiol Dis. 2010;37:455–460. doi: 10.1016/j.nbd.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona S, Bassas N, Rovira M, Gispert JD, Soliva JC, Prado M, Tomas J, Bulbena A, Vilarroya O. Pediatric OCD structural brain deficits in conflict monitoring circuits: A voxel-based morphometry study. Neurosci Lett. 2007;421:218–223. doi: 10.1016/j.neulet.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Chan KH, Morell JR, Jarrard LE, Davidson TL. Reconsideration of the role of the hippocampus in learned inhibition. Behav Brain Res. 2001;119:111–130. doi: 10.1016/s0166-4328(00)00363-6. [DOI] [PubMed] [Google Scholar]
- Channon S, Pratt P, Robertson MM. Executive function, memory, and learning in Tourette's syndrome. Neuropsychology. 2003;17:247–254. doi: 10.1037/0894-4105.17.2.247. [DOI] [PubMed] [Google Scholar]
- Charlop-Christy MH, Kelso SE. Teaching children with autism conversational speech using a cue card/written script program. Education and Treament of Children. 2003;26:108–127. [Google Scholar]
- Cherney LR. Aphasia treatment: Intensity, dose parameters, and script training. Int J Speech Lang Pathol. 2012;14:424–431. doi: 10.3109/17549507.2012.686629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney LR, Halper AS. Novel technology for treating individuals with aphasia and concomitant cognitive deficits. Topics in stroke rehabilitation. 2008;15:542–554. doi: 10.1310/tsr1506-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney LR, Halper AS, Holland AL, Cole R. Computerized script training for aphasia: Preliminary results. Am J Speech Lang Pathol. 2008a;17:19–34. doi: 10.1044/1058-0360(2008/003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney LR, Patterson JP, Raymer A, Frymark T, Schooling T. Evidence-based systematic review: effects of intensity of treatment and constraint-induced language therapy for individuals with stroke-induced aphasia. J Speech Lang Hear Res. 2008b;51:1282–1299. doi: 10.1044/1092-4388(2008/07-0206). [DOI] [PubMed] [Google Scholar]
- Chiarello C, Lombardino LJ, Kacinik NA, Otto R, Leonard CM. Neuroanatomical and behavioral asymmetry in an adult compensated dyslexic. Brain Lang. 2006;98:169–181. doi: 10.1016/j.bandl.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Chun MM. Contextual cueing of visual attention. Trends in Cognitive Sciences. 2000;4:170–178. doi: 10.1016/s1364-6613(00)01476-5. [DOI] [PubMed] [Google Scholar]
- Crozier S, Tincani M. Effects of social stories on prosocial behavior of preschool children with autism spectrum disorders. J Autism Dev Disord. 2007;37:1803–1814. doi: 10.1007/s10803-006-0315-7. [DOI] [PubMed] [Google Scholar]
- Dagher A, Owen A, Boecker H, Brooks D. The role of the striatum and hippocampus in planning: A PET activation study in Parkinson's disease. Brain. 2001;124:1020–1032. doi: 10.1093/brain/124.5.1020. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Otto M, Savage C, Baer L, Jenike MA. The relationship between semantic organization and memory in obsessive-compulsive disorder. Psychother Psychosom. 2000;69:101–107. doi: 10.1159/000012373. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage CR, Reilly-Harrington N, Clark L, Sachs G, Rauch SL. Episodic memory impairment in bipolar disorder and obsessive-compulsive disorder: The role of memory strategies. Bipolar Disord. 2004;6:233–244. doi: 10.1111/j.1399-5618.2004.00118.x. [DOI] [PubMed] [Google Scholar]
- Delaney PF, Knowles ME. Encoding strategy changes and spacing effects in the free recall of unmixed lists. Journal of Memory and Language. 2005;52:120–130. [Google Scholar]
- Denys D, Zohar J, Westenberg HGM. The role of dopamine in obsessive-compulsive disorder: Preclinical and clinical evidence. J Clin Psychiatry. 2004;65:11–17. [PubMed] [Google Scholar]
- Dewey D, Wall K. Praxis and memory deficits in language-impaired children. Developmental Neuropsychology. 1997;13:507–512. [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord. 2012;42:147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbinson S, Perkins M, Boucher J. The interactional significance of formulas in autistic language. Clin Linguist Phon. 2003;17:299–307. doi: 10.1080/0269920031000080046. [DOI] [PubMed] [Google Scholar]
- Dommett EJ, Henderson EL, Westwell MS, Greenfield SA. Methylphenidate amplifies long-term plasticity in the hippocampus via noradrenergic mechanisms. Learn Mem. 2008;15:580–586. doi: 10.1101/lm.1092608. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury JE, Ullman MT. The memorization of complex forms in aphasia: Implications for recovery. Brain Lang. 2002;83:139–141. [Google Scholar]
- Eales MJ. Pragmatic impairments in adults with childhood diagnoses of autism or developmental receptive language disorder. Joumal of Autism and Developmental Disorders. 1993;23:593–617. doi: 10.1007/BF01046104. [DOI] [PubMed] [Google Scholar]
- Eckert ME, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, Berninger V. Anatomical signatures of dyslexia in children: Unique information from manual and voxel based morphometry brain measure. Cortex. 2005;41:304–315. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- Eden G, Jones K, Cappell K, Gareau L, Wood F, Zeffiro T, Dietz N, Agnew J, Flowers D. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44:411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Ehri LC, Nunes SR. Systematic phonics instruction helps students learn to read: Evidence from the National Reading Panel's meta-analysis. Rev Educ Res. 2001;71:393–447. [Google Scholar]
- Eichenbaum H. The Cognitive Neuroscience of Memory:An Introduction. 2. Oxford University Press; Oxford; 2012. [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36:1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Risk tables for parkinsonism and Parkinson's disease. J Clin Epidemiol. 2002;55:25–31. doi: 10.1016/s0895-4356(01)00425-5. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. PNAS. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton RH, Wood FB, Brown IS, Campbell SK, Harter MR. Separate verbal memory and naming deficits in attention deficit disorder and reading disability. Brain Lang. 1987;31:171–184. doi: 10.1016/0093-934x(87)90067-8. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Stephenson CME, Carpenter TA, Donovan T, Bullmore ET. Regional brain activations predicting subsequent memory success: An event-related fMRI study of the influence of encoding tasks. Cortex. 2003;39:1009–1026. doi: 10.1016/s0010-9452(08)70875-x. [DOI] [PubMed] [Google Scholar]
- Fonteneau E, van der Lely HKJ. Electrical brain responses in language-impaired children reveal grammar specific deficits. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0001832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Cavanna AE. Behavioural treatments for Tourette syndrome: An evidence-based review. Behavioural neurology. 2013;27:105–117. doi: 10.3233/BEN-120309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freo U, Pizzolato G, Dam M, Ori C, Battistin L. A short review of cognitive and functional neuroimaging studies of cholinergic drugs: Implications for therapeutic potentials. J Neural Transm. 2002;109:857–870. doi: 10.1007/s007020200070. [DOI] [PubMed] [Google Scholar]
- Frith U. Emanuel Miller lecture: Confusions and controversies about Asperger syndrome. J Child Psychol Psychiatry. 2004;45:672–686. doi: 10.1111/j.1469-7610.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161:857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- Ganz JB, Kaylor M, Bourgeois B, Hadden K. The impact of social scripts and visual cues on verbal communication in three children with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities. 2008;23:79–94. [Google Scholar]
- Gebauer D, Fink A, Kargl R, Reishofer G, Koschutnig K, Purgstaller C, Fazekas F, Enzinger C. Differences in brain function and changes with intervention in children with poor spelling and reading abilities. PLoS ONE. 2012;7:e38201. doi: 10.1371/journal.pone.0038201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller DA. Obsessive-compulsive and spectrum disorders in children and adolescents. The Psychiatric clinics of North America. 2006;29:353–370. doi: 10.1016/j.psc.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Gillberg C. The ESSENCE in child psychiatry: Early ymptomatic Syndromes Eliciting Neurodevelopmental Clinical Examinations. Res Dev Disabil. 2010;31:1543–1551. doi: 10.1016/j.ridd.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Pienaar IS, Vohra S, Qamhawi Z. Sex differences in Parkinson's disease. Front Neuroendocrinol. 2014;35:370–384. doi: 10.1016/j.yfrne.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel EW, Blomeke K, Zadikoff C, Simuni T, Weintraub S, Reber PJ. Implicit perceptual-motor skill learning in mild cognitive impairment and Parkinson's disease. Neuropsychology. 2013;27:314–321. doi: 10.1037/a0032305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorhuis-Brouwer SM, Wijnberg-Williams BJ. Specificity of specific language impairment. Folia Phoniatr Logop. 1996;48:269–274. doi: 10.1159/000266421. [DOI] [PubMed] [Google Scholar]
- Green AE, Dunbar KN. Mental Function as Genetic Expression: Emerging insights from cognitive neurogenetics. In: Holyoak KJ, Morrison RG, editors. The Oxford handbook of thinking and reasoning. Oxford University Press; Oxford: 2012. pp. 90–111. [Google Scholar]
- Greisberg S, McKay D. Neuropsychology of obsessive-compulsive disorder: a review and treatment implications. Clin Psychol Rev. 2003;23:95–117. doi: 10.1016/s0272-7358(02)00232-5. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Wassenaar M, Brown C. Real-time semantic compensation in patients with agrammatic comprehension: Electrophysiological evidence for multiple-route plasticity. PNAS. 2003;100:4340–4345. doi: 10.1073/pnas.0230613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung CM, Widiger TA. Gender differences in the diagnosis of mental disorders: Conclusions and controversies of the DSM-IV. Psychol Bull. 1998;123:260–278. doi: 10.1037/0033-2909.123.3.260. [DOI] [PubMed] [Google Scholar]
- Hawke JL, Olson RK, Willcut EG, Wadsworth SJ, DeFries JC. Gender ratios for reading difficulties. Dyslexia. 2009;15:239–242. doi: 10.1002/dys.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedenius M, Ullman MT, Alm P, Jennische M, Persson J. Enhanced recognition memory after incidental encoding in children with developmental dyslexia. PLoS ONE. 2013;8:e63998. doi: 10.1371/journal.pone.0063998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K. A model for memory systems based on processing modes rather than consciousness. Nature Reviews Neuroscience. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- Himle MB, Woods DW, Piacentini JC, Walkup JT. Brief review of habit reversal training for Tourette syndrome. J Child Neurol. 2006;21:719–725. doi: 10.1177/08830738060210080101. [DOI] [PubMed] [Google Scholar]
- Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra P, Anderson G, Limburg P, Korf J, Kallenberg C, Minderaa R. Neurobiology and neuroimmunology of Tourette's syndrome: an update. Cell Mol Life Sci. 2004;61:886–898. doi: 10.1007/s00018-003-3320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, Rettenbacher MA, Widschwendter CG, Verius M, Golaszewski SM, Koppelstaetter F, Felber S, Wolfgang Fleischhacker W. The neural regions sustaining episodic encoding and recognition of objects. Brain Cogn. 2007;63:159–166. doi: 10.1016/j.bandc.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Holden C. Sex and the suffering brain. Science. 2005;308:1574. doi: 10.1126/science.308.5728.1574. [DOI] [PubMed] [Google Scholar]
- Howard JHJ, Howard DV, Japikse KC, Eden GF. Dyslexics are impaired on implicit higher-order sequence learning, but not on implicit spatial context learning. Neuropsychologia. 2006;44:1131–1144. doi: 10.1016/j.neuropsychologia.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Howlin P, Goode S, Hutton J, Rutter M. Savant skills in autism: Psychometric approaches and parental reports. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1359–1357. doi: 10.1098/rstb.2008.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey ML, Heflin LJ, Alberto P. The use of social stories to promote independent behaviors in novel events for children with PDD-NOS. Focus on Autism and Other Developmental Disabilities. 2004;19:164–176. [Google Scholar]
- Jacobs E, D'Esposito M. Estrogen shapes dopamine-dependent cognitive processes: Implications for women's health. The Journal of Neuroscience. 2011;31:5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johari K, Walenski M, Ullman MT. Language, sex and tools: An investigation of syntax, morphology and tool knowledge in Parkinson's disease In Preparation. [Google Scholar]
- Jorm AF. Specific reading retardation and working memory: A review. Br J Psychol. 1983;74:311–342. doi: 10.1111/j.2044-8295.1983.tb01865.x. [DOI] [PubMed] [Google Scholar]
- Keisler A, Shadmehr R. A shared resource between declarative memory and motor memory. J Neurosci. 2010;30:14817–14823. doi: 10.1523/JNEUROSCI.4160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibby MY. Memory functioning in developmental dyslexia: An analysis using two clinical memory measures. Arch Clin Neuropsychol. 2009;24:245–254. doi: 10.1093/arclin/acp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibby MY, Cohen MJ. Memory functions in children with reading disabilities and/or attention deficit/hyperactivity disorder: A clinical investigation of their working memory and long-term memory functioning. Child Neuropsychology. 2008;14:525–546. doi: 10.1080/09297040701821752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger LG, Dawson G. Prototype formation in autism. Dev Psychopathol. 2001;13:111–124. doi: 10.1017/s0954579401001080. [DOI] [PubMed] [Google Scholar]
- Klinger LG, Klinger MR, Pohlig RL. Implicit Learning Impairments in Autism Spectrum Disorders: Implications for Treatment. In: Peréz JM, González PM, Comí ML, Nieto C, editors. New Developments in Autism: The Future is Today. Jessica Kinglsey Publishers; Philadelphia: 2007. pp. 76–103. [Google Scholar]
- Knowlton BJ, Squire LR. The learning of categories: Parallel brain systems for item memory and category knowledge. Science. 1993;262:1747–1749. doi: 10.1126/science.8259522. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kompoliti K, Goetz C, Leurgans S, Raman R, Comella C. Estrogen, progesterone, and tic severity in women with Gilles de la Tourette syndrome. Neurology. 2001;57:1519. doi: 10.1212/wnl.57.8.1519. [DOI] [PubMed] [Google Scholar]
- Krafnick AJ, Flowers DL, Napoliello EM, Eden GF. Gray matter volume changes following reading intervention in dyslexic children. Neuroimage. 2011;57:733–741. doi: 10.1016/j.neuroimage.2010.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006;26:433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Knee K, Delis D. Verbal memory impairments in dyslexia. Arch Clin Neuropsychol. 2000;15:83–93. [PubMed] [Google Scholar]
- Krantz PJ, McClannahan LE. Teaching children with autism to initiate to peers: Effects of a script-fading procedure. J Appl Behav Anal. 1993;26:121–132. doi: 10.1901/jaba.1993.26-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz PJ, McClannahan LE. Social interaction skills for children with autism: A script-fading procedure for beginning readers. J Appl Behav Anal. 1998;31:191–202. doi: 10.1901/jaba.1998.31-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annu Rev Psychol. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, Kim JJ, Lee DW, Lee JS, Lee DS, Kim MS, Lyoob IK, Chob MJ, Leea MC. Neural correlates of clinical symptoms and cognitive dysfunctions in obsessive-compulsive disorder. Psychiatry Research Neuroimaging. 2003;122:37–47. doi: 10.1016/s0925-4927(02)00104-x. [DOI] [PubMed] [Google Scholar]
- Landa R. Social language use in Asperger syndrome and high-functioning autism. In: Klin A, Volkmar FR, Sparrow SS, editors. Asperger syndrome. The Guilford Press; New York, NY: 2000. [Google Scholar]
- Lang R, Regester A, Lauderdale S, Ashbaugh K, Haring A. Treatment of anxiety in autism spectrum disorders using cognitive behaviour therapy: A systematic review. Developmental neurorehabilitation. 2010;13:53–63. doi: 10.3109/17518420903236288. [DOI] [PubMed] [Google Scholar]
- Leonard LB. Children with specific language impairment. 2nd. MIT Press; 2014. [Google Scholar]
- Leonard LB. Children with specific language impairment. MIT Press; Cambridge, MA: 1998. [Google Scholar]
- Levy D, Ronemus M, Yamrom B, Lee Yh, Leotta A, Kendall J, Marks S, Lakshmi B, Pai D, Ye K, Buja A, Krieger A, Yoon S, Troge J, Rodgers L, Iossifov I, Wigler M. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Lukacs A, Kemeny F, Ullman MT. Enhanced declarative memory consolidation in children with SLI In Preparation. [Google Scholar]
- Lum JA, Conti-Ramsden G. Long-term memory: A review and meta-analysis of studies of declarative and procedural memory in specific language impairment. Topics in Language Disorders. 2013;33:282–297. doi: 10.1097/01.tld.0000437939.01237.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum J, Hedenius M, Tomblin JB, Ullman MT. Recognition memory in children with SLI In Preparation. [Google Scholar]
- Lum JAG, Conti-Ramsden G, Page D, Ullman MT. Working, declarative and procedural memory in specific language impairment. Cortex. 2012;48:1138–1154. doi: 10.1016/j.cortex.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JAG, Ullman MT, Conti-Ramsden G. Verbal declarative memory impairments in specific language impairment are related to working memory deficits. Brain Lang. doi: 10.1016/j.bandl.2015.01.008. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon GR, Shaywitz SE, Shaywitz BA. A definition of dyslexia. Annals of dyslexia. 2003;53:1–14. [Google Scholar]
- Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging. 2000;21:373–383. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- March JS. Cognitive-behavioral psychotherapy for children and adolescents with OCD: A review and recommendations for treatment. Journal of American Academy of Child and Adolescent Psychiatry. 1995;34:7–18. doi: 10.1097/00004583-199501000-00008. [DOI] [PubMed] [Google Scholar]
- Marsh R, Alexander GM, Packard MG, Zhu H, Wingard JC, Quackenbush G, Peterson BS. Habit learning in Tourette Syndrome, a translational neuroscience approach to a developmental psychopathology. Arch Gen Psychiatry. 2004;61:1259–1268. doi: 10.1001/archpsyc.61.12.1259. [DOI] [PubMed] [Google Scholar]
- Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends in Cognitive Sciences. 2007;11:442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Massand E, Bowler DM, Mottron L, Hosein A, Jemel B. ERP correlates of recognition memory in Autism Spectrum Disorder. J Autism Dev Disord. 2013;43:2038–2047. doi: 10.1007/s10803-012-1755-x. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior-medial temporal lobe: I. Intracranial distribution and neural generators. J Neurosci. 1995;15:1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Mohammadi S, Kugel H, Schiffbauer H, Floel A, Albers J, Kramer K, Menke R, Baumgartner A, Knecht S, Breitenstein C, Deppe M. Integrity of the hippocampus and surrounding white matter is correlated with language training success in aphasia. Neuroimage. 2010;53:283–290. doi: 10.1016/j.neuroimage.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Menke R, Meinzer M, Kugel H, Deppe M, Baumgartner A, Schiffbauer H, Thomas M, Kramer K, Lohmann H, Floel A, Knecht S, Breitenstein C. Imaging short- and long-term training success in chronic aphasia. BMC Neurosci. 2009;10:118. doi: 10.1186/1471-2202-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk ML, van Tol MJ, Penninx BW, van der Wee NJA, Aleman A, Veltman DJ, Spinhoven P, Elzinga BM. BDNF val66met affects hippocampal volume and emotion-related hippocampal memory activity. Translational psychiatry. 2012;2:e74. doi: 10.1038/tp.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody TD, Bookheimer SY, Vanek Z, Knowlton BJ. An Implicit Learning Task Activates Medial Temporal Lobe in Patients With Parkinson's Disease. Behav Neurosci. 2004;118:438–442. doi: 10.1037/0735-7044.118.2.438. [DOI] [PubMed] [Google Scholar]
- Mormann F, Fell J, Axmacher N, Weber B, Lehnertz K, Elger CE, Fernández G. Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus. 2005;15:890–900. doi: 10.1002/hipo.20117. [DOI] [PubMed] [Google Scholar]
- Mottron L, Bouvet L, Bonnel A, Samson F, Burack JA, Dawson M, Heaton P. Veridical mapping in the development of exceptional autistic abilities. Neurosci Biobehav Rev. 2013;37:209–228. doi: 10.1016/j.neubiorev.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Nation K, Snowling MJ. Individual differences in contextual facilitation: Evidence from dyslexia and poor reading comprehension. Child Dev. 1998;69:996–1011. [PubMed] [Google Scholar]
- Neville HJ, Coffey SA, Holcomb PJ, Tallal P. The neurobiology of sensory and language processing in language-impaired children. J Cogn Neurosci. 1993;5:235–253. doi: 10.1162/jocn.1993.5.2.235. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Procedural learning difficulties: Reuniting the developmental disorders? Trends in Neuroscience. 2007;30:135–141. doi: 10.1016/j.tins.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Oetting JB, Horohov JE. Past tense marking by children with and without specific language impairment. J Speech Hear Res. 1997;40:62–74. doi: 10.1044/jslhr.4001.62. [DOI] [PubMed] [Google Scholar]
- Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. J Affect Disord. 2007;104:15–23. doi: 10.1016/j.jad.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Pauc R. Comorbidity of dyslexia, dyspraxia, attention deficit disorder (ADD), attention deficit hyperactive disorder (ADHD), obsessive compulsive disorder (OCD) and Tourette's syndrome in children: A prospective epidemiological study. Clinical Chiropractic. 2005;8:189–198. [Google Scholar]
- Pedersen PM, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Aphasia in acute stroke: Incidence, determinants, and recovery. Ann Neurol. 1995;38:659–666. doi: 10.1002/ana.410380416. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Bishop DVM. Relations among speech, language, and reading disorders. Annu Rev Psychol. 2009;60:283–306. doi: 10.1146/annurev.psych.60.110707.163548. [DOI] [PubMed] [Google Scholar]
- Pernet CR, Poline JB, Demonet JF, Rousselet GA. Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neurosci. 2009;10:67. doi: 10.1186/1471-2202-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Choi HA, Hao X, Amat JA, Zhu H, Whiteman R, Liu J, Xu D, Bansal R. Morphologic Features of the Amygdala and Hippocampus in Children and Adults With Tourette Syndrome. Arch Gen Psychiatry. 2007;64 doi: 10.1001/archpsyc.64.11.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The Brain-Derived Neurotrophic Factor val66met Polymorphism and Variation in Human Cortical Morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Pizzamiglio L, Mammucari A, Razzano C. Evidence for sex differences in brain organization in recovery in aphasia. Brain Lang. 1985;25:213–223. doi: 10.1016/0093-934x(85)90081-1. [DOI] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Peterson BS. Imaging evidence for anatomical disturbances and neuroplastic compensation in persons with Tourette syndrome. J Psychosom Res. 2009;67:559–573. doi: 10.1016/j.jpsychores.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J, Hugdahl K, Peterson BS. Hippocampus and amygdala morphology in Attention-Deficit/Hyperactivity Disorder. Arch Gen Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Portman EC. A social competence intervention program for children with high functioning autism and asperger's syndrome: A qualitative study, Philosopy. University of Texas at Austin; Austin: 2006. p. 212. [Google Scholar]
- Prado E, Ullman MT. Can imageability help us draw the line between storage and composition? Journal of Experimental Psychology: Language, Memory, and Cognition. 2009;35:849–866. doi: 10.1037/a0015286. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, McEwen B, Silbersweig D, Stern E. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18:985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Neininger B, Elbert T, Mohr B, Rockstroh B, Koebbel P, Taub E. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32:1621–1626. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- Quinn NP, Marsden CD. Menstrual-related fluctuations in Parkinson's disease. Mov Disord. 1986;1:85–87. doi: 10.1002/mds.870010112. [DOI] [PubMed] [Google Scholar]
- Quinn PO. Treating adolescent girls and women with ADHD: Gender-specific issues. J Clin Psychol. 2005;61:579–587. doi: 10.1002/jclp.20121. [DOI] [PubMed] [Google Scholar]
- Rack JP, Snowling MJ, Olson RK. The nonword reading deficit in developmental dyslexia: a review. Read Res Quart. 1992;27:29–53. [Google Scholar]
- Rauch SL, Savage C. Investigating cortico-striatal pathophysiology in obsessive–compulsive disorders: Procedural learning and imaging probes. In: Goodman W, Rudorfer M, Maser JD, editors. Obsessive–compulsive disorder: Contemporary issues in treatment. Erlbaum; Mahwah, NJ: 2000. pp. 133–154. [Google Scholar]
- Rauch SL, Savage CR, Alpert NM, Dougherty D, Kendrick A, Curran T, Brown HD, Manzo P, Fischman AJ, Jenike MA. Probing striatal function in obsessive-compulsive disorder: A PET study of implicit sequence learning. J Neuropsychiatry Clin Neurosci. 1997;9:568–573. doi: 10.1176/jnp.9.4.568. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, Cannistraro PA, Wilhelm S. Functional magnetic resonance imaging study of regional brain activation during implicit sequence learning in obsessive-compulsive disorder. Biol Psychiatry. 2007;61:330–336. doi: 10.1016/j.biopsych.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Curran T, Shin LM, Coffey BJ, Savage CR, McInerney SC, Baer L, Jenike MA. Probing striato-thalamic function in obsessive-compulsive disorder and Tourette syndrome using neuroimaging methods. Adv Neurol. 2001;85:207–224. [PubMed] [Google Scholar]
- Raz S, Goldstein R, Hopkins TL, Lauterbach MD, Shah F, Porter CL, Riggs WW, Magill LH, Sander CJ. Sex differences in early vulnerability to cerebral injury and their neurodevelopmental implications. Psychobiology. 1994;22:244–253. [Google Scholar]
- Repantis D, Laisney O, Heuser I. Acetylcholinesterase inhibitors and memantine for neuroenhancement in healthy individuals: A systematic review. Pharmacol Res. 2010;61:473–481. doi: 10.1016/j.phrs.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Richardson U, Thomson JM, Scott SK, Goswami U. Auditory processing skills and phonological representation in dyslexic children. Dyslexia. 2004;10:215–233. doi: 10.1002/dys.276. [DOI] [PubMed] [Google Scholar]
- Rieckmann A, Backman L. Implicit learning in aging: Extant patterns and new directions. Neuropsychol Rev. 2009;19:490–503. doi: 10.1007/s11065-009-9117-y. [DOI] [PubMed] [Google Scholar]
- Rieckmann A, Fischer H, Backman L. Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: Relations to performance. Neuroimage. 2010;50:1303–1312. doi: 10.1016/j.neuroimage.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Roth RM, Baribeau J, Milovan D, O'Connor K, Todorov C. Procedural and declarative memory in obsessive-compulsive disorder. J Int Neuropsychol Soc. 2004;10:647–654. doi: 10.1017/S1355617704105018. [DOI] [PubMed] [Google Scholar]
- Roth RM, Saykin AJ, Wishart HA, Flashman LA, Ward J, Mamourian AC. An fMRI study of brain activation during implicit learning in OCD. J Neuropsychiatry Clin Neurosci. 2003;15:262. [Google Scholar]
- Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: Unifying issues and research strategies. J Child Psychol Psyc. 2003;44:1092–1115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- Sandyk R, Bamford CR, Iacono RP. Menstrual-related fluctuations in Tourette's syndrome. Int J Neurosci. 1987;35:237–239. doi: 10.3109/00207458708987133. [DOI] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Wilhelm S, Rauch SL, Baer L, Reid T, Jenike MA. Strategic processing and episodic memory impairment in obsessive compulsive disorder. Neuropsychology. 2000;14:141–151. doi: 10.1037//0894-4105.14.1.141. [DOI] [PubMed] [Google Scholar]
- Sawada H, Shimohama S. Neuroprotective effects of estradiol in mesencephalic dopaminergic neurons. Neurosci Biobehav Rev. 2000;24:143–147. doi: 10.1016/s0149-7634(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Scharf JM, Miller LL, Mathews CA, Ben-Shlomo Y. Prevalence of Tourette syndrome and chronic tics in the population-based Avon longitudinal study of parents and children cohort. J Am Acad Child Adolesc Psychiatry. 2012;51:192–201. doi: 10.1016/j.jaac.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendan H, Searl M, Melrose R, Stern C. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Scheurholz LJ, Baumgardner TL, Singer HS, Reiss AL, Denckla MB. Neuropsychological status of children with Tourette's Syndrome with and without attention deficit hyperactive disorder. Neurology. 1996;46:958–965. doi: 10.1212/wnl.46.4.958. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annual Review of Neuroscience. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Schofield PR, Williams LM, Paul RH, Gatt JM, Brown K, Luty A, Cooper N, Grieve S, Dobson-Stone C, Morris C, Kuan SA, Gordon E. Disturbances in selective information processing associated with the BDNF Val66Met polymorphism: Evidence from cognition, the P300 and fronto-hippocampal systems. Biol Psychol. 2009;80:176–188. doi: 10.1016/j.biopsycho.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Schwabe MJ, Konkol RJ. Menstrual cycle-related fluctuations of tics in Tourette syndrome. Pediatr Neurol. 1992;8:43–46. doi: 10.1016/0887-8994(92)90051-y. [DOI] [PubMed] [Google Scholar]
- Senju A, Southgate V, White S, Frith U. Mindblind eyes: An absence of spontaneous theory of mind in Asperger syndrome. Science. 2009;325:883–885. doi: 10.1126/science.1176170. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Pugh KR, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biol Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women. Proc Soc Exp Biol Med. 1998;217:17–22. doi: 10.3181/00379727-217-44200. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers C, Grossman S, Sage J, Gluck M, Poldrack R. Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. Brain. 2004;127:851–859. doi: 10.1093/brain/awh100. [DOI] [PubMed] [Google Scholar]
- Snowling M, Hulme C, Goulandris N. Word recognition in developmental dyslexia: a connectionist interpretation. The Quarterly Journal of Experimental Psychology. 1994;47:895–916. doi: 10.1080/14640749408401101. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JC, Williams DC. Parental investment, self-control, and sex differences in the expression of ADHD. Human Nature. 2000;11:405–422. doi: 10.1007/s12110-000-1010-6. [DOI] [PubMed] [Google Scholar]
- Sutherland R, Kolb B, Schoel W, Whishaw I, Davies D. Neuropsychological assessment of children and adults with Tourette syndrome: a comparison with learning disabilities and schizophrenia. Adv Neurol. 1982;35:311–322. [PubMed] [Google Scholar]
- Swan D, Goswami U. Picture naming deficits in developmental dyslexia: the phonological representations hypothesis. Brain Lang. 1997;56:334–353. doi: 10.1006/brln.1997.1855. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Calkins S. Does imitation facilitate the acquisition of grammar? Evidence from a study of autistic, Down's syndrome and normal children. J Child Lang. 1990;17:591–606. doi: 10.1017/s0305000900010898. [DOI] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Pidikiti R. Constraint-induced movement therapy: a new family of techniques with broad application to physical rehabilitation--a clinical review. J Rehabil Res Dev. 1999;36:237–251. [PubMed] [Google Scholar]
- Temple E, Deutsch G, Poldrack R, Miller S, Tallal P, Merzenich M, Gabrieli J. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proceedings of the National Academy of Science. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Protopapas A, Nagarajan S, Salz T, Tallal P, Merzenich MM, Gabrieli JD. Disruption of the neural response to rapid acoustic stimuli in dyslexia: Evidence from functional MRI. Proceedings of the National Academy of Science U S A. 2000;97:13907–13912. doi: 10.1073/pnas.240461697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesak Jr, Code C. Milestones in the history of aphasia: Theories and protagonists. Psychology Press; New York: 2008. [Google Scholar]
- Thiemann KS, Goldstein H. Social stories, written text cues, and video feedback: effects on social communication of children with autism. J Appl Behav Anal. 2001;34:425–446. doi: 10.1901/jaba.2001.34-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordardottir E, Ellis Weismer S. Verb argument structure weakness in specific language impairment in relation to age and utterance length. Clin Linguist Phon. 2002;16:233–250. doi: 10.1080/02699200110116462. [DOI] [PubMed] [Google Scholar]
- Toichi M, Kamio Y. Long-term memory and levels-of-processing in autism. Neuropsychologia. 2002;40:964–969. doi: 10.1016/s0028-3932(01)00163-4. [DOI] [PubMed] [Google Scholar]
- Ullman MT. The neural basis of lexicon and grammar in first and second language: The declarative/procedural model. Bilingualism: Language and Cognition. 2001;4:105–122. [Google Scholar]
- Ullman MT. Contributions of memory circuits to language: The declarative/procedural model. Cognition. 2004;92:231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ullman MT. A cognitive neuroscience perspective on second language acquisition: The declarative/procedural model. In: Sanz C, editor. Mind and context in adult second language acquisition: Methods, theory and practice. Georgetown University Press; Washington, DC: 2005a. pp. 141–178. [Google Scholar]
- Ullman MT. More is sometimes more: Redundant mechanisms in the mind and brain. APS Observer. 2005b;18:7, 46. [Google Scholar]
- Ullman MT. The Declarative/Procedural Model: A Neurobiologically-Motivated Theory of First and Second Language. In: VanPatten B, Williams J, editors. Theories in Second Language Acquisition. 2nd. Routledge; New York, London: In Press-a. [Google Scholar]
- Ullman MT. The declarative/procedural model: A neurobiological model of language learning, knowledge and use. In: Hickok G, Small SA, editors. The Neurobiology of Language. Elsevier; In Press-b. [Google Scholar]
- Ullman MT, Estabrooke IV. Grammar, tools and sex. J Cogn Neurosci, Supplement. 2004:67. [Google Scholar]
- Ullman MT, Gopnik M. Inflectional morphology in a family with inherited specific language impairment. Appl Psycholinguist. 1999;20:51–117. [Google Scholar]
- Ullman MT, Miranda RA, Travers ML. Sex differences in the neurocognition of language. In: Becker JB, Berkley KJ, Geary N, Hampson E, Herman J, Young E, editors. Sex on the brain: From genes to behavior. Oxford University Press; New York: 2008. pp. 291–309. [Google Scholar]
- Ullman MT, Pierpont EI. Specific language impairment is not specific to language: The procedural deficit hypothesis. Cortex. 2005;41:399–433. doi: 10.1016/s0010-9452(08)70276-4. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Pullman M, Lovelett JT, McQuaid GA, Pierpont EI, Turkeltaub PE. Brain abnormalities in Specific Language Impairment are localized to frontal regions and the caudate nucleus. Neurosci Biobehav Rev Under Review. [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: Insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unoki K, Kasuga T, Matsushima E, Ohta K. Attentional processing of emotional information in obsessive-compulsive disorder. Psychiatry Clin Neurosci. 1999;53:535–542. doi: 10.1046/j.1440-1819.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- Vaidya C, Foss-Feig J, Shook D, Kaplan L, Kenworthy L, Gaillard W. Controlling attention to gaze and arrows in childhood: an fMRI study of typical development and Autism Spectrum Disorders. Developmental Science. 2011;14:911–924. doi: 10.1111/j.1467-7687.2011.01041.x. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Remijnse PL, Mataix-Cols D, Vrenken H, Groenewegen HJ, Uylings HBM, van Balkom AJLM, Veltman DJ. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132:853–868. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, Barkhof F, van Dyck R. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry. 2005;62:301–309. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- van der Lely HKJ, Ullman MT. Past tense morphology in specifically language impaired and normally developing children. Lang Cognitive Proc. 2001;16:177–217. [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Vicari S, Marotta L, Menghiai D, Molinari M, Petrosini L. Implicit learning deficit in children with developmental dyslexia. Neuropsychologia. 2003;41:108–114. doi: 10.1016/s0028-3932(02)00082-9. [DOI] [PubMed] [Google Scholar]
- Vulink NCC, Denys D, Bus L, Westenberg HGM. Female hormones affect symptom severity in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2006;21:171–175. doi: 10.1097/01.yic.0000199454.62423.99. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Walenski M, Mostofsky SH, Gidley-Larson JC, Ullman MT. Brief Report: Enhanced picture naming in autism. J Autism Dev Disord. 2008;38:1395–1399. doi: 10.1007/s10803-007-0513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenski M, Mostofsky SH, Ullman MT. Speeded processing of grammar and tool knowledge in Tourette's syndrome. Neuropsychologia. 2007;45:2447–2460. doi: 10.1016/j.neuropsychologia.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenski M, Tager-Flusberg H, Ullman MT. Language in Autism. In: Moldin SO, Rubenstein JLR, editors. Understanding Autism: From Basic Neuroscience to Treatment. Taylor and Francis Books; Boca Raton, FL: 2006. pp. 175–203. [Google Scholar]
- Watson HJ, Rees CS. Meta-analysis of randomized, controlled treatment trials for pediatric obsessive-compulsive disorder. Journal of child psychology and psychiatry, and allied disciplines. 2008;49:489–498. doi: 10.1111/j.1469-7610.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M. Memory Transformation and Systems Consolidation. J Int Neuropsychol Soc. 2011;17:766–780. doi: 10.1017/S1355617711000683. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. The medial temporal lobe and the attributes of memory. Trends in Cognitive Sciences. 2011;15:210–217. doi: 10.1016/j.tics.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT. Stress and memory in humans: Twelve years of progress? Brain Res. 2009;1293:142–154. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Woollett K, Maguire EA. Acquiring “the Knowledge” of London's layout drives structural brain changes. Curr Biol. 2011;21:2109–2114. doi: 10.1016/j.cub.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y, Gerardin E, Hartmann A, Valabregue R, Chupin M, Tremblay L, Vidailhet M, Colliot O, Lehericy S. Distinct structural changes underpin clinical phenotypes in patients with Gilles de la Tourette syndrome. Brain. 2010;133:3649–3660. doi: 10.1093/brain/awq293. [DOI] [PubMed] [Google Scholar]
- Zwicker JG, Missiuna CC, Boyd LA. Neural correlates of developmental coordination disorder: A review of hypotheses. J Child Neurol. 2009;24:1273–1281. doi: 10.1177/0883073809333537. [DOI] [PubMed] [Google Scholar]
- Zwicker JG, Missiuna CC, Boyd LA. Brain activation of children with developmental coordination disorder is different than peers. Pediatrics. 2010;126:678–686. doi: 10.1542/peds.2010-0059. [DOI] [PubMed] [Google Scholar]


