Abstract
Background
Hunger enhances sensitivity to reward, yet individuals with anorexia nervosa (AN) are not motivated to eat when starved. This study examined whether diminished response to reward could underlie food restriction in AN by investigating brain response to rewards during hunger and satiated states.
Methods
Using a delay discounting monetary decision task known to discriminate brain regions contributing to processing of immediate rewards and cognitive control important for decision making regarding future rewards, we compared 23 adults remitted from AN (to reduce the confounding effects of starvation [RAN]) to 17 healthy women (CW). Monetary rewards were used because the rewarding value of food may be confounded by anxiety in AN.
Results
Interactions of group (RAN, CW) × visit (hunger, satiety) revealed that, for CW, hunger significantly increased activation in reward salience circuitry (ventral striatum, dorsal caudate, anterior cingulate cortex) during processing of immediate reward, whereas satiety increased activation in cognitive control circuitry (ventrolateral prefrontal cortex, insula) during decision-making. In contrast, brain response in reward and cognitive neurocircuitry did not differ during hunger and satiety in RAN. A main effect of group revealed elevated response in the middle frontal gyrus for RAN.
Conclusions
RAN failed to increase activation of reward valuation circuitry when hungry and showed elevated response in cognitive control circuitry independent of metabolic state. Decreased sensitivity to hunger’s motivational drive may explain AN individuals’ ability to restrict food when emaciated. Moreover, difficulties in valuating emotional salience may contribute to inabilities to appreciate the risks inherent in this deadly disorder.
Keywords: anorexia nervosa, decision making, delay discounting, eating disorders, functional MRI, reward processing
INTRODUCTION
Anorexia nervosa (AN) is characterized by restricted eating, severe emaciation, and disturbed body image.(1) Individuals with AN can severely restrict their caloric intake for years. In contrast, most people have difficulty adhering to a diet, with a high rate of recidivism after losing weight. How are AN individuals able to ignore signals regarding hunger that otherwise motivate eating, even when severely emaciated?
One clue may be the tendency for AN individuals to be anhedonic, finding little in life that is rewarding aside from the pursuit of weight loss. AN individuals are often temperamentally inhibited, constrained, and over-concerned with consequences.(2) Such behaviors suggest that disturbances of reward or pleasure,(3, 4) coupled with alterations in neurocircuitry supporting inhibition and cognitive control, underlie AN behavior,(2, 5, 6) such as a propensity to override signals regarding hunger and energy deficits. For example, ill AN adolescents(4) and recovered AN adults(3) failed to differentiate monetary wins and losses in ventral striatal regions, suggesting an impaired ability to identify the emotional significance of salient stimuli. This is consistent with studies showing limbic regions are underactive for motivational behavior in ill AN.(6)
Delay discounting tasks are a common behavioral metric for examining decision making in relation to rewarding stimuli because they assess the degree to which participants suppress the desire for smaller-sooner rewards in order to obtain larger rewards at a later time. Behavioral studies of delay discounting have shown that both ill AN adults and individuals with obsessive compulsive personality disorder(7) have an enhanced ability to delay reward compared to healthy peers,(8) whereas most disorders (e.g., substance abuse, ADHD, obesity) demonstrate increased discounting.
Functional neuroimaging studies of delay discounting(9) have identified several brain systems involved in emotional and cognitive valuation of a range of salient stimuli, including food, money, and drugs.(10, 11) The ventral striatum, rostral (i.e., ventromedial prefrontal cortex [VMPFC]) and dorsal anterior cingulate cortex (ACC), caudate nucleus, and posterior cingulate cortex (PCC) are associated with reward valuation, especially for more immediate rewards.(9, 12, 13) Another network that includes the dorsolateral (DLPFC; including the middle frontal gyrus [MFG]) and ventrolateral prefrontal cortex (VLPFC), insula, and posterior parietal cortex, is associated with cognitive control and is consistently engaged in delay discounting tasks, with less dependence on whether rewards are immediate or not.(9, 12, 13)
This study is the first to investigate delay discounting neural processing in individuals remitted from AN. Because the response to food in AN may be confounded by poorly understood factors such as anxiety, obsessions, or body image distortions, we reasoned that response to money might be a better test of response to salient rewarding stimuli. We examined only adults who were remitted from AN to avoid the confounding effects of malnutrition, and because studies(2) show traits contributing to disordered eating (e.g., anxiety, harm avoidance) persist after recovery.
Hunger and satiety may influence behavioral choice by manipulating the appetitiveness of food and monetary cues in healthy participants. Imaging studies(14, 15) suggest that hunger increases the motivational aspects of stimuli by activating regions associated with reward or reducing top-down inhibitory control. In contrast, satiety may reduce the rewarding value of stimuli, perhaps through decreased responsiveness of limbic circuitry or greater cognitive control. Hunger in healthy participants increases rates of delay discounting,(16) reduces risk-aversion,(17) and can lead to overvaluation of unhealthy, higher-calorie foods.(18) In animals, food deprivation enhances sensitivity to drugs of abuse,(19, 20) suggesting hunger enhances preference for more immediate rewards.
The effects of fasting on frontostriatally-mediated neural substrates of decision-making have not been assessed in AN or healthy volunteers. To examine whether diminished response to reward could underlie food restriction in AN, this study used fMRI to investigate brain activation during delay discounting in healthy women (CW) and adults remitted from AN (RAN) when hungry and satiated. The purpose of the current study was to: 1) elucidate the modulation of activation in regions involved in delay discounting by hunger and satiety, and 2) determine whether CW and RAN differ in their response to hunger and satiety during delay discounting. We hypothesized an interaction between group and metabolic state whereby RAN would show reduced response to immediate reward when hungry in regions associated with reward valuation, and increased response to decision-making independent of hunger state in regions associated with cognitive control. Revealing brain reward mechanisms in RAN will advance understanding of the neurobiology underlying the puzzling symptoms of AN and help guide disease-specific treatment development strategies.
METHODS AND MATERIALS
Subjects
Twenty-three women remitted from AN (RAN; 16 restricting subtype, 7 restricting-purging subtype), with remittance defined (see Wagner(3)) as maintaining a weight above 85% of average body weight, regular menstrual cycles, and no binge eating, purging, or restrictive eating patterns for at least 1 year prior to study, were compared (Table 1) to 17 age- and weight-matched healthy comparison women (CW). RAN participants were recruited from a larger eating disorder study at UCSD, and CW were recruited from the community through local advertisements. Any previous life-time DSM-IV Axis I diagnosis was determined (see Table 1), but no subject had a current DSM-IV diagnosis, or history of alcohol or drug abuse or dependence 3 months prior to study; medical or neurologic concerns; or conditions contraindicative to MRI. None of the participants took psychotropic medication within 3 months prior to the study. The study was conducted according to the IRB regulations of the University of California, San Diego, and, after complete description of the study to the subjects, written informed consent was obtained.
Table 1.
Participant demographics and characteristics.
| Characteristic | CW (N=17) | RAN (N=23) |
t or χ2 value |
DF |
p value |
Cohen's d |
|---|---|---|---|---|---|---|
| Scanner | 0.00 | 1.00 | ||||
| Signa Excite | 10 | 14 | ||||
| MR 750 | 7 | 9 | ||||
| Demographics | ||||||
| Age | 25.3 ± 1.4 [20.6–40.9] | 27.7 ± 1.6 [19.1–45.7] | −1.2 | 38.0 | 0.3 | −0.4 |
| BMI | 22.6 ± 0.7 [18.5–29.5] | 21.6 ± 0.3 [18.9–24.2] | 1.4 | 24.6 | 0.2 | 0.5 |
| Education (years) | 15.6 ± 0.3 [13–19] | 16.8 ± 0.7 [13–27] | −1.6 | 29.3 | 0.1 | −0.5 |
| WASI IQ estimate | 112.1 ± 3.0 [89–136] | 112.7 ±2.9 [85–133] | −0.2 | 35.9 | 0.9 | −0.1 |
| Estradiol (pg/mL)a | 15.3 ± 3.1 [1.0–37.0] | 19.5 ± 3.3 [1.0–45.0] | −0.9 | 35.4 | 0.4 | −0.3 |
| Lifetime Diagnoses (n) | ||||||
| MDD | 0 | 17 | 18.9 | 1 | <0.001 | |
| Any anxiety disorder | 0 | 8 | 5.4 | 1 | 0.02 | |
| OCD | 0 | 4 | 1.6 | 1 | 0.20 | |
| Past drug/alcohol abuse/dependencea | 0 | 3 | 0.9 | 1 | 0.4 | |
| Alcohol (%)a | 0 | 7.9 | 0.9 | 1 | 0.4 | |
| Cannabis (%)a | 0 | 2.6 | 0.0 | 1 | 1.0 |
Note: Entries are of the form mean +/− SEM [min-max]. Statistical comparisons were either by means of Welsh t-tests or χ2 test for equality of proportions.
BMI: body mass index; WASI: Wechsler Abbreviated Scale of Intelligence; CW: healthy comparison women; DF: degrees of freedom; MDD: major depressive disorder; OCD: obsessive-compulsive disorder; RAN: women remitted from anorexia nervosa.
Any anxiety disorder defined as having had at least one prior episode of panic disorder, phobia, post-traumatic stress disorder, generalized anxiety disorder, or any anxiety disorder NOS. Any alcohol defined as any history of abuse or dependent per DSM-IV criteria.
One CW and 1 RAN did not complete this assessment.
Experimental design
Participants performed a delay discounting task(9) (Supplemental Figure S1) during fMRI on 1 of 2 scanners on 2 visits 24 hours apart. For the hungry state, participants fasted for 16 hours prior to the scan session. During the satiated state, participants consumed a personalized, standardized breakfast (determined by the individual’s BMI and containing 30% of overall daily caloric needs or approximately 450 kilocalories, with a macronutrient distribution of 53% carbohydrates, 32% fat, and 15% protein) 2 hours prior to the 9 am scan session. Subjects were housed and meals were provided by the UCSD Clinical & Translational Research Institute to ensure 100% compliance with this diet. The order of visits was randomized across participants and performed in the early follicular phase.
Delay discounting task
Two functional runs of 488 sec each were performed during each visit. For each 15 sec trial, participants were presented with two choices on either side of the screen; each choice included a monetary amount and a time delay for receiving this amount (Supplemental Figure S1). The first two trials within each run were fixed to allow participants to acclimate to the task. The first trial required participants to choose between the same dollar amount available at two different delays (i.e., $27.10 available in 1 week vs. $27.10 available in 1 month), and two dollar amounts in which the smaller earlier amount was less than 1% of the delayed value (i.e., $0.16 today vs. $34.04 in 6 weeks). The remaining 30 trials within each run were randomly ordered. The following parameters were used: the delay to the early reward, d, was selected from the set {today, 2 weeks, 4 weeks}. The delay between the late reward, d’, and the early reward (i.e., d’-d) was selected from the set (2 weeks, 4 weeks), provided that the late reward occurred no more than 6 weeks from the time of the study (that is, trials with the early choice at 4 weeks and with a 4 week delay for the later choice were excluded). The percent difference in amount between the two rewards (i.e., ($R’-$R)/$R) was selected from the set {3%, 5%, 10%, 15%, 25%, 35%}. Consistent with McClure,(9) at the end of the experiment, one completed trial was chosen at random, and the participant received the selected reward at the specified temporal delay.
Delay discounting task performance
To determine whether choice behavior differed between the two groups, a Group × Visit × Percent Monetary Difference linear mixed-effects (LME) analysis was computed using the nlme package in R (http://www.r-project.org). To examine group differences in response time due to choice difficulty, data were submitted to a Group × Visit × Difficulty (Hard, Easy) LME analysis.
MRI Protocol
Functional images were acquired axially using T2* weighted echo planar imaging (EPI) with an 8-channel head coil. Imaging data were collected on one of two scanners: a 3T Signa HDx (GE Medical Systems, Milwaukee, WI) (TR = 2000 ms, TE = 30 ms, flip angle = 80°, 64 × 64 matrix, ASSET factor = 2, 40 2.6-mm ascending interleaved slices with a 0.4-mm gap, 244 volumes) and a 3T GE Discovery MR 750 (GE Medical Systems, Milwaukee, WI) (TR = 2000 ms, TE = 30 ms, flip angle = 80°, 64 × 64 matrix, ASSET factor = 2, 40 3.0-mm ascending interleaved slices, 244 volumes). The first four volumes of each run were discarded to discount T1 saturation. EPI-based field maps were acquired to correct for susceptibility-induced geometric distortions. High-resolution T1-weighted fast spoiled gradient echo (FSPGR) anatomical images (Signa HDx: TR=7.7 ms, TE=2.98 ms, flip angle=8°, 192 × 256 matrix, 172 1 mm slices; MR 750: TR=8.1s, TE=3.17ms, flip angle=8°, 256×256 matrix, 172 1 mm slices) were obtained sagittally for subsequent spatial normalization and activation localization. Multisite imaging studies suggest that inter-participant variance far outweighs that of site or magnet variance. To control for potential differences due to magnet hardware, groups were balanced across magnets (Table 1), each participant was scanned on the same scanner for both visits, and subject was nested within scanner and treated as a random effect in subsequent analyses.
MRI statistical analysis
Functional images were preprocessed and analyzed using Analysis of Functional NeuroImages (AFNI) software, and group analyses were performed in R. EPI images were motion-corrected and aligned to high-resolution anatomical images with align_epi_anat.py. Outliers were generated using AFNI’s 3dToutcount. Volumes with more than 10% of the voxels marked as outliers were censored from subsequent analyses. Approximately 2.3% of all volumes were censored overall (for all subjects: M = 11.0 volumes; SD = 4.5; range = 1–25). Registration to the MNI-152 atlas was performed using FMRIB's Non-linear Image Registration Tool (FNIRT), part of FSL (http://fsl.fmrib.ox.ac.uk/fsl/). The modeled hemodynamic responses were subsequently scaled so that beta weights would be equivalent to percent signal change (PSC). Data were spatially blurred with a 4.2 mm FWHM spatial filter.
Statistical analyses were performed based on the approach in McClure et al.(9) using 2 separate general linear models (GLMs), with individual events (i.e., onset of each choice trial) modeled using AFNI’s SPMG3 function, which convolves the hemodynamic response with a gamma variate basis function. To model reward valuation response (e.g., incentive of immediate rewards or impatience), the first GLM (i.e., beta regressor) included only decision trials in which the early reward option was available immediately (i.e., “Today”). To model cognitive control response (e.g., deliberate decision-making or patience), a second GLM (i.e., delta regressor) included all decision trials. Six motion parameters (3 rotations and 3 translations) were used as nuisance regressors to account for motion artifact.
Regions of interest (ROIs) were based upon prior findings.(9, 12, 13) ROIs associated with valuation included the ventral striatum, dorsal anterior caudate, rostral (aka VMPFC) and dorsal ACC, and PCC. The ROIs associated with cognitive control included the superior posterior parietal cortex, MFG (including the DLPFC and premotor cortex), insula, and VLPFC (see Supplement for details).
We employed a Group (RAN, CW) × Visit (Hungry, Satiated) LME analysis in R for the valuation and cognitive models separately within their respective ROIs. Each ROI was treated as a search region. Subjects were nested within scanner and treated as random effects, with Group and Visit as fixed effects. Small volume correction was determined with Monte-Carlo simulations (via AFNI’s 3dClustSim) to guard against false positives. Minimum cluster sizes required to achieve an a posteriori ROI-wise probability of p < 0.05, with an a priori voxel-wise probability of p < 0.05 are listed in Table 2. Post hoc analyses were conducted using glht from the multcomp package in R to calculate general linear hypotheses using Tukey’s all-pair comparisons.(21) Within the RAN group, exploratory logistic regressions using the mean PSC within each significant cluster resulting from the Group × Visit LME analysis and presence/absence of a lifetime history of major depressive disorder (MDD) or anxiety disorder were performed separately for each visit to determine whether past psychiatric morbidity influenced current results.
Table 2.
Analysis of variance results within regions of interest demonstrating a main effect of group, a main effect of visit, and an interaction of group with visit for the valuation and cognition circuitry. Coordinates are presented in RAI format.
| Analysis of variance | Post hoc Comparisons |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak MNI Coordinates |
||||||||||||
| Region | L/R | BA | Volume (µL) |
Min cluster size (µL) |
x | y | z |
peak F |
Cohen’s d |
Contrast | z | p |
| VALUATION CIRCUITRY (BETA REGRESSOR) | ||||||||||||
| Group (RAN vs CW) | ||||||||||||
| Anterior Cingulate | L | 33/24 | 1080 | 240 | −12 | 30 | 18 | 13.3 | 1.2 | n.s. | ||
| R | 33/24 | 376 | 240 | 16 | 18 | 28 | 6.7 | 0.8 | n.s. | |||
| Posterior Cingulate | L | 23 | 296 | 226 | −8 | −26 | 32 | 9.0 | 1.0 | n.s. | ||
| R | 31 | 880 | 218 | 14 | −24 | 34 | 9.2 | 1.0 | n.s. | |||
| Visit (Hungry vs Satiated) | ||||||||||||
| None | ||||||||||||
| Group × Visit | ||||||||||||
| Ventral Striatum | L | 576 | 128 | −6 | 12 | −4 | 9.4 | 1.0 | Satiated: RAN > CW | 2.2 | 0.1 | |
| R | 1136 | 128 | 16 | 22 | −8 | 8.1 | 0.9 | CW: Hungry > Satiated | 2.7 | 0.04 | ||
| Satiated: RAN > CW | 2.8 | 0.03 | ||||||||||
| Dorsal Anterior Caudate | L | 3192 | 168 | −18 | 22 | 8 | 15.5 | 1.3 | CW: Hungry > Satiated | 2.4 | 0.08 | |
| Satiated: RAN > CW | 2.7 | 0.03 | ||||||||||
| R | 4096 | 168 | 16 | 14 | 6 | 16.2 | 1.3 | CW: Hungry > Satiated | 2.8 | 0.02 | ||
| Satiated: RAN > CW | 3.2 | 0.008 | ||||||||||
| Anterior Cingulate | L | 32/24 | 1832 | 240 | −6 | 36 | −4 | 9.7 | 1.0 | CW: Hungry > Satiated | 2.4 | 0.07 |
| Satiated: RAN > CW | 3.1 | 0.01 | ||||||||||
| 33/24 | 1800 | −8 | 18 | 22 | 11.0 | 1.1 | CW: Hungry > Satiated | 2.8 | 0.02 | |||
| Satiated: RAN > CW | 3.3 | 0.01 | ||||||||||
| 24 | 984 | −2 | 0 | 46 | 7.1 | 0.8 | CW: Hungry > Satiated | 2.8 | 0.03 | |||
| Satiated: RAN > CW | 3.0 | 0.01 | ||||||||||
| R | 33/24/32 | 6408 | 240 | 10 | 14 | 28 | 18.7 | 1.4 | CW: Hungry > Satiated | 3.4 | 0.003 | |
| Satiated: RAN > CW | 3.8 | <0.001 | ||||||||||
| Posterior Cingulate | L | 31 | 2112 | 226 | −6 | −32 | 34 | 7.6 | 0.9 | n.s. | ||
| R | 31/23/24 | 4296 | 218 | 4 | −38 | 30 | 8.8 | 1.0 | n.s. | |||
| COGNITIVE CIRCUITRY (DELTA REGRESSOR) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group (RAN vs CW) | ||||||||||||
| Middle Frontal Gyrus | L | 8 | 1144 | 304 | −38 | 30 | 30 | 10.6 | 1.0 | RAN > CW | 2.8 | 0.005 |
| 6 | 528 | −48 | 8 | 40 | 7.1 | 1.0 | n .s. | |||||
| 6 | 376 | −38 | 20 | 54 | 13.1 | 1.2 | n.s. | |||||
| R | 6/8 | 2040 | 304 | 34 | 22 | 60 | 11.4 | 1.1 | RAN > CW | 2.9 | 0.004 | |
| 6 | 384 | 50 | 8 | 42 | 8.7 | 0.9 | n.s. | |||||
| Visit (Hungry vs Satiated) | ||||||||||||
| Middle Frontal Gyrus | L | 6/8 | 4920 | 304 | −32 | 12 | 42 | 15.3 | 1.3 | Satiated > Hungry | 2.9 | 0.004 |
| R | 8 | 312 | 304 | 38 | 24 | 34 | 7.4 | 0.9 | n.s. | |||
| Insula | L | 13 | 568 | 224 | −37 | 2 | 2 | 13.2 | 1.2 | Satiated > Hungry | 3.6 | <0.001 |
| 13 | 384 | −26 | 20 | −4 | 8.1 | 0.9 | Satiated > Hungry | 2.7 | 0.008 | |||
| 13 | 248 | −32 | −16 | 0 | 9.1 | 1.0 | Satiated > Hungry | 2.9 | 0.004 | |||
| Ventrolateral Prefrontal Cortex | L | 44/45 | 3000 | 240 | −54 | 18 | 14 | 14.0 | 1.2 | Satiated > Hungry | 2.8 | 0.005 |
| R | 45 | 240 | 224 | 56 | 26 | 20 | 7.6 | 0.9 | n.s. | |||
| Group × Visit | ||||||||||||
| Middle Frontal Gyrus | L | 6 | 600 | 304 | −30 | 8 | 62 | 11.1 | 1.1 | CW: Hungry > Satiated | 2.6 | 0.04 |
| Satiated: RAN > CW | 2.7 | 0.03 | ||||||||||
| Insula | L | 13 | 264 | 224 | −40 | −2 | 8 | 9.5 | 1.0 | CW: Satiated > Hungry | 3.6 | 0.002 |
| R | 13 | 456 | 224 | 40 | 10 | −4 | 8.5 | 0.9 | CW: Satiated > Hungry | 3.1 | 0.009 | |
| 13 | 320 | 34 | 8 | 8 | 10.0 | 1.0 | n.s. | |||||
| Ventrolateral Prefrontal Cortex | R | 45/47 | 264 | 224 | 38 | 26 | −6 | 8.7 | 0.9 | CW: Satiated > Hungry | 2.9 | 0.02 |
| Superior Parietal Cortex | L | 544 | 256 | −22 | −50 | 38 | 13.0 | 1.2 | n.s. | |||
| R | 408 | 248 | 22 | −54 | 48 | 9.0 | 1.0 | n.s. | ||||
Note: BA: Brodmann area; CW: healthy comparison women; L: left; n.s.: not significant; R: right; RAN: women recovered from anorexia nervosa.
Small volume correction was determined with Monte-Carlo simulations (via AFNI’s 3dClustSim) to guard against false positives. Post hoc analyses were conducted using glht from the multcomp package in R to calculate general linear hypotheses using Tukey’s all-pair comparisons.
RESULTS
Demographics and clinical assessments
Individuals within the RAN and CW groups were of similar age, body mass index, education, intelligence, and history of alcohol/drug use (Table 1). Consistent with previous findings,(2) the RAN group had a significantly higher frequency of lifetime MDD or anxiety disorder.
Behavioral analysis
Pre- and post-scan assessments
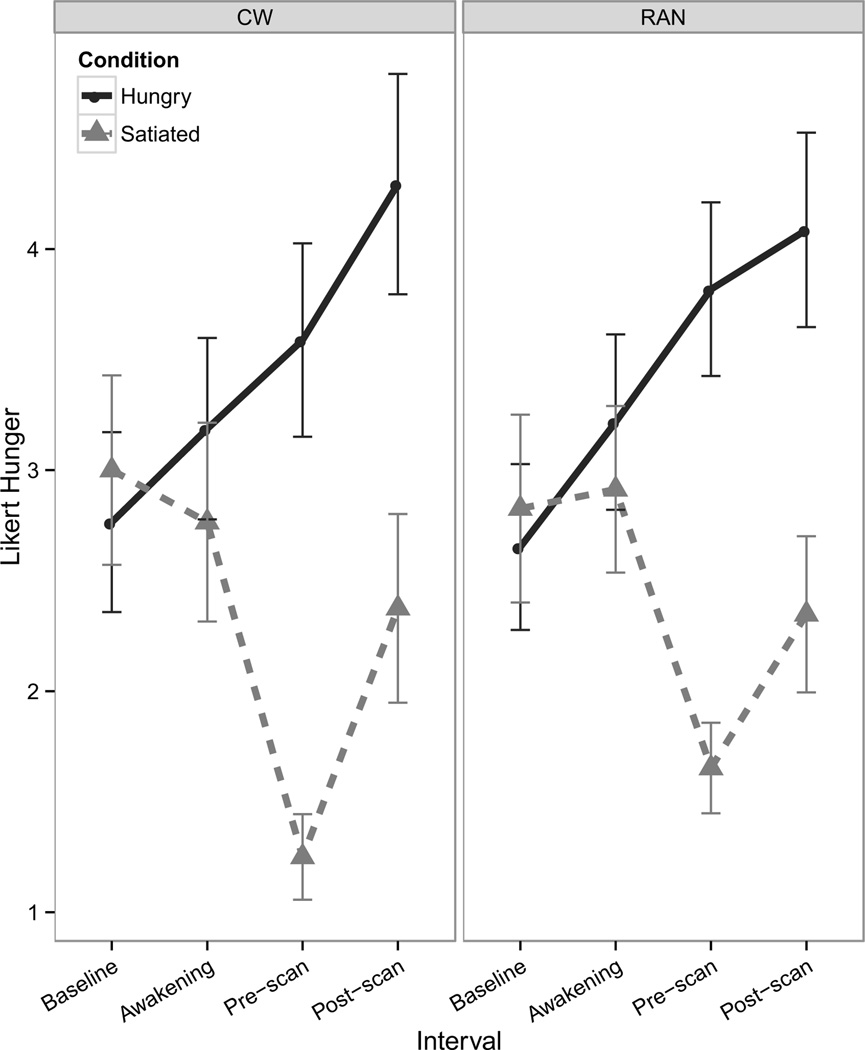
Participants reported significantly greater hunger during the Hungry condition relative to the Satiated condition (Figure 1; Supplemental Table S1).
Figure 1.
Line graphs reflecting self-report Likert visual analog scale values. Line graph of pre- and post-scan self-report measures of hunger shows a main effect of visit [F(1,111)=123.2, p<0.001, d=3.6] and of interval [F(1,111)=12.7, p=<0.001, d=1.1] resulting from a group (RAN, CW) × visit (Hungry, Satiated) × interval period (pre-scan, post-scan) LME. Participants reported greater hunger during the hungry condition relative to the satiated condition [z=6.0, p<0.001]; participants also tended to be more hungry at the post-scan interval relative to the pre-scan interval [z=1.8, p=0.08]. Error bars represent the standard error. CW: healthy comparison women; RAN: women recovered from anorexia nervosa.
Delay discounting task performance
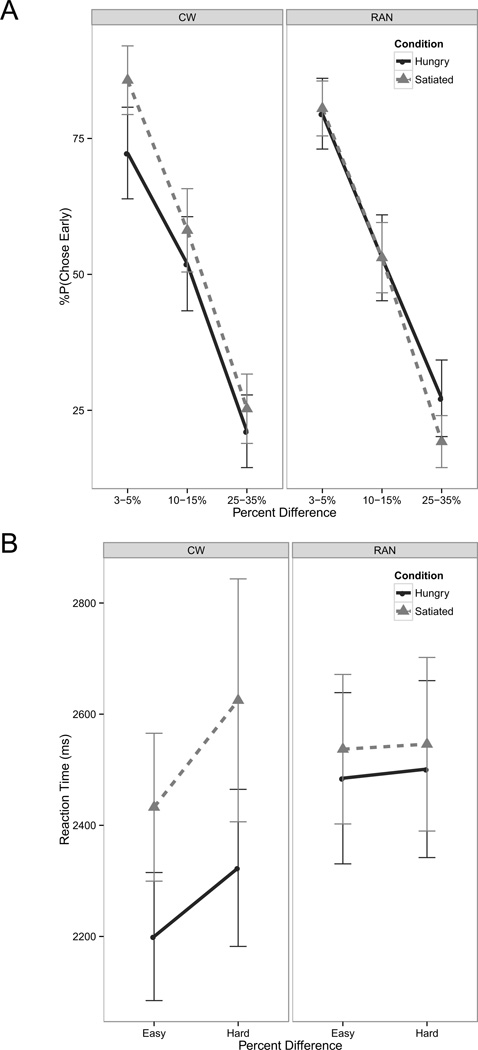
Participants were significantly less likely to choose the early option for choices with larger differences in the size of the monetary outcomes (Figure 2A). No significant group differences were found in choice behavior. CW responded significantly more slowly when satiated than when hungry, indicating greater deliberation to make a choice (Figure 2B). Response time in RAN when satiated was similar to CW, but was not significantly faster when hungry.
Figure 2.
Plots showing group differences in behavioral choice and their modulation by satiety. Hard choices were defined as those in which the probability of choosing the smaller-sooner was approximately 50%, and corresponded to difference in dollar amounts between 10 and 15%; all other choices were defined as easy. A) We examined the probability of choosing the early reward with respect to the percent difference in amount between the early and later choices. Participants showed a main effect of percent difference [F(2,190)=173.0, p<0.001, d=4.2], such that participants were less likely to choose the early option as the percent monetary difference between choices grew larger [3–5% – 10–15%: z=3.1, p=0.006; 3–5% – 25–35%: z=7.8, p<0.001; 10–15% – 25–35%: z=4.7, p<0.001]. There was also a significant interaction of group with visit [F(1,190)=4.2, p=0.04, d=0.7], but the post hoc analyses were not significant [all p>0.6]. B) For reaction time, there was a main effect of visit [F(1,114)=5.1, p=0.03, d=0.7], with participants showing a tendency for faster response times when hungry than when satiated [z=1.7, p=0.09]. There was also a trend for an interaction of group with satiety [F(1,114)=3.0, p=0.09, d=0.6]. Post hoc analyses found that this was due to CW having a faster response time when hungry [z=2.8, p=0.02]. RAN did not show this effect. RAN: women recovered from anorexia nervosa; CW: healthy comparison women.
ROI analyses
Valuation circuitry
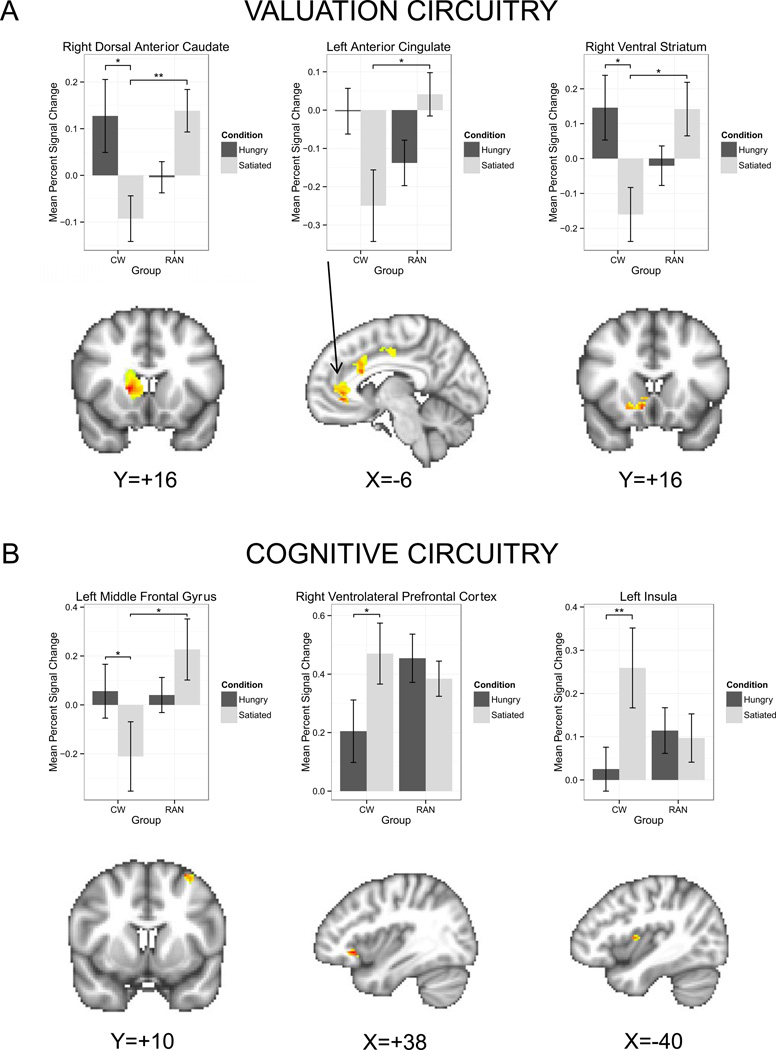
For the valuation circuitry (e.g., modeled brain response for choices including an immediate reward) we found a significant interaction of group with visit within the bilateral ventral striatum, dorsal anterior caudate, rostral and dorsal aspects of the ACC, and PCC (Table 2, Figure 3A). Post-hoc analyses revealed that for all but the left ventral striatum, the CW activated significantly more to immediate reward when hungry relative to when satiated, and CW response was less than RAN response when satiated in all ROIs. RAN response did not significantly differ between hunger and satiety (all p > 0.14), suggesting that these brain areas are less sensitive to metabolic state when determining the value of rewarding stimuli. Main effects of group and visit did not reach statistical significance in any ROI based on post-hoc analysis.
Figure 3.
Plots demonstrating a significant group × visit interaction within representative regions of interest. A) Valuation-related ROIs for the beta (“Today”) regressor. Left: Within the right dorsal anterior caudate, CW had an elevated response when hungry relative to when satiated [z=2.8, p=0.02], and, when satiated, RAN had a greater response relative to CW [z=3.2, p=0.008]. Middle: Within the rostral zone of the left anterior cingulate, CW had an elevated response when hungry relative to when satiated [z=2.4, p=0.07], and, when satiated, RAN had a greater response relative to CW [z=3.3, p=0.01]. Right: Within the right ventral striatum, CW had a greater response when hungry than when satiated [z=2.7, p=0.04], and RAN had a greater response than CW when satiated [z=2.8, p=0.03]. B) Cognitive-related ROIs for the delta (“All Decisions”) regressor. Left: Within the left middle frontal gyrus, CW responded more strongly when hungry than when satiated [z=2.6, p=0.04], and RAN responded more robustly than CW when satiated [z=2.7, p=0.03]. Middle: Within the right ventrolateral prefrontal cortex, CW responded more strongly when satiated than when hungry [z=2.9, p=0.02]. Right: Within the left insula, CW responded more strongly when satiated than when hungry [z=3.6, p=0.002]. Error bars represent the standard error for each group. CW: healthy comparison women; RAN: women remitted from anorexia nervosa. *p<0.05; **p<0.01.
Cognitive circuitry
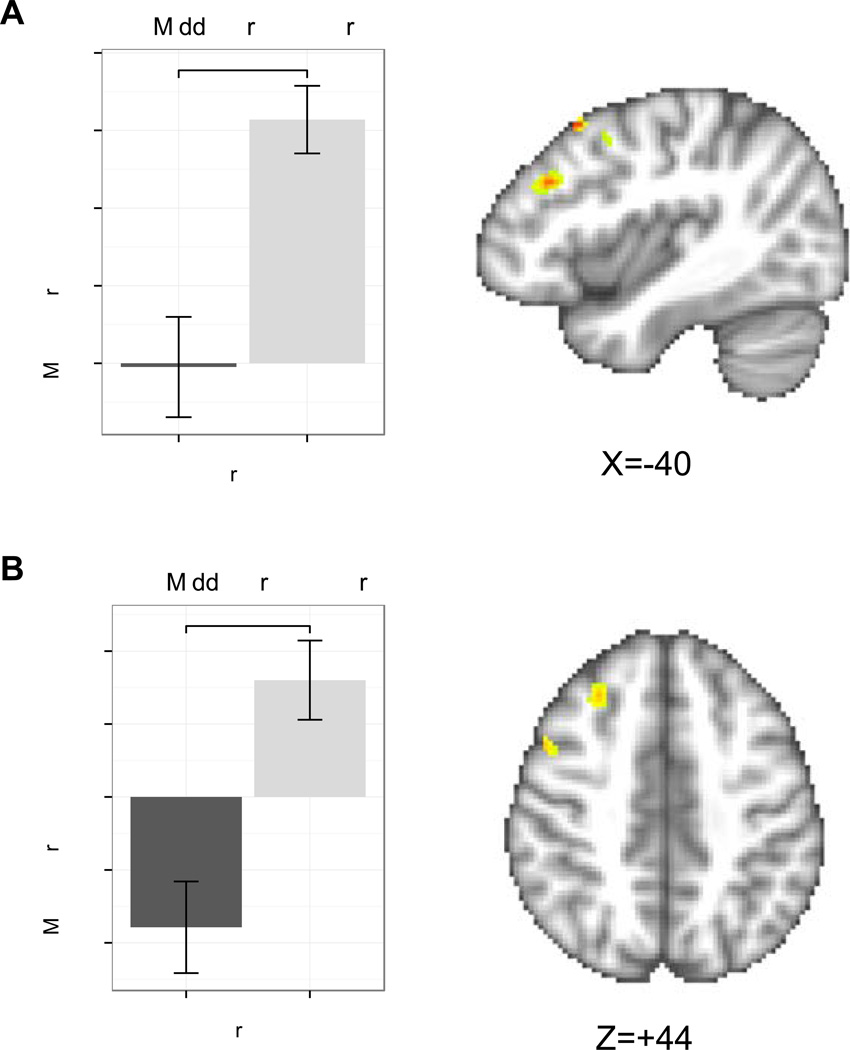
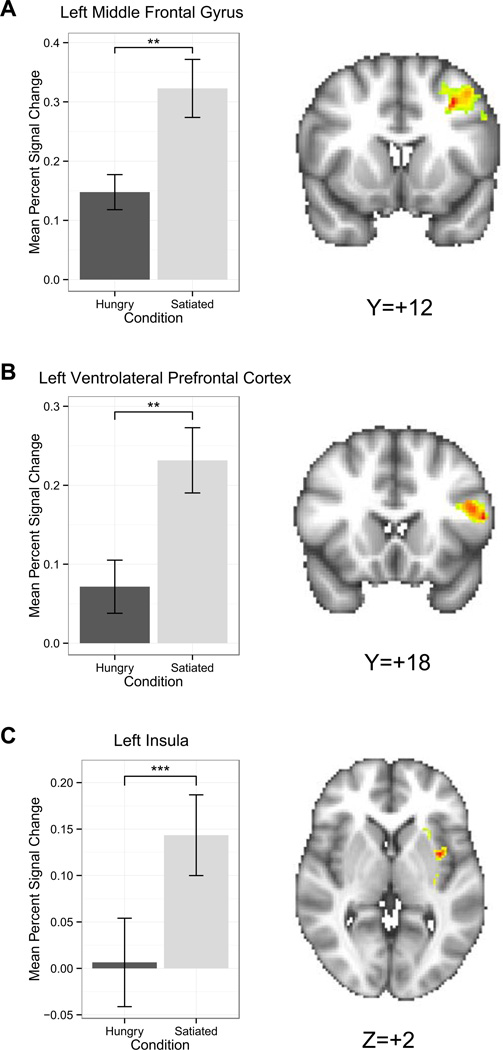
Satiety differentially modulated cognitive control response by group during intertemporal choice across all trials. We found a significant interaction of group with visit for the left MFG, bilateral insula, right VLPFC, and bilateral superior parietal cortex (Table 2, Figure 3B). Post-hoc analyses revealed within the bilateral insula and right VLPFC, CW responded significantly more strongly when satiated relative to when hungry, suggesting greater cognitive control when sated. Within the left MFG, the significant interaction was associated both with stronger response for CW when hungry relative to when satiated, and stronger response for RAN than CW when satiated. Post hoc analyses for the parietal cortex ROI were not significant. Several clusters within the MFG demonstrated a significant main effect of group (Figure 4); RAN responded more strongly than CW, suggesting elevated cognitive control in RAN regardless of satiety or hunger. Finally, a main effect of visit was detected within the bilateral MFG, the left insula, and the bilateral VLPFC (Figure 5) due to a greater response to decision trials when satiated relative to when hungry.
Figure 4.
Regions of interest associated with cognition showing a main effect of group for the delta regressor. A) Within the left middle frontal gyrus, RAN responded more robustly than CW [z=2.8, p=0.005]. B) Similarly, RAN responded more robustly than CW within the right middle frontal gyrus [z=2.9, p=0.004]. Error bars represent the standard error for each group. CW: healthy comparison women; RAN: women remitted from anorexia nervosa. **p<0.01
Figure 5.
Regions of interest associated with cognition showing a main effect of visit for the delta regressor. A) Within the left middle frontal gyrus, there was a significantly greater response when satiated than when hungry [z=2.9, p=0.004]. B) Within the left ventrolateral prefrontal cortex, there was a greater response when participants were satiated than when hungry [z=2.8, p=0.005]. C) Similarly, participants exhibited a greater response within the left insula when satiated than when hungry [z=3.6, p<0.001]. Error bars represent the standard error for each group. **p<0.01; ***p<0.001.
Relationship between BOLD response and psychiatric history
Logistic regressions between BOLD response during delayed discounting and presence of a lifetime diagnosis of either MDD or an anxiety disorder did not survive correction for multiple comparisons.
DISCUSSION
Metabolic state had a differential effect on brain response to delay discounting in CW compared to RAN women. For CW, hunger increased brain response in reward salience circuitry whereas satiety increased response in circuitry responsible for cognitive control during decision making. This finding is consistent with behavioral studies showing hunger enhances preference for immediate reward and reduces risk-averse behavior.(17) In contrast, hunger and satiety in RAN did not result in significant changes in valuation or cognitive neural circuitry, revealing insensitivity to metabolic state during delay discounting. This suggests RAN are less influenced by motivation of hunger when making decisions about salient stimuli.
VALUATION CIRCUITRY
Increased activation in valuation-related brain regions in CW when hungry suggests that metabolic state influences decision making by making immediate rewards more appetitive. CW were quicker to make a choice (Figure 2B) when hungry, suggesting that they engaged in less deliberation when making decisions. Limbic and paralimbic regions, such as the ventral striatum, anterior caudate, rostral ACC, and PCC have been associated with the preferential valuation of immediate outcomes in delay discounting(9, 12) specifically related to signaling reward expectancy for monetary, social, or taste rewards,(22, 23) conflict monitoring, and encoding valence.(24, 25)
Neuroimaging studies that manipulate satiety have demonstrated hunger-enhanced responses to appetitive tastes or food pictures in several limbic regions, including the orbitofrontal cortex and insula.(26, 27) People naturally favor larger over smaller rewards and rewards received sooner rather than later.(28) This is the first imaging study to show that hunger can also elevate the valuation response to financial cues, further demonstrating that metabolic state plays an important role in the modulation of the brain’s response to reward. The powerful effect of hunger on neural circuits may have important implications for treatment of substance abuse or obesity. People with these disorders, which may be related to enhanced reward or reduced cognitive control,(29) may be particularly susceptible to hungry states.
The lack of difference between brain responses in valuation or cognitive regions in RAN when hungry versus satiated suggests a failure to integrate homeostatic state into decision making. The finding of altered response to salient stimuli in RAN is consistent with other studies that have not controlled for hunger and satiety, but which show limbic regions do not differentiate between positive and negative monetary outcomes in RAN(3) and are underactive for motivational behavior in ill AN.(6, 30) Altered reward activation in RAN also occurs in response to tastes of palatable foods.(31) The lack of susceptibility to hunger-driven reward seeking behavior raises the possibility that this pathophysiology may play a critical role in successful food restriction in AN, in that hunger does not make salient stimuli more appetitive in RAN.
How does metabolic state drive reward? There is evidence that peptides involved in energy balance such as leptin, insulin, orexin, ghrelin and PYY, also provide signals to reward processes, in the service of modulating feeding in response to changes in energy states.(29) For example, ghrelin has orexigenic effects,(32) and ghrelin levels rise before meals and during fasting to prompt food seeking. Ghrelin also alters the function of areas involved in reward and incentive motivation (e.g., ventral striatum) and decision making (e.g., prefrontal cortex), suggesting a role in food reward. Ill and weight restored women with AN failed to show the expected association between ghrelin levels and BOLD response to visual food cues in limbic regions (e.g., amygdala, hippocampus, insula, and orbitofrontal cortex).(33) Thus, aberrant peptide function provides a possible pathway that may mediate the relationship between hunger and diminished reward response in AN. In addition, several neurotransmitters, including dopamine (DA), cannabinoids, opioids and serotonin, have been implicated in the rewarding effects of food.(29) Adults remitted from AN have altered DA and serotonin activity, raising the possibility that monoamine dysregulation contributes to a diminished response to reward.(34) For example, remitted AN show altered ventral striatal function that is consistent with diminished endogenous DA activity.(35, 36) Whether the abnormal mechanism is peptide-based, and/or located in reward processes related to DA or serotonin systems remains to be determined. Still, this study defines a paradigm that can be used to discriminate and test altered reward response to fasting in AN, both to systematically dissect contributing neurotransmitter, peptide, and neural circuitry mechanisms, and also to identify and test new drugs that might enhance reward response to fasting in AN.
COGNITIVE CIRCUITRY
CW showed the opposite pattern within cortical areas responsible for cognitive control and decision making; they had greater brain responses in the MFG, VLPFC and insula when satiated compared to when hungry. The DLPFC subregion of the MFG, and the VLPFC are often associated with cognitive control, including numeric computation, future planning, and inhibition,(37) and the insula is involved in the perception of time(38) and codes the selection of options for immediate versus delayed gratification.(39)
RAN did not show different brain responses during decision-making when hungry versus satiated. Instead, they exhibited elevated brain response compared to CW independent of hunger state in regions of the MFG including the DLPFC and premotor cortex. This insensitivity to hunger is also reflected in their response time: RAN, unlike the CW, showed similar response times regardless of decision difficulty or hunger.
FMRI studies in RAN demonstrate elevated frontoparietal activation, relative to CW,(3, 6) suggesting a more strategic approach during task performance. Elevated DLPFC activation has been observed in RAN, relative to CW, in response to aversive tastes,(40) and elevated resting state connectivity has been shown between the DLPFC and precuneus in RAN relative to CW.(41) Behavioral studies point to impaired decision making,(42) reflecting cognitive inflexibility. Our findings provide further support for enhanced cognitive control in RAN that is insensitive to hunger. In conclusion, enhanced inhibition, self-control, and/or insensitivity to interoceptive signals such as hunger in reward circuits may facilitate persistent food restriction. CW and RAN may use different strategies to evaluate choice, with RAN relying on cognitive evaluation to compensate for impaired reward processing.
LIMITATIONS
Unlike prior work,(8) we did not show behavioral choice differences in discounting between groups, though recent findings suggest discounting rate may normalize with weight restoration.(43) Because performance differences between groups can obscure whether differences in brain activation reflect biological differences or individual differences in ability, designs that equate performance are often preferable.(44) The similar performance between RAN and CW thus strengthens conclusions regarding group differences in brain systems involved during delay discounting.
We studied individuals remitted from AN to avoid confounding effects of malnutrition. The premorbid occurrence of similar temperament traits, such as altered response to reward and risk avoidance, supports the notion that these findings in RAN reflect neurobiological underpinnings of heritable traits that contribute to the anorectic phenotype and a vulnerability for pathological eating that persists even after nutrition and weight normalizes. Alternatively, recent studies in animals raise the question of whether extremes of food ingestion produce chronic effects on the reward system.(45) Given the frequency of dieting and weight loss in our culture, if extreme dieting produced powerful brain changes, the incidence of AN would be much higher, or weight loss in obesity would be much easier. Lastly, response to reward was higher for RAN than CW when satiated, raising the possibility that satiety is associated with abnormal motivation in RAN.
CLINICAL IMPLICATIONS
A lack of understanding of AN pathophysiology has hindered development of effective treatments. For example, AN individuals tend to lack motivation to engage in treatment(46) or appropriately balance the risk of emaciation versus the benefits of a healthy weight. This study shows that, consistent with the literature,(3, 4) RAN may have difficulty in valuating everyday choices due to altered brain response, recognition, or coding of reward. Limbic and cognitive circuits interact to code stimulus-reward value, maintain representations of predicted future reward and future behavioral choice, and play a role in integrating and evaluating reward prediction to guide decisions. These data support the possibility that AN individuals have an inherent altered ability to identify the emotional significance of stimuli, which may translate to an inability to make appropriate decisions to engage in treatment or appreciate the consequences of their behaviors. This study examined response to monetary choice and raises the question of whether the RAN failure to appropriately value monetary reward generalizes to valuation of food when hungry. As shown in CW, hunger enhances neural mechanisms that heighten the valuation of salient stimuli. Holsen(33) investigated the effects of hunger and satiety on response to images of food and found hypoactivation in food motivation regions involved in the assessment of food’s reward value in ill and remitted AN. Together, data support the likelihood that AN individuals fail to increase valuation of salient stimuli when hungry and overly rely on cognitive appraisal, thus explaining their ability to restrict food even though emaciated and their lack of motivation to seek treatment.
Supplementary Material
ACKNOWLEDGEMENTS
Supported by NIH grants R01-MH042984-17A1, R01-MH042984-18S1, and the Price Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Some of these data were presented at the American College of Neuropsychopharmacology annual meeting, December 8–12, 2013 and at the Society of Biological Psychiatry annual meeting, May 8–10, 2014.
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.American Psychiatric Association Workgroup on Eating Disorders. Practice guideline for the treatment of patients with eating disorders (revision) Am J Psychiatry. 2000;157:1–39. [PubMed] [Google Scholar]
- 2.Kaye W, Wierenga C, Bailer U, Simmons A, Bischoff-Grethe A. Nothing tastes as good as skinny feels: The neurobiology of anorexia nervosa. Trends in Neuroscience: Special Issue on Neural Control of Appetite. 2013;36:110–120. doi: 10.1016/j.tins.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner A, Aizenstein H, Venkatraman M, Fudge J, May J, Mazurkewicz L, et al. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry. 2007;164:1842–1849. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff-Grethe A, McCurdy D, Grenesko-Stevens E, Irvine L, Wagner A, Yau W-Y, et al. Altered brain response to reward and punishment in adolescents with anorexia nervosa. Psych Res. 2013;214:331–340. doi: 10.1016/j.pscychresns.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberndorfer T, Kaye W, Simmons A, Strigo I, Matthews S. Demand-specific alteration of medial prefrontal cortex response during an ihhibition task in recovered anorexic women. Int J Eat Disord. 2011;44:1–8. doi: 10.1002/eat.20750. [DOI] [PubMed] [Google Scholar]
- 6.Zastrow A, Kaiser SSC, Walthe S, Herzog W, Tchanturia K, Belger A, et al. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. Am J Psychiatry. 2009;166:608–616. doi: 10.1176/appi.ajp.2008.08050775. [DOI] [PubMed] [Google Scholar]
- 7.Pinto A, Steinglass J, Greene A, Weber E, Simpson H. Capacity to delay reward differentiates obsessive-compulsive disorder and obsessive-compulsive personality disorder. Biol Psychiatry. 2014;75:653–659. doi: 10.1016/j.biopsych.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinglass J, Figner B, Berkowitz S, Simpson H, Weber E, Walsh B. Increased capacity to delay reward in anorexia nervosa. Journal of the International Neuropsychological Society : JINS. 2012;18:773–780. doi: 10.1017/S1355617712000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClure S, Laibson D, Loewenstein G, Cohen J. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 10.Feil J, Sheppard D, Fitzgerald P, Yucel M, Lubman D, Bradshaw J. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neuroscience and biobehavioral reviews. 2010;35:248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Shimojo S, O'Doherty J. Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cereb Cortex. 2011;21:769–776. doi: 10.1093/cercor/bhq145. [DOI] [PubMed] [Google Scholar]
- 12.McClure S, Ericson K, Laibson D, Loewenstein G, Cohan J. Time discounting for primary rewards. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wittman MLKL, Lane S, Paulus M. Now or later? Striatum and insula activation to immediate versus delayed rewards. J Neurosci Psychol Econ. 2010;3:15–26. doi: 10.1037/a0017252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstone A, Prechtl de Hernandez C, Beaver J, Muhammed K, Croese C, Bell G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30:1625–1635. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 15.Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Dvorak R. Sweet future: fluctuating blood glucose levels affect future discounting. Psychol Sci. 2010;21:183–188. doi: 10.1177/0956797609358096. [DOI] [PubMed] [Google Scholar]
- 17.Levy D, Thavikulwat A, Glimcher P. State dependent valuation: the effect of deprivation on risk preferences. PLoS One. 2013;8:e53978. doi: 10.1371/journal.pone.0053978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tal A, Wansink B. Fattening fasting: hungry grocery shoppers buy more calories, not more food. JAMA Intern Med. 2013;173:1146–1148. doi: 10.1001/jamainternmed.2013.650. [DOI] [PubMed] [Google Scholar]
- 19.Carr K. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- 20.Carroll M, France C, Meisch R. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205:319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- 21.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical Journal. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 22.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 23.O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 24.Carter C, Braver T, Barch D, Botvinick M, Noll D, Cohen J. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 25.Mattfeld A, Gluck M, Stark C. Functional specialization within the striatum along both the dorsal/ventral and anterior/posterior axes during associative learning via reward and punishment. Learn Mem. 2011;18:703–711. doi: 10.1101/lm.022889.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage. 2009;44:1008–1021. doi: 10.1016/j.neuroimage.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaBar K, Gitelman D, Parrish T, Kim Y, Nobre A, Mesulam M. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 28.Hariri A, Brown S, Williamson D, Flory J, de Wit H, Manuck S. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. Neuroscience. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkow N, Wang G, Fowler J, Tomasi A, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Current topics in behavioral neurosciences. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- 30.Frank G, Shott M, Hagman J, Mittal V. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry. 2013;170:1152–1160. doi: 10.1176/appi.ajp.2013.12101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberndorfer T, Frank G, Fudge J, Simmons A, Paulus M, Wagner A, et al. Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. Am J Psychiatry. 2013;170:1143–1151. doi: 10.1176/appi.ajp.2013.11111745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skibicka K, Dickson S. Ghrelin and food reward: the story of potential underlying substrates. Peptides. 2011;32:2265–2273. doi: 10.1016/j.peptides.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Holsen L, Lawson E, Blum K, Ko E, Makris N, Fazeli P. Food motivation circuitry hypoactivation related to hedonic and nonhedonic aspects of hunger and satiety in women with active anorexia nervosa and weight-restored women with anorexia nervosa. J Psychiatry Neurosci. 2012;37:322–332. doi: 10.1503/jpn.110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaye W, Fudge J, Paulus M. New insight into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 35.Frank G, Bailer UF, Henry S, Drevets W, Meltzer CC, Price JC, et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11C]raclopride. Biological Psychiatry. 2005;58:908–912. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Bailer U, Frank G, Price J, Meltzer C, Becker C, Mathis C, et al. Interaction between serotonin transporter and dopamine D2/D3 receptor radioligand measures is associated with harm avoidant symptoms in anorexia and bulimia nervosa. Psych Res Neuroimaging. 2012;211:160–168. doi: 10.1016/j.pscychresns.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller E, Cohen J. An integrative theory of prefrontal cortex function. Ann Rev Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 38.Craig A. How do you feel--now? The anterior insula and human awareness. Nature Rev Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka S, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 40.Cowdrey F, Park R, Harmer C, McCabe C. Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol Psych. 2011;70:736–743. doi: 10.1016/j.biopsych.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 41.Cowdrey F, Filippini N, Park R, Smith S, McCabe C. Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22202. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts M, Tchanturia K, Stahl D, Southgate L, Treasure J. A systematic review and meta-analysis of set-shifting ability in eating disorders. Psychol Med. 2007;37:1075–1084. doi: 10.1017/S0033291707009877. [DOI] [PubMed] [Google Scholar]
- 43.Steinglass J, Decker H, Figner B, Walsh B. Societ of Biological Psychiatry. New York NY: New York Hilton Midtown; 2014. Is Delay of Gratification a Stable Trait of Anorexia Nervosa? [Google Scholar]
- 44.Gazzaley A, D'Esposito M. Considerations for the Application of BOLD Functional Magnetic Resonance Imaging to Neurologically Impaired Populations. In: Hillary F, DeLuca J, editors. Functional Neuroimaging in Clinical Populations. New York: New York: 2007. [Google Scholar]
- 45.Frank G. Altered Brain Reward Circuits in Eating Disorders: Chicken or Egg? Curr Psychiatry Rep. 2013;15:396. doi: 10.1007/s11920-013-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halmi K, Agras WS, Crow S, Mitchell J, Wilson G, Bryson S, et al. Predictors of treatment acceptance and completion in anorexia nervosa. Archives of general psychiatry. 2005;62:776–781. doi: 10.1001/archpsyc.62.7.776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.