Abstract
Inflammation is a key part of central nervous system pathophysiology. However, inflammatory factors are now thought to have both beneficial and deleterious effects. Here, we examine the hypothesis that lipocalin-2, an inflammatory molecule that can be upregulated in the distressed central nervous system, may enhance angiogenesis in brain endothelial cells. Adding lipocalin-2 (0.5–2.0µg/ml) to RBE.4 rat brain endothelial cells significantly increased matrigel tube formation and scratch migration, and also elevated levels of iron and reactive oxygen species (ROS). Co-treatment with a radical scavenger (UE83836E), a Nox inhibitor (apocynin) and an iron chelating agent (deferiprone) significantly dampened the ability of lipocalin-2 to enhance tube formation and scratch migration in brain endothelial cells. These findings provide in vitro proof of the concept that lipocalin-2 can promote angiogenesis via iron- and ROS-related pathways, and support the idea that lipocalin-2 may contribute to the neurovascular recovery aspects of inflammation.
Keywords: iron, migration, neurovascular remodeling, reactive oxygen species, stroke, tube formation
INTRODUCTION
Neurovascular remodeling is an essential part of stroke recovery (Liu et al. 2014). Within the surviving peri-infarct zone, angiogenesis may assist with endogenous repair and reorganization. Regrowth of brain blood vessels not only restores perfusion, but newly reconstructed microvessels may also serve as conduits for neuroblast migration and neural remodeling. As expected, these endogenous angiogenic mechanisms should involve canonical vascular endothelial growth factor (VEGF)-mediated pathways (Greenberg and Jin 2013). Blockade of VEGF signaling interferes with post-stroke angiogenesis, whereas promoting VEGF signaling enhances stroke recovery.
More recently, however, emerging data suggest that beyond VEGF, the vascular remodeling process may also recruit a wider array of other factors. For example, it is now recognized that molecules and pathways responsible for neural development significantly overlap with those that regulate angiogenesis (Xiong et al. 2010). Brain derived neurotrophic factor (BDNF) also possess pro-angiogenic properties (Kermani and Hempstead 2007), netrins regulate both axon guidance as well as vessel formation (Park et al. 2004), and matrix metalloproteinases are essential for both neural and vascular remodeling (Yang et al. 2011).
Within the context of stroke, one potential linking theme may be centered on injury response mechanisms, because inflammation may also contribute to tissue repair in both neural and vascular compartments. At lower concentrations, the inflammatory cytokine tumor necrosis factor alpha (TNF-α), which is released by damaged neurons, may in fact promote angiogenesis (Fajardo et al. 1992). The damage-associated-molecular-pattern (DAMP) mediator HMGB1 can attract both endothelial progenitor cells and augment microvascular recovery (Yang et al. 2014). Thus, inflammation possesses both deleterious and beneficial properties, and the damaged CNS upregulates a wide spectrum of distress signals and substrates that may initiate the process of self-healing within all cell-types of the extended neurovascular unit. In this regard, recent data implicating lipocalin-2 (LCN2) in stroke may be relevant. Lipocalin-2, also known as neutrophil gelatinase-associated lipocalin or 24p3, is a secretory glycoprotein belonging to the lipocalin superfamily. Although initially identified as an antibacterial factor released from neutrophils (Flo et al. 2004), LCN2 is now known to be upregulated in the CNS after injury (Jin et al. 2014; Rathore et al. 2011; Xing et al. 2014). Is it possible that LCN2 may also contribute to endogenous recovery via pro-angiogenic mechanisms? In this proof-of-principle study, we assess this hypothesis by testing LCN2 in rat brain endothelial cells. Our results suggest that within narrow concentration windows, LCN2 enhances angiogenesis via reactive oxygen species and iron-dependent mechanisms. Therefore, LCN2 may contribute to potentially beneficial effects of inflammation in the injured CNS.
MATERIALS AND MEDTHODS
Rat Brain Endothelial Cell Culture
RBE.4 cells, a rat brain endothelial cell line, were cultured in flasks coated with rat tail collagen I (Corning, Bedford, MA) and maintained in endothelial basal medium (EBM)-2 (Lonza, Hopkinton, MA) supplemented with fetal bovine serum, fibroblast growth factor-2, epidermal endothelial growth factor, hydrocortisone, insulin-like growth factor, ascorbic acid, VEGF, and amphotericin B. RBE.4 cells were treated with different concentrations (0.5, 1, or 2µg/ml) of recombinant rat LCN2 (R&D, Minneapolis, MN) for 24hr. Cytotoxicity was measured by a standard lactate dehydrogenase (LDH) release assay (Roche, Germany) and Annexin V apoptosis detection kit (BD, San Jose, CA), and proliferation was evaluated by standard 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl-tetrazolium bromide (MTT) assay. The expression of LCN2 receptors (LRP2 and 24p3R) was detected by Western Blot assay. The primary antibodies of LRP2 and 24p3R were purchased from Abcam (Cambridge, MA) and Bioss (Woburn, MA), respectively. All experiments were approved by Massachusetts General Hospital, Harvard Medical School.
In Vitro Angiogenesis Assays
Tube formation and scratch migration assays were used to assess in vitro angiogenesis. The standard Matrigel assay was used to assess the spontaneous formation of capillary-like structures of endothelial cells. Briefly, RBE.4 cells (4×104 cells/well) were seeded in 48-well plates coated with growth factor reduced Matrigel (Corning), and incubated with different concentrations of LCN2 (0.5, 1, or 2µg/ml) at 37°C for 18–24hr in the absent or present of 5µM of reactive oxygen species (ROS) scavenger U83836E (Calbiochem, San Diego, CA), 100µM of NADPH oxidase (Nox) inhibitor apocynin (Sigma, St. Louis, MO) or 50µM of iron chelator deferiprone (Sigma). The number of tubes was counted in two random fields from each well.
Scratch migration assay was used to measure cell migration across a scratch gap in vitro. RBE.4 cells were plated on 6-well plates until a confluent monolayer formed. The cells were scratched using a 10µl pipette tip, and treated with or without different doses of LCN2 (0.5, 1, and 2µg/ml) in the absence or presence of U83836E, apocynin or deferiprone for 2 days. Progression of cell migration into the gap field was monitored each day by phase contrast microscopy. Two areas were randomly selected and imaged in each well. The cell migration was evaluated by calculating the percentage of areas occupied by migrated cells.
Determination of Labile Iron
The level of labile iron was determined by a fluorescence technique with the Fe sensor calcein. RBE.4 cells were 70–80% confluent, and then exposed to 1µg/ml of LCN2 for various times (1hr, 3hr, or 6hr). After addition of 2µM of calcein-acetomethoxy (calcein-AM) (Molecular Probes, Eugene, OR) for 30min at 37°C in the dark, fluorescence intensity was measured using an excitation wavelength of 485nm and an emission wavelength of 535nm by a microplate reader.
Measurement of Reactive Oxygen Species
Levels of intracellular ROS were measured using CM-H2DCFDA (Molecular Probes). Briefly, after RBE.4 cells were 70–80% confluent, CM-H2DCFDA was added to the cultures at a final concentration of 10µM for 45min in the dark. Then the cultures were treated with 1µg/ml of LCN2 for various times (1hr, 3hr, or 6hr). Fluorescence intensity was measured at an excitation wavelength of 495nm and an emission wavelength of 525nm using the microplate reader.
Statistical Analysis
All experiments were repeated at least 3 times independently. Data were expressed as mean±SD. Quantitative data were analyzed using one-way or two-way ANOVA followed by inter-group Tukey-Kramer tests as needed (SPSS version 16.0). Values of P<0.05 were considered statistically significant.
RESULTS
LCN2 Enhanced Angiogenesis in Rat Brain Endothelial Cells
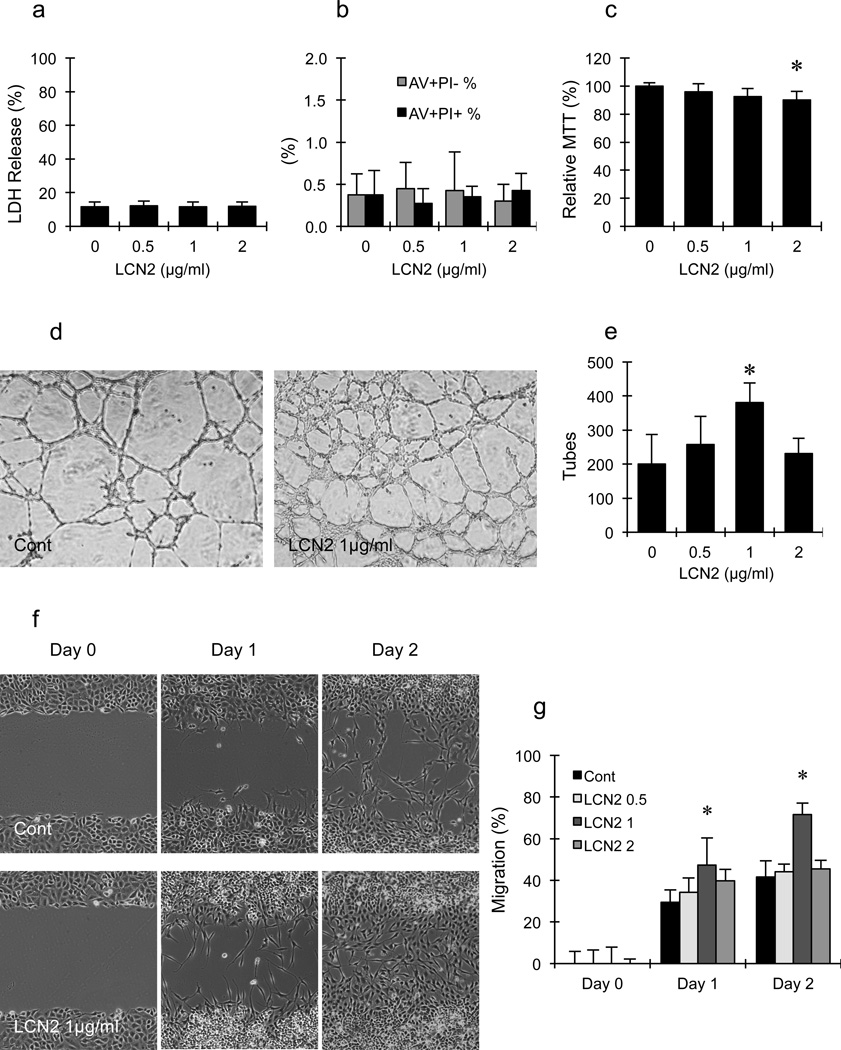
LCN2 treatment was not toxic to rat brain endothelial cells, and did not induce cell death as measured by the standard LDH release assay (Fig. 1a) and flow-cytometry for cell death markers (propidium iodide and annexin V) (Fig. 1b). However, at the highest concentration tested (2µg/ml), LCN2 slightly decreased metabolic activity and cell proliferation, as measured by the standard MTT assay (Fig. 1c).
Figure 1.
(a–c) Effects of lipocalin-2 (LCN2) on cell death and proliferation in rat brain endothelial cells. (a) LDH assay showed that LCN2 treatment was not toxic to RBE.4 cells. (b) Flow cytometry showed that LCN2 treatment did not increase the percentage of early apoptosis (Annexin V+PI−) and late apoptosis/necrosis (AV+PI+) in RBE.4 cells. (c) MTT assay showed that 2µg/ml of LCN2 slightly inhibited the proliferation of RBE.4 cells. *, p<0.05 compared with control group. (d–g) Effects of LCN2 on tube formation and scratch migration in rat brain endothelial cells. (d, f) Representative pictures of tube formation (d) and scratch migration (f). (e, g) Bar graph of the number of tubes (e) and migration (g). Treatment with 1µg/ml of LCN2 significantly increased the number of tubes, reduced the area of gap field and enhanced endothelial migration. *, p<0.05 compared with the control group.
Matrigel tube formation and scratch migration were performed as two independent in vitro assays of angiogenesis. First, LCN2 was added to rat brain endothelial cells seeded onto matrigel and tube formation was quantified. LCN2 (0.5–1µg/ml) induced tube formation in a dose-dependent manner. Compared with the control group, 1µg/ml of LCN2 significantly increased the number of tubes by about 70–80% (Fig. 1d and 1e). But this effect appeared to have a narrow concentration window since the higher level of LCN2 (2µg/ml) no longer appeared to promote endothelial tube formation.
Based on these results of cellular viability and matrigel tube formation, the effects of LCN2 on endothelial migration was examined next. Rat brain endothelial cells were plated and then a mechanical scratch was used to remove cells in a linear gap. By 1 to 2 days later, endothelial cells were observed to migrate into the denuded gaps (Fig. 1f). One day after scratching, all three different doses of LCN2 seemed to slightly increase cell migration compared to the control group. However, two days after scratching, there is no difference in migration between control and LCN2-treated groups except at the dose of 1µg/ml. In control cells, gaps were closed by 40%; LCN2 (1µg/ml) significantly enhanced endothelial migration to 70% (Fig. 1g). The apparent dose-response for endothelial migration was consistent with the effects of LCN2 on tube formation.
LCN2 Increased Intracellular Iron Levels and Induced ROS Generation
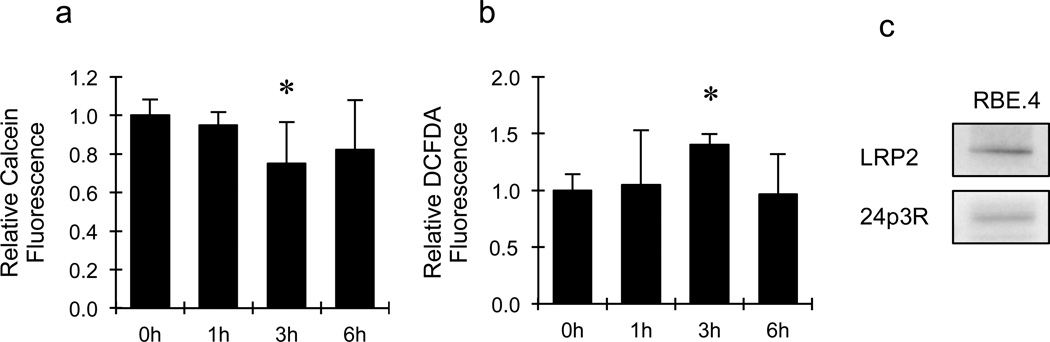
LCN2 is known to regulate cellular iron transport. So we asked whether pro-angiogenic effects of LCN2 also occurred in the context of altered iron levels. The intracellular labile iron pool was measured by the use of calcein-AM that is quenched upon binding to ‘free’ iron. These measurements showed that intracellular labile iron was significantly elevated by about 25% after exposing to 1µg/ml of LCN2 for 3hr (Fig. 2a).
Figure 2.
Effects of lipocalin-2 (LCN2) on intracellular levels of labile iron and ROS generation in rat brain endothelial cells. Treatment with 1µg/ml of LCN2 for 3hrs significantly reduced fluorescent intensity of calcein-AM (a) and increased fluorescent intensity of CM-H2DCFDA (b) in RBE.4 cells. *, p<0.05 compared with the control group. (c) Western blot showed that RBE.4 cells expressed the two major receptors of LCN2, including LRP2 and 24p3R.
Since iron accumulation may generate ROS, which may be involved in cell signaling, we also asked whether LCN2 affected endothelial ROS generation. Treatment with 1µg/ml of LCN2 for 3hr significantly increased ROS levels, as measured by a standard H2DCFDA-sensitive fluorescent assay (Fig. 2b). Compared with the control group, ROS levels were increased by about 40% after LCN2 treatment.
Finally, it has been reported that lipocalin-2 signaling may involve interactions with specific membrane receptors 24p3R and LRP2 (Devireddy et al. 2005; Hvidberg et al. 2005). Therefore we used western blotting to assess the expression of 24p3R and LRP2. The blots showed that our rat brain endothelial cell system could express both LCN2 receptors (Fig. 2c).
Iron chelation and ROS Inhibition Abrogated LCN2-Induced Angiogenesis
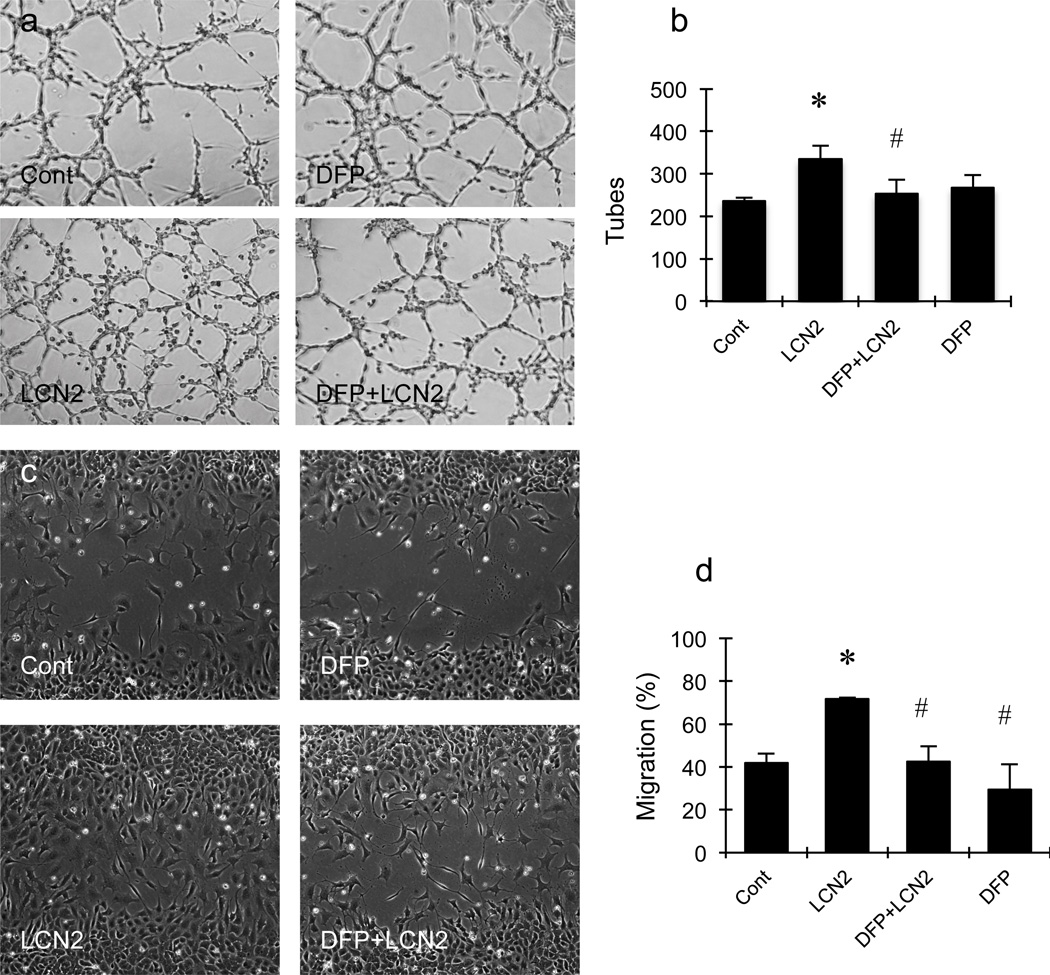
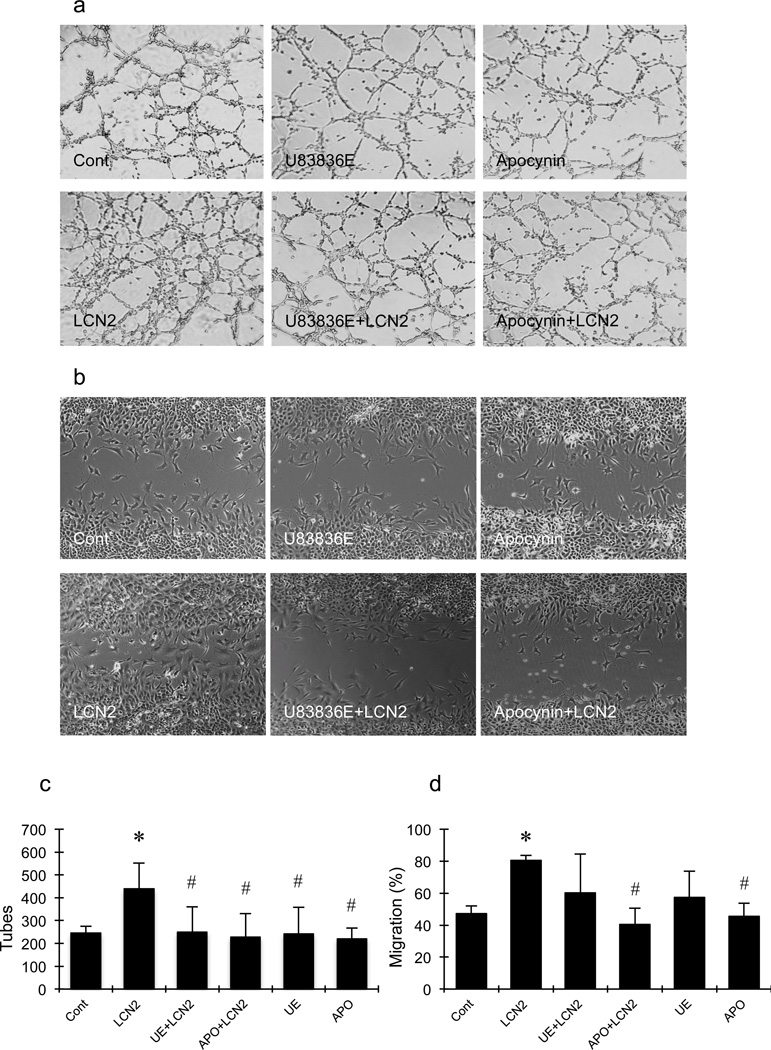
To connect iron accumulation and ROS generation with LCN2-mediated angiogenesis, pharmacologic blockade experiments were performed. Exposure to 50µM of the iron chelator deferiprone significantly blocked LCN2-induced tube formation (Fig. 3a and 3b) and cell migration (Fig. 3c and 3d). Similarly, the lazaroid-based ROS scavenger U83836E also significantly prevented LCN2 from upregulating tube formation and migration (Fig. 4a–4d). Finally, because endothelial ROS generation is typically mediated by the Nox system, we tested the effects of apocynin as a standard Nox inhibitor. As expected, apocynin significantly suppressed both LCN2-induced endothelial tube formation and migration (Fig. 4a–4d).
Figure 3.
Effects of deferiprone (DFP) on lipocalin-2 (LCN2)-induced tube formation and scratch migration in rat brain endothelial cells. (a, c) Representative pictures of tube formation (a) and migration (c). (b, d) Bar graphs of the number of tubes (b) and migration (d). Deferiprone significantly inhibited LCN2-induced increment of the number of tubes (b) and cell migration (d) in RBE.4 cells. *, p<0.05 compared with the control group; #, p<0.05 compared with LCN2-treated group.
Figure 4.
Effects of U83836E (UE) and apocynin (APO) on lipocalin-2 (LCN2)-induced tube formation and scratch migration in rat brain endothelial cells. (a, b) Representative pictures of tube formation (a) and cell migration (b). (c, d) Bar graphs of the number of tubes (c) and migration (d). U83836E and apocynin significantly inhibited LCN2-induced increment of the number of tubes (c) and cell migration (d) in RBE.4 cells. *, p<0.05 compared with the control group; #, p<0.05 compared with LCN2-treated group.
DISCUSSION
Angiogenesis and neurogenesis provide critical substrates for recovery after stroke. Although the time courses of angiogenesis and neurogenesis overlap, some data suggest that angiogenesis occurs first, and then newborn microvessels promote neuroblast migration and axonal remodeling in experimental models of ischemic brain injury (Liu et al. 2014; Xiong et al. 2010). Thus, understanding the signals for angiogenesis in damaged brain may allow us to design pro-recovery therapies for stroke. Although angiogenic mechanisms will surely be centered on VEGF, an emerging literature now implicates a broader network of signals, including those recruited from inflammatory cascades (Liu et al. 2014). In this proof-of-concept study, we demonstrate that the inflammatory mediator LCN2 increased tube formation and cell migration in rat brain endothelial cells via iron and ROS-dependent mechanisms. Therefore, further investigations might be warranted to explore how LCN2 contributes to the angiogenic response after stroke.
Recently, we showed that LCN2 is upregulated in damaged brain as part of an endogenous repair response (Xing et al. 2014). After oxygen-glucose deprivation in vitro or cerebral ischemia in vivo, injured-but-not-dead neurons appeared to release low levels of LCN2 as a “help-me” signal that shifted astrocytes and microglia into beneficial phenotypes. The present study may extend this concept. LCN2 may also promote recovery by enhancing angiogenesis.
LCN2 was originally identified as an inflammatory factor released by activated neutrophils (Flo et al. 2004). In general, neutrophil infiltration and inflammation after stroke is thought to be deleterious. Our findings here raise the possibility that these phenomena may be nuanced. Indeed, recent studies propose that, analogous to the M1-vs-M2 concept of biphasic macrophages, both damaging N1 and beneficial N2 neutrophils may also be triggered after stroke (Cuartero et al. 2013). If LCN2 can induce pro-recovery glia (Xing et al. 2014) and enhance angiogenesis (present study), these findings may provide at least one potential mechanism for biphasic neutrophil inflammation. As a general principle, the biphasic nature of many mediators in neurobiology is well known. For example, nerve growth factor and BDNF promote cellular survival via their primary receptors TrkA and TrkB respectively. However, at high concentrations, both factors can also be neurotoxic via overactivation of the p75NTR receptor (Blochl and Blochl 2007). The same may be true for LCN2. At high concentrations or with prolonged exposure, LCN2 may induce neuronal death (Bi et al. 2013). But for the present study, 0.5 to 1.0µg/ml levels of LCN2 appeared to have beneficial actions without cytotoxicity in either endothelial cells or neurons.
Our initial data implicate iron- and ROS-dependent pathways. Considering the initial role of LCN2 as an iron transporter, we believed that the intracellular iron accumulation induced by LCN2 could stimulate low-dose ROS generation that further enhanced angiogenesis in brain endothelial cells. Transition metals such as iron generate hydroxyl radicals and play an important role in cell growth and adaptation to imbalanced microenvironment change in the tissue (Kim et al. 2009; Piloni et al. 2013). Enhanced total Fe levels, as well as the labile iron pool concentrations of the brain, were associated with a significant increase in ROS generation and oxidative stress condition (Piloni et al. 2013). A role of iron is consistent with LCN2’s known ability to transport iron (Flo et al. 2004). The involvement of ROS may be more surprising. Overexpression of LCN2 enhanced cell proliferation and decreased senescence induced by H2O2 treatment in bone-marrow-derived mesenchymal stem cells, suggesting that LCN2 might be protective against oxidative stress (Bahmani et al. 2014). Alternatively, LCN2-induced elevations in iron may increase ROS via Fenton-like reactions. High ROS levels damage cells, but low ROS levels can act as intracellular signaling molecules. During angiogenesis, multiple steps including endothelial progenitor recruitment, endothelial differentiation and migration, and vessel sprouting and branching, are all modulated by redox signaling via Nox pathways (Lassegue et al. 2012). Our study may be consistent with these ideas. Low levels of LCN2 moderately increased ROS in brain endothelial cells without toxicity, and ROS scavenging or Nox inhibition inhibited LCN2-induced tube formation and migration. In a canine model of cardiac ischemia, ROS scavengers abrogated coronary collateral growth (Gu et al. 2003). Is it possible that excessive iron chelation or radical scavenging may also interfere with LCN2-mediated angiogenesis during stroke recovery?
Taken together, our findings suggest that LCN2 may promote angiogenesis in brain endothelial cells via iron- and ROS-dependent mechanisms. However, many caveats remain. First, although low levels of LCN2 may be a “help-me” signal for beneficial glial activation (Xing et al. 2014) and angiogenesis, higher levels may be deleterious (Bi et al. 2013). The angiogenic effects seem to fall within a rather narrow concentration window. In this study, the highest dose tested showed a slight effect on metabolic suppression, albeit without overt cell death. Future studies are needed to carefully assess dose- and time-dependence of angiogenesis versus potential cytotoxicity under conditions that mimic stroke. Second, it is always difficult to correlate concentrations in cell culture versus what happens in vivo. We previously showed that LCN2 levels ranged between 5 and 10ng/mg protein in ischemic brain tissue, and calculated comparable LCN2 concentrations in cell cultures in the range of 0.5 to 1µg/ml (Jin et al. 2014; Rathore et al. 2011; Xing et al. 2014). Hence, the range of LCN2 tested in our endothelial cells is consistent with our previous findings in astrocytes and microglia (Jin et al. 2014; Rathore et al. 2011; Xing et al. 2014), but future studies are required to confirm angiogenic concentrations in vivo. Third, cellular roles for LCN2 may be model- and species-dependent. In a rat model of focal ischemia, injured-but-not-dead neurons produce potentially beneficial LCN2 (Xing et al. 2014), whereas in mouse cerebral ischemia or spinal cord injury, astrocytes may produce damaging levels of LCN2 (Jin et al. 2014; Rathore et al. 2011). Our initial angiogenic findings here are obtained in RBE.4 cells, a well-characterized rat brain endothelial cell line that is widely used for cerebrovascular biology. Nevertheless, further investigations are needed to assess how LCN2 contributes to the balance between injury and repair in different species and cell types, especially in terms of delineating crosstalk between neuronal, glial and vascular elements in primary cell cultures. Fourth, our data implicate iron and ROS signaling, and we also confirm the presence of the two major LCN2 receptors 24p3R and LRP2. But more rigorous studies are required to define specific receptor mechanisms since iron and ROS pathways are involved in multiple systems during physiology and pathophysiology. Finally, these initial results only provide in vitro proof-of-concept. Further in vivo studies are warranted to completely understand the effects of LCN2 on neovascularization as well as its signaling pathways in stroke recovery.
ACKNOWLEDGEMENTS
The study was supported in part by grants from the NIH, AHA and the Rappaport Foundation.
Abbreviations
- AM
acetomethoxy
- APO
apocynin
- BDNF
Brain derived neurotrophic factor
- DAMP
damage-associated-molecular-pattern
- DFP
deferiprone
- EBM
endothelial basal medium
- LCN2
lipocalin-2
- LDH
lactate dehydrogenase
- MTT
3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl-tetrazolium bromide
- Nox
NADPH oxidase
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor alpha
- UE
U83836E
- VEGF
vascular endothelial growth factor
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- Bahmani B, Roudkenar MH, Halabian R, Jahanian-Najafabadi A, Amiri F, Jalili MA. Lipocalin 2 decreases senescence of bone marrow-derived mesenchymal stem cells under sub-lethal doses of oxidative stress. Cell Stress Chaperones. 2014 doi: 10.1007/s12192-014-0496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi F, Huang C, Tong J, Qiu G, Huang B, Wu Q, Li F, Xu Z, Bowser R, Xia XG, Zhou H. Reactive astrocytes secrete lcn2 to promote neuron death. Proc Natl Acad Sci U S A. 2013;110:4069–4074. doi: 10.1073/pnas.1218497110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blochl A, Blochl R. A cell-biological model of p75NTR signaling. J Neurochem. 2007;102:289–305. doi: 10.1111/j.1471-4159.2007.04496.x. [DOI] [PubMed] [Google Scholar]
- Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, Corbi AL, Lizasoain I, Moro MA. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARgamma agonist rosiglitazone. Stroke. 2013;44:3498–3508. doi: 10.1161/STROKEAHA.113.002470. [DOI] [PubMed] [Google Scholar]
- Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–1305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol. 1992;140:539–544. [PMC free article] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci. 2013;70:1753–1761. doi: 10.1007/s00018-013-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Weihrauch D, Tanaka K, Tessmer JP, Pagel PS, Kersten JR, Chilian WM, Warltier DC. Reactive oxygen species are critical mediators of coronary collateral development in a canine model. Am J Physiol Heart Circ Physiol. 2003;285:H1582–H1589. doi: 10.1152/ajpheart.00318.2003. [DOI] [PubMed] [Google Scholar]
- Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005;579:773–777. doi: 10.1016/j.febslet.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Jin M, Kim JH, Jang E, Lee YM, Soo Han H, Woo DK, Park DH, Kook H, Suk K. Lipocalin-2 deficiency attenuates neuroinflammation and brain injury after transient middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2014 doi: 10.1038/jcbfm.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc Med. 2007;17:140–143. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Kim JH, Kim EH, Na HK, Cha YN, Chung JH, Surh YJ. 15-Deoxy-Delta12,14-prostaglandin J2 upregulates the expression of heme oxygenase-1 and subsequently matrix metalloproteinase-1 in human breast cancer cells: possible roles of iron and ROS. Carcinogenesis. 2009;30:645–654. doi: 10.1093/carcin/bgp012. [DOI] [PubMed] [Google Scholar]
- Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler RA, Lawton MT, Yang GY. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol. 2014;115:138–156. doi: 10.1016/j.pneurobio.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, Kuo CJ, Li DY. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci U S A. 2004;101:16210–16215. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piloni NE, Fermandez V, Videla LA, Puntarulo S. Acute iron overload and oxidative stress in brain. Toxicology. 2013;314:174–182. doi: 10.1016/j.tox.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Rathore KI, Berard JL, Redensek A, Chierzi S, Lopez-Vales R, Santos M, Akira S, David S. Lipocalin 2 plays an immunomodulatory role and has detrimental effects after spinal cord injury. J Neurosci. 2011;31:13412–13419. doi: 10.1523/JNEUROSCI.0116-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C, Wang X, Cheng C, Montaner J, Mandeville E, Leung W, van Leyen K, Lok J, Lo EH. Neuronal production of lipocalin-2 as a help-me signal for glial activation. Stroke. 2014;45:2085–2092. doi: 10.1161/STROKEAHA.114.005733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11:298–308. [PMC free article] [PubMed] [Google Scholar]
- Yang S, Xu L, Yang T, Wang F. High-mobility group box-1 and its role in angiogenesis. J Leukoc Biol. 2014;95:563–574. doi: 10.1189/jlb.0713412. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hill JW, Rosenberg GA. Multiple roles of metalloproteinases in neurological disorders. Prog Mol Biol Transl Sci. 2011;99:241–263. doi: 10.1016/B978-0-12-385504-6.00006-3. [DOI] [PubMed] [Google Scholar]