Abstract
Many species, including humans, engage in a series of behaviors that are preparatory to the arrival of offspring. Such "nesting behaviors" are of obvious importance, but relevant neuroendocrine mechanisms remain little studied. We here focus on the potential roles of vasoactive intestinal polypeptide (VIP) in the performance of appetitive and consummatory nesting behaviors in male and female zebra finches (Taeniopygia guttata). Using combined immunocytochemistry for Fos and in situ hybridization for VIP, we now show that many VIP cell groups show increased transcriptional activity in response to nest building in male and female zebra finches. Particularly strong data come from the preoptic area (medial preoptic area and medial preoptic nucleus), where VIP-Fos co-expression correlates positively with three different measures of nesting behavior, as does the number of VIP-expressing cells. Remarkably, we find that VIP mRNA and/or VIP-Fos co-expression is correlated with nesting behavior in virtually every brain area that we examined, including the medial amygdala (anterior and posterior), medial bed nucleus of the stria terminalis, medial preoptic area, medial preoptic nucleus, anterior hypothalamus, ventromedial hypothalamus, periaqueductal gray complex (central gray and nucleus intercollicularis), and ventral tegmental area. Near-significant effects are also obtained in the tuberoinfundibular hypothalamus. Although most correlations are positive, negative correlations are observed for the VIP cell group of the anterior hypothalamus, a population that selectively promotes aggression, and also the periaqueductal gray complex. These data demonstrate a network-wide relationship between peptide production and social behavior that is, to our knowledge, unparalleled by other peptidergic modulators.
Introduction
Nesting behavior takes a variety of forms across vertebrates -- such as the location of a den, provisioning of a human nursery, simple excavation of a depression, or highly complex behaviors such as the construction of large communal nests, as in some weavers. Importantly, unlike all other aspects of parental care, these nesting behaviors are exhibited prior to, and in the absence of, the stimuli associated with parturition, egg laying or offspring. Hence, the neuroendocrine mechanisms that underlie nesting behavior may be quite different from the mechanisms of actual offspring care. However, whereas the neural mechanisms of offspring care have been extensively investigated, comparatively little is known about the neural mechanisms of nesting. We here focus on the potential role of vasoactive intestinal polypeptide (VIP) in the performance of nesting behaviors in male and female zebra finches. VIP and its cognate VPAC receptors are essential for prolactin (PRL) secretion (Macnamee et al., 1986; Lam, 1991; El Halawani et al., 1996; Chaiseha et al., 1998; Maney et al., 1999; Vleck and Patrick, 1999; Christensen and Vleck, 2008; Chaiyachet et al., 2013) and are present in virtually every brain area that is known to be important for social behavior, including all nodes of the core "social behavior network (social behavior network: Newman, 1999; Goodson and Kingsbury, 2013; VIP/VPAC: Sims et al., 1980; Roberts et al., 1982; Antonopoulos et al., 1987; Kuenzel and Blahser, 1994; Aste et al., 1995; Kuenzel et al., 1997; Chaiseha et al., 1998; Joo et al., 2004; Goodson et al., 2006; Chaiyachet et al., 2013). These features suggest that VIP may be an important modulator of nesting behavior, yet the social functions of VIP circuits remain little known.
PRL regulates many aspects of reproductive behavior and parental care in a variety of species, including nesting building in rabbits (Gonzalez-Mariscal et al., 1996; Gonzalez-Mariscal, 2001), crop-sac development and parental feeding in ring doves (Buntin et al., 1991; Lea et al., 1991; Buntin et al., 1999), lactation in mammals (Grattan and Bridges, 2009), parental egg fanning in fish (de Ruiter et al., 1986), incubation behavior in bantam hens (Sharp et al., 1989) and turkeys (El Halawani et al., 1996), and chick rearing in native Thai hens (Chaiyachet et al., 2013). It is well established that VIP is a major PRL-releasing hormone for a variety of mammals (Lam, 1991; Watanobe and Sasaki, 1995; Christian et al., 2007) and birds (El Halawani, 1997), such as domestic fowl (Lea and Vowles, 1986; Macnamee et al., 1986; Kulick et al., 2005) and songbirds (Maney et al., 1999; Vleck and Patrick, 1999), including the opportunisticly breeding zebra finches studied here (Vleck and Patrick, 1999; Christensen and Vleck, 2008). In addition, changes in VIP portal blood levels closely mirror changes in plasma PRL levels across the reproductive cycle, as do VIP expression in the tuberoinfundibular hypothalamus (Inf) and VIP immunoreactivity in both the Inf and median eminence, (Cloues et al., 1990; Youngren et al., 1996; Chaiseha et al., 1998; Chaiyachet et al., 2013), suggesting a role for VIP in the control of PRL-mediated breeding behaviors. Finally, immunization against VIP blocks VIP-induced increases in plasma PRL levels and reduces nest visits/incubation behavior in turkeys (El Halawani et al., 1996) and increases nest desertion in incubating bantam hens (Sharp et al., 1989). Interestingly, dopamine regulates the VIP-mediated release of PRL in turkeys via actions on its receptors (Youngren et al., 1998; Al Kahtane et al., 2003; Bhatt et al., 2003) and changes in hypothalamic VIP during incubation and nest deprivation in native Thai hens are associated with changes in tyrosine hydroxylase-immunolabeling (Prakobsaeng et al., 2011), providing evidence for a role for DA in the regulation of VIP/PRL secretion and the associated reproductive behaviors.
Beyond PRL secretion, an accumulating body of evidence demonstrates that VIP is involved in the modulation of a variety of social behaviors, consistent with the widespread distribution of VIP elements across brain areas that are essential for the expression of parental, aggressive, sexual and affiliative behaviors. To date, VIP has been shown to be important for aggressive and agonistic communication in emberizid sparrows and estrildid finches (Goodson, 1998a, b; Goodson et al., 2012a); social contact, gregariousness, and pair bonding in zebra finches (Kingsbury et al., 2013; Kingsbury and Goodson, 2014); and social recognition and sociability in mice (Hill et al., 2007a; Hill et al., 2007b). Indirect evidence suggests that VIP is also important for seasonal flocking in sparrows (Goodson et al., 2012b). Notably, the effects of VIP signaling on behavior are often site specific and in some instances, sex-specific.
To specifically explore VIP’s role in preparatory nesting, we here combined Fos immunocytochemistry (ICC) and VIP in situ hybridization (ISH) in order to examine Fos induction in VIP cells across the "social behavior network" (Newman, 1999; Goodson and Kingsbury, 2013) in response to nesting. ISH was chosen for the detection of VIP because some VIP populations are not detectable with ICC due to rapid peptide release (Goodson et al., 2012a). Consistent with VIP’s widespread distribution and its importance for a variety of social behaviors, we find that VIP mRNA and/or VIP-Fos co-expression is correlated with nesting behavior in virtually every socially relevant brain area that we examined.
Methods
Subjects and behavioral testing
Zebra finches were housed in male-female pairs in cages measuring 91 cm W×43 cm H×36 cm D. Subjects were kept on a 14L:10D photoperiod with full spectrum lighting and were provided finch seed mix, cuttlebone, grit, and water ad libitum. Experimentation was conducted in a humane manner and approved by the Institutional Animal Care and Use Committee of Indiana University.
Behavioral observations were conducted as previously described (Klatt and Goodson, 2013). Birds were prescreened for nesting behavior over a period of 4 days, during which unlimited access to nesting material (shredded burlap) and a nest cup was provided. A millet stalk was also placed in the cage, given that some subjects would be provided with a millet stalk as a control stimulus in the Fos experiment. At the end of prescreening, all nesting material and nests were removed from the cages. Only pairs that built complete nests were retained (n = 21 pairs). Nests and nesting material were removed for a period of 4–5 days prior to the Fos experiment in an effort to promote nesting behavior.
On the morning of testing, subjects received a nest cup and nesting material (n = 7 pairs), a nest cup and a stalk of millet (n = 7 pairs) or only a nest cup (n = 7 pairs). The millet was provided to control for Fos induction related to general activity and oral manipulation. However, no differences were observed between the data collected for the millet group and the control group that received no stimulus, and thus these groups were combined into a single control group (n = 14 pairs) for analyses. Subjects were video recorded for 30 min and we quantified the amount of time spent in the nest cup, the number of nest items picked up ("pick-ups"), and the number of nest items carried to the nest ("deliveries"). Subjects were sacrificed 90 min after the initiation of testing. Because all pairs were provided with a nest cup, any between-groups differences in Fos induction must reflect group differences in actual nesting (i.e, consummatory behavior), rather than appetitive attraction to the nest cup. This design allows us to correlate time in the nest cup (possibly representing appetitive nesting behavior) with VIP and Fos labeling across the entire subject pool.
Cloning of finch VIP
Total RNA was isolated from brain using RNApure (GenHunter, Nashville, TN). For standard and 3’ RACE PCR reactions, cDNA was synthesized using Superscript II (Invitrogen; 3’RACE System for Rapid Amplification of cDNA Ends). Conserved regions of VIP mRNA sequence were identified by ClustalW comparison of VIP mRNA sequences from chicken (Genebank NM205366) and sparrow (AB292731). Conserved regions were used to design PCR primers for finch VIP. For 3’ RACE reactions, 500 nM gene-specific upstream primers (ZFvip410F 5’TAGAAATGCAAGGCATGCTG3’ and ZFvip510F 5’CGAGTTAGCTCCCAGGACAG3’) were included in PCR reactions containing 200 nM 3’ RACE downstream primers (either the Universal or Abridged Universal Amplification Primers, Invitrogen, Carlsbad, CA); 0.5µl cDNA and 1X concentration REDTaq Ready Mix PCR Mix (Sigma, St. Louis, MO) in a 25 µl reaction. Reactions were cycled 35X using a touchdown PCR program [30 sec denaturation 95°C step, 1 min annealing step (65°C during cycle 1, and a 0.5°C decrease for each subsequent cycle until a 60°C annealing temperature, 60°C annealing temperature for all subsequent cycles)] and a 3 min 72°C extension step. Products were cloned using the TOPO TA Cloning Kit (Invitrogen). To select for plasmids with cloned PCR inserts, bacteria were grown overnight on LB plates containing 50 µg/ml kanamycin) and 80 µg/ml 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside (X-gal). Plasmids were purified using QIAprep Spin Miniprep Kit (QIAGEN, Valencia, CA). Sequencing reactions were performed by the Mount Desert Island Biological Laboratory (Salisbury Cove, ME). Sequence traces were analyzed using Finch TV 1.4 chromatogram viewer (Geospiza, www.geospiza.com/finchtv). The NCBI BLAST database and ORF Finder (NCBI) were used for sequence analysis and sequence translation. A 558 bp fragment was found that shared 98% identity with sparrow VIP and 92% identity with chicken VIP. The fragment contained the 3’ region of the transcript, including 81 amino acids of the coding region, the stop codon, the 3’ untranslated region and 2 polyadenylation sites.
Riboprobe preparation
Plasmid DNA containing the 558 bp fragment was grown from bacterial stocks, isolated with Wizard Plus SV Minipreps DNA Purification System (Promega, Madison, WI), precipitated with 3M sodium acetate (pH 5.2) and 100% EtOH, and linearized with either FastDigest NotI restriction enzyme (Thermo Scientific, Waltham, MA) or HindIII (Roche Diagnostics, Indianapolis, IN) to generate antisense and sense riboprobes. Linearized plasmid DNA was purified with UltraClean PCR Clean-Up DNA purification Kit (MO BIO Laboratories, Carlsbad, CA) or Agencourt AMPure XP beads (Beckman Coulter, Pasadena, CA) and a magnetic separation rack (New England Biolabs, Ipswich, MA). Transcription was performed using 10X Digoxigenin (DIG) RNA dNTP labeling mix (Roche Diagnostics), RNAse inhibitor (Roche Diagnostics), SP6 or T7 RNA polymerases (New England Biolabs) and 10X transcription buffer (New England Biolabs). Following transcription, RNA was precipitated with 4M LiCl and 2.5 volumes of 100% EtOH and then washed in 70% EtOH to remove unincorporated DIG dNTPs. Ribroprobe concentration was assessed on a Dot Blot using serial dilutions of probe and control RNA (100 ng/µl, Roche Diagnostics). Visualization of probe and control RNA was performed using anti-DIG-AP antibody (1:1000, Roche Diagnostics), followed by a detection reaction with 4-Nitro blue tetrazoium chloride and 5-Bromo-4-chloro-3-indolyl-phosphate (Roche Diagnostics).
Histology and ICC-ISH
Subjects were sacrificed 90 min after the initiation of testing and brains were processed as previously described (Klatt and Goodson, 2013). One of the three 40 µm tissue series was used here for ICC-ISH. Sections through areas of interest were first immunofluorescently labeled for Fos using standard lab protocols (Goodson et al., 2012a) followed by fluorescent ISH for VIP. Tissue was immunolabeled using the primary antibody, rabbit anti-Fos (5:1000; Santa Cruz Biotechnology, Santa Cruz, CA) and a donkey anti-rabbit secondary conjugated to Alexa Fluor 594 (5:1000, Invitrogen). Following two 15 min rinses in PBS, sections were then processed for ISH under RNAse-free conditions as described previously (Goodson et al., 2012a). Tissue was rinsed twice for 5 min in PBS + 0.1% Triton (PBST, pH 7.4), followed by a rinse in 0.1M Triethanolamine-HCL (TEA, pH 8.0) for 5 min. Sections underwent acetylation in TEA + 0.25% acetic anhydride for 10 min with agitation, followed by two additional 5 min rinses in PBST. Sections were then placed in hybridization buffer consisting of 50% deionized formamide, 10% dextran sulfate, 4X sodium saline citrate (SSC), 1X Denhardt’s solution and1µl transfer RNA (10.5 mg/ml) per 40 ml, to which 50 µl salmon sperm DNA/ml hybe buffer was added for prehybridization at 37°C for 30 min. Riboporobe was diluted to 750 ng/ml hybe buffer, denatured at 75°C for 5 min and immediately placed on ice. Sections were hybridized with probe solution (500 µl probe solution per well containing ~6 sections) at 52°C for 48 h in a humidified chamber protected from light. Following hybridization, tissue sections underwent two 10 min rinses in 2X SSC, two 10 min rinses in 1X SSC, and two 10 min rinses in 0.1X SSC, all at 37°C with agitation. Tissue was then rinsed in TN buffer (100 mM Tris HCl, 150 mM NaCl, pH 7.5) for 10 min at RT and blocked for 30 min in TNB buffer consisting of TN buffer + 0.5% blocking reagent (Roche Diagnostics). Tissue was incubated with anti-DIG-POD, Fab fragments (Roche Diagnostics) diluted in TNB buffer (1:500) for 1 h at RT. Tissue was rinsed twice for 15 min each at RT in TNT buffer consisting of TN buffer + 0.05% Tween-20. Tissue was then processed for Tyramide Signal Amplification using the TSA Plus Biotin Kit (PerkinElmer, Waltham, MA). Tissue was incubated with Biotin amplification reagent diluted 1:200 in 1X Plus amplification diluent for 10 min with agitation. Tissue was then rinsed twice for 10 min each in TNT buffer and incubated for 2 h with streptavidin conjugated to Alexa Fluor 488 (Molecular Probes, Eugene, OR) diluted in TNB buffer (3:1000) for visualization of VIP mRNA. Sections were mounted on subbed slides and coverslipped with ProLong Gold antifade reagent containing DAPI nuclear stain (Invitrogen) and immediately photographed. The specificity of the VIP riboprobe was confirmed by in situ hybridization of adjacent sections with antisense and sense strands and is presented in Supplementary Figure 1.
Double ICC for Fos and VIP
We observed poor resolution of Fos- immunoreactive (-ir) neurons along the midline of the Inf in tissue processed for ICC-ISH. We therefore processed an additional series for both VIP and Fos ICC. Tissue was labeled for ICC as described above, with the addition of a primary goat anti-VIP antibody (1:1000; Santa Cruz Biotechnology) alongside the primary rabbit anti-Fos. To visualize VIP-ir cells, tissue was incubated with a donkey anti-goat secondary conjugated to Alexa Fluor 488 (3:1000, Invitrogen), in addition to the donkey anti-rabbit secondary conjugated to Alexa Fluor 594 for visualization of Fos-ir cells. The specificity of the primary goat anti-VIP antibody was demonstrated by the pre-absorption test and is presented in Supplementary Figure 2.
Quantification
Images were acquired as previously described (Klatt and Goodson, 2013) and cell counts were conducted according to standard lab protocols (Goodson and Wang, 2006; Goodson et al., 2009; Goodson et al., 2012b). Labeling was quantified in the medial amygdala (MeA; anterior, capping the solid band of the ventral amygdalofugal tract, and posterior, ventromedial to the fascicles of the ventral amygdalofugal tract; (see Goodson et al., 2012b); medial bed nucleus of the stria terminalis (BSTm; supracommissural); medial preoptic nucleus and medial preoptic area (MPO and MPA, respectively; both rostral, medial to the septomesencephalic tract); anterior hypothalamus (AH; delineated as in (Goodson et al., 2012a; Goodson and Kingsbury, 2013); ventromedial hypothalamus (VMH); tuberoinfundibular hypothalamus (Inf); periaqueductal gray (PAG) complex including the central gray, CG, and nucleus intercollicularis, ICo; see (Kingsbury et al., 2011) ventral tegmental area (VTA; rostral and caudal; see Goodson et al., 2009).
Statistical analyses
Group differences in VIP-Fos colocalization (i.e., the percent of VIP-expressing neurons that were Fos-ir-positive) were examined using Sex×Condition ANOVAs. No significant differences were observed between the two control conditions (nest cup plus millet versus nest cup alone) and thus those conditions were combined to form a single Control group. For the Nesting subjects only, linear regressions were conducted that each included one of the behavioral variables (time spent in the nest cup; pick-ups; and deliveries) and one of the three anatomical variables -- VIP-Fos colocalization, as just defined; the total number of VIP-expressing neurons; and the raw number of neurons double-labeled for VIP and Fos. Finally, because all subjects were given nest cups, regressions for time spent in the nest cup were performed with Nesting and Control subjects combined.
An obvious concern with this analytical approach is that the potential for type 1 error is high. However, calculating that probability is not straightforward, given that the multiple analyses for any given brain area are not independent. For instance, the number of VIP-expressing neurons is not independent of the raw number of double-labeled neurons, and the raw number of double-labeled neurons is not independent of VIP-Fos colocalization (defined as the percent of VIP neurons that co-express Fos). Thus, in our interpretations of the data, we consider findings for a given brain area to be significant only if 2 or more analyses yield a consistent pattern of results. Importantly, even if all analyses for each brain area were independent, only a single significant effect should be expected by chance. The probability that 2 significant effects would be obtained by chance is much lower, and the probability that those 2 (or more) effects would all fall in the same direction is lower yet. Notably, for several brain areas, numerous significant effects are obtained that all fall in the same direction, and we observe no effects for a given brain area that are contradictory (i.e., all correlations for a given brain area are either positive or negative). In fact, with the exception of the AH cell group and cell groups of the PAG complex, all other cell groups exhibit positive relationships to nesting. As addressed in the Discussion, the negative relationship between the AH cell group and nesting behavior is expected a priori based on previous experimental evidence and on recent Fos data on nesting behavior, as are the negative relationships for the PAG complex (i.e. medial ICo and CG), based on circuit models of maternal care (Numan, 2007).
Results
Overview of results
Representative VIP-Fos labeling is shown in Fig. 1. Multiple and consistent significant effects are observed for most of the brain regions examined, including the MeA, BSTm, MPA, MPO, AH, VMH, CG, and ICo. Strong trends were also observed for the Inf. Single effects are observed for the VTA and lateral ICo (putative dorsomedial PAG), but for reasons addressed above ("Statistical Analyses"), we regard single effects of p < 0.05 as insufficient evidence for a functional relationship to behavior. Virtually all significant effects are obtained from regressions with behavior, and thus unless explicitly addressed below, all ANOVA models are not significant (p > 0.10). This general lack of group differences is not unexpected, given that all subjects had nest cups and could therefore exhibit appetitive behavior towards the cup, even if they were not offered nesting material. Indeed, as described below, we observe numerous significant effects for the time spent in the nest cup with all subjects pooled, suggesting extensive relationships between VIP cell groups and appetitive aspects of nesting behavior. Finally, most significant regressions for nesting behaviors reveal positive relationships, with the exceptions of models for the AH, a brain region where VIP production strongly promotes aggression, and the avian PAG (CG-ICo complex). Results of significant regressions for nesting behavior and the number of VIP-expressing cells, the number of VIP-expressing cells co-labeled with Fos and VIP-Fos colocalization are presented in Tables 1, 2 and 3, respectively.
Fig. 1.
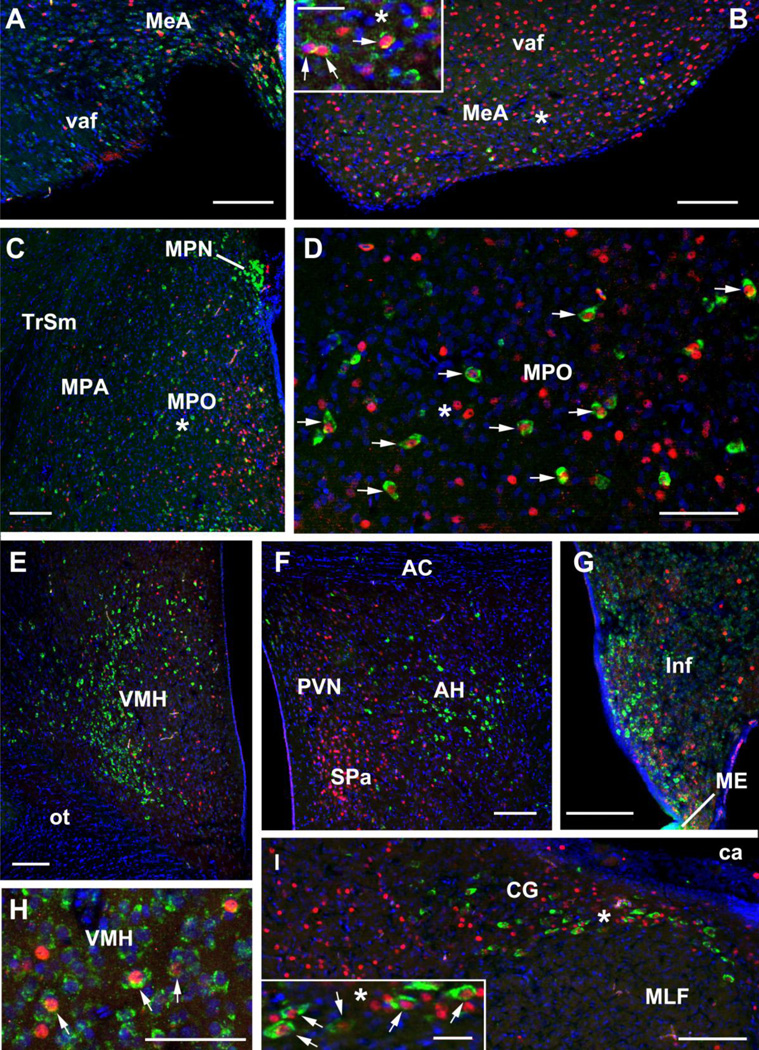
Representative labeling for VIP mRNA (green) and Fos immunoreactivity (red) in cross-sections from a nesting male (A, H, I) and nesting female (B-G) zebra finch. DAPI nuclear stain is shown in blue. (A) Labeling in the anterior portion of the MeA, where the MeA caps the ventral amygdalofugal pathway (vaf; also known as the occipitomesencephalic tract). (B) Labeling in the mid-caudal portion of the MeA, where the MeA is situated ventromedial to fascicles of the vaf. Asterisks denote matching locations in the main panel and inset. As shown in the inset, many VIP cells in the MeA express low levels of mRNA, although numerous cells are strongly labeled, as well. Arrows highlight VIP-Fos double-labeled neurons. (C-D) Labeling in the MPO and MPA. The asterisk in panel C corresponds to the location of the asterisk in panel D. (EG) Representative labeling in the VMH (E), AH (F) and Inf (G). (H) VIP-Fos double-labeled neurons in the VMH, as indicated by arrows. (I) Labeling in the CG, putative homologue of the mammalian ventrolateral PAG. Asterisks denote matching locations in the main panel and inset. All scale bars = 100 µm except insets, which = 25 µm.
Additional abbreviations: AC, anterior commissure; ca, cerebral aqueduct; MPO, medial preoptic area; ot, optic tract; ME, median eminence; MLF, medial longitudinal fasciculus; MPN, median preoptic nucleus; PVN, paraventricular nucleus; SPa, subparaventricular hypothalamus; TrSM, septomesecephalic tract; VMH, ventromedial hypothalamus.
Table 1.
Results of simple regressions between the number of VIP-labeled neurons and nesting behaviors (time in nest, pick-ups and deliveries).
| Area | Subjects | Behavior | r 2 | P |
|---|---|---|---|---|
| Extended amygdala: | ||||
| MeA, anterior | all nesting | pick-ups | 0.43 | 0.01 |
| all nesting | deliveries | 0.42 | 0.02 | |
| nesting males | pick-ups | 0.81 | <0.01 | |
| nesting males | deliveries | 0.79 | <0.01 | |
| MeA, posterior | all nesting | pick-ups | 0.41 | 0.01 |
| all nesting | deliveries | 0.42 | 0.01 | |
| nesting males | pick-ups | 0.75 | 0.01 | |
| nesting males | deliveries | 0.73 | 0.01 | |
| all subjects | time in nest | 0.14 | 0.01 | |
| nesting females | time in nest | 0.76 | 0.01 | |
| BSTm | all males | time in nest | 0.35 | 0.03 |
| POA-hypothalamus: | ||||
| MPA (medial preoptic area) | nesting males | pick-ups | 0.65 | 0.05 |
| nesting males | deliveries | 0.66 | 0.05 | |
| all nesting | time in nest | 0.38 | 0.04 | |
| MPO (medial preoptic nucleus) | all nesting | time in nest | 0.48 | 0.02 |
| nesting females | time in nest | 0.94 | <0.01 | |
| Midbrain: | ||||
| VTA, caudal | all females | time in nest | 0.19 | 0.05 |
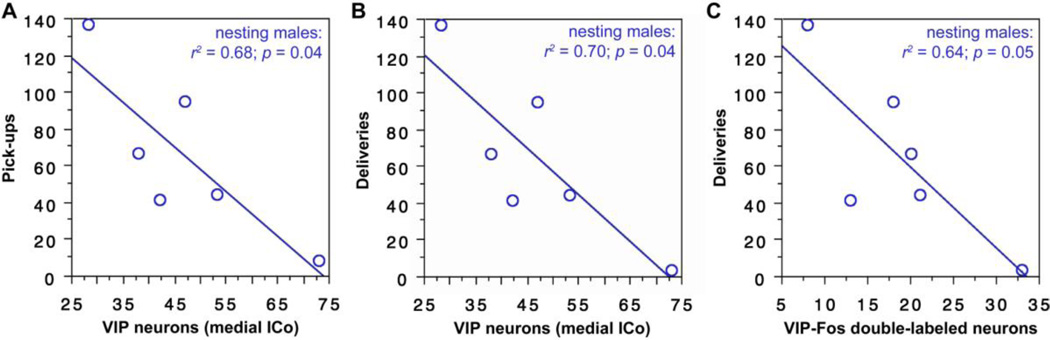
| ICo, medial | nesting males | pick-ups | 0.68 | 0.04 |
| nesting males | deliveries | 0.70 | 0.04 |
Table 2.
Results of simple regressions between the number of VIP-Fos co-labeled neurons and nesting behaviors (time in nest, pick-ups and deliveries).
| Area | Subjects | Behavior | r 2 | P |
|---|---|---|---|---|
| Extended amygdala: | ||||
| MeA, anterior | all nesting | pick-ups | 0.33 | 0.03 |
| all nesting | deliveries | 0.32 | 0.03 | |
| MeA, posterior | all nesting | pick-ups | 0.46 | <0.01 |
| all nesting | deliveries | 0.46 | <0.01 | |
| nesting females | time in nest | 0.56 | 0.05 | |
| POA-hypothalamus: | ||||
| MPA (medial preoptic area) | nesting males | pick-ups | 0.85 | <0.01 |
| nesting males | deliveries | 0.84 | 0.01 | |
| all nesting | time in nest | 0.35 | 0.06 | |
| MPO (medial preoptic nucleus) | all nesting | pick-ups | 0.37 | 0.05 |
| all nesting | deliveries | 0.39 | 0.04 | |
| all nesting | time in nest | 0.49 | 0.02 | |
| Inf (tuberoinfundibular) | all nesting | time in nest | 0.25 | 0.07 |
| Midbrain: | ||||
| ICo, medial | nesting males | pick-ups | 0.62 | 0.06 |
| nesting males | deliveries | 0.64 | 0.05 |
Table 3.
Results of simple regressions between the percent of VIP-Fos co-labeled neurons and nesting behaviors (time in nest, pick-ups and deliveries).
| Subjects | Behavior | r 2 | P | |
|---|---|---|---|---|
| Extended amygdala: | ||||
| BSTm | all males | time in nest | 0.35 | 0.03 |
| POA-hypoth alamus: | ||||
| MPO (medial preoptic nucleus) | all males | time in nest | 0.38 | 0.02 |
| VMH | all males | time in nest | 0.29 | 0.03 |
| AH | all subjects | time in nest | 0.16 | 0.05 |
| nesting males | time in nest | 0.81 | 0.04 | |
| Inf (tuberoinfundibular) | all nesting | time in nest | 0.25 | 0.07 |
| Midbrain: | ||||
| ICo, lateral | nesting females | deliveries | 0.59 | 0.04 |
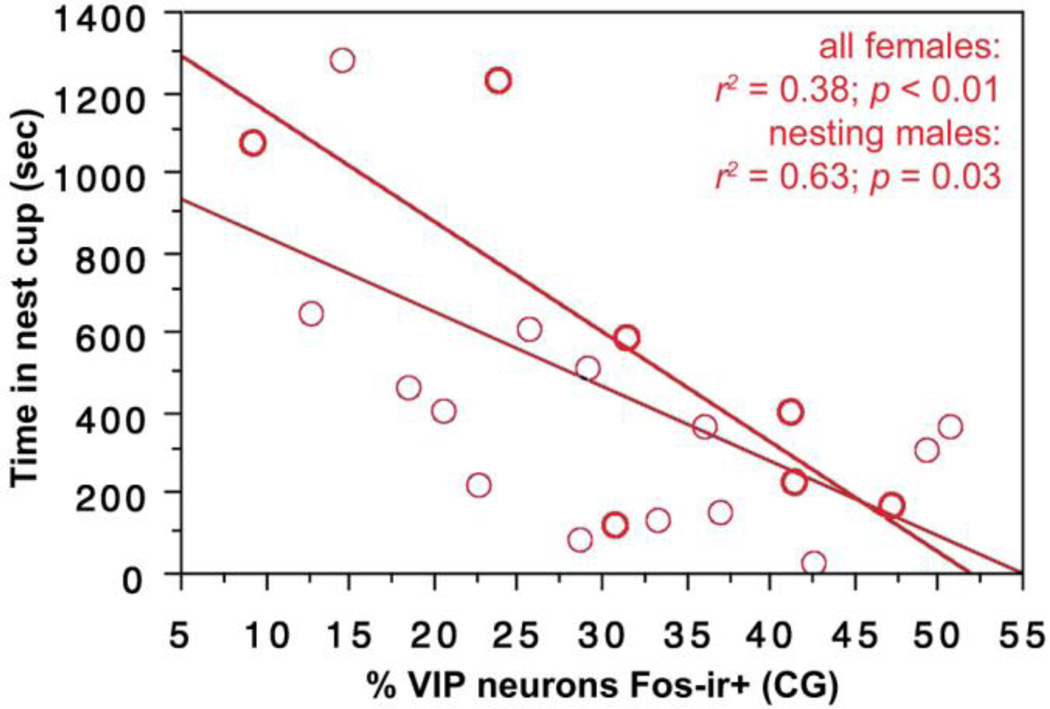
| CG | all females | time in nest | 0.38 | <0.01 |
| nesting females | time in nest | 0.63 | 0.03 |
Extended amygdala
MeA
Previous data suggest that, as in mammals (Newman, 1999), the anterior portion of the avian MeA (i.e., the portion of the MeA capping the ventral amydalofugal, or "occcipitomesencephalic," tract) is functionally distinct from the caudal portions of this nucleus (Goodson et al., 2012b). We therefore analyzed anterior and posterior regions separately, although the results were extensively comparable.
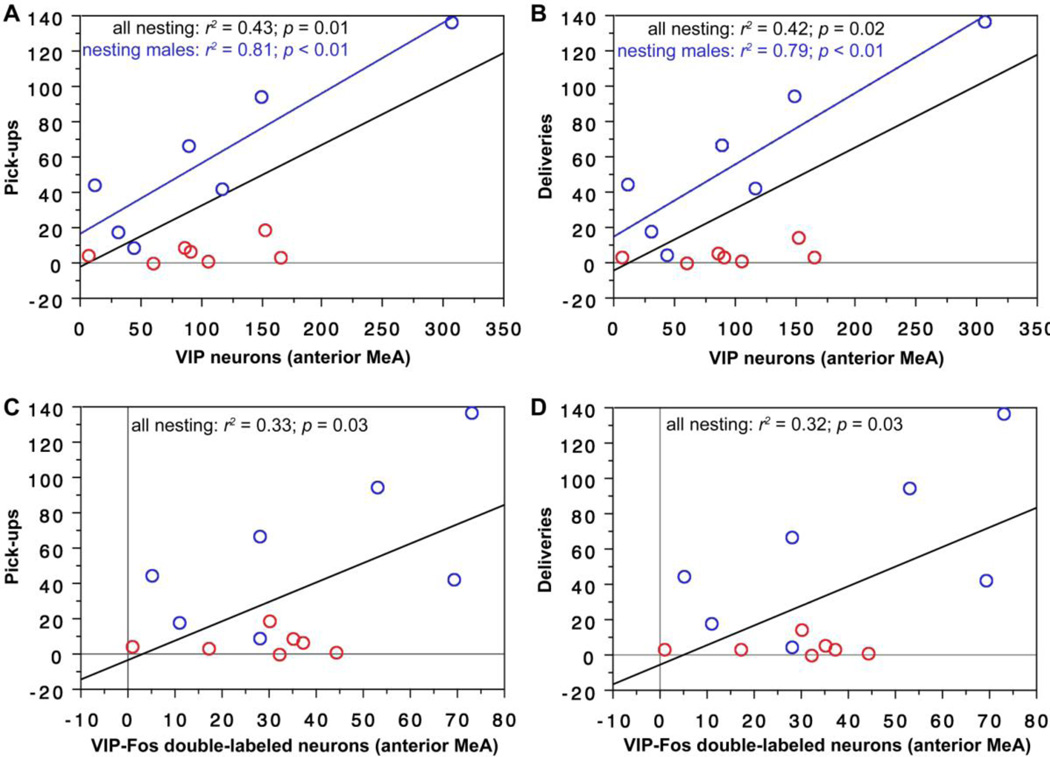
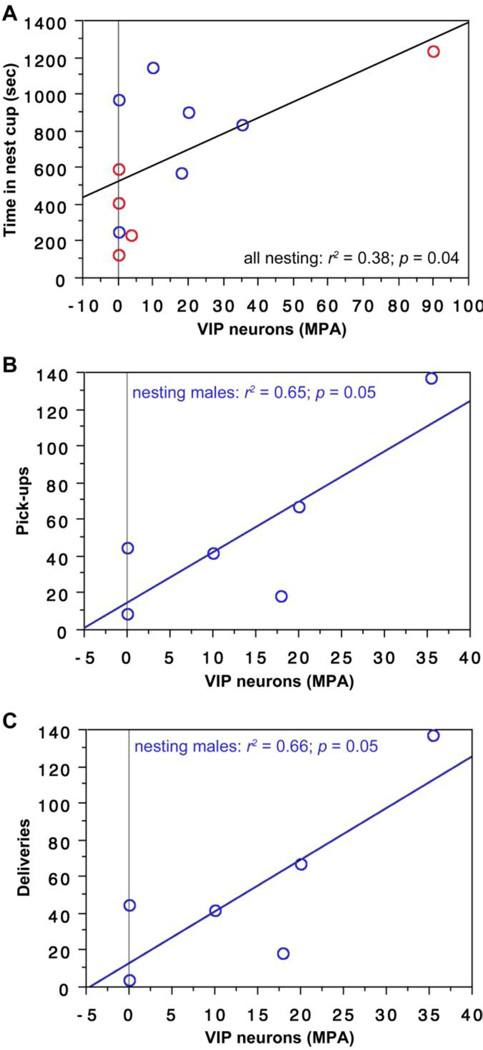
As shown in Fig. 2, the number of cells expressing VIP in the anterior MeA significantly predicts pick-ups with sexes pooled and deliveries with sexes pooled. Similar results are obtained for analyses with nesting males alone. In addition, the number of VIP-Fos double-labeled neurons significantly predicts pick-ups with sexes pooled and deliveries with sexes pooled.
Fig. 2.
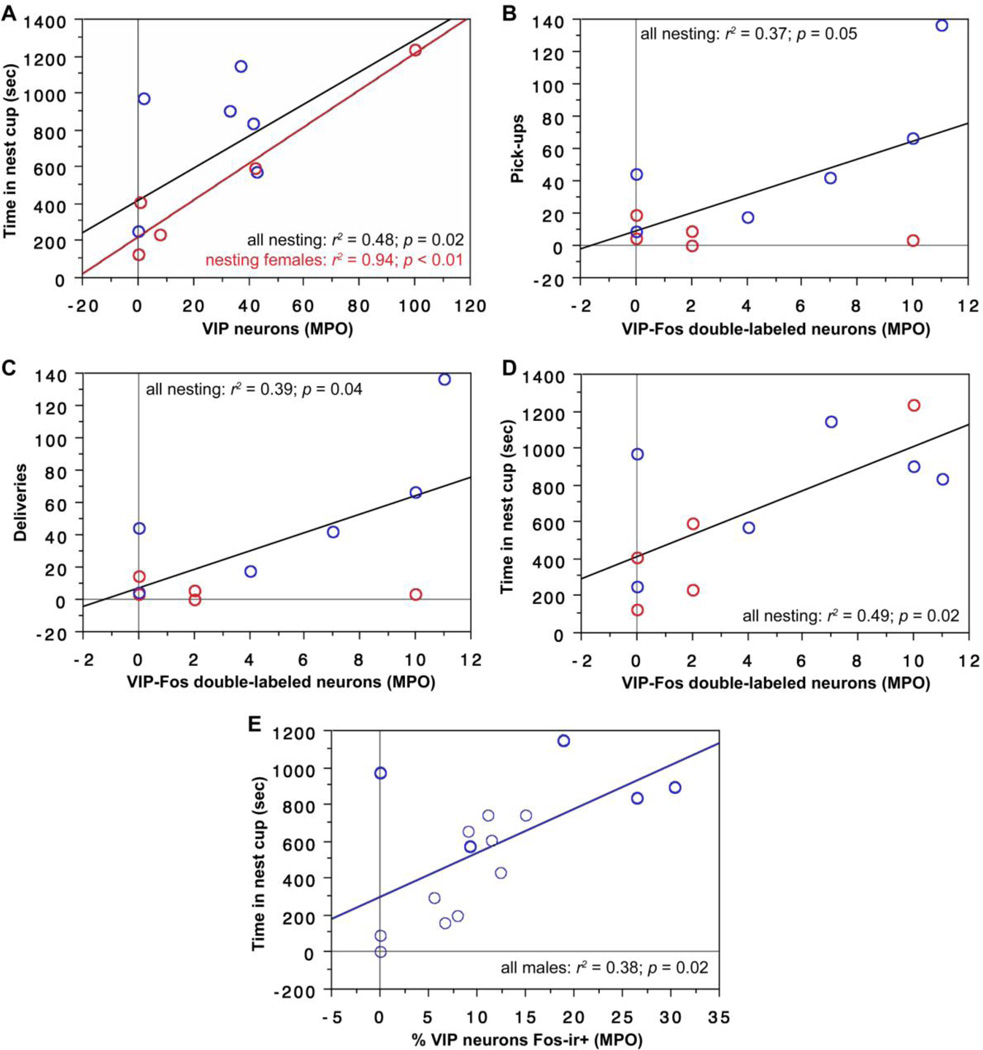
VIP expression and VIP-Fos double-labeling in the anterior MeA correlate positively with nesting behavior in male and female finches. Note that very similar data are obtained for the posterior MeA (see Results). (A,B) The number of VIP-expressing neurons (per 40 µm section) in the anterior MeA correlates positively with pick-ups of nesting items (A) and deliveries of nesting items to the nest (B), both with sexes pooled and in males alone. Males are shown in blue; females in red. Black regression lines are from analyses with sexes pooled. (C,D) The number of VIP-Fos doubled-labeled neurons also correlates positively with pick-ups (C) and deliveries (D) (sexes pooled).
As in the anterior MeA, the number of cells expressing VIP in the posterior MeA significantly predicts pick-ups and deliveries in nesting subjects (Table 1). Similar results are obtained for analyses with nesting males alone. The number of VIP-Fos double-labeled neurons also significantly predicts pick-ups with sexes pooled and deliveries with sexes pooled (Table 2).
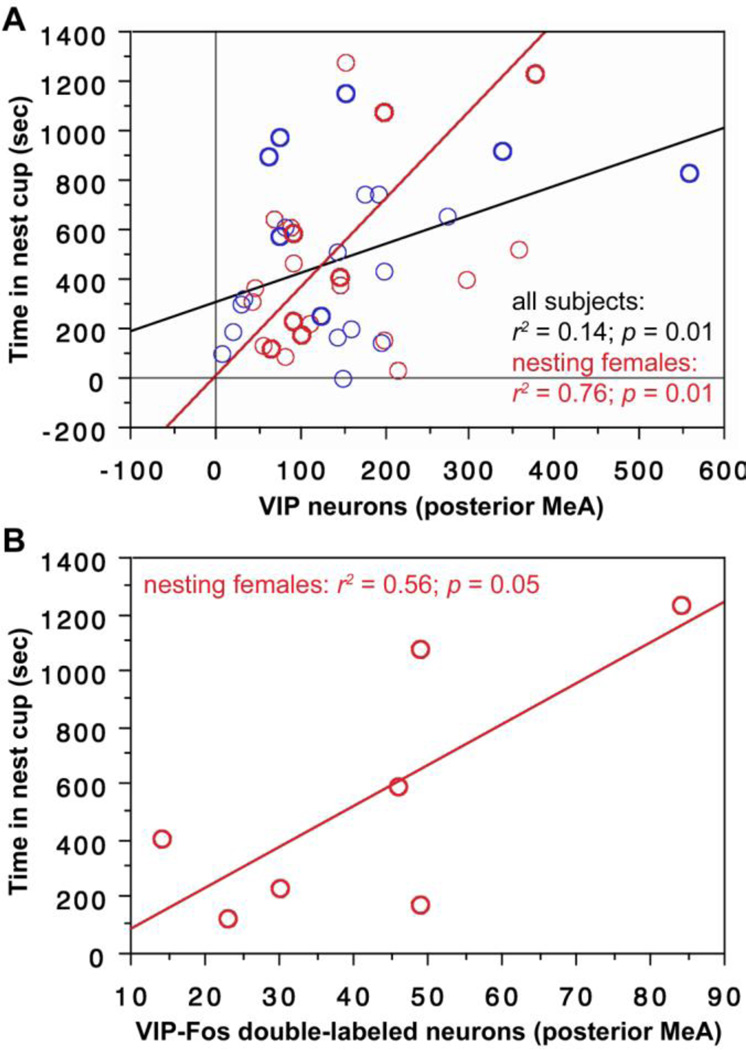
In addition to these similarities with the anterior MeA, we further find that the number of VIP-expressing cells in the posterior MeA significantly predicts the amount of time spent in the nest cup for the entire subject pool (Fig. 3A) and for nesting females alone (Fig. 3A). Finally, the number of VIP-Fos double-labeled neurons also predicts the amount of time that nesting females spent in the nest cup (Fig. 3B).
Fig. 3.
VIP expression and VIP-Fos double-labeling in the posterior MeA correlate positively with time spent in the nest cup. (A) The number of VIP neurons (per 40 µm section) in the posterior MeA correlates positively with time spent in the nest cup for all subjects (i.e., including nesting subjects and those that were not given nesting material; black regression line) and in nesting females alone (red line). Males are shown in blue; females in red. Data points from nesting subjects are shown in heavy circles; data points from subjects with nest cups only are shown in lighter circles. (B) The number of VIP-Fos doubled-labeled neurons also correlates positively with the time spent in the nest for nesting females.
BSTm
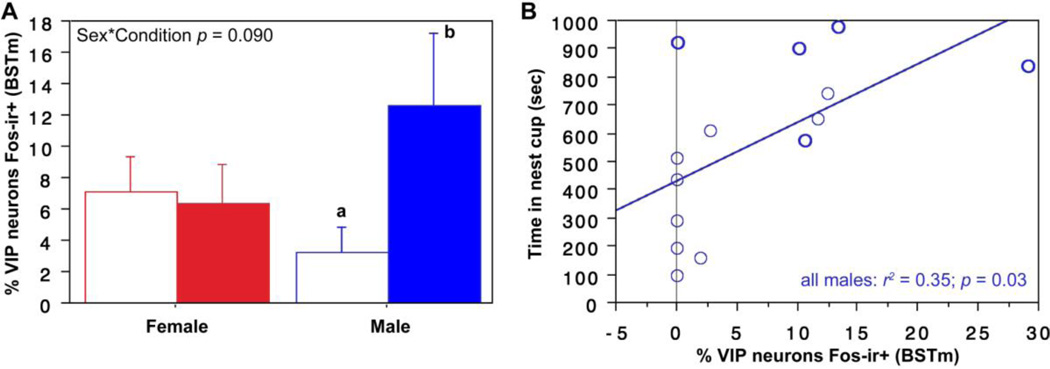
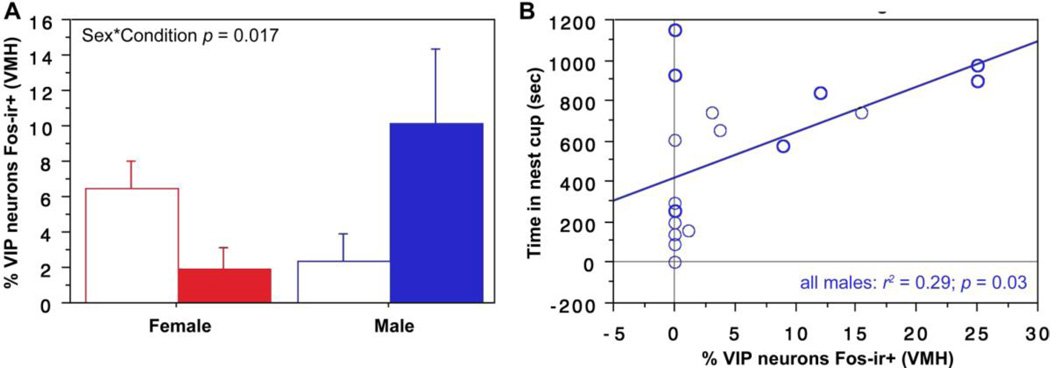
As shown in Fig. 4A, a weak Sex*Condition effect is observed for VIP-Fos colocalization in the BSTm (F(1, 23) = 3.129, p = 0.09, partial eta squared = 0.120), with a clear tendency for nesting males, but not nesting females, to exhibit greater colocalization than control subjects that were not offered nesting material. Given this trend, we conducted sex-specific t-tests and find that nesting males do exhibit greater VIP-Fos colocalization than control males (unpaired t = 2.28, p = 0.04, Cohen’s d = 1.134). Consistent with this effect, we find that VIP-Fos colocalization correlates positively with the amount of time that all male subjects (nesting and those with nest cups only) spent in the nest cup (Fig. 4B).
Fig. 4.
VIP-Fos colocalization (% of VIP neurons co-expressing Fos) increases in the BSTm of nesting males and correlates with the amount of time that males spend in the nest cup. (A) ANOVA shows a trend for a Sex*Condition interaction for VIP-Fos colocalization in the BSTm, with male nesting subjects (solid bar) showing greater colocalization than males that were not given nesting material (open bar). This difference is confirmed in a pairwise comparison (different letters above the error bars indicate p < 0.05; unpaired t-test). Nesting subjects are shown in solid bars. (B) VIP-Fos colocalization correlates positively with time in the nest cup for all males. Data points from nesting subjects are shown in heavy circles; data points from subjects with nest cups only are shown in lighter circles.
Preoptic area
MPO
The number of cells expressing VIP in the MPO significantly predicts time spent in the nest cup for all nesting subjects (i.e., with sexes pooled; Fig. 5A) and more strongly for nesting females alone (Fig. 5A). In addition, the number of VIP-Fos double-labeled neurons significantly predicts pick-ups with sexes pooled (Fig. 5B), deliveries with sexes pooled (Fig. 5C), and the amount of time that nesting subjects spent in the nest cup (sexes pooled; Fig. 5D). Finally, VIP-Fos colocalization significantly predicts the amount of time that male subjects spent in the nest cup (Fig. 5E).
Fig. 5.
VIP expression, VIP-Fos double-labeling, and VIP-Fos colocalization (% of VIP neurons co-expressing Fos) in the MPO correlate positively with nesting behavior in male and female finches. (A) The number of VIP neurons (per 40 µm section) in the MPO correlates positively with time spent in the nest cup by nesting subjects, both with sexes pooled and in females alone. Males are shown in blue; females in red. Black regression lines are from analyses with sexes pooled. (C-E) The number of VIP-Fos doubled-labeled neurons also correlates positively with pick-ups (B), deliveries (C) and time spent in the nest cup (D) by nesting males and females (sexes pooled). (E) VIP-Fos colocalization correlates positively with time in the nest cup for all males. Data points from nesting subjects are shown in heavy circles; data points from subjects with nest cups only are shown in lighter circles.
MPA
The number of cells expressing VIP in the MPA significantly predicts time spent in the nest cup for all nesting subjects (i.e., with sexes pooled; Fig. 6A) and significantly predicts pick-ups and deliveries by nesting males (Fig. 6B-C). The number of VIP-Fos double-labeled neurons also predicts pick-ups and deliveries by nesting males and tends to predict the amount of time that nesting subjects spent in the nest cup (Table 2).
Fig. 6.
VIP expression in the MPA correlates positively with nesting behavior. (A) The number of VIP neurons (per 40 µm section) in the MPA correlates positively with time spent in the nest cup by nesting subjects (sexes pooled). Males are shown in blue; females in red. (B,C) VIP expression also correlates positively with pick-ups of nesting items (B) and deliveries of nesting items to the nest cup (C) in males.
Hypothalamus
VMH
A significant Sex*Condition effect is observed for VIP-Fos colocalization in the VMH (F(1,32) = 6.129, p = 0.02, partial eta squared 0.161; Fig. 7A), reflecting a tendency for colocalization to decrease in nesting females relative to controls, but increase in nesting males. Consistent with this male-specific increase, we find that VIP-Fos colocalization correlates positively with the amount of time that all male subjects (nesting and those with nest cups only) spent in the nest cup (Fig. 7B).
Fig. 7.
Nesting induces sex-specific effects on VIP-Fos colocalization (% of VIP neurons co-expressing Fos) in the VMH. (A) A significant Sex*Condition interaction (ANOVA) is observed for VIP-Fos colocalization in the VMH. (B) VIP-Fos colocalization also correlates positively with time in nest for males. Data points from nesting subjects are shown in heavy circles; data points from subjects with nest cups only are shown in lighter circles.
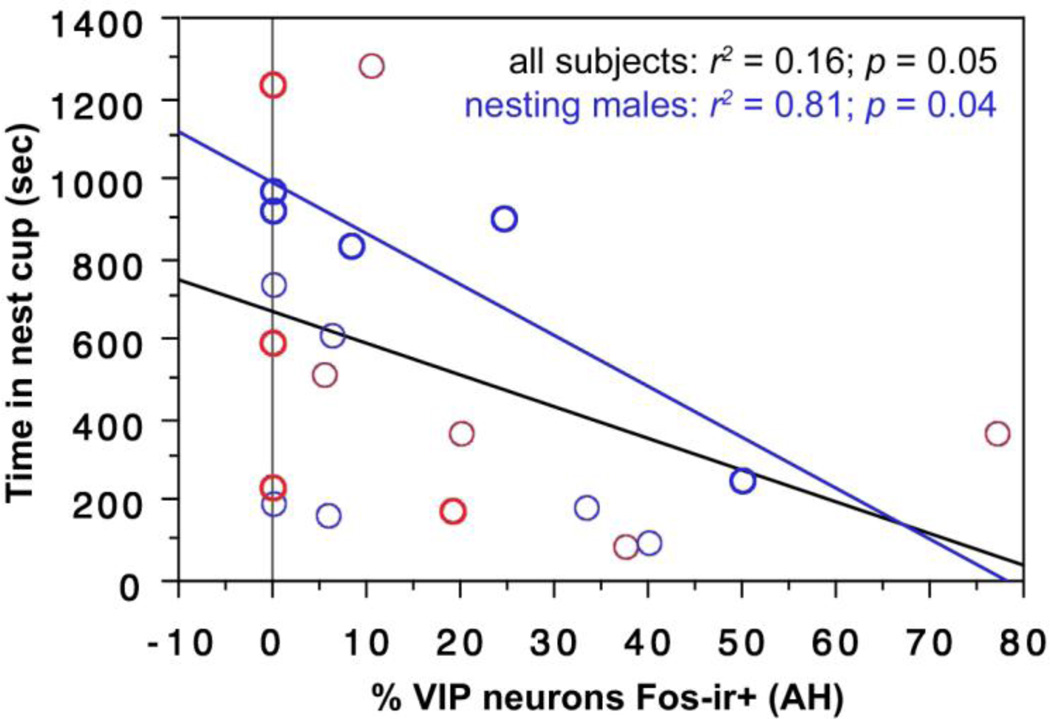
AH
VIP-Fos colocalization in the AH correlates negatively with the amount of time spent in the nest cup by all subjects (Fig. 8) and by nesting males alone (Fig. 8).
Fig. 8.
VIP-Fos colocalization (% of VIP neurons co-expressing Fos) in the AH correlates negatively with the amount of time spent in the nest cup by all subjects (black regression line) and by nesting males alone (blue regression line). Males are shown in blue; females in red. Data points from nesting subjects are shown in heavy circles; data points from subjects with nest cups only are shown in lighter circles.
Inf
As addressed in the Methods, we obtained poor resolution of Fos-ir neurons along the midline of the Inf (where the majority of VIP-expressing cells are located) in the ICC-ISH material. We therefore conducted analyses based on ICC for both VIP and Fos. These analyses show that the amount of time that nesting subjects spent in the nest cup tends to correlate positively with the number of VIP-Fos double-labeled neurons (Table 2) and with VIP-Fos colocalization (Table 3).
Midbrain
VTA
Because functional variation is observed along the rostrocaudal axis of the VTA in both birds and mammals (Lammel et al., 2008; Goodson et al., 2009; Alger et al., 2011), we analyzed data separately for a far-rostral level and for a far-caudal level. No effects are obtained for the rostral VTA. A single significant effect is obtained for the caudal VTA, with the number of VIP-expressing cells predicting the amount of time that female subjects spent in the nest (Table 1).
PAG complex (CG-ICo)
Due to the hypertrophy and lateral displacement of the avian optic tectum, regions that lie in the dorsal portion of the mammalian PAG (i.e., under the optic tectum, or superior colliculus) lie laterally in birds, medial to the optic tectum, within territory classically defined as ICo. In addition, the ICo region medial to the auditory torus is likely homologous to the lateral and dorsolateral columns in the mammalian dorsal PAG, whereas the ICo region lateral to the auditory torus is likely homologous to the dorsomedial column of the mammalian PAG. The avian CG (or "griseum centrale") is clearly homologous to the ventrolateral PAG (Kingsbury et al., 2011; Goodson and Kingsbury, 2013). Hence, we here conducted separated analyses for the lateral ICO (putative dorsomedial PAG), medial ICo (putative lateral-dorsolateral PAG) and CG (ventrolateral PAG).
Only a single significant effect was obtained for the lateral ICo (putative dorsomedial PAG), with VIP-Fos colocalization correlating positively with deliveries in nesting females (Table 3).
The number of cells expressing VIP in the medial ICo (putative lateral-dorsolateral PAG) significantly predicts pick-ups and deliveries by nesting males (Fig. 9A-B), although these correlations are negative. Similarly, the number of VIP-Fos double-labeled neurons tends to correlate negatively with pick-ups and correlates negatively with deliveries by nesting males (Fig. 9C).
Fig. 9.
(A-B) The number of cells expressing VIP in the medial ICo (putative lateral-dorsolateral PAG) significantly predicts pick-ups (A) and deliveries (B) by nesting males. (C) The number of VIP-Fos double-labeled neurons also correlates negatively with deliveries by nesting males.
VIP-Fos co-localization in the CG (ventrolateral PAG) correlates negatively with the amount of time spent in the nest cup by all females (Fig. 10) and by nesting females alone (Fig. 10).
Fig. 10.
VIP-Fos colocalization in the CG (ventrolateral PAG) correlates negatively with the amount of time spent in the nest cup by all females (light regression line) and by nesting females alone (heavy regression line). Data points from nesting subjects are shown in heavy circles; data points from subjects with nest cups only are shown in lighter circles.
Because these three subdivisions of the PAG are functionally distinct in birds and mammals (Bandler and Shipley, 1994; Kingsbury et al., 2011), we will not consider the lateral ICo (dorsomedial PAG) further below, as only a single effect is observed for this area.
Discussion
The present experiments demonstrate that VIP expression and VIP-Fos colabeling is associated with nesting behaviors across brain regions that comprise an evolutionarily conserved "social behavior network" (Newman, 1999; Goodson and Kingsbury, 2013). Although other neuropeptides exert effects throughout this network, they tend to be produced in only a subset of loci (e.g., the vasopressin-oxytocin peptides; Goodson and Kingsbury, 2013). In contrast, we here show that virtually all nodes of the social behavior network contain VIP-expressing neurons that show relationships to nesting behavior. The extent of this association is, to our knowledge, unparalleled by any other neuromodulator. As addressed in the sections below, the present findings suggest that VIP neurons relate to individual differences in nesting readiness (i.e., "motivation") in addition to nesting performance, and to both appetitive and consummatory aspects of behavior. In addition, the results reported here substantially expand the number of brain areas that are potentially involved in nesting behavior.
It is important to note that for several areas (i.e. MPA, MPO and VMH), 36–54% of animals in the nesting condition showed no or very little VIP cell labeling and/or VIP-Fos co-labeling (Fig. 5A-D, Fig. 6A and Fig. 7B). One possible explanation for these results is individual variation in response to nesting material, coupled with the short time interval in which individuals had access to nest material. Thirty minutes of exposure to nest material and a nest cup may not be enough time to induce VIP expression in these brain areas in every animal. Previous literature demonstrates that immunolabeling for VIP is dynamic across the reproductive cycle. For instance, in ring doves, greater amounts of VIP-immunoreactivity are observed during incubation, hatching and brooding as compared to isolated birds or newly paired birds (Cloues et al., 1990). VIP transcription in the hypothalamus of turkeys and VIP-immunolabeling in the hypothalamus of native Thai hens also varies across the reproductive cycle with the greatest expression and number of cells during incubation (Chaiseha et al., 1998) and hatching (Chaiyachet et al., 2013), respectively. Thus we might predict to find more VIP-labeled cells in the MPO, MPA and VMH of nesting zebra finches during the reproductive stages of incubation and chick care as apposed to the beginning of nesting. We might also predict that the longer zebra finches are together with their nest, the greater the labeling of VIP cells in the MPO and MPA and VMH. Furthermore, zebra finches in the present study were forced paired and did not self-pair. Thus, individual variation in VIP cell labeling may be due to how well and how fast a male and female exhibit pairing behavior and begin to build a nest as latency to pair-bond is variable (Kingsbury and Goodson, 2014).
Relationship of VIP neurons to nesting readiness versus performance
We here examined the relationships of nesting behavior to 1) the number of VIP-expressing neurons; 2) the number of VIP-Fos double-labeled neurons; and 3) the percent of VIP neurons that were positive for Fos, which we have referred to as "VIP-Fos colocalization." Unless we assume that VIP expression changes on the timescale of an immediate early gene such as c-fos (i.e., over 90 min in the present experiment), which is unlikely, all correlations between VIP expression and behavior must reflect a relationship between VIP and a readiness, or "motivation," to engage in nesting behavior. Such relationships may be specific to nesting, or may reflect more general aspects of reproductive readiness. Of note is that zebra finches are opportunistic breeders, photoperiodic insensitive and will breed whenever they deem conditions to be permissive (Christensen and Vleck, 2008). In contrast, relationships between VIP-Fos colocalization should reflect rapid, phasic shifts in behavioral state and/or motor performance. The raw number of VIP-Fos double-labeled neurons represents a somewhat hybrid measure, and likely provides the most accurate indicator of the total neuromodulatory impact of each cell group. We observe the fewest effects for VIP-Fos colocalization, the most effects for the numbers of VIP-expressing neurons, and an intermediate number of effects for the numbers of VIP-Fos double-labeled neurons. Hence, we can conclude that VIP is most strongly related to individual differences in nesting readiness/motivation and more modestly involved in the phasic performance of nesting. However, this varies substantially across cell groups.
Of the cell groups that exhibit relationships to nesting readiness, the MeA and MPA are clear standouts. Results are largely similar for the anterior MeA, posterior MeA, and MPA, with positive correlations observed for VIP expression and all three behaviors measured in nesting subjects (time spent in the nest cup, pick-ups and deliveries; with the exception that no effects are observed in the anterior MeA for time spent in the nest cup). Time spent in the nest cup by all subjects combined (i.e., in nesting subjects and control subjects with nest cups only or nesting females and males combined) also correlates positively with VIP expression in the posterior MeA and MPA, as it does in the MPO. Although not all of the effects just described are observed in separate analyses of males and females, most are nonetheless significant with sexes pooled, and thus there is not strong evidence for sex-specificity. However, correlations in the MPA for pick-ups and deliveries are indeed male-specific. This fact should not be over-interpreted, however, given that males perform these behaviors at much higher rates than females (Zann, 1996). Finally, VIP expression correlates negatively with pick-ups and deliveries by nesting males in the medial ICo (putative lateral-dorsolateral PAG).
Relationship of VIP neurons to appetitive versus consummatory aspects of nesting
Behaviors are often categorized as appetitive or consummatory. Appetitive behaviors are anticipatory and serve to bring an animal into contact with a goal object, often in the absence of direct stimulation from that goal object. In contrast, consummatory behaviors are those behaviors that represent the realization of the goal through direct interaction with the goal object (e.g., copulation or ingestion of food). As discussed by Ball and Balthazart (2008), the appetitive-consummatory distinction has been criticized for a variety of reasons. One of these is that the distinction is often not clear, such that a given aspect of behavior cannot readily be described as appetitive or consummatory. However, as Ball and Balthazart (2008) point out, the distinction is still quite useful if viewed as a continuum rather than a dichotomy (also see Pfaus, 1996). As an example, male Japanese quail (Japonix japonica) crow in the absence of females, which serves to bring females into close proximity (Goodson and Adkins-Regan, 1997). At this point the males cease to crow (Potash, 1974) and begin to strut in front of the female prior to copulation (Balthazart and Ball, 2007; Ball and Balthazart, 2008). In this case, crowing is clearly appetitive, copulation is clearly consummatory, and strutting is likely somewhere in between. The question then is whether this behavioral continuum is "real," in the sense that it is reflected in functional aspects of the nervous system. Opponents of the appetitive-consummatory distinction have argued that the terms are historically rooted in models that were flawed in their views of neural processes, but regardless of this history, behaviors that can be clearly categorized as appetitive or consummatory are indeed differentially regulated within the brain, as shown for sexual behaviors in male quail (Balthazart et al., 1998; Taziaux et al., 2006; Balthazart and Ball, 2007).
In contrast to the example above, appetitive aspects of nesting behavior are less clear. Certainly searching for an appropriate nest site could be viewed as appetitive, but once a pair begins to investigate a potential site (e.g., a cluster of forking branches or a plastic cup), the distinction of appetitive versus consummatory becomes less obvious, as with strutting in male quail. In order to address this problem, we here sought to determine whether any VIP cell groups exhibit only positive relationships to time spent in the nest cup (potentially appetitive), but not to pick-ups and deliveries (consummatory), thus differentiating cell groups that may selectively promote appetitive aspects of nesting. The strongest evidence that we obtained for such a pattern was in the BSTm and VMH, but for males only, where VIP-Fos colocalization correlates positively with time in the nest for all males combined (i.e., including those without nesting material). In addition, we observed trends for the number of VIP-Fos double-labeled neurons and VIP-Fos colocalization in the Inf to correlate positively with time spent in the nest cup by nesting subjects. Although not quite significant (both p = 0.07), these findings are worth noting, given that these neurons are involved in the regulation of PRL secretion and may therefore "prime" animals to exhibit an interest in nesting.
VIP cell groups of the AH and CG (homologue of the ventrolateral PAG; (Kingsbury et al., 2011) also exhibit relationships only to time in the nest cup, but these relationships are negative. Notably, extensive behavioral testing demonstrates that VIP knockdown in the AH selectively reduces overt aggression in territorial waxbills, and selectively reduces aggression in zebra finches -- primarily in the context of nest defense, but not mate competition (Goodson et al., 2012a). Given this pattern, it seems likely that at the same time that AH VIP neurons promote aggression, they concomitantly reduce interest or attention to other aspects of social and reproductive behavior. Similarly, the ventrolateral PAG of mammals promotes quiescence and hyporeactivity (Bandler and Shipley, 1994), and must therefore exert inhibitory effects on exploratory and seeking aspects of behavior.
In sum then, with the possible exception of VIP cells in the BSTm, VMH and perhaps the Inf, we find no evidence that VIP cell groups promote only appetitive aspects of nesting behavior. However, the present experimental design is not optimal for the study of appetitive nesting behavior, which could be more readily identified through conditioning studies such as those used in Japanese quail (Domjan and Hall, 1986; Balthazart and Ball, 2007). This issue notwithstanding, we provide evidence that several cell groups are involved in behaviors that are clearly consummatory (pick-ups and deliveries; MeA, MPA, MPO and medial ICo), in addition to behavior that is potentially appetitive (time spent in the nest cup; MeA, MPA and MPO).
Sex differences: real or artifact?
A variety of sex-specific effects are observed in the present dataset. However, because males and females do not exhibit all nesting behaviors at the same frequencies, it is difficult to determine whether these findings truly reflect sex-specific aspects of VIP neuronal function. Males tend to perform the majority of the gathering and delivery of nest material, whereas females tend to spend more time in the nest arranging of the material (Zann, 1996). Hence, our measures of gathering nest material (pick-ups) and delivering it to the nest (deliveries) are apt to be more robust in males, whereas our measure of time spent in the nest is apt to be more robust in females. Indeed, of the 11 male-specific correlations obtained here, all but 3 are observed for pick-ups and deliveries. These are observed for correlations of pick-ups and deliveries with VIP expression and VIP-Fos double-labeling in the MPA and medial ICo, with no effects for VIP-Fos colocalization. Within the MeA, the number of VIP-expressing cells and VIP-Fos double-labeled neurons significantly predicts pick-ups and deliveries with sexes pooled, however one nesting female may account for the significant correlations as this female was unusual in her frequency of pickups and deliveries compared to the other nesting females (Fig. 2A-D). Thus, like the VIP-Fos double-labeling in the MPA and medial ICo, VIP expression in the MeA may primarily correlate with pick-ups and deliveries in males. Indeed similar results are obtained for analyses with nesting males alone for the MeA. Support for the idea that time in nest may be a more robust behavior in females are the findings that the only two female-specific correlations we observed were for time spent in the nest cup (VIP-Fos double-labeling in the posterior MeA and VIP-Fos colocalization in the CG).
More intriguing are male-specific effects in the BSTm and VMH. In both areas, nesting males exhibit significant increases in VIP-Fos colocalization relative to controls that are not exhibited by females. This might be expected if those areas are particularly important for the male-biased behaviors of gathering and delivering nest material, but in both areas, colocalization is significantly correlated with time spent in the nest, not with pick-ups and deliveries. Furthermore, significant correlations are obtained only with all males pooled; that is, including control males that could spend time in the nest cup but had no nest material. Hence, the present data suggest that VIP neurons of the BSTm and VMH contribute to appetitive aspects of nesting in a manner that is indeed sex-specific. In addition, because the only effects in these brain areas are obtained for colocalization, the sex-specificity likely relates to the phasic performance of behavior rather than the constitutive readiness to nest.
These sex differences in the BSTm and VMH are certainly not unique. For instance, vasotocin/vasopressin production in the BSTm is sexually dimorphic (male-biased) across a wide variety of species (Goodson and Bass, 2001; De Vries and Panzica, 2006), and recent experiments in zebra finches and Angolan blue waxbills (Uraeginthus angolensis) demonstrate that this sex difference is associated with male-specific and/or male-biased effects on courtship, aggression and gregariousness (Kelly and Goodson, 2013a, b). However, we observe no sex differences in VIP expression within the BSTm (present study), and local densities of VIP binding sites are likewise not sexually differentiated (Goodson et al., 2006). In the case of the VMH, arguably the most notable sex difference is in the stronger requirement of this brain area for sexual behavior in females than males, a pattern that is associated with greater post-copulatory Fos induction in the VMH of females than males. This is observed in both mammals (Newman, 1999) and birds (Meddle et al., 1997; Meddle et al., 1999), although the VMH is still important for male mating and aggression (e.g., (Lin et al., 2011). Regardless, we are aware of no VMH functions that are male-biased, as suggested here for male nesting behavior.
Nesting circuits in the brain
Despite the fact that nesting behaviors are essential for reproduction in many species -- and that humans likewise invest a great deal of time and resources into preparation for the arrival of offspring -- the neural mechanisms of nesting have been studied far less than other aspects of parental behavior. However, reproductive nesting behavior is somewhat more difficult to study in laboratory rodents (e.g., rats and mice) than are other forms of parental behavior, given that rodents build nests for both reproductive and thermoregulatory purposes (Deacon, 2006). In fact, several of the brain areas that regulate parental behavior (e.g., (Gonzalez-Mariscal, 2001; Numan, 2007) are also involved in thermoregulation (Crawshaw et al., 1985; Morrison and Nakamura, 2011).
From the standpoint of differentiating thermoregulatory and reproductive nest building, data from rabbits are particularly useful, given that female rabbits dig burrows and line them with straw for strictly reproductive purposes. Numerous hormones influence nest building in rabbits, particularly estradiol, which promotes nest building when implanted into the preoptic region, nucleus accumbens, and BSTm (Gonzalez-Mariscal, 2001; Gonzalez-Mariscal et al., 2005).
As described below, the medial portion of the preoptic region is particularly important for nesting behavior in rodents, as well -- a finding that is very consistent with the present data in finches. Note, however, that whereas the medial portion of the preoptic region in birds is subdivided into the MPA and MPO, the medial preoptic area of mammals (MPOA) typically is not. The recognition of an MPO subdivision in birds is based largely on its sexually dimorphic size (males > females; Panzica et al., 1996). Similar sexual dimorphisms have been found in various mammals (e.g., rats; Gorski et al., 1980), but these appear to be species-specific and are not necessarily functionally equivalent to the avian MPO (Panzica et al., 1996; De Vries and Södersten, 2009). Regardless, our data suggest that VIP neurons of both the MPA and MPO are important for nesting behavior, allowing fairly direct comparison to the mammalian MPOA.
Numerous lines of evidence demonstrate that the MPOA is essential for nest building in rats, Syrian hamsters (Mesocricetus auratus), and mice. The most extensive data come from rats, in which the importance of the MPOA has been demonstrated through knife cuts lateral to the MPOA, and both electrolytic and cytotoxic lesions (Terkel et al., 1979; Jacobson et al., 1980; Miceli et al., 1983; Kalinichev et al., 2000; Oxley and Fleming, 2000; Olazabal et al., 2002). These manipulations virtually abolish nesting behavior and concomitantly impair other aspects of maternal behavior. Also consistent with the present data, nesting is reduced by lesions to the MPOA but not the adjacent AH (Gray and Brooks, 1984). Knife cuts lateral to the MPOA in hamsters likewise disrupt nest building (Miceli and Malsbury, 1982), as does genetic ablation of MPOA galanin neurons in mice (Wu et al., 2014).
The MPOA (or MPA/MPO in birds) is interconnected with several other areas of the social behavior network and mesolimbic dopamine system that are important for the regulation of maternal care, including the lateral septum (LS), MeA, BSTm, PAG, and VTA (Balthazart et al., 1994; Newman, 1999; Numan, 2007; O'Connell and Hofmann, 2011; Goodson and Kingsbury, 2013). All of these areas also influence nesting behavior in mammals (Slotnick and Nigrosh, 1975; Numan and Smith, 1984; Flannelly et al., 1986; Oxley and Fleming, 2000; Gonzalez-Mariscal et al., 2005; Moura et al., 2010), although amygdalar lesions have not been restricted to the MeA and have produced inconsistent results (Oxley and Fleming, 2000; Sheehan et al., 2001). In contrast, lesions of the LS consistently abolish nesting (Slotnick and Nigrosh, 1975; Flannelly et al., 1986). We did not reliably observe VIP expression in the LS and therefore did not conduct relevant quantification in this area. However, the LS expresses high densities of VIP binding sites (putative VPAC receptors; (Goodson et al., 2006) and VIP innervation of the ventrolateral LS is significantly denser in the more parental morph of the polymorphic white-throated sparrow (Zonotrichia albicollis) (Maney et al., 2005). The present findings are also consistent with findings in rats for the PAG (see below) and BSTm (Gonzalez-Mariscal et al., 2005), although we here obtained nesting-related effects in the BSTm only for males. Experimental evidence in rats further demonstrates that projections of the MPOA to the VTA are essential for the normal display of nest building (Numan and Smith, 1984). Although we here obtained only a single significant correlation for the VTA (a positive correlation between VIP neuron number and female nest time), the finding is nonetheless consistent with the data in rats.
As noted above, lesions of the amygdala do not produce consistent effects on nesting, although Oxley and Fleming (2000) have shown that lesions facilitate nest building in rats. This finding is consistent with the fact that MeA lesions facilitate other aspects of maternal care in rats, as well (Sheehan et al., 2001). In contrast, we here obtained numerous positive correlations for the MeA. These seemingly inconsistent results may reflect the fact that amydgalar regions tend to express intercalated populations of functionally opposed neurons, such as intermingled neurons that respond to positive and negative valence (e.g., (Goodson and Wang, 2006; Paton et al., 2006)).
In contrast to the areas discussed above, we here observe negative correlations between nesting behavior and VIP expression and/or VIP-Fos co-expression in the AH and PAG complex (CG-ICo). Interestingly, indirect evidence suggests that both of these areas inhibit maternal care in rats (Numan, 2007), which may reflect the fact that these areas promote aspects of behavior such as aggression and defense that are inconsistent with affiliation behaviors. Indeed, a variety of findings in birds directly demonstrate or indirectly suggest that the dorsal AH and ICo promote aggression, whereas the ventral AH and CG are associated with defense (Kingsbury et al., 2011; Goodson et al., 2012a; Goodson et al., 2012b). Most notably, antisense knockdown of VIP production in the dorsal AH virtually abolishes offensive aggression in zebra finches and the territorial violet-eared waxbill (Uraeginthus granatina), and does so in a highly selective manner (Goodson et al., 2012a). Consistent with this finding, 1) VIP-ir cell numbers in the AH (quantified following colchicine treatment) correlate positively with aggression in waxbills (Goodson et al., 2012a), and 2) VIP immunolabeling in the AH correlates positively with individual and species differences in aggression in sparrows (Goodson et al., 2012b). Similarly, aggression is positively related to Fos induction in the ICo (putative lateral-dorsolateral PAG) of violet-eared waxbills and zebra finches, and VIP immunolabeling in the ICo tends to correlate positively with aggression in sparrows (p = 0.09, Table S13, (Goodson et al., 2012b). In contrast, Fos induction in the ventral AH correlates positively with the amount of aggression received by subordinate waxbills (Goodson et al., 2012a) and Fos induction in the CG (ventrolateral PAG) is observed following aggressive subjugation and pursuit by a human hand (i.e., following the expression of defensive behaviors; (Kingsbury et al., 2011).
Finally, the present results are somewhat consistent with a recent study of Fos induction following in zebra finches (Hall et al., 2014), in which several nodes of the social behavior network were examined (MPO, LS, BSTm, VMH, AH, and CG) in addition to the VTA and other areas not examined here. Surprisingly, Hall et al. obtained no significant effects for the MPO, although significant effects were obtained for the VTA, BSTm and AH. The AH data are of particular interest, given that they show a negative relationship between Fos induction and the amount of time that females spend in the nest (regressions for other areas are positive). Hence, converging lines of evidence from zebra finches and rats indicate that the AH exhibits a negative relationship to nesting and parental care (Numan, 2007; Hall et al., 2014;). The lack of effects for the MPO in Hall et al. (2014) may reflect the fact that they focused their analysis on the caudal MPO (at the level of the anterior commissure), whereas we focused our own analysis on the rostral MPO where the majority of VIP-expressing neurons are located. Notably, maternal care in rats is more substantially impaired by small lesions of the rostral MPOA as compared to the caudal MPOA (Gray and Brooks, 1984).
Roles of other hormones, neurotransmitters and neuropeptides in nesting
In rabbits, discrete stages of nest-building are correlated with unique combinations and levels of particular hormones. For instance, nest-digging is correlated with high levels of estradiol (E) and progesterone (P), straw carrying with decreasing P, increasing PRL and high E, and hair pulling/hair lining of the nest with low P and high PRL (Gonzalez-Mariscal et al., 1994). Subsequent studies showed that blocking PRL with bromocriptine did not affect digging but abolished straw carrying and hair-pulling, supporting a role for PRL in these stages of nest-building in rabbits (Gonzalez-Mariscal et al., 1996). In turkeys, a photosensitive species, gonadal stimulative photoperiods induce PRL release (Burke and Dennison, 1980) and this release of PRL is dependent on hypothalamic VIP since VIP immunoneutralization blocks photo-induced PRL secretion (El Halawani et al., 1996). Furthermore, incubation behavior is blocked in VIP-immunized female turkeys compared to control, non-immunized birds (El Halawani et al., 1996). DA also plays a role in PRL secretion. For instance, the stimulatory role of DA in PRL release does not occur in VIP-immunized turkeys (Youngren et al., 1996). Incubation behavior is also disrupted in VIP-immunized chickens (Sharp et al., 1989) but is unaffected in VIP-immunized ring doves (Lea et al., 1991). Thus, it remains to be seen whether the association of VIP with nesting behavior in zebra finches is mediated through PRL release. Most recently, a role for the vasopressin-oxytocin (VP-OT) peptides in nest building in zebra finches has been shown (Klatt and Goodson, 2013). Peripheral OT receptor antagonists greatly reduce time in nest for females while peripheral antagonism of V1a receptors reduces time on nest for both sexes. Because 1) these effects are not observed after central receptor antagonist infusions and 2) Fos induction is increased in paraventricular (PVN) vasotocin (VT; homologue to mammalian VP) neurons within females in response to nesting conditions, it is hypothesized that peptide release from PVN VT neurons stimulates nesting behavior through peripheral feedback of OTR binding in the oviduct (Klatt and Goodson, 2013).
Conclusions
We now demonstrate that VIP expression and VIP-Fos co-expression are correlated with nesting behavior in all nodes of the social behavior network except the LS, which was not examined due to low numbers of VIP-expressing neurons. Thus, the present data suggest that VIP neurons in the MPO/MPA, MeA, BSTm, AH, VMH, and PAG complex may play a role in nesting. Hence, the relationship between VIP and nesting behavior appears to span the entire social behavior network, and may also extend to the mesolimbic dopamine system. Furthermore, several brain areas appear to exhibit relationships to both appetitive and consummatory aspects of nesting. As discussed above, many of our results are consistent with findings for maternal and nesting behavior in mammals (i.e., in terms of the relevant brain areas and their positive or negative relationships to behavior), and thus the present data may productively inform further research in a wide variety of species. Future experiments using VPAC receptor antagonism and/or knockdown of VIP production in specific brain areas are now needed to explore a causal link between VIP signaling and nesting behavior.
Supplementary Material
Research Highlights.
VIP expression and Fos co-expression are extensively associated with nesting behavior
VIP neurons and nesting are associated throughout the social behavior network
VIP neurons relate to individual differences in nesting readiness and performance
VIP and Fos co-expression relates to appetitive and consummatory aspects of behavior
Acknowledgements
We thank Sara E. Schrock with assistance with histology. This work was supported by NIH grant RO1 MH092331. We would like to dedicate this paper in memory of Jim Goodson, beloved husband and colleague and extraordinary neuroendocrinologist.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Kahtane A, Chaiseha Y, El Halawani M. Dopaminergic regulation of avian prolactin gene transcription. J. Mol. Endocrinol. 2003;31:185–196. doi: 10.1677/jme.0.0310185. [DOI] [PubMed] [Google Scholar]
- Alger SJ, Juang C, Riters LV. Social affiliation relates to tyrosine hydroxylase immunolabeling in male and female zebra finches (Taeniopygia guttata) J. Chem. Neuroanat. 2011;42:45–55. doi: 10.1016/j.jchemneu.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonopoulos J, Papadopoulos GC, Karamanlidis AN, Parnavelas JG, Dinopoulos A, Michaloudi H. VIP- and CCK-like-immunoreactive neurons in the hedgehog (Erinaceus europaeus) and sheep (Ovis aries) brain. J. Comp. Neurol. 1987;263:290–307. doi: 10.1002/cne.902630211. [DOI] [PubMed] [Google Scholar]
- Aste N, Viglietti-Panzica C, Fasolo A, Panzica GC. Mapping of neurochemical markers in quail central nervous system: VIP- and SP-like immunoreactivity. J. Chem. Neuroanat. 1995;8:87–102. doi: 10.1016/0891-0618(94)00031-n. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. How useful is the appetitive and consummatory distinction for our understanding of the neuroendocrine control of sexual behavior? Horm. Behav. 2008;53:307–311. doi: 10.1016/j.yhbeh.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gerard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J. Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front. Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Dupiereux V, Aste N, Viglietti-Panzica C, Barrese M, Panzica GC. Afferent and efferent connections of the sexually dimorphic medial preoptic nucleus of the male quail revealed by in vitro transport of DiI. Cell Tiss. Res. 1994;276:455–475. doi: 10.1007/BF00343944. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: Modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Bhatt R, Youngren O, Kang S, El Halawani M. Dopamine infusion into the third ventricle increases gene expression of hypothalamic vasoactive intestinal peptide and pituitary prolactin and luteinizing hormone beta subunit in the turkey. Gen. Comp. Endocrinol. 2003;130:41–47. doi: 10.1016/s0016-6480(02)00533-6. [DOI] [PubMed] [Google Scholar]
- Buntin JD, Becker GM, Ruzycki E. Facilitation of parental behavior in ring doves by systemic or intracranial injections of prolactin. Horm. Behav. 1991;25:424–444. doi: 10.1016/0018-506x(91)90012-7. [DOI] [PubMed] [Google Scholar]
- Buntin JD, Hnasko RM, Zuzick PH. Role of the ventromedial hypothalamus in prolactin-induced hyperphagia in ring doves. Physiol. Behav. 1999;66:255–261. doi: 10.1016/s0031-9384(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Burke WH, Dennison PT. Prolactin and luteinizing hormone levels in female turkeys (Meleagris gallopavo) during a photoinduced reproductive cycle and broodiness. Gen. Comp. Endocrinol. 1980;41:92–100. doi: 10.1016/0016-6480(80)90037-4. [DOI] [PubMed] [Google Scholar]
- Chaiseha Y, Tong Z, Youngren OM, El Halawani ME. Transcriptional changes in hypothalamic vasoactive intestinal peptide during a photo-induced reproductive cycle in the turkey. J. Mol. Endocrinol. 1998;21:267–275. doi: 10.1677/jme.0.0210267. [DOI] [PubMed] [Google Scholar]
- Chaiyachet OA, Chokchaloemwong D, Prakobsaeng N, Sartsoongnoen N, Kosonsiriluk S, Rozenboim I, El Halawani ME, Porter TE, Chaiseha Y. Neuroendocrine regulation of rearing behavior in the native Thai hen. Acta histochemica. 2013;115:209–218. doi: 10.1016/j.acthis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Christensen D, Vleck CM. Prolactin release and response to vasoactive intestinal peptide in an opportunistic breeder, the zebra finch (Taeniopygia guttata) Gen. Comp. Endocrinol. 2008;157:91–98. doi: 10.1016/j.ygcen.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Christian HC, Chapman LP, Morris JF. Thyrotrophin-releasing hormone, vasoactive intestinal peptide, prolactin-releasing peptide and dopamine regulation of prolactin secretion by different lactotroph morphological subtypes in the rat. J. Neuroendocrinol. 2007;19:605–613. doi: 10.1111/j.1365-2826.2007.01567.x. [DOI] [PubMed] [Google Scholar]
- Cloues R, Ramos C, Silver R. Vasoactive intestinal polypeptide-like immunoreactivity during reproduction in doves: influence of experience and number of offspring. Horm. Behav. 1990;24:215–231. doi: 10.1016/0018-506x(90)90006-j. [DOI] [PubMed] [Google Scholar]
- Crawshaw L, Grahn D, Wollmuth L, Simpson L. Central nervous regulation of body temperature in vertebrates: comparative aspects. Pharmacol. Ther. 1985;30:19–30. doi: 10.1016/0163-7258(85)90045-2. [DOI] [PubMed] [Google Scholar]
- de Ruiter AJ, Wendelaar Bonga SE, Slijkhuis H, Baggerman B. The effect of prolactin on fanning behavior in the male three-spined stickleback, Gasterosteus aculeatus L. Gen. Comp. Endocrinol. 1986;64:273–283. doi: 10.1016/0016-6480(86)90014-6. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: Different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Södersten P. Sex differences in the brain: The relation between structure and function. Horm. Behav. 2009;55:589–596. doi: 10.1016/j.yhbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM. Assessing nest building in mice. Nat. Protoc. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- Domjan M, Hall S. Determinants of social proximity in Japanese quail (Coturnix coturnix japonica): male behavior. J. Comp. Psychol. 1986;100:59–67. [PubMed] [Google Scholar]
- El Halawani M. Vasoactive intestinal peptide as the avian prolactin-releasing factor. In: Harvey S, Etches RJ, editors. Perspectives in avian endocrinology. 1997. pp. 403–416. [Google Scholar]
- El Halawani ME, Pitts GR, Sun S, Silsby JL, Sivanandan V. Active immunization against vasoactive intestinal peptide prevents photo-induced prolactin secretion in turkeys. Gen. Comp. Endocrinol. 1996;104:76–83. doi: 10.1006/gcen.1996.0143. [DOI] [PubMed] [Google Scholar]
- Flannelly KJ, Kemble ED, Blanchard DC, Blanchard RJ. Effects of septal-forebrain lesions on maternal aggression and maternal care. Behav. Neural Biol. 1986;45:17–30. doi: 10.1016/s0163-1047(86)80002-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal G. Neuroendocrinology of maternal behavior in the rabbit. Horm. Behav. 2001;40:125–132. doi: 10.1006/hbeh.2001.1692. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal G, Chirino R, Rosenblatt JS, Beyer C. Forebrain implants of estradiol stimulate maternal nest-building in ovariectomized rabbits. Horm. Behav. 2005;47:272–279. doi: 10.1016/j.yhbeh.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal G, Diaz-Sanchez V, Melo AI, Beyer C, Rosenblatt JS. Maternal behavior in New Zealand white rabbits: quantification of somatic events, motor patterns, and steroid plasma levels. Physiol. Behav. 1994;55:1081–1089. doi: 10.1016/0031-9384(94)90391-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal G, Melo AI, Jimenez P, Beyer C, Rosenblatt JS. Estradiol, progesterone, and prolactin regulate maternal nest-building in rabbits. J. Neuroendocrinol. 1996;8:901–907. doi: 10.1111/j.1365-2826.1996.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm. Behav. 1998a;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina) Gen. Comp. Endocrinol. 1998b;111:233–244. doi: 10.1006/gcen.1998.7112. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Playback of crows of male Japanese quail elicits female phonotaxis. Condor. 1997;99:990–993. [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res. Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm. Behav. 2006;50:223–236. doi: 10.1016/j.yhbeh.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc. Nat. Acad. Sci. U. S. A. 2009;106:8737–8742. doi: 10.1073/pnas.0811821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kelly AM, Kingsbury MA, Thompson RR. An aggression-specific cell type in the anterior hypothalamus of finches. Proc. Nat. Acad. Sci. U. S. A. 2012a;109:13847–13852. doi: 10.1073/pnas.1207995109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kingsbury MA. What's in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm. Behav. 2013;64:103–112. doi: 10.1016/j.yhbeh.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc. Nat. Acad. Sci. U. S. A. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Wilson LC, Schrock SE. To flock or fight: Neurochemical signatures of divergent life histories in sparrows. Proc. Nat. Acad. Sci. U. S. A. 2012b;109(Suppl 1):10685–10692. doi: 10.1073/pnas.1203394109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J. Comp. Neurol. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- Gray P, Brooks PJ. Effect of lesion location within the medial preoptic-anterior hypothalamic continuum on maternal and male sexual behaviors in female rats. Behav. Neurosci. 1984;98:703–711. doi: 10.1037//0735-7044.98.4.703. [DOI] [PubMed] [Google Scholar]
- Hall ZJ, Bertin M, Bailey IE, Meddle SL, Healy SD. Neural correlates of nesting behavior in zebra finches (Taeniopygia guttata) Behav. Brain Res. 2014;264:26–33. doi: 10.1016/j.bbr.2014.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Cuasay K, Abebe DT. Vasoactive intestinal peptide antagonist treatment during mouse embryogenesis impairs social behavior and cognitive function of adult male offspring. Exp. Neurol. 2007a;206:101–113. doi: 10.1016/j.expneurol.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Hill JM, Hauser JM, Sheppard LM, Abebe D, Spivak-Pohis I, Kushnir M, Deitch I, Gozes I. Blockage of VIP during mouse embryogenesis modifies adult behavior and results in permanent changes in brain chemistry. J. Mol. Neurosci. 2007b;31:183–200. doi: 10.1385/jmn:31:03:185. [DOI] [PubMed] [Google Scholar]
- Jacobson CD, Terkel J, Gorski RA, Sawyer CH. Effects of small medial preoptic area lesions on maternal behavior: Retrieving and nest building in the rat. Brain Res. 1980;194:471–478. doi: 10.1016/0006-8993(80)91226-3. [DOI] [PubMed] [Google Scholar]
- Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J. Comp. Neurol. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Rosenblatt JS, Morrell JI. The medial preoptic area, necessary for adult maternal behavior in rats, is only partially established as a component of the neural circuit that supports maternal behavior in juvenile rats. Behav. Neurosci. 2000;114:196–210. doi: 10.1037//0735-7044.114.1.196. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL. Behavioral relevance of species-specific vasotocin anatomy in gregarious finches. Front. Neurosci. 2013a;7:242. doi: 10.3389/fnins.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL. Functional significance of a phylogenetically widespread sexual dimorphism in vasotocin/vasopressin production. Horm. Behav. 2013b;64:840–846. doi: 10.1016/j.yhbeh.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Kingsbury MA, Goodson JL. Pair bond formation is impaired by VPAC receptor antagonism in the socially monogamous zebra finch. Behav. Brain Res. 2014;272:264–268. doi: 10.1016/j.bbr.2014.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Kelly AK, Schrock SE, Goodson JL. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS One. 2011;6:e20720. doi: 10.1371/journal.pone.0020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Miller KM, Goodson JL. VPAC receptor signaling modulates grouping behavior and social responses to contextual novelty in a gregarious finch: A role for a putative prefrontal cortex homologue. Horm. Behav. 2013;64:511–518. doi: 10.1016/j.yhbeh.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt JD, Goodson JL. Sex-specific activity and function of hypothalamic nonapeptide neurons during nest-building in zebra finches. Horm. Behav. 2013;64:818–824. doi: 10.1016/j.yhbeh.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Kuenzel WJ, Blahser S. Vasoactive intestinal polypeptide (VIP)-containing neurons: distribution throughout the brain of the chick (Gallus domesticus) with focus upon the lateral septal organ. Cell Tissue Res. 1994;275:91–107. doi: 10.1007/BF00305378. [DOI] [PubMed] [Google Scholar]
- Kuenzel WJ, McCune SK, Talbot RT, Sharp PJ, Hill JM. Sites of gene expression for vasoactive intestinal polypeptide throughout the brain of the chick (Gallus domesticus) J. Comp. Neurol. 1997;381:101–118. doi: 10.1002/(sici)1096-9861(19970428)381:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kulick RS, Chaiseha Y, Kang SW, Rozenboim I, El Halawani ME. The relative importance of vasoactive intestinal peptide and peptide histidine isoleucine as physiological regulators of prolactin in the domestic turkey. Gen. Comp. Endocrinol. 2005;142:267–273. doi: 10.1016/j.ygcen.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Lam KS. Vasoactive intestinal peptide in the hypothalamus and pituitary. Neuroendocrinology. 1991;53(Suppl 1):45–51. doi: 10.1159/000125795. [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Haeckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lea RW, Talbot RT, Sharp PJ. Passive immunization against chicken vasoactive intestinal polypeptide suppresses plasma prolactin and crop sac development in incubating ring doves. Horm. Behav. 1991;25:283–294. doi: 10.1016/0018-506x(91)90002-y. [DOI] [PubMed] [Google Scholar]
- Lea RW, Vowles DM. Vasoactive intestinal polypeptide stimulates prolactin release in vivo in the ring dove (Streptopelia risoria) Experientia. 1986;42:420–422. doi: 10.1007/BF02118639. [DOI] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnamee MC, Sharp PJ, Lea RW, Sterling RJ, Harvey S. Evidence that vasoactive intestinal polypeptide is a physiological prolactin-releasing factor in the bantam hen. Gen. Comp. Endocrinol. 1986;62:470–478. doi: 10.1016/0016-6480(86)90057-2. [DOI] [PubMed] [Google Scholar]
- Maney DL, Erwin KL, Goode CT. Neuroendocrine correlates of behavioral polymorphism in white-throated sparrows. Horm. Behav. 2005;48:196–206. doi: 10.1016/j.yhbeh.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Maney DL, Schoech SJ, Sharp PJ, Wingfield JC. Effects of vasoactive intestinal peptide on plasma prolactin in passerines. Gen. Comp. Endocrinol. 1999;113:323–330. doi: 10.1006/gcen.1998.7220. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Foidart A, Wingfield JC, Ramenofskyand M, Balthazart J. Effects of sexual interactions with a male on fos-like immunoreactivity in the female quail brain. J. Neuroendocrinol. 1999;11:771–784. doi: 10.1046/j.1365-2826.1999.00384.x. [DOI] [PubMed] [Google Scholar]
- Meddle SL, King VM, Follett BK, Wingfield JC, Ramenofsky M, Foidart A, Balthazart J. Copulation activates Fos-like immunoreactivity in the male quail forebrain. Behav. Brain Res. 1997;85:143–159. doi: 10.1016/s0166-4328(97)87581-x. [DOI] [PubMed] [Google Scholar]
- Miceli MO, Fleming AS, Malsbury CW. Disruption of maternal behaviour in virgin and postparturient rats following sagittal plane knife cuts in the preoptic area-hypothalamus. Behav. Brain Res. 1983;9:337–860. doi: 10.1016/0166-4328(83)90137-7. [DOI] [PubMed] [Google Scholar]
- Miceli MO, Malsbury CW. Sagittal knife cuts in the near and far lateral preoptic area-hypothalamus disrupt maternal behaviour in female hamsters. Physiol. Behav. 1982;28:856–867. [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front. Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura LM, Canteras NS, Sukikara MH, Felicio LF. Morphine infusions into the rostrolateral periaqueductal gray affect maternal behaviors. Braz. J. Med. Biol. Res. 2010;43:899–905. doi: 10.1590/s0100-879x2010007500085. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev. Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav. Neurosci. 1984;98:712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J. Comp. Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Kalinichev M, Morrell JI, Rosenblatt JS. MPOA cytotoxic lesions and maternal behavior in the rat: effects of midpubertal lesions on maternal behavior and the role of ovarian hormones in maturation of MPOA control of maternal behavior. Horm. Behav. 2002;41:126–138. doi: 10.1006/hbeh.2001.1753. [DOI] [PubMed] [Google Scholar]
- Oxley G, Fleming AS. The effects of medial preoptic area and amygdala lesions on maternal behavior in the juvenile rat. Dev. Psychobiol. 2000;37:253–265. [PubMed] [Google Scholar]
- Panzica GC, Viglietta-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: A key brain area mediating steroid action on male sexual behavior. Front. Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Frank A. Beach Award—homologies of animal and human sexual behaviors. Horm. Behav. 1996;30:187–200. doi: 10.1006/hbeh.1996.0024. [DOI] [PubMed] [Google Scholar]
- Potash LM. An experimental analysis of the use of location calls by Japanese quail, Coturnix coturnix japonica. Behaviour. 1974;54:153–180. [Google Scholar]
- Prakobsaeng N, Sartsoongnoen N, Kosonsiriluk S, Chaiyachet OA, Chokchaloemwong D, Rozenboim I, El Halawani M, Porter TE, Chaiseha Y. Changes in vasoactive intestinal peptide and tyrosine hydroxylase immunoreactivity in the brain of nest-deprived native Thai hen. Gen. Comp. Endocrinol. 2011;171:189–196. doi: 10.1016/j.ygcen.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Woodhams PL, Polak JM, Crow TJ. Distribution of neuropeptides in the limbic system of the rat: the amygdaloid complex. Neuroscience. 1982;7:99–131. doi: 10.1016/0306-4522(82)90156-7. [DOI] [PubMed] [Google Scholar]
- Sharp PJ, Sterling RJ, Talbot RT, Huskisson NS. The role of hypothalamic vasoactive intestinal polypeptide in the maintenance of prolactin secretion in incubating bantam hens: observations using passive immunization, radioimmunoassay and immunohistochemistry. J. Endocrinol. 1989;122:5–13. doi: 10.1677/joe.0.1220005. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Paul M, Amaral E, Numan MJ, Numan M. Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience. 2001;106:341–356. doi: 10.1016/s0306-4522(01)00286-x. [DOI] [PubMed] [Google Scholar]
- Sims KB, Hoffman DL, Said SI, Zimmerman EA. Vasoactive intestinal polypeptide (VIP) in mouse and rat brain: an immunocytochemical study. Brain Res. 1980;186:165–183. doi: 10.1016/0006-8993(80)90263-2. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Nigrosh BJ. Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. J. Comp. Physiol. Psychol. 1975;88:118–127. doi: 10.1037/h0076200. [DOI] [PubMed] [Google Scholar]
- Taziaux M, Cornil CA, Dejace C, Arckens L, Ball GF, Balthazart J. Neuroanatomical specificity in the expression of the immediate early gene c-fos following expression of appetitive and consummatory male sexual behaviour in Japanese quail. Eur. J. Neurosci. 2006;23:1869–1887. doi: 10.1111/j.1460-9568.2006.04719.x. [DOI] [PubMed] [Google Scholar]
- Terkel J, Bridges RS, Sawyer CH. Effects of transecting lateral neural connections of the medial preoptic area on maternal behavior in the rat: nest building, pup retrieval and prolactin secretion. Brain Res. 1979;169:369–380. doi: 10.1016/0006-8993(79)91037-0. [DOI] [PubMed] [Google Scholar]
- Vleck CM, Patrick DJ. Effects of vasoactive intestinal peptide on prolactin secretion in three species of passerine birds. Gen. Comp. Endocrinol. 1999;113:146–154. doi: 10.1006/gcen.1998.7191. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Sasaki S. Effect of thyroid status on the prolactin-releasing action of vasoactive-intestinal-peptide in humans - comparison with the action of thyrotropin-releasing- hormone. Neuroendocrinology. 1995;61:207–212. doi: 10.1159/000126842. [DOI] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509:325–330. doi: 10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngren OM, Chaiseha Y, El Halawani ME. Regulation of prolactin secretion by dopamine and vasoactive intestinal peptide at the level of the pituitary in the turkey. Neuroendocrinology. 1998;68:319–325. doi: 10.1159/000054380. [DOI] [PubMed] [Google Scholar]
- Youngren OM, Pitts GR, Phillips RE, el Halawani ME. Dopaminergic control of prolactin secretion in the turkey. Gen. Comp. Endocrinol. 1996;104:225–230. doi: 10.1006/gcen.1996.0165. [DOI] [PubMed] [Google Scholar]
- Zann RA. The zebra finch: a synthesis of field and laboratory studies. Oxford: Oxford University Press; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.