Abstract
The opioid system is involved in infant-mother bonds and adult-adult bonds in many species. We have previously shown that μ opioid receptors (MOR) and κ opioid receptors (KOR) are involved in regulating the adult attachment of the monogamous titi monkey. The present study sought to determine the distribution of MOR and KOR in the titi monkey brain using receptor autoradiography. We used [3H]DAMGO to label MORs and [3H]U69,593 to label KORs. MOR binding was heterogeneous throughout the titi monkey brain. Specifically, MOR binding was observed in the cingulate gyrus, striatum, septal regions, diagonal band, amygdala, hypothalamus, hippocampus, and thalamus. Binding was particularly dense in the septum, medial amygdala, paraventricular nucleus of the hypothalamus, mediodorsal thalamus with moderate binding in the nucleus accumbens. Consistent with other primate species, MOR were also observed in “neurochemically unique domains of the accumbens and putamen” (NUDAPs). In general KOR binding was more homogenous. KORs were primarily found in the cingulate gyrus, striatum, amygdala and hippocampus. Dense KOR binding was observed in the claustrum. Relative MOR and KOR binding in the titi monkey striatum was similar to other humans and primates, but was much lower compared to rodents. Relative MOR binding in the titi monkey hypothalamus was much greater than that found in rodents. This study was the first to examine MOR and KOR binding in a monogamous primate. The location of these receptors gives insight into where ligands may be acting to regulate social behavior and endocrine function.
Keywords: titi monkey, mu opioid receptor, kappa opioid receptor, monogamy, autoradiography, DAMGO
INTRODUCTION
The opioid system is implicated in social behavior and social attachment. Most of the research on opioids and social behavior has focused on the μ opioid receptor (MOR) and its role in offspring-mother relationships, as well as adult relationships in non-monogamous primates. MOR manipulation affects the separation distress response between infant and mothers (Herman and Panksepp, 1978, Panksepp et al., 1978a, Panksepp et al., 1978b, Panksepp et al., 1980, Kalin et al., 1988), as well as physical contact (Fabre-Nys et al., 1982, Keverne et al., 1989, Kalin et al., 1995, Martel et al., 1995). There is also evidence that the κ opioid receptor (KOR) regulates separation related ultrasonic vocalizations in rat pups, in that KOR activation results in an increase in ultrasonic vocalizations (Carden et al., 1991, Carden et al., 1994).
There has been a recent surge in studies examining how the opioid system regulates adult attachment as well as social reward and social pain (Hsu et al., 2013). Studies in the monogamous prairie vole have found that MOR aid in pair-bond formation, and the KOR regulates pair-bond maintenance (Resendez and Aragona, 2013). In the same species, systemic opioid blockade or MOR blockade in the dorsal striatum or dorso-medial nucleus accumbens (NAcc) shell prevents pair-bond formation, which is expressed as a failure to develop a partner preference (Burkett et al., 2011, Resendez et al., 2013). Even though MOR activation aids in pair-bond formation, overactivation by a preferential MOR agonist, morphine, in established pair-bonds can inhibit behaviors associated with its expression (i.e. side-by-side contact) (Shapiro et al., 1989). With regards to the involvement of KOR in pair-bond maintenance, administration of the KOR antagonist, nor-BNI, peripherally or locally into the NAcc shell decreases attack frequency in prairie voles (Resendez et al., 2012). This mate-guarding behavior is exhibited by selective aggression towards unknown conspecifics, a hallmark of an established prairie vole pair-bond (Aragona and Wang, 2004).
In our previous research on the monogamous titi monkey (Callicebus cupreus), we found that the presence of a pair-mate modulates the results of pharmacological manipulation of the opioid system (Ragen et al., 2013). When a male receives the opioid antagonist naloxone, the presence of a female pair-mate results in a buffering of the drug's aversive effects; specifically the ability of naloxone to increase locomotion, cortisol, and vasopressin is attenuated in the female's presence. Interestingly, neither morphine nor naloxone affects overall physical contact between pair-mates (Ragen et al., 2013). However, we have also shown that morphine administration prior to a reunion after a 30-minute separation reduced the number of times the male approached and initiated contact with a female without an overall change in physical contact indicating that the female is compensating for the male's decrease in social motivation (Ragen et al., 2015). This result is similar to the findings in prairie voles, in that over-activating MORs also appears to reduce social motivation expressed as a reduction of social contact; however, MOR blockade has no effect (Shapiro et al., 1989, Burkett et al., 2011, Resendez et al., 2012). In contrast to titi monkeys, blocking opioid receptors with naloxone or naltrexone in other nonhuman primate species results in an increase in social contact (Fabre-Nys et al., 1982, Martel et al., 1995). Similar to prairie voles, the KOR appears to regulate pair-bond maintenance in titi monkeys. Specifically, peripheral administration of the KOR antagonist, GNTI, buffers the behavioral component of the social separation distress response (Ragen et al., 2015). It is possible that involuntary separation activates KOR, which induces a negative affective state. By remaining in proximity to an attachment figure, that dysphoric state can be averted. That same dysphoric state can be ameliorated with a KOR antagonist.
Pharmacological manipulations of behavior in prairie voles have been supplemented by analyses of the distribution of MOR mRNA, MOR binding and KOR binding (Resendez et al., 2012, Inoue et al., 2013, Resendez et al., 2013). In most brain areas, prairie voles have greater MOR receptor binding and MOR mRNA compared to non-monogamous male meadow voles (Inoue et al., 2013). This difference in MOR binding includes the dorsal striatum and NAcc shell — brain regions involved in pair-bond formation in prairie voles.
Compared to rodents, there has been limited examination of opioid receptor distribution in brain tissue from nonhuman primates. Daunais et al. (2001) performed autoradiography in cynomologus macaques (Macaca fascicularis) to examine MOR binding as well as MOR-stimulated [35S]GTPγS, which gives a measure of the G-protein activation of MORs after exposure to an agonist. KORs have been mapped in vivo in rhesus macaques (Macaca mulatta) (Schoultz et al., 2010) and baboons (Papio anubis) (Talbot et al., 2005) via positron emission tomography (PET) imaging with the radiolabeled KOR agonist, [11C]GR103545, and autoradiography has been used to map KOR-stimulated [35S]GTPγS in cynomologus macaques (Sim-Selley et al., 1999) and squirrel monkeys (Chen et al., 2005). At present, however, no studies have examined the distribution of either MOR or KOR in a monogamous primate.
Thus, the goal of the current study is to establish the neuroanatomical distribution of MOR and KOR binding in the monogamous, New World coppery titi monkey. We narrowed our study to MOR and KOR and did not include δ opioid receptors (DOR) because the growing research on opioids and pair-bonding has focused on MOR and KOR. This would give us insight into the relationship between receptor binding and current knowledge on pair-bonding and physiology. The results of this study will give insight into where both exogenous and endogenous opiates may be acting in the titi monkey brain to exert behavioral and physiological effects.
METHODS
Animals
Brains were collected from nine titi monkeys (Mean age = 7.0 years; Age range = 4.3-12.1 years). All subjects were sexually mature, had the ability to be sexually active, and were not considered geriatric. Three of the subjects were females that were living with their male pair-mate when euthanized. Three of the subjects were males that were living with their female pair-mate when euthanized. Three of the subjects were unpaired males. Two of these males had been living alone for 11-13 months. One male had been unpaired for one month but was living with two infants. These three lone males had all been previously paired. Brains were removed, cut into two hemispheres, blocked into four sections, flash frozen and stored at -80° C. Brain hemispheres were sliced into 20 μm sections on a cryostat at -20° C and mounted on Fisher Frost-plus slides. Slides were stored at -80° C in sealed slide boxes with desiccant.
All animals were euthanized due to an adenovirus associated with fulminant pneumonia (Chen et al., 2011), which had no neurological symptoms. It is important to note that even though neurological symptoms were not present, there is some evidence that the cytokine, interleukin-6 (IL-6), can increase MOR expression in neural tissue (Bianchi et al., 1999, Borner et al., 2004). IL-6 knock-out mice have decreased MOR expression in midbrain grey matter (Bianchi et al., 1999). Additionally, in vitro research has shown that there is a temporary down-regulation of MOR in neuronal cell lines in response to the cytokine, interferon-γ (Kraus et al., 2006). Since these cytokines are released in response to illness it is possible that relative MOR binding density in the brains used may be different compared to healthy subjects.
Receptor Autoradiography
MOR autoradiography
MOR autoradiography, including concentration of each ligand, was based on Daunais et al. (2001). Slides were brought up to room temperature. Sections were dipped in 0.1% paraformaldehyde for 2 minutes. Sections then underwent four 5-minute washes in Tris buffer (50mM Tris-HCl, 120mM NaCl, 5mM KCl; pH 7.4) at room temperature. Sections were then incubated for 60 minutes in 4.0 nM MOR agonist [3H][D-Ala2,N-Me-Phe4,Gly5-ol]enkephalin (DAMGO) (PerkinElmer, Inc., Boston, MA). Although DAMGO is a highly selective MOR agonist, we wanted to ensure that DOR and KORs were not being labeled. Table 1 presents the Ki for all ligands used. To block DORs and KORs, sections were co-incubated with 400nM of the specific DOR agonist, [D-Pen2,5]-enkephalin (DPDPE) (Tocris Bioscience, Minneapolis, MN), and 400nM of the specific KOR agonist, U69,593 (Sigma-Aldrich, St. Louis, MO), respectively. For non-specific binding, select sections were co-incubated with 10 μM of naloxone, a non-selective opioid antagonist. Concentrations of [3H]DAMGO and competitors were based on previous studies that utilized autoradiography to map opioid receptor binding in macaques and humans (Hiller and Fan, 1996, Daunais et al., 2001). Sections underwent six 20-second washes in 4° C Tris buffer then were dipped in 4° C ddH20 and air-dried. Slides were placed on Amersham Hyperfilm MP (Fisher Scientific, Suwanee, GA) for 21 weeks and then developed with Kodak Developer and Fixer.
Table 1.
| Compound | MOR | KOR | DOR |
|---|---|---|---|
| DAMGO | 1.23 | 534 | 634 |
| U69,593 | 260 | 0.89 | >2500 |
| DPDPE | 457 | >2500 | 1.41 |
| naloxone | 0.62 | 1.95 | 49 |
Ki (nM) values that are based on macaque brain membranes (Emmerson et al., 1994)
KOR Autoradiography
KOR autoradiography was based on deLanerolle et al. (1997). Slides were brought up to room temperature. Sections were dipped in Tris buffer (50mM Tris base; pH 7.4) for 60 minutes at room temperature then incubated for 90 minutes in 3.0 nM [3H]U69,593 (PerkinElmer, Inc., Boston, MA) and co-incubated with 400nM of DPDPE and 400nM DAMGO (Tocris Bioscience, Minneapolis, MN) at room temperature to block DOR and MOR, respectively. Select slides were co-incubated with 10 μM of naloxone for non-specific binding. Concentrations of [3H]U69,593 and competitors were based on previous studies that utilized autoradiography to map opioid receptor in humans (Hiller and Fan, 1996, deLanerolle et al., 1997). Slides underwent two 60-second washes in 4° C Tris buffer. They underwent a 10 second wash in 4° C ddH2O and were then dried. Slides were placed on Amersham Hyperfilm MP for 21 weeks and then developed with Kodak Developer and Fixer.
Acetylcholinesterase (AChE) Staining
Following receptor autoradiography and film development, select slides were counterstained for AChE to delineate the brain regions for image analysis. AChE staining technique was based on Lim et al. (2004). Slides were incubated for 6 hours in Solution A (0.072 mg/mL ethopropazine hydrochloride, 0.5 mg/ mL copper(II) sulfate pentahydrate, 0.75 mg/mL glycine, 1.2 mg/mL acetylthiocholine iodide, 6.8 mg/ mL sodium acetate trihydrate; pH 5.0). Slides were then rinsed three times in ddH2O and developed for 30 minutes in Solution B (7.7 mg/mL sodium sulfide nonahydrate; pH 7.8). Slides were rinsed again in ddH2O three times and were then exposed to Solution C (10 mg/mL silver nitrate) for 10 minutes while slowly agitated and completely protected from light. Finally, slides were rinsed three times in ddH2O, air-dried overnight, and cover slipped. Images of the resulting counterstained sections were compared with images from a red-bellied titi monkey brain atlas (www.brainmuseum.org), a rhesus macaque brain atlas (Paxinos et al., 1999), and a common marmoset brain atlas (Newman et al., 2009) to determine neuroanatomical landmarks and identify regions.
Quantification
Due to differences in the location of the midline between each brain as well as some tissue damage due to blocking the brains, we were not able to quantify all brain areas in all brains. Furthermore, due to assay size only brain areas from the prefrontal cortex (rostral) to the mediodorsal thalamus (caudal) were analyzed..
For quantification of optical binding density (OBD), developed film was scanned with an Epson Perfection V500 Photo scanner. Quantification was done with NIH ImageJ 64. Regions of interest (ROI) included brain areas involved in social behavior and endocrine functioning. These brain regions were chosen because the recent research on MOR and KOR manipulation in titi monkeys and prairie voles have focused on endocrine and social outcomes. OBD was calculated via background subtraction from a brain region where no binding was detected. Quantification was aided by obtaining the optical binding values from a set of tritium standards (American Radiolabeled Chemicals, Inc., St. Louis, MO), to produce a standard curve that allowed for the extrapolation of OBD values of the ROIs. To obtain the OBD of an ROI, two to four values from each animal were averaged. Those values were then averaged to obtain a mean ± SEM, which are found in Table 2. Table 2 also includes the number of brains in which the OBD could be obtained.
Table 2.
| Brain Region | MOR (dpm/mg) | N | KOR (dpm/mg) | N |
|---|---|---|---|---|
| Cingulate Gyrus | 828.0 ± 146.7 | 8 | 1512.0 ± 153.1 | 8 |
| Striatum | ||||
| Caudate | 937.4 ± 292.6 | 8 | 715.9 ± 107.8 | 9 |
| Putamen | 519.8 ± 55.9 | 8 | 779.7 ± 111.7 | 9 |
| Nucleus Accumbens Shell | 2173.5 ± 379.1 | 9 | 764.7 ± 102.9 | 9 |
| Nucleus Accumbens Core | 1482.3 ± 266.7 | 9 | 781.3 ± 98.2 | 9 |
| NUDAP | 3352.5 ± 494.1 | 7 | ND | N/A |
| Claustrum | ND | N/A | 3348.84 ± 454.54 | 9 |
| Globus Pallidus Internal | ND | N/A | 909.0 ± 141.3 | 3 |
| Ventral Pallidum | ND | N/A | 1299.70 | 1 |
| Amygdala/Extended Amygdala | ||||
| Anterior Amygdala | 3396.5 ± 508.3 | 7 | 612.5 ± 168.7 | 9 |
| Basolateral Amygdala | 2420.5 ± 254.8 | 9 | 1061.2 ± 104.1 | 9 |
| Basomedial Amygdala | 1925.4 ± 274.1 | 9 | 997.2 ± 111.6 | 9 |
| Cortical Amygdala | 2250.8 ± 324.7 | 7 | 804.7 ± 109.5 | 9 |
| Medial Amygdala | 4356.4 ± 572.9 | 9 | 753.3 ± 93.0 | 9 |
| Central Amygdala | ND | 689.3 ± 107.8 | 9 | |
| Paralaminar n. | 3498.5 ± 359.0 | 5 | ND | N/A |
| Lateral Amygdala | 751.9 ± 63.57 | 9 | 1030.4 ± 95.8 | |
| Bed Nucleus of the Stria | ||||
| Terminalis | 2491.6 ± 378.0 | 5 | ND | N/A |
| Septal Region | ||||
| Medial Septum | 2732.1 ± 319.2 | 6 | 476.30 | 1 |
| Lateral Septum | 1568.5 ± 233.1 | 6 | 439.8 ± 64.0 | 3 |
| Diagonal Band | 1662.3 ± 231.8 | 6 | 626.2 ± 192.7 | 2 |
| Hippocampus | ||||
| Septohippocampal n. | 3229.5 ± 824.2 | 4 | ND | N/A |
| Presubiculum | 1382.8 ±352.2 | 5 | 885.4 ± 132.6 | 9 |
| Entorhinal cortex | 484.6 ± 244.1 | 2 | 975.7 ± 171.9 | 7 |
| CA1 | ND | N/A | 555.5 ± 91.2 | 9 |
| CA3 | ND | N/A | 433.9 ± 116.4 | 9 |
| Hypothalamus | 693.98 ± 84.62 | 4 | ||
| Anterior hypothalamic n. | 3024.6 ± 812.0 | 5 | ||
| Arcuate N. | 2456.7 ± 444.2 | 7 | ||
| Dorsomedial hypothalamic n. | 2404.9 ± 518.5 | 7 | ||
| Lateral hypothalamic n. | 1783.8 ± 184.6 | 3 | ||
| Medial preoptic area | 2928.9 ± 745.5 | 5 | ||
| Paraventricular hypothalamic n. | 44518. ± 1232.7 | 5 | ||
| Suprachiasmatic hypothalamic n. | 3238.3 ± 824.2 | 3 | ||
| Supraoptic hypothalamic. N. | 3368.768 ± 750.3 | 4 | ||
| Ventromedial hypothalamic n. | 2515.3 ± 521.3 | 6 | ||
| Septohypothalamic n. | 2267.9 ± 4.3 | 2 | ||
| MammiHary body | 1932.3 ±799.8 | 4 | ND | N/A |
| Substantia Nigra | 1151.6 ± 325.3 | 4 | ND | N/A |
| Thalamus | ||||
| Anteroventral thalamic n. | 1892.3 ± 246.1 | 8 | ND | N/A |
| Ventrolateral thalamic n. | 1653.9 ± 654.7 | 2 | ND | N/A |
| ventral anterior thalamic n. | 1077.61 ± 116.0 | 3 | ND | N/A |
| Mediodorsal thalamic n. | 2688.4 ± 394.2 | 8 | ND | N/A |
| Pulvinar | 2109.9 | 1 | ND | N/A |
| Interpeduncular n. | 5661.312 | 1 | ND | N/A |
ND: Not Detectable; N/A: Not Applicable
RESULTS
Nonspecific Binding
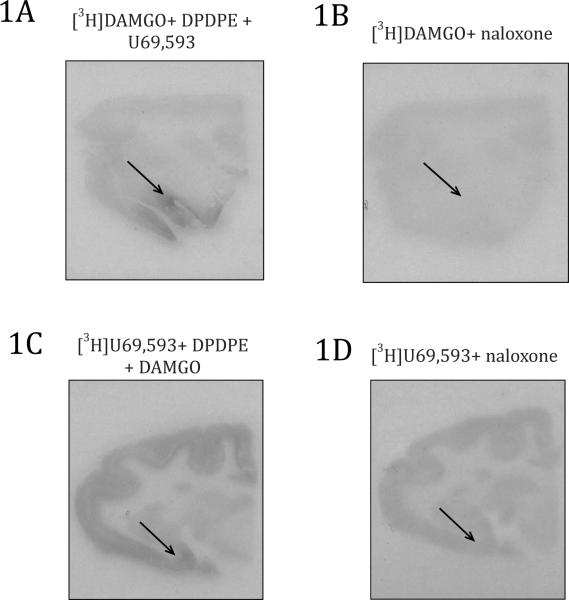
Slides adjacent to those used for receptor mapping were co-incubated with large concentrations of naloxone. Incubation with naloxone prevented [3H]DAMGO and [3H]U69,593 binding (Figure 1). Coincubation of naloxone with either radioactive ligand was even able to displace sparse binding such as the binding of KOR in the striatum. In membrane homogenates from rhesus brain tissue, naloxone has great enough binding affinity for MOR and KOR demonstrated by the ability to displace both [3H]DAMGO and [3H]U69,593, respectively (Emmerson et al., 1994).
Figure 1.
Selective and non-specific binding for MOR and KOR autoradiography. 1A) Selective binding of MOR using [3H]DAMGO blocking DOR and KOR by co-incubating with DPDPE and U69,593, respectively 1B) Non-specific binding of MOR with [3H]DAMGO and co-incubating with naloxone 1C) Selective binding of KOR using [3H]U69,593 blocking DOR and MOR by co-incubating with DPDPE and DAMGO, respectively 1D) Non-specific binding of KOR with [3H]U69,593 and co-incubating with naloxone
MOR Distribution
MOR binding was detected through the use of the tritiated labeled specific MOR agonist DAMGO and co-incubation of the slides with DPDPE and U69,593 to block DOR and KOR, respectively. The high selectivity of these opioid ligands has been demonstrated with rhesus monkey brain membranes (Emmerson et al., 1994), indicating that the signal we detected in the titi monkey brain has specific binding to MOR.
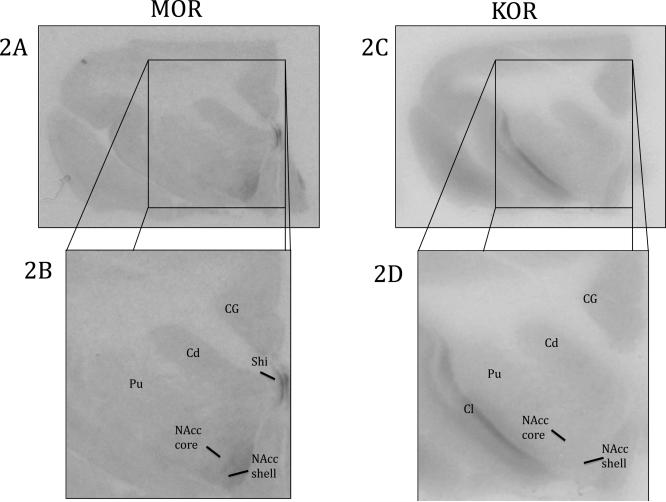
MOR ligand binding was found throughout various forebrain structures (Table 2 and Figure 2). There was sparse binding in the cingulate gyrus (CG). MOR binding was found throughout the striatum with sparse binding in the caudate (Cd) and putamen (Pu) and moderate binding in the NAcc core and NAcc shell. The densest binding in the striatum was in the NAcc shell.
Figure 2.
Opioid binding in the striatum and surrounding regions. 2A) MOR binding viewed on a coronal slice of at the level of the striatum 2B) Enlarged view of MOR in the striatum, CG, and Shi 2C) KOR binding viewed on a coronal slice of at the level of the striatum 2D) Enlarged view of KOR in the striatum, CG and Cl. Cd: Caudate, CG: Cingulate Gyrus, Cl: Claustrum, NAcc core: Nucleus Accumbens Core, NAcc shell: Nucleus Accumbens Shell, Pu: Putamen, Shi: Septohippocampal nucleus
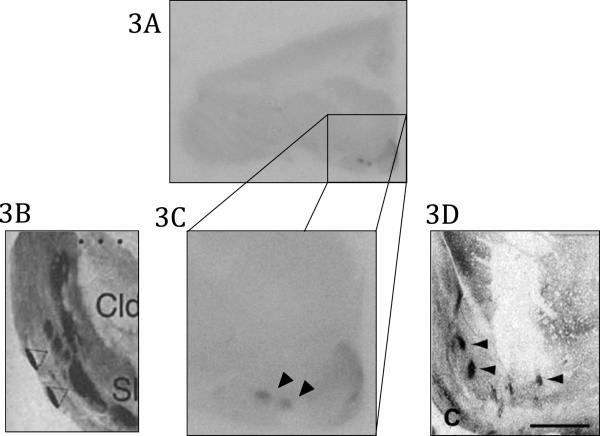
In the seven of the nine brains we found small patches of dense binding that were on the border of the NAcc and the Pu (Figure 3). Similar binding patterns have been discovered in macaques and humans and have been labeled “neurochemically unique domains of the accumbens and putamen” (NUDAPs) (Voorn et al., 1996, Daunais et al., 2001) (Figure 3). Because the pattern of binding in titi monkeys is similar to that of humans and macaques, we feel confident in categorizing these dense patches of MOR binding in the titi monkey as NUDAPs.
Figure 3.
Neurochemically distinct areas of the accumbens and putamen (NUDAPs). 3A Coronal view of the striatum of the titi monkey expressing NUDAPs 3B) Enlarged view of human NUDAPs (Voorn et al., 1996; permission from authors) 3C) Enlarged view of titi monkey NUDAPs 3D) Close of view of NUDAPs in the macaque brain (Daunais et al., 2001; permission from authors)
Other forebrain structures with MOR binding were located in the medial septum (MS), lateral septum (LS), diagonal band (DB), and the septohippocampal nucleus (Shi) (Table 2). The densest binding was in the MS with more moderate binding in the LS and DB. Binding in the Shi was relatively dense.
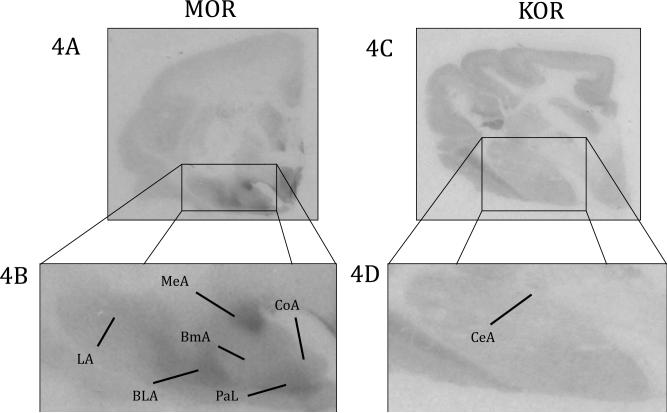
MOR binding was also found in the amygdala and extended amygdala (Figures 4, 5 and Table 2). There was relatively heterogeneous binding in the amygdala. The densest binding was located in the medial amygdala (MeA) and the paralaminar nucleus (PaL). There was moderate to dense binding in rostral regions of the anterior amygdala (AAA). There was moderate binding in the basolateral amygdala (BLA), basomedial amygdala (BmA), and cortical amygdala (CoA), with sparse binding in the lateral amygdala (LA). MOR binding was completely absent in the central amygdala (CeA). There was also moderate to dense binding in the bed nucleus of the stria terminalis (BNST).
Figure 4.
Opioid binding in the amygdala. 4A) MOR binding viewed on a coronal slice at the level of the amygdala 4B) Enlarged view of MOR in the amygdala 4C) KOR binding viewed on a coronal slice at the level of the amygdala 4D) Enlarged view of KOR in the amygdala, BLA: Basolateral Amygdala, BmA: Basomedial Amygdala, CeA: Central Amygdala, CoA: Cortical Amygdala, LA: Lateral Amygdala, MeA: Medial Amygdala, PaL: Paralaminar Amygdala
Figure 5.
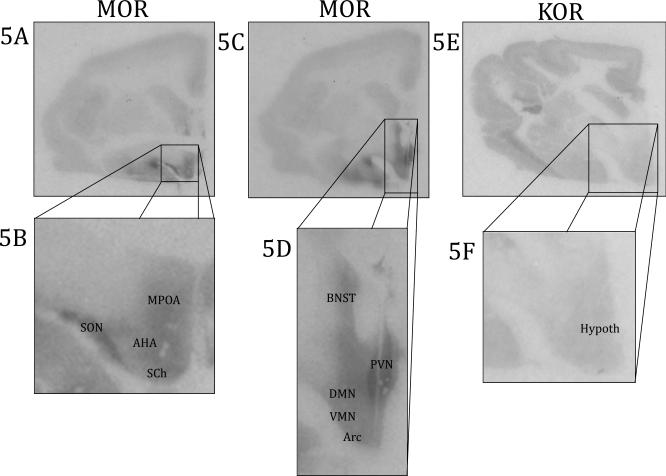
Opioid binding in the hypothalamus. 5A) MOR binding viewed on a coronal slice at the level of a rostral section of the hypothalamus 5B) Enlarged view of MOR of a rostral section of the hypothalamus 5C) MOR binding viewed on a coronal slice at the level of a caudal section of the hypothalamus 5D) Enlarged view of MOR of a caudal section of the hypothalamus 5E) KOR binding viewed on a coronal slice of the hypothalamus 5F) Enlarged view of KOR binding of the hypothalamus AHA: anterior hypothalamic nucleus, Arc: arcuate nucleus, BNST: bed nucleus of the stria terminalis, DMN: dorsomedial nucleus, Hypoth: hypothalamus, MPOA: medial preoptic area, PVN: paraventricular nucleus, SCh: suprachiasmatic nucleus, SON: supraoptic nucleus, VMN: ventromedial nucleus
There was MOR binding in various hypothalamic nuclei (Figure 5 and Table 2). Binding in hypothalamic regions were relatively homogenous and included the anterior hypothalamus (AH), arcuate nucleus (Arc), dorsomedial hypothalamus (DMH), lateral hypothalamus (LH), medial preoptic area (MPOA), suprachiasmatic nucleus (SCN), supraoptic nucleus (SON), ventromedial hypothalamus (VMH), and septohypothalamic nucleus (Shy). The one brain region that had very dense MOR binding was the paraventricular nucleus (PVN).
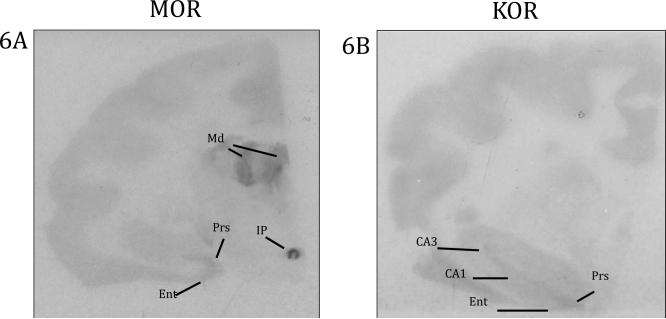
There was sparse MOR binding in the hippocampus (Figure 6 and Table 2) but primarily localized in nearby structures, specifically the presubiculum (Prs) and entorhinal cortex (Ent). Sparse to moderate binding was also found in the mammillary body (MM) and substantia nigra (SNR). The interpeduncular nucleus (IP) was present in one subject, and the binding in this brain area was extremely dense.
Figure 6.
Opioid binding in the hippocampus and thalamus. 6A) MOR binding in the hippocampus, thalamus, and IP 6B) KOR binding in the hippocampus, Ent: entorhinal cortex, IP: interpeduncular nucleus, Md: mediodorsal thalamus, Prs: presubiculum
Finally, there was MOR binding in thalamic nuclei (Figure 6 and Table 2). Moderate to sparse binding was found in the anteroventral thalamic nucleus (AV), ventrolateral thalamic nucleus (VL), and the ventral anterior thalamic nucleus (VA). There was very dense binding in the mediodorsal thalamic nucleus (MD). One subject had binding in the pulvinar (Pul).
There were no evident sex differences in MOR binding. However, we did not perform additional statistical comparisons due to the small sample size and large number of brain areas that were analyzed.
KOR Distribution
KOR ligand binding was observed through the use of [3H]U69,593 and the blockade of DORs and MORs with DPDPE and DAMGO, respectively. As with MOR binding, we feel confident that due to the specificity of these ligands, we are only measuring KOR binding. In general, KOR binding was very sparse throughout the titi monkey brain. The one exception was the dense binding in the claustrum (Cl), which was an area that lacked MOR binding (Figure 2 and Table 2). There was KOR binding in the Cd, Pu, NAcc core, and NAcc shell, but binding in the striatum was homogenous and very sparse (Figure 2 and Table 2). We also found very sparse, homogenous binding in the amygdala. In contrast to MOR binding there was sparse KOR binding in the CeA and no binding in the PaL (Figure 4 and Table 2). There was also no binding in the BNST.
KOR binding in the hypothalamus was very sparse and only above background in four of the nine brains. Since KOR binding in the hypothalamus was uniform, specific hypothalamic nuclei were not quantified (Figure 5 and Table 2). KOR binding in the thalamus was at background levels, which is in contrast to MOR binding.
In one subject we found sparse binding in the MS, in three subjects we observed sparse binding in the LS, and in two subjects we observed sparse binding in the DB. We did observe some clear, but sparse KOR binding in hippocampal regions (Figure 6 and Table 2). Like MORs there was binding in the Prs and Ent, however in contrast to MOR binding, there was KOR binding in CA1 and CA3.
There were no evident sex differences in KOR binding. As with MOR, we did not perform statistical comparisons due to the small sample size and large number of brain areas that were analyzed.
DISCUSSION
This study is the first to map MOR and KOR binding in a monogamous primate. We observed MOR and KOR binding throughout the brain with the locations of binding similar to those of other species. Importantly, both MOR and KOR were observed in the CG, striatum, hypothalamus, and amygdala, which are brain regions involved in social behavior, affect, and hormone functioning.
The distribution of MOR binding in the titi monkey brain is similar to that of macaques (Daunais et al., 2001), squirrel monkeys (Chen et al., 2005), and humans (Voorn et al., 1996). Despite these similarities some slight differences in distribution and relative density are present and could differ due to species or methodological differences. For example, some of these studies have used less specific radioligands (i.e. [3H]naloxone). Additionally, the autoradiography study in squirrel monkeys only looked at MOR-stimulated [35S]GTPγS (Chen et al., 2005). Studies which used the specific radiolabeled MOR agonist, [3H]DAMGO, did demonstrate similarities between titi monkeys and other primates. However, there are some differences in relative MOR binding between titi monkeys and rodents (Table 3). MOR binding in the titi monkey striatum is relatively much sparser compared to rats (Mansour et al., 1987, Sharif and Hughes, 1989), voles (Resendez et al., 2012, Inoue et al., 2013), and guinea pigs (Sharif and Hughes, 1989). In contrast, MOR binding using [3H]DAMGO in the rodent hypothalamus is extremely low and sometimes undetectable (Mansour et al., 1987, Sharif and Hughes, 1989, Inoue et al., 2013). This is different in the titi monkey hypothalamus, where relative MOR binding was moderate or dense. It is unknown how relative MOR binding in the titi monkey hypothalamus compares to humans and macaques because studies have only examined mRNA (Peckys and Landwehrmeyer, 1999) or utilized the less specific [3H]naloxone (Lewis et al., 1984).
Table 3.
| Brain Region | [3H]DAMGO MOR Relative Binding | ||||||
|---|---|---|---|---|---|---|---|
| Titi Monkey | Human1,2,3 | Macaque4 | Rat5,6 | Prairie Vole7,8 | Montane Vole7 | Guinea Pig6 | |
| Cingulate Gyrus | + | +++ | ++ | + | |||
| Striatum | |||||||
| Caudate | + | ++ | + | ++++ | ++++ | ++ | ++++ |
| Putamen | + | ++ | + | ++++ | ++++ | ++ | ++++ |
| Nucleus Accumbens Shell | ++ | + | + | ++++ | +++ | ++ | +++ |
| Nucleus Accumbens Core | ++ | ++ | + | ++++ | +++ | ++ | +++ |
| NUDAP | +++ | ++++ | +++ | N/A | N/A | N/A | N/A |
| Claustrum | 0 | + | 0 | 0 | 0 | 0 | |
| Globus Pallidus Internal | 0 | + | + | + | + | ||
| Ventral Pallidum | 0 | ||||||
| Amygdala/Extended Amygdala | +++ | ||||||
| Anterior Amygdala | +++ | ++ | |||||
| Basolateral Amygdala | ++ | +++ | ++++ | ++ | |||
| Basomedial Amygdala | ++ | +++ | |||||
| Cortical Amygdala | ++ | +++ | ++++ | ++ | |||
| Medial Amygdala | ++++ | ++++ | +++ | ++ | ++ | ++ | |
| Central Amygdala | 0 | + | + | + | |||
| Paralaminar n. | +++ | ++++ | |||||
| Lateral Amygdala | + | ++ | ++++ | ++ | |||
| Bed Nucleus of the Stria | |||||||
| Terminalis | ++ | ++ | ++ | + | + | ++ | |
| Septal Region | |||||||
| Medial Septum | ++ | +++ | |||||
| Lateral Septum | ++ | 0 | ++ | + | |||
| Diagonal Band | ++ | ++ | |||||
| Hippocampus | |||||||
| Septohippocampal n. | +++ | ++ | + | ||||
| Presubiculum | + | ++++ | |||||
| Entorhinal cortex | + | + | |||||
| CA1 | 0 | 0 | + | ||||
| CA3 | 0 | +++ | |||||
| Hypothalamus | |||||||
| Anterior hypothalamic n. | +++ | 0 | + | + | |||
| Arcuate N. | ++ | 0 | |||||
| Dorsomedial hypothalamic n. | ++ | + | + | ||||
| Lateral hypothalamic n. | ++ | + | + | + | |||
| Medial preoptic area | +++ | + | |||||
| Paraventricular hypothalamic n. | ++++ | 0 | |||||
| Suprachiasmatic hypothalamic n. | +++ | ||||||
| Supraoptic hypothalamic. N. | +++ | 0 | |||||
| Ventromedial hypothalamic n. | ++ | 0 | ++ | + | |||
| Septohypothalamic n. | ++ | ||||||
| Mammillary body | ++ | +++ | ++ | + | |||
| Substantia Nigra | + | +++ | + | + | |||
| Thalamus | |||||||
| Anteroventral thalamic n. | ++ | +++ | + | ||||
| Ventrolateral thalamic n. | ++ | 0 | |||||
| ventral anterior thalamic n. | + | ||||||
| Mediodorsal thalamic n. | ++ | +++ | ++++ | + | + | ||
| Pulvinar | ++ | ||||||
| Interpeduncular n. | ++++ | ++++ | ++++ | ++++ | |||
++++, very dense
+++ dense
++ moderate
+ light, 0 not detectable, N/A not applicable, blank cells = no data
The distribution of KOR in the titi monkey is also similar to that of macaques (Sim-Selley et al., 1999, Schoultz et al., 2010), humans (Pilapil et al., 1987, Vonkeman et al., 1996, deLanerolle et al., 1997), squirrel monkeys (Chen et al., 2005), and baboons (Talbot et al., 2005); however, relative binding density is difficult to compare. Most of these studies have used the non-specific KOR agonist bremazocine, PET imaging, or KOR-simulated [35S]GTPγS (Sim-Selley et al., 1999, Chen et al., 2005). It has been shown that [3H]bremazocine labels a different population of KORs compared to the more specific [3H]U69,593 (Unterwald et al., 1991). There is evidence that suggests [3H]bremazocine is labeling KOR-DOR heterodimers while [3H]U69,593 is labeling KOR monomers (Jordan and Devi, 1999). Table 4 compares KOR binding between species, but only for those studies using [3H]U69,593. Relative KOR binding in titi monkeys using [3H]U69,593 is similar to that in humans and rats. However, one striking species difference is sparse striatal binding in titi monkeys compared to the dense KOR binding in guinea pigs and prairie voles.
Table 4.
| Brain Region | [3H]U69,593 KOR Relative Binding | ||||
|---|---|---|---|---|---|
| Titi Monkey | Human9,10 | Rat11,12 | Guinea Pig11 | Prairie Vole8 | |
| Cingulate Gyrus | + | ||||
| Striatum | |||||
| Caudate | + | ++ | + | +++ | +++ |
| Putamen | + | ++ | + | +++ | +++ |
| Nucleus Accumbens Shell | + | ++ | ++ | +++ | ++++ |
| Nucleus Accumbens Core | + | ++ | ++ | +++ | +++ |
| NUDAP | 0 | N/A | N/A | N/A | |
| Claustrum | ++++ | ++++ | ++ | ++++ | +++ |
| Globus Pallidus Internal | + | + | + | + | |
| Ventral Pallidum | ++ | ++ | |||
| Amygdala/Extended Amygdala | +++ | + | + | ||
| Anterior Amygdala | + | ||||
| Basolateral Amygdala | + | ||||
| Basomedial Amygdala | + | ||||
| Cortical Amygdala | + | ||||
| Medial Amygdala | + | ||||
| Central Amygdala | + | ||||
| Paralaminar n. | 0 | ||||
| Lateral Amygdala | + | ||||
| Bed Nucleus of the Stria | |||||
| Terminalis | 0 | + | + | ||
| Septal Region | |||||
| Medial Septum | + | + | + | ||
| Lateral Septum | + | + | + | ||
| Diagonal Band | + | ||||
| Hippocampus | 0 | ||||
| Septohippocampal n. | 0 | ||||
| Presubiculum | + | ||||
| Entorhinal cortex | + | ||||
| CA1 | + | +++ | |||
| CA3 | + | + | |||
| Hypothalamus | + | +++*10 | + | + | 0 |
| Anterior hypothalamic n. | |||||
| Arcuate N. | |||||
| Dorsomedial hypothalamic n. | |||||
| Lateral hypothalamic n. | + | + | |||
| Medial preoptic area | |||||
| Paraventricular hypothalamic n. | |||||
| Suprachiasmatic hypothalamic n. | |||||
| Supraoptic hypothalamic. N. | |||||
| Ventromedial hypothalamic n. | + | ||||
| Septohypothalamic n. | |||||
| Mammillary body | 0 | + | + | ||
| Substantia Nigra | 0 | + | + | +++ | |
| Thalamus | 0 | +++*10 | |||
| Anteroventral thalamic n. | |||||
| Ventrolateral thalamic n. | |||||
| ventral anterior thalamic n. | |||||
| Mediodorsal thalamic n. | + | + | |||
| Pulvinar | |||||
| Interpeduncular n. | 0 | + | |||
++++, very dense; +++ dense, ++ moderate, + light, 0 not detectable, N/A not applicable, blank cells = no data, *10Quirion et al., 1987 does not specify nuclei or supply images
Conserved Brain Regions
We found binding in brain regions that are found in other species, indicating evolutionary conservation of opioid receptor binding. An example was the strong KOR binding in the Cl. Strong KOR binding in the Cl has been found in humans, macaques, rats, prairie voles, and guinea pigs (Pilapil et al., 1987, Unterwald et al., 1991, Sim-Selley et al., 1999, Chen et al., 2005, Resendez et al., 2012). Since strong KOR binding in the Cl is found in a wide range of species, it provides external validity for our binding results in the titi monkey.
We also found moderate MOR binding in the MD. MOR binding in the MD has been observed in humans (Berthele et al., 2005), macaques (Sim-Selley et al., 1999), rats (Mansour et al., 1987), and mice (Diaz et al., 2006) Again, this similarity of binding pattern provides evidence for the validity of our MOR assay.
A very interesting finding was the presence of “NUDAPs”, which are dense patchy areas of MOR binding on the border area of the nucleus accumbens shell and the putamen. This MOR binding pattern has only been observed in humans (Voorn et al., 1996) and macaques (Daunais et al., 2001) (Figure 3), and not in rodents. It has been suggested that these patches are only found in primates. It is currently unknown whether these unique neuroanatomical binding patterns serve a function. The fact that this binding pattern is also found in titi monkeys suggests that this binding pattern is conserved in New World primates, Old World primates, and apes.
Social Affect and Pair-Bonding
The opioid system plays a crucial role in social reward and social pain. MOR binding in titi monkeys is present in the amygdala, MD, NAcc and CG. Humans who were exposed to social rejection experience an increase in MOR activation in amygdalar nuclei, NAcc, and the midline thalamus, as measured by PET. In addition, exposure to social acceptance results in an increase in MOR activation in the amygdala and deactivation in the midline thalamus and anterior CG (Hsu et al., 2013). Social rejection in humans also has been associated with increases in fMRI BOLD signal in the dorsal CG (Way et al., 2009). It is possible that MORs in these brain regions in titi monkeys may also process social pleasure and social pain.
Another brain area that has been implicated in the processing of social reward is the striatum. In the current study we found moderate levels of MOR and sparse levels of KOR expression in this brain region in titi monkeys. It is possible that MORs in the striatum may also be crucial for pair-bond formation in titi monkeys. Research analyzing glucose uptake in titi monkeys has found that compared to males living alone there are differences in glucose uptake in the NAcc in males who are recently paired and males in long-term pair-bonds (Bales et al., 2007). Comparative evidence comes from studies in prairie voles, where blockade of MORs in either the dorsal striatum or dorso-medial shell of the NAcc prevents pair-bond formation (Burkett et al., 2011, Resendez et al., 2013). Oxytocin receptors (Breier et al.) in the prairie vole striatum are important for pair-bond formation in prairie voles (Liu and Wang, 2003); however it has recently been discovered that titi monkeys do not express OTR in the striatum (Freeman et al., 2014). MOR activation in the striatum may be the alternate system that promotes pair-bond formation in titi monkeys.
In contrast to MOR and OTR involvement in pair-bond formation, there is evidence that striatal KORs are important for pair-bond maintenance. In titi monkeys, individuals may be motivated to maintain proximity with their pair-mate to prevent KOR activation upon separation and a subsequent dysphoric state. This hypothesis is demonstrated by an attenuation of their separation distress response after administration of the KOR antagonist, GNTI (Ragen et al., 2015). Furthermore, KORs in the BLA have been shown to mediate anxiety in rats (Bruchas et al., 2009, Knoll et al., 2011) and the titi monkey BLA expresses KORs. Thus, KORs in the titi monkey BLA may be activated when titi monkeys are anxious as a result of separation from their pair-mate. Blockade of these receptors could attenuate the negative affective experience of being separated from their pair-mate. KORs in NAcc shell may also be involved in titi monkey pair-bond maintenance since comparative work with prairie voles has found that KOR antagonist administration in the NAcc shell to a paired male attenuates aggression towards a stranger conspecific (Resendez et al., 2012).
Endocrine functioning
In the present study we found that MOR and KOR were found throughout the entire hypothalamus. The hypothalamus integrates signals from diverse brain areas to regulate many endocrine systems. In general, hypothalamic MOR binding was relatively homogenous, except for the PVN where there was very dense binding. Strong binding of MORs in the PVN is of significance due to its role in regulation of the hypothalamic-pituitary-adrenal (HPA) axis. It has been proposed that MORs inhibit corticotropin releasing hormone (CRH) neurons in the PVN which would likely affect the subsequent release of cortisol (Wand et al., 1998). The presence of MORs in the PVN in the titi monkey brain lends support to the argument that MORs are present on CRH neurons, although future studies are needed to confirm this hypothesis. This would then have functional significance on HPA activity. For example, it is known that systemic administration of naloxone or naltrexone to block MORs results in increases in cortisol in humans (Wand et al., 1998), sheep (Parrott and Thornton, 1989), talapoin monkeys (Fabre-Nys et al., 1982), and titi monkeys (Ragen et al., 2013). It is possible that naloxone may be blocking MORs in the PVN to release any inhibitory tone thereby allowing activational components of the HPA axis to be expressed. Furthermore, MOR agonists such as morphine or fentanyl inhibit or decrease cortisol release (Zis et al., 1984, Parrott and Thornton, 1989, Broadbear et al., 2004, Ragen et al., 2013). This may be a result of MOR activation causing a further inhibition of CRH neurons and preventing of any excitatory mechanisms that could be acting upon the PVN.
The MOR also impacts neuropeptides such as vasopressin (AVP) and oxytocin (OT) and the presence of MORs in the PVN and SON, regions that produce OT and AVP (Buijs et al., 1978), could explain these effects. In titi monkeys, naloxone administration results in an increase in AVP, specifically when a male is separated from his pair-mate although MOR manipulation does not affect baseline OT in male titi monkeys (Ragen et al., 2013). Naloxone also has no effect on baseline OT in men (Honer et al., 1986), but does attenuate the increase in OT when men orgasm (Murphy et al., 1990). It is possible that MOR manipulation could affect OT release in female titi monkeys. There is evidence in baboons and humans that MOR activation via morphine decreases plasma OT during parturition and lactation (Lindow et al., 1992, Kowalski et al., 1998, Lindow et al., 1999).
KOR agonists have been observed to increase plasma ACTH and cortisol (Calogero et al., 1996, Ranganathan et al., 2012). The KOR agonist U50,488 increases ACTH and cortisol in rhesus macaques (Pascoe et al., 2008). A KOR agonist could be working at one or multiple places to produce the increase in cortisol in titi monkeys. A possible candidate would be the hypothalamus. Although U50,488 increases cortisol in titi monkeys, the effects are weak (Ragen et al., 2015), and could be explained by the very sparse KOR binding in the titi monkey hypothalamus. Buckingham and Cooper (1986) exposed rat hypothalami in vitro to U50,488 to measure CRH release. There was an increase in CRH but the effect was weak, which could possibly reflect the low levels of KOR binding in the rat hypothalamus (Unterwald et al., 1991). It is possible that very large doses are needed to produce a pronounced increase in cortisol. Both MOR and KOR binding were found in the amygdala, and the HPA activating effects of U50,488 could also be working on the titi monkey amygdala where KORs were found sparsely distributed. Activation of certain amygdalar nuclei has been found to activate the HPA axis. The CeA, MeA, and BLA have all been found to regulate HPA activity (Herman et al., 2003). In particular CeA activation results in increases in CRH production and the resulting release of ACTH and glucocorticoids. Finally, U50,488 could also be acting directly on the adrenal cortex to release cortisol. KOR binding has been detected in the rat adrenal cortex using [3H]EKC (Quirion et al., 1983).
Hippocampus
The present study found that there was MOR and KOR binding in the titi monkey hippocampal formation and related structures. Of potential importance, KOR binding was found specifically in the Ent, Pr, CA1, and CA3. No KOR binding was found in the DG, which differs from humans where there is both KOR mRNA and KOR binding (deLanerolle et al., 1997, Peckys and Landwehrmeyer, 1999). There was also MOR binding in the titi monkey hippocampal formation. Like KORs, there was binding found in the Ent and Prs, however unlike KORs there was binding in the Shi and there was no binding in CA1 or CA3. There was no binding in the DG, where MOR mRNA is present in humans (Peckys and Landwehrmeyer, 1999).
KOR binding in CA1 in titi monkeys could be related to social stress. Rats raised under social isolation experience an attenuation of induced long-term potentiation (LTP) in CA1 as well as impairment in learning of spatial memory tasks (Lu et al., 2003). KOR in the hippocampus regulates LTP when animals experience stress (Keralapurath et al., 2014). Titi monkey males engage in significantly more arousal behaviors and locomotion when housed in isolation compared to when housed with a pair-mate suggesting a possible state of chronic stress (Ragen et al., 2012). The behavioral results of isolated housing may reflect the natural titi monkey bachelor phase when they range the forest alone looking for a pair-mate (Bossuyt, 2002). It is possible that KOR in the hippocampus may regulate LTP and spatial memory when titi monkeys are living in different social situations.
Conclusion
The present study is one of the first to map radioligand binding for the MOR and KOR in a New World monkey as well as a monogamous primate. We found that distribution and binding densities of these receptors are similar to that of other primate species but differ in certain regions compared to rodents. MOR and KOR binding in the striatum and cingulate gyrus gives insight into possible neuroanatomical locations where these opioid receptors may be functioning to aid in pair-bond formation and maintenance in the titi monkey. The presence of MOR and KOR binding in amygdalar nuclei and the hypothalamus could be brain areas where opioid ligands are acting to regulate endocrine functioning. Previous studies have administered exogenous opiates to manipulate endocrine functioning and social behavior and this study provides possible explanations for where in the brain these drugs may be working to have their effects.
HIGHLIGHTS.
κ and μ opioid receptors were located in limbic regions associated to pair-bonding
μ opioid receptor binding in the titi monkey brain is heterogeneous
κ opioid receptor binding is relatively light and homogeneous
There is evidence that μ and κ opioid receptor binding is conserved across species
μ opioid receptor inding was dense in the hypothalamus
ACKNOWLEDGMENTS
Funding was provided by NICHD: HD053555 and the Office of Research Infrastructure Programs: Grant P51OD01107. I would like to acknowledge Dr. Michael Jarcho and Rebecca Larke for help with slicing tissue.
ABBREVIATIONS
- AAA
anterior amygdala
- AH
anterior hypothalamic nucleus
- AV
anteroventral thalamic nucleus
- Arc
arcuate nucleus
- BLA
basolateral amygdala
- BmA
basomedial amygdala
- BNST
bed nucleus of the stria terminalis
- CA1
CA1 field of the hippocampus
- CA3
CA3 field of the hippocampus
- Cd
caudate
- CeA
central amygdala
- CG
cingulate gyrus
- Cl
claustrum
- CoA
cortical amygdala
- DAMGO
[D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- DB
diagonal band
- DMH
dorsomedial hypothalamic nucleus
- DPDPE
[D-Pen2,5]-enkephalin
- Ent
entorhinal cortex
- Gpi
globus pallidus internal
- IP
interpeduncular nucleus
- LA
lateral amygdala
- LH
lateral hypothalamic nucleus
- LS
lateral septum
- MM
mammillary body
- MeA
medial amygdala
- MPOA
medial preoptic area
- MS
medial septum
- MD
mediodorsal thalamic nucleus
- NAcc
core nucleus accumbens core
- NAcc
shell nucleus accumbens shell
- NUDAP
neurochemically unique domains of the accumbens and putamen
- PaL
paralaminar nucleus
- PVN
paraventricular hypothalamic nucleus
- PrS
presubiculum
- Pul
pulvinar
- Pu
putamen
- Shi
septohippocampal nucleus
- Shy
septohypothalamic nucleus
- SNR
substantia nigra
- SCN
suprachiasmatic hypothalamic nucleus
- SON
supraoptic hypothalamic nucleus
- VA
ventral anterior thalamic nucleus
- VP
ventral pallidum
- VL
ventrolateral thalamic nucleus
- VMN
ventromedial hypothalamic nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aragona BJ, Wang ZX. The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. Ilar J. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Res. 2007;1184:245–253. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthele A, Platzer S, Jochim B, Boecker H, Buettner A, Conrad B, Riemenschneider M, Toelle TR. COMT Val(108/158) Met genotype affects the mu-opioid receptor system in the human brain: Evidence from ligand-binding, G-protein activation and preproenkephalin mRNA expression. Neuroimage. 2005;28:185–193. doi: 10.1016/j.neuroimage.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Maggi R, Pimpinelli F, Rubino T, Parolaro D, Poli V, Ciliberto G, Panerai AE, Sacerdote P. Presence of a reduced opioid response in interleukin-6 knock out mice. Eur J Neurosci. 1999;11:1501–1507. doi: 10.1046/j.1460-9568.1999.00563.x. [DOI] [PubMed] [Google Scholar]
- Borner C, Kraus J, Schroder H, Ammer H, Hollt V. Transcriptional regulation of the human mu-opioid receptor gene by interleukin-6. Mol Pharmacol. 2004;66:1719–1726. doi: 10.1124/mol.104.003806. [DOI] [PubMed] [Google Scholar]
- Bossuyt F. Natal dispersal of titi monkeys (Callicebus moloch) at Cocha Cashu, Manu National Park, Peru. American Journal of Physical Anthropology. 2002:47–47. [Google Scholar]
- Breier A, Kestler L, Adler C, Elman I, Wiesenfeld N, Malhotra A, Pickar D. Dopamine D2 receptor density and personal detachment in healthy subjects. Am J Psychiatry. 1998;155:1440–1442. doi: 10.1176/ajp.155.10.1440. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Woods JH. Self-administration of fentanyl, cocaine and ketamine: effects on the pituitary-adrenal axis in rhesus monkeys. Psychopharmacology. 2004;176:398–406. doi: 10.1007/s00213-004-1891-x. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Lemos JC, Chavkin C. CRF1-R Activation of the Dynorphin/Kappa Opioid System in the Mouse Basolateral Amygdala Mediates Anxiety-Like Behavior. PLoS One. 2009;4:9. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA. Pharmacological characterization of opioid receptors influencing the secretion of corticotropin releasing factor in the rat. Neuroendocrinology. 1986;44:36–40. doi: 10.1159/000124618. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Swaab DF, Dogterom J, Vanleeuwen FW. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Cell Tissue Res. 1978;186:423–433. doi: 10.1007/BF00224932. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, Young LJ. Activation of μ-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology. 2011;36:2200–2210. doi: 10.1038/npp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogero AE, Scaccianoce S, Burrello N, Nicolai R, Muscolo LAA, Kling MA, Angelucci L, Dagata R. The kappa-opioid receptor agonist MR-2034 stimulates the rat hypothalamic-pituitary-adrenal axis: Studies in vivo and in vitro. J Neuroendocrinol. 1996;8:579–585. [PubMed] [Google Scholar]
- Carden SE, Barr GA, Hofer MA. Differential effects of specific opioid receptor agonists on rat pup isolation calls. Dev Brain Res. 1991;62:17–22. doi: 10.1016/0165-3806(91)90185-l. [DOI] [PubMed] [Google Scholar]
- Carden SE, Davachi L, Hofer MA. U50,488 increases ultrasonic vocalizations in 3-day-old, 10-day-old and 18-day-old rat pups in isolation and the home cage. Dev Psychobiol. 1994;27:65–83. doi: 10.1002/dev.420270107. [DOI] [PubMed] [Google Scholar]
- Chen EC, Yagi S, Kelly KR, Mendoza SP, Maninger N, Rosenthal A, Spinner A, Bales KL, Schnurr DP, Lerche NW, Chiu CY. Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony. PLoS Pathog. 2011;7:16. doi: 10.1371/journal.ppat.1002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Togasaki DM, Langston JW, Di Monte DA, Quik M. Enhanced striatal opioid receptor-mediated G-protein activation in L-dopa-treated dyskinetic monkeys. Neuroscience. 2005;132:409–420. doi: 10.1016/j.neuroscience.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Letchworth SR, Sim-Selley LJ, Smith HR, Childers SR, Porrino LJ. Functional and anatomical localization of mu opioid receptors in the striatum, amygdala, and extended amygdala of the nonhuman primate. J Comp Neurol. 2001;433:471–485. doi: 10.1002/cne.1154. [DOI] [PubMed] [Google Scholar]
- deLanerolle NC, Williamson A, Meredith C, Kim JH, Tabuteau H, Spencer DD, Brines ML. Dynorphin and the kappa 1 ligand H-3 U69,593 binding in the human epileptogenic hippocampus. Epilepsy Res. 1997;28:189–205. doi: 10.1016/s0920-1211(97)00044-2. [DOI] [PubMed] [Google Scholar]
- Diaz SL, Barros VG, Antonelli MC, Rubio MC, Balerio GN. Morphine withdrawal syndrome and its prevention with baclofen: Autoradiographic study of mu-opioid receptors in prepubertal male and female mice. Synapse. 2006;60:132–140. doi: 10.1002/syn.20279. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Liu MR, Woods JH, Medzihradsky F. Binding affinity and selectivity of opioids at mu, delta, and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther. 1994;271:1630–1637. [PubMed] [Google Scholar]
- Fabre-Nys C, Meller RE, Keverne EB. Opiate antagonists stimulate affiliative behaviour in monkeys. Pharmacol Biochem Behav. 1982;16:653–659. doi: 10.1016/0091-3057(82)90432-4. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus). Neuroscience. 2014;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: Evidence for opiate mediation of social affect. Pharmacol Biochem Behav. 1978;9:213–220. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hiller JM, Fan LQ. Laminar distribution of the multiple opioid receptors in the human cerebral cortex. Neurochem Res. 1996;21:1333–1345. doi: 10.1007/BF02532374. [DOI] [PubMed] [Google Scholar]
- Honer WG, Thompson C, Lightman SL, Williams TDM, Checkley SA. No effect of naloxone on plasma oxytocin in normal men. Psychoneuroendocrinology. 1986;11:245–248. doi: 10.1016/0306-4530(86)90061-2. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H, Ni L, Walker SJ, Mickey BJ, Korycinski ST, Koeppe RA, Crocker JK, Langenecker SA, Zubieta JK. Response of the mu-opioid system to social rejection and acceptance. Mol Psychiatr. 2013;18:1211–1217. doi: 10.1038/mp.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Burkett JP, Young LJ. Neuroanatomical distribution of mu-opioid receptor mRNA and binding in monogamous prairie voles (Microtus ochrogaster) and non-monogamous meadow voles (Microtus pennsylvanicus). Neuroscience. 2013;244:122–133. doi: 10.1016/j.neuroscience.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Lynn DE. Opiate systems in mother and infant primates coordinate intimate contact during reunion. Psychoneuroendocrinology. 1995;20:735–742. doi: 10.1016/0306-4530(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Keralapurath MM, Clark JK, Hammond S, Wagner JJ. Cocaine- or stress-induced metaplasticity of LTP in the dorsal and ventral hippocampus. Hippocampus. 2014;24:577–590. doi: 10.1002/hipo.22250. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Martensz ND, Tuite B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology. 1989;14:155–161. doi: 10.1016/0306-4530(89)90065-6. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, Carroll FI, Nestler EJ, Konradi C, Carlezon WA. Kappa Opioid Receptor Signaling in the Basolateral Amygdala Regulates Conditioned Fear and Anxiety in Rats. Biol Psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski WB, Parsons MT, Pak SC, Wilson L. Morphine inhibits nocturnal oxytocin secretion and uterine contractions in the pregnant baboon. Biol Reprod. 1998;58:971–976. doi: 10.1095/biolreprod58.4.971. [DOI] [PubMed] [Google Scholar]
- Kraus J, Borner C, Lendeckel U, Hollt V. Interferon-gamma down-regulates transcription of the mu-opioid receptor gene in neuronal and immune cells. J Neuroimmunol. 2006;181:13–18. doi: 10.1016/j.jneuroim.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Lewis ME, Khachaturian H, Akil H, Watson SJ. Anatomical relationship between opioid peptides and receptors in rhesus monkey brain. Brain Res Bull. 1984;13:801–812. doi: 10.1016/0361-9230(84)90238-7. [DOI] [PubMed] [Google Scholar]
- Lim MM, Hammock EAD, Young LJ. A method for acetylcholinesterase staining of brain sections previously processed for receptor autoradiography. Biotech Histochem. 2004;79:11–16. doi: 10.1080/10520290410001671344. [DOI] [PubMed] [Google Scholar]
- Lindow SW, Hendricks MS, Nugent FA, Dunne TT, van der Spuy ZM. Morphine suppresses the oxytocin response in breast-feeding women. Gynecol Obstet Invest. 1999;48:33–37. doi: 10.1159/000010130. [DOI] [PubMed] [Google Scholar]
- Lindow SW, Vanderspuy ZM, Hendricks MS, Rosselli AP, Lombard C, Leng G. The effect of morphine and naloxone administration on plasma oxytocin concentrations in the first stage of labor. Clin Endocrinol. 1992;37:349–353. doi: 10.1111/j.1365-2265.1992.tb02337.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Bao GB, Chen H, Xia P, Fan XL, Zhang JS, Pei G, Ma L. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol. 2003;183:600–609. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Martel FL, Nevison CM, Simpson MJ, Keverne EB. Effects of opioid receptor blockade on the social behavior of rhesus monkeys living in large family groups. Dev Psychobiol. 1995;28:71–84. doi: 10.1002/dev.420280202. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Checkley SA, Seckl JR, Lightman SL. Naloxone inhibit oxytocin release at orgasm in man. J Clin Endocrinol Metab. 1990;71:1056–1058. doi: 10.1210/jcem-71-4-1056. [DOI] [PubMed] [Google Scholar]
- Newman JD, Kenkel WM, Aronoff EC, Bock NA, Zametkin MR, Silva AC. A combined histological and MRI brain atlas of the common marmoset monkey, Callithrix jacchus. Brain Res Rev. 2009;62:1–18. doi: 10.1016/j.brainresrev.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott JP. Biology of social attachments: Opiates alleviate separation distress. Biol Psychiatry. 1978a;13:607–618. [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neurosci Biobehav Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Vilberg T, Bean NJ, Coy DH, Kastin AJ. Reduction of distress vocalization in chicks by opiate-like peptides. Brain Res Bull. 1978b;3:663–667. doi: 10.1016/0361-9230(78)90014-x. [DOI] [PubMed] [Google Scholar]
- Parrott RF, Thornton SN. Opioid influences on pituitary function in sheep under basal conditions and during psychological stress. Psychoneuroendocrinology. 1989;14:451–459. doi: 10.1016/0306-4530(89)90044-9. [DOI] [PubMed] [Google Scholar]
- Pascoe JE, Williams KL, Mukhopadhyay P, Rice KC, Woods JH, Ko MC. Effects of mu, kappa, and delta opioid receptor agonists on the function of hypothalamic-pituitary-adrenal axis in monkeys. Psychoneuroendocrinology. 2008;33:478–486. doi: 10.1016/j.psyneuen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga AW. The rhesus monkey brain in stereotaxic coordinates. Academic Press; Sand Diego: 1999. [Google Scholar]
- Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: A P-33 in situ hybridization study. Neuroscience. 1999;88:1093–1135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- Pilapil C, Welner S, Magnan J, Gauthier S, Quirion R. Autoradiographic distribution of multiple classes of opioid receptor binding sites in human forebrain. Brain Res Bull. 1987;19:611–615. doi: 10.1016/0361-9230(87)90080-3. [DOI] [PubMed] [Google Scholar]
- Quirion R, Finkel MS, Mendelsohn FAO, Zamir N. Localization of opiate binding sites in the kidney and adrenal gland of the rat. Life Sci. 1983;33:299–302. doi: 10.1016/0024-3205(83)90502-7. [DOI] [PubMed] [Google Scholar]
- Quirion R, Pilapil C, Magnan J. Localization of kappa opioid receptor binding sites in human forebrain using [3H]U69,593: Comparison with [3H]bremazoncine. Cell Mol Neurobiol. 1987;7:303–307. doi: 10.1007/BF00711306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Bales KL. The effects of morphine, naloxone, and κ opioid manipulation on endocrine functioning and social behavior in monogamous titi monkeys (Callilcebus cupreus). Neuroscience. 2015;287:32–42. doi: 10.1016/j.neuroscience.2014.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Jarcho MR, Bales KL. Presence of a pair-mate regulates the behavioral and physiological effects of opioid manipulation in the monogamous titi monkey (Callicebus cupreus). Psychoneuroendocrinology. 2013;38:2448–2461. doi: 10.1016/j.psyneuen.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Mendoza SP, Mason WA, Bales KL. Differences in titi monkey (Callicebus cupreus) social bonds affect arousal, affiliation, and response to reward. Am J Primatol. 2012;74:758–769. doi: 10.1002/ajp.22026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, Schnakenberg A, Skosnik PD, Cohen BM, Pittman B, Sewell RA, D'Souza DC. Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the κ opioid agonist Salvinorin A in humans. Biol Psychiatry. 2012;72:871–879. doi: 10.1016/j.biopsych.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Aragona BJ. Aversive motivation and the maintenance of monogamous pair bonding. Rev Neurosci. 2013;24:51–60. doi: 10.1515/revneuro-2012-0068. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Dome M, Gormley G, Franco D, Nevarez N, Hamid AA, Aragona BJ. μ-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. J Neurosci. 2013;33:9140–9149. doi: 10.1523/JNEUROSCI.4123-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. κ-opioids receptors within the nucleus accumbens shell mediate pair bond maintenance. J Neurosci. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G, Troisi A. Opiate receptor blockade in juvenile macaques: effect on affiliative interactions with their mothers and group companions. Brain Res. 1992;576:125–130. doi: 10.1016/0006-8993(92)90617-i. [DOI] [PubMed] [Google Scholar]
- Schoultz BW, Hjornevik T, Willoch F, Marton J, Noda A, Murakami Y, Miyoshi S, Nishimura S, Arstad E, Drzezga A, Matsunari I, Henriksen G. Evaluation of the kappa-opioid receptor-selective tracer [C-11]GR103545 in awake rhesus macaques. Eur J Nucl Med Mol Imaging. 2010;37:1174–1180. doi: 10.1007/s00259-010-1384-6. [DOI] [PubMed] [Google Scholar]
- Shapiro LE, Meyer ME, Dewsbury DA. Affiliative behavior in voles: effects of morphine, naloxone, and cross-fostering. Physiol Behav. 1989;46:719–723. doi: 10.1016/0031-9384(89)90357-0. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Hughes J. Discrete mapping of brain mu and delta opioid receptors using selective peptides: Quantitative autoradiography, species differences and comparison with kappa receptors. Peptides. 1989;10:499–522. doi: 10.1016/0196-9781(89)90135-6. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Daunais JB, Porrino LJ, Childers SR. Mu and kappa-1 opioid-stimulated [S-35]guanylyl-5 '-O-(gamma-thio)-triphosphate binding in cynomolgus monkey brain. Neuroscience. 1999;94:651–662. doi: 10.1016/s0306-4522(99)00344-9. [DOI] [PubMed] [Google Scholar]
- Talbot PS, Narendran R, Butelman ER, Huang YY, Ngo K, Slifstein M, Martinez D, Laruelle M, Hwang DR. C-11-GR103545, a radiotracer for imaging kappa-opioid receptors in vivo with PET: Synthesis and evaluation in baboons. J Nucl Med. 2005;46:484–494. [PubMed] [Google Scholar]
- Unterwald EM, Knapp C, Zukin RS. Neuroanatomical localization of κ1 and κ2 opioid receptors in rat and guinea pig brain. Brain Res. 1991;562:57–65. doi: 10.1016/0006-8993(91)91186-5. [DOI] [PubMed] [Google Scholar]
- Vonkeman HE, Voorn P, Brady LS, Berendse HW, Richfield EK. Opioid receptor ligand binding in the human striatum: II. Heterogeneous distribution of kappa opioid receptor labeled with [H-3]bremazocine. J Comp Neurol. 1996;374:223–229. doi: 10.1002/(SICI)1096-9861(19961014)374:2<223::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Voorn P, Brady LS, Berendse HW, Richfield EK. Densitometrical analysis of opioid receptor ligand binding in the human striatum .1. Distribution of mu opioid receptor defines shell and core of the ventral striatum. Neuroscience. 1996;75:777–792. doi: 10.1016/0306-4522(96)00271-0. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, El Deiry S, McCaul ME, Hoover D. Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry. 1998;55:1114–1119. doi: 10.1001/archpsyc.55.12.1114. [DOI] [PubMed] [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc Natl Acad Sci U S A. 2009;106:15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zis AP, Haskett RF, Albala AA, Carroll BJ. Morphine inhibits cortisol and stimulates prolactin secretion in man. Psychoneuroendocrinology. 1984;9:423–427. doi: 10.1016/0306-4530(84)90050-7. [DOI] [PubMed] [Google Scholar]
- Zukin RS, Eghbali M, Olive D, Unterwald EM, Tempel A. Characterization and visualization of rat and guinea pig brain K opioid receptors: Evidence for κ1 and κ2 opioid receptors. Proc Natl Acad Sci U S A. 1988;85:4061–4065. doi: 10.1073/pnas.85.11.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]