Abstract
Objective
Septic shock is associated with increased long-term morbidity and mortality. However, little is known about the use of hospital-based acute care in survivors after hospital discharge. The objectives of the study were to examine the frequency, timing, causes, and risk factors associated with Emergency Department (ED) visits and hospital readmissions within 30 days of discharge.
Design
Retrospective cohort study.
Setting
Tertiary, academic hospital in the United States.
Patients
Patients admitted with septic shock (serum lactate ≥ 4 mmol/L or refractory hypotension) and discharged alive to a non-hospice setting between 2007 and 2010.
Interventions
None.
Measurements and Main Results
The co-primary outcomes were all-cause hospital readmission and ED visits (treat-and-release encounters) within 30 days to any of the three health system hospitals. Of 269 at-risk survivors, 63 (23.4%, 95% confidence interval (CI): 18.2, 28.5) were readmitted within 30 days of discharge and another 12 (4.5%, 95% CI: 2.3, 7.7) returned to the ED for a treat-and-release visit. Readmissions occurred within 15 days of discharge in 75% of cases and were more likely in oncology patients (p=0.001) and patients with a longer hospital length of stay (p=0.04). Readmissions were frequently due to another life-threatening condition and resulted in death or discharge to hospice in 16% of cases. The reasons for readmission were deemed potentially related to the index septic shock hospitalization in 78% (49/63) of cases. The most common cause was infection-related, accounting for 46% of all 30-day readmissions, followed by cardiovascular or thromboembolic events (18%).
Conclusions
The use of hospital-based acute care appeared to be common in septic shock survivors. Encounters often led to readmission within 15 days of discharge, were frequently due to another acute condition, and appeared to result in substantial morbidity and mortality. Given the potential public health implications of these findings, validation studies are needed.
Keywords: septic shock, infection, hospital readmission, emergency department use
Sepsis has a tremendous impact on our health care system. The incidence of sepsis in the U.S. has been rising (1) and the associated cost has been estimated at $24.3 billion (2). Although the incidence has increased, substantially more patients are surviving their hospitalization (1, 3–8).
Sepsis survivorship is associated with cognitive and physical impairments, decreased quality of life, and increased long-term mortality (9–13). While evidence has highlighted the importance of understanding the trajectories of care after critical illness (14), little is known about the healthcare needs of septic shock survivors. Hospital-based, acute care use after a hospitalization is common and costly (15–17). Emergency department (ED) visits and hospital readmissions may reflect the quality and coordination of care provided during the index hospitalization (15–20). To patients and family members, these are important and stressful events that can disrupt the recovery process (21). Recent studies using administrative data have shown that sepsis survivors may be particularly vulnerable (22–24). Therefore, urgent investigation is justified to determine how often these events occur and, importantly, why.
We evaluated septic shock survivors, defined by several recent clinical trials as a serum lactate level of 4 mmol/L or higher or refractory hypotension (5–7), to determine the frequency, timing, and causes of ED visits and hospital readmissions. We hypothesized that hospital-based acute care use in the 30 days following a hospitalization for septic shock would be common, occurring in one in five cases or more based on other high-risk conditions (15–17), and the reasons for and outcomes related to these encounters may explain the increased mortality that survivors experience.
Materials and Methods
Study Design
This was a retrospective cohort study of adult survivors of septic shock discharged between December, 2007 and January, 2010. The institutional review board of the University of Pennsylvania approved the study (#819400) with an informed consent exemption.
Study Setting and Population
We conducted the study at the Hospital of the University of Pennsylvania (HUP), a tertiary care university hospital within the three hospital University of Pennsylvania Health System (UPHS). We studied patients admitted from the ED with septic shock, defined as a serum lactate level ≥4 mmol/L or systolic blood pressure <90 mm Hg after volume resuscitation or use of a vasoactive agent (5–7). The details of our validated strategy to identify cases of severe sepsis and quality assurance have been described previously (25–28). To examine hospital-based, acute care use in survivors and maintain the assumption of independent observations, we limited the study to patients discharged to a non-hospice setting after an index septic shock admission. We excluded patients who did not fulfill criteria for septic shock (29–30) and those who left against medical advice (AMA) or were transferred to another institution.
Data Collection
As part of the HUP Severe Sepsis database, trained investigators collected ED and hospitalization data from the electronic medical record (EMR) using a pre-drafted case abstraction form (25–28), permitting calculation of the Charlson Comorbidity Index (31). Consistent with prior work (25), missing data (>5% per variable) was rare and limited to laboratory measurements (e.g., coagulation measures). The Acute Physiology and Chronic Health Evaluation (APACHE) II score was calculated at hospital admission (25, 32).
We abstracted the following from the EMR using a separate abstraction form to examine hospital-based, acute care use within UPHS: disposition and follow-up at discharge from the index hospitalization, prior hospitalization within 30 days of the index hospitalization, and dates, timing, cause, and outcome of an ED encounter and/or hospital readmission within 30 days (33). We identified readmissions occurring after 30 days to calculate 60- and 90-day readmission rates. We did not collect data on ED visits or readmissions outside of UPHS, nor did we abstract data on subsequent ED visit(s) or readmission(s), with the exception of ED encounters that led to readmission. Consistent with the parent registry (25), post-discharge data was verified by a separate investigator for accuracy.
Our co-primary outcomes were ED visits (treat-and-release) and all-cause hospital readmission to any UPHS hospital within 30 days of discharge after the index hospitalization for septic shock (15–17). We included patients readmitted via inter-hospital transfer to minimize the risk of underestimating the hospital readmission rate. As a reference, using 2010 administrative data, we calculated the 30-day readmission rate for 10,985 index medical and surgical admissions at HUP to be 13.4% (95% confidence interval (CI): 12.8, 14.1). Secondary outcomes included 60- and 90-day hospital readmission rate and a composite outcome of ED visit and hospital readmission within 30 days of discharge.
A focus of the study was to understand why survivors returned to the hospital. Consistent with the readmission literature (34–35), we considered a 30-day readmission to be potentially related to the index septic shock admission when the readmission was due to an unresolved, recurrent, or new infection or was due to a clinical deterioration or complication potentially related to care provided or a consequence of sepsis. We a priori categorized readmissions as related to: acute kidney injury, cognitive impairment (e.g., medication error), complications of tubes and lines, cardiovascular and thromboembolic events, infection, physical impairment (e.g., falls), and when the readmission was potentially related to the index septic shock admission but not captured in the above categories, as other (9–13, 36–44). When the readmission was attributed exclusively to the underlying disease and/or was elective, it was deemed unavoidable.
Two independent investigators reviewed the discharge summary, including primary and secondary diagnoses, and EMR to determine the readmission cause. Due to the infrequent number of readmissions attributed to cognitive and physical impairment, these were collapsed into the other category, resulting in 6 final response options. The inter-rater reliability for diagnoses was good, with a Kappa statistic of 0.79 (95% confidence interval (CI): 0.67 – 0.92). Adjudication, necessary for 9 readmissions, was performed by a third investigator blinded to prior assessments. The cause of an ED treat-and-release visit was determined by its ICD-9 code.
Last, we examined the relationship between clinical risk factors and 30-day hospital readmission. Using the general readmission (33–35) and sepsis literature, we examined sociodemographics, comorbid conditions, illness severity at presentation and during the hospitalization, source of sepsis, hospital length of stay, and discharge disposition. To assess whether hospital readmission was associated with quality of care provided at presentation, we tested time to antibiotics (27), volume of resuscitation (5–7), and initiation of early goal-directed therapy (5–7, 26). We considered year of admission and prior hospitalization within 30 days of the index hospitalization as potential confounders (28, 34).
Statistical Analyses
We compared continuous variables across groups using Student’s t-test or Wilcoxon’s rank-sum test and compared categorical variables across groups using the chi-squared test or Fisher’s exact test, as appropriate. We used Stata 13.0 IC (Stata Datacorp, College Station, TX) to perform analyses and considered p-values ≤ 0.05 as significant.
We used multivariable logistic regression to adjust for potential confounding in the associations between risk factors and 30-day hospital readmission. In primary analyses, factors associated with the dependent variable at a significance level of p<0.05 in bivariate analyses were included to create parsimonious multivariable models. Potential confounding variables associated at a significance level of p<0.20 were added one-at-a-time to the base model and maintained if there was an alteration by >10% in the point estimate for the odds ratio (OR) of any risk factor (45). Multicollinearity was assessed using variance inflation factors. In primary analyses, we excluded variables with ≤10 observations per cell to avoid overfitting. In secondary analyses, we included these variables one-at-a-time. In separate secondary analyses, we included factors associated with the dependent variable at a significance level of p<0.20 in bivariate analyses into the multivariable models.
Results
Baseline characteristics
Of 997 unique severe sepsis patients over the study interval, we examined 414 patients admitted from the ED with septic shock. Of these 414 patients, 23.2% expired in-hospital and 8.7% were discharged to hospice (see Figure 1). After excluding 13 survivors who left AMA or were transferred, there were 269 survivors at-risk for hospital readmission (Table 1).
Figure 1.
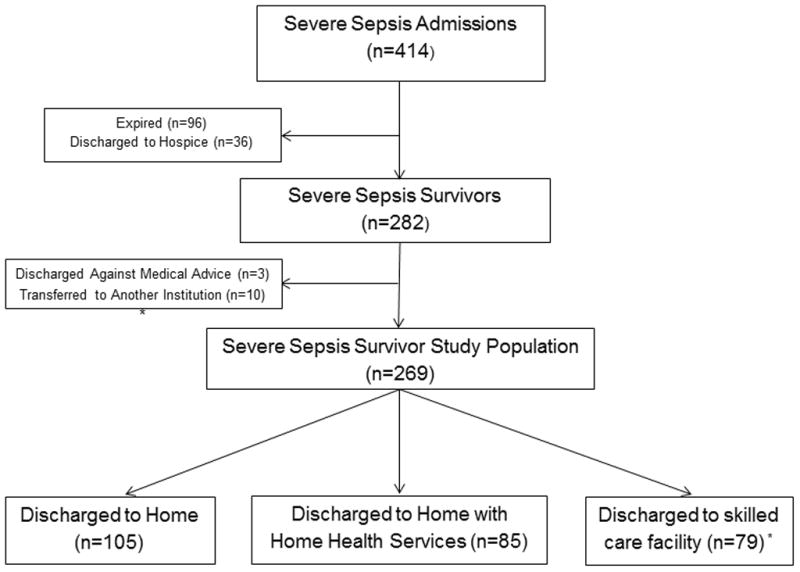
Flow diagram of use of post-acute care in survivors of sepsis sepsis. * Of 79 survivors discharged to a skilled care facility, 25 were discharged to acute rehabilitation, 47 to a skilled nursing facility, and 7 to a long-term acute care hospital.
Table 1.
Baseline characteristics of septic shock survivor cohort.
| At-Risk Survivors (N=269) | |
|---|---|
| Clinical Factors at Presentation
| |
| Age, years | 59 ± 18 |
| Female sex (n, %) | 135 (50.2) |
| Race (n, %) | |
| White | 112 (42.6) |
| Black | 144 (54.8) |
| Other | 7 (2.7) |
| Charlson Comorbidity Index | 2 (1 – 3) |
| Illness Severity at Presentation | |
| Initial serum lactate (mmol/L) | 4.6 (3.7 – 6.1) |
| Hypotension (n, %) | 184 (68.4) |
| Refractory hypotension (n, %) | 95 (35.3) |
| Admission to ICU (n, %) | 207 (77.0) |
| APACHE II score | 19 ± 7 |
| Admission to ICU (n, %) | 207 (77.0) |
| Mechanical ventilation use (n, %) | 54 (20.1) |
| Source of sepsis (n, %) | |
| Bacteremia | 27 (10.0) |
| Pneumonia | 50 (18.6) |
| Genitourinary | 57 (21.2) |
| Gastrointestinal | 53 (19.7) |
| Skin or soft-tissue | 21 (7.8) |
Definition of abbreviation: APACHE=acute physiology and chronic health evaluation.
Categorical variables are presented as counts and percentages. Continuous variables are presented as means and standard deviations or median and interquartile ranges, as determined by their distribution.
Hypotension defined as systolic blood pressure ≤ 90 mm Hg. Refractory hypotension defined as systolic blood pressure ≤ 90 mm Hg after fluid resuscitation or use of vasoactive agents.
Rate, Timing, and Cause of 30-Day Emergency Department Visits and 30-Day Readmission
Of 269 at-risk survivors, 63 (23.4%, 95% CI: 18.2, 28.5) were readmitted within 30 days and another 12 (4.5%, 95% CI: 2.3, 7.7) returned to the ED for a treat-and-release visit (readmission rates presented in Table 2). The median time to an ED encounter was 8 days, with interquartile range (IQR) from 4 to 15 days. Of the 56 ED encounters within 30 days, the conversion rate to hospital admission was 75% (42/56). The reasons for a treat-and-release ED visit varied, with the most common reasons being infection (n=6), device complications (n=3) and fall injuries (n=2) (see Table 2).
Table 2.
Hospital-based acute care use in 269 survivors of septic shock.
| Outcomes | |
|---|---|
| Hospital Readmissions | |
|
| |
| 30-day hospital readmission (n, %) | 63 (23.4) |
| 60-day hospital readmission (n, %) | 89 (33.1) |
| 90-day hospital readmission (n, %) | 100 (37.2) |
|
| |
| ED Visits (Treat – and – Release Encounters) | |
|
| |
| 30-day ED visit (n, %) * | 14 (5.2) |
|
| |
| Hospital-Based Acute Care Post-Discharge | |
|
| |
| 30-day ED visit or readmission (n, %) | 75 (27.9)† |
Definition of abbreviation: ED=emergency department.
Categorical variables are reported as a count and percentage.
Of 56 ED encounters within 30 days, 42 (75%) led to an admission. The reasons for the 14 ED encounters not resulting in an admission were: cellulitis or surgical site infection (n=4), complication of a device (n=3), head injury after a fall (n=2), altered mental status (n=1), post-operative pain (n=1), dyspnea (n=1), gastroenteritis (n=1), and pneumonia (n=1).
One patient returned for a treat-and-release ED visit for altered mental status and subsequently was readmitted, both within 30 days of discharge; one patient was readmitted and subsequently returned for a treat-and-release ED visit for a head injury after a fall, both within 30 days. These 2 instances explain why the sum of the ED visits and 30-day hospital readmissions do not total 75.
The majority of 30-day readmissions presented to UPHS through the ED (68%), with the remainder presenting as a direct admission (24%) or outside hospital transfer (8%). The median time to 30-day hospital readmission was 7 days, IQR: 3, 15 (Figure 2) and the median length of stay for readmissions was 5 days, IQR: 2, 14. Of 30-day readmissions, 21 (33%) survivors required ICU care and 10 (16%) expired or were transitioned to hospice during the readmission. Follow-up appointments were not arranged for 26 readmitted patients, and 17 were readmitted prior to their scheduled follow-up.
Figure 2.
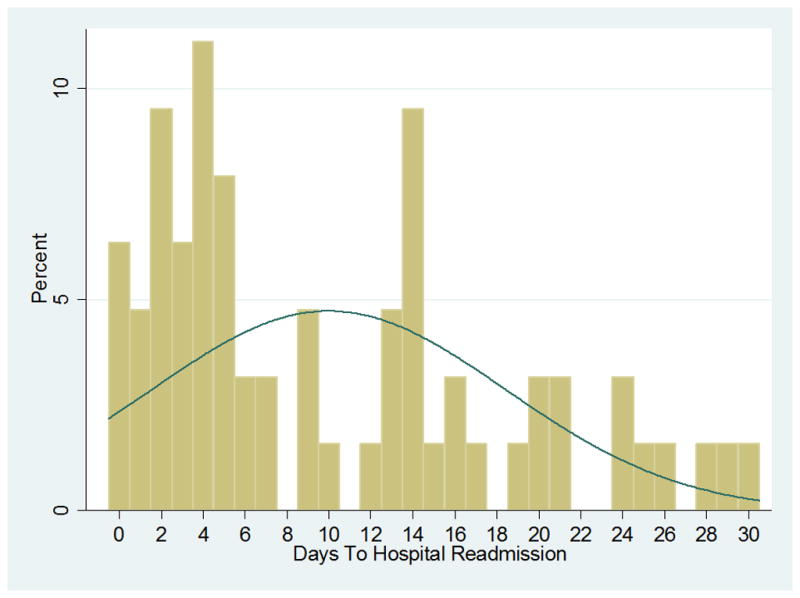
Timing of 30-day hospital readmission in severe sepsis survivors.
The reasons for readmission were potentially related to the index septic shock hospitalization in 78% (49/63) of cases (Table 3). The most common cause was infection, accounting for 46% of 30-day readmissions, followed by cardiovascular or thromboembolic at 18%. The infectious causes for readmission varied, with cellulitis and soft tissue infections being the most common (28%), followed by pneumonia (24%), gastrointestinal infections (17%), and bacteremia (17%).
Table 3.
Readmission diagnoses following hospitalization for septic shock.
| Readmission Diagnoses Potentially Related to Prior Sepsis Hospitalization, n(%) | |
| Infection * | 29 (46.0) |
| Cardiovascular and thromboembolic † | 11 (17.5) |
| Acute kidney injury‡ | 4 (6.4) |
| Complications of devices | 2 (3.2) |
| Other£ | 3 (4.8) |
| Readmission Unrelated to Prior Hospitalization | |
| Related to comorbid condition** | 14 (22.2) |
| Total: N = 63 | |
Infectious cases included: 8 skin or soft-tissue infections (3 cellulitis cases and 5 abscess cases), 7 respiratory infections (6 pneumonia cases and 1 empyema case), 5 gastrointestinal infections (2 new clostridium difficile cases, 2 recurrent cases, and 1 spontaneous bacterial peritonitis case), 5 bacteremia infections (including 3 line-related cases), 2 urinary tract infections, and 2 systemic infections (one culture-negative sepsis case and one disseminated candidiasis case).
Cardiovascular and thromboembolic cases included 4 cases of venous thromboembolism, 3 cases of congestive heart failure, 1 cerebrovascular accident, 1 case of venous sinus thrombosis, 1 acute coronary syndrome, and 1 cardiac arrest.
Acute kidney injury includes 1 case of acute interstitial nephritis due to treatment for endocarditis diagnosed during prior hospitalization and 1 case of concomitant hypotension at time of readmission.
Other diagnoses include subarachnoid haemorrhage status post fall, altered mental status due to medication error, and anemia requiring transfusion.
Categorization includes 5 oncology cases readmitted for failure to thrive or chemotherapy, 2 cases of progressive liver disease, 2 cases of chronic pain, 1 case of malignant pericardial effusion, 1 case of gout, 1 case of nephrolithiasis, 1 case of recurrent small bowel obstruction after clostridium difficile enterocolitis, and 1 case of hydrocephalus requiring the placement of a shunt.
Characteristics of Patients Readmitted Within 30 Days
Compared to those not readmitted, readmitted patients were more likely to have been hospitalized within the prior 30 days of the index septic shock hospitalization (p<0.001), have cirrhosis (p=0.05), an oncology diagnosis (p<0.001), lower initial serum lactate levels (p=0.02), higher APACHE II scores (p=0.05), longer hospital lengths of stay (p=0.01), and were more likely to be discharged with a PICC (p=0.02) (Table 4). Although readmitted patients had more comorbidities as measured by the Charlson (p=0.001), the association was non-significant when the oncology subscore was removed (p=0.67). There were no significant differences between the two groups in ED quality measures, other metrics of illness severity, or admission year (p=0.46).
Table 4.
Patient-level factors associated with 30-day hospital readmission in survivors of septic shock.
| No Readmission (N=206) | Readmissions (N=63) | p- value | |
|---|---|---|---|
| Clinical Factors
| |||
| Age, years | 59 ± 18 | 60 ± 16 | 0.75 |
| Female sex (n, %) | 100 (48.5) | 35 (55.6) | 0.33 |
| Race (n, %) | |||
| White | 84 (41.4) | 28 (46.7) | 0.74 |
| Black | 113 (55.7) | 31 (51.7) | |
| Other | 6 (3.0) | 1 (1.7) | |
| Comorbidities (n, %) | |||
| CAD | 25 (12.1) | 8 (12.7) | 0.90 |
| CHF | 26 (12.6) | 9 (14.3) | 0.73 |
| Cirrhosis | 7 (3.4) | 6 (9.5) | 0.05 |
| Chronic renal disease | 23 (11.2) | 8 (12.7) | 0.74 |
| COPD | 16 (7.8) | 5 (7.9) | 0.96 |
| Diabetes mellitus | 68 (33.0) | 17 (27.0) | 0.37 |
| ESRD | 16 (7.8) | 4 (6.4) | 0.71 |
| HIV | 9 (4.4) | 2 (3.2) | 1.00 |
| Hypertension | 95 (46.8) | 31 (49.2) | 0.74 |
| Oncology | 43 (20.9) | 29 (46.0) | <0.001 |
| Transplant | 29 (14.1) | 8 (12.7) | 0.78 |
| Charlson Comorbidity Index | 1 (1 – 3) | 2 (1 – 3) | 0.001 |
| Prior hospitalization within 30 days | 43 (20.9) | 28 (44.4) | <0.001 |
| Illness Severity at Presentation | |||
| Organ dysfunction | |||
| Acute kidney injury (n, %) * | 87 (42.2) | 23 (36.5) | 0.42 |
| Change in mental status | 57 (27.7) | 17 (27.0) | 0.92 |
| Coagulation failure * | 34 (16.5) | 15 (23.8) | 0.19 |
| Hematologic failure * | 18 (8.7) | 7 (11.1) | 0.57 |
| Hepatic failure * | 8 (3.9) | 4 (6.4) | 0.48 |
| Hypoperfusion, lactate (mmol/L) | 4.8 (4.0 – 6.3) | 4.2 (2.5 – 5.3) | 0.02 |
| Refractory hypotension | 70 (34.0) | 25 (39.7) | 0.41 |
| Admission to ICU | 157 (76.2) | 50 (79.4) | 0.60 |
| APACHE II score | 18 ± 7 | 20 ± 6 | 0.05 |
| ED Processes of Care | |||
| Time to antibiotics, minutes | 114 (62 – 198) | 142 (81 – 224) | 0.29 |
| Volume resuscitation, cc | 3150 (2050 – 4350) | 3100 (2050 – 4150) | 0.44 |
| Transfusion (n, %) | 10 (4.8) | 2 (3.2) | 0.57 |
| EGDT initiated | 58 (28.2) | 22 (34.9) | 0.30 |
| Source of sepsis (n, %) | |||
| Bacteremia | 20 (9.7) | 7 (11.1) | 0.75 |
| Pneumonia | 37 (18.0) | 13 (20.6) | 0.63 |
| Genitourinary | 47 (22.8) | 10 (15.9) | 0.24 |
| Gastrointestinal | 40 (19.4) | 13 (20.6) | 0.83 |
| Skin or soft-tissue | 13 (6.3) | 8 (12.7) | 0.10 |
| Hospitalization | |||
| Hospital length of stay | 6 (4 – 13) | 10 (5 – 15) | 0.01 |
| ICU length of stay† | 3 (1 – 5) | 2 (2 – 4) | 0.32 |
| Mechanical ventilation use (n, %) | 46 (22.3) | 8 (12.7) | 0.10 |
| PICC present at discharge | 15 (7.3) | 11 (17.5) | 0.02 |
| Discharge disposition | |||
| Home | 87 (42.2) | 18 (28.6) | 0.15 |
| Home with home services | 61 (29.6) | 24 (38.1) | |
| Skilled care facility | 58 (28.2) | 21 (33.3) | |
Definition of abbreviation: APACHE=acute physiology and chronic health evaluation; CAD=coronary artery disease; CHF=congestive heart failure; COPD=chronic obstructive pulmonary disease; HIV=human immunodeficiency virus; ICU=Intensive Care Unit; PICC=peripherally-inserted central catheter.
Categorical variables are presented as counts and percentages. Continuous variables are presented as means and standard deviations or median and interquartile ranges, as determined by their distribution.
Acute kidney injury defined as serum creatinine ≥ 0.5 mg/dL from baseline. Coagulation failure defined as international normalized ratio > 1.5 or PTT > 60 and hematologic failure as platelets < 100, and hepatic failure as total bilirubin > 4.0 mg/dL (25, 30). Coagulation measures and hepatic function measures were obtained in 78% and 90% of the cohort, respectively; when not assessed, they were presumed to be normal in accord with APACHE II score calculations (32). Refractory hypotension defined as systolic blood pressure ≤ 90 mm Hg after fluid resuscitation or use of vasoactive agents (5–7, 30).
ICU length of stay in subgroup of patients requiring ICU admission.
In multivariable models, independent risk factors associated with 30-day hospital readmission included: an oncology diagnosis (p=0.001), hospitalization within 30 days of the index septic shock hospitalization (p=0.01), and length of stay (p=0.04) (Table 5). Initial serum lactate levels were collinear with APACHE II scores. Neither initial lactate levels (p=0.07), nor APACHE II when substituted for lactate levels in multivariable models (p=0.58), were associated with 30-day readmission. In secondary analyses including variables with ≤10 observations per cell, cirrhosis was independently associated with 30-dayreadmission (p=0.04). In separate secondary analyses, including discharge disposition, use of mechanical ventilation, and coagulation failure as variables associated with the dependent variable at a less conservative significance level, an oncology diagnosis (p=0.001) and a hospitalization within 30 days of the index septic shock hospitalization (p=0.02) were the lone factors associated with 30-day readmission.
Table 5.
Clinical risk factors associated with 30-day hospital readmission in survivors of septic shock.
| Independent Variable * | Adjusted Odds Ratio (95% CI) | p-value |
|---|---|---|
| Oncology | 3.02 (1.60 – 5.68) | 0.001 |
| Recent hospitalization | 2.26 (1.19 – 4.29) | 0.01 |
| Length of stay, > 4 days | 2.18 (1.02 – 4.64) | 0.04 |
| Initial lactate levels (mmol/L) | 0.89 (0.78 – 1.01) | 0.07 |
| PICC at discharge | 1.68 (0.66 – 4.25) | 0.27 |
Definition of abbreviation: CI=confidence interval; PICC=peripherally-inserted central catheter.
An adjusted odds ratio of greater than 1 represents an increased risk of 30-day hospital readmission. The potential confounder, year of admission, was neither associated with the dependent variable nor did its inclusion alter the odds ratios of any of the candidate risk factors significantly. Conversely, a recent hospitalization attenuated the association between length of stay and 30-day readmission significantly.
Discussion
In this observational study of survivors of septic shock, our principal finding was that there was a high rate of hospital-based acute care use among patients discharged after an admission for septic shock. Three-quarters of hospital-based, acute care occurred within 15 days of discharge and these encounters often led to readmission. In sum, we found that 23% of at-risk survivors were readmitted within 30 days, substantially higher than the general 30-day readmission rate at HUP, and nearly one out of six readmissions resulted in death or a transition to hospice. Furthermore, an additional 5% returned to the ED for a treat-and-release visit.
These findings support and complement the recent findings of Liu et al (23) and Prescott et al (24). Using a claims-based approach to identify cases of sepsis at an integrated community-based healthcare system, Liu et al observed that 18% of sepsis survivors were rehospitalized within 30 days, with a median time to readmission of 11 days. The rate of readmission was observed to be as high as 22% in survivors in the highest predicted mortality quartile (23). In a separate claims-based investigation, Prescott et al. examined the post-discharge healthcare use of elderly survivors of severe sepsis within a longitudinal cohort (1998–2005) study. Using a design that would result in a slightly higher rate of readmission (i.e., repeat severe sepsis hospitalizations were included), Prescott and colleagues reported a rate of 30- and 90-day readmission rate of 26% and 41%, respectively, after a severe sepsis hospitalization (24). Survivors, in comparison to their pre-sepsis state and matched non-sepsis hospitalizations, experienced a substantial increase in healthcare use and greater post-discharge mortality in the year post-discharge (24).
Collectively, these findings suggest that sepsis survivors are a high-risk group that frequently requires hospital-based acute care after discharge. Importantly, whether these apparent risks are concentrated in the most severely ill survivors remains unclear. Nevertheless, the rate and pattern observed in these studies mirrors those seen in readmissions after heart failure, acute myocardial infarction, and pneumonia (33). While it is possible that high-quality discharge planning and short-term follow-up after discharge could play a substantial role in lowering the rate of readmissions after sepsis (46), two critical questions require examination to focus efforts: who is at risk to be readmitted and why?
Consistent with Liu et al (23), we found that a higher burden of comorbid conditions and a lengthy hospitalization, as a measure of illness severity, were associated with readmission. Specifically, and in line with hospital readmissions in general (33–35), we found that an oncologic diagnosis was both prevalent amongst septic shock survivors and independently associated with an increased risk of hospital readmission. Further, many septic shock survivors had been hospitalized prior to the index septic shock admission and this exposure was a risk factor for subsequent hospital readmission. Given evidence of a bidirectional relationship between cognitive and physical decline and an acute infectious insult (9, 47), further investigation is required to elucidate the role that hospital-based, acute care plays in the path to decline or recovery in survivors (48).
As to why survivors are readmitted, the initial suggestion that infection played a pivotal role emanated from a recent Healthcare Cost and Utilization Project (49). Therein, Sutton et al. reported that amongst hospital admissions with a primary or secondary diagnosis of septicemia, 16% of survivors were rehospitalized with the same condition and this scenario appeared to be increasing (46). More recently, while data was limited to 79% of readmissions, Liu et al found infection was the cause in 28–43% of cases (23). We found that septic shock survivors were frequently readmitted within 30 days with life-threatening conditions. Infections caused, or at least contributed to, readmission in 46% of cases. The source of infection varied, with a skin or soft-tissue infection or pneumonia accounting for approximately 50% of the infectious cases, and an additional 34% due to gastrointestinal infections or bacteremia. These observations complement the report by Liu et al (23), and are consistent with a small, recent study that suggested that infectious risk is increased in sepsis survivors (50). Additional common causes included cardiovascular and thromboembolic events and acute kidney injury. These findings, in concert with the available evidence (23–24), suggest that readmissions are the result of a synergistic process between pre-sepsis health conditions and sepsis-related sequelae. The latter influence, which requires direct examination, may be related to provisions of sepsis care or to residual organ dysfunction, new or progressive functional impairments, and/or the enduring immunosuppressive, inflammatory, and procoagulant response of sepsis (36–44).
Innovative interventions will be required to accelerate recovery and mitigate the apparent readmission risk amongst survivors. The optimal strategy will likely require a coordinated, comprehensive approach from diagnosis to discharge planning and follow-up. Components with the potential to reduce readmissions include an antibiotic stewardship program during the hospitalization and post-discharge (51–52), a longitudinal rehabilitation program to mitigate against functional impairments (53), early and frequent follow-up, and timely access to providers to effectively manage new conditions and complications (54). Comparative studies, designed to test the various potential strategies, will be essential.
There are several limitations to discuss. First, our study focused on septic shock patients admitted through the ED. Further studies, designed to examine the full spectrum of sepsis and those who develop sepsis during the hospitalization, are required to determine whether post-acute care needs are concentrated in the most severely ill. Second, while we designed our study to identify ED visits and hospital readmissions to any UPHS hospital and captured patients readmitted through inter-hospital transfer, we were unable to capture care provided outside of UPHS and therefore may have underestimated the rate of post-discharge resource utilization and ED visits specifically. Third, we did not fully account for the burden experienced by septic shock survivors. Future studies, designed to complement the recent work of Liu et al (23) and Prescott et al (24), will be necessary to more completely describe the survivor’s experience, utilization of services, and associated costs. Fourth, amongst the readmissions categorized as unavoidable, septic shock may have accelerated the decline. Therefore, our classification schema may have underestimated the deleterious effects of septic shock. Fifth, the risk factor analyses were limited by power and therefore the potential for a Type II error exists. Although we examined a multitude of factors at presentation and during the hospitalization, we acknowledge the threat of residual confounding and the potential for a Type I error. Additional study is warranted to examine factors (e.g., infectious source, provisions of care (e.g., duration of antibiotics, transfusions) associated with post-acute care use at discharge and readmission and how discharge disposition may modify this relationship.
In conclusion, the use of hospital-based acute care appears to be common in septic shock survivors. Encounters often occurred within 15 days of discharge, frequently led to readmission with a life-threatening condition, and appeared to result in substantial morbidity and mortality. Further studies are necessary to validate these findings and to identify effective strategies to address the post-acute care needs of survivors.
Acknowledgments
Funding: The study was supported in part by National Institutes of Health, National Heart, Lung and Blood Institute (NIH NHLBI) Loan Repayment Program, Bethesda, MD
Footnotes
Disclosures: For each of the above authors, no financial or other potential conflicts of interest exist related to the work.
Copyright form disclosures: Dr. Mikkelsen received support for article research from the National Institutes of Health (NIH), received support as a NIH Loan Repayment Program Awardee, and consulted for AnsunBiopharma. His institution received grant support from the NIH U01 Trial (site primary investigator). Dr. Ortego is employed by Bellevue Hospital. Dr. Halpern’s institution received grant support from the NIH. Dr. Christie received support for article research from the NIH. His institution received grant support from Glaxosmithkline (funding for clinical trials and an ongoing sepsis study called Galaxy ALI) and the NHLBI. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the Incidence and Mortality of Severe Sepsis in the United States. Crit Care Med. 2013;41:1167–74. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 2.Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson EK, Rubenstein AR, Radin GT, et al. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. 2014;42(3):625–31. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014 doi: 10.10001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 5.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 6.Jones AE, Shapiro NI, Trzeciak S, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. New Engl J Med. 2014 doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis among older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winters BD, Eberlein M, Leung J, et al. Long-term mortality and quality of life in sepsis; A systematic review. Crit Care Med. 2010;38(5):1276–1283. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 11.Cuthbertson BH, Elders A, Hall S, et al. Mortality and quality of life in the five years after severe sepsis. Crit Care. 2013;17(2):R70. doi: 10.1186/cc12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson S, Ruokonen E, Varpula T, et al. Long-term outcome and quality-adjusted life years after severe sepsis. Crit Care Med. 2009;37(4):1268–1274. doi: 10.1097/CCM.0b013e31819c13ac. [DOI] [PubMed] [Google Scholar]
- 13.Hofhuis JG, Spronk PE, van Stel HF, et al. The impact of severe sepsis on health-related quality of life: a long-term follow-up study. Anesth Analg. 2008;107(6):1957–64. doi: 10.1213/ane.0b013e318187bbd8. [DOI] [PubMed] [Google Scholar]
- 14.Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation. A cohort study. Ann Intern Med. 2010;153:167–75. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rising KL, White LF, Fernandez WG. Emergency Department Visits After Hospital Discharge: A Missing Part of the Equation. Annals Emerg Med. 2013;62(2):145–150. doi: 10.1016/j.annemergmed.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Vashi AA, Fox JP, Carr BG, et al. Use of hospital-based acute care among patients recently discharged from the hospital. JAMA. 2013;309(4):364–371. doi: 10.1001/jama.2012.216219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mechanic R. Post-acute care – the next frontier for controlling medicare spending. N Engl J Med. 2014;370(8):692–694. doi: 10.1056/NEJMp1315607. [DOI] [PubMed] [Google Scholar]
- 18.Ashton CM, Kuykendall DH, Johnson ML, et al. The association between the quality of inpatient care and early readmission. Ann Intern Med. 1995;122(6):415–421. doi: 10.7326/0003-4819-122-6-199503150-00003. [DOI] [PubMed] [Google Scholar]
- 19.Ashton CM, Del Junco DJ, Souchek J, et al. The association between the quality of inpatient care and early readmission: a meta-analysis of the evidence. Med Care. 1997;35(10):1044–1059. doi: 10.1097/00005650-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Medicare & Medicaid Services. Medicare Hospital Quality Chartbook 2011: Performance Report on Readmission Measures for Acute Myocardial Infarction, Heart Failure, and Pneumonia. Washington, DC: Centers for Medicare & Medicaid Services; 2011. [Google Scholar]
- 21.Lee CM, Herridge MS, Matte A, et al. Education and support needs during recovery in acute respiratory distress syndrome. Crit Care. 2009;13:R153. doi: 10.1186/cc8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elixhauser A, Steiner C. HCUP Statistical Brief #161. Agency for Healthcare Research and Quality; Rockville, MD: Apr, 2013. Readmissions to U.S. hospital by diagnosis, 2010. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb153.pdf. [PubMed] [Google Scholar]
- 23.Liu V, Lei X, Prescott HC, et al. Hospital readmission and healthcare utilization following sepsis in community settings. J Hosp Med. 2014 doi: 10.1002/jhm.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prescott HC, Langa KM, Liu V, et al. Increased one-year health care utilization in survivors of severe sepsis. Amer J Respir Crit Care Med. 2014 doi: 10.1164/rccm.201403-0471OC. [DOI] [PMC free article] [PubMed]
- 25.Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–1675. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 26.Mikkelsen ME, Gaieski DF, Goyal M, et al. Factors associated with non-adherence with early goal-directed therapy in the emergency department. Chest. 2010;138:551–558. doi: 10.1378/chest.09-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045–1053. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 28.Whittaker SA, Mikkelsen ME, Gaieski DF, Koshy S, Kean C, Fuchs BD. Severe sepsis cohorts derived from claims-based strategies appear to be biased toward a more severely ill patient population. Crit Care Med. 2013;41:945–953. doi: 10.1097/CCM.0b013e31827466f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 30.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 33.Dharmarajan K, et al. Diagnosis and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309 (4):355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donze J, Aujesky D, Williams D, et al. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638. doi: 10.1001/jamainternmed.2013.3023. [DOI] [PubMed] [Google Scholar]
- 35.Goldfield NI, McCullough EC, Hughes JS, et al. Identifying potentially preventable readmissions. Health Care Finan Review. 2008;30(1):75–91. [PMC free article] [PubMed] [Google Scholar]
- 36.Yende S, Angus DC. Long-term outcomes from sepsis. Curr Infect Dis Rep. 2007;9(5):382–386. doi: 10.1007/s11908-007-0059-3. [DOI] [PubMed] [Google Scholar]
- 37.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77(6):527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yende S, D’Angelo G, Mayr F, et al. Elevated hemostasis markers after pneumonia increases one-year risk of all-cause and cardiovascular deaths. PLoS One. 2011;6(8):e22847. doi: 10.1371/journal.pone.0022847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yende S, D’Angelo G, Kellum JA, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177(11):1242–1247. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon PM, Delude RL, Lee M, et al. Duration and magnitude of hypotension and monocyte deactivation in patients with community-acquired pneumonia. Shock. 2011;36(6):553–559. doi: 10.1097/SHK.0b013e318235331e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward PA. Immunosuppression in sepsis. JAMA. 2011;306(23):2618–2619. doi: 10.1001/jama.2011.1831. [DOI] [PubMed] [Google Scholar]
- 42.Yende S, Linde-Zwirble W, Mayr F, et al. Risk of cardiovascular events in severe sepsis survivors. Am J Respir Crit Care Med. 2014;189(9):1065–1074. doi: 10.1164/rccm.201307-1321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwashyna TJ, Netzer G, Langa KM, et al. Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185(8):835–841. doi: 10.1164/rccm.201109-1660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walkey AJ, Wiener RS, Ghobrial JM, et al. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306(20):2248–2254. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Amer J of Epid. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 46.Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281:613–620. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- 47.Shah FA, Pike F, Alvarez K, et al. Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med. 2013;188(5):586–592. doi: 10.1164/rccm.201212-2154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwashyna TJ. Trajectories of recovery and dysfunction after acute illness, with implications for clinical trial design. Am J Respir Crit Care Med. 2012;186(4):302–304. doi: 10.1164/rccm.201206-1138ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sutton J, Friedman B. HCUP Statistical Brief #161. Agency for Healthcare Research and Quality; Rockville, MD: Sep, 2013. Trends in septicemia hospitalizations and readmissions in selected HCUP states, 2005 and 2010. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb161.pdf. [PubMed] [Google Scholar]
- 50.Wang T, Derhovanessian A, De Cruz S, et al. Subsequent infections in survivors of sepsis: epidemiology and outcomes. J Intensive Care Med. 2014;29(2):87–95. doi: 10.1177/0885066612467162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 52.Keller SC, Ciuffetelli D, Bilker W, et al. The impact of an infectious diseases transition service on the care of outpatients on parenteral antimicrobial therapy. J Pharm Tech. 2013;29(5):205–214. doi: 10.1177/8755122513500922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomized, controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin CY, Barnato AE, Degenholtz HB. Physician follow-up visits after acute care hospitalization for elderly Medicare beneficiaries discharged to non-institutional settings. J Am Geriatr Soc. 2011;59(10):1947–1954. doi: 10.1111/j.1532-5415.2011.03572.x. [DOI] [PubMed] [Google Scholar]


