Abstract
Reduced catechol-O-methyltransferase (COMT) activity resulting from genetic variation or pharmacological depletion results in enhanced pain perception in humans and nociceptive behaviors in animals. Using phasic mechanical and thermal reflex tests (e.g. von Frey, Hargreaves), recent studies show that acute COMT-dependent pain in rats is mediated by β-adrenergic receptors (βARs). In order to more closely mimic the characteristics of human chronic pain conditions associated with prolonged reductions in COMT, the present study sought to determine volitional pain-related and anxiety-like behavioral responses following sustained as well as acute COMT inhibition using an operant 10–45°C thermal place preference task and a light/dark preference test. In addition, we sought to evaluate the effects of sustained COMT inhibition on generalized body pain by measuring tactile sensory thresholds of the abdominal region. Results demonstrated that acute and sustained administration of the COMT inhibitor OR486 increased pain behavior in response to thermal heat. Further, sustained administration of OR486 increased anxiety behavior in response to bright light, as well as abdominal mechanosensation. Finally, all pain-related behaviors were blocked by the non-selective βAR antagonist propranolol. Collectively, these findings provide the first evidence that stimulation of ARs following acute or chronic COMT inhibition drives cognitive-affective behaviors associated with heightened pain that affects multiple body sites.
Keywords: COMT, catecholamines, hyperalgesia, allodynia, anxiety, thermal place preference, operant
Introduction
Catechol-O-methyltransferase (COMT) is an enzyme that inactivates catecholamines and plays a key role in a subset of common chronic pain conditions. Variants of the COMT gene that result in reduced activity of the corresponding enzyme are associated with experimental pain as well as risk of temporomandibular disorder, irritable bowel syndrome, fibromyalgia and low back pain (Diatchenko et al., 2005, Karling et al., 2011, Jacobsen et al., 2012, Martinez-Jauand et al., 2013). These functional genetic variants are also associated with anxiety, depression, and other psychological traits that influence the perception of pain (Fernandez-de-Las-Penas et al., 2012). In line with the results of human association studies, COMT-deficient animals exhibit increased pain and anxiety. Mice with system-wide deletion of COMT exhibit exaggerated behavioral responses evoked by thermal heat stimuli as well as by stressful acoustic stimuli (Papaleo et al., 2008, Walsh et al., 2010). Similarly, rats receiving an acute dose of the COMT inhibitor OR486 exhibit exaggerated responses evoked by thermal heat and mechanical stimuli alongside increased circulating levels of pro-inflammatory cytokines and nitric oxide (NO), which are molecules known to sensitize nociceptors (Brenman and Bredt, 1997, Millan, 1999, Kress, 2004, Nackley et al., 2007, Kambur et al., 2010, Hartung et al., 2014). Acute COMT-dependent evoked pain as well as increased levels of cytokines and NO are mediated by stimulation of β-adrenergic receptors (βARs) (Nackley et al., 2007, Hartung et al., 2014). These findings implicate βARs in the pathophysiology of pain conditions associated with reduced COMT activity. Before these findings can be extrapolated to individuals suffering from chronic pain, however, additional work in a more clinically-relevant animal model is required.
Many existing animal models of chronic pain initiate pain through application of inflammatory agents, chemical irritants, or tissue injury and then measure evoked responses to standard experimental stimuli applied to the hindpaw. The initiation of pain in these models is relevant to conditions such as arthritis, visceral pain, and neuropathic pain, but not to conditions such as temporomandibular disorder and fibromyalgia. In order to more closely mimic the etiology of human chronic pain conditions associated with prolonged reductions in COMT activity, sustained systemic inhibition of COMT is required. Also, while chronic pain conditions often result in increased evoked responses to standard experimental stimuli, assessments of these behaviors do not capture the ongoing and perhaps more clinically-relevant nature of the pain (Wall et al., 2006, Vierck et al., 2008b). Paw withdrawal reflexes to phasic sensations are subserved through spinal and spinal-bulbo-spinal loops, while perception of pain requires a cerebral cortex (Coghill et al., 1999, Vierck et al., 2002). Operant algesia assays require decision making behavior in order to terminate a perceived nociceptive stimulus and have been successfully translated from primates to rodents for hindpaw and visceral testing (e.g. operant escape, thermal place preference and conditioned place preference) (Mauderli et al., 2000, Neubert et al., 2005, Deyama et al., 2009). A tremendous advantage of operant algesia testing is that the results better parallel those obtained in humans, providing a valid indicator of clinical nociceptive sensitivity (Yezierski et al., 2010).
Therefore, the present study sought to determine volitional pain and anxiety-related behavioral responses following sustained as well as acute COMT inhibition using an operant 10–45°C thermal place preference task and a light/dark preference test. We hypothesized that animals receiving the COMT inhibitor OR486 would exhibit hypersensitivity to the noxious 45°C surface and to bright light in a βAR-dependent manner. Further, we sought to evaluate the effects of sustained COMT inhibition on abdominal tactile sensory thresholds, hypothesizing that animals receiving OR486 would exhibit evoked hypersensitivity at a remote body site in a βAR-dependent manner.
As predicted, acute and sustained COMT inhibition produced consciously-motivated reductions in occupancy for the chamber with a floor heated to 45°C. Sustained COMT inhibition also produced enhanced aversion to bright light and abdominal tactile sensitivity. All pain and anxiety-related behaviors were blocked by the non-selective βAR antagonist propranolol. Together, these results demonstrate that sustained administration of the COMT inhibitor OR486 leads to changes in sensory and affective nociceptive responses and induces widespread cutaneous hypersensitivity.
Experimental Procedures
Animals and General Protocol
A total of 34 adult male Sprague Dawley rats weighing (325–425g), (Harlan Industries, Indianapolis IN) were housed two per shoebox cage with a reverse 12 h light/dark cycle and ad libitum access to food and water. Thermal and light/dark preference was evaluated in 16 animals receiving intraperitoneal (i.p.) injections of either propranolol (3 mg/kg) or saline 30 minutes prior to OR486 (30 mg/kg) or saline. Thermal and light/dark preference as well as abdominal sensitivity was evaluated in a separate set of 18 animals implanted with osmotic minipumps that delivered either propranolol (9 mg/kg/day) or saline alongside OR486 (15 mg/kg/day) or saline. All procedures were approved by the University of Kentucky Institutional Use and Animal Care Committee and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
The COMT inhibitor OR486 was selected based on its 1) ability to cross the blood-brain barrier (Nissinen et al., 1988, Kambur et al., 2010), 2) good absorption and long duration of action (Nissinen et al., 1988), and 3) previously reported pro-nociceptive effects in rodent models (Diatchenko et al., 2005, Nackley et al., 2007, Kambur et al., 2010, Hartung et al., 2014). OR486 (R&D Systems, Minneapolis, MN) was dissolved in a vehicle consisting of a 5:2:3 ratio of dimethylsulfoxide (DMSO), ethanol and 0.9% saline. As acute i.p. or sustained 14 day systemic delivery of this vehicle to rats has no effect on evoked mechanical/thermal sensitivity or non-evoked measures such as locomotor activity (Nackley et al., 2007, Ciszek and Nackley, 2012, Hartung et al., 2014), control groups received 0.9% saline. The general β-adrenergic antagonist propranolol (Sigma, St. Louis MO) was dissolved in 0.9% saline.
Surgery
Rats were anesthetized by isoflurane inhalation and surgical site prepared for aseptic implantation of two osmotic minipumps with a 0.5μl/hr infusion rate with a reservoir volume of 200μl (model 2002, Durect Corp, Cupertino, CA) slightly posterior and between the scapulae. Three groups of six animals received the following two-pump combinations; saline/saline, saline/OR486, and propranolol/OR486. The incisions (approximately 1cm) were closed with sutures and removed after 7 days.
Behavioral Testing
Rats were thoroughly adapted to handling and to the experimental test chambers before thermal and mechanical stimuli were introduced into the training regimen. Testing was performed in a dimly lit room with continuous white noise background following 30–60 minutes of acclimation to the testing room. Animals were trained to asymptotic performance on thermal place preference during the first week prior to acute or chronic OR486 administration. For the acute study, rats received an i.p. injection of propranolol or vehicle 30 min prior to the COMT inhibitor OR486 or vehicle (i.p). Previous work has demonstrated that OR486 induces up to 2.25 hours of hypersensitivity beginning 30 minutes after injection (Nackley et al., 2007). This rapid onset and prolonged duration of hypersensitivity allowed for the consecutive assessment of light dark and thermal place preference 45 and 60 min after OR486 or vehicle injection, respectively in the same cohort. Similarly, in the chronic study, light/dark preference immediately preceded thermal place preference testing and was observed once weekly for two weeks. Experimenters were blind to all experimental conditions.
Thermal Place Preference
Thermal place preference testing is an operant assay that requires learned apportioning of hot or cold stimulation to determine relative thermal sensitivity. Rats resolve this conflict by alternating occupancy between the two compartments, with time on the cold plate positively related to the increasingly aversive intensity of the heat stimulus. The test apparatus consisted of a 27x27cm clear Plexiglas box divided by a partition into two equal halves. A 2.5″ x 2.5″ opening in the center of the partition permitted free movement between sides. The floor of each side was a separate aluminum thermal plate which allowed independent temperature control. The position of the rats was monitored in real time for 10 minutes by continuous observation using a custom-designed computer program which captured the number of crossings and amount of time spent on each side (Vierck et al., 2002). It was determined during initial training that if the floor on one side was 10°C and the other side was at 45°C rats spent about equal amounts of time on either side. Occupancy of the heated plate is known to be noxious since low dose morphine increases occupancy for the 45°C surface (Vierck et al., 2002). Dependent measures were: 1) cumulative heat – cold occupancy, 2) total amount of rearing behavior and 3) the initial hot-to-cold transition latency. Surface temperature of the floor plates was monitored with an electronic thermometer (model 4000, YSI Inc., Yellow Springs, OH). Cumulative thermal preference was plotted, response by response, as difference scores (45°C - 10°C plate durations for the first occupancy, then 45°C - 10°C plate durations for the second occupancy etc.). The cumulative response format provides advantages by emphasizing the 1) effective illustration of continuous decision based behavior between two sensory stimuli and 2) that the assay provides and displays responses to repetitive thermal stimuli which indicates pain tolerance rather than a sensory threshold. These temporally summative response formats are similar to the pain-score formats of clinical studies, which also applied continuously repetitive stimuli, necessary to evaluate the temporal modification of tonic stimuli (Vierck et al., 2001). This conventional method of display has been consistently reported for both operant escape and thermal place preference tests (Vierck et al., 2008a, Yezierski et al., 2010, Vierck et al., 2013, Yezierski et al., 2013). For both acute and chronic studies, thermal place preference testing was preceded immediately by a light/dark preference test.
Light/Dark Preference
The light/dark box test is a widely used method for detection of anxiety-like behaviors based upon the dark preference of animals when paired with a brightly-lit alternative. The test apparatus consisted of a 27x27cm Plexiglas box divided by a partition into two equal halves, one half was opaque (dark) while the other half was transparent. A 2.5″ x 2.5″ opening in the center of the opaque partition permitted free movement between the light and dark chambers. A 12 inch 15 watt fluorescent bulb lamp (725 lumens) was located along the exterior top horizontal edge of the transparent side for illumination. One half of the test chamber lid was transparent while the other half was black, allowing for near complete darkness. Dependent measures were: 1) cumulative dark – light occupancy, 2) total amount of light-side activity (rearing) and 4) the initial dark-to-light transition latency over the 10 minute place preference test (Parent et al., 2012).. Cumulative light/dark preference was plotted, response by response, as difference scores (dark - light chamber durations for the first occupancy, then dark - light chamber durations for the second occupancy etc.). As with thermal place preference, light/dark preference data are presented as a cumulative preference which displays a summative result consequent to multiple trips into and out of the bright light. For the chronic study, light/dark preference testing was always preceded by abdominal mechanical allodynia testing after a 1 hour room acclimation.
Abdominal von Frey
Tactile allodynia was determined using low to high bending force calibrated nylon filaments, measuring the withdrawal frequency (%) of 10 repeated abdominal applications to 3.61, 4.08 and 4.31g respectively. Applications were approximately 1 s in duration with an interstimulus interval of 1–30 s. Tactile allodynia was defined as an increase in the percentage frequency ([No. of abdominal withdrawals/10]X100) of evoked abdominal withdrawal. Positive abdominal withdrawal responses consisted of raising or flexion of the abdomen from a rested position with floor contact. Abdominal skin was shaved with clippers once each week one day prior to testing. Rats were handled and habituated to the test environment (7cm x 4 cm x 4cm clear Plexiglas enclosures on a raised metal mesh platform) during 3 baseline sessions one week prior to osmotic pump implantation. Behavioral responses to mechanical stimuli were assessed at baseline and 14 days after osmotic pump implantation. The intraperitoneal injection route of delivery precluded the assessment of OR486 on abdominal sensitivity.
Statistical Procedures
T-test, One and Two-way ANOVA were used for comparison of 1) thermal place preference hot-to-cold transitions, plate occupancies, transition latencies and exploratory activity (rearing), 2) light/dark box light-to-dark transitions, light occupancy, transition latencies and light side activity (rearing) and 3) frequency of withdrawal to abdominal application of von Frey filaments with a minimum significance level of p<0.05 to reject the null hypothesis using SigmaPlot software (SPSS, Chicago, IL). Tukey’s test was used for pairwise post-hoc comparisons within the ANOVA analysis.
Results
Effects of acute and sustained OR486 on thermal place preference
To determine if acute and chronic COMT inhibition preferentially affect heat or cold sensitivity, we assessed 10°C vs. 45°C thermal place preference responses in the acute cohort one hour after OR486 injection or 7 and 14 days after continuous OR486 infusion in the chronic cohort. Stable baseline performance was noted as none of the occupancies for heat in the last three baseline sessions (48.0+3.4%, 55.6+3.7%, and 44.4+2.1%, respectively) were significantly different from one another by one-way ANOVA (P > 0.05; data not shown). As shown in Figure 1A, animals receiving acute OR486 exhibited a significant increase in preference for the 10°C plate compared to those receiving saline or OR486 with propranolol pre-treatment over the 10 minute test duration (P < 0.001, df = 2, F = 33.12, by Two-way ANOVA). Furthermore, animals receiving propranolol prior to OR486 did not differ from saline controls (P=0.065, Two-way ANOVA). As shown in Figure 1B, animals receiving sustained administration of OR486 exhibited increased preference for the 10°C plate after 7 days compared to those receiving saline (P < 0.001) or OR486 with propranolol pre-treatment (P < 0.005, df = 2, F = 8.23, by Two-way ANOVA). There were no significant differences between treatment groups 14 days after continuous infusion (data not shown). We attribute the differences in the 45°C plate occupancies between the acute and chronic cohorts possibly resulting from the difference in the experimental procedures; the acute cohort underwent repeated i.p. injection while the chronic cohort underwent surgical implantation of osmotic minipumps. Thermal preference is shown as difference scores of the cumulative responses. (heat – cold duration for the first occupancy within each side of the test chamber, plus heat – cold duration for the second occupancy, etc). These observations demonstrate that both acute and chronic COMT inhibition decrease thermal place preference for noxious 45°C heat via βAR signaling at day 7.
Figure 1.
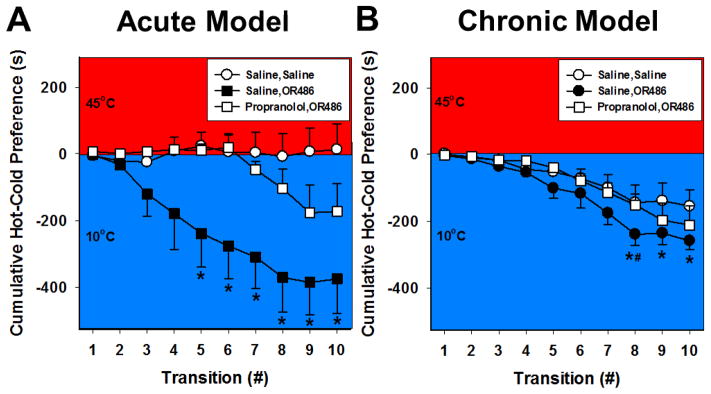
Acute and chronic COMT inhibition produces thermal hyperalgesia in a 10°–45°C thermal place preference task of 10 minutes duration. Administration of (A) a single dose of OR486 (30mg/kg) effective for one hour or (B) continuous delivery of OR486 (15 mg/kg/day) for 7 days, led to increased preference for 10°C. COMT-dependent thermal hyperalgesia was blocked by a single dose of propranolol (3 mg/kg) prior to acute OR486 or by continuous delivery of propranolol (9mg/kg/day) concurrently with sustained OR486. The cumulative plots were constructed by summing the first time of occupancy on the cold plate with the first duration on the hot plate, response by response, in order to generate the group means shown. N= 8–16 per group. *p<.001 compared to Saline, Saline and Propranolol, OR486. #p<0.005 compared to Propranolol, OR486.
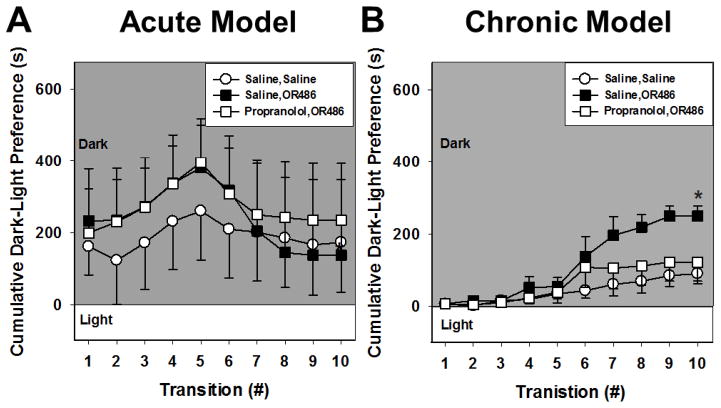
Effects of acute and sustained OR486 on light/dark preference
To determine the effects of acute and chronic COMT inhibition on anxiety-like behavior, we assessed behavior in a light/dark preference test 45 min after OR486 injection in the acute cohort or 7 and 14 days after continuous OR486 infusion in the chronic cohort. As shown in Figure 2A, animals receiving a single dose of OR486 did not exhibit differences in light/dark preference (P = 0.26, by Two-way ANOVA). This insignificant trend continued even after 7 days of continuous OR486 infusion (data not shown). In contrast, animals receiving sustained administration of OR486 for 14 days had increased preference for dark compared to those receiving saline or OR486 with propranolol (P < 0.001, df = 2, F = 12.2, by Two-way ANOVA; Figure 2B). We attribute the increased dark side occupancy in the acute cohort compared to the chronic cohort, as anxiety-like behavior possibly resulting from two sequential i.p. injections as a single i.p. saline injection is capable of producing pain-like and anxiety-like behavior (Lapin, 1995). Light/dark preference is shown response by response as difference scores (dark - light duration for the first occupancy within each side of the test chamber, plus dark - light duration for the second occupancy, etc.). These data indicate that chronic, but not acute COMT inhibition, produces anxiety-like behavior that is mediated by βARs.
Figure 2.
Chronic, but not acute COMT inhibition, produces anxiety-like behavior. (A) Acute OR486 (30mg/kg) administration did not significantly increase occupancy for the dark chamber. (B) Continuous delivery of OR486 (15mg/kg/day) for 14 days increased preference for the dark chamber in the 10 minute light/dark place preference test. This effect was blocked by continuous co-administration of propranolol (9mg/kg/day). The cumulative plots were constructed by summing the first time of occupancy on the light side from the first duration on the dark side, response by response, in order to generate the group means shown. N= 6–16 per group. *p<.01 compared to Saline, Saline.
Effects of acute and sustained OR486 on exploratory activity
OR486 administration had minimal effects on exploratory behavior during thermal and light/dark preference testing. In the thermal preference test, acute but not chronic OR486 significantly reduced the first hot to cold transition latency compared to acute saline, consistent with sensitivity to 45°C heat (P <0.01, T-test) (Table 1). Increased rearing behavior and decreased transition latencies were observed in both chronic treatment groups compared to acute treatment groups (P < 0.001, T-test). Likewise in the light/dark preference test, increased rearing behavior and decreased transition latencies were observed in both chronic treatment groups compared to acute treatment groups (P < 0.001, T-test) (Table 1). Prolonged latencies to enter the light chamber in the acute cohort compared to the chronic cohort further supports the conclusion that repetitive i.p. injections produced anxiety-like behavior. Similar changes in behavior in chronic study animals, saline and OR486, were seen compared to acute study animals. Propranolol either co- or pre-administered with OR486 had no effect on these exploratory behaviors compared to OR486/saline co-administration indicating no adverse effects. We attribute the overall differences in exploratory activity between the acute and chronic cohorts possibly due to the anxiety-provoking effects of repetitive i.p. injections in the acute but not the chronic cohort (Lapin, 1995).
Table 1.
Rearing and transition latency behaviors during thermal and light-dark preference testing
| Thermal Preference Testing | ||||
|---|---|---|---|---|
| Acute | Chronic | |||
| Saline | OR486 | Saline | OR486 | |
| Rearing | 31.25 (3.8) | 32.8 (3.3) | 67.0 (4.5) # | 49.1 (4.3)*# |
| Latency | 16.9 (2.9) | 9.6 (2.8)# | 4.3 (0.9)# | 5.6 (1.3)# |
| Light-Dark Preference Testing | ||||
|---|---|---|---|---|
| Acute | Chronic | |||
| Saline | OR486 | Saline | OR486 | |
| Rearing | 5.2 (1.1) | 5.8 (1.1) | 17.2 (2.3) # | 14.2 (1.5)# |
| Latency | 203.1 (81) | 167.9 (80) | 20.0 (5.1) # | 18.3 (2.6)# |
N=6–16 per group.
p<0.01 vs saline and
p<0.001 acute vs chronic. Results are expressed as mean ± SEM.
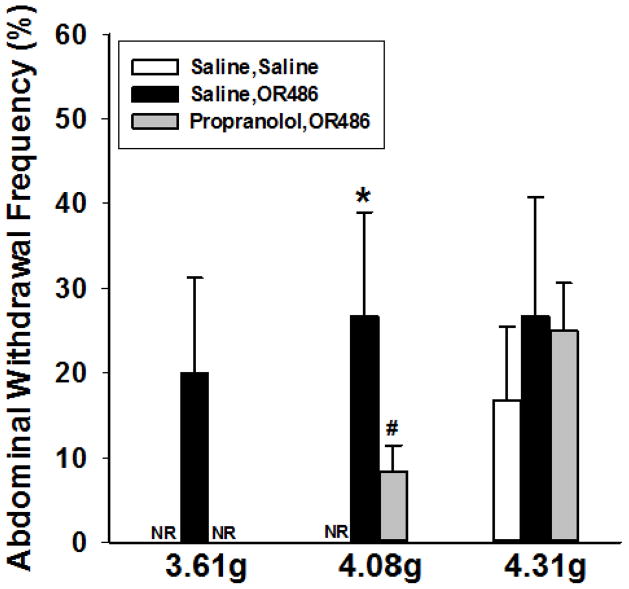
Effects of sustained OR486 on abdominal sensitivity
To determine the effects of chronic COMT inhibition on mechanosensation of the abdominal skin, we assessed the abdominal withdrawal frequency to von Frey filament application after 14 days of continuous OR486 infusion. Figure 3 demonstrates that animals receiving OR486 exhibit a significant increase in abdominal withdrawal frequency compared to those receiving saline (P < 0.006, df = 2, F = 6.47, by Two-way ANOVA). Animals receiving continuous propranolol and OR486 had attenuated responses to the 3.61 and 4.08 g stimuli compared to those receiving saline and OR486 (P < 0.02, df=2, F = 6.47, by Two-way ANOVA). These observations demonstrate that chronic COMT inhibition produces abdominal mechanical hypersensitivity that is mediated by βARs.
Figure 3.
Chronic COMT inhibition produces innocuous abdominal tactile hypersensitivity in the 10 minute light/dark place preference test. Continuous delivery of OR486 for 14 days (15mg/kg/day) resulted in increased abdominal withdrawal frequency to 3.61, 4.08 and 4.31g von Frey filaments. Co-administration of propranolol (9mg/kg/day) blocked OR486-induced increases in withdrawal frequency to the 3.61 and 4.08, but not the 4.31g filaments. Results are expressed as mean ± SEM. N= 6 per group. *p<.01 compared to Saline, OR486. #p<0.01 compared to Saline, Saline.
Discussion
Here, we provide the first demonstration that stimulation of βARs following acute or chronic COMT inhibition drives cognitive-affective behaviors associated with heightened pain. Compared to animals receiving saline, those receiving an acute injection or continuous infusion of the COMT inhibitor OR486 exhibited pronounced sensitivity to noxious 45°C heat, preferring the 10°C cold. Animals receiving continuous infusion of OR486 also exhibited a greater sensitivity to bright light, preferring the dark. Furthermore, we show that chronic COMT inhibition leads to increased mechanosensation on the abdominal skin. COMT-dependent increases in pain and anxiety-like behaviors were blocked by the nonselective β-adrenergic antagonist propranolol.
These findings are in line with those from earlier studies showing that stimulation of βARs following acute COMT inhibition leads to increased pain evoked by thermal heat or punctate mechanical stimuli on the footpad (Nackley et al., 2007, Hartung et al., 2014). Compared to assays of evoked withdrawal responses, the operant assays used herein require decision making behavior in order to terminate a perceived threatening stimulus. Thus, reduced occupancy on the heated plate following OR486 administration may be interpreted as enhanced perception of peripheral noxious heat. In support of this interpretation, subjects with the low (158met) versus high (158val) COMT activity allele exhibit stronger functional Magnetic Resonance Imaging (fMRI) signals in pain-relevant cortical and sub-cortical regions during presentation of noxious thermal heat (Loggia et al., 2011). Of note, the thermal heat intensities used in earlier Hargreaves and hot plate assays of evoked pain were higher (47°–50°C) than used here (Nackley et al., 2007, Kambur et al., 2010, Hartung et al., 2014), likely stimulating different populations of nociceptors (Yeomans and Proudfit, 1996). Higher temperatures preferentially activate Aδ nociceptors, while temperatures ranging 43°–45°C preferentially activate C-thermal nociceptors (Yeomans and Proudfit, 1996, Vierck et al., 2002). Thus, past and present studies together provide behavioral evidence for COMT-dependent sensitization of both Aδ and C-thermal nociceptors. Further characterization of operant responses and nociceptor physiology during periods of low COMT activity may prove useful to the study of chronic pain, as patients with temporomandibular disorders and fibromyalgia have abnormal nociceptor function in response to a similar range of moderate to high intensity temperatures (Maixner et al., 1995, Maixner et al., 1998, Park et al., 2010, Vierck et al., 2014).
In addition to enhanced pain amplification, patients with chronic pain often experience high levels of stress and anxiety, which are traits also mediated by COMT (Vassend et al., 1995, Thieme et al., 2004, Fernandez-de-Las-Penas et al., 2012). Using a light/dark assay, we found that sustained, but not acute, administration of OR486 resulted in anxiety-like behavior defined by reduced light side activity. These data are in agreement with those published by Desbonnet and colleagues, who show that life-long inhibition of COMT in COMT null mice produces anxiety-like behavior, while acute administration of the COMT inhibitor tolcapone to wildtype mice fails to alter light/dark performance (Desbonnet et al., 2012). Furthermore, COMT null mice display increased acoustic startle amplitude along with decreased novel object exploration and decreased open arm entries in an elevated plus maze (Papaleo et al., 2008). Consistent with genetic studies in mice, fibromyalgia patients with the low (158met) COMT activity allele experience increased levels of depression and anxiety (Fernandez-de-Las-Penas et al., 2012). The influence of COMT on psychological traits such as anxiety is generally thought to be mediated by dopaminergic signaling in the pre-frontal cortex. However, the ability of propranolol to normalize light-dark preference in the chronic COMT inhibition model suggests that this view needs to be expanded to include adrenergic systems (Smolka et al., 2005).
The present findings describe a novel relationship between COMT inhibition and volitional pain-related behaviors. As these measures are sensitive to changes in exploratory behavior (Mauderli et al., 2000), we also evaluated the effects of acute and sustained OR486 administration on rearing during the thermal preference assay and transition latency during the light/dark preference assay. No deleterious effects of OR486 were observed on exploratory activity during either of these assays. In support of this association, pharmacologic inhibition or genetic knockdown of COMT in mice does not affect ambulation or exploratory activity (Papaleo et al., 2008, Desbonnet et al., 2012). We, therefore, conclude that COMT-dependent decreases in heat and light preference are indeed due to increased nociception.
Another hallmark of chronic musculoskeletal pain conditions is that patients often experience generalized sensory abnormalities at remote bodily sites (Gracely et al., 1992, Gracely et al., 2003, Giesecke et al., 2004, Vierck, 2006). In order to evaluate the generalized nature of chronic COMT inhibition, we evaluated withdrawal responses to innocuous tactile stimuli applied to the abdominal region. Our findings extend earlier work by demonstrating that COMT inhibition produces increased mechanosensation on the hairy skin of the abdomen as well as the glabrous skin of the hindpaw (Nackley et al., 2007, Kambur et al., 2010, Hartung et al., 2014). This phenomenon is likely driven by neurophysiologic changes in the central nervous system that increase central responses to peripheral stimuli (Gracely et al., 1992, Bohm-Starke et al., 1998, 1999, Giesecke et al., 2004). Indeed, work by Desmeules and colleagues demonstrate that fibromyalgia patients exhibit a linear relationship between COMT val158met genotype and magnitude of spinal nociceptive flexion reflex, a physiologic correlate of central sensitization, such that patients with two copies of the val allele have the highest reflex thresholds and those with two copies of the met allele have the lowest reflex thresholds (Desmeules et al., 2012). Additionally, patients using COMT inhibitors for the treatment of Parkinson’s disease report heightened visceral and generalized pain (Gordin et al., 2004).
The ability of propranolol to block both volitional pain and anxiety-related behaviors as well as evoked pain responses at multiple sites following prolonged reductions in COMT is consistent with clinical observations that βAR antagonists provide pain relief for patients with FM and TMD (Wood et al., 2005, Light et al., 2009, Tchivileva et al., 2010). Furthermore, results from a randomized controlled trial demonstrate that the βAR antagonist propranolol provides significant pain relief for TMD patients who carry the low activity, but not high activity, COMT genotype (Tchivileva et al., 2010). However, more work is required to evaluate the role of specific βAR subtypes in mediating the development and maintenance of volitional COMT-dependent pain behaviors.
Propranolol is a nonselective βAR antagonist able to block β1-, β2-, and β3AR subtypes at doses similar to the one used herein (O’Donnell et al., 1994, Tsujii and Bray, 1998). Previously, our group has shown that evoked COMT-dependent pain behaviors following OR486 administration are mediated by β2- and β3AR subtypes (Hartung et al., 2014). β2- and β3ARs are G protein-coupled receptors expressed in peripheral, spinal, and central regions where they could potentially mediate pain sensitivity. In the periphery, β2ARs are located on mononuclear leukocytes (Landmann, 1992), adipocytes (Kobilka et al., 1987), vascular, uterine, and airway smooth muscle cells (Dixon et al., 1986). In the central nervous system, β2ARs are located on thalamic, cerebellar (Rainbow et al., 1984, Nicholas et al., 1996), and spinal dorsal horn neurons (Nicholson et al., 2005) as well as glial cells (Stone and Ariano, 1989, Salm and McCarthy, 1992). The contribution of β2ARs to enhanced pain sensitivity is in line with results from previous studies demonstrating that epinephrine activates β2ARs located on primary afferent nociceptors and produces a hyperalgesic state in rats (Khasar et al., 1999a, Khasar et al., 1999b, Aley et al., 2001, Khasar et al., 2003). Additionally, common variants of the human β2AR gene, coding for differences in receptor expression and internalization, are associated with the onset of TMD (Diatchenko et al., 2006). β3ARs are mainly expressed in brown and white adipose tissue, where their activation produces robust increases in pro-inflammatory cytokines (Mohamed-Ali et al., 2001). Recently, they have been shown to drive norepinephrine-induced ATP release in dorsal root ganglia (DRG) as a contributor to neuropathic pain behavior (Kanno et al., 2010). β2- and β3ARs drive COMT-dependent pain, at least in part, by promoting the release of NO and the innate immunity cytokines tumor necrosis factorα, interleukine-1β, and interleukin-6, which in turn produce heightened pain sensitivity. While β2- and β3ARs and their downstream effectors may also mediate the development and maintenance of volitional COMT-dependent pain behaviors, additional pharmacologic studies are required to determine if this is the case.
Furthermore, additional pharmacologic studies are required to determine the potential effects of the OR486 vehicle on volitional pain behaviors. Acute i.p. or sustained 14 day systemic delivery of this vehicle to rats has no effect on evoked mechanical/thermal sensitivity or non-evoked measures such as locomotor activity (Nackley et al., 2007, Ciszek and Nackley, 2012, Hartung et al., 2014), thus control groups herein received 0.9% saline. This represents a limitation of our study as it is possible that vehicle effects might change as a function of different experimental protocols.
Finally, it is important to note that the influence of COMT on pain and anxiety-related behaviors is likely modified by sex. It is well-established that males and females differ with respect to COMT activity levels, with males exhibiting higher levels in brain (Chen et al., 2004) and peripheral tissues (Boudikova et al., 1990). A growing literature continues to demonstrate that the effects of COMT on nociception, cognition, and behavioral traits such as anxiety are sexually dimorphic, with the magnitude and direction of the effect being highly assay-dependent (Gogos et al., 1998, Harrison and Tunbridge, 2008, Papaleo et al., 2012, Belfer et al., 2013, Sannino et al., 2014, White et al., 2014). As the present study only included males, future studies should also be conducted to explore interactions between sex and COMT/adrenergic signaling on pain and cognitive-affective behaviors.
Conclusions
Taken together, these findings provide the first evidence that stimulation of βARs following acute or chronic COMT inhibition drives cognitive-affective behaviors associated with heightened pain that affects multiple body sites. Moreover, these data support the chronic COMT inhibition model as a novel animal model that 1) mimics genetic and phenotypic characteristics of human persistent pain conditions and 2) has utility to elucidate the key mechanisms underlying chronic pain conditions associated with abnormalities in COMT and catecholamine signaling. Finally, these data have important clinical implications, suggesting that βAR antagonist therapy may prove useful in suppressing pain at multiple levels associated with the transmission and perception of pain.
The chronic COMT inhibition model mimics genetic and phenotypic traits of human pain
Chronic COMT inhibition leads to heightened cognitive-affective pain behaviors
Chronic COMT-dependent pain affects multiple body sites
Chronic COMT-dependent pain is mediated by β-adrenergic receptors
Acknowledgments
The authors wish to thank Mr. Ikenna Chukwudolue for his support in the animal behavior studies. This study was supported by NIH R01 NS072205 to AGN and NIH R01 NS039041 to KNW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci. 2001;21:6933–6939. doi: 10.1523/JNEUROSCI.21-17-06933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfer I, Schreiber KL, Shaffer JR, Shnol H, Blaney K, Morando A, Englert D, Greco C, Brufsky A, Ahrendt G, Kehlet H, Edwards RR, Bovbjerg DH. Persistent postmastectomy pain in breast cancer survivors: analysis of clinical, demographic, and psychosocial factors. The journal of pain: official journal of the American Pain Society. 2013;14:1185–1195. doi: 10.1016/j.jpain.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Bohm-Starke N, Hilliges M, Falconer C, Rylander E. Increased intraepithelial innervation in women with vulvar vestibulitis syndrome. Gynecologic and obstetric investigation. 1998;46:256–260. doi: 10.1159/000010045. [DOI] [PubMed] [Google Scholar]
- Bohm-Starke N, Hilliges M, Falconer C, Rylander E. Neurochemical characterization of the vestibular nerves in women with vulvar vestibulitis syndrome. Gynecologic and obstetric investigation. 1999;48:270–275. doi: 10.1159/000010198. [DOI] [PubMed] [Google Scholar]
- Boudikova B, Szumlanski C, Maidak B, Weinshilboum R. Human liver catechol-O-methyltransferase pharmacogenetics. Clinical pharmacology and therapeutics. 1990;48:381–389. doi: 10.1038/clpt.1990.166. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Bredt DS. Synaptic signaling by nitric oxide. Curr Opin Neurobiol. 1997;7:374–378. doi: 10.1016/s0959-4388(97)80065-7. [DOI] [PubMed] [Google Scholar]
- Chen LX, Fang Q, Chen Q, Guo J, Wang ZZ, Chen Y, Wang R. Study in vitro and in vivo of nociceptin/orphanin FQ(1–13)NH2 analogues substituting N-Me-Gly for Gly2 or Gly3. Peptides. 2004;25:1349–1354. doi: 10.1016/j.peptides.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Ciszek BP, Nackley A. Society for Neuroscience Abstract Viewer/Itinerary Planner CD-ROM. Society for Neuroscience; New Orleans, LA: 2012. Chronic catechol-O-methyltransferase-dependent pain: a peripheral contribution. [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. Journal of neurophysiology. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Tighe O, Karayiorgou M, Gogos JA, Waddington JL, O’Tuathaigh CM. Physiological and behavioural responsivity to stress and anxiogenic stimuli in COMT-deficient mice. Behavioural brain research. 2012;228:351–358. doi: 10.1016/j.bbr.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Desmeules J, Piguet V, Besson M, Chabert J, Rapiti E, Rebsamen M, Rossier MF, Curtin F, Dayer P, Cedraschi C. Psychological distress in fibromyalgia patients: a role for catechol-O-methyl-transferase Val158met polymorphism. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2012;31:242–249. doi: 10.1037/a0025223. [DOI] [PubMed] [Google Scholar]
- Deyama S, Katayama T, Kondoh N, Nakagawa T, Kaneko S, Yamaguchi T, Yoshioka M, Minami M. Role of enhanced noradrenergic transmission within the ventral bed nucleus of the stria terminalis in visceral pain-induced aversion in rats. Behavioural brain research. 2009;197:279–283. doi: 10.1016/j.bbr.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Anderson AD, Slade GD, Fillingim RB, Shabalina SA, Higgins T, Sama S, Belfer I, Goldman D, Max MB, Weir BS, Maixner W. Three major haplotypes of the β2 adrenergic receptor define psychological profile, blood pressure, and the risk for development of a common musculoskeletal pain disorder. American Journal of Medical Genetics. 2006 doi: 10.1002/ajmg.b.30324. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Kobilka BK, Strader DJ, Benovic JL, Dohlman HG, Frielle T, Bolanowski MA, Bennett CD, Rands E, Diehl RE, et al. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986;321:75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Fernandez-de-Las-Penas C, Ambite-Quesada S, Gil-Crujera A, Cigaran-Mendez M, Penacoba-Puente C. Catechol-O-methyltransferase Val158Met polymorphism influences anxiety, depression, and disability, but not pressure pain sensitivity, in women with fibromyalgia syndrome. J Pain. 2012;13:1068–1074. doi: 10.1016/j.jpain.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis and rheumatism. 2004;50:613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordin A, Kaakkola S, Teravainen H. Clinical advantages of COMT inhibition with entacapone - a review. Journal of neural transmission. 2004;111:1343–1363. doi: 10.1007/s00702-004-0190-3. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Grant MA, Giesecke T. Evoked pain measures in fibromyalgia. Best Pract Res Clin Rheumatol. 2003;17:593–609. doi: 10.1016/s1521-6942(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–194. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:3037–3045. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Hartung JE, Ciszek BP, Nackley AG. beta2- and beta3-adrenergic receptors drive COMT-dependent pain by increasing production of nitric oxide and cytokines. Pain. 2014;155:1346–1355. doi: 10.1016/j.pain.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LM, Schistad EI, Storesund A, Pedersen LM, Rygh LJ, Roe C, Gjerstad J. The COMT rs4680 Met allele contributes to long-lasting low back pain, sciatica and disability after lumbar disc herniation. Eur J Pain. 2012;16:1064–1069. doi: 10.1002/j.1532-2149.2011.00102.x. [DOI] [PubMed] [Google Scholar]
- Kambur O, Talka R, Ansah OB, Kontinen VK, Pertovaara A, Kalso E, Mannisto PT. Inhibitors of catechol-O-methyltransferase sensitize mice to pain. Br J Pharmacol. 2010;161:1553–1565. doi: 10.1111/j.1476-5381.2010.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T, Yaguchi T, Nishizaki T. Noradrenaline stimulates ATP release from DRG neurons by targeting beta(3) adrenoceptors as a factor of neuropathic pain. J Cell Physiol. 2010;224:345–351. doi: 10.1002/jcp.22114. [DOI] [PubMed] [Google Scholar]
- Karling P, Danielsson A, Wikgren M, Soderstrom I, Del-Favero J, Adolfsson R, Norrback KF. The relationship between the val158met catechol-O-methyltransferase (COMT) polymorphism and irritable bowel syndrome. PLoS One. 2011;6:e18035. doi: 10.1371/journal.pone.0018035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999a;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999b;81:1104–1112. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Miao FJ, Gear RW, Green PG, Levine JD. Vagal modulation of bradykinin-induced mechanical hyperalgesia in the female rat. J Pain. 2003;4:278–283. doi: 10.1016/s1526-5900(03)00631-x. [DOI] [PubMed] [Google Scholar]
- Kobilka BK, Dixon RA, Frielle T, Dohlman HG, Bolanowski MA, Sigal IS, Yang-Feng TL, Francke U, Caron MG, Lefkowitz RJ. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A. 1987;84:46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress MaS C. Neuroimmunology and Pain: Peripheral Effects of Proinflammatory Cytokines. In: Brune KaHHO., editor. Hyperalgesia: Molecular Mechanisms and Clinical Implications, Progress in Pain Research Management. Vol. 30. Seattle: IASP Press; 2004. pp. 57–65. [Google Scholar]
- Landmann R. Beta-adrenergic receptors in human leukocyte subpopulations. Eur J Clin Invest. 1992;22(Suppl 1):30–36. [PubMed] [Google Scholar]
- Lapin IP. Only controls: effect of handling, sham injection, and intraperitoneal injection of saline on behavior of mice in an elevated plus-maze. Journal of pharmacological and toxicological methods. 1995;34:73–77. doi: 10.1016/1056-8719(95)00025-d. [DOI] [PubMed] [Google Scholar]
- Light KC, Bragdon EE, Grewen KM, Brownley KA, Girdler SS, Maixner W. Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder. J Pain. 2009;10:542–552. doi: 10.1016/j.jpain.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Jensen K, Gollub RL, Wasan AD, Edwards RR, Kong J. The catechol-O-methyltransferase (COMT) val158met polymorphism affects brain responses to repeated painful stimuli. PLoS One. 2011;6:e27764. doi: 10.1371/journal.pone.0027764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63:341–351. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Martinez-Jauand M, Sitges C, Rodriguez V, Picornell A, Ramon M, Buskila D, Montoya P. Pain sensitivity in fibromyalgia is associated with catechol-O-methyltransferase (COMT) gene. Eur J Pain. 2013;17:16–27. doi: 10.1002/j.1532-2149.2012.00153.x. [DOI] [PubMed] [Google Scholar]
- Mauderli AP, Acosta-Rua A, Vierck CJ. An operant assay of thermal pain in conscious, unrestrained rats. J Neurosci Methods. 2000;97:19–29. doi: 10.1016/s0165-0270(00)00160-6. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Flower L, Sethi J, Hotamisligil G, Gray R, Humphries SE, York DA, Pinkney J. beta-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. J Clin Endocrinol Metab. 2001;86:5864–5869. doi: 10.1210/jcem.86.12.8104. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain. 2007;128:199–208. doi: 10.1016/j.pain.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Jr, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Hokfelt T, Pieribone VA. The distribution and significance of CNS adrenoceptors examined with in situ hybridization. Trends Pharmacol Sci. 1996;17:245–255. doi: 10.1016/0165-6147(96)10022-5. [DOI] [PubMed] [Google Scholar]
- Nicholson R, Dixon AK, Spanswick D, Lee K. Noradrenergic receptor mRNA expression in adult rat superficial dorsal horn and dorsal root ganglion neurons. Neurosci Lett. 2005;380:316–321. doi: 10.1016/j.neulet.2005.01.079. [DOI] [PubMed] [Google Scholar]
- Nissinen E, Linden IB, Schultz E, Kaakkola S, Mannisto PT, Pohto P. Inhibition of catechol-O-methyltransferase activity by two novel disubstituted catechols in the rat. Eur J Pharmacol. 1988;153:263–269. doi: 10.1016/0014-2999(88)90614-0. [DOI] [PubMed] [Google Scholar]
- O’Donnell JM, Frith S, Wilkins J. Involvement of beta-1 and beta-2 adrenergic receptors in the antidepressant-like effects of centrally administered isoproterenol. J Pharmacol Exp Ther. 1994;271:246–254. [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, Chen J. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Erickson L, Liu G, Chen J, Weinberger DR. Effects of sex and COMT genotype on environmentally modulated cognitive control in mice. Proc Natl Acad Sci U S A. 2012;109:20160–20165. doi: 10.1073/pnas.1214397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent AJ, Beaudet N, Beaudry H, Bergeron J, Berube P, Drolet G, Sarret P, Gendron L. Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behavioural brain research. 2012;229:160–167. doi: 10.1016/j.bbr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Clark GT, Kim YK, Chung JW. Analysis of thermal pain sensitivity and psychological profiles in different subgroups of TMD patients. International journal of oral and maxillofacial surgery. 2010;39:968–974. doi: 10.1016/j.ijom.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A. 1984;81:1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salm AK, McCarthy KD. The evidence for astrocytes as a target for central noradrenergic activity: expression of adrenergic receptors. Brain Res Bull. 1992;29:265–275. doi: 10.1016/0361-9230(92)90056-4. [DOI] [PubMed] [Google Scholar]
- Sannino S, Gozzi A, Cerasa A, Piras F, Scheggia D, Manago F, Damiano M, Galbusera A, Erickson LC, De Pietri Tonelli D, Bifone A, Tsaftaris SA, Caltagirone C, Weinberger DR, Spalletta G, Papaleo F. COMT Genetic Reduction Produces Sexually Divergent Effects on Cortical Anatomy and Working Memory in Mice and Humans. Cerebral cortex. 2014 doi: 10.1093/cercor/bhu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Braus DF, Goldman D, Buchel C, Heinz A. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Ariano MA. Are glial cells targets of the central noradrenergic system? A review of the evidence. Brain Res Brain Res Rev. 1989;14:297–309. doi: 10.1016/0165-0173(89)90015-5. [DOI] [PubMed] [Google Scholar]
- Tchivileva IE, Lim PF, Smith SB, Slade GD, Diatchenko L, McLean SA, Maixner W. Effect of catechol-Omethyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: a randomized, double-blind, placebo-controlled, crossover pilot study. Pharmacogenetics and genomics. 2010;20:239–248. doi: 10.1097/FPC.0b013e328337f9ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: relationship to somatic and psychosocial variables. Psychosom Med. 2004;66:837–844. doi: 10.1097/01.psy.0000146329.63158.40. [DOI] [PubMed] [Google Scholar]
- Tsujii S, Bray GA. A beta-3 adrenergic agonist (BRL-37,344) decreases food intake. Physiology & behavior. 1998;63:723–728. doi: 10.1016/s0031-9384(97)00518-0. [DOI] [PubMed] [Google Scholar]
- Vassend O, Krogstad BS, Dahl BL. Negative affectivity, somatic complaints, and symptoms of temporomandibular disorders. Journal of psychosomatic research. 1995;39:889–899. doi: 10.1016/0022-3999(95)00041-9. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Acosta-Rua A, Nelligan R, Tester N, Mauderli A. Low dose systemic morphine attenuates operant escape but facilitates innate reflex responses to thermal stimulation. J Pain. 2002;3:309–319. doi: 10.1054/jpai.2002.125186. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Acosta-Rua AJ, Rossi HL, Neubert JK. Sex differences in thermal pain sensitivity and sympathetic reactivity for two strains of rat. J Pain. 2008a;9:739–749. doi: 10.1016/j.jpain.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008b;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Vierck CJ., Jr Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia) Pain. 2006;124:242–263. doi: 10.1016/j.pain.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Jr, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, King CD, Berens SA, Yezierski RP. Excitotoxic injury to thoracolumbar gray matter alters sympathetic activation and thermal pain sensitivity. Experimental brain research. 2013;231:19–26. doi: 10.1007/s00221-013-3666-2. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Wong F, King CD, Mauderli AP, Schmidt S, Riley JL., 3rd Characteristics of sensitization associated with chronic pain conditions. Clin J Pain. 2014;30:119–128. doi: 10.1097/AJP.0b013e318287aac7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, McMahon SB, Koltzenburg M. Wall and Melzack’s textbook of pain. Philadelphia: Elsevier/Churchill Livingstone; 2006. [Google Scholar]
- Walsh J, Tighe O, Lai D, Harvey R, Karayiorgou M, Gogos JA, Waddington JL, O’Tuathaigh CM. Disruption of thermal nociceptive behaviour in mice mutant for the schizophrenia-associated genes NRG1, COMT and DISC1. Brain Res. 2010;1348:114–119. doi: 10.1016/j.brainres.2010.06.027. [DOI] [PubMed] [Google Scholar]
- White TP, Loth E, Rubia K, Krabbendam L, Whelan R, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Conrod P, Fauth-Buhler M, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Lawrence C, Mann K, Paillere ML, Nees F, Paus T, Pausova Z, Rietschel M, Robbins T, Smolka MN, Shergill SS, Schumann G, Consortium I. Sex differences in COMT polymorphism effects on prefrontal inhibitory control in adolescence. Neuropsychopharmacology. 2014;39:2560–2569. doi: 10.1038/npp.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PB, Kablinger AS, Caldito GS. Open trial of pindolol in the treatment of fibromyalgia. Ann Pharmacother. 2005;39:1812–1816. doi: 10.1345/aph.1G014. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: Electrophysiological evidence. Pain. 1996;68:141–150. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
- Yezierski RP, Green M, Murphy K, Vierck CJ. Effects of gabapentin on thermal sensitivity following spinal nerve ligation or spinal cord compression. Behavioural pharmacology. 2013 doi: 10.1097/FBP.0b013e3283656d9b. [DOI] [PubMed] [Google Scholar]
- Yezierski RP, King CD, Morgan D, Carter CS, Vierck CJ. Effects of age on thermal sensitivity in the rat. The journals of gerontology Series A, Biological sciences and medical sciences. 2010;65:353–362. doi: 10.1093/gerona/glq024. [DOI] [PMC free article] [PubMed] [Google Scholar]