Abstract
Objective
Vital signs and composite scores, such as the Modified Early Warning Score (MEWS), are used to identify high-risk ward patients and trigger rapid response teams. Although age-related vital sign changes are known to occur, little is known about the differences in vital signs between elderly and non-elderly patients prior to ward cardiac arrest (CA). We aimed to compare the accuracy of vital signs for detecting CA between elderly and non-elderly patients.
Design
Observational cohort study.
Setting
Five hospitals in the United States.
Patients
A total of 269,956 patient admissions to the wards with documented age, including 422 index ward CAs.
Interventions
None.
Measurements and Main Results
Patient characteristics and vital signs prior to CA were compared between elderly (age 65 years or older) and non-elderly (age less than 65 years) patients. The area under the receiver operating characteristic curve (AUC) for vital signs and the MEWS were also compared. Elderly patients had a higher CA rate (2.2 vs. 1.0 per 1000 ward admissions; P<0.001) and in-hospital mortality (2.9% vs. 0.7%; P<0.001) than non-elderly patients. Within four hours of CA, elderly patients had significantly lower mean heart rate (88 vs. 99 beats per minute; P<0.001), diastolic blood pressure (60 vs. 66 mm Hg; P=0.007), shock index (0.82 vs. 0.93; P<0.001), and MEWS (2.6 vs. 3.3; P<0.001), and higher pulse pressure index (0.45 vs. 0.41; P<0.001) and temperature (36.4 vs. 36.3 °C; P=0.047). The AUCs for all vital signs and the MEWS were higher for non-elderly patients than elderly patients (MEWS AUC 0.85 (95% CI 0.82-0.88) vs. 0.71 (95% CI 0.68-0.75); P<0.001).
Conclusions
Vital signs more accurately detect CA in non-elderly patients compared to elderly patients, which has important implications for how they are used for identifying critically ill patients. More accurate methods for risk stratification of elderly patients are necessary to decrease the occurrence of this devastating event.
Keywords: heart arrest, hospital rapid response team, aged, physiologic monitoring, quality improvement, early diagnosis
Introduction
In-hospital cardiac arrest causes a substantial healthcare burden, and some of these events are thought to be preventable.(1-3) In particular, arrests that occur on the general hospital wards are often due to errors in triage, diagnosis, and treatment of the underlying condition.(3) Vital signs are often an important component of the decision-making process regarding whether to transfer a critically ill patient to the intensive care unit (ICU) or allow them to remain on the wards. In addition, composite scores of vital sign derangement, such as the Modified Early Warning Score (MEWS),(4) are often used to trigger calls to the Rapid Response Team (RRT) and aid with these decisions. However, the utility of these scores has been called into question given their variable accuracy and the mixed results of the RRT literature for their effect on important outcomes, such as in-hospital mortality.(5)
Increasing age is known to be an independent risk factor for adverse events in the hospital for critically ill patients.(6, 7) In addition, changes in vital signs are known to occur with age.(8, 9) However, the implications of these changes on vital signs prior to ward cardiac arrest are poorly characterized. If differences between elderly and non-elderly patients were discovered, it could have important implications regarding how vital signs and early warning scores are used for the identification and triage of high-risk ward patients. Therefore, we aimed to investigate the differences in vital signs between elderly and non-elderly patients in a multicenter cohort of hospitalized patients. We hypothesized that vital signs would be less deranged and therefore less accurate before cardiac arrest in elderly patients.
Methods
Study population and setting
We conducted an observational cohort study at five hospitals (the University of Chicago and four NorthShore HealthSystem Hospitals (Evanston, Glenbrook, Highland Park, and Skokie) that included adult patients hospitalized on the wards from the period of November 2008 to January 2013. The University of Chicago is an urban tertiary-care university hospital, Evanston and Glenbrook are suburban teaching hospitals, and Highland Park and Skokie are community non-teaching hospitals. All hospitals had nurse-led RRTs in place during the study period. The study protocol was approved by the University of Chicago Institutional Review Board, with a waiver of consent, which was granted based on minimal harm and general impracticability (IRB #16995A).
Data collection
Cardiac arrest, defined as the loss of a palpable pulse with attempted resuscitation, was collected via a prospective quality improvement database at the University of Chicago that has been previously described,(10) and through a prospectively collected log at NorthShore University HealthSystem. All arrests underwent manual review to ensure data quality, and only a patient's index arrest on the wards was used in the analyses if they suffered more than one event. Patients who died on the wards without attempted resuscitation were not counted as cardiac arrests because these were most likely expected deaths in patients with do-not-resuscitate orders. Routinely collected vital signs (temperature, respiratory rate, heart rate, arterial blood pressure, and peripheral oxygen saturation) were collected from the electronic health record at the University of Chicago (Epic; Verona, WI) and an electronic data warehouse at NorthShore. All event and vital sign data were time and location stamped. Patient characteristics were obtained from administrative databases at all hospitals.
Statistical Analysis
Patient characteristics were compared between elderly (age 65 years or older) and non-elderly (age less than 65 years) patients using t-tests and chi-squared tests for continuous and categorical data, with the exception of MEWS, oxygen saturation, and length of stay, which were presented as median (IQR 25-75) and compared using the Wilcoxon rank sum due to their skewed distributions. Ward vital signs in the entire dataset were compared between elderly and non-elderly patients using a mixed-effects regression model with patient-level random effects to account for the correlation among values from the same patient. Vital signs within four hours of a patient's index ward cardiac arrest were then compared between these groups using the same method. The four hour time period was chosen because patients in this study typically had vital signs checked every four hours while on the wards. Vital sign trajectories in the twenty-four hours prior to arrest were then compared visually using restricted cubic splines with three knots. Mixed-effects models were fit to test the difference in vital sign trajectory using a model with a random intercept at the patient level and an autoregressive correlation structure. This method not only accounts for the correlation of values from the same patient but also the fact that vital signs measured closer together in time are more likely to be highly correlated than those measured farther apart.(11) Each vital sign was separately modeled as the outcome variable with time and a time-age group interaction variable in the model. A statistically significant interaction term was used to define a difference in the vital sign slope between elderly and non-elderly patients.
A MEWS score was then calculated for each observation time for each patient in the entire dataset.(4) If a vital sign was missing that was necessary for the calculation at a specific time point then the previous value was carried forward. If no values were available then a median value was imputed because these values are likely to be normal.(6) The MEWS was then compared between elderly and non-elderly patients in its overall distribution in the dataset, the values four hours prior to cardiac arrest, and trajectory prior to the event using the same methods as for the individual vital signs.
Finally, the area under the receiver operating characteristic curve (AUC) was calculated for each individual vital sign for elderly and non-elderly ward patients for cardiac arrest by using the highest and lowest value during the ward admission for those who suffered an arrest compared to those discharged alive without suffering an arrest or ward to ICU transfer during their admission.(12) A similar analysis was performed for the MEWS using the highest value for each patient during their ward admission. Patients were then separated into deciles of age, and the MEWS AUC analysis was repeated and compared to the incidence of cardiac arrest in each age group. Finally, a sensitivity analysis was performed where age ≥75 was considered elderly instead of age ≥65 for the MEWS comparisons. All analyses were performed using Stata version 12.1 (StataCorps; College Station, Texas), with a two-tailed p-value <0.05 denoting statistical significance.
Results
A total of 269,999 patient admissions occurred during the study period, of which 43 patients did not have age documented during their stay. This resulted in a total of 269,956 patient admissions and 422 index ward cardiac arrests for study inclusion. All vital signs had less than 5% missing except for oxygen saturation (12%) and AVPU (28%). Variable missingness was similar between elderly and non-elderly patients except that elderly patients were more likely to be missing blood pressure (4% vs. 3%) and less likely to be missing AVPU (23% vs. 32%) than non-elderly patients. Elderly patients accounted for 46% of the study population (n=123,671) and 65% of the ward cardiac arrests (n=273). Elderly patients were less likely to be female (56% vs. 64%; P<0.001), less likely to be black (13% vs. 23%; P<0.001), and had a higher cardiac arrest rate (2.2 vs. 1.0 per 1000 ward admissions; P<0.001), and in-hospital mortality (2.9% vs. 0.7%; P<0.001) compared to non-elderly patients (Table 1). In addition, elderly patients presented more often with asystole than non-elderly patients (23% vs. 14%) but these differences in initial rhythm were not statistically significant between the two groups (P=0.06). Comparisons between the vital signs of elderly and non-elderly patients in the entire dataset are shown in Table 2, which were all significantly different (p<0.001). Of note, although statistically significant, the MEWS and its distribution were similar in both patient groups using the entire dataset (median MEWS of 1 (IQR 1-2)). In addition, the MEWS was similar at the time of ICU transfer for non-elderly (median MEWS of 2 (IQR 1-4)) and elderly (median MEWS of 2 (IQR 1-3)) patients.
Table 1.
Comparisons of patient characteristics between elderly and non-elderly patients.
| Elderly patients (n=123,671) | Non-elderly patients (n=146,285) | Total (n=269,956) | |
|---|---|---|---|
| Age, mean (SD), years | 79 (8)* | 45 (13) | 60 (20) |
| Female sex, n (%) | 69,276 (56)* | 93,005 (64) | 162,281 (60) |
| Race | |||
| Black, n (%) | 16,049 (13)* | 33,621 (23) | 49,670 (18) |
| White, n (%) | 75,142 (61)* | 65,288 (45) | 140,430 (52) |
| Other/ Unknown, n (%) | 32,480 (26)* | 47,376 (32) | 79,899 (30) |
| Cardiac arrests, n (# per 1000 ward admissions) | 273 (2.2)* | 149 (1.0) | 422 (1.6) |
| Initial rhythm of cardiac arrest, n (% out of 422 index arrests) | |||
| PEA | 136 (50%) | 93 (62%) | 229 (54%) |
| VF/VT | 61 (22%) | 30 (20%) | 91 (21%) |
| Asystole | 63 (23%) | 21 (14%) | 84 (20%) |
| Unknown | 14 (5%) | 6 (4%) | 20 (5%) |
| Length of ward stay prior to arrest, median (IQR), hours (n=422) | 58 (28-152) | 57 (23-112) | 57 (24-120) |
| In-hospital mortality, % | 2.9%* | 0.7% | 1.7% |
P-value <0.05 for comparison between elderly and non-elderly patients
Abbreviations: PEA = pulseless electrical activity; VF/VT = ventricular fibrillation or ventricular tachycardia
Table 2.
Comparisons of mean vital signs and Modified Early Warning Score between elderly and non-elderly patients in entire dataset.
| Elderly patients (n=123,671) | Non-elderly patients (n=146,285) | All patients (n=269,956) | |
|---|---|---|---|
| Temperature, °C | 36.6 (0.6)* | 36.6 (0.6) | 36.6 (0.6) |
| Respiratory rate, breaths/min | 19 (3)* | 18 (2) | 19 (3) |
| Heart rate, beats/min | 80 (16)* | 84 (16) | 82 (16) |
| Systolic blood pressure, mmHg¶ | 129 (24)* | 123 (21) | 126 (22) |
| Diastolic blood pressure, mmHg¶ | 66 (13)* | 71 (13) | 69 (13) |
| Pulse pressure index | 0.48 (0.09)* | 0.42 (0.08) | 0.45 (0.09) |
| Shock index | 0.64 (0.18)* | 0.70 (0.18) | 0.67 (0.18) |
| Oxygen saturation, median (IQR), % | 97 (95-98)* | 98 (96-99) | 97 (95-99) |
| MEWS, median (IQR) | 1 (1-2)* | 1 (1-2) | 1 (1-2) |
P-value <0.001 for comparison between elderly and non-elderly patients
Blood pressure was non-invasively measured arterial blood pressure
Pulse pressure index = (systolic blood pressure – diastolic blood pressure)/(systolic blood pressure); Shock index = systolic blood pressure/heart rate
Abbreviations: MEWS = Modified Early Warning Score
In the four hours before cardiac arrest, elderly patients had lower mean heart rate (88 vs. 99 beats per minute; P<0.001), diastolic blood pressure (60 vs. 66 mm Hg; P=0.007), shock index (0.82 vs. 0.93; P<0.001), respiratory rate (22 vs. 23 breaths/min; P=0.05), and MEWS (median 2 vs. 3; P<0.001), and higher pulse pressure index (0.45 vs. 0.41; P<0.001) and temperature (36.4 vs. 36.3 °C; P=0.047) (Table 3). In addition, respiratory rate and pulse pressure index trajectories were steeper in the twenty-four hours prior to cardiac arrest for non-elderly compared to elderly patients (P=0.001 and P=0.05). The MEWS trajectory was similar between the two groups prior to the event (Figure 1). The AUCs for the highest and lowest vital signs during the ward stay were greater for non-elderly compared to elderly patients with the exception of the minimum respiratory rate (Table 4). The most accurate vital signs for non-elderly patients were the maximum respiratory rate (AUC 0.82 (95% CI 0.79-0.86)) and heart rate (AUC 0.77 (95% CI 0.73-0.81)). For elderly patients, the maximum respiratory rate (AUC 0.67 (95% CI 0.64-0.71)) and the shock index (AUC 0.67 (95% CI 0.63-0.70)) were the most accurate. The MEWS was significantly more accurate for detecting ward cardiac arrest in non-elderly patients compared to elderly patients (AUC 0.85 (95% CI 0.82-0.88) vs. 0.71 (0.68-0.75); p<0.001). A sensitivity analysis performed by changing the definition of elderly to age 75 or greater resulted in similar findings (AUC 0.81 (95% CI 0.78-0.83) vs. 0.71 (95% CI 0.66-0.75); p<0.001). Separating the patients into deciles by age demonstrated an increasing incidence of cardiac arrest and a decreasing accuracy of the MEWS with increasing age (Figure 2).
Table 3.
Differences in vital signs within four hours of cardiac arrest between elderly and non-elderly patients
| Elderly patients (n=273) | Non-elderly patients (n=149) | P-value | |
|---|---|---|---|
| Temperature, °C | 36.4 (0.7) | 36.3 (0.9) | 0.047 |
| Respiratory rate, breaths/min | 22 (5) | 23 (7) | 0.050 |
| Heart rate, beats/min | 88 (23) | 99 (25) | <0.001 |
| Systolic blood pressure, mmHg* | 112 (30) | 113 (28) | 0.08 |
| Diastolic blood pressure, mmHg* | 60 (16) | 66 (17) | 0.007 |
| Pulse pressure index | 0.45 (0.1) | 0.41 (0.12) | <0.001 |
| Shock index | 0.82 (0.31) | 0.93 (0.32) | <0.001 |
| Oxygen saturation, median (IQR), % | 97 (94-99) | 96 (93-99) | 0.696 |
| MEWS, median (IQR) | 2 (1-3) | 3 (2-5) | <0.001 |
Blood pressure was non-invasively measured arterial blood pressure
Pulse pressure index = (systolic blood pressure – diastolic blood pressure)/(systolic blood pressure); Shock index = systolic blood pressure/heart rate
Abbreviations: MEWS = Modified Early Warning Score
Figure 1.
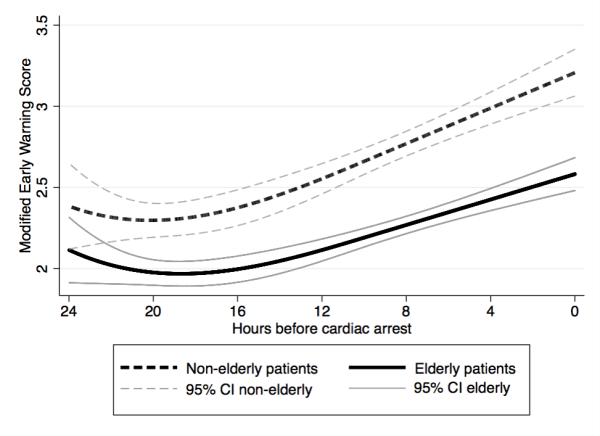
Trajectory of the Modified Early Warning Score in the twenty-four hours prior to cardiac arrest for elderly and non-elderly patients.
Table 4.
Comparisons of areas under the receiver operating characteristic curves between elderly and non-elderly patients for whether a cardiac arrest occurred using the highest and lowest values for individual vital signs and the Modified Early Warning Score.*
| Highest value | Lowest value | |||
|---|---|---|---|---|
| Elderly | Non-elderly | Elderly | Non-elderly | |
| Temperature | - | 0.53 (0.47-0.58) | 0.56 (0.52-0.60) | 0.65 (0.60-0.70) |
| Respiratory rate | 0.67 (0.64-0.71) | 0.82 (0.79-0.86) | 0.57 (0.53-0.61) | 0.54 (0.49-0.59) |
| Heart rate | 0.63 (0.60-0.67) | 0.77 (0.73-0.81) | - | - |
| Systolic blood pressure¶ | - | 0.57 (0.52-0.62) | 0.65 (0.61-0.69) | 0.67 (0.62-0.73) |
| Diastolic blood pressure¶ | - | 0.59 (0.54-0.64) | 0.60 (0.56-0.63) | 0.65 (0.60-0.70) |
| Pulse pressure index | 0.48 (0.44-0.52) | 0.60 (0.54-0.66) | 0.57 (0.54-0.61) | 0.68 (0.63-0.73) |
| Shock index | 0.67 (0.63-0.70) | 0.76 (0.72-0.81) | - | - |
| Oxygen saturation | - | - | 0.55 (0.51-0.59) | 0.69 (0.64-0.74) |
| MEWS | 0.71 (0.68-0.75) | 0.85 (0.82-0.88) | - | - |
Data presented as area under the receiver operating characteristic curve (95% CI).
- Denotes that the variable's area under the receiver operating characteristic curve was statistically worse than 0.50 and thus not predictive in the noted direction.
Blood pressure was non-invasively measured arterial blood pressure Pulse pressure index = (systolic blood pressure – diastolic blood pressure)/(systolic blood pressure); Shock index = systolic blood pressure/heart rate
Abbreviations: MEWS = Modified Early Warning Score
Figure 2.
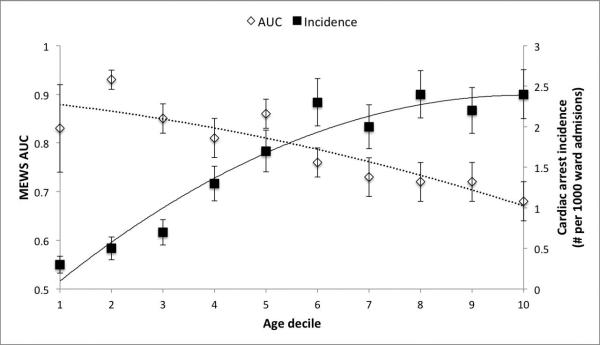
Area under the receiver operating characteristic curve of the Modified Early Warning Score and the incidence of cardiac arrest by deciles of increasing age.* *Lines represent best-fit curves for the trend in AUCs (dotted) and cardiac arrest incidence (solid) across age deciles.
Discussion
In this multicenter observational study, we found dramatic differences in the accuracy of vital signs and the MEWS between elderly and non-elderly patients. Importantly, while the incidence of cardiac arrest increases with age the accuracy of the MEWS decreases. Consistent with this finding was the fact that almost all vital signs more accurately detected cardiac arrest in the non-elderly patients compared to elderly patients. In addition, there were differences in which vital signs were most accurate between these groups. These findings have important implications for the risk stratification of hospitalized patients and suggest that those at highest risk of cardiac arrest are the most challenging to detect using routine physiologic measurements. Our work also suggests that, similar to pediatric patients,(13-17) the age of the patient should be considered when interpreting vital sign derangements in order to make appropriate triage decisions.
These results add to the literature regarding the association between age, vital signs, and adverse outcomes for adult patients on the wards. For example, Smith and colleagues investigated the relationship between age, vital signs, early warning scores, and in-hospital mortality in a cohort of 9,987 patient admissions.(18) They found that inhospital mortality was higher for older patients at a given vital sign and MEWS value. In addition, Bleyer et al. also showed that in-hospital mortality increased with age for a given number of vital sign derangements.(19) Several studies, including those by Subbe et al., Duckitt et al., and our group, have shown that adding age to early warning scores can increase their accuracy for detecting adverse outcomes.(4, 20) This is consistent with the inclusion of age in other well-validated risk scores such as the APACHE algorithms.(6) Finally, developers of pediatric early warning scores have recognized the need for age-specific scoring systems given the fact that normal values change with the age of the patient.(14-17)
Vital signs are routinely and intermittently collected on ward patients and are often used to make ICU triage decisions and to trigger RRT calls. Age-related changes in vital signs are known to occur, particularly decreases in maximum heart rate and the arterial partial pressure of oxygen.(8, 9, 21) In addition, the decrease in vascular compliance and capacitance associated with ageing would be expected to increase systolic blood pressure and decrease diastolic blood pressure for any given stroke volume, and hence have an impact on the pulse pressure index dependent upon the relative changes in systolic and diastolic pressures.(8) In addition, medications taken for medical conditions, such as beta-blockers, can blunt the physiologic response to stress and are more commonly used by older patients.(22) Another contributing factor to our findings may be that elderly patients have a more limited physiologic reserve compared to non-elderly patients and thus cannot tolerate the same level of derangement in vital signs. For example, cardiac output, vital capacity, creatinine clearance, and the ability to maintain glucose homeostasis are all known to decrease with age.(9) Finally, elderly patients were more likely to have a cardiac arrest with asystole as the presenting rhythm in our dataset than non-elderly patients (23% vs. 14%). These events are likely less predictable, on average, than cardiac arrests due other causes, such as the progression of sepsis or respiratory failure. Regardless of the net effects of these wide ranging physiologic explanations, it would not be surprising if different predictive tools based on vital signs would need to be age-adjusted.
Our findings have important implications for how vital signs are used for detecting high-risk ward patients and suggest that a “one-size fits all” approach to early warning scores may not be optimal. This is consistent with findings from the pediatric literature, where the different normal ranges for vital signs are known to vary by age.(13) First, the differences in relative accuracy of vital signs within each patient group suggests that having separate early warning scores for elderly and non-elderly patients would be more accurate than a unified risk score for all patients. Second, the fact that the prevalence of cardiac arrest increases with age while vital sign accuracy decreases suggests that the addition of age to risk scores might help mitigate this disparity. In fact, we have previously shown that age is an independent risk factor for ward cardiac arrest in two single-center studies,(23, 24) similar to other groups.(18-20) Finally, the poor accuracy of the MEWS for elderly patients demonstrates the need to find other predictors of cardiac arrest that could supplement early warning scores for these patients. For example, co-morbidities, use of medications, such as beta-blockers, and admitting diagnosis are all potential variables that could improve the accuracy of these systems.
Our study has several strengths compared to previous investigations. First, our patient population comes from a diverse set of hospitals, including an urban tertiary care center, two suburban teaching hospitals, and two community non-teaching hospitals. Second, we focused on ward cardiac arrest patients. In our hospital system, these are the patients who would benefit most from an accurate early warning score given the fact that they remained on the wards instead of being transferred to the ICU. Patients who die on the wards would include both these patients and patients who were comfort care and who would not have benefitted from early detection for life-saving interventions, thereby making mortality a less favorable outcome to investigate. Finally, we not only demonstrated that the risk of arrest increases with age but also investigated the accuracy of vital signs and the MEWS within each age group. This allowed us to highlight the poor accuracy of vital signs for the patients with the highest risk of the event.
Our study also has several limitations. First, we did not have access to patient medication use and co-morbidities, which may impact both physiology and a patient's risk of cardiac arrest. However, the vast majority of early warning scores in use today do not account for these variables so our study provides insight into how vital signs and the MEWS perform in “real world” use. Second, the cut-off of age 65 is a somewhat arbitrary one for investigating physiological changes. However, this distinction is commonly used in the medical literature, and it has particular relevance in the United States because patients who acquire Medicare coverage are more likely to have access to medications for chronic diseases. In addition, we showed that the accuracy of the MEWS decreased overall across deciles of age and that our findings did not change when altering the definition of elderly to only include patients at least 75 years of age. Third, our study involved five hospitals in Illinois and the results may not be generalizable to other settings or countries. Finally, the exact values of the AUC for different vital signs and the MEWS for cardiac arrest will likely vary across hospitals and countries with varying resources and ICU bed availability. However, our findings have greater generalizability than previous studies given the diverse nature of our multicenter dataset.
Conclusions
In conclusion, there are significant differences in vital signs and their accuracy prior to cardiac arrest on the wards, which has important implications for how they are used for ICU triage decisions and triggering RRTs. Importantly, the poor accuracy for the MEWS in elderly patients suggests that additional predictors of cardiac arrest, such as comorbidities, are needed to accurately identify these patients. In addition, the fact that the most accurate vital signs were different for elderly compared to non-elderly patients suggests that age-specific early warning scores may improve accuracy over current systems.
Acknowledgments
We would like to thank Donald Saner, MS, Justin Lakeman, and Contessa Hsu for assistance with data extraction and technical support, Poome Chamnankit, MS, CNP, Kelly Bhatia, MSN, ACNP, and Audrey Seitman, MSN, ACNP for performing manual chart review of cardiac arrest patients, and Nicole Twu, MS for administrative support.
Financial Disclosures
This research was funded in part by an institutional Clinical and Translational Science Award grant (UL1 RR024999; PI: Dr. Julian Solway). Drs. Churpek and Edelson are both supported by career development awards from the National Heart, Lung, and Blood Institute (K08 HL121080 and K23 HL097157, respectively). Drs. Churpek and Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. In addition, Dr. Edelson has received research support and honoraria from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and an honorarium from Early Sense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, IL), which is developing products for risk stratification of hospitalized patients.
Footnotes
Preliminary versions of these data were presented at the 2012 meeting of the American Thoracic Society (May 20, 2012; San Francisco, CA).
Copyright form disclosures: Dr. Churpek provided expert testimony for Rothenburg law firm, disclosed a patent pending (ARCD. P0535US.P2 - for risk stratification algorithms for hospitalized patients), and received support for article research from the National Institutes of Health (NIH). His institution received grant support from the NIH- NHLBI (Career development award from the National Heart, Lung, and Blood Institute (K08 HL121080)). Dr. Winslow's institution received grant support from the University of Chicago Pilot and Collaborative Translations and Clinical Studies Award. Dr. Hall provided expert testimony for lawfirms for patients, hospitals, and health providers; received royalties from McGraw Hill and ACCP (textbooks); and has stock options with Vanguard (Health industry mutual funds). Dr. Edelson disclosed a patent pending (ARCD. P0535US.P2 - for risk stratification algorithms for hospitalized patients); ahs ownership interested in QuantHC (Chicago, IL - which is developing products for risk stratification of hospitalized patients); and received support for article research from the NIH. His institution consulted for Early Sense and received grant support from the National Heart, Lung, and Blood Institute (K23 HL097157); Philips Healthcare (Andover, MA),; the American Heart Association (Dallas, TX); and Laerdal Medical (Stavanger, Norway). Dr. Trevor disclosed that he does not have any potential conflicts of interest.
References
- 1.Merchant RM, Yang L, Becker LB, Berg RA, Nadkarni V, Nichol G, et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39(11):2401–6. doi: 10.1097/CCM.0b013e3182257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith AF, Wood J. Can some in-hospital cardio-respiratory arrests be prevented? A prospective survey. Resuscitation. 1998;37(3):133–7. doi: 10.1016/s0300-9572(98)00056-2. [DOI] [PubMed] [Google Scholar]
- 3.Hodgetts TJ, Kenward G, Vlackonikolis I, Payne S, Castle N, Crouch R, et al. Incidence, location and reasons for avoidable in-hospital cardiac arrest in a district general hospital. Resuscitation. 2002;54(2):115–23. doi: 10.1016/s0300-9572(02)00098-9. [DOI] [PubMed] [Google Scholar]
- 4.Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521–6. doi: 10.1093/qjmed/94.10.521. [DOI] [PubMed] [Google Scholar]
- 5.Chan PS, Khalid A, Longmore LS, Berg RA, Kosiborod M, Spertus JA. Hospital-wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300(21):2506–13. doi: 10.1001/jama.2008.715. [DOI] [PubMed] [Google Scholar]
- 6.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–36. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 7.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 8.Chester JG, Rudolph JL. Vital signs in older patients: age-related changes. J Am Med Dir Assoc. 2011;12(5):337–43. doi: 10.1016/j.jamda.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;135(6):434–40. [PMC free article] [PubMed] [Google Scholar]
- 10.Churpek MM, Yuen TC, Huber MT, Park SY, Hall JB, Edelson DP. Predicting cardiac arrest on the wards: a nested case-control study. Chest. 2012;141(5):1170–6. doi: 10.1378/chest.11-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedeker DR, Gibbons RD. Longitudinal data analysis. J. Wiley; Hoboken, N.J.: 2006. [Google Scholar]
- 12.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 13.Fleming S, Thompson M, Stevens R, Heneghan C, Pluddemann A, Maconochie I, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377(9770):1011–8. doi: 10.1016/S0140-6736(10)62226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan H, Hutchison J, Parshuram CS. The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006;21(3):271–8. doi: 10.1016/j.jcrc.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Edwards ED, Powell CV, Mason BW, Oliver A. Prospective cohort study to test the predictability of the Cardiff and Vale paediatric early warning system. Arch Dis Child. 2009;94(8):602–6. doi: 10.1136/adc.2008.142026. [DOI] [PubMed] [Google Scholar]
- 16.Haines C, Perrott M, Weir P. Promoting care for acutely ill children-development and evaluation of a paediatric early warning tool. Intensive Crit Care Nurs. 2006;22(2):73–81. doi: 10.1016/j.iccn.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Tibballs J, Kinney S, Duke T, Oakley E, Hennessy M. Reduction of paediatric in-patient cardiac arrest and death with a medical emergency team: preliminary results. Arch Dis Child. 2005;90(11):1148–52. doi: 10.1136/adc.2004.069401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith GB, Prytherch DR, Schmidt PE, Featherstone PI, Kellett J, Deane B, et al. Should age be included as a component of track and trigger systems used to identify sick adult patients? Resuscitation. 2008;78(2):109–15. doi: 10.1016/j.resuscitation.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Bleyer AJ, Vidya S, Russell GB, Jones CM, Sujata L, Daeihagh P, et al. Longitudinal analysis of one million vital signs in patients in an academic medical center. Resuscitation. 2011;82(11):1387–92. doi: 10.1016/j.resuscitation.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 20.Duckitt RW, Buxton-Thomas R, Walker J, Cheek E, Bewick V, Venn R, et al. Worthing physiological scoring system: derivation and validation of a physiological early-warning system for medical admissions. An observational, population-based single-centre study. Br J Anaesth. 2007;98(6):769–74. doi: 10.1093/bja/aem097. [DOI] [PubMed] [Google Scholar]
- 21.Nes BM, Janszky I, Wisloff U, Stoylen A, Karlsen T. Age-predicted maximal heart rate in healthy subjects: The HUNT Fitness Study. Scand J Med Sci Sports. 2013;23(6):697–704. doi: 10.1111/j.1600-0838.2012.01445.x. [DOI] [PubMed] [Google Scholar]
- 22.Brawner CA, Ehrman JK, Schairer JR, Cao JJ, Keteyian SJ. Predicting maximum heart rate among patients with coronary heart disease receiving beta-adrenergic blockade therapy. Am Heart J. 2004;148(5):910–4. doi: 10.1016/j.ahj.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 23.Churpek MM, Yuen TC, Park SY, Meltzer DO, Hall JB, Edelson DP. Derivation of a cardiac arrest prediction model using ward vital signs*. Crit Care Med. 2012;40(7):2102–8. doi: 10.1097/CCM.0b013e318250aa5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Churpek MM, Yuen TC, Park SY, Gibbons R, Edelson DP. Using electronic health record data to develop and validate a prediction model for adverse outcomes in the wards*. Crit Care Med. 2014;42(4):841–8. doi: 10.1097/CCM.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]


