Abstract
Background
Disturbed sleep and pain often co-exist and the relationship between the two conditions is complex and likely reciprocal. This 5-year prospective study examines whether disturbed sleep can predict the onset of multi-site pain, and whether non-disturbed sleep can predict the resolution of multi-site pain.
Methods
The cohort (n = 1599) was stratified by the number of self-reported pain sites: no pain, pain from 1–2 sites and multi-site pain (≥3 pain sites). Sleep was categorized by self-reported sleep disturbance: sleep A (best sleep), sleep B and sleep C (worst sleep). In the no-pain and pain-from-1–2 sites strata, the association between sleep (A, B and C) and multi-site pain 5 years later was analysed. Further, the prognostic value of sleep for the resolution of multi-site pain at follow-up was calculated for the stratum with multi-site pain at baseline. In the analyses, gender, age, body mass index, smoking, physical activity and work-related exposures were treated as potential confounders.
Results
For individuals with no pain at baseline, a significantly higher odds ratio for multi-site pain 5 years later was seen for the tertile reporting worst sleep [odds ratio (OR) 4.55; 95% confidence interval (CI) 1.28–16.12]. Non-disturbed (or less disturbed) sleep had a significant effect when predicting the resolution of multi-site pain (to no pain) (OR 3.96; 95% CI 1.69–9.31).
Conclusion
In conclusion, sleep could be relevant for predicting both the onset and the resolution of multi-site pain. It seems to be a significant factor to include in research on multi-site pain and when conducting or evaluating intervention programmes for pain.
What's already known about this topic?
Disturbed sleep and pain are often concurrent and the relationship between them is reciprocal.
Sleep seems to predict the onset and resolution from chronic widespread pain.
What does this study add?
Disturbed sleep seems to be a predictor for the onset of multi-site pain 5 years later among individuals free from pain at baseline.
Non-disturbed sleep is a potential predictor of the resolution from multi-site pain 5 years later.
1. Introduction
Low back pain (LBP) is often concurrent with pain from other body sites. Individuals with localized LBP report better perceived health, less pain severity, shorter pain duration and shorter time with pain the previous year compared with individuals with LBP accompanied by widespread pain (Natvig et al., 2001). This group also reports their sleep to be better than those with LBP and widespread pain, defined as LBP and, in addition, pain from four or more sites of the body (Natvig et al., 2001).
The number of painful locations has been shown to be more strongly associated with health-related functioning than chronicity or location of the pain (Saastamoinen et al., 2006). Multi-site pain is associated with an increased risk of sickness absence (Haukka et al., 2013), disability pension (Kamaleri et al., 2009b) and poor self-rated work ability (Miranda et al., 2010).
Sleep problems is a factor associated with both pain (Smith and Haythornthwaite, 2004; Fishbain et al., 2010; Alsaadi et al., 2011) and sickness absence (Sivertsen et al., 2006, 2009; Akerstedt et al., 2007; Salo et al., 2010).
In a review from 2010, investigating the relationship between chronic pain and sleep disorder, the presence of a bidirectional relationship between sleep and pain could not be excluded (Fishbain et al., 2010). Insufficient sleep or short sleep duration has shown to be associated with subsequently increased self-reported pain intensity (Edwards et al., 2008; Haack et al., 2012) and inadequate pain inhibition (Edwards et al., 2009; Campbell et al., 2011; Haack et al., 2012). There are, however, few longitudinal studies that have investigated the effect of disturbed sleep on pain. A 28-year follow-up study showed that disturbed sleep at baseline was a significant predictive factor for hospitalization due to back pain, even after excluding participants with back pain at baseline (Kaila-Kangas et al., 2006). Gupta and colleagues found, after controlling for pain at baseline, that self-reported sleep problems, somatic symptoms and illness behaviour all predicted the onset of chronic widespread pain (CWP), defined as pain present from two contralateral quadrants of the body, above and below the waist, and in the axial skeleton (Gupta et al., 2007).
It is of clinical value to know more about predictive factors for multi-site pain. Previously having been in pain is a strong predictor of multi-site pain (Kamaleri et al., 2009a) and CWP (Gupta et al., 2007). Self-reported problems with sleep have shown to be predictive of the onset of CWP (Gupta et al., 2007) and restorative sleep has been shown to predict resolution of CWP (Davies et al., 2008). To the best of our knowledge, there is no prospective study that has investigated disturbed sleep as a predictor of the onset and resolution of multi-site musculoskeletal pain in which the cohort has been stratified by the number of pain sites. We hypothesize that disturbed sleep (including the dimensions, difficulties falling asleep, repeated awakenings, premature final awakening, not feeling well-rested at awakening and disturbed/restless sleep) predicts the onset of multi-site (musculoskeletal) pain 5 years later among individuals free from pain at baseline, and that good sleep predicts the resolution of multi-site pain 5 years later.
2. Methods and materials
2.1 Data
The present study is based upon data from the MUSIC (Musculoskeletal Intervention Centre) – Norrtälje Study, a study initiated in order to identify and quantify risk and protective factors for LBP and NSP, and to investigate pain prognoses. In the MUSIC-Norrtälje Study, data were assessed at two time points. The study was designed as a prospective case-referent study with one 5-year follow-up of all cases and referents that participated in the baseline study. The total response rate of the 2812 individuals recruited was 83%. The baseline study was conducted during 1994–1996. Approximately 17,000 men and women, 20–59 years of age, who were living and working in the rural municipality of Norrtälje in Sweden, comprised the study population. The cases were recruited when participants sought care for LBP or NSP from 1 of approximately 70 caregivers in the area, including physicians, physiotherapists and alternative caregivers, such as chiropractors, osteopaths and homeopaths. Referents, matched by age and gender, were chosen at random via the population register. The inclusion criteria for the MUSIC-Norrtälje study were to be working, and to not have sought care for, or been treated for LBP or NSP, for at least 6 months prior to baseline assessment. Individual and demographic data, information on LBP and NSP, sleep disturbance and other medical conditions, and also on work-related exposures were assessed through self-administered questionnaires and interviews among both cases and referents (Grooten et al., 2004b, 2007). The 5-year follow-up was conducted using postal questionnaires. Census data on sickness absence (>14 consecutive days) were taken from the National Social Insurance Board and linked to each of the 2329 subjects who participated in both the baseline study and at follow-up.
The MUSIC-Norrtälje study was approved by the Ethics Committee of Karolinska Institutet (Dnr 03-139; Dnr 93-255).
The present study is designed as a 5-year prospective cohort study, which considers all participants with data from baseline and follow-up regardless of whether they were cases or referents in the original MUSIC-Norrtälje study.
An exclusion criterion based upon some diseases associated with chronic pain was however added. In the baseline questionnaire, the participants were asked to state whether they had (1) vascular pain from legs; (2) disease from nerves (brain, spinal cord, peripheral nerves); (3) joint reconstruction (arthroplasty) in hip, knee or other joint; (4) any congenital defect in joints, muscles or back; (5) been diagnosed with rheumatoid arthritis; and (6) been diagnosed with ankylosing spondylitis (Bechterew's disease). If the answer was yes for any one of these items, the individual was excluded from the study (resulting in exclusion of 10% of the cohort). The exclusion criterion was based upon the assumption that having any one of these conditions would make it more likely for individuals to have chronic multi-site pain where sleep might well influence their general health, but would be less likely to have an impact on the onset or resolution of pain. The cohort included all subjects with valid data from baseline and follow-up, not reporting any of the above medical conditions.
2.2 Baseline data
Sleep disturbance was assessed through a modified version of the Karolinska Sleep Questionnaire, using a 5-item Likert scale (Akerstedt et al., 2002a, 2008). To obtain a value for sleep disturbance, a sleep disturbance index (Akerstedt et al., 2002b, 2008) with the following five questions was used: During the last 6 months have you had (1) Difficulties falling asleep?; (2) Repeated awakenings with difficulties going back to sleep?; (3) The feeling of not being well-rested when waking up?; (4) Premature (final) awakening?; (5) Disturbed/restless sleep? The respondents could select between 1 – never; 2 – seldom/a few times per year; 3 – sometimes/several times per month; 4 – most of the time/several times per week; 5 – always, every day. The eligible sum of score ranged from 5 to 25.
The distribution of sleep scores (including all 2329 individuals with baseline data) enabled us to divide sleep into tertiles with the following cut-offs: <10 (sleep A), 11–13 (sleep B) and >14 (sleep C). Sleep A represents the tertile with best sleep and sleep C represents the tertile with worst sleep.
Multi-site pain was defined using items from a modified version of the Standard Nordic Questionnaire (Kuorinka et al., 1987): Have you had discomfort (pain, ache, discomfort) at any time during the last 6 months from (1) neck; (2) shoulder/shoulders; (3) elbow/elbows; (4) hand/hands/wrist/wrists; (5) upper back (thoracic); (6) lower back; (7) hip/hips; (8) knee/knees; and (9) foot/feet? We made no distinction between unilateral and bilateral pain, entailing, e.g., that if pain was reported from both elbows, the two elbows were treated as just one pain location. Thus, the total number of pain locations was nine.
The same items for assessment of pain sites were used at both baseline and follow-up. In both questionnaires, the questions were supplemented with an illustration of the body parts in question. The expression ‘multisite pain’ has been used in other studies (Kamaleri et al., 2009b; Solidaki et al., 2010) but with different specifications of how many sites should, in general, form a cut-off. The cut-off used in the present study (≥3 sites) is based upon the results in a study by Kamaleri and colleagues, using the same questionnaire. In that study, individuals with three or more pain sites were found to show an increased risk for work disability (Kamaleri et al., 2009b). To establish whether the same cut-off was reasonable for the present study, a binary logistic regression analysis of sickness absence (for >14 consecutive days at any time during the 5-year study period) was made with the numbers of pain sites as independent factors adjusted for age and gender. The analysis showed an OR of 1.9 (p = 0.001) at the level of three pain sites (for two pain sites OR = 1.4; p = 0.04 and for four pain sites OR = 3.0; p = 0.000).
2.3 Potential confounders
High physical (biomechanical) (Haukka et al., 2012) and psychosocial (Haukka et al., 2011) workload have been shown to predict the onset of multi-site pain. Apart from the already-mentioned risk factors, commonly used covariates in research investigating pain are age, gender, physical activity, smoking, body mass index (BMI) and psychosocial circumstances.
In the present study, age (continuous), gender, inclusion as case or referent in MUSIC-Norrtälje, BMI (dichotomized into <25 and ≥25 kg/m2) smoking, physical activity, psychosocial exposures at work and biomechanical exposures at work were considered as potential confounders and assessed in questionnaires and interviews at baseline.
Smoking was categorized into (1) non-smoker; (2) smoking sometimes, e.g., at parties; and (3) smoker.
Physical activity comprised two variables, medium-intensity exercise and high-intensity exercise, and was assessed by the questions: (1) To what extent do you at the present perform regular medium intensity exercise (e.g., jogging, riding, gymnastics, dancing)? (2) To what extent do you at the present perform regular high intensity exercise (e.g., running, football, squash)? The variables were allocated to five categories according to their frequency: (1) 5 days or more per week; (2) 2–4 days/week; (3) 1 day/week; (4) 1–3 days/month; (5) almost never/never.
Biomechanical exposures at work were treated in four variables: working with hands above shoulder level; prolonged sitting (≥75% of the working time); manual handling (≥50N2 ≥60 min/day); and working with vibrating tools (≥60 min/day).
Psychosocial exposures at work were considered in eight variables: (1) few opportunities to learn and develop at work; (2) high mental demands; (3) low decision latitude; (4) job strain (a combination of high mental demands and low decision latitude); (5) poor general support at work; (6) low meaningfulness; (7) high time pressure; and (8) hindrances at work (including poor work task clarity, poor material or personnel resources, and/or poor work task support affecting the quality of work or leading to regular overtime working or neglecting safety rules to accomplish the work). The variables treating work-related exposures were assessed in questionnaires and interviews. Each of the psychosocial and biomechanical exposures was dichotomized into exposed or non-exposed. The construction of the psychosocial and biomechanical exposures, and of the variables and cut-offs for dichotomization, is described in detail in previous MUSIC-Norrtälje studies (Tornqvist et al., 2001; Grooten et al., 2004a).
The distribution of former cases and referents are presented in the study as a baseline characteristic since cases and referents might have different pain characteristics. To be included as a case or referent in the study has been treated as a confounder in the analysis of the two strata including individuals reporting pain at baseline.
Further, the prevalence of each pain location was identified in each pain stratum.
2.4 Statistics
Pain at baseline is considered a strong predictive factor for an increase in the number of pain sites (Kamaleri et al., 2009a). Three pain strata were thus formed: no pain, pain from 1–2 sites and multi-site pain. The stratification was used both at baseline and for the outcomes at follow-up. The design of the stratification and research questions are described in Fig. 1.
Figure 1.
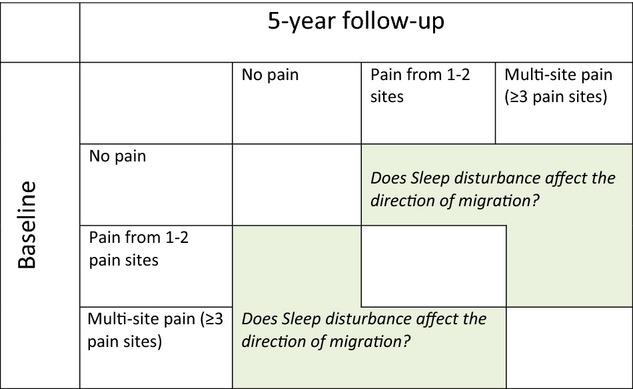
Flowchart describing the study design. The study is designed to study individuals migrating from one stratum at baseline to a different one at follow-up. Does disturbed sleep affect the direction of the change in pain sites?
The effect of sleep disturbance in the three pain strata was analysed for the three outcomes (no pain, 1–2 pain sites and ≥3 pain sites) in multinomial logistic regression analyses.
The potential confounders were tested one by one in a stratified multinomial logistic regression analysis, and included in the modelling if, after adjustment for age and gender, they were significantly associated with outcome at a 0.05 level and altered the beta coefficient by more than 10%.
3. Results
In all, 1777 participants had valid data on all the parameters included (baseline and follow-up). Of these, 1599 fulfilled the added inclusion criterion (not having any of the identified medical conditions associated with increased musculoskeletal pain). There were more women (60%) than men in the group included in the study, and 58% were under 45 years of age at baseline. Among the participants included in the study, 622 (39%) had best sleep (sleep A), 481 (30%) had medium sleep (sleep B) and 496 (31%) had worst sleep (sleep C).
Further, 881 participants reported multi-site pain at baseline, whereas 188 reported no pain (Table 1). Women were more common in the multi-site pain stratum than in the other two strata. Among the individuals who reported multi-site pain at baseline, more also reported the worst sleep (sleep C) than in the other two pain strata.
Table 1.
Background characteristics showing the number of individuals (n) and percentage of individuals (%) within each pain stratum
| Baseline characteristics | No pain | Pain from 1–2 sites | Multi-site pain |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| All | 188 (11) | 530 (33) | 881 (56) |
| Referents | 186 (99) | 367 (69) | 434 (49) |
| Women | 95 (51) | 278 (53) | 595 (68) |
| Age, mean (SD) | 42 (11) | 41 (10) | 42 (10) |
| BMI, mean (SD) | 24 (4) | 25 (14) | 26 (21) |
| BMI ≥ 25 kg/m2 | 72 (39) | 207 (40) | 380 (44) |
| Sleep | |||
| Sleep Aa | 106 (56) | 254 (48) | 262 (30) |
| Sleep Bb | 53 (28) | 147 (28) | 281 (32) |
| Sleep Cc | 29 (15) | 129 (24) | 338 (38) |
| Smoking | |||
| Yes | 28 (15) | 91 (17) | 217 (25) |
| Yes, sometimes | 16 (9) | 40 (8) | 63 (7) |
| No | 144 (77) | 399 (75) | 600 (68) |
| Regular medium intensive exercise | |||
| ≥5 days/week | 17 (9) | 70 (13) | 103 (12) |
| 2–4 days/week | 58 (31) | 167 (32) | 258 (29) |
| 1 day/week | 40 (21) | 113 (21) | 170 (19) |
| 1–3 days/month | 19 (10) | 68 (13) | 113 (13) |
| Almost never/never | 53 (28) | 112 (21) | 232 (26) |
| Regular high-intensity exercise | |||
| ≥5 days/week | 5 (3) | 12 (2) | 16 (2) |
| 2–4 days/week | 38 (20) | 126 (24) | 162 (18) |
| 1 day/week | 24 (13) | 90 (17) | 135 (15) |
| 1–3 days/month | 19 (10) | 47 (9) | 81 (9) |
| Almost never/never | 101 (54) | 254 (48) | 486 (55) |
| Exposed to work-related exposures | |||
| Manual handling | 14 (7) | 63 (12) | 88 (10) |
| Hands above shoulder | 36 (19) | 94 (18) | 148 (17) |
| Prolonged sitting | 34 (18) | 102 (19) | 170 (19) |
| Vibrating tools | 21 (11) | 77 (15) | 111 (13) |
| High mental demands | 37 (20) | 113 (21) | 240 (27) |
| Low decision latitude | 30 (16) | 90 (17) | 184 (21) |
| Poor general support at work | 48 (26) | 172 (33) | 311 (35) |
| Low meaningfulness | 20 (11) | 70 (13) | 122 (14) |
| High job strain | 3 (2) | 18 (3) | 56 (6) |
| High time pressure | 20 (11) | 73 (14) | 139 (16) |
| Low possibilities to learn | 28 (15) | 103 (19) | 225 (26) |
| High hindrances | 52 (28) | 166 (31) | 311 (35) |
Best sleep.
Medium sleep.
Worst sleep.
3.1 Onset of multi-site pain
Among individuals with no pain at baseline (n = 188), 51% were women and 55% were under 45 years of age.
Among the 11% (n = 21) who reported no pain at baseline and multi-site pain at follow-up, the most frequently reported pain sites at follow-up were shoulder/shoulders (86%), neck (76%), lower back (71%) and thoracic spine (43%). These 21 participants were rather evenly distributed across the three sleep categories (sleep A 33%; sleep B 29%; sleep C 38%) (Table 2).
Table 2.
Pain locations reported by participants with no pain at baseline, but reporting multi-site pain at follow-up, number of individuals (n), percentage within each sleep group (%)
| Pain site | Sleep Aa | Sleep Bb | Sleep Cc | Total |
|---|---|---|---|---|
| n = 7 | n = 6 | n = 8 | n = 21 | |
| Neck, n (%) | 6 (86) | 5 (83) | 5 (63) | 16 (76) |
| Shoulder(s), n (%) | 6 (86) | 6 (100) | 6 (75) | 18 (86) |
| Elbow(s), n (%) | 0 (0) | 1 (17) | 1 (13) | 2 (10) |
| Hand/wrist, n (%) | 1 (14) | 1 (17) | 2 (25) | 4 (20) |
| Thoracic spine, n (%) | 3 (43) | 4 (67) | 2 (25) | 9 (43) |
| LBP, n (%) | 4 (57) | 4 (67) | 7 (88) | 15 (71) |
| Hip(s), n (%) | 4 (57) | 1 (17) | 3 (38) | 8 (38) |
| Knee(s), n (%) | 1 (14) | 2 (33) | 4 (50) | 7 (33) |
| Foot/ankle, n (%) | 1 (14) | 0 (0) | 1 (13) | 2 (10) |
LBP, low back pain.
Best sleep.
Medium sleep.
Worst sleep.
BMI fulfilled the criteria for confounding and was therefore included in the full model. BMI ≥ 25 kg/m2 showed a negative association with multi-site pain at follow-up, but no association with 1–2 pain sites at follow-up (data not shown).
In the multinomial logistic regression analysis, the worst sleep tertile (sleep C) showed a significantly higher odds ratio (OR 4.55; 95% CI 1.28–16.12 in the full model) for reporting multi-site pain (pain from ≥3 sites) in the no-pain-at-baseline stratum. The medium sleep tertile (sleep B) also showed a higher odds ratio for reporting multi-site pain at follow-up, but the increase was non-significant (OR 2.31; 95% CI 0.66–8.09 in the full model). The same trend for sleep A–sleep C was not seen for the pain-from-1-2-sites outcome at follow-up (Table 3).
Table 3.
Multinomial regression analysis presenting odds ratios for reporting 1–2 pain sites or ≥3 pain sites at follow-up, stratified by no pain or 1–2 pain sites at baseline
| 1–2 pain sites |
≥3 pain sites |
||||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| No pain | Model 1a | ||||
| Sleep Ab | 1 | ||||
| Sleep Bc | 1.43 | 0.71–2.89 | 2.19 | 0.65–7.31 | |
| Sleep Cd | 0.80 | 0.31–2.06 | 4.86* | 1.47–16.02 | |
| Model 2e | |||||
| Sleep Ab | 1 | 1 | |||
| Sleep Bc | 1.44 | 0.71–2.92 | 2.24 | 0.65–7.70 | |
| Sleep Cd | 0.81 | 0.31–2.11 | 5.27* | 1.52–18.23 | |
| Model 3f | |||||
| Sleep Ab | 1 | 1 | |||
| Sleep Bc | 1.43 | 0.70–2.90 | 2.31 | 0.66–8.09 | |
| Sleep Cd | 0.90 | 0.34–2.40 | 4.55* | 1.28–16.12 | |
| Pain from 1–2 sites | Model 1a | ||||
| Sleep Ab | 1 | 1 | |||
| Sleep Bc | 1.29 | 0.77–2.17 | 1.53 | 0.85–2.73 | |
| Sleep Cd | 0.92 | 0.53–1.59 | 1.98* | 1.11–3.52 | |
| Model 2e | |||||
| Sleep Ab | 1 | 1 | |||
| Sleep Bc | 1.30 | 0.77–2.19 | 1.54 | 0.86–2.77 | |
| Sleep Cd | 0.91 | 0.52–1.58 | 1.90* | 1.10–3.39 | |
| Model 3g | |||||
| Sleep Ab | 1 | ||||
| Sleep Bc | 1.31 | 0.78–2.21 | 1.55 | 0.86–2.79 | |
| Sleep Cd | 0.93 | 0.53–1.62 | 1.94* | 1.08–3.49 | |
CI, confidence interval; OR, odds ratio.
Significant result at the 0.05 level.
Crude.
Best sleep.
Medium sleep
Worst sleep
Adjusted for age and gender.
Adjusted for age, gender and body mass index.
Adjusted for age, gender and inclusion as case or referent.
3.2 Resolution of multi-site pain
Among the individuals with multi-site pain at baseline (n = 881), 68% were women and 57% were under 45 of age. A majority of those who reported multi-site pain at baseline also reported multi-site pain at follow-up (69%), and very few (6%) reported no pain at follow-up.
The most frequently reported pain sites among the participants with multi-site pain at baseline were lower back (82%), shoulder/shoulders (79%) and neck (78%). Pain in the lower back and neck were reported by 62% of the participants included in the stratum.
Among the 6% (n = 53) who reported multi-site pain at baseline but no pain at follow-up, the most common pain sites in this group were neck (74%), lower back (74%), shoulder/shoulders (68%) and knees (38%) (Table 4).
Table 4.
Pain locations reported by participants with multi-site pain at baseline, but reporting no pain at follow-up, number of individuals (n), percentage of each sleep group and of total (%). Prevalence is presented for the three tertiles of sleep disturbance and in total
| Pain site | Sleep Aa | Sleep Bb | Sleep Cc | Total |
|---|---|---|---|---|
| n = 23 | n = 20 | n = 10 | n = 53 | |
| Neck, n (%) | 15 (65) | 16 (80) | 8 (80) | 39 (74) |
| Shoulder(s), n (%) | 13 (57) | 15 (75) | 8 (80) | 36 (68) |
| Elbow(s), n (%) | 6 (26) | 3 (15) | 3 (30) | 12 (23) |
| Hand/wrist, n (%) | 6 (26) | 6 (30) | 3 (30) | 15 (28) |
| Thoracic spine, n (%) | 4 (17) | 6 (30) | 3 (30) | 13 (25) |
| LBP, n (%) | 19 (83) | 13 (65) | 7 (70) | 39 (74) |
| Hip(s), n (%) | 5 (22) | 6 (30) | 3 (30) | 14 (26) |
| Knee(s), n (%) | 11 (48) | 5 (25) | 4 (40) | 20 (38) |
| Foot/ankle, n (%) | 8 (35) | 7 (35) | 4 (40) | 19 (36) |
LBP, low back pain.
Best sleep.
Medium sleep.
Worst sleep.
Manual handling (≥50N2 ≥60 min/day) and prolonged sitting (≥75% of working time) both fulfilled the criteria for confounding factors and were therefore included in the full model for the multinomial logistic regression analysis (data not shown).
Sleep A and sleep B showed higher odds ratios for no pain at follow-up compared with more disturbed sleep (sleep C) (OR 3.48; 95% CI 1.61–7.53 for sleep A, and OR 2.44; 95% CI 1.11–5.36 for sleep B) after adjusting for age and gender. In the full model, the OR and the confidence interval increased for both sleep A (OR 3.96) and sleep B (OR 2.40), and remained significant for sleep A at a 0.05 level (Table 5).
Table 5.
Multinomial regression analysis showing the odds ratios for reporting fewer pain sites at follow-up (1–2 pain sites or no pain) among individuals with multi-site pain at baseline
| 1–2 pain sites |
No pain |
||||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| Multi-site pain | Model 1a | ||||
| Sleep Cb | 1 | ||||
| Sleep Bc | 1.23 | 0.84–1.80 | 2.64* | 1.21–5.76 | |
| Sleep Ad | 1.82* | 1.25–2.65 | 3.72* | 1.72–8.02 | |
| Model 2e | |||||
| Sleep Cb | 1 | 1 | |||
| Sleep Bc | 1.17 | 0.79–1.72 | 2.44* | 1.11–5.36 | |
| Sleep Ad | 1.75* | 1.20–2.56 | 3.48* | 1.61–7.53 | |
| Model 3f | |||||
| Sleep Cb | 1 | 1 | |||
| Sleep Bc | 1.11 | 0.75–1.64 | 2.26* | 1.02–4.98 | |
| Sleep Ad | 1.69* | 1.15–2.47 | 3.25* | 1.50–7.07 | |
| Model 4g | |||||
| Sleep Cb | 1 | 1 | |||
| Sleep Bc | 1.19 | 0.79–1.78 | 2.40 | 1.00–5.76 | |
| Sleep Ad | 1.72* | 1.15–2.57 | 3.96* | 1.69–9.31 | |
CI, confidence interval; OR, odds ratio.
Significant result at the 0.05 significance level.
Crude.
Worst sleep.
Medium sleep
Best sleep.
Adjusted for age and gender.
Adjusted for age, gender and inclusion as case or referent.
Adjusted for age, gender, inclusion as case or referent, manual handling, prolonged sitting.
4. Discussion
Both hypotheses in this study, (1) that disturbed sleep predicts (musculoskeletal) multi-site pain 5 years later among individuals free from pain at baseline and (2) that good sleep predicts resolution of (musculoskeletal) multi-site pain 5 years later, are supported by the results.
That sleep is a predictor of multi-site pain is in line with previous research (Kamaleri et al., 2009a), and the present study implies that the association is also significant for initially pain-free individuals.
The results indicate that reporting better sleep (or having less disturbed sleep) is one factor relevant to predicting the resolution of multi-site pain. To the best of our knowledge, no study has specifically looked at this relationship before, although restorative sleep has previously been shown to be a predictive factor in the resolution of CWP (Davies et al., 2008).
High physical workload (Haukka et al., 2012), psychosocial work exposures (Haukka et al., 2011) and obesity(Haukka et al., 2012; Magnusson et al., 2014) have, in previous research, been shown to be associated with the onset of multi-site pain. The potential confounders had very little influence on the main findings in our study.
For the present study, sleep disturbance was categorized into tertiles to study sleep disturbance in a general sense, including several dimensions of sleep problems.
The stratification by pain and the cut-off used for multi-site pain were, as in previous studies in this field, somewhat arbitrary. There are no clear definitions of multi-site pain, and one common way of establishing a cut-off is to divide the cohort into percentiles. Since the cohort in the original MUSIC-Norrtälje study comprised both cases and referents, a division of this kind would not have been interpretable. Our cut-off, with ≥3 pain sites, is based upon the results in previous research (Kamaleri et al., 2009b) where an increased risk for work disability was seen if the number of pain sites was ≥3 (when adjusting for age and gender). Furthermore, we chose to establish the relevance of the chosen cut-off for multi-site pain in our study group by calculating the odds ratios for sickness absence when individuals were exposed to various numbers of pain sites. In a supplementary analysis (data not shown), we included a variable for number of pain sites in a model alongside sleep (for the multi-site pain-at-baseline stratum). This influenced the results to some extent, but did not change their trend or significance. To stratify, rather than to include a number of pain sites in the modeling, was an approach adopted in order to make the results easier to interpret even in clinical settings. To stratify into more than three strata was not an option because of limited statistical power. We wanted the three strata to reflect three different pain characteristics, with one pain-free stratum and one multi-site pain stratum, similar to previous research (Kamaleri et al., 2009b). We found that, with our multi-site pain cut-off, 55% reported multi-site pain. Since the original study included both cases (recruited when seeking care for LBP or NSP) and referents, it is likely that the ratio reporting multi-site pain is higher in the present study than it is in a general population.
The definition of multi-site pain is vague and primarily refers to concurrent musculoskeletal pain (sometimes including also headache) from several body sites. We were able to exclude individuals with medical conditions associated with pain (e.g., rheumatoid arthritis, claudication intermittence, nervous disorder or stroke), which gave us better control over the nature of the included participants pain complaints. We found it relevant to investigate the locations of the pain reported by the individuals with multi-site pain at baseline, but no pain at follow-up. Our presumption was that pain from the extremities might not be of the same kind as LBP or neck pain (NP). There was a somewhat lower prevalence of NP, shoulder pain and pain from the thoracic spine among the individuals who slept well than among those who slept medium well or worst. This is worth considering in future studies.
Due to a paucity of research on multi-site pain, we have referred to research on CWP in this paper. Multi-site pain is clinically different from CWP, not only due to chronicity, but also because CWP traditionally shares diagnostic criteria with fibromyalgia, i.e., pain present from two contralateral quadrants of the body, above and below the waist, and in the axial skeleton (Gupta et al., 2007; McBeth et al., 2007).
Even though our study indicates a clear trend, some caution has to be observed in interpreting the results. Generally, the odds ratios had wide confidence intervals, which indicate limited statistical power and uncertainty in risk estimation. When stratifying the cohort into three strata, the size of the group entering each analysis became small. When investigating migration from one extreme (e.g., no pain at baseline) to another (e.g., multi-site pain at follow-up), the number of events was few. The trends are however consistent, which strengthens the presumption that there is an association. Further research is needed to establish the strength and size of the association. The high prevalence of multi-site pain in our study might have resulted in overestimated odds ratios (Zhang and Yu, 1998), which is another reason for caution in interpreting the results.
It is possible that there are other confounders that should have been included in the analysis. Due to stratification by pain site, statistical power was lost, and, accordingly, we chose to be very careful in including confounders, so as not to lose more power than necessary. We had no information on the psychosocial factors (except for work-related ones) that might have been worth testing in the model, which is a limitation of the study. Also, it would have been relevant to investigate the variables in terms of pain severity (pain intensity and disability), so as to exclude the possibility that the participants who slept well were actually the ones who had less severe pain or lower pain intensity. We used a modified version of the Standard Nordic Questionnaire, which did not include any items on pain intensity or disability, and could therefore not exclude the possibility of an effect of pain severity on sleep and the resolution of multi-site pain. In a previous study of the cohort (submitted for publication), however, we saw that the differences in pain intensity in the lower back and neck/shoulder(s) for the three sleep categories were small.
The two questionnaires used for assessments of sleep disturbance and pain sites were both referring to respective symptom occurrences during the last 6 months. With regard to pain, this could have overestimated the events classified as multi-site pain. In previous studies on multi-site pain, questionnaires including items on pain during the last 12 months (Kamaleri et al., 2009a,b), 3 months (Haukka et al., 2011, 2012) or 1 month (Haukka et al., 2013) have been used.
The MUSIC-Norrtälje study was, in its design, a case-referent study, where cases were recruited when participants sought care for LBP or NSP. Thus, our data are limited in terms of generalizability in the sense that, there in our cohort, was a larger proportion of individuals with pain who had also sought care for their pain than there is in a general population. Cote and colleagues found that only one quarter of individuals with LBP or NP sought care, and that individuals seeking care had worse pain, more disabling pain, worse physical-role-functioning, and an over-all poorer health status (Cote et al., 2001). It is also possible that there is a difference between the cases and referent with regard to coping strategies. In the present study, the distribution of cases and referents in the different sleep tertiles was fairly even and the effect of sleep remained after adjusting for ‘inclusion as case or referent’.
Disturbed sleep has been associated with many disorders, among of which pain is just one. Nevertheless, sleep is rarely discussed as a factor to consider in the evaluation of intervention programmes, e.g., in multimodal rehabilitation programmes for chronic pain patients. In clinical settings in Sweden, there are recommendations to take psychosocial factors into account when meeting patients with pain, but sleep is not yet a factor mentioned in these recommendations (Socialstyrelsen [English: National Board of Health and Welfare, 2007). We believe that sleep should be investigated further as a factor in research on individuals with pain in order to investigate whether supplementation of the clinical recommendations is warranted.
In this study, we did not focus on the different possible causes of disturbed sleep, but rather on disturbed sleep in general as a predictive factor for the onset or resolution of pain. Data were assessed at two time points with 5 years in between.
In order to better understand the relationship between disturbed sleep and pain, prospective studies should be performed with repeated measurements. There are some truly interesting research yet to be performed in order to inquire into possible mechanisms underlying the risk factors for the severance and maintenance of pain. Intervention studies may investigate the clinical value of also addressing disturbed sleep in rehabilitation programmes for individuals with pain
4.1 Conclusion
The results show that sleep disturbance could be a relevant factor to consider when predicting multi-site pain and that non-disturbed (or less disturbed) sleep might be relevant when predicting the resolution of multi-site pain. Further research on larger study populations is needed to better understand the impact of sleep on the onset and resolution of multi-site pain. Sleep disturbance should be considered when evaluating rehabilitation programmes for patients with multi-site pain so as further to investigate its significance in clinical settings.
Author contributions
All authors have contributed by discussing the results and and commenting on the manuscript.
Acknowledgments
Special thanks to the MUSIC-Norrtälje study group for providing extensive data. Special thanks also to our statistician, Natalja Balliu, for her advice. The study was performed under the auspices of the Stockholm Stress Center and the Future Occupational Health Services. The MUSIC-Norrtälje study was approved by the Ethics Committee of Karolinska Institutet (Dnr 03-139; Dnr 93-255).
Conflicts of interest
None declared.
Web references
Socialstyrelsen [English: National Board of Health and Welfare], (2007). Sjukskrivning vid risk för långvarig icke-malign smärta i rörelseapparaten, inklusive fibromyalgi som rör rörelseapparaten [Swedish]. Retrieved from: http://www.socialstyrelsen.se/riktlinjer/forsakringsmedicinsktbeslutsstod/riskforlangvarigicke-malignsma (accessed 20 May 2013)
References
- Akerstedt T, Ingre M, Broman JE, Kecklund G. Disturbed sleep in shift workers, day workers, and insomniacs. Chronobiol Int. 2008;25:333–348. doi: 10.1080/07420520802113922. [DOI] [PubMed] [Google Scholar]
- Akerstedt T, Kecklund G, Alfredsson L, Selen J. Predicting long-term sickness absence from sleep and fatigue. J Sleep Res. 2007;16:341–345. doi: 10.1111/j.1365-2869.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Akerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, Kecklund G. Sleep disturbances, work stress and work hours – a cross-sectional study. J Psychosom Res. 2002a;53:741–748. doi: 10.1016/s0022-3999(02)00333-1. [DOI] [PubMed] [Google Scholar]
- Akerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, Kecklund G. Work organisation and unintentional sleep: Results from the WOLF study. Occup Environ Med. 2002b;59:595–600. doi: 10.1136/oem.59.9.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaadi SM, McAuley JH, Hush JM, Maher CG. Prevalence of sleep disturbance in patients with low back pain. Eur Spine J. 2011;20:737–743. doi: 10.1007/s00586-010-1661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CM, Bounds SC, Simango MB, Witmer KR, Campbell JN, Edwards RR, Haythornthwaite JA, Smith MT. Self-reported sleep duration associated with distraction analgesia, hyperemia, and secondary hyperalgesia in the heat-capsaicin nociceptive model. Eur J Pain. 2011;15:561–567. doi: 10.1016/j.ejpain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote P, Cassidy JD, Carroll L. The treatment of neck and low back pain: Who seeks care? Who goes where? Med Care. 2001;39:956–967. doi: 10.1097/00005650-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Davies KA, Macfarlane GJ, Nicholl BI, Dickens C, Morriss R, Ray D, McBeth J. Restorative sleep predicts the resolution of chronic widespread pain: Results from the EPIFUND study. Rheumatology (Oxford) 2008;47:1809–1813. doi: 10.1093/rheumatology/ken389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Almeida DM, Klick B, Haythornthwaite JA, Smith MT. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202–207. doi: 10.1016/j.pain.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: Associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009;13:1043–1047. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbain DA, Cole B, Lewis JE, Gao JR. What is the evidence for chronic pain being etiologically associated with the DSM-IV category of sleep disorder due to a general medical condition? A structured evidence-based review. Pain Med. 2010;11:158–179. doi: 10.1111/j.1526-4637.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- Grooten WJ, Wiktorin C, Norrman L, Josephson M, Tornqvist EW, Alfredsson L. Seeking care for neck/shoulder pain: A prospective study of work-related risk factors in a healthy population. J Occup Environ Med. 2004a;46:138–146. doi: 10.1097/01.jom.0000112181.06324.42. [DOI] [PubMed] [Google Scholar]
- Grooten WJA, Mulder M, Josephson M, Alfredsson L, Wiktorin C. The influence of work-related exposures on the prognosis of neck/shoulder pain. Eur Spine J. 2007;16:2083–2091. doi: 10.1007/s00586-007-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooten WJA, Wiktorin C, Norrman L, Josephson M, Tornqvist EW, Alfredsson L, Grp MNS. Seeking care for neck/shoulder pain: A prospective study of work-related risk factors in a healthy population. J Occup Environ Med. 2004b;46:138–146. doi: 10.1097/01.jom.0000112181.06324.42. [DOI] [PubMed] [Google Scholar]
- Gupta A, Silman AJ, Ray D, Morriss R, Dickens C, MacFarlane GJ, Chiu YH, Nicholl B, McBeth J. The role of psychosocial factors in predicting the onset of chronic widespread pain: Results from a prospective population-based study. Rheumatology (Oxford) 2007;46:666–671. doi: 10.1093/rheumatology/kel363. [DOI] [PubMed] [Google Scholar]
- Haack M, Scott-Sutherland J, Santangelo G, Simpson NS, Sethna N, Mullington JM. Pain sensitivity and modulation in primary insomnia. Eur J Pain. 2012;16:522–533. doi: 10.1016/j.ejpain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukka E, Kaila-Kangas L, Ojajarvi A, Miranda H, Karppinen J, Viikari-Juntura E, Heliovaara M, Leino-Arjas P. Pain in multiple sites and sickness absence trajectories: A prospective study among Finns. Pain. 2013;154:306–312. doi: 10.1016/j.pain.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Haukka E, Leino-Arjas P, Ojajarvi A, Takala EP, Viikari-Juntura E, Riihimaki H. Mental stress and psychosocial factors at work in relation to multiple-site musculoskeletal pain: A longitudinal study of kitchen workers. Eur J Pain. 2011;15:432–438. doi: 10.1016/j.ejpain.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Haukka E, Ojajarvi A, Takala EP, Viikari-Juntura E, Leino-Arjas P. Physical workload, leisure-time physical activity, obesity and smoking as predictors of multisite musculoskeletal pain. A 2-year prospective study of kitchen workers. Occup Environ Med. 2012;69:485–492. doi: 10.1136/oemed-2011-100453. [DOI] [PubMed] [Google Scholar]
- Kaila-Kangas L, Kivimaki M, Harma M, Riihimaki H, Luukkonen R, Kirjonen J, Leino-Arjas P. Sleep disturbances as predictors of hospitalization for back disorders – a 28-year follow-up of industrial employees. Spine. 2006;31:51–56. doi: 10.1097/01.brs.0000193902.45315.e5. [DOI] [PubMed] [Google Scholar]
- Kamaleri Y, Natvig B, Ihlebaek CM, Benth JS, Bruusgaard D. Change in the number of musculoskeletal pain sites: A 14-year prospective study. Pain. 2009a;141:25–30. doi: 10.1016/j.pain.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Kamaleri Y, Natvig B, Ihlebaek CM, Bruusgaard D. Does the number of musculoskeletal pain sites predict work disability? A 14-year prospective study. Eur J Pain. 2009b;13:426–430. doi: 10.1016/j.ejpain.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Kuorinka I, Jonsson B, Kilbom A, Vinterberg H, Bieringsorensen F, Andersson G, Jorgensen K. Standardized Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon. 1987;18:233–237. doi: 10.1016/0003-6870(87)90010-x. [DOI] [PubMed] [Google Scholar]
- Magnusson K, Osteras N, Mowinckel P, Natvig B, Hagen KB. No strong temporal relationship between obesity and multisite pain–results from a population-based 20-year follow-up study. Eur J Pain. 2014;18:120–127. doi: 10.1002/j.1532-2149.2013.00338.x. [DOI] [PubMed] [Google Scholar]
- McBeth J, Silman AJ, Gupta A, Chiu YH, Ray D, Morriss R, Dickens C, King Y, Macfarlane GJ. Moderation of psychosocial risk factors through dysfunction of the hypothalamic-pituitary-adrenal stress axis in the onset of chronic widespread musculoskeletal pain: Findings of a population-based prospective cohort study. Arthritis Rheum. 2007;56:360–371. doi: 10.1002/art.22336. [DOI] [PubMed] [Google Scholar]
- Miranda H, Kaila-Kangas L, Heliovaara M, Leino-Arjas P, Haukka E, Liira J, Viikari-Juntura E. Musculoskeletal pain at multiple sites and its effects on work ability in a general working population. Occup Environ Med. 2010;67:449–455. doi: 10.1136/oem.2009.048249. [DOI] [PubMed] [Google Scholar]
- Natvig B, Bruusgaard D, Eriksen W. Localized low back pain and low back pain as part of widespread musculoskeletal pain: Two different disorders? A cross-sectional population study. J Rehabil Med. 2001;33:21–25. doi: 10.1080/165019701300006498. [DOI] [PubMed] [Google Scholar]
- Saastamoinen P, Leino-Arjas P, Laaksonen M, Martikainen P, Lahelma E. Pain and health related functioning among employees. J Epidemiol Community Health. 2006;60:793–798. doi: 10.1136/jech.2005.043976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo P, Oksanen T, Sivertsen B, Hall M, Pentti J, Virtanen M, Vahtera J, Kivimaki M. Sleep disturbances as a predictor of cause-specific work disability and delayed return to work. Sleep. 2010;33:1323–1331. doi: 10.1093/sleep/33.10.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivertsen B, Overland S, Bjorvatn B, Maeland JG, Mykletun A. Does insomnia predict sick leave? The Hordaland Health Study. J Psychosom Res. 2009;66:67–74. doi: 10.1016/j.jpsychores.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Overland S, Neckelmann D, Glozier N, Krokstad S, Pallesen S, Nordhus IH, Bjorvatn B, Mykletun A. The long-term effect of insomnia on work disability – the HUNT-2 historical cohort study. Am J Epidemiol. 2006;163:1018–1024. doi: 10.1093/aje/kwj145. [DOI] [PubMed] [Google Scholar]
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Solidaki E, Chatzi L, Bitsios P, Markatzi I, Plana E, Castro F, Palmer K, Coggon D, Kogevinas M. Work-related and psychological determinants of multisite musculoskeletal pain. Scand J Work Environ Health. 2010;36:54–61. doi: 10.5271/sjweh.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornqvist EW, Kilbom A, Vingard E, Alfredsson L, Hagberg M, Theorell T, Waldenstrom M, Wiktorin C, Hogstedt C. The influence on seeking care because of neck and shoulder disorders from work-related exposures. Epidemiology. 2001;12:537–545. doi: 10.1097/00001648-200109000-00013. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]


