Abstract
TRPM8 has been implicated in pain and migraine based on dorsal root- and trigeminal ganglion-enriched expression, upregulation in preclinical models of pain, knockout mouse studies, and human genetics. Here, we evaluated the therapeutic potential in pain of AMG2850 ((R)-8-(4-(trifluoromethyl)phenyl)-N-((S)-1,1,1-trifluoropropan-2-yl)-5,6-dihydro-1,7-naphthyridine-7(8H)-carboxamide), a small molecule antagonist of TRPM8 by in vitro and in vivo characterization. AMG2850 is potent in vitro at rat TRPM8 (IC90 against icilin activation of 204 ± 28 nM), highly selective (>100-fold IC90 over TRPV1 and TRPA1 channels), and orally bioavailable (F po > 40 %). When tested in a skin-nerve preparation, AMG2850 blocked menthol-induced action potentials but not mechanical activation in C fibers. AMG2850 exhibited significant target coverage in vivo in a TRPM8-mediated icilin-induced wet-dog shake (WDS) model in rats (at 10 mg/kg p.o.). However, AMG2850 did not produce a significant therapeutic effect in rat models of inflammatory mechanical hypersensitivity or neuropathic tactile allodynia at doses up to 100 mg/kg. The lack of efficacy suggests that either TRPM8 does not play a role in mediating pain in these models or that a higher level of target coverage is required. The potential of TRPM8 antagonists as migraine therapeutics is yet to be determined.
Keywords: TRPM8, AMG2850, Pain, Inflammatory, Neuropathic, Hypersensitivity, Small molecule, Therapeutic, Rat
Introduction
The transient receptor potential melastatin 8 (TRPM8) is a nonselective cation channel known as the “cool receptor” stemming from early work describing the cooling-activated current (Reid and Flonta 2001). Later, the localization of TRPM8 in subpopulations of small-diameter, cold-sensitive peripheral sensory neurons confirmed its activation by cool temperatures or compounds known to evoke cooling sensations such as menthol and icilin (McKemy et al. 2002; Peier et al. 2002; Nieto-Posadas et al. 2011). Further evidence of the role of TRPM8 in thermal sensation and even thermoregulation was generated with TRPM8 knockout (KO) mice. TRPM8 KO mice do not show the normal warm temperature preference that is typical in mice (Dhaka et al. 2006; Bautista et al. 2007; Colburn et al. 2007; Dhaka et al. 2007; Knowlton et al. 2010; Knowlton and McKemy 2011) nor do they show normal adaptive responses to cold or menthol-induced thermoregulatory challenges (Tajino et al. 2007, 2011; Almeida et al. 2012; Gavva et al. 2012).
Recent genome-wide association studies (GWAS) involving migraine patients have demonstrated a strong association of TRPM8 with migraine. Single nucleotide polymorphs (SNPs) within the TRPM8 gene have consistently been found to show a protective association with migraine in multiple studies as well as in a meta-analysis of all migraine GWAS suggesting that TRPM8 modulators may act as migraine therapeutics (Chasman et al. 2011; Schurks 2011; Freilinger et al. 2012; Anttila et al. 2013; Esserlind et al. 2013; Chasman et al. 2014).
Besides interest in TRPM8 as a temperature sensor and target for migraine, it has also been considered as a potential target for chronic pain based on results in KO mice, receptor expression, and early pharmacology (Daniels and McKemy 2007; Stucky et al. 2009; Liu and Qin 2011; Malkia et al. 2011; McCoy et al. 2011; Almaraz et al. 2014). KO mice have also been used to demonstrate a significant role for TRPM8 in the inflammatory and neuropathic injury-related increases in cold sensitivity (Colburn et al. 2007; Xing et al. 2007; Descoeur et al. 2011) and also that TRPM8 is required for cooling-evoked analgesia (Proudfoot et al. 2006; Dhaka et al. 2008). TRPM8 expression in free nerve terminals of skin, and localization in Aδ and C neurons in the dorsal root and trigeminal ganglia, is consistent with a role in both thermosensation as well as cold hypersensitivity (McKemy et al. 2002; Park et al. 2006; Proudfoot et al. 2006; Fleetwood-Walker et al. 2007; Takashima et al. 2007; Belmonte et al. 2009; Knowlton and McKemy 2011; McCoy et al. 2011).
Although delivery of a therapeutic that addresses the clinical need for reduction of cold hypersensitivity in conditions such as joint pathology or chemotherapy-induced peripheral neuropathy would be beneficial for a large number of patients, a drug that additionally mitigates the mechanical hypersensitivity and spontaneous pain in these disorders would have even broader clinical utility. Thus, TRPM8 upregulation in preclinical models of pain that are characterized by mechanical hypersensitivity and ongoing pain, including chronic constriction injury, complete Freund’s adjuvant (CFA) model, and oxaliplatin-induced hypersensitivity (Gauchan et al. 2009), implicates TRPM8 as an ideal target for mediating not only cold-evoked hypersensitivities but also inflammatory- or neuropathic-induced pathophysiology itself. As interest increases in TRPM8 as a more widespread pain target (Malkia et al. 2009; Ferrer-Montiel et al. 2012; Tamayo et al. 2012; Almaraz et al. 2014; Horne et al. 2014), a large number of patents for potent TRPM8 antagonists are emerging.
To assess the therapeutic potential of TRPM8 antagonists for chronic pain, we characterized AMG2850 as a potent, selective, orally bioavailable antagonist of TRPM8 and profiled it in models of inflammatory and neuropathic pain.
Methods
Compounds and reagents
AMG2850, which was synthesized at Amgen Inc (Thousand Oaks, CA), resulted from an internal medicinal chemistry effort (Horne et al. 2014). All the cell culture reagents were purchased from Invitrogen (Carlsbad, CA).
In vitro characterization
Luminescence readout assay for measuring intracellular calcium
Stable Chinese hamster ovary (CHO) cell lines expressing rat TRPA1, rat TRPM8, rat TRPV3, and human TRPV4 were generated using the tetracycline-inducible T-REx™ expression system from Invitrogen (Carlsbad, CA) and a stable CHO cell line expressing rat TRPV1 was generated using a constitutive expression system (Klionsky et al. 2007). To enable a luminescence readout based on intracellular increase in calcium (Le Poul et al. 2002), each cell line was also co-transfected with pcDNA3.1 plasmid containing jelly fish aequorin cDNA. Twenty-four hours before the assay, cells were seeded in 96-well plates and all TRP channel expression, except for TRPV1, was induced with 0.5 ug/mL tetracycline. On the day of the assay, culture media were removed and cells were incubated for 2 h with pH 7.2 assay buffer (F12 containing 30 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) for TRPV1, TRPA1, TRPM8, and TRPV3; F12 containing 30 mM HEPES, 1 mM CaCl2, and 0.3 % BSA for TRPV4) containing 15 uM coelenterazine (P.J.K GmbH, Germany). AMG2850 was added 2.5 min prior to the addition of an agonist. Luminescence was measured by a CCD camera-based FLASH-luminometer built by Amgen, Inc. The following agonists were used to activate TRP channels: 0.5 uM capsaicin for TRPV1, 80 uM allyl isothiocyanate (AITC) for TRPA1, 1 uM icilin or cold buffer (12 °C) for TRPM8, 200 uM aminoethoxydiphenyl borate (2-APB) for TRPV3, and 1 uM 4α-phorbol 12,13-didecanoate (4α-PDD) for TRPV4. Antagonist IC50 values were calculated using GraphPad Prism 4.01 (GraphPad Software Inc, San Diego, CA).
Agonist-induced 45Ca2+ uptake assay
Two days prior to the assay, cells were seeded in Cytostar 96-well plates (Amersham) at a density of 20,000 cells/well. The activation of TRPM8 was followed as a function of cellular uptake of radioactive calcium (45Ca2+) upon cold or icilin stimulation. To determine the ability to block agonist activation of TRPM8, AMG2850 was incubated with CHO cells expressing the TRP channel for 2 min before the addition of agonist and 45Ca2+ and cells were washed after a further incubation of 2 min to determine the 45Ca2+ uptake. All antagonist 45Ca2+ uptake assays were conducted as reported previously (Gavva et al. 2007), with a final 45Ca2+ concentration of 10 μCi/mL. Radioactivity was measured using a MicroBeta Jet (PerkinElmer, Inc.). Data were analyzed using GraphPad Prism 4.01 (GraphPad Software Inc, San Diego, CA).
Pharmacokinetics in plasma and brain
Plasma pharmacokinetics of each compound in male Sprague-Dawley rats (n = 3 animals per study) were determined after intravenous dosing at 2 mg/kg in 100 % dimethylsulfoxide (DMSO) or after administration of 5 mg/kg by oral gavage with test article formulated in 5 % Tween 80 in OraPlus®. At designated time points, blood was collected via the femoral artery and processed for plasma by centrifugation. Plasma was then transferred into a 96-well container and stored in a freezer maintained at −70 °C. Similar bioanalytical methods were used to determine plasma and brain exposure in animals tested in pharmacodynamic and pain models. Brain samples were homogenized with a 4:1 ratio of water (mL) for every gram of brain tissue. All analytical methods utilized protein precipitation, with the addition of a structurally similar compound to function as an internal standard. Calibration standards were prepared from a 1 mg/mL stock solution of each compound in DMSO with a serial dilution into a blank matrix (plasma or brain homogenate). Liquid chromatography with tandem mass spectrometry was used for the quantitation of each compound in rat plasma and brain. Non-compartmental pharmacokinetic analysis of plasma concentrations was conducted using WinNonlin Enterprise v.5.1.1 (Pharsight Corporation, Mountain View, CA). Brain uptake was calculated as a ratio of concentration in whole brain tissue to that in plasma taken from the same animal at approximately the same time.
Plasma protein binding
The unbound fraction in plasma was determined by ultracentrifugation. Test compound (5 ug/mL) was incubated in plasma from male rats at 37 °C for 15 min. Samples were transferred to a centrifuge tube and centrifuged at 16,128×g for 3 h at 37 °C. After protein precipitation using acetonitrile containing an internal standard (2:1 acetonitrile to plasma), supernatants were dried under a stream of nitrogen gas, with residues reconstituted in methanol/water (50:50, v/v) prior to analysis by liquid chromatography with tandem mass spectrometry. The concentration was determined from a linear regression of peak area ratios (analyte peak area/internal standard peak area) relative to calibration standards. The unbound fraction in plasma (C u) was calculated as a ratio of the concentration measured relative to the nominal concentration.
Skin nerve preparation and fiber analysis
Adult C57BL/6 mice were obtained from Jackson Laboratories and used for ex vivo skin-nerve preparations. Mice were allowed to acclimate to the animal facility at the Medical College of Wisconsin, an Association for the Assessment and Accreditation of Laboratory Animal Committee (AALAC)-accredited facility, for approximately 1 week following shipment. Animals were housed with up to four other cagemates and had ad libitum access to food pellets and hyper-chlorinated water. Mice were housed on a 14:10 h light-dark cycle in cages containing aspen bedding and paper nesting material. Room temperatures averaged 21 °C. Prior to use, animals were briefly anesthetized with inhaled isoflurane and then sacrificed via cervical dislocation. All procedures were approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin.
The mouse saphenous skin-nerve in vitro preparation (Koltzenburg et al. 1997; Stucky et al. 1999) was used for electrophysiological recordings of cutaneous terminals of primary afferent fibers in situ. Recordings were conducted on adult male C57BL/6 mice, aged 7–18 weeks. Mice were anesthetized with isoflurane and sacrificed by cervical dislocation. The saphenous nerve and skin from the medial dorsum of the hind paw were then rapidly dissected free and placed corium side up into a bath superfused with oxygen-saturated synthetic interstitial fluid containing (in mM) 123 NaCl, 3.5 KCl, 0.7 MgSO4, 1.7 NaH2PO4, 2.0 CaCl2, 9.5 sodium gluconate, 5.5 glucose, 7.5 sucrose, and 10 HEPES, 290 mOsm, at pH 7.45 ± 0.05, and temperature 32.0 ± 0.5 °C. The saphenous nerve was desheathed and teased into fine filaments for extracellular recordings, as described (Kwan et al. 2009). Single afferent units were identified using a mechanical search stimulus (blunt glass rod). C fibers were identified by conduction velocities slower than 1.2 m/s. Once a unit with a signal to noise ratio >2 was identified, the mechanical threshold was determined using calibrated von Frey filaments (range, 0.44–147.0 mN).
For treatment with TRPM8 inhibitors, the receptive field of each C fiber was isolated with a metal ring (4-mm diameter) sealed to the skin and incubated in vehicle (0.1 % DMSO or 0.003 % DMSO) or 10 μM AMG2850 for 10 min. AMG2850 was dissolved to the final concentration in 0.1 % DMSO. A baseline recording was taken during the final 2 min of compound incubation to measure any ongoing action potentials.
For mechanical stimulation in the presence of test compounds, the receptive field of each C fiber was tested with a series of increasing mechanical forces (5, 10, 20, 40, 100, 150, 200, 245 mN; 10-s sustained force; 1-min inter-stimulus interval). For chemical stimulation, the receptive field of each C fiber was stimulated with 300 μM menthol in the presence of the respective compound and action potentials were recorded for 2 min. C fibers were considered to be responsive to menthol if they fired at least three action potentials above baseline during the 2-min incubation period.
Wave forms of action potentials evoked by mechanical stimuli were saved on an oscilloscope for comparison of shape and profile. Data were collected using a Powerlab 4.0 system and LabChart software (ADInstruments, Colorado Springs, CO) and saved for off-line analysis. Action potentials were discriminated and counted off-line using a spike histogram software extension. For C fibers that exhibited ongoing activity in the presence of TRPM8 inhibitors, the frequency of action potentials during baseline was subtracted from action potentials evoked during mechanical or menthol stimulation. The operator was blinded to chemical treatment applied to each receptive field. All data was also analyzed in a blinded manner.
In vivo pharmacodynamic and pain models
Adult male Sprague-Dawley rats weighing 220–300 g (Harlan, San Diego) were cared for in accordance to the Guide for the Care and Use of Laboratory Animals, 8th Edition (National Research Council Committee for the Update of the Guide for the C and Use of Laboratory A 2011). Animals were group housed at an Association for Assessment and Accreditation of Laboratory Animal Committee-accredited facility in non-sterile ventilated micro-isolator housing on corn cob bedding. All research protocols were approved by the Institutional Animal Care and Use Committee. Animals had ad libitum access to pelleted feed (Harlan Teklad 2020X, Indianapolis, IN) and water (on-site generated reverse osmosis) via automatic watering system. Animals were maintained on a 12:12 h light-dark cycle in rooms at 21 ± 3 °C, 50 ± 20 % room humidity, and had access to enrichment opportunities (nesting materials and plastic domes). All animals were sourced from approved vendors who meet or exceed animal health specifications for the exclusion of specific pathogens (i.e., mouse parvovirus, Helicobacter). Animals were allowed at least 1-week acclimation to the facility prior to any procedures. Following completion of behavioral or blood pressure measurements, animals were euthanized with carbon dioxide followed by immediate blood collection via cardiac puncture for pharmacokinetic analysis. All behavioral data was scored by an observer blind to dosing condition or through an automated device.
Icilin-induced wet-dog shake (WDS) model
The TRPM8 antagonist (1, 3, or 10 mg/kg) or vehicle control (5 % Tween 80 in OraPlus®) was administered p.o. 90 min prior to administration of icilin (0.5 mg/kg, 1 ml/kg, i.p., 100 % PEG 400). The number of shakes exhibited by the rats (wet-dog shakes; WDS) was counted for a duration of 30 min post icilin administration (Werkheiser et al. 2006). Rats were viewed through transparent Plexiglas® observation cylinders that were placed on a custom, opaque, plastic apparatus such that one rat could not view another rat. The 0.5 mg/kg dose of icilin was chosen based on an in-house dose-response effect over 0.3–1.0 mg/kg in which nonlinear dose-response curve analysis yielded a goodness of fit r 2 of 0.84, and the resulting ED90 was 0.46 mg/kg (0.28–0.75; data not shown).
Cold-induced increase in blood pressure—cold pressor test (CPT)
Rats were treated with the TRPM8 antagonist (0.3, 1, or 3 mg/kg) or vehicle control (5 % Tween 80 in OraPlus®) p.o. 2 h prior to anesthesia with sodium pentobarbital at 60 mg/kg i.p. Blood pressure was recorded for 5 min for “pre-cold baseline” and an additional 5 min during immersion of the paws and ventral half of the body in ice water. Mean blood pressure (MBP) was derived electronically using Digi-Med Blood Pressure Analyzer (Model 400). Plasma was collected through the artery catheter immediately after the cold pressor test for pharmacokinetic analysis. Percent increase from baseline and percent inhibition attributed to treatment with test compound were then determined using the following formula: (1) percent increase from baseline (mmHg) cold pressor effect = [(cold-evoked change in MBP)/MBP pre-cold] × 100; (2) percent inhibition of cold pressor effect = [1 − (cold-evoked change in MBP post-test compound/cold-evoked change in MBP post-vehicle)] × 100.
Open field activity
Rats were habituated in a reversed light cycle room in home cages for at least 1 week and acclimated to the testing room for 1 h prior to dosing. TRPM8 antagonist (10, 30, or 100 mg/kg) or vehicle (5 % Tween 80 in OraPlus®) was injected 2 h before placing an animal in the open-field apparatus. Open-field activity was measured using a system that counts interruptions of a set of photobeams for the course of 60 min (Kinder Scientific, Poway, CA). To begin a session, animals were removed from the home cage and placed individually into an independent Plexiglas box (41 cm L × 41 cm W × 38 cm H) surrounded by a frame consisting of 32 photocells (16Y and 16X) that track the movement of the animal. Photobeam breaks were used as an indication of activity and were measured as the following parameters per minute: basic movements (beam breaks), distance traveled (cm), time spent (s), and number of repetitive beam breaks (i.e., stereotypic movement). Chlordiazepoxide, which consistently elicits a decrease in open field activity, was used as a positive control. Chlordiazepoxide was formulated in saline at 5.6 mg/kg i.p. and dosed 30 min before the start of the open field assay.
CFA-induced hypersensitivity
Rats were habituated in a reversed light cycle room for at least 1 week and to the testing room for 1 h prior to dosing. To begin the study, rats received an injection of CFA into the left hind paw (100 μL of 0.1 % CFA in saline) based on previous demonstration that this causes a decrease in rearing. Since this rearing deficit can be significantly reversed by treatment with indomethacin, it is presumed to represent mechanical and/or tactile hypersensitivity and rats are rearing less in order to avoid weight bearing on the inflamed hindpaw (Youngblood et al. 2008). Twenty-one hours after the CFA injection, animals were treated (p.o.) with vehicle (5 % Tween 80 in OraPlus®) or TRPM8 antagonist. Two hours after drug dosing (23 h after CFA injection), rats were placed in the same Kinder Scientific open field boxes as described above but with customized prickly floor inserts placed inside. Rearing time and rearing counts were measured for 60 min. Indomethacin at a dose of 1 mg/kg dosed p.o. in 5 % Tween 80 in OraPlus® was chosen as a positive control based on reliable efficacy demonstrated in previous experiments (Youngblood et al. 2008).
Sciatic nerve ligation (SNL)-induced tactile allodynia
SNL surgery was performed using aseptic surgical techniques and a stereomicroscope (Kim and Chung 1992). Spinal nerve injury was caused by ligating the left L5 and L6 spinal nerves, with special care to avoid damage to the L4 spinal nerve or surrounding area. More specifically, under gaseous anesthesia with a mixture of O2 and isoflurane (3 % for induction and 2 % for maintenance), skin was excised and the longissimus lumborum muscle, part of articular processes (L4-S1), and the fascia above L6 spinal nerve were carefully removed. This procedure provided a clean and spacious working area to enable complete resection of the L6 transverse process and to separate the L5 spinal nerve from the L4 spinal nerve without damage to L4. The L5 and L6 spinal nerves were each tightly ligated with 6-0 silk thread and then L5 was cut. The entire surgery procedure beginning from anesthesia and ending with wound clipping of the outside skin lasted 15 min or less.
Surgical procedure
Behavioral testing Two weeks post surgery, mechanical sensitivity was measured by determining the median 50 % foot withdrawal threshold for von Frey filaments using the up-down method (Chaplan et al. 1994). Rats were placed under a plastic cover (9 × 9 × 20 cm) on a metal mesh floor. von Frey filaments (Semmes-Weinstein monofilaments from Stoelting) were applied to the middle glabrous area between the footpads of the plantar surface of the injured hind paw. This plantar area was touched with a series of nine recently calibrated von Frey filaments with approximately exponentially incremental bending forces (von Frey filament numbers 3.61, 3.8, 4.0, 4.2, 4.41, 4.6, 4.8, 5.0, and 5.2; equivalent to 0.41, 0.63, 1.0, 1.58, 2.51, 4.07, 6.31, 10, and 15.8 g). The von Frey filament was presented perpendicular to the plantar surface with sufficient force to cause slight bending and held for approximately 3–4 s. To avoid possible reflex responses, only abrupt withdrawal of the foot accompanied by pain indicative behaviors (namely paw flinching, shaking, or licking for more than 2 s) was recorded as a response. Any post-surgery rat that displayed a mechanical threshold of more than 3.16 g or less than 0.7 g was eliminated from the study. After measuring basal threshold, animals were treated (p.o.) with vehicle (5 % Tween 80 in OraPlus®) or TRPM8 antagonist (AMG2850 at 100 mg/kg), or gabapentin (200 mg/kg; Sigma, St. Louis). The measurement of the tactile threshold was reassessed at 1 and 2 h after drug administration in the same animals.
Statistical analyses
In vivo IC50 and IC90 estimates using WDS model data were determined by fitting a sigmoidal dose-response curve to individual animal number of WDS versus resulting plasma concentration. Behavioral and electrophysiological data are expressed as mean ± standard error of the mean (S.E.M.). Results were analyzed using one-way analysis of variance (ANOVA) with Dunnett’s multiple-comparisons post-hoc test for significance relative to vehicle. Since the von Frey filament set was calibrated on a logarithmic scale by the vendor (Stoelting), our selection of nine filaments for the up-down method was also based on near-equal logarithmic intervals (Dixon 1980), and because it is our experience that variability noticeably increases with threshold value and also variances are statistically different using Bartlett’s test of equal variances, thus violating the assumptions of ANOVA, data were analyzed following logarithmic transformation prior to statistical analysis. Actual gram values are plotted on a logarithmic scale Y-axis of the figures for convenience. Statistical calculations and graphs were made using GraphPad Prism 5.01 (GraphPad Software Inc, San Diego, CA). Percent responders to menthol stimulation in the ex vivo skin-nerve preparation were compared under vehicle and antagonist conditions through the use of Fisher’s exact test. Responses of C fibers to increasing mechanical forces under vehicle or antagonist conditions were compared using a two-way ANOVA with a Bonferonni post-hoc analysis.
Results
AMG2850 acts as a potent and selective TRPM8 antagonists in vitro
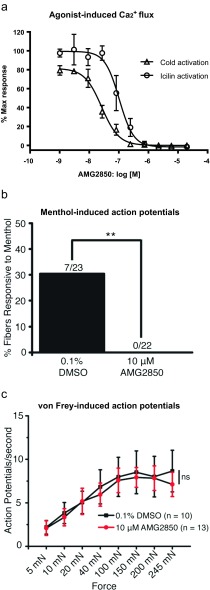
TRPM8 antagonist hits were identified by high throughput screening of compound libraries and subsequent medicinal chemistry efforts yielded AMG2850 (Horne et al. 2014). AMG2850 blocks both icilin and cold activation of rat TRPM8 potently. The IC50 estimates of AMG2850 are 41 ± 8 nM against cold activation and 204 ± 28 nM against icilin activation assays (Fig. 1a). AMG2850 is >600-fold selective for TRPM8 versus TRPA1 and >100-fold selective for TRPM8 over rat TRPV1, rat TRPV3, and human TRPV4 (see Table 1).
Fig. 1.
a AMG2850 potently inhibits both cold temperature- and icilin-induced increase in luminescence believed to reflect intracellular calcium in CHO cells stably expressing rat TRPM8. After a 2-h incubation with coelenterazine, cells were incubated with compounds for 2.5 min prior to the addition of either icilin (1 μM) or cold buffer (12 °C). Luminescence was measured by a CCD camera-based FLASH-luminometer. Concentration-response curves were generated using GraphPad Prism 4.01. Each point in the graphs is an average ± SEM of an experiment conducted in triplicate. Responses of agonists used were normalized to 100 % of maximum. b In a skin-nerve preparation, total percentage of C fibers responding to 300 μM menthol after incubation with vehicle or AMG2850. A response to menthol was defined as three action potentials above baseline over a 2-min interval. Incubation with AMG2850 significantly decreased the percentage of C fibers responding to menthol as compared to vehicle controls (**p < 0.005). c In a skin-nerve preparation, AMG2850 has no effect on C fiber firing in response to sustained mechanical force of increasing intensities. Note an increasing number of action potentials with increased force that is not altered by preincubation of AMG2850
Table 1.
Summary of AMG2850 properties (where indicated using mean ± standard deviation)
| Assay | AMG2850 |
|---|---|
| rTRPM8 (cold) IC90 (nM) | 41 ± 8 |
| rTRPM8 (icilin) IC90 (nM) | 204 ± 28 |
| rTRPA1 IC50 (nM) | >25,000 |
| rTRPA1 agonism IC50 (nM) | >25,000 |
| rTRP(V1, V3) IC50 (nM) | >25,000 |
| Rat clearance (L/h/kg) | 0.47 |
| Oral bioavailability (%) | 47 |
| B/P ratio | 0.8–1.5 |
| Protein binding | 88.1 % |
| Rat PD ED50 (mg/kg) | 1.8 |
| Rat PD IC90 (nM) | 99 |
AMG2850 exhibits suitable pharmacokinetic properties for in vivo studies
AMG2850 exhibited good pharmacokinetic properties including low plasma clearance 0.47 L/h/kg and good oral bioavailability 47 % in male Sprague-Dawley rat (see Table 1). Total brain to plasma ratio of AMG2850 is in the range of 0.8–1.5 which demonstrates brain uptake and would be expected to provide coverage if there are brain TRPM8 channels that play a role in pain. We have used unbound plasma concentrations (C u; also sometimes referred to as “exposure”) in calculating the target coverage since “protein-bound” fraction of compounds are considered unavailable for TRPM8 occupancy.
AMG2850 blocks menthol-evoked activation of C fibers in mouse
In order to better understand how the TRPM8 antagonists affect primary afferent firing, we assessed the effects of these compounds in an ex vivo skin-nerve preparation. First, it was assessed whether the compounds could block a TRPM8 agonist response. We focused on C fibers as most TRPM8-expressing afferents are C fibers (Bautista et al. 2007). C fibers treated with the vehicle control (0.1 % DMSO) responded to menthol (300 μM) with action potential firing (8.6 ± 2.9 action potentials per 2 min). In contrast, pretreatment with AMG2850 (10 μM) completely inhibited all menthol-evoked firing (n = 22; p = 0.005; Fig. 1b) demonstrating that AMG2850 completely blocks chemical activation of TRPM8 on cutaneous nerve terminals in situ.
TRPM8 inhibition has no effect on mechanically induced firing in cutaneous C fibers
AMG2850 (10 μM) was evaluated in mechanical activation of cutaneous C fibers following a range of increasing, sustained force in the presence of the vehicle or TRPM8 antagonist. AMG2850 had no significant effect on mechanical firing in C fibers compared to vehicle controls (Fig. 1c). The C fibers treated with vehicle or AMG2850 did not differ in average conduction velocity or von Frey mechanical thresholds (data not shown). These data indicate that the TRPM8 channel does not contribute to mechanical firing following von Frey stimulation in cutaneous C fiber nociceptors.
AMG2850 dose-dependently inhibits icilin-induced WDS in rats
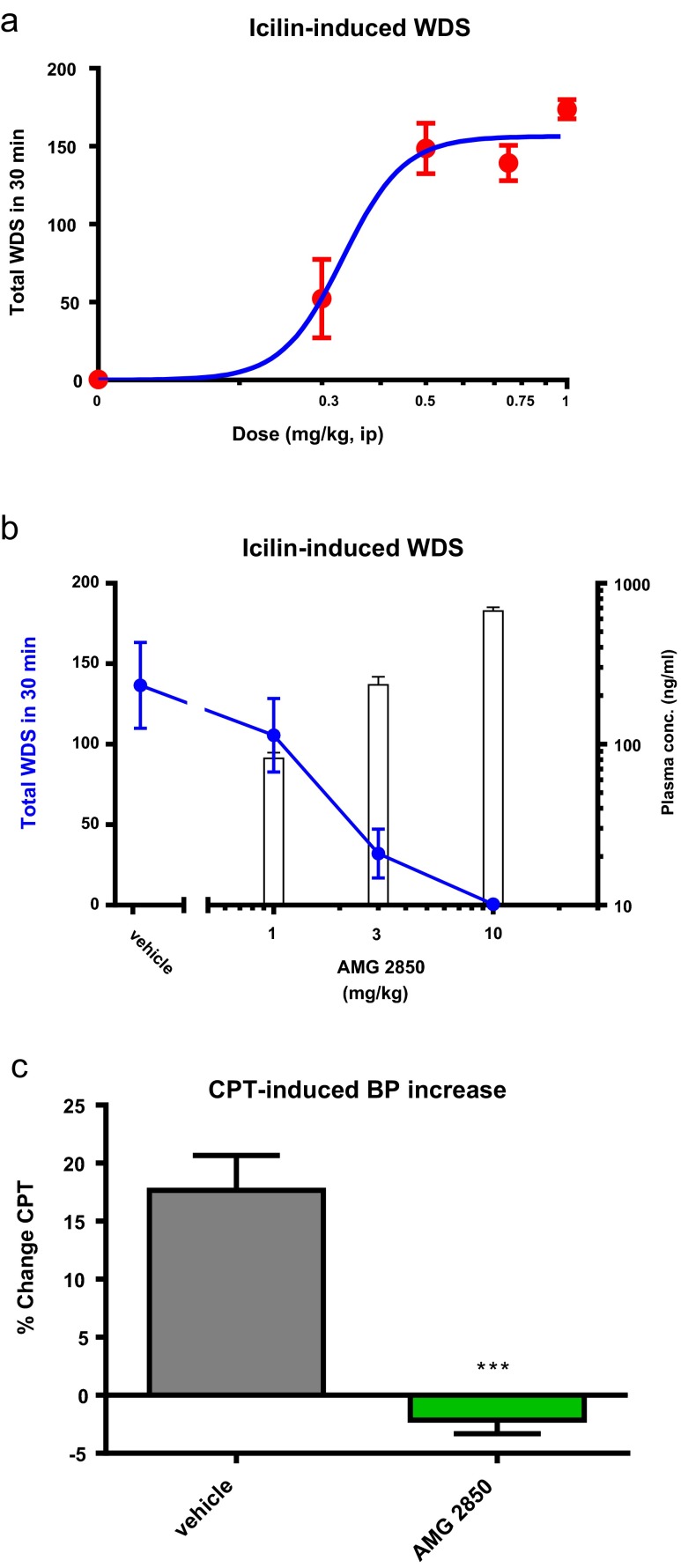
To choose the icilin concentration for reversal studies, the number of WDS was evaluated following 0.3, 0.5. 0.75, and 1 mg/kg of icilin. As shown in Fig. 2a, the ED80 is just less than 0.5 mg/kg. In the experiments that evaluated AMG2850, 0.5 mg/kg of icilin showed a comparable 137 ± 27 WDS in the vehicle-treated groups. AMG2850 exhibited dose-dependent and full prevention of icilin-induced WDS (Fig. 2b). Full prevention occurred at 10 mg/kg with a C u of 192 ± 26 nM and calculated unbound mean in vivo IC90 value of 99 nM.
Fig. 2.
a Intraperitoneal injection of icilin induces wet-dog shakes (WDS) in a dose-dependent manner. b AMG2850 reduces WDS in a dose- and plasma concentration-dependent manner. c AMG2850 (10 mg/kg, p.o.) significantly and fully blocked the cold pressor effect (***p < 0.001)
AMG2850 suppresses cold-induced increase in blood pressure
Since TRPM8 can be activated by cold in addition to icilin, we evaluated a dose near the WDS ED90 of AMG2850 in the cold pressor model (CPT; Fig. 2c). When tested at 10 mg/kg, AMG2850 also fully blocked the cold pressor response with a resulting C u of 488 ± 48 nM.
AMG2850 did not affect open field activity
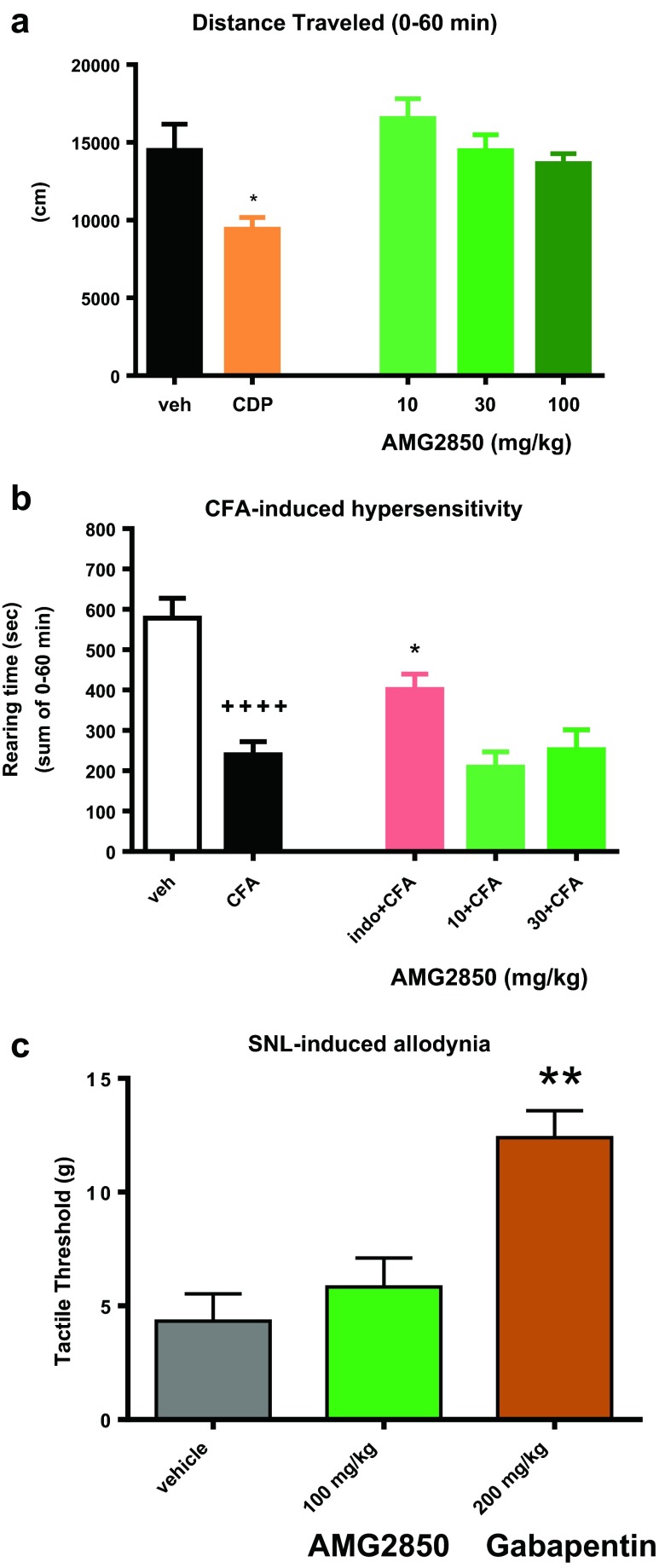
While assessing the potential analgesic effects of a compound, conclusions could be confounded if compounds also produce sedative or motor side effects, resulting in false positives. Thus, prior to testing in pain behavioral models, we evaluated AMG2850 in the open field assay along with a positive control of 5.6 mg/kg chlordiazepoxide (CDP; Fig. 3a). When AMG2850 was administered at 100 mg/kg (mean C u concentration was of 2.59 μM), total distance traveled was 13,640 ± 623 cm, which was not significantly different relative to the vehicle-treated group (14,484 ± 1686 cm; F 3,20 = 1.0, p > 0.05). As expected, total distance traveled was significantly reduced to 9423 ± 745 cm in rats receiving chlordiazepoxide (t 10 = 2.8, p < 0.05; Fig. 3a).
Fig. 3.
a AMG2850 did not affect the total distance traveled in reversed light cycle rat open field boxes (p > 0.05) while the positive control compound chlordiazepoxide (CDP; 5.6 mg/kg) significantly reduced distance traveled (*p < 0.05). b Unilateral hindpaw injection of complete Freund’s adjuvant (CFA) causes a reduction in rearing behavior. The positive control indomethacin (indo; 1 mg/kg) significantly (++++ p < 0.0001) reversed the CFA-induced reduction in rearing behavior. AMG2850 (100 mg/kg) produced no significant effect (*p > 0.05). c SNL produces a reduction in tactile threshold from approximately 15 g to approximately 5 g. The positive control, gabapentin (200 mg/kg), significantly reversed the reduction in tactile threshold (**p < 0.01). AMG2850 (100 mg/kg) produced no significant effect at 2.5 h post treatment (p > 0.05)
AMG2850 did not reverse CFA-induced mechanical hypersensitivity
We previously validated an automated, high-throughput assay to measure spontaneous/ongoing mechanical hypersensitivity (Youngblood et al. 2008). In this assay, rats injected with CFA to one hindpaw show a decrease in rearing behavior as measured 24 h later in an open field apparatus with a prickly floor insert. This window of presumed hypersensitivity behavior can be reversed by standard nonsteroidal anti-inflammatory drugs (Youngblood et al. 2008). In addition to automated scoring, a key advantage of this model is that reduction of pain behavior is quantified as a recovery of normal rearing instead of a decrease in an evoked response; thus, compounds that reduce general movement would not falsely be interpreted as efficacious.
In the experiment to evaluate the effects of AMG2850, there was also a significant window of hypersensitivity as measured by the rearing time response of animals administered with CFA in the hindpaw (204 ± 21 s) as compared to vehicle control in the hindpaw (525 ± 35 s; t 38 = 7.9, p < 0.01). There was an overall significant effect on hypersensitivity (F 3,36 = 4.6) with the reduction in rearing inhibited by 1 mg/kg indomethacin (388 ± 32 cm; p < 0.05 by Dunnett’s multiple comparisons; Fig. 3b). AMG2850 dosed at 100 mg/kg produced no significant effect on rearing time relative to vehicle (157 ± 25 s; p > 0.05 by Dunnett’s multiple comparisons; Fig. 3b) despite a mean C u plasma concentration of 4.3 μM.
AMG2850 did not reverse SNL-induced tactile allodynia
Two weeks post SNL surgery, rats exhibited a tactile threshold of 4.4 ± 1.2 g (vehicle-administered group; Fig. 3c). A logarithmic transformation of the data was performed prior to analysis, though actual g values are referred to in the text and shown on graphs. By Dunnett’s multiple-comparisons test relative to vehicle control, the positive control, gabapentin, significantly reversed SNL-induced mechanical allodynia with a significant increase in the threshold to 12.4 ± 1.2 g (F 2,31 = 11.99, p < 0.05). AMG2850 at 100 mg/kg p.o. produced no significant effect on von Frey threshold relative to vehicle (p > 0.05) with tactile threshold of 5.8 ± 1.3 g. The mean C u plasma concentration in this group was 2.1 μM.
Discussion
Here, we report the pharmacology of AMG2850 as a potent and selective antagonist of TRPM8 channels in vitro and in vivo. AMG2850 demonstrated significant TRPM8 antagonism in vivo in two distinct target coverage models (icilin-induced wet-dog shake behavior (WDS) and cold-induced blood pressure increase in CPT model). AMG2850 significantly blocked menthol-induced, but not mechanically induced, activation of C fibers in situ. AMG2850 did not show efficacy in either the CFA model of mechanical hypersensitivity or the SNL-induced tactile allodynia model of neuropathic pain at plasma unbound concentrations in excess of 21-fold of an IC90 concentration in the TRPM8-specific WDS model. This suggests that either TRPM8 does not play a role in mechanical pain behaviors measured or a much higher target coverage is required for efficacy. Here, we discuss the results of this study in the context of antagonist exposure in vivo and TRPM8 function in different in vivo models.
Comparison of exposure in different models: relationship to target coverage and efficacy
Protein unbound concentrations of antagonists are used for comparison between different studies. In the WDS in vivo target coverage model, resulting unbound IC90 value for AMG2850 is 99 nM. Compared to the WDS IC90 value, unbound concentration of AMG2850 in CFA and SNL models was 43- and 21-fold higher, respectively, than the WDS IC90 value. Lack of efficacy in both CFA and SNL models with unbound concentrations in excess of 21-fold the IC90 coverage in WDS model either suggests that IC90 coverage in WDS model is an underestimate of actual target coverage or TRPM8 does not play a role in pain behaviors measured in these models.
“Molecular sensors” such as TRPM8 (a cold temperature sensor) play a role in body temperature homeostasis by inducing autonomic and behavioral cold defenses upon activation by either cold or chemical ligands (Almeida et al. 2012; Gavva et al. 2012). WDS behavior induced by icilin probably represents a skeletal muscle-mediated heat generation mechanism and such a response may only need activation of a small subpopulation of TRPM8 channels; hence, target coverage measured in WDS model may be an underestimate relative to target coverage required in pain models in which TRPM8 channels are reportedly upregulated.
A second potential underestimation could come from the way the icilin dose is chosen for an antagonist evaluation. Typically, an ED80 of an agonist dose is identified based on a dose response assuming that the behavioral endpoint such as WDS is linear. However, behavioral endpoints may not be linear due to different reasons such as physical limitations, resulting in a lower ED80 value that may not cover the target at 80 % occupancy. This may be the case with AMG2850 where 0.5-fold of in vitro IC90 concentration is equivalent to WDS IC90 concentration.
Role of TRPM8 antagonists on cold sensing and cold hypersensitivity
The role of TRPM8 in cold sensing and cold hypersensitivity is unequivocally proven with KO mouse studies as well as with antagonists (Knowlton et al. 2011; McCoy et al. 2011; Parks et al. 2011; Patel et al. 2014). In particular, TRPM8 antagonists representing different chemotypes such as compound 496 (Parks et al. 2011), PBMC (Knowlton et al. 2011), and M8-An (Patel et al. 2014) attenuated cold hypersensitivity after CFA or peripheral nerve injury models suggesting that TRPM8 antagonists may act as therapeutics for indications where cold hypersensitivity is an issue such as chemotherapy and other neuropathic conditions. Although we have not tested antagonists in these models, the overlap between published results and our studies is that TRPM8 antagonists cover the target in WDS model as well as suppress cold-induced increase in blood pressure (CPT model). Additionally, electrophysiological data generated using an ex vivo skin-nerve preparation demonstrates that AMG2850 is effective in blocking C fiber action potentials evoked by a TRPM8 agonist, menthol. Thus, cold-related behavioral endpoints, whether at baseline, post-inflammatory, or post-neuropathic, appear to serve as additional target coverage models for TRPM8 antagonists and are separable from efficacy models with non-cold endpoints.
TRPM8 role in tactile allodynia
While initial reports using TRPM8 KO mice indicated that TRPM8 is not involved in mechanical sensitivity (Bautista et al. 2007; Knowlton et al. 2013), another study found increased von Frey thresholds among C fibers in exogenous skin-nerve preparations taken from TRPM8 KO mice as compared to wild-type controls (Zimmermann et al. 2011). Additionally, behavioral studies using icilin or menthol have also shown that agonist activation of TRPM8 may cause sensitization or desensitization/inhibition of responses to mechanical stimuli under naïve conditions (Brignell et al. 2008; Klein et al. 2010; Harrington et al. 2011) or after injury (Proudfoot et al. 2006; Brignell et al. 2008; Su et al. 2011; Liu et al. 2013). However, we observed no effect of AMG2850 on mechanical sensitivity using both inflammatory and neuropathic pain models, despite a robust effect on cold behaviors. Additionally, ex vivo teased fiber recordings indicated no effect of AMG2850 on mechanical sensitivity from naïve C fibers. Thus, two possibilities exist: either TRPM8 does not play a role in mechanosensation before or after injury, or AMG2850 binds TRPM8 in such a way as to inhibit ligand activation by menthol, but not its role in mechanosensation. Regardless, it can be concluded that AMG2850 is not an effective modulator of mechanical hypersensitivity or tactile allodynia, despite target coverage in vivo.
Although many have suggested TRPM8 as a therapeutic target for chronic pain, there are no examples of TRPM8 antagonists that reverse non-cold-evoked behavioral endpoints of inflammatory or neuropathic pain. One recent example is M8-An, a potent small molecule antagonist of TRPM8 shown to attenuate SNL-induced cold hypersensitivity, but was shown to be ineffective at reducing tactile allodynia (Patel et al. 2014). Similarly, AMG2850 potently blocks cold-induced physiological changes but not inflammatory- or neuropathic-induced non-cold endpoints such as mechanical hypersensitivity or tactile allodynia.
An indication for which antagonism of TRPM8 may still hold therapeutic promise is migraine, though the lack of validated preclinical migraine models leaves the promise to be revealed in the clinic. Additional indications for which new data is emerging such as urogenital diseases and pathophysiologies related to lacrimation, airway, and vasculature await further evaluation (for recent reviews, see Knowlton and McKemy 2011; Liu and Qin 2011; Malkia et al. 2011; Almaraz et al. 2014).
Acknowledgments
Authors would like to thank our colleagues, Jeff Clarine, Dan Horne, Mark Norman, Nuria Tamayo, and Sara Rao, for their help and input.
Author contributions
Davis, Gavva, Lehto, Stucky, and Weyer participated in the research design while Wild advised on research designs. Kerstein, Wang, Wang, Weyer, Youngblood, and Zhang conducted the experiments. Davis, Gavva, Lehto, Stucky, Wang, Wang, Weyer, Wild, Youngblood, and Zhang wrote or contributed to the writing of the manuscript.
Abbreviations
- TRPM8
Transient receptor potential melastatin 8
- TRPV1
Transient receptor potential vanilloid type 1
- TRPA1
Transient receptor potential ankyrin 1
- CHO
Chinese hamster ovary
- CNS
Central nervous system
- AMG2850
(R)-8-(4-(Trifluoromethyl)phenyl)-N-((S)-1,1,1-trifluoropropan-2-yl)-5,6-dihydro-1,7-naphthyridine-7(8H)-carboxamide
Contributor Information
Sonya G. Lehto, Email: slehto@amgen.com
Narender R. Gavva, Email: ngavva@amgen.com
References
- Almaraz L, Manenschijn JA, de la Pena E, Viana F. TRPM8. Handb Exp Pharmacol. 2014;222:547–579. doi: 10.1007/978-3-642-54215-2_22. [DOI] [PubMed] [Google Scholar]
- Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, Garami A, Bautista D, Gavva NR, Romanovsky AA. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci: Off J Soc Neurosci. 2012;32:2086–2099. doi: 10.1523/JNEUROSCI.5606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila V, Winsvold BS, Gormley P, Kurth T, Bettella F, McMahon G, Kallela M, Malik R, de Vries B, Terwindt G, Medland SE, Todt U, McArdle WL, Quaye L, Koiranen M, Ikram MA, Lehtimaki T, Stam AH, Ligthart L, Wedenoja J, Dunham I, Neale BM, Palta P, Hamalainen E, Schurks M, Rose LM, Buring JE, Ridker PM, Steinberg S, Stefansson H, Jakobsson F, Lawlor DA, Evans DM, Ring SM, Farkkila M, Artto V, Kaunisto MA, Freilinger T, Schoenen J, Frants RR, Pelzer N, Weller CM, Zielman R, Heath AC, Madden PA, Montgomery GW, Martin NG, Borck G, Gobel H, Heinze A, Heinze-Kuhn K, Williams FM, Hartikainen AL, Pouta A, van den Ende J, Uitterlinden AG, Hofman A, Amin N, Hottenga JJ, Vink JM, Heikkila K, Alexander M, Muller-Myhsok B, Schreiber S, Meitinger T, Wichmann HE, Aromaa A, Eriksson JG, Traynor BJ, Trabzuni D, Rossin E, Lage K, Jacobs SB, Gibbs JR, Birney E, Kaprio J, Penninx BW, Boomsma DI, van Duijn C, Raitakari O, Jarvelin MR, Zwart JA, Cherkas L, Strachan DP, Kubisch C, Ferrari MD, van den Maagdenberg AM, Dichgans M, Wessman M, Smith GD, Stefansson K, Daly MJ, Nyholt DR, Chasman DI, Palotie A. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet. 2013;45:912–917. doi: 10.1038/ng.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Brock JA, Viana F. Converting cold into pain. Exp Brain Res Exp Hirnforschung Exp Cerebrale. 2009;196:13–30. doi: 10.1007/s00221-009-1797-2. [DOI] [PubMed] [Google Scholar]
- Brignell JL, Chapman V, Kendall DA. Comparison of icilin- and cold-evoked responses of spinal neurones, and their modulation of mechanical activity, in a model of neuropathic pain. Brain Res. 2008;1215:87–96. doi: 10.1016/j.brainres.2008.03.072. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chasman DI, Schurks M, Anttila V, de Vries B, Schminke U, Launer LJ, Terwindt GM, van den Maagdenberg AM, Fendrich K, Volzke H, Ernst F, Griffiths LR, Buring JE, Kallela M, Freilinger T, Kubisch C, Ridker PM, Palotie A, Ferrari MD, Hoffmann W, Zee RY, Kurth T. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet. 2011;43:695–698. doi: 10.1038/ng.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasman DI, Anttila V, Buring JE, Ridker PM, Schurks M, Kurth T. Selectivity in genetic association with sub-classified migraine in women. PLoS Genet. 2014;10:e1004366. doi: 10.1371/journal.pgen.1004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Daniels RL, McKemy DD. Mice left out in the cold: commentary on the phenotype of TRPM8-nulls. Mol Pain. 2007;3:23. doi: 10.1186/1744-8069-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoeur J, Pereira V, Pizzoccaro A, Francois A, Ling B, Maffre V, Couette B, Busserolles J, Courteix C, Noel J, Lazdunski M, Eschalier A, Authier N, Bourinet E. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med. 2011;3:266–278. doi: 10.1002/emmm.201100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci: Off J Soc Neurosci. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Esserlind AL, Christensen AF, Le H, Kirchmann M, Hauge AW, Toyserkani NM, Hansen T, Grarup N, Werge T, Steinberg S, Bettella F, Stefansson H, Olesen J. Replication and meta-analysis of common variants identifies a genome-wide significant locus in migraine. Eur J Neurol: Off J Eur Fed Neurol Soc. 2013;20:765–772. doi: 10.1111/ene.12055. [DOI] [PubMed] [Google Scholar]
- Ferrer-Montiel A, Fernandez-Carvajal A, Planells-Cases R, Fernandez-Ballester G, Gonzalez-Ros JM, Messeguer A, Gonzalez-Muniz R. Advances in modulating thermosensory TRP channels. Exp Opin Ther Patents. 2012;22:999–1017. doi: 10.1517/13543776.2012.711320. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker SM, Proudfoot CW, Garry EM, Allchorne A, Vinuela-Fernandez I, Mitchell R. Cold comfort pharm. Trends Pharmacol Sci. 2007;28:621–628. doi: 10.1016/j.tips.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Freilinger T, Anttila V, de Vries B, Malik R, Kallela M, Terwindt GM, Pozo-Rosich P, Winsvold B, Nyholt DR, van Oosterhout WP, Artto V, Todt U, Hamalainen E, Fernandez-Morales J, Louter MA, Kaunisto MA, Schoenen J, Raitakari O, Lehtimaki T, Vila-Pueyo M, Gobel H, Wichmann E, Sintas C, Uitterlinden AG, Hofman A, Rivadeneira F, Heinze A, Tronvik E, van Duijn CM, Kaprio J, Cormand B, Wessman M, Frants RR, Meitinger T, Muller-Myhsok B, Zwart JA, Farkkila M, Macaya A, Ferrari MD, Kubisch C, Palotie A, Dichgans M, van den Maagdenberg AM. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat Genet. 2012;44:777–782. doi: 10.1038/ng.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauchan P, Andoh T, Kato A, Kuraishi Y. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neurosci Lett. 2009;458:93–95. doi: 10.1016/j.neulet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Hovland DN, Jr, Lehto SG, Klionsky L, Surapaneni S, Immke DC, Henley C, Arik L, Bak A, Davis J, Ernst N, Hever G, Kuang R, Shi L, Tamir R, Wang J, Wang W, Zajic G, Zhu D, Norman MH, Louis JC, Magal E, Treanor JJ. Repeated administration of vanilloid receptor TRPV1 antagonists attenuates hyperthermia elicited by TRPV1 blockade. J Pharmacol Exp Ther. 2007;323:128–137. doi: 10.1124/jpet.107.125674. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Davis C, Lehto SG, Rao S, Wang W, Zhu DX. Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol Pain. 2012;8:36. doi: 10.1186/1744-8069-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AM, Hughes PA, Martin CM, Yang J, Castro J, Isaacs NJ, Blackshaw LA, Brierley SM. A novel role for TRPM8 in visceral afferent function. Pain. 2011;152:1459–1468. doi: 10.1016/j.pain.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Horne DB, Tamayo NA, Bartberger MD, Bo Y, Clarine J, Davis CD, Gore VK, Kaller MR, Lehto SG, Ma VV, Nishimura N, Nguyen TT, Tang P, Wang W, Youngblood BD, Zhang M, Gavva NR, Monenschein H and Norman MH (2014) Optimization of potency and pharmacokinetic properties of tetrahydroisoquinoline transient receptor potential melastatin 8 (TRPM8) Antagonists. J Med Chem [DOI] [PubMed]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Klein AH, Sawyer CM, Carstens MI, Tsagareli MG, Tsiklauri N, Carstens E. Topical application of L-menthol induces heat analgesia, mechanical allodynia, and a biphasic effect on cold sensitivity in rats. Behav Brain Res. 2010;212:179–186. doi: 10.1016/j.bbr.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky L, Tamir R, Gao B, Wang W, Immke DC, Nishimura N, Gavva NR. Species-specific pharmacology of trichloro(sulfanyl)ethyl benzamides as transient receptor potential ankyrin 1 (TRPA1) antagonists. Mol Pain. 2007;3:39. doi: 10.1186/1744-8069-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68–77. doi: 10.2174/138920111793937961. [DOI] [PubMed] [Google Scholar]
- Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One. 2011;6:e25894. doi: 10.1371/journal.pone.0025894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci: Off J Soc Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL (2009) TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci 29:4808–4819 [DOI] [PMC free article] [PubMed]
- Le Poul E, Hisada S, Mizuguchi Y, Dupriez VJ, Burgeon E, Detheux M. Adaptation of aequorin functional assay to high throughput screening. J Biomol Screen. 2002;7:57–65. doi: 10.1177/108705710200700108. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qin N. TRPM8 in health and disease: cold sensing and beyond. Adv Exp Med Biol. 2011;704:185–208. doi: 10.1007/978-94-007-0265-3_10. [DOI] [PubMed] [Google Scholar]
- Liu B, Fan L, Balakrishna S, Sui A, Morris JB, Jordt SE. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain. 2013;154:2169–2177. doi: 10.1016/j.pain.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkia A, Pertusa M, Fernandez-Ballester G, Ferrer-Montiel A, Viana F. Differential role of the menthol-binding residue Y745 in the antagonism of thermally gated TRPM8 channels. Mol Pain. 2009;5:62. doi: 10.1186/1744-8069-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkia A, Morenilla-Palao C, Viana F. The emerging pharmacology of TRPM8 channels: hidden therapeutic potential underneath a cold surface. Curr Pharm Biotechnol. 2011;12:54–67. doi: 10.2174/138920111793937916. [DOI] [PubMed] [Google Scholar]
- McCoy DD, Knowlton WM, McKemy DD. Scraping through the ice: uncovering the role of TRPM8 in cold transduction. Am J Physiol Regul, Integrative Comp Physiol. 2011;300:R1278–1287. doi: 10.1152/ajpregu.00631.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- National Research Council Committee for the Update of the Guide for the C and Use of Laboratory A . The National Academies Collection: reports funded by National Institutes of Health, in guide for the care and use of laboratory animals. Washington: National Academies Press (US) National Academy of Sciences; 2011. [Google Scholar]
- Nieto-Posadas A, Jara-Oseguera A, Rosenbaum T. TRP channel gating physiology. Curr Top Med Chem. 2011;11:2131–2150. doi: 10.2174/156802611796904870. [DOI] [PubMed] [Google Scholar]
- Park CK, Kim MS, Fang Z, Li HY, Jung SJ, Choi SY, Lee SJ, Park K, Kim JS, Oh SB. Functional expression of thermo-transient receptor potential channels in dental primary afferent neurons: implication for tooth pain. J Biol Chem. 2006;281:17304–17311. doi: 10.1074/jbc.M511072200. [DOI] [PubMed] [Google Scholar]
- Parks DJ, Parsons WH, Colburn RW, Meegalla SK, Ballentine SK, Illig CR, Qin N, Liu Y, Hutchinson TL, Lubin ML, Stone DJ, Jr, Baker JF, Schneider CR, Ma J, Damiano BP, Flores CM, Player MR. Design and optimization of benzimidazole-containing transient receptor potential melastatin 8 (TRPM8) antagonists. J Med Chem. 2011;54:233–247. doi: 10.1021/jm101075v. [DOI] [PubMed] [Google Scholar]
- Patel R, Goncalves L, Newman R, Jiang FL, Goldby A, Reeve J, Hendrick A, Teall M, Hannah D, Almond S, Brice N, Dickenson AH. Novel TRPM8 antagonist attenuates cold hypersensitivity after peripheral nerve injury in rats. J Pharmacol Exp Ther. 2014;349:47–55. doi: 10.1124/jpet.113.211243. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/S0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Current Biol: CB. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- Reid G, Flonta ML. Physiology. Cold current in thermoreceptive neurons. Nature. 2001;413:480. doi: 10.1038/35097164. [DOI] [PubMed] [Google Scholar]
- Schurks M (2011) Genetics of migraine in the age of genome-wide association studies. J Headache Pain [DOI] [PMC free article] [PubMed]
- Stucky CL, Koltzenburg M, Schneider M, Engle MG, Albers KM, Davis BM. Overexpression of nerve growth factor in skin selectively affects the survival and functional properties of nociceptors. J Neurosci. 1999;19:8509–8516. doi: 10.1523/JNEUROSCI.19-19-08509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, Dubin AE, Jeske NA, Malin SA, McKemy DD, Story GM. Roles of transient receptor potential channels in pain. Brain Res Rev. 2009;60:2–23. doi: 10.1016/j.brainresrev.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Wang C, Yu Y-h, Ren Y-y, Xie K-l, Wang G-l. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 2011;12:120. doi: 10.1186/1471-2202-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajino K, Matsumura K, Kosada K, Shibakusa T, Inoue K, Fushiki T, Hosokawa H, Kobayashi S. Application of menthol to the skin of whole trunk in mice induces autonomic and behavioral heat-gain responses. Am J Physiol Regul, Integrative Comp Physiol. 2007;293:R2128–2135. doi: 10.1152/ajpregu.00377.2007. [DOI] [PubMed] [Google Scholar]
- Tajino K, Hosokawa H, Maegawa S, Matsumura K, Dhaka A, Kobayashi S. Cooling-sensitive TRPM8 is thermostat of skin temperature against cooling. PLoS One. 2011;6:e17504. doi: 10.1371/journal.pone.0017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci: Off J Soc Neurosci. 2007;27:14147–14157. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo NA, Bo Y, Gore V, Ma V, Nishimura N, Tang P, Deng H, Klionsky L, Lehto SG, Wang W, Youngblood B, Chen J, Correll TL, Bartberger MD, Gavva NR, Norman MH. Fused piperidines as a novel class of potent and orally available transient receptor potential melastatin type 8 (TRPM8) antagonists. J Med Chem. 2012;55:1593–1611. doi: 10.1021/jm2013634. [DOI] [PubMed] [Google Scholar]
- Werkheiser JL, Rawls SM, Cowan A. Mu and kappa opioid receptor agonists antagonize icilin-induced wet-dog shaking in rats. Eur J Pharmacol. 2006;547:101–105. doi: 10.1016/j.ejphar.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci: Off J Soc Neurosci. 2007;27:13680–13690. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B, Hever G, Zhang M, Sun H, Willee A, Rubino J, Zhu D, Deng H, Wang T, Magal E, Lee D, Manning B, Wild K, Lehto SG. Validation of a novel, automated assay of inflammation-induced allodynia in rats. Soc Neurosci Abstracts. 2008;468:13. [Google Scholar]
- Zimmermann K, Lennerz JK, Hein A, Link AS, Kaczmarek JS, Delling M, Uysal S, Pfeifer JD, Riccio A, Clapham DE. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc Natl Acad Sci U S A. 2011;108:18114–18119. doi: 10.1073/pnas.1115387108. [DOI] [PMC free article] [PubMed] [Google Scholar]