Abstract
Variations in cord blood manufacturing and administration are common, and the optimal practice, not known. We compared processing and banking practices at 16 public cord blood banks (CBB) in the United States, and assessed transplant outcomes on 530 single umbilical cord blood (UCB) myeloablative transplantations for hematologic malignancies, facilitated by these banks. UCB banking practices were separated into three mutually exclusive groups based on whether processing was automated or manual; units were plasma and red blood cell reduced or buffy coat production method or plasma reduced. Compared to the automated processing system for units, the day-28 neutrophil recovery was significantly lower after transplantation of units that were manually processed and plasma reduced (red cell replete) (odds ratio [OR] 0.19 p=0.001) or plasma and red cell reduced (OR 0.54, p=0.05). Day-100 survival did not differ by CBB. However, day-100 survival was better with units that were thawed with the dextran-albumin wash method compared to the “no wash” or “dilution only” techniques (OR 1.82, p=0.04). In conclusion, CBB processing has no significant effect on early (day 100) survival despite differences in kinetics of neutrophil recovery.
INTRODUCTION
Umbilical cord blood (UCB) transplantation has extended access to hematopoietic stem cell transplantation (HCT) to a diverse racial and ethnic population.1 Recent data has suggested comparable results between UCB and grafts from matched adult unrelated donor transplant in both the myeloablative and reduced intensity (RIC) setting.2–5 However, unlike bone marrow or peripheral blood, UCB units are collected, cryopreserved and when needed, thawed and infused.
Although the Food and Drug Administration (FDA) has issued guidance for manufacturing of cord blood for banking for unrelated transplantation, and the American Association of Blood Banks (AABB) and the Foundation for Accreditation of Cellular Therapy (FACT) have established standards for product manufacturing, practices at individual Cord Blood Banks (CBB) vary tremendously. For example, UCB can either be collected in utero by trained obstetrical personnel and/or ex utero by trained staff of the UCB bank. The American Red Cross reported no difference in total nucleated cell (TNC) count or post processing CD34+ or colony forming units (CFU-GM) between the two methods, but transplant outcomes were not assessed.6 Similarly, processing of the UCB unit varies widely among and within the CBBs. In the earliest years of UCB banking, CBBs did not manipulate the product, other than diluting and adding dimethyl sulfoxide (DMSO), prior to freezing.7 Today, most CBBs employ some form of volume reduction which is generally achieved by depleting red blood cells, plasma or both.8 Each CBB has its own procedures, some of which may have evolved over the history of the bank. Most CBBs have adopted the plasma and red blood cell reduction method.9 An alternative method is to deplete plasma but not red blood cells so that entrapment of nucleated cells, and possibly progenitor and stem cells is avoided, with some degree of volume reduction associated with the removal of plasma.10,11
Appropriate handling and thawing of UCB units at transplant centers are equally important to successful transplant outcomes. Pablo Rubinstein described a thawing procedure using a dextran and albumin solution, to remove dimethyl sulfoxide (DMSO). The majority of transplant centers adopted this approach, and nucleated cell count recoveries of 75–90% have been reported.12,13 More recently, Barker and colleagues described a dilution only “no wash” method with reconstitution in dextran-albumin for a final 5% DMSO concentration.14 Nucleated cell count recovery was 86%, and there were no serious adverse infusion events. Finally, some centers have used a nonvolume reduced (unmanipulated) thawing strategy, and have demonstrated adequate engraftment.15 The report of several life threatening infusion reactions with UCB infusion have intensified the need to determine the optimal thawing practice.16
The optimal processing techniques for UCB units are not established, and whether transplant outcomes differ by techniques is not clear. Therefore, we collected information on UCB processing at the CBBs and examined for an effect of processing methods at CBBs in patients who had undergone a single UCB transplantation for acute leukemia or myelodysplastic syndrome, the most common indications for allogeneic HCT. This report, the first of its kind, provides additional knowledge on whether practices at CBBs techniques influence hematopoietic recovery and early survival after UCB transplantation.
MATERIALS AND METHODS
Data Source
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a working group of over 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous HCT to a Statistical Center at the Medical College of Wisconsin or the Data Coordinating Center at the National Marrow Donor Program. Banking practices at CBBs were obtained using a short survey, which addressed UCB unit processing at the bank. Data on UCB unit thawing at transplant centers were obtained through standardized data collection forms developed by the CIBMTR. Patients provide written informed consent for participation in accordance with the Declaration of Helsinki. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved the study.
Patients
Included are 530 patients with acute myeloid leukemia (AML), acute lymphoid leukemia (ALL) or myelodysplasia (MDS) who received single unit unrelated UCB transplant in the United States with a UCB unit from one of the 16 participating CBBs. All transplants occurred in the United States between the years 2000 to 2011. Only recipients of myeloablative regimens, defined as having received total body irradiation dose of 1000 cGy or higher or busulfan dose greater than 9 mg/kg or melphalan dose greater than 150 mg/m2, are included.17 Recipients of multiple or expanded UCB units, reduced intensity conditioning regimens, and transplantations for non-malignant diseases were excluded.
Cord Blood Bank Practices
Sixteen publically funded CBBs in the United States participated in the survey. Using banking practices reported, three mutually exclusive groups were created (Table 1) based on the following: automated or manual processing at the CBB and whether units were plasma and red blood cell reduced, used the buffy coat production method or plasma reduced. All units contained DMSO and an hyperosmolar agent. Group 1 included units that were processed using an automated method, that were plasma and red blood cell reduced (n=84) or buffy coat production method (n=34). Group 2 included manually processed units that were plasma and red blood cell reduced (n=274) or buffy coat production method (n=5). Group 3 included manually processed units that were plasma reduced. Of note, as the groups were created based on self reported practices and some CBBs are represented in more than one group, as banking practices evolved over the study period. Further, Group 3 represents a single CBB and the buffy coat production method is implemented at three CBBs and represented in Group 1 (n=34 from a single CBB) and Group 2 (n=5 from two CBBs).
Table 1.
Cord bank practices
|
Group 1 Automated processing Plasma and red cell reduced or buffy coat production method |
Group 2 Manual processing Plasma and red cell reduced or buffy coat production method |
Group 3 Manual processing Plasma reduction |
|
|---|---|---|---|
| Number of banks* | 6 | 13 | 1 |
| Type of processing system | |||
| Automated | 6 | ||
| Manual | 13 | 1 | |
| Product processing method at banks | |||
| Plasma and red cell reduction | 5 | 11 | |
| Buffy coat production | 1 | 2 | |
| Plasma reduction | 1 | ||
| Anticoagulant | |||
| ACD | 1 | ||
| CPD | 4 | 12 | 1 |
| CPDA | 1 | 1 | |
| Storage method at bank | |||
| Vapor phase | 1 | 6 | 1 |
| Liquid nitrogen | 5 | 8 | |
| Years of existence of bank | |||
| ≤10 y | 2 | 2 | |
| 11 – 15 y | 2 | 7 | 1 |
| 15 – 20 y | 2 | 4 |
Number of banks exceeds N = 16 because practice changes occurred over time and those banks are represented in the Table more than once
Outcomes
The primary endpoint was hematopoietic recovery; neutrophil recovery was defined as achieving an absolute neutrophil count (ANC) ≥0.5 × 109 /L for three consecutive days and platelet recovery as achieving platelets ≥20 × 109 /L, unsupported for 7 days. Death from any cause was considered an event.
Statistical Methods
The characteristics of patients, their disease and transplantation grouped according to CBB practice were compared using the chi-square test for categorical variables.18 The incidence of neutrophil and platelet recovery was calculated using the cumulative incidence estimator; death without an event was considered a competing risk.19 The day- 100 probability of overall survival was calculated using the Kaplan-Meier estimator.18 Generalized linear mixed models were used to fit random effect logistic regression models for day-28, day-42 neutrophil and day-90 platelet recovery and, day-100 overall survival. These models included random effects for cord blood bank and for transplant center in order to account for within bank or within center correlation when examining CBB specific variables or transplant center specific variables.20 The following variables were tested in a multivariate analysis. CBB practice groups (1, 2 and 3) and its effect on outcomes was the primary interest. Consequently, the variable for CBB practice group was held in all steps of model building regardless of the level of significance attained. Other CBB variables tested included: vapor vs. liquid storage, year of collection (1996 – 1999 vs. 2000 – 2005 vs. 2006 – 2011), and length of existence of the CBB (≤10 vs. 11 – 15 vs. >15 years). Transplant center practice was tested as follows: no DMSO dilution/no wash vs. reconstitution in dextran/plasmalyte A and, interval between thaw and completion of infusion (<2 vs. 2 – 4 vs. >4 hours). In addition to CBB practice and transplant center practice, patient (age, gender, performance score, CMV serostatus, race), disease and disease status at transplantation, and transplant characteristics (conditioning regimen, in vivo T-cell depletion, GVHD prophylaxis, HLA-match [6/6 vs. 5/6 vs. 4/6], total nucleated cell dose [<3 vs. 3 – 5 vs. >5 × 107/kg], donor-recipient race match [donor/recipient same race vs. other], interval between unit collection and transplantation [<1 vs. 1 – 3 vs. >3 – 5 vs. > 5 years], and transplant period [2000–2004 vs. 2005–2011]) were tested in all multivariate models such that the effects of CBB and transplant center practice were adjusted for known clinical characteristics associated with outcome. P-value of 0.05 or less was considered significant; all p-values were two-sided. Analyses were performed with SAS 9.3 (Cary, NC).
RESULTS
Patient, Disease, and Transplant Characteristics
Table 2 shows the characteristics of the 530 patients grouped by processing methods at the CBBs. Overall, the characteristics of patients, their disease and transplantation were similar across the three groups except for patient race, TNC, thaw procedures at transplant centers, transplant-conditioning regimen, in vivo T-cell depletion, planned growth factor treatment, and transplantation period. Caucasians were less likely to have received units that were manually processed and plasma reduced. The manually processed units were more likely to contain pre-freeze TNC greater than 5 × 107/kg but there were no differences in post-thaw TNC recovery between the three groups. During the thawing process, the automated processed units, which account for the more recent transplantations were less likely to have been washed and reconstituted in dextran. Total body irradiation transplant conditioning regimens were more commonly used in patients who received units that were processed using an automated technique. On the other hand, in vivo T-cell depletion was common with manually processed units and reflect clinical practice in an earlier period. Planned growth factor therapy was less common for units that were manually processed and plasma reduced.
Table 2.
Patients, their disease and transplantation and transplant center practices
| Variable |
Group 1 Automated processing plasma and red cell reduced or buffy coat production method |
Group 2 Manual processing plasma and red cell reduced or buffy coat production method |
Group3 Manual processing plasma reduced |
P-value |
|---|---|---|---|---|
| Number of patients | 118 | 279 | 133 | |
| Age | 0.47 | |||
| ≤16 years | 89 (75) | 225 (81) | 107 (80) | |
| >16 years | 29 (25) | 54 (19) | 26 (20) | |
| Sex | 0.76 | |||
| Male | 55 (47) | 141 (51) | 67 (50) | |
| Female | 63 (53) | 138 (46) | 66 (50) | |
| Performance score | 0.21 | |||
| <90 | 20 (17) | 51 (18) | 33 (25) | |
| 90 – 100 | 97 (82) | 218 (78) | 97 (73) | |
| Not reported | 1 (<1) | 10 (4) | 3 (2) | |
| Recipient CMV serostatus | 0.06 | |||
| Negative | 46 (39) | 137 (49) | 53 (40) | |
| Positive | 71 (60) | 137 (49) | 79 (59) | |
| Not reported | 1 (<1) | 5 (1) | 1 (1) | |
| Recipient race | <0.001 | |||
| Caucasian | 61 (52) | 181 (65) | 55 (41) | |
| Non-Caucasian | 57 (48) | 92 (33) | 77 (58) | |
| Not reported | 0 | 6 (2) | 1 (<1) | |
| Disease status at transplant | 0.02 | |||
| CR1/RA | 52 (44) | 92 (33) | 53 (40) | |
| CR2 | 53 (45) | 127 (46) | 48 (36) | |
| Relapse/RAEB | 13 (11) | 60 (21) | 32 (24) | |
| Conditioning regimen | 0.04 | |||
| TBI-containing | 96 (81) | 193 (69) | 100 (75) | |
| Non-TBI containing | 22 (19) | 86 (31) | 33 (25) | |
| GVHD prophylaxis | 0.26 | |||
| Tacrolimus-containing | 33 (28) | 77 (28) | 47 (35) | |
| Cyclosporine-containing | 83 (70) | 199 (71) | 85 (64) | |
| Other | 2 (2) | 3 (1) | 1 (1) | |
| HLA match low resolution at A, B, and allele-level DRB1 | 0.42 | |||
| 4/6 | 42 (36) | 88 (32) | 55 (41) | |
| 5/6 | 57 (48) | 141 (51) | 58 (44) | |
| 6/6 | 19 (16) | 50 (17) | 20 (15) | |
| Donor recipient race match | 0.02 | |||
| Donor recipient same race | 71 (60) | 150 (54) | 70 (53) | |
| Donor recipient different race | 47 (40) | 72 (26) | 62 (47) | |
| Unknown | 0 | 57 (20) | 1 (1) | |
| In vivo T depletion | <0.001 | |||
| No | 76 (64) | 116 (42) | 58 (44) | |
| Yes | 42 (36) | 163 (58) | 75 (56) | |
| Total nucleated cells (107/kg) | ||||
| ≤3.0 | 11 (9) | 32 (11) | 17 (13) | 0.01 |
| 3.0 – 5.0 | 44 (37) | 74 (27) | 24 (18) | |
| > 5.0 | 61 (52) | 172 (62) | 92 (69) | |
| Not reported | 2 (2) | 1 (<1) | 0 | |
| Post-thaw total nucleated cells recovery | 0.77 | |||
| <70% | 21 (18) | 56 (20) | 27 (20) | |
| 70% – 90% | 53 (45) | 120 (43) | 62 (47) | |
| >90% | 23 (19) | 67 (24) | 28 (21) | |
| Not reported | 21 (18) | 36 (13) | 16 (12) | |
| Thawing method at transplant center | <0.001 | |||
| No Wash/DMSO dilution | 44 (37) | 42 (15) | 24 (18) | |
| Washed, reconstituted in dextran/hespan/plasmalyte | 71 (60) | 219 (78) | 95 (71) | |
| Not reported | 3 (3) | 17 (7) | 14 (11) | |
| Interval between thaw to completing of infusion | 0.62 | |||
| <2 h | 38 (32) | 91 (33) | 45 (34) | |
| 2 – 4 h | 56 (47) | 127 (46) | 56 (42) | |
| >4 h | 11 (9) | 32 (11) | 22 (17) | |
| Not reported | 13 (11) | 29 (10) | 10 (8) | |
| Interval from unit collection to transplant | <0.001 | |||
| ≤1 y | 27 (23) | 22 (8) | 21 (16) | |
| 1–3 y | 64 (54) | 97 (35) | 48 (36) | |
| 3–5 y | 19 (16) | 76 (27) | 33 (25) | |
| >5 y | 8 (7) | 84 (30) | 31 (23) | |
| Planned growth factor therapy | 0.02 | |||
| None | 34 (29) | 87 (31) | 60 (45) | |
| Yes | 68 (58) | 152 (54) | 52 (39) | |
| Not reported | 16 (14) | 40 (15) | 21 (16) | |
| Year of transplant | <0.001 | |||
| 2000–2004 | 6 (5) | 57 (20) | 21 (16) | |
| 2005–2011 | 112 (95) | 222 (80) | 112 (84) |
Neutrophil and Platelet Recovery
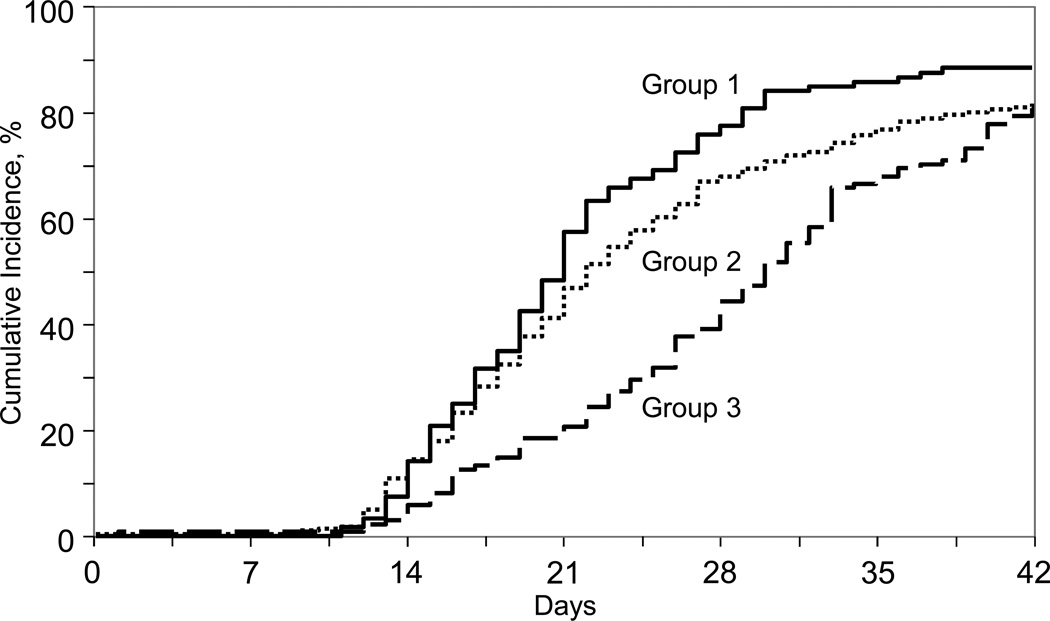
The primary endpoint of the study was hematopoietic recovery: day-28 neutrophil recovery and day-90 platelet recovery (Table 3). The likelihood of neutrophil recovery was lower after transplantation of UCB units that were processed manually and plasma reduced (red blood cell replete) or plasma and red cell depleted (Table 3; Figure 1). Further, neutrophil recovery was lower after transplantation of manually processed units with plasma reduction compared to plasma and red cell reduction (OR 0.34, 95% CI 0.16 – 0.74, p=0.04). The day-28 cumulative incidence of neutrophil recovery was 40% (95% CI 32 – 48) after transplantation of UCB units that were processed manually and plasma reduced; median time to recovery was 29 days. Corresponding probabilities for units that were processed using automated and manual methods with plasma and red blood cell reduction or buffy coat production method were 77% (95% CI 69–84) and 68% (95% CI 63–73), respectively; median time to neutrophil recovery was 20 and 21 days, respectively. Independent of processing methods, the likelihood of neutrophil recovery was higher after transplantation of units with TNC greater than 5 × 107/kg compared to units with TNC <3 × 107/kg and older patients (Table 3). Despite early differences in recovery rates, by 6 weeks after transplantation, there were no appreciable differences between the three groups (Table 3).
Table 3.
Risk factors associated with neutrophil recovery at day-28 and day-42 platelet recovery at day-90
| N1/N2 | Odds Ratio | p-value | |
|---|---|---|---|
| Neutrophil recovery at day-28 | |||
| Banking practice* | |||
| Group 1 | 92 /116 | 1.00 | |
| Group 2 | 188/270 | 0.54 (0.29 – 1.00) | 0.05 |
| Group 3 | 60/132 | 0.19 (0.08 – 0.44) | <0.001 |
| Age | |||
| ≤16 years | 269/412 | 1.00 | |
| >16 years | 71/106 | 2.01 (1.11 – 3.67) | 0.02 |
| Total nucleated cell dose | |||
| ≤ 3 × 107/kg | 31/58 | 1.00 | |
| > 3 – 5 | 89/140 | 1.65 (0.82 – 3.31) | 0.16 |
| > 5 | 220/320 | 3.26 (1.57 – 6.77) | 0.002 |
| Neutrophil recovery at day-42 | |||
| Group 1 | 104 /116 | 1.00 | |
| Group 2 | 225/270 | 0.61 (0.31 – 1.21) | 0.16 |
| Group 3 | 108/132 | 0.50 (0.23 – 1.06) | 0.07 |
| Total nucleated cell dose | |||
| ≤ 3 × 107/kg | 40/58 | 1.00 | |
| > 3 – 5 | 117/140 | 2.11 (1.03 – 4.33) | 0.04 |
| > 5 | 280/320 | 3.74 (1.90 – 7.35) | <0.001 |
| Conditioning regimen | |||
| TBI + cyclophosphamide | 327/379 | 1.00 | |
| Busulfan + cyclophosphamide | 110/139 | 0.52 (0.30 – 0.88) | 0.02 |
| Platelet recovery at day-90 | |||
| Group 1 | 76/116 | 1.00 | |
| Group 2 | 171/270 | 0.98 (0.61 – 1.58) | 0.93 |
| Group 3 | 69/132 | 0.61 (0.36 – 1.05) | 0.08 |
| Gender | |||
| Female | 145/260 | 1.00 | |
| Male | 171/258 | 1.58 (1.09 – 2.28) | 0.02 |
| Total nucleated cell dose | |||
| ≤ 3 × 107/kg | 23/58 | 1.00 | |
| > 3 – 5 | 88/140 | 2.43 (1.27 – 4.66) | 0.008 |
| > 5 | 205/320 | 2.64 (1.46 – 4.78) | 0.002 |
| Disease status | |||
| Early | 128/193 | 1.00 | |
| Intermediate | 141/222 | 0.82 (0.54 – 1.25) | 0.36 |
| Advanced | 47/103 | 0.45 (0.27 – 0.75) | 0.002 |
Odds ratios >1 indicate better outcome
N1 = number of events; N2 = number evaluable
TBI = total body irradiation
Other comparisons:
Day-28 neutrophil recovery:
Group 3 vs. Group 2, OR= 0.34 (0.16 – 0.74), p-value=0.04
Day-42 neutrophil recovery:
Group 3 vs. Group 2, OR=0.81 (0.47– 1.42), p-value= 0.46
Group 1 : automated processing, units were plasma and red blood cell reduced or buffy coat production method
Group 2 : manual processing, units were plasma and red blood cell reduced or buffy coat production method Group 3 : manual processing, units were plasma reduced
Early risk = patients in first complete remission / refractory anemia
Intermediate risk = patients in second complete remission
Advanced = patients in relapse / refractory anemia with excess blasts
Figure 1.
Neutrophil Recovery
Group 1: automated processing, units were plasma and red blood cell reduced or buffy coat production method
Group 2: manual processing, units were plasma and red blood cell reduced or buffy coat production method
Group 3: manual processing, units were plasma reduced
There was no difference in platelet recovery at day-90 among the three groups, (Table 3). The day-90 probabilities of platelet recovery were 65% (95% CI 57 – 74), 64% (95% CI 58 – 69) and 53% (95% CI 44 – 61) for Groups 1, 2 and 3 respectively. The median time to platelet recovery was 46, 48 and 61 days, respectively. Additionally, platelet recovery was better after transplantation of units with TNC greater than 5 × 107/kg compared to units with TNC 3 × 107/kg or lower and in males compared to females. Platelet recovery was lower in patients transplanted with active disease compared to those transplanted in first remission. The effect of TNC and disease status on platelet recovery was independent of CBB processing methods.
Of note, we carefully examined for an effect of duration of storage UCB units and its effect on hematopoietic recovery. Neutrophil recovery at day-28 and day-42 were not associated with duration of storage (p=0.87 and p=0.51, respectively). Similarly, platelet recovery at day-90 was also not associated with duration of storage of UCB units (p=0.89).
Overall Survival
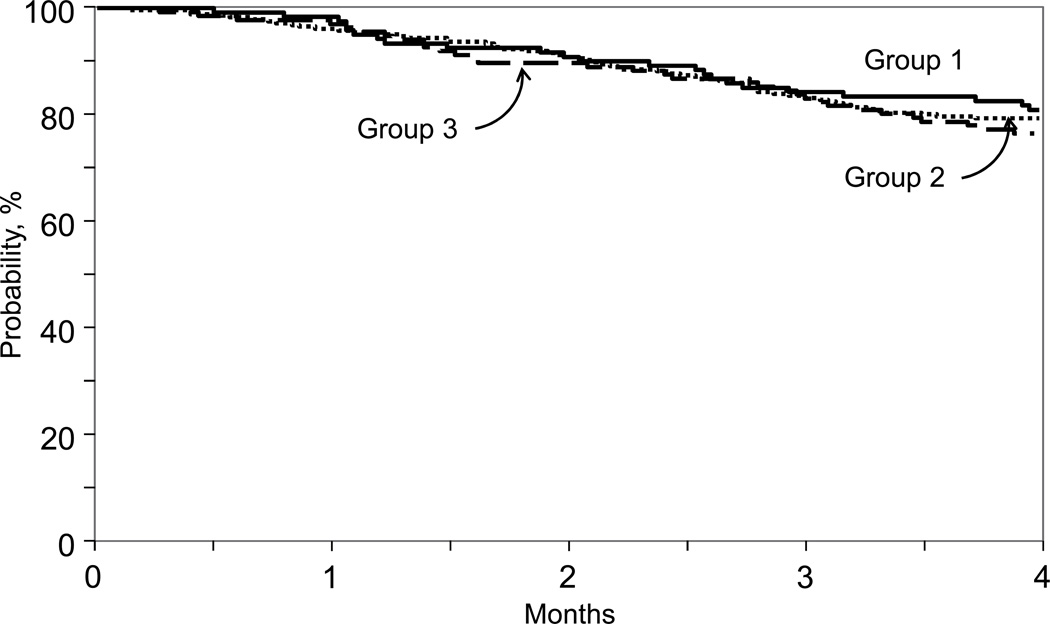
Despite a lower threshold of early neutrophil recovery after transplantation of manually processed units, there were no differences among the groups with respect to overall survival at day-100 (Table 4, Figure 2). The day-100 survival probabilities for Groups 1, 2 and 3 were 83% (95% CI 76–89), 82% (95% CI 74–88) and 80% (95% CI 73 – 87), respectively. However, there were other factors associated with early survival that were independent of CBB processing methods. Survival was higher for males, for transplantations in first complete remission/refractory anemia, and after 2004. Independent of CBB practice, thawing practice at transplant centers also influenced early survival; survival was higher after transplantation of units that were thawed, washed and reconstituted in dextran compared to units that were not washed or underwent DMSO dilution only (Table 4). We did not observe differences in early survival after transplantation of units that were not washed (n= 40; 78% [95% CI 63–89]) and DMSO dilution only (n=70; 77% [95% CI 67–86]).
Table 4.
Risk factors associated with overall survival at day-100
| N1/N2 | Odds Ratio | p-value | |
|---|---|---|---|
| Banking practice | |||
| Group 1 | 96/116 | 1.00 | |
| Group 2 | 218/270 | 0.94 (0.51 – 1.73) | 0.85 |
| Group 3 | 106/132 | 0.93 (0.47 – 1.84) | 0.84 |
| Gender | |||
| Female | 199/260 | 1.00 | |
| Male | 221/258 | 1.84 (1.16 – 2.91) | 0.01 |
| Disease status | |||
| Early | 165/193 | 1.00 | |
| Intermediate | 183/222 | 0.82 (0.48 – 1.40) | 0.47 |
| Advanced | 72/103 | 0.44 (0.24 – 0.80) | 0.007 |
| Unit processing at transplant center | |||
| No wash / DMSO dilution | 84/108 | 1.00 | |
| Thaw/wash + dextran reconstitution | 314/376 | 1.82 (1.02 – 3.23) | 0.04 |
| Transplant period | |||
| 2000–2004 | 61/83 | ||
| 2005 – 2011 | 359/435 | 2.06 (1.14 – 3.72) | 0.02 |
Odds ratios >1 indicate better outcome
N1 = number alive; N2 = number evaluable
Group 1 : automated processing, units were plasma and red blood cell reduced or buffy coat production method
Group 2 : manual processing, units were plasma and red blood cell reduced or buffy coat production method
Group 3 : manual processing, units were plasma reduced
Early risk = patients in first complete remission / refractory anemia
Intermediate risk = patients in second complete remission
Advanced = patients in relapse / refractory anemia with excess blasts
Figure 2.
Overall Survival
Group 1: automated processing, units were plasma and red blood cell reduced or buffy coat production method
Group 2: manual processing, units were plasma and red blood cell reduced or buffy coat production method
Group 3: manual processing, units were plasma reduced
Toxicities associated with infusion
There were no life-threatening or serious or suspected serious adverse events as defined by 21CFR 312.32, reported for any of the transplantations in the current analysis. One of the infused units was infected as defined by a positive product culture (organism not reported). This patient achieved neutrophil and platelet recovery and is alive, two years after transplantation.
DISCUSSION
In this study, the first of its kind, we explored whether differences exist in hematopoietic recovery and early survival by processing methods at the CBBs. The current analysis was prompted by the concern that variations in processing and banking have never been fully studied and that these practice variations might impact the early outcomes of UCB transplantation. In a carefully controlled analysis and adjusting for known patient, disease and transplant characteristics, we identified two banking practices with adverse effects on early neutrophil recovery (i.e., at day-28). Neutrophil recovery was lower after transplantation of manually processed UCB units that were plasma reduced (red blood cell replete) or plasma and red cell depleted compared to automated methods of processing with plasma and red cell depletion. Automated processing is relatively recent, and only 118 UCB units were processed using automated techniques in this study.21 Further, among the manually processed units, neutrophil recovery was less likely with plasma reduction. Lower rates of neutrophil recovery within four weeks after transplantation did not translate into higher early overall mortality implying advances in supportive care during the early neutropenic period in part negated its adverse effects on early survival. Additionally, thawing methods were associated with early survival. Survival was better when units were washed and reconstituted in dextran, hespan or plasmalyte implying thawing techniques at transplant centers may influence early survival. The observed adverse effect of thawing techniques at transplant centers was independent of unit processing and banking at CBBs nor was it associated with hematopoietic recovery. The results of the bedside thaw approach in 26 transplant recipients and that from the thaw and dilute approach in 54 patients from single institutions demonstrated these approaches were safe and, with sustained hematopoietic recovery.14,15 To our knowledge the current analysis is the first to have compared the three approaches to UCB thaw. It is worth noting that we do not have detailed information on handling and processing of UCB units at the transplant centers or information as to whether the transplant centers followed the recommendations for thawing by the CBB all of which may have an effect on survival. With the data available it is not possible to recommend one approach over another other than recommend UCB unit thaw that either adhere to the recommendations from the CBB or adopt techniques that have been validated by the institution’s Cell Processing laboratory. We did not identifiy differences in hematopoietic recovery or overall survival based on the years of banking experience, year of collection of UCB unit, storage condition (vapor vs liquid phase), and the length of time the UCB unit was stored at the CBB.
Our findings differ from that reported by others on the effects of transplantation of UCB units that were plasma reduced.11 The report by Chow and colleagues11 demonstrated neutrophil recovery rates higher than reported in the current analysis. An important difference between that analysis and the current analysis is that in the former, neutrophil recovery rates were calculated with the Kaplan-Meier method whereas the current analysis used the cumulative incidence estimator, which considers the competing risk for the event of interest, i.e., death without recovery.
As expected from other studies in the literature, day-100 survival was improved for patients transplanted in first complete remision and for those patients transplanted after 2004.22,23 Although higher performance status, younger age, and higher cell dose were associated with improved survival in other larger studies on single UCB transplantation, these factors did not impact survival in this current study and likley attributed to that fact that we explored factors associated with survival within 3 months after transplantation.24 Only plausible explanations include the fact that the current analysis is limited to is primarily a pediatric cohort with about 80% of pateints aged ≤16 years and that their performance scores were 90 or 100. Similarly, almost 90% of patients received UCB units with TNC dose in excess of 3 × 107/kg.
The current analysis has several limitations. First, our analysis is limited to approximately 500 and largely pediatric recipients of single unit UCBT and comprise approximately 10% of UCB units distributed by the participating CBBs. In order to allows us to carefully examine for CBB processing and banking, we limited the cohort to a relatively homogenous group of patients who received a single UCB unit whch explains the relatively small numbers of transplant recipients in the current analysis; we arbitrarily assigned CBB practices into four broad groups. The second, while we adjusted for known prognostic factors there may be several unknown and unmeasured practices both at CBBs and transplant centers that may have influenced outcome. Third, we were not able to study storage temperature at CBBs,25 cell viability or the Cord Blood Apgar score.26 Fourth, CBBs were surveyed for their banking practice with reliance on self reporting. All other data were collected on standardized CIBMTR reporting Forms with appropriate Data Management Manuals and subject to audit.
Despite the limitations, our observations have clinical relevance. Banking practices are not associated with early survival but manual methods of UCB unit processing lower the odds of neutrophil recovery during the very early post-transplantation period. Although only about of 40% of CBBs were using automated processing methods over a year ago, it is possible several CBBs have switched to the automated methods. These data support favoring selecting an adeqautely dosed and HLA-matched or HLA-mismatched UCB unit that was processed using automated methods when such a unit is available.
Highlights.
Banking procedures at the Cord Blood Banks are associated with lower likelihoods of neutrophil recovery but was not associated with early mortality
Acknowledgement
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; *Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement: The authors do not have any conflicts to disclose.
REFERENCES
- 1.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends access to allogeneic hematopoietic stem cell transplant to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16:1541–1548. doi: 10.1016/j.bbmt.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen YB, Aldridge J, Kim HT, et al. Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. Biol Blood Marrow Transplant. 2012;18:805–812. doi: 10.1016/j.bbmt.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Brunstein CG, Barker JN, Wesidorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor hematopoietic stem-cell transplantation in adults with acute leukemia: a retrospective analysis. Lancet Oncology. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasky LC, Lane T, Miller J, et al. In utero or ex utero cord blood collection: which is better? Transfusion. 2002;42:1261–1267. doi: 10.1046/j.1537-2995.2002.t01-1-00177.x. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman E, Broxmeyer HE, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi anemia by means of umbilical cord blood from a HLA-identical sibling. N Engl J Med. 1989;321(17):1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 8.Broxmeyer HE, Gordon GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86.10:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstiution. Proc Natl Acad Sci USA. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow R, Nademanee M, Rosenthal J, et al. Analysis of hematopoietic cell transplants using plasma-depleted cord blood products that are not red blood cell reduced. Biology Blood and Marrow Transplant. 2007;13:1346–1357. doi: 10.1016/j.bbmt.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Chow R, Lin A, Tonai R, et al. Cell recovery comparison between plasma depletion/reduction- and red cell reduction-processing of umbilical cord blood. Cytotherapy. 2011;13.9:1105–1119. doi: 10.3109/14653249.2011.592524. [DOI] [PubMed] [Google Scholar]
- 12.Laroche V, McKenna DH, Moroff G, et al. Cell loss and recovery in umbilical cord blood processing: a comparison of postthaw and postwash samples. Transfusion. 2005;45:1909–1916. doi: 10.1111/j.1537-2995.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- 13.Zinno F, Landi F, Aureli V, et al. Pre-transplant manipulation processing of umbilical cord blood units: Efficacy of Rubinstein's thawing technique used in 40 transplantation procedures. Transfus Apher Sci. 2010;43(2):173–178. doi: 10.1016/j.transci.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Barker JN, Abboud M, Rice RD, et al. A "No-wash" albumin-dextran dilution strategy for cord blood unit thaw: high rate of engraftment and a low incidence of serious infusion reactions. Biol Blood Marrow Transplant. 2009;15:1596–1602. doi: 10.1016/j.bbmt.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn T, Bunworasate U, George MC, et al. Use of no volume-reduced (unmanipulated after thawing) umbilical cord blood stem cells for allogeneic transplantation results in safe engraftment. Bone Marrow Transplant. 2003;32(2):145–150. doi: 10.1038/sj.bmt.1704091. [DOI] [PubMed] [Google Scholar]
- 16.Choi S, Hoffmann S, Cooley L. Another case of acute cardiopulmonary toxicity with cord blood infusion: is dextran the culprit? Transfusion. 2012;52(1):207–208. doi: 10.1111/j.1537-2995.2011.03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacigalupo A, Ballen KK, Rizzo D, et al. Defining the intensity of the conditioning regimen: Working Definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein JP, Moeschberger ML. Survival Analysis: Statistical Methods for Censored and Truncated Data. 2nd ed. New York, NY: Springer-Verlag; 2003. [Google Scholar]
- 19.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risk: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Wolfinger R, O’Connell M. Generalized linear mixed models: A pseudo-likelihood approach. Journal of Statistical Computation and Simulation. 1993;48:233–243. [Google Scholar]
- 21.Lapierre V, Pellegrini N, Bradley I, et al. Cord blood volume reduction using an automated system (Sepax) versus a semi-automated system (Optipress II) and a manual method (hydroxyethyl starch sedimentation) for routine cord blood banking: a comparative study. Cytotherapy. 2007;9:165–169. doi: 10.1080/14653240701196811. [DOI] [PubMed] [Google Scholar]
- 22.Ballen KK, Gluckman E, Broxmeyer H. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122(4):491–498. doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peffault de Latour R, Brunstein CG, Porcher R, et al. Similar overall survival using sibling, unrelated donor and cord blood grafts after reduced-intensity conditioning for older patients with acute myeloid leukemia. Biol Blood Marrow Transplant. 2013;19(9):1355–1360. doi: 10.1016/j.bbmt.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Ballen KK, Koreth J, Chen YB, et al. Selection of optimal alternative graft source: mismatched unrelated donor, umbilical cord blood, or haploidentical transplant. Blood. 2012;119:1972–1980. doi: 10.1182/blood-2011-11-354563. [DOI] [PubMed] [Google Scholar]
- 25.Louis I, Wagner E, Dieng MM, et al. Impact of storage temperature and processing delays on cord blood quality: discrepancy between functional in vitro and in vivo assays. Transfusion. 2012;52:2401–2405. doi: 10.1111/j.1537-2995.2012.03650.x. [DOI] [PubMed] [Google Scholar]
- 26.Page KM, Zhang L, Mendizabal A, et al. The Cord Blood Apgar: a novel scoring system to optimize selection of banked cord blood grafts for transplantation. Transfusion. 2012;52:272–283. doi: 10.1111/j.1537-2995.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]