Abstract
The emergence of multidrug resistance (MDR) and extensively drug resistance (XDR) in Acinetobacter baumannii has made an important challenge in the treatment of infections caused by this organism. The ability of carbapenemase production is one of the main mechanisms for the emergence of MDR and/or XDR in A. baumannii. The aim of this study was to detect carbapenemase producer A. baumannii. In this study, 65 imipenem resistant A. baumannii were collected from burned patients. Biochemical identification, antibiotic susceptibility test and multiplex polymerase chain reactions for the detection of carbapenemases genes were performed. The results showed that all strains carried blaOXA-51. 83%, 12.5% and 9.23% strains harbored blaOXA-23, blaVIM and blaKPC genes, respectively. None of the isolates carried blaIMP, blaOXA-48, blaNDM-1 and blaSPM-1 genes. The results of this study indicate the emergence of Klebsiella pneumoniae Carbapenemase (KPC) in A. baumannii causing nosocomial infections in burned patients which can be important for hospital infection prevention systems in Iran.
Key Words: Acinetobacter baumannii, carbapenemases, KPC
Acinetobacter baumannii has been recognized as one of the important causes of nosocomial infections in hospitalized patients, particularly in burned ones in recent years (1- 3). During the past decade, in some countries, this opportunistic pathogen had high rate of infection in burned patients and was reported to be the second most common cause of nosocomial infections in burned patients (1, 2, 4-7). A. baumannii has been shown to acquire fast antibiotic resistance elements (8, 9). Recently, this Gram negative bacilli has shown resistance to the most available antibiotics followed by the emergence of multiple (MDR) and extensive drug resistance (XDR) strains (2, 8-10). This has partly been due to the extensive use of broad spectrum antibiotics especially in burned patients (9). Carbapenems are generally used for antibiotic therapy in infections due to MDR A. baumannii. However, the emergence of carbapenem resistant isolates has made it difficult to treat such infections (2, 3, 8, 11-14). One of the most common and important mechanisms in carbapenem resistance in A. baumannii is their ability to produce carbapenemase enzymes (2, 3, 6-8). Among the carbapenem hydrolyzing β-lactamases, class D oxacillinases (OXA type) is the most prevalent in A. baumannii strains (3, 9, 14). On the other hand, some studies have reported class B beta lactamases (metallo- beta lactamases) in A. baumannii as the most prevalent carbapenemase after OXA- type (8). The significant importance is the worldwide prevalence of Klebsiella pneumoniae Carbapenemase (KPC) producers (15, 16). Gram negative KPC producers can be resistant to all beta lactam antibiotics except aztreonam (17). Most of these carbapenemases have been located in transferable genetic elements and can spread among A. baumannii and even into other Gram- negative bacilli (2, 8). Therefore, the determination of carbapenemase- producing strains in healthcare systems could help in the eradication of A. baumannii antibiotic resistant strains.
Materials and methods
Bacterial isolates
This cross-sectional study was conducted in Motahari Hospital which is the only referral burn center in Tehran, Iran. Sixty-five imipenem resistant A. baumannii isolated from burn wound were collected from admitted patients from April to July 2013. Primary identification of these strains was performed by conventional biochemical tests such as oxidase, TSI, SIM and growing at 44 oC as A. baumannii. The identification of isolates was confirmed by API 20 NE (Bio-Merieux, Lyon, France) strip test.
Antimicrobial susceptibility testing
The antibiotic susceptibility testing was performed by disc diffusion method on Mueller–Hinton agar using cefotaxime (30 µg), ceftazidime (30 µg), imipenem (10 µg), ticarcillin (75 µg), ticarcillin- clavulanic acid (75/10 µg), piperacillin (100 µg), piperacillin- tazobactam (100/10 µg), ciprofloxacin (5 µg), gentamicin (10 µg), tobramycin (10 µg), amikacin (30 µg), tetracycline (30 µg), trimethoprim (5 μg) and trimethoprim- sulfamethoxazole (1.25/23.75 μg) according to clinical and laboratory standards institute (CLSI) 2011. The standard antibiotic disks used in this study were from MAST Company (Mast Diagnostics, UK). P. aeruginosa ATCC 27853 was used as control strain in the antibiotic susceptibility testing. Detection of extended spectrum beta lactamase (ESBL) has been conducted by combination disc method by using ceftazidime and ceftazidime plus clavulanic acid. Strains with increasing at least 5 mm in the diameter of inhibition zone around ceftazidime plus clavulanic acid in contrast of ceftazidime alone were considered as an ESBL producer.
PCR amplification for carbapenemases produ-ction genes
Extraction of bacterial DNA was performed with a plasmid Minikit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions.
Three different sets of multiplex PCR for blaVIM, blaIMP and blaOXA-23, blaOXA-48 and blaNDM-1, blaSPM-1 genes and common PCR for blaKPC and blaOXA-51genes were designed. The lists of primers used are shown in Table 1 and amplifivation conditions are represented in Table 2.
Table 1.
Sequence of primers
| Primer | Sequence (5 ' →3 ' ) | PCR pdoduct size (bp) | References |
| VIM F | TTGACACTCCATTTACDG | 390 | |
| VIM R | GATYGAGAATTAAGCCACYCT | ||
| Imp F | GATGGTGTTTGGTCGCATA | 139 | 8 |
| Imp R | CGAATGCGCAGCACCAG | ||
| OXA-23 F | GATGTGTCATAGTATTCGTCGT | 1050 | |
| OXA-23 R | TCACAACAACTAAAAGCACTGT | 9 | |
| OXA-48-ike F | CCAAGCATTTTTACCCGCATCKACC | 389 | |
| OXA-48-like R | GYT TGA CCA TAC GCT GRC TGC G | ||
| NDM-1 F | CCCGGCCACACCAGTGACA | 129 | |
| NDM-1 R | GTAGTGCTCAGTGTCGGCAT | 9 | |
| SPM-1 F | GGGTGGCTAAGACTATGAAGCC | 447 | |
| SPM-1 R | GCCGCCGAGCTGAATCGG | ||
| OXA-51-like F | TAATGCTTTGATCGGCCTTG | 353 | 7 |
| OXA-51-like R | TGGATTGCACTTCATCTTGG | ||
| KPC F | GTATCGCCGTCTAGTTCTGC | 636 | 18 |
| KPC R | GGTCGTGTTTCCCTTTAGCC |
Table 2.
PCR conditions for bla genes amplification
| No. Cycles | Final extension Temperature (time) | Extension Temperature (time) | Annealing Temperature (time) |
Denaturation
Temperature (time) |
Initial denaturation Temperature (time) | Gene |
|---|---|---|---|---|---|---|
| 30 | 72 oC (7 min) | 72 oC (1 min) | 60 oC (40 sec) | 94 oC (40 sec) | 94 oC (10 min) | bla VIM & blaIM: |
| 30 | 72 oC (7 min) | 72 oC (1 min) | 55 oC (1 min) | 94 oC (30 sec) | 94 oC (1 min) |
bla
OXA-23 & blaOXA-48 |
| 30 | 72 oC (1 min) | 72 oC (1 min) | 60 oC (40 sec) | 94 oC (30 sec) | 94 oC (1 min) |
bla
NDM-1 & blaSPM-1 |
| 30 | 72 oC (5 min) | 72 oC (1 min) | 56 oC (1 min) | 94 oC (1 min) | 94 oC (5 min) | bla KPC |
| 30 | 72 oC (5 min) | 72 oC (1 min) | 58 oC (1 min) | 94 oC (45 sec) | 94 oC (5 min) | bla OXA-51 |
PCR products were analyzed by electrophoresis on 1.5% agarose gel with SYBR safe staining. Direct sequencing of amplicons was carried out using ABI 3730X capillary sequencer (Genfanavaran, Macrogen, Seoul, Korea).
Results
A total of 65 A. baumannii were isolated from 65 hospitalized burned patients, 15 (23%) females and 50 (77%) males. The age of patients were between 9 months to 72 years and total body burned surface area (TBSA) was more than 6%. Causes of burn were classified as follows: fire 6 (11%), hot water 6 (11%), electricity 10 (17%) gas 10 (17%), 6 (11%) chemical materials and others 19 (33%). Based on antibiotic susceptibility test, 97% of strains were resistant to all tested beta lactam antibiotics and 80% of them showed resistance to all tested aminoglycoside antibiotics.
The results of antibiotic susceptibility tests indicated seven antibiotic resistance patterns (Table 3). Pattern one was observed in 55% of isolates. Those strains were resistant to all tested antibiotics except colistin and tetracycline. In pattern two, all strains were resistant to the tested antibiotics which were observed in 25% of strains. In 20% strains, resistance to at least one of aminoglycoside antibiotics were observed according to antibiotic resistance patterns.
Table 3.
Antibiotic resistance patterns
| patterns | Antibiotic Resistance patterns | Number | Percentage |
|---|---|---|---|
| 1 | CTX CAZ CEF IMI PTZ PYR TC TC-C GM AK TO TM SXT CI | 36 | 55 |
| 2 | CTX CAZ CEF IMI PTZ PYR TC TC-C GM AK TO TM SXT CI T | 16 | 25 |
| 3 | CTX CAZ CEF IMI PTZ PYR TC TC-C AK TM SXT CI T | 7 | 11 |
| 4 | CTX CAZ CEF IMI PTZ PYR TC TC-C GM TO TM SXT CI | 3 | 5 |
| 5 | CTX CAZ CEF IMI PTZ PYR TC TC-C AK TO TM SXT CI T | 2 | 3 |
| 7 | CTX CAZ CEF IMI PTZ PYR TC TC-C AK TO TM SXT CI | 1 | 1 |
CTX: cefotaxime, CAZ: ceftazidime, CEF: CEFEPIME, IMI: imipenem, PTZ: piperacillin-Tazobactam, PRL: piperacillin, TC: ticarcillin, TC-c: ticarcillin- clavulanic acid, GM: gentamicin, AK: amikacin, TO: tobramycin, TM: trimethoprim, SXT: trimethoprim- sulfamexazole, CI: ciprofloxacin, T: tetracycline
The Verona integron-encoded metallo-β-lactamase (VIM) and KPC producing strains were observed just in two antibiotic resistance patterns. OXA-23 and OXA-51 producing strains showed all seven antibiotic resistance patterns.
The ability of ESBL production was indicated just in one strain in combination disc method.
Sequence analysis showed that blaOXA-51 gene was detected in all isolates, 54 (83%) isolates included blaOXA-23 gene. Eight (12.5%) and 6 (9.23%) strains carried blaVIM and blaKPC genes respectively (Figures 1-4). Six out of eight VIM- producing strains contained both blaOXA-23 and blavim genes (Table 4). All of KPC producer isolates were combined by OXA-23. On the other hand, none of them contained blaIMP, blaOXA-48, blaNDM-1 and blaSPM-1 genes.
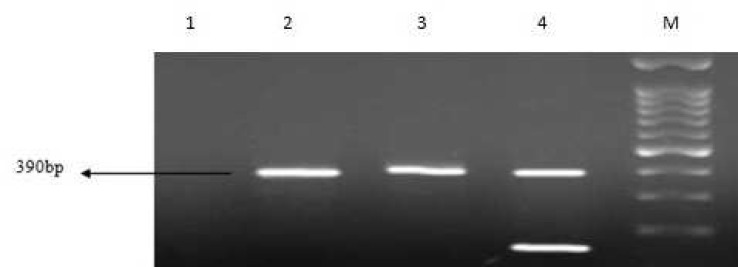
Fig. 1.
Multiplex PCR amplification fragments for the detection of vim and imp gene among Acinetobacter baumannii isolates. Lane 1: negative control, lanes 2, 3: positive vim strains, lane 4: positive control of vim and imp genes and M: 1kb DNA size marker
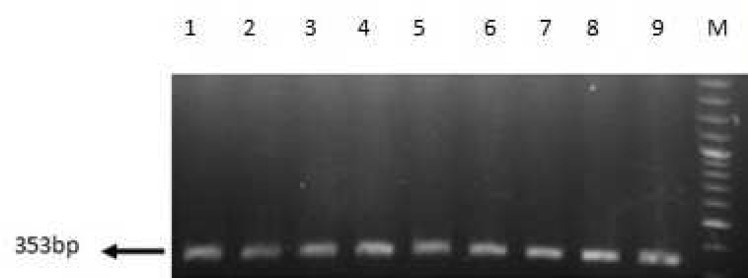
Fig 4.
PCR amplification fragments for the detection of oxa-51 gene among Acinetobacter baumannii isolates. Lane 1: positive control of oxa-51, lanes 2- 9: positive strains of oxa-51.and M: 1kb DNA size marke
Table 4.
Number and percentages of detected bla genes
| bla VIM and bla OXA_23 | bla KPC and bla OXA_23 | bla VIM | bla OXA_23 | bla OXA-51 | genes |
| 6(9.52%) | 6(9.52%) | 2(3.1%) | 42(66.6%) | 63(100%) | NO.(%) |
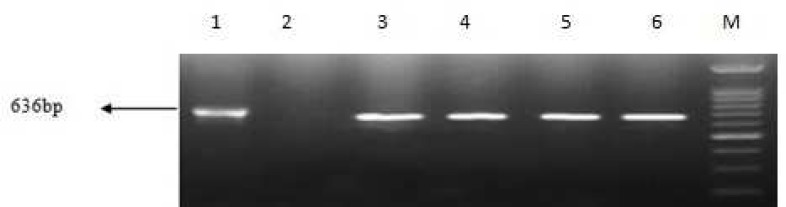
Fig. 2.
PCR amplification fragments for the detection of kpc gene among Acinetobacter baumannii isolates. Lane 1: positive control of kpc gene, lane 2: negative control, lanes 3- 6: positive kpc strains and M: 1kb DNA size marker
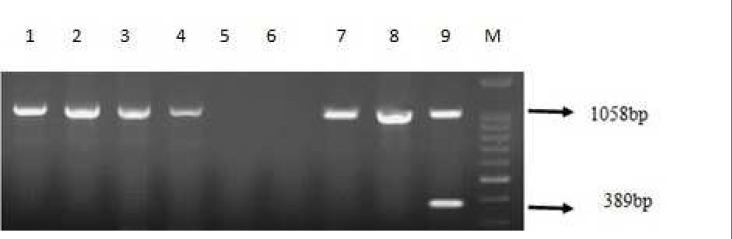
Fig. 3.
PCR amplification fragments for the detection of oxa-23 and oxa-48 genes among Acinetobacter baumannii isolates. Lane 1- 4 and 7, 8: positive oxa-23 strains, lanes 5, 6: negative control of oxa-23 and oxa-48 genes, lane 9: positive control of oxa-23 and oxa-48 genes and M: 1kb DNA size marker
Discussion
According to many studies, A. baumannii is known as one of the most common Gram negative bacteria that can cause nosocomial infection in health care centers especially in burned hospitalized patients (2, 3, 8). Treatment of infection due to XDR A. baumannii is extremely difficult and causes more morbidity and motility in hospitalized burn patients (13, 19). The results of this study indicated that 98.4% of A. baumannii were resistant to all tested beta lactam antibiotics and 55% of them remained susceptible just to colistin and tetracycline. Generally, carbapenemases producer strains of A. baumannii convert to XDR strains and can be resistant to most antibiotics used for the treatment of A. baumannii infections. Wounds of burned patients generally infected by multiple bacteria harboring antibiotic resistance genes located in transferable genetic elements, can be a disaster in burn care units. Resistance to all tested antibiotics in 50% of isolated KPC- producer A. baumannii and to all tested antibiotics except tetracycline in the remaining isolates is a significant point and creates limitation for treatment choices of this kind of infection. OXA type is the common carbapenemase in A. baumannii and OXA-23 is the common one in Iran (3, 8, 14, 20). Outbreaks of OXA-23 producing A. baumannii have been reported in several Asian countries such as China, Thailand, Taiwan (8, 6, 11). On the other hand, metallo- bata lactamase (MBL) can be considered as a second common carbapenemase in A. baumannii. In the research which was carried out by Szejbach in 2013 in Poland on MBL- producer A. baumannii, it was shown that 10.3% of isolates carried blaIMP-like gene. No blaVIM-4 was detected in the isolates (7). On the contrary, in the same study in India in 2012, 47% of isolated A. baumannii carried the blaVIM and 0.9% of them harbored blaIMP. The variants result may be related to different ecology situation, antibiotic therapy program and variants antibiotype patterns in two different countries (21). In 2013 in China, OXA-51 gene was detected in all isolated A. baumannii and 94% carried oxa-23 gene (22). Also, the results of a study in 2012 on 68 imipenem-resistant A. baumannii in Iran indicated that OXA-51 and OXA-23 types were detected in all strains (20). But in the current study, 83% of isolates were identified as an OXA-23-producer which can be associated to the source of isolated bacteria. In this study, all isolates were collected from wounds of burned patients but in the study carried out in 2012 in Iran (20), the isolates were obtained from different medical specimens. On the other hand, in 2012 in another study in Iran, 88.7% of imipenem resistant A. baumannii carried blaOXA-23-like gene (14) and these results are similar to ours.
In Korea in 2013, 97% of Acinetobacter isolates harbored the blaOXA-23-like gene (23).
Mutation in OXA type enzymes because of overuse and/or abuses of carbapenem antibiotics in clinic can lead to high carbapenem activity by OXA type carbapenemase enzymes. Because they generally have low activity of carbapenem hydrolyzing activity (9, 24). In addition, we had a report from the prevalence of OXA-48- producer K. pneumonia at the same time and same burn unit and this gene was located in mobile genetic elements. Thus, we have detected specifically oxa-48 gene in isolated A. baumannii (25). Consequently, it can be an alarming threat in health in Iran. Moreover, as the gene of this carbapenemase is located in a mobile genetic element and can be transferred to A. baumannii, it can impose more complications in burn patient's therapy. In the current study, 97% of carbapemase producer strains subsequently were resistant to all beta lactam antibiotics and 80% of them were resistant to aminoglycoside. These results can be a concern for physicians because 78% of the strains were sensitive to colistin and tetracycline only. The results of this study indicated that the ability of carbapenemase production can be one of the most important reasons for the emergence of XDR A. baumannii in nosocomial infections. The infection is important in burned patients because their skin lose a first protective barrier, putting the patients at high risk for multiple infections.
Regarding to the mentioned above study and our results, it seems that A. baumannii is becoming one of the important and challengeable Gram-negative microorganism and P. aeruginosa is replaced by A. baumannii in nosocomial infection. The emergence of their XDR type makes significant therapeutic problem especially in burned patients. According to our results and another study (26), colistin remains a more effective antibiotic for the treatment of infection caused by these XDR and/or MDR microorganisms. The spread of carbapene-mase producer A. baumannii, as a second important pathogen that can make nosocomial infection in burned patients can be alarming for public health organization and health care systems.
Acknowledgment
This study was supported by a grant (M/T 91-04-134-20187) from IranUniversity of Medical Sciences, Tehran, Iran.
Conflict of interest
The authors declared no conflicts of interest.
References
- 1.Alaghehbandan R, Azimi L, Rastegar Lari A. Nosocomial infections among burn patients in Teheran, Iran: a decade later. Ann Burns Fire Disasters. 2012;25:3–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Owlia P, Azimi L, Gholami A, et al. ESBL- and MBL-mediated resistance in Acinetobacter baumannii: a global threat to burn patients. Infez Med. 2012;20:182–7. [PubMed] [Google Scholar]
- 3.Sari AN, Bicmen M, Gulay Z. The first report on the outbreak of OXA-24/40-like carbapenemase-producing Acinetobacter baumannii in Turkey. Jpn J Infect Dis. 2013;66:439–42. [PubMed] [Google Scholar]
- 4.Lari AR, Alaghehbandan R. Nosocomial infections in an Iranian burn care center. Burns. 2000;26:737–40. doi: 10.1016/s0305-4179(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 5.Azimi L, Motevallian A, Ebrahimzadeh Namvar A, et al. Nosocomial infections in burned patients in motahari hospital, tehran, iran. Dermatol Res Pract. 2011;2011:436952. doi: 10.1155/2011/436952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ku WW, Kung CH, Lee CH, et al. Evolution of carbapenem resistance in Acinetobacter baumannii: An 18-year longitudinal study from a medical center in northern Taiwan. J Microbiol Immunol Infect. 2013 doi: 10.1016/j.jmii.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Szejbach A, Mikucka A, Bogiel T, et al. Usefulness of phenotypic and genotypic methods for metallo-beta-lactamases detection in carbapenem-resistant Acinetobacter baumannii strains. Med Sci Monit Basic Res. 2013;19:32–6. doi: 10.12659/MSMBR.883744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowak P, Paluchowska P, Budak A. Distribution of blaOXA genes among carbapenem-resistant Acinetobacter baumannii nosocomial strains in Poland. New Microbiol. 2012;35:317–25. [PubMed] [Google Scholar]
- 9.Liu S, Wang Y, Xu J, et al. Genome sequence of an OXA23-producing, carbapenem-resistant Acinetobacter baumannii strain of sequence type ST75. J Bacteriol. 2012;194:6000–1. doi: 10.1128/JB.01440-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarrilli R, Di Popolo A, Bagattini M, et al. Clonal spread and patient risk factors for acquisition of extensively drug-resistant Acinetobacter baumannii in a neonatal intensive care unit in Italy. J Hosp Infect. 2012;82:260–5. doi: 10.1016/j.jhin.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Thapa B, Tribuddharat C, Srifuengfung S, et al. High prevalence of bla(OXA)-23 in oligoclonal carbapenem-resistant Acinetobacter baumannii from Siriraj Hospital, Mahidol University, Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 2010;41:625–35. [PubMed] [Google Scholar]
- 12.Dallenne C, Da Costa A, Decre D, et al. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65:490–5. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 13.Voets GM, Fluit AC, Scharringa J, et al. A set of multiplex PCRs for genotypic detection of extended-spectrum beta-lactamases, carbapenemases, plasmid-mediated AmpC beta-lactamases and OXA beta-lactamases. Int J Antimicrob Agents. 2011;37:356–9. doi: 10.1016/j.ijantimicag.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Sohrabi N, Farajnia S, Akhi MT, et al. Prevalence of OXA-type beta-lactamases among Acinetobacter baumannii isolates from Northwest of Iran. Microb Drug Resist. 2012;18:385–9. doi: 10.1089/mdr.2011.0077. [DOI] [PubMed] [Google Scholar]
- 15.Roth AL, Hanson ND. Rapid detection and statistical differentiation of KPC gene variants in Gram-negative pathogens by use of high-resolution melting and ScreenClust analyses. J Clin Microbiol. 2013;51:61–5. doi: 10.1128/JCM.02193-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribeiro VB, Andrade LN, Linhares AR, et al. Molecular characterization of Klebsiella pneumoniae carbapenemase-producing isolates in southern Brazil. J Med Microbiol. 2013;62:1721–7. doi: 10.1099/jmm.0.062141-0. [DOI] [PubMed] [Google Scholar]
- 17.Rastegar Lari A, Azimi L, Rahbar M, et al. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns. 2013;39:174–6. doi: 10.1016/j.burns.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Wolter DJ, Khalaf N, Robledo IE, et al. Surveillance of carbapenem-resistant Pseudomonas aeruginosa isolates from Puerto Rican Medical Center Hospitals: dissemination of KPC and IMP-18 beta-lactamases. Antimicrob Agents Chemother. 2009;53:1660–4. doi: 10.1128/AAC.01172-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shields RK, Kwak EJ, Potoski BA, et al. High mortality rates among solid organ transplant recipients infected with extensively drug-resistant Acinetobacter baumannii: using in vitro antibiotic combination testing to identify the combination of a carbapenem and colistin as an effective treatment regimen. Diagn Microbiol Infect Dis. 2011;70:246–52. doi: 10.1016/j.diagmicrobio.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Peymani A, Higgins PG, Nahaei MR, et al. Characterisation and clonal dissemination of OXA-23-producing Acinetobacter baumannii in Tabriz, northwest Iran. Int J Antimicrob Agents. 2012;39:526–8. doi: 10.1016/j.ijantimicag.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Amudhan MS, Sekar U, Kamalanathan A, et al. bla(IMP) and bla(VIM) mediated carbapenem resistance in Pseudomonas and Acinetobacter species in India. J Infect Dev Ctries. 2012;6:757–62. doi: 10.3855/jidc.2268. [DOI] [PubMed] [Google Scholar]
- 22.Gao J, Zhao X, Bao Y, et al. Antibiotic resistance and OXA-type carbapenemases-encoding genes in airborne Acinetobacter baumannii isolated from burn wards. Burns. 2014;40:295–9. doi: 10.1016/j.burns.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Hong SB, Shin KS, Ha J, et al. Co-existence of blaOXA-23 and armA in multidrug-resistant Acinetobacter baumannii isolated from a hospital in South Korea. J Med Microbiol. 2013;62:836–44. doi: 10.1099/jmm.0.055384-0. [DOI] [PubMed] [Google Scholar]
- 24.Walther-Rasmussen J, Hoiby N. OXA-type carbapenemases. J Antimicrob Chemother. 2006;57:373–83. doi: 10.1093/jac/dki482. [DOI] [PubMed] [Google Scholar]
- 25.Azimi L, Nordmann P, Lari AR, et al. First report of OXA-48-producing Klebsiella pneumoniae strains in Iran. GMS Hyg Infect Control. 2014;9:Doc07. doi: 10.3205/dgkh000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mezzatesta ML, Caio C, Gona F, et al. Carbapenem and multidrug resistance in Gram-negative bacteria in a single centre in Italy: considerations on in vitro assay of active drugs. Int J Antimicrob Agents. 2014;44:112–6. doi: 10.1016/j.ijantimicag.2014.04.014. [DOI] [PubMed] [Google Scholar]