ABSTRACT
Crumbs proteins are important regulators of epithelial polarity. In C. elegans, no essential role for the two described Crumbs homologs has been uncovered. Here, we identify and characterize an additional Crumbs family member in C. elegans, which we termed CRB-3 based on its similarity in size and sequence to mammalian CRB3. We visualized CRB-3 subcellular localization by expressing a translational GFP fusion. CRB-3::GFP was expressed in several polarized tissues in the embryo and larval stages, and showed apical localization in the intestine and pharynx. To identify the function of the Crumbs family in C. elegans development, we generated a triple Crumbs deletion mutant by sequentially removing the entire coding sequence for each crumbs homolog using a CRISPR/Cas9-based approach. Remarkably, animals lacking all three Crumbs homologs are viable and show normal epithelial polarity. Thus, the three C. elegans Crumbs family members do not appear to play an essential role in epithelial polarity establishment.
Keywords: C. elegans, cell polarity, Crumbs, CRB
INTRODUCTION
Cell polarity is of vital importance for the proper development and functioning of epithelial tissues. Epithelial cells are polarized into distinct apical and basolateral plasma membrane domains, separated by the apical junctional complex (AJC). Studies in Caenorhabditis elegans and Drosophila melanogaster identified three evolutionarily conserved groups of proteins that control the establishment and maintenance of apical and basolateral membrane domains (St Johnston and Ahringer, 2010). Members of the Scribble group (SCRIB/DLG/LGL) localize to the basolateral side and promote basolateral identity, while the apically localized PAR (PAR-3/PAR-6/aPKC) and Crumbs (CRB/PALS1/LIN-7/PATJ) complexes define apical identity.
The Crumbs protein was originally identified in Drosophila, where it plays an important role in the establishment of epithelial polarity, specification of apical membrane identity, and the formation of adherens junctions (AJs) (Tepass, 2012). In addition, Crumbs may contribute to the control of tissue growth by regulating the Hippo and Notch signaling pathways (Chen et al., 2010; Grusche et al., 2010; Grzeschik et al., 2010). Crumbs is a transmembrane protein with a large extracellular domain, and a short intracellular domain. Interestingly, the intracellular domain appears to mediate much of the functioning of Crumbs, as expression of only the intracellular domain coupled to a transmembrane domain is sufficient to rescue most of the phenotypes observed in crumbs mutant flies (Klebes and Knust, 2000; Wodarz et al., 1995). The intracellular domain contains a band 4.1 protein/Ezrin/Radixin/Moesin (FERM)-domain binding site and a C-terminal PSD-95/Discs large/ZO-1 (PDZ)-domain binding motif (Klebes and Knust, 2000). The PDZ-domain binding motif mediates binding to the Crumbs complex component Stardust/PALS1, as well as to Par6, and is essential for the establishment of cell polarity (Bulgakova and Knust, 2009; Klose et al., 2013; Morais-de-Sá et al., 2010; Walther and Pichaud, 2010). The FERM-domain binding motif mediates interactions with several FERM domain proteins, including Yurt, a negative regulator of Crumbs (Laprise et al., 2006), Expanded, an upstream component in the Hippo pathway (Ling et al., 2010; Robinson et al., 2010), the cytoskeletal protein βH-spectrin (Médina et al., 2002), and the cytoskeletal linker protein Moesin (Médina et al., 2002). The FERM-domain binding motif however is dispensable for polarity establishment in Drosophila embryonic epithelia (Klose et al., 2013).
Mammals have three Crumbs family members (CRB1-3) which all contain the conserved intracellular domain containing the FERM- and PDZ-domain binding motifs. However, CRB3 lacks the large extracellular domain present in the other family members and Drosophila Crumbs. Expression of CRB1 in human and mice is limited to the retina and parts of the brain (den Hollander et al., 1999; den Hollander et al., 2002; van Rossum et al., 2006). Mutations in human CRB1 cause retinitis pigmentosa (RP), while Crb1 knockout mice show more limited retinal defects (den Hollander et al., 1999; van de Pavert et al., 2004). CRB2 is expressed in the retina and kidney, while mouse Crb2 is also broadly expressed during early embryonic development (van den Hurk et al., 2005; Xiao et al., 2011). Mice lacking Crb2 die during gastrulation, likely due to disrupted polarity of epiblast cells, and conditional knockout of Crb2 in the retina causes defects similar to RP (Alves et al., 2013; van de Pavert et al., 2004; Xiao et al., 2011). CRB3 is broadly expressed in embryonic and adult epithelial tissues (Lemmers et al., 2004; Makarova et al., 2003; Yin et al., 2014). Knockdown and overexpression studies of CRB3 in MDCK cells, frog blastomeres, and human mammary cells indicate an important role for CRB3 in epithelial polarity establishment and junction formation (Chalmers et al., 2005; Lemmers et al., 2004; Roh et al., 2003; Schlüter et al., 2009; Whiteman et al., 2014). Crb3 knockout mice die shortly after birth from epithelial defects, such as cystic kidneys and abnormal intestine with apical membrane blebs and disrupted microvilli (Whiteman et al., 2014).
In C. elegans, two Crumbs family members have been described: CRB-1 and EAT-20. CRB-1 localizes to the apical domain of intestinal and pharyngeal cells, starting in embryonic development (Bossinger et al., 2001; Segbert et al., 2004). In the embryonic intestine, CRB-1 localizes just apical of the junctional protein DLG-1 (Segbert et al., 2004). Loss of crb-1 does not cause overt defects in polarity. However, an indication for a more subtle role in cell polarity for CRB-1 comes from studies examining the roles of the C. elegans Scribble homolog LET-413 and the C. elegans α-catenin homolog HMP-1 in positioning of DLG-1. Depletion of LET-413 results in disrupted positioning of DLG-1, while DLG-1 localization appears normal in let-413 hmp-1 double knock down embryos and in crb-1 knock down animals. Triple let-413 hmp-1 crb-1 RNAi leads to a similar phenotype as let-413 RNAi (Segbert et al., 2004). These results indicate a role for CRB-1 as a redundant mechanism for the correct positioning of DLG-1. EAT-20 is expressed in the pharynx, intestine, seam cells, a subset of neurons, and hypodermal cells (Achilleos et al., 2010; Shibata et al., 2000). A presumed null mutant of eat-20 has a mild phenotype due to reduced pharyngeal pumping. The mutant worms have a starved appearance, a smaller brood size, and a prolonged egg-laying period (Shibata et al., 2000). crb-1 and/or eat-20 RNAi embryos develop normal epithelial identity (Bossinger et al., 2001; Segbert et al., 2004). Thus, although CRB-1 and EAT-20 localize apically, no essential role in polarity regulation has been uncovered for the Crumbs complex in C. elegans.
Here, we identify a third C. elegans Crumbs homolog, which is highly similar to mammalian CRB3 in size and domain structure. We show that this homolog of Crumbs is expressed in several polarized tissues in the embryo and larval stages and that the protein localizes apically in the intestine and pharynx. We used CRISPR/Cas9 technology to target all three Crumbs homologs for deletion, which did not result in apparent disruption of epithelial polarity. These results show that C. elegans contains an expanded Crumbs family consisting of three homologs, as is the case in mammals. However, the Crumbs complex does not appear to play an essential role in the establishment of epithelial polarity in C. elegans, and may instead contribute a more subtle or redundant function.
MATERIALS AND METHODS
Culture conditions and strains
C. elegans strains were maintained under standard culture conditions as previously described (Brenner, 1974). The wild-type strain used was Bristol N2. Unless otherwise indicated, strains were maintained at 15°C. The following strains were used: ST6: eat-20(nc4)X, BOX41: mibIs23[lgl-1::GFP-Avi, Pmyo-3::mCherry]V, BOX42: mibIs24[crb-3::GFP-Avi, Pmyo-3::mCherry]IV, BOX56: mibIs31[dlg-1::GFP-Avi, Pmyo-3::mCherry]V, BOX66: mibIs41[crb-3::GFP-Avi, Pmyo-3::mCherry]III, BOX51: mibIs26[par-3::GFP-Avi, Pmyo-3::mCherry]V, BOX142: crb-1(mib3) eat-20(mib5) crb-3(mib4)X, BOX143: crb-3(tm6075)X, BOX144: mibIs31[dlg-1::GFP-Avi, Pmyo-3::mCherry]V; crb-1(mib3) eat-20(mib5) crb-3(mib4)X, BOX145: mibIs23[lgl-1::GFP-Avi, Pmyo-3::mCherry]V; crb-1(mib3) eat-20(mib5) crb-3(mib4)X, BOX146: mibIs26[par-3::GFP-Avi, Pmyo-3::mCherry]V; crb-1(mib3) eat-20(mib5) crb-3(mib4)X
Protein domain prediction and homology searches
To predict protein domains, we used the InterPro online prediction tool (http://www.ebi.ac.uk/interpro/) (Hunter et al., 2012). BLAST searches were performed through NCBI (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi) and iterative HMMER searches were performed using the jackhammer online interface at Janelia Farms (http://hmmer.janelia.org/search/jackhmmer).
Phylogenetic analysis
First, we identified candidate Crumbs proteins through an iterative HMMER search against the UniProtKB database. As the extracellular domain is highly variable in length and contains multiple EGF-like domains, which are present in a large number of proteins, we used the conserved transmembrane and intracellular domains for our search. The Human CRB1 C-terminal 73 amino acids were used as the starting sequence. From a list of homologous sequences obtained after 3 iterations of the search, we removed duplicate sequences. Next, the sequences were aligned using the online version of MAFFT with default settings (http://mafft.cbrc.jp/alignment/server/) (Katoh and Standley, 2013). From the aligned sequences, a phylogenetic tree was produced using the online version of PhyML with default settings (http://atgc.lirmm.fr/phyml/) (Guindon et al., 2010). Finally, the online Interactive Tree of Life tool was used to visualize the phylogenetic tree (http://itol.embl.de/) (Letunic and Bork, 2011). We rooted the resulting gene tree such that it minimizes the number of gene duplication events.
Generation of GFP fusion constructs
To generate the GFP fusion constructs, we used the recombineering procedure previously described (Tursun et al., 2009). The sequences inserted by recombineering consist of C. elegans codon-optimized GFP containing FRT flanked GalK, derived from vector pBALU1 (Tursun et al., 2009), to which we added an Avi-tag (de Boer et al., 2003; Schatz, 1993) for future purification efforts. The GFP-Avi tag was inserted at the 3′ ends of the predicted genes. For crb-3, we amplified GFP-Avi using primers crb-3-F (5′-ACGCAAAAGACCTACCATATCTTCAACCTCCGAATGTAGAAGGACTTATCGGAGGGATCTGAGGAGGATCTGGAGGAGGA-3′) and crb-3-R (5′-CACATATAAAAGCGCCCAATTTGATTGAAATGAATAATAAAAATATTTTATTCATGCCATTCAATCTTCTGAGCTTCG-3′), for dlg-1 we used primers dlg-1-F (5′-ACTCCATCATCAGCCGTGAATCGCAGACGCCAATTTGGGTGCCACGTCATGGAGGAGGATCTGGAGGAGGAGGATCTGGAGGAGGA-3′) and dlg-1-R (5′-ACATATTTCTTGAAGAAACGATTATTTGTCTAAAAAATATCCAATTTCATCTATTCATGCCATTCAATCTTCTGAGCTTCG-3′), for lgl-1 we used primers lgl-1-F (5′- GAAGTACGGTGAATTTGAACTTTCGCGGTTGGAGCAGTACGCACAAGTCAGGAGGAGGATCTGGAGGAGGAGGATCTGGAGGAGGA-3′) and lgl-1-R (5′-AAAATTAATATATATCAACAGGAAAACGATTTTTAAAAAAAATGCATCTATTCATGCCATTCAATCTTCTGAGCTTCG-3′, and for par-3 we used primers par-3-F (5′- GCCAATACCGTCGCAGAGATCAGGGACCGCCTCATCGTTTTCCCCAGTACGGAGGAGGATCTGGAGGAGGAGGATCTGGAGGAGGA-3′) and par-3-R (5′-GATTCCGTATTTTTCGCGGCTGCGTAATATAACTTTGAGAAAAAACTGACCTATTCATGCCATTCAATCTTCTGAGCTTCG-3′). Fosmids used were WRM0628dH07 carrying crb-3, WRM067dB05 carrying dlg-1, WRM065bB11 carrying lgl-1, and WRM064bG02 carrying par-3. All PCR amplifications were done with KOD hot start polymerase (Manufactured by Toyobo and distributed by Merck Millipore, Billerica, MA, USA) according to manufacturer's instructions, and an annealing temperature of 70°C. As an example of the final sequence, supplementary material Fig. S1 shows the final crb-3::GFP-Avi coding sequence.
Generation of transgenic lines
Plasmid injections were performed using standard C. elegans injection procedures (Berkowitz et al., 2008). For γ–irradiation mediated integration, 100–150 late L4 stage animals carrying an extrachromosomal array transmitting at a rate of 20–60% were placed on a 6 cm NGM agar plate seeded with E. coli strain OP50. Next, a Cesium-137 source was used to deliver a dose of 4000 Gy of radiation. Following irradiation, animals were transferred to 9 cm NGM plates seeded with OP50, 10 animals per plate. Plates were allowed to starve for 7 days at 20°C. From each starved plate a large chunk (1/4 plate) was placed on a fresh seeded 9 cm NGM plate. After 1–3 days, 20 animals were transferred from each plate to individual 6 cm seeded NGM plates (200 animals total). After 4–5 days incubation at 20°C, plates were examined for 100% transmission rate. Integrated lines were backcrossed with N2 at least twice.
CRISPR/Cas9
To generate deletion alleles of crb-1, eat-20, and crb-3, we simultaneously targeted a site near the start codon and a site near the stop codon of each gene with CRISPR/Cas9. To clone the sequences of the target sites into the sgRNA expression vector, we first annealed pairs of oligonucleotides crb-1_CRISPR_1_F (5′-AATTGACAATACACCTGGCTCTCT-3′) with crb-1_CRISPR_1_R (5′-AAACAGAGAGCCAGGTGTATTGTC-3′), crb-1_CRISPR_2_F (5′-AATTGAGAAAAGACACAGATGAAC-3′) with crb-1_CRISPR_2_R (5′-AAACGTTCATCTGTGTCTTTTCTC-3′), eat-20_CRISPR_1_F (5′-AATTGACAAAACTCCACTGAGAAA-3′) with eat-20_CRISPR_1_R (5′-AAACTTTCTCAGTGGAGTTTTGTC-3′), eat-20_CRISPR_2_F (5′-AATTGCTCGTGTACTCCCAAGTGA-3′) with eat-20_CRISPR_2_R (5′-AAACTCACTTGGGAGTACACGAGC-3′), crb-3_CRISPR_1_F (5′-AATTGAAAATGGCGTCAAACAGTA-3′) with crb-3_CRISPR_1_R (5′-AAACTACTGTTTGACGCCATTTTC-3′), and crb-3_CRISPR_2_F (5′-AATTGAATTAGTCTCGCTTTGCCT-3′) with crb-3_CRISPR_2_R (5′-AAACAGGCAAAGCGAGACTAATTC-3′). The resulting linkers were ligated into the BsaI digested U6::sgRNA expression vector pMB70 (Waaijers et al., 2013). For each deletion, we injected 30 animals with a mixture containing 5 ng/µl Pmyo-3::mCherry (pCFJ104, Addgene #19328), 50 ng/µl of each of the two sgRNAs and 50 ng/µl Phsp-16.48::Cas9 using standard C. elegans microinjection procedures. To induce expression from the hsp-16.48 promoter, injected animals were heat shocked for 1 h at 34°C on agar plates floating in a water bath, 30 min after injection. From transgenic F1 animals expressing mCherry, we PCR amplified a region surrounding the target site using primers crb-1_CRISPR_check_F (5′-GTCGCTTGTTATGGGATAAAAC-3′) and crb-1_CRISPR_check_R (5′-GGTACCAGTGACAACATTTGCT-3′) for crb-1, eat-20_CRISPR_check_F (5′-GTGTGACCAAACTTATTGCTTTC-3′) and eat-20_CRISPR_check_R (5′-GCTCTCCAAGTCAAAAAGTTCTTA-3′) for eat-20, and crb-3_CRISPR_check_F (5′-GGAGACGGAGATGGTCAAGT-3′) and crb-3_CRISPR_check_R (5′-ACGTGTAGTACTCGGTGTTCAGG-3′) for crb-3. We established homozygous mutant lines by isolating single F2 animals and determining their genotype by PCR and sequence analysis. In addition to sequence analysis of each deletion, we verified that the deleted sequences had not inserted elsewhere in the genome using multiple sets of internal primers for each deletion (supplementary material Fig. S2). Internal primer sets used were crb-1_CF1 (5′-TTGCAGCCCATCTCTTCTTT-3′) and crb-1_CR1 (5′-CACTGAAACCCTTCGGACAT-3′), crb-1_CF2 (5′-GAGCGTCGAATGTTGTAGCA-3′) and crb-1_CR2 (5′-TTGCAGGTGCTAGAAGAGCA-3′), crb-1_CF3 (5′-GATTGAGAAAAACCGCGAAG-3′) and crb-1_CR3 (5′-ACACGATGACAACCGCAATA-3′), eat-20_CF1 (5′-GACCCCTCGGTTCTAGGAAG-3′) and eat-20_CR1 (5′-GGTGAAACCCTGACGACACT-3′), eat-20_CF2 (5′-GCCCAACACCAATGGTTATC-3′) and eat-20_CR2 (5′-CCACTCGAGGTGTGATGATG-3′), crb-3_CF1 (5′-GCATGGTTACTGAAGCGACA-3′) and crb-3_CR1 (5′-AACGTTTTCCCAGTTCCGTA-3′), crb-3_CF2 (5′-ATTACGGAACTGGGAAAACG-3′) and crb-3_CR2 (5′-GGTCTTTTGCGTGATGTTGTT-3′).
Progeny counting and scoring of embryonic lethality
Starting at the L4 stage, individual animals were cultured at 20°C and transferred to a fresh plate every 24 h. Hatched and unhatched progeny were counted 24 h after removal of the P0.
Embryo staining with MH27
For antibody staining, embryos were released from gravid adult animals by bleaching, and allowed to develop for 6 h at 20°C in M9 (0.22 M KH2PO4, 0.42 M Na2HPO4, 0.85 M NaCl, 0.001 M MgSO4). Embryos were then washed once in water, and a 10 µl drop of embryos was placed on a slide coated with poly-L-Lysine (Sigma-Aldrich Corp., St. Louis, MO, USA). Embryos were permeabilized by freeze-cracking, and fixed with methanol (5 minutes at −20°C) and acetone (20 minutes at −20°C). Embryos were stained on-slide as described (Duerr, 2006). Antibodies used were anti-AJM-1 MH27 mouse monoclonal supernatant (Developmental Studies Hybridoma Bank, Iowa City, IA, USA) diluted 1:20, and Alexa-Fluor 488 goat-anti-mouse (Life Technologies Europe, Bleiswijk, The Netherlands) diluted 1:500. Worms were mounted in Prolong Anti-Fade Gold (Life Technologies Europe, Bleiswijk, The Netherlands) supplemented with 2 µg/ml 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich Corp., St. Louis, MO, USA).
Microscopy and image processing
Microscopy of living animals was performed on a spinning disc platform consisting of a Nikon Ti-U inverted microscope with a motorized XY stage and a Piezo Z stage, 60× and 100× PLAN APO 1.4 NA oil objectives, a Yokogawa CSU-X1 spinning disk unit equipped with a dual dichroic mirror set for laser wavelengths 488 nm and 561 nm, 488 nm and 561 nm solid state 50 mW lasers controlled by an Andor revolution 500 series AOTF Laser modulator and combiner, Semrock 512/23 + 630/91 dual band pass emission filter, Semrock 525/30 single band pass emission filter, Semrock 617/73 single band pass filter, Semrock 4800 long pass filter (500–1200 pass), and an Andor iXON DU-885 monochrome EMCCD+ camera. All components are controlled by MetaMorph Microscopy Automation & Image Analysis Software. Microscopy of fixed samples was performed on a Zeiss LSM700 laser scanning confocal microscope equipped with a 63× Plan-Apochromat 1.4 NA objective, 405 nm, 488 nm, 555 nm, and 633 nm lasers, and the following emission filters: SP490 (400–490 nm), SP555 (400–555 nm), SP640 (400–640 nm), BP490-555 (490–555 nm), LP560 (560–750 nm), LP640 (640–750 nm) and BP592-662 (592–662 nm). The LSM700 is controlled by the Zen software package. All Z-stacks were taken with an 0.5 µm spacing, and maximum projections were generated with ImageJ. Final figures were produced using Adobe Photoshop CS6 and Adobe Illustrator CS6.
RESULTS
Identification of a candidate C. elegans CRB3 homolog
Thus far two Crumbs homologs have been described in C. elegans: crb-1 and eat-20. Both consist of a long extracellular region, a transmembrane (TM) domain, and a short intracellular region. CRB-1 is most similar in size and protein domain composition to Drosophila Crumbs (Fig. 1). CRB-1 consists of 1722 amino acids and contains 26 EGF repeats and two Laminin G-like domains in its extracellular region. The EAT-20 protein is 808 amino acids long and comprises three EGF repeats in its extracellular region. In both CRB-1 and EAT-20, the essential residues of the FERM-binding motif and the PDZ-domain binding motif are conserved in the intracellular region (Klebes and Knust, 2000; Klose et al., 2013). To identify potential additional Crumbs homologs, we searched the predicted C. elegans proteome for candidate homologs of Crumbs proteins by BLAST and HMMER. Searches with the human CRB3 sequence or intracellular domain of Drosophila Crumbs yielded a third significant hit, C35B8.4, in addition to CRB-1 and EAT-20. Large-scale expression profiling experiments indicate that the C35B8.4 gene is expressed (Levin et al., 2012; Spencer et al., 2011). The predicted protein encoded by C35B8.4 is 100 amino acids long, similar in length to mammalian CRB3, and consists of a short extracellular tail without recognizable domains, followed by a transmembrane domain and an intracellular part. The essential residues of the FERM-domain binding site and most residues of the PDZ-domain binding motif in the intracellular part are conserved (Fig. 1). A tyrosine at position 10 and a glutamic acid at position 16 of the intracellular part are part of the FERM-domain binding site and were shown to be essential for rescuing crumbs null phenotypes in Drosophila (Klebes and Knust, 2000; Klose et al., 2013). Both of these essential residues are conserved in C. elegans C35B8.4. The final four amino acids of CRB-3 are EGLI, and hence differ from the canonical ERLI PDZ-domain binding motif present in most Crumbs proteins. However, an alternative splice variant of human CRB3 also contains an alternative C-terminus (CLPI) and was shown to function in spindle assembly, cilia formation, and cell division. This alternative splice variant binds to importin β-1, unlike the ERLI isoform (Fan et al., 2007). Thus, the final four amino acids of C35B8.4 could potentially have a different binding specificity. In contrast to CRB-1, EAT-20, and the mammalian Crumbs proteins, C35B8.4 lacks a predicted N-terminal signal peptide. The transmembrane domain presumably acts as an internal ER signal sequence, and the positive charge of the residues following the transmembrane domain is consistent with a cytosolic C-terminus (Hartmann et al., 1989; Sipos and von Heijne, 1993). Phylogenetic analysis indicated that C35B8.4 is more similar to mammalian Crumbs3 proteins than to Crumbs1 or Crumbs2 proteins (supplementary material Fig. S3). Based on the similarity of C35B8.4 to human CRB3 and the apical localization of the protein described below, we assigned C35B8.4 the name crb-3.
Fig. 1. Homology between Drosophila, human, and C. elegans Crumbs proteins.
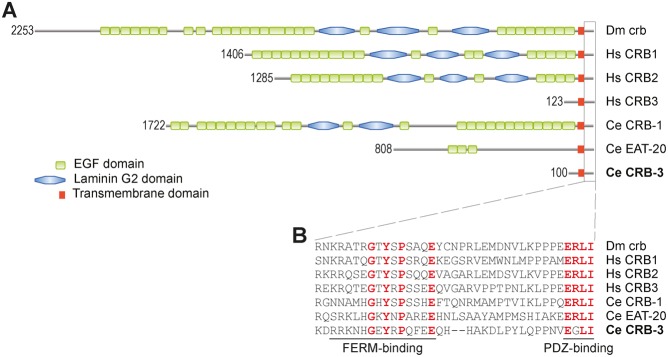
(A) Protein domain structure of the Crumbs proteins. The number in front of the protein corresponds to the length of the protein in amino acids. (B) Intracellular part with conserved residues of the FERM-domain binding site and the PDZ-domain binding site depicted in red. The tyrosine and glutamic acid of the FERM-domain binding site were shown to be essential for Drosophila Crumbs (Klebes and Knust, 2000; Klose et al., 2013). Dm = Drosophila melanogaster, Hs = Homo sapiens, Ce = Caenorhabditis elegans.
CRB-3 localizes apically in multiple polarized tissues
To determine a potential role for CRB-3 in establishing epithelial polarity, we first determined its expression pattern and subcellular localization. If CRB-3 acts as a regulator of epithelial polarity similar to Crumbs proteins in other organisms, we expect localization at the apical membrane domain of epithelial cells. To visualize the expression and localization pattern of CRB-3, we generated a translational CRB-3::GFP fusion. We made use of fosmid-based recombineering, to mimic the endogenous expression pattern as closely as possible (Tursun et al., 2009). We inserted the GFP-encoding sequence at the predicted 3′ end of the crb-3 gene, and generated transgenic lines carrying an integrated copy of this construct by gamma-irradiation mediated integration of an extrachromosomal array. Two independently integrated strains showed the same expression pattern. CRB-3::GFP was first detected in embryonic pharyngeal and intestinal precursor cells (Fig. 2A). Throughout the larval stages the fusion protein localized to the apical membrane domain of pharyngeal cells, to the excretory canal, to the apical membrane domain of intestinal cells, to a circumferential pattern resembling the pattern of commissural axons, to neurons in the dorsal and ventral nerve cords, to the coelomocytes, and in a fraction of animals (n = 4/6) to the apical membrane domain of the rectal epithelium (Fig. 2). During the fourth larval stage, CRB-3::GFP became visible in the uterus (Fig. 2H). No fusion protein was detected in the seam cells or vulval epithelial cells, two tissues in which EAT-20 was shown to be expressed (Shibata et al., 2000) (Fig. 2H,J). The expression of CRB-3 in polarized tissues together with its apical localization in the pharynx and intestine strengthens our hypothesis that CRB-3 is a bona fide Crumbs homolog and third C. elegans Crumbs family member.
Fig. 2. Expression and localization of CRB-3::GFP throughout development.
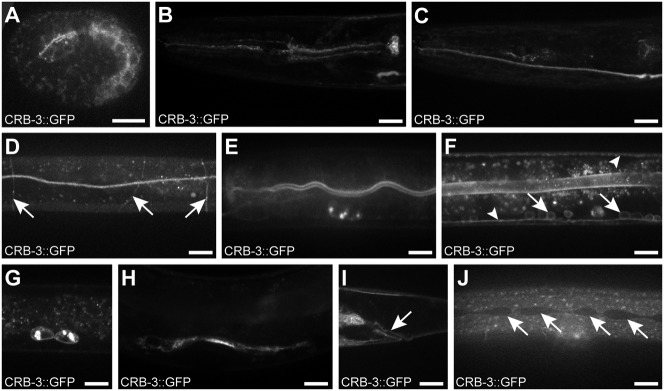
(A) 1.5-fold embryo, (B–G,J) third larval stage, (H,I) fourth larval stage. (B) pharynx, (C) excretory canal, (D) circumferential pattern, indicated by arrows, resembling the pattern of commissural axons, (E) apical localization in the intestine (expression is also visible in a coelomocyte), (F) dorsal cord and ventral nerve cords indicated by arrowheads and cell bodies of the ventral nerve cord motor neurons indicated by arrows, (G) coelomocytes, (H) uterine epithelial cells, (I) rectal epithelium as indicated by the arrow, (J) seam cells indicated by arrows. Panels B and C show the head region of the same animal at different Z heights. Regions in panels D–F, H, and J are located along the middle of the body. Scale bars are 10 µm.
The C. elegans Crumbs family is not essential for epithelial polarity
To investigate the role of crb-3 in polarity establishment, we analyzed a deletion mutant (tm6075) obtained from the National Bioresource Project in Japan. This mutation is predicted to result in a frame shift and premature stop in crb-3. The corresponding protein lacks the transmembrane domain and intracellular region. Animals homozygous for the tm6075 allele appeared healthy, and exhibited no embryonic or larval lethality. Examination of crb-3(tm6075) animals by Nomarski DIC microscopy also revealed no obvious developmental defects (not shown). These results indicate that crb-3 is not essential for the establishment of epithelial polarity.
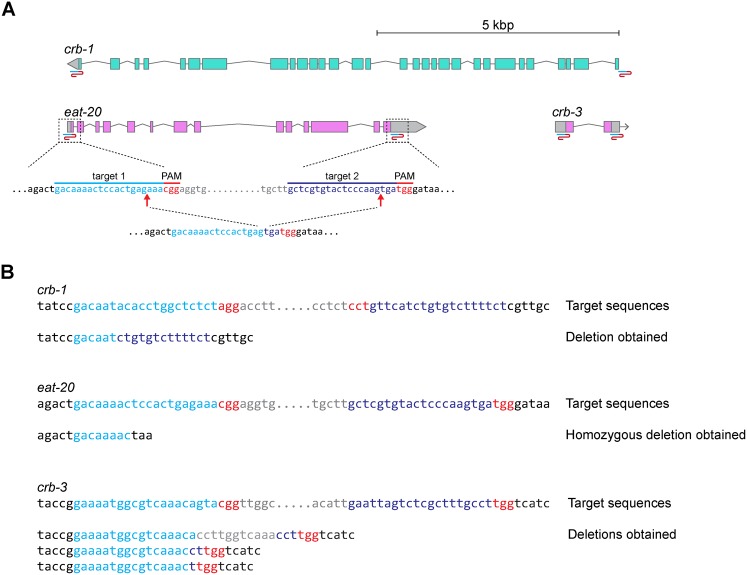
The expression pattern of crb-3 shows extensive overlap with that of crb-1 and eat-20, including expression of all three proteins in the intestine and pharynx of the developing embryo, and expression of at least crb-3 and eat-20 in larval tissues such as the pharynx, anal hypodermis, and coelomocytes (Bossinger et al., 2001; Shibata et al., 2000). One possible explanation for the limited defects we observed in crb-3(tm6075) animals and that were reported for crb-1 and eat-20 (Bossinger et al., 2001; Segbert et al., 2004; Shibata et al., 2000) is that functional redundancy exists between these genes. To investigate this possibility, we generated a strain lacking all three genes. For both eat-20 and crb-1, deletion alleles exist as well. However, neither the eat-20(nc4) nor the crb-1(ok931) allele removes the entire gene coding sequences, and crb-1(ok931) is an in-frame deletion of part of the extracellular domain. Thus, it is possible that residual gene function remains due to e.g. alternative splicing, alternative start codons, or the remaining crb-1 regions. To completely rule out the possibility of residual gene function, we decided to generate a triple knock-out strain in which we removed the entire predicted coding sequence of crb-1, eat-20, and crb-3. We used a CRISPR/Cas9-based approach to delete entire loci (Fig. 3). Previously, we used CRISPR/Cas9 to target a single DSB to specific loci in the genome, which results in the generation of small insertions or deletions due to errors during non-homologous end joining (Waaijers et al., 2013). By using two sgRNAs, one targeting a sequence before the start codon of the gene and the other targeting a sequence after the stop codon, the intervening sequence can be lost during DNA repair. Deletions of genes can easily be detected in the F1 generation by PCR with primers flanking the desired deletion. To generate a triple Crumbs knockout strain, we started from the eat-20(nc4) background, which has already lost part of eat-20. To delete the ∼11 kb crb-1 coding sequence, we injected expression constructs for the two sgRNAs (U6::sgRNA), Cas9 controlled by the heat shock promoter (Phsp-16.48::Cas9) and a co-injection marker (Pmyo-3::mCherry) in the gonad of 30 P0 animals and exposed the injected animals to a 1 h heat shock at 34°C. We screened 89 transgenic F1 worms for deletion of the gene by PCR and obtained one deletion mutant. DNA sequence analysis confirmed the presence of a deletion with boundaries close to the predicted Cas9 cut sites, thus eliminating the entire crb-1 coding region (Fig. 3B). The homozygous eat-20 crb-1 double mutant did not show embryonic or larval lethality. Next, we used this double mutant as a background to delete the crb-3 coding sequences using the same CRISPR/Cas9 approach. Out of 84 transgenic F1 worms, we obtained 3 crb-3 deletion alleles that lacked the entire crb-3 coding region. We sequenced the alleles and confirmed that each was a deletion with boundaries close to the predicted Cas9 cut sites (Fig. 3B). Finally, to ensure that no functional EAT-20 protein is produced, we deleted the remaining eat-20 sequences, with a success rate of 13 deletion mutants out of 32 transgenic worms. We sequenced one allele that appeared to be homozygous in the F1 generation (Fig. 3B). We compared the brood size, egg laying period, and embryonic lethality of the homozygous triple crumbs deletion mutant with that of wild-type N2 animals and eat-20(nc4) animals. We observed no increase in embryonic lethality compared to N2 (supplementary material Fig. S4). As previously reported (Shibata et al., 2000), we observed a reduction in brood size and extension in egg laying period for eat-20(nc4) animals, which was not exacerbated in the crb-1 eat-20 crb-3 triple deletion mutant (supplementary material Fig. S4).
Fig. 3. Generation of crb-1, eat-20, and crb-3 deletions by CRISPR/Cas9.
(A) Gene predictions with sgRNA target sites indicated by blue/red inverted S shape symbol. For eat-20, sequences of the target sites and expected deletion are shown. Genes on the forward strand are in pink, and on the reverse strand in blue. Gray boxes indicate untranslated regions. (B) Sequences of deletions obtained. Light blue = left Cas9 target site, dark blue = right Cas9 target site, red = PAM sequences, gray = sequence between targeted sites.
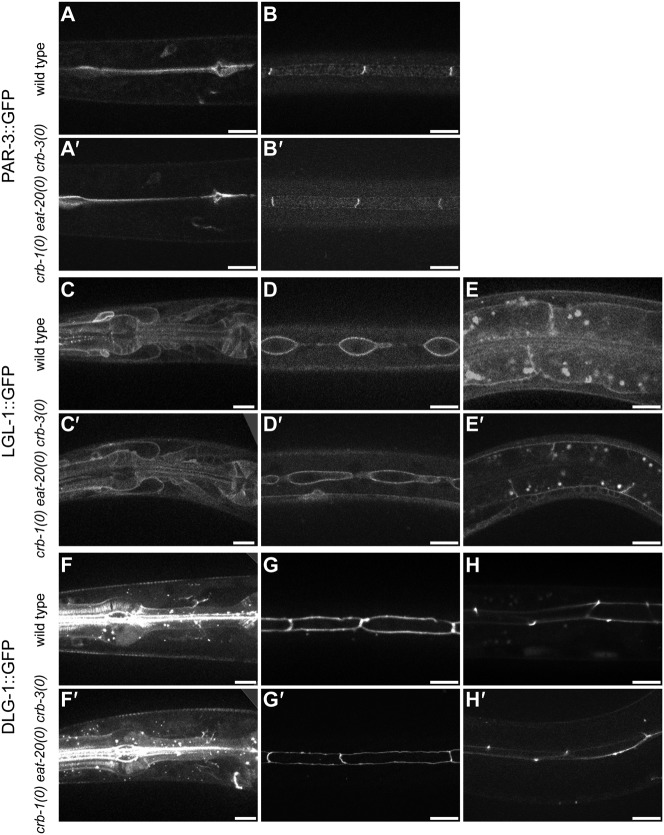
To be able to analyze the effects of simultaneous loss of crb-1, eat-20, and crb-3 on polarity in more detail, we created marker lines that express apically localized PAR-3::GFP, basolaterally localized LGL-1::GFP, or junctionally localized DLG-1::GFP. The expression constructs were generated by fosmid-based recombineering, and integrated into the genome by γ-irradiation. Each of the marker lines was crossed into the crb-1(mib3) eat-20(mib5) crb-3(mib4) triple deletion strain. The resulting strain was subsequently examined for effects of crb-family deletion on localization of polarity proteins in larval epithelia. For LGL-1 and DLG-1, we examined the pharyngeal epithelium, seam cells, and intestine. For PAR-3, which was not expressed in the intestine, we examined the pharyngeal epithelium and seam cells. In all cases, the localization of the GFP-tagged polarity proteins was similar to the wild type localization pattern (Fig. 4). Because of the previously described possible contribution of crb-1 to junction assembly (Segbert et al., 2004), we also examined the formation of apical junctions in the crb-1(mib3) eat-20(mib5) crb-3(mib4) triple deletion mutant strain. To visualize apical junctions, we stained 1.5-fold embryos with the MH27 antibody, which recognizes the junctional component AJM-1. We compared the MH27 staining pattern in the triple mutants to the characteristic pattern of cell junctions in wild-type embryos and, again, observed no abnormalities (Fig. 5, compare A to C and B to D). Taken together, our analysis of a triple crumbs deletion mutant indicates that the function of the three Crumbs family members is not critical for establishment of apical-basal polarity in C. elegans epithelia.
Fig. 4. Expression of apical, basolateral, and junctional markers in wild type animals and the triple crb-1 eat-20 crb-3 deletion strain.
(A–H) wild type, (A′–H′) crb-1(mib3) eat-20(mib5) crb-3(mib4) triple deletion strain. (A,A′) pharyngeal expression of PAR-3::GFP, (B,B′) apical expression of PAR-3::GFP in the seam cells (confocal image taken at level of apical membrane), (C,C′) pharyngeal expression of LGL-1::GFP, (D,D′) basolateral expression of LGL-1::GFP in the seam cells (confocal image taken at level below the cell junctions), (E,E′) basolateral expression of LGL-1::GFP in the intestine, (F,F′) junctional localization of DLG-1::GFP in the pharynx, (G,G′) junctional localization of DLG-1::GFP in the seam cells, (H,H′) junctional localization of DLG-1::GFP in the intestine. Scale bars represent 10 µm.
Fig. 5. Localization of AJM-1 in wild-type and triple crb-1 eat-20 crb-3 deletion embryos.
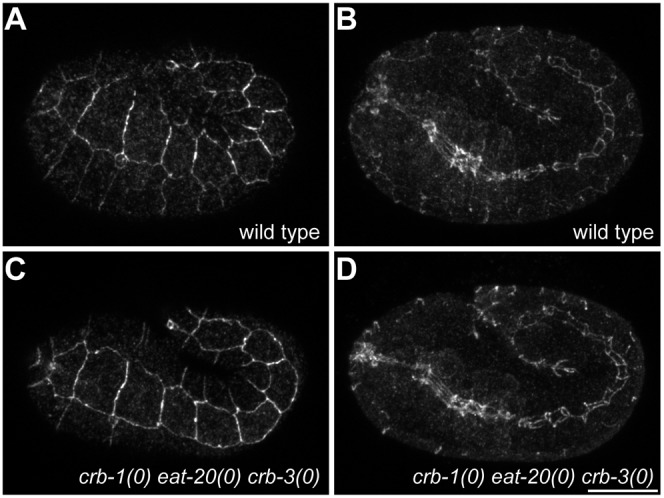
Embryos were fixed and stained with the MH27 antibody, and imaged by confocal microscopy. All images are maximum intensity projections of slices taken 0.5 µm apart. (A,B) Wild-type 1.5-fold embryo. (C,D) crb-1(mib3) eat-20(mib5) crb-3(mib4) 1.5-fold embryo. (A,C) Projections of the outer 3 µm showing junctions between hypodermal cells. (B,D) Projections of the central 8 µm showing AJM-1 localization in the pharynx and intestine. Scale bars represent 10 µm.
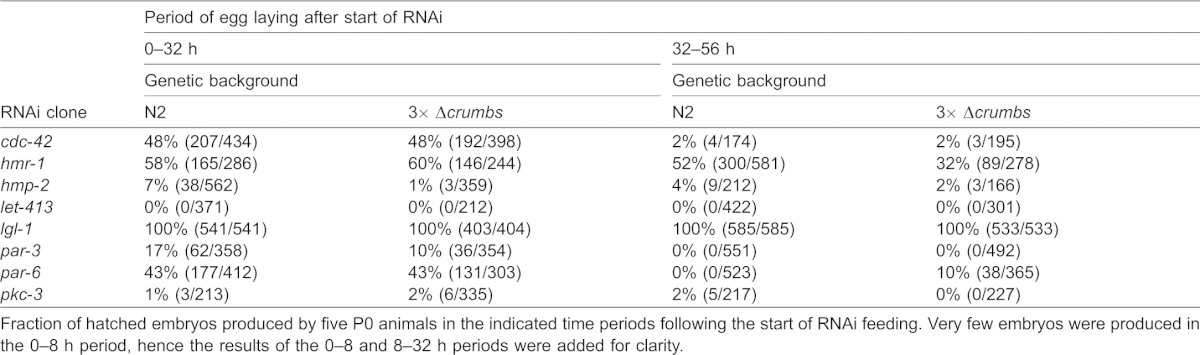
One possibility for the observed lack of phenotype in the triple crumbs deletion strain is that C. elegans Crumbs proteins function redundantly with other polarizing mechanisms. To investigate this possibility, we inactivated cdc-42, par-3, par-6, pkc-3, hmr-1, hmp-2, let-413 or lgl-1 by RNAi in the triple crumbs deletion strain. Feeding RNAi was started at the L4 stage, and we counted the number of hatched and unhatched embryos produced 0–8 h, 8–32 h, and 32–56 h after the start of RNAi. Inactivation of pkc-3 and let-413 resulted in 100% embryonic lethality, precluding observation of any synergistic effect of the triple crumbs deletion (Table 1). RNAi for cdc-42, par-6, hmr-1 resulted in partial lethality, while lgl-1 RNAi did not induce embryonic lethality. Additional inactivation of the C. elegans crumbs family did not result in increased embryonic lethality for any of these genes. Finally, RNAi for par-3 and hmp-2 resulted in a very limited number of hatched progeny produced in the first 32 h after the start of RNAi feeding. For both genes, additional inactivation of the crumbs genes reduced the fraction of hatched progeny produced in this time period. However, the already small number of hatching progeny in the wild-type background makes it difficult to draw a firm conclusion regarding a redundant function of par-3 or hmp-2 with the crumbs genes.
Table 1. Embryonic survival after RNAi in wild type or 3× Δcrumbs background.
DISCUSSION
Here, we identified a third C. elegans Crumbs family member, which we termed CRB-3, based on similarity to mammalian CRB3. Using a translational CRB-3::GFP fusion, we observed expression of C. elegans crb-3 in several polarized tissues in the embryo and in larval stages, with clear apical localization of CRB-3::GFP in the intestine and pharynx. Our results show that the Crumbs family in C. elegans consists of at least three members, similar as in mammals and in contrast to the single Drosophila crumbs gene. We deleted all three C. elegans Crumbs homologs using a CRISPR/Cas9-based approach. Surprisingly, given the importance of the Crumbs family in other organisms, animals that lack all three crumbs homologs were viable and showed no more severe defects than the starved appearance, reduced brood size, and extended egg laying period previously described for eat-20(nc4) (Shibata et al., 2000). Moreover, localization of PAR-3::GFP, LGL-1::GFP, or DLG-1::GFP was unaffected in the triple crumbs homolog deletion strain. These results show that, despite evolutionary conservation, the Crumbs family has no essential role in C. elegans.
One possible explanation for the observed lack of a phenotype is that the C. elegans Crumbs proteins control specific aspects of epithelial cells, rather than critically regulating apical polarity. For example, CRB-1, EAT-20, or CRB-3 may act through FERM-domain containing proteins like ERM-1 or SMA-1, the C. elegans homologs of Moesin and βH-Spectrin respectively, to contribute to the regulation of the actin cytoskeleton in tissues like the intestine or excretory canal. Subtle defects in these tissues would not have been uncovered using the approaches employed here. Alternatively, the C. elegans Crumbs proteins may function redundantly with other polarizing mechanisms. In support of this hypothesis, a previous study observed that CRB-1 can provide a positional cue for the localization of DLG-1 after inactivation of the basolateral regulator let-413 Scribble and the junctional component hmp-1 α-Catenin (Segbert et al., 2004). Thus, LET-413 and the Cadherin/Catenin complex (CCC) are likely candidates for a redundant mechanism. Another possible candidate for acting redundantly with the Crumbs proteins is the apical PAR complex, which is known to act together with Crumbs to establish apical identity in Drosophila (Krahn et al., 2010; Walther and Pichaud, 2010). We investigated potential redundancy by examining if inactivation of PAR or CCC components by RNAi caused enhanced embryonic lethality in the triple crumbs deletion strain. We did not observe a synergistic effect between deletion of the Crumbs family and inactivation of the PAR complex component PAR-6, or the CCC component HMR-1. Similarly, we observed no redundancy between the Crumbs family and CDC-42 or LGL-1. We were not able to extend this analysis to PAR-3, PKC-3, LET-413, or HMR-1, as RNAi for the corresponding genes already causes extensive embryonic lethality in the wild-type background. Thus, it remains possible that the Crumbs family acts redundantly with these, or other polarity regulators.
The composition of the C. elegans Crumbs complex has not been further investigated to date. The core Crumbs complex in Drosophila consist of Crumbs, Stardust (PALS1 in mammals), PATJ, and Lin-7 (Bulgakova and Knust, 2009). Of these, only Crumbs and Stardust are broadly required for epithelial polarity, while PATJ and Lin-7 have more specific functions (Bachmann et al., 2008; Nam and Choi, 2006). The C. elegans genome encodes three candidate Stardust homologs: MAGU-1, MAGU-2, and MAGU-3 (Assémat et al., 2008; Knust and Bossinger, 2002), of which MAGU-2 is most similar to PALS1 and Stardust. The subcellular localization pattern of these proteins has not been determined. The likely null allele magu-2(gk218) is reported to be homozygous viable. No good candidate homolog of PATJ exists in C. elegans. The closest homolog, MPZ-1, resembles both PATJ and MPDZ/MUPP1 in that it contains a high number of PDZ domains. However, MPZ-1 lacks the characteristic L27 domain present in PATJ and Stardust, and functional analysis of MPZ-1 suggests that it is more likely to represent a homolog of MPDZ (Xiao et al., 2006). Finally, LIN-7 was originally identified in C. elegans, where it acts in a complex with LIN-2 and LIN-10 to control the basolateral localization of the EGF receptor LET-23 in vulval epithelial cells (Kaech et al., 1998; Simske et al., 1996). A potential role for LIN-7 as a component of a Crumbs complex has not been investigated.
Together with the PAR and Scribble groups, the Crumbs complex regulates cell polarity in a variety of different epithelial cell types. However, it is clear that the mechanisms through which these evolutionarily conserved proteins establish polarity vary markedly in different cell types or conditions. In Drosophila, not all epithelia in which Crb is expressed require Crb to maintain epithelial polarity (Tepass, 2012). Similarly, even though mouse Crb3 is widely expressed in embryonic tissues, Crb3 knockout mice complete embryogenesis and die shortly after birth (Whiteman et al., 2014). During development of the Drosophila embryo, at least three groups of basolateral regulators function at different times (Tepass, 2012). In the C. elegans embryo, PAR-3 is required for the assembly of cell junctions in intestinal cells, but apical junctions still form in the absence of PAR-3 in epidermal epithelia (Achilleos et al., 2010). It is important therefore to study the functioning of polarity regulators in a range of different systems and organisms. Though it remains unclear what the exact role of the Crumbs proteins is in C. elegans, our identification of a Crumbs3 homolog provides further insight into the C. elegans Crumbs family.
Supplementary Material
Acknowledgments
We thank Sander van den Heuvel, Adri Thomas, and Suzan Ruijtenberg for critically reading this manuscript, Berend Snel for assistance with the phylogenetic analysis, and Olaf Bossinger for helpful discussions on the role of Crumbs proteins in C. elegans, and the sharing of unpublished data.
Footnotes
Author Contributions: M.B. and S.W. conceived and designed the experiments. S.W., J.J.R., T.K., and E.K. performed the experiments. M.B., S.W., J.J.R., T.K., and E.K. analyzed the data. M.B. and S.W. wrote the paper.
Competing interests: The authors have no competing or financial interests to declare.
Funding
Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). The crb-3(tm6075) mutant allele was provided by the MITANI Lab through the National BioResource Project of the MEXT, Japan. This work is supported by Innovational Research Incentives Scheme Vidi grant 864.09.008, financed by the Netherlands Organization for Scientific Research (NWO/ALW).
References
- Achilleos A., Wehman A. M., Nance J. (2010). PAR-3 mediates the initial clustering and apical localization of junction and polarity proteins during C. elegans intestinal epithelial cell polarization. Development 137, 1833–1842 10.1242/dev.047647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves C. H., Sanz A. S., Park B., Pellissier L. P., Tanimoto N., Beck S. C., Huber G., Murtaza M., Richard F., Sridevi Gurubaran I. et al. (2013). Loss of CRB2 in the mouse retina mimics human retinitis pigmentosa due to mutations in the CRB1 gene. Hum. Mol. Genet. 22, 35–50 10.1093/hmg/dds398 [DOI] [PubMed] [Google Scholar]
- Assémat E., Bazellières E., Pallesi-Pocachard E., Le Bivic A., Massey-Harroche D. (2008). Polarity complex proteins. Biochim. Biophys. Acta 1778, 614–630 10.1016/j.bbamem.2007.08.029 [DOI] [PubMed] [Google Scholar]
- Bachmann A., Grawe F., Johnson K., Knust E. (2008). Drosophila Lin-7 is a component of the Crumbs complex in epithelia and photoreceptor cells and prevents light-induced retinal degeneration. Eur. J. Cell Biol. 87, 123–136 10.1016/j.ejcb.2007.11.002 [DOI] [PubMed] [Google Scholar]
- Berkowitz L. A., Knight A. L., Caldwell G. A., Caldwell K. A. (2008). Generation of stable transgenic C. elegans using microinjection. J. Vis. Exp. 2008, e833 10.3791/833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossinger O., Klebes A., Segbert C., Theres C., Knust E. (2001). Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev. Biol. 230, 29–42 10.1006/dbio.2000.0113 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgakova N. A., Knust E. (2009). The Crumbs complex: from epithelial-cell polarity to retinal degeneration. J. Cell Sci. 122, 2587–2596 10.1242/jcs.023648 [DOI] [PubMed] [Google Scholar]
- Chalmers A. D., Pambos M., Mason J., Lang S., Wylie C., Papalopulu N. (2005). aPKC, Crumbs3 and Lgl2 control apicobasal polarity in early vertebrate development. Development 132, 977–986 10.1242/dev.01645 [DOI] [PubMed] [Google Scholar]
- Chen C.-L., Gajewski K. M., Hamaratoglu F., Bossuyt W., Sansores-Garcia L., Tao C., Halder G. (2010). The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc. Natl. Acad. Sci. USA 107, 15810–15815 10.1073/pnas.1004060107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer E., Rodriguez P., Bonte E., Krijgsveld J., Katsantoni E., Heck A., Grosveld F., Strouboulis J. (2003). Efficient biotinylation and single-step purification of tagged transcription factors in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. USA 100, 7480–7485 10.1073/pnas.1332608100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander A. I., ten Brink J. B., de Kok Y. J., van Soest S., van den Born L. I., van Driel M. A., van de Pol D. J., Payne A. M., Bhattacharya S. S., Kellner U. et al. (1999). Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat. Genet. 23, 217–221 10.1038/13848 [DOI] [PubMed] [Google Scholar]
- den Hollander A. I., Ghiani M., de Kok Y. J. M., Wijnholds J., Ballabio A., Cremers F. P. M., Broccoli V. (2002). Isolation of Crb1, a mouse homologue of Drosophila crumbs, and analysis of its expression pattern in eye and brain. Mech. Dev. 110, 203–207 10.1016/S0925-4773(01)00568-8 [DOI] [PubMed] [Google Scholar]
- Duerr J. S. (2006). Immunohistochemistry. WormBook: The Online Review of C. Elegans Biology Pasadena, CA: WormBook, Available at: http://www.ncbi.nlm.nih.gov/books/NBK19743/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S., Fogg V., Wang Q., Chen X.-W., Liu C.-J., Margolis B. (2007). A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin beta interactions. J. Cell Biol. 178, 387–398 10.1083/jcb.200609096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusche F. A., Richardson H. E., Harvey K. F. (2010). Upstream regulation of the hippo size control pathway. Curr. Biol. 20, R574–R582 10.1016/j.cub.2010.05.023 [DOI] [PubMed] [Google Scholar]
- Grzeschik N. A., Parsons L. M., Allott M. L., Harvey K. F., Richardson H. E. (2010). Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr. Biol. 20, 573–581 10.1016/j.cub.2010.01.055 [DOI] [PubMed] [Google Scholar]
- Guindon S., Dufayard J.-F., Lefort V., Anisimova M., Hordijk W., Gascuel O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. (1989). Predicting the orientation of eukaryotic membrane-spanning proteins. Proc. Natl. Acad. Sci. USA 86, 5786–5790 10.1073/pnas.86.15.5786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S., Jones P., Mitchell A., Apweiler R., Attwood T. K., Bateman A., Bernard T., Binns D., Bork P., Burge S. et al. (2012). InterPro in 2011: new developments in the family and domain prediction database. Nucleic Acids Res. 40, D306–D312 10.1093/nar/gkr948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S. M., Whitfield C. W., Kim S. K. (1998). The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell 94, 761–771 10.1016/S0092-8674(00)81735-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebes A., Knust E. (2000). A conserved motif in Crumbs is required for E-cadherin localisation and zonula adherens formation in Drosophila. Curr. Biol. 10, 76–85 10.1016/S0960-9822(99)00277-8 [DOI] [PubMed] [Google Scholar]
- Klose S., Flores-Benitez D., Riedel F., Knust E. (2013). Fosmid-based structure-function analysis reveals functionally distinct domains in the cytoplasmic domain of Drosophila crumbs. G3 (Bethesda) 3, 153–165 10.1534/g3.112.005074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E., Bossinger O. (2002). Composition and formation of intercellular junctions in epithelial cells. Science 298, 1955–1959 10.1126/science.1072161 [DOI] [PubMed] [Google Scholar]
- Krahn M. P., Bückers J., Kastrup L., Wodarz A. (2010). Formation of a Bazooka-Stardust complex is essential for plasma membrane polarity in epithelia. J. Cell Biol. 190, 751–760 10.1083/jcb.201006029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise P., Beronja S., Silva-Gagliardi N. F., Pellikka M., Jensen A. M., McGlade C. J., Tepass U. (2006). The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev. Cell 11, 363–374 10.1016/j.devcel.2006.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers C., Michel D., Lane-guermonprez L., Delgrossi M.-H., Médina E., Arsanto J., Le Bivic A. (2004). CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol. Biol. Cell 15, 1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I., Bork P. (2011). Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 39, W475–W478 10.1093/nar/gkr201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Hashimshony T., Wagner F., Yanai I. (2012). Developmental milestones punctuate gene expression in the Caenorhabditis embryo. Dev. Cell 22, 1101–1108 10.1016/j.devcel.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Ling C., Zheng Y., Yin F., Yu J., Huang J., Hong Y., Wu S., Pan D. (2010). The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc. Natl. Acad. Sci. USA 107, 10532–10537 10.1073/pnas.1004279107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova O., Roh M. H., Liu C.-J., Laurinec S., Margolis B. (2003). Mammalian Crumbs3 is a small transmembrane protein linked to protein associated with Lin-7 (Pals1). Gene 302, 21–29 10.1016/S0378111902010843 [DOI] [PubMed] [Google Scholar]
- Médina E., Williams J., Klipfell E., Zarnescu D., Thomas G., Le Bivic A. (2002). Crumbs interacts with moesin and beta(Heavy)-spectrin in the apical membrane skeleton of Drosophila. J. Cell Biol. 158, 941–951 10.1083/jcb.200203080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-de-Sá E., Mirouse V., St Johnston D. (2010). aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell 141, 509–523 10.1016/j.cell.2010.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S.-C., Choi K.-W. (2006). Domain-specific early and late function of Dpatj in Drosophila photoreceptor cells. Dev. Dyn. 235, 1501–1507 10.1002/dvdy.20726 [DOI] [PubMed] [Google Scholar]
- Robinson B. S., Huang J., Hong Y., Moberg K. H. (2010). Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr. Biol. 20, 582–590 10.1016/j.cub.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh M. H., Fan S., Liu C.-J., Margolis B. (2003). The Crumbs3-Pals1 complex participates in the establishment of polarity in mammalian epithelial cells. J. Cell Sci. 116, 2895–2906 10.1242/jcs.00500 [DOI] [PubMed] [Google Scholar]
- Schatz P. J. (1993). Use of peptide libraries to map the substrate specificity of a peptide-modifying enzyme: a 13 residue consensus peptide specifies biotinylation in Escherichia coli. Biotechnology 11, 1138–1143 10.1038/nbt1093-1138 [DOI] [PubMed] [Google Scholar]
- Schlüter M. A., Pfarr C. S., Pieczynski J., Whiteman E. L., Hurd T. W., Fan S., Liu C.-J., Margolis B. (2009). Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol. Biol. Cell 20, 4652–4663 10.1091/mbc.E09-02-0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segbert C., Johnson K., Theres C., van Fürden D., Bossinger O. (2004). Molecular and functional analysis of apical junction formation in the gut epithelium of Caenorhabditis elegans. Dev. Biol. 266, 17–26 10.1016/j.ydbio.2003.10.019 [DOI] [PubMed] [Google Scholar]
- Shibata Y., Fujii T., Dent J. A., Fujisawa H., Takagi S. (2000). EAT-20, a novel transmembrane protein with EGF motifs, is required for efficient feeding in Caenorhabditis elegans. Genetics 154, 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simske J. S., Kaech S. M., Harp S. A., Kim S. K. (1996). LET-23 receptor localization by the cell junction protein LIN-7 during C. elegans vulval induction. Cell 85, 195–204 10.1016/S0092-8674(00)81096-X [DOI] [PubMed] [Google Scholar]
- Sipos L., von Heijne G. (1993). Predicting the topology of eukaryotic membrane proteins. Eur. J. Biochem. 213, 1333–1340 10.1111/j.1432-1033.1993.tb17885.x [DOI] [PubMed] [Google Scholar]
- Spencer W. C., Zeller G., Watson J. D., Henz S. R., Watkins K. L., McWhirter R. D., Petersen S., Sreedharan V. T., Widmer C., Jo J. et al. (2011). A spatial and temporal map of C. elegans gene expression. Genome Res. 21, 325–341 10.1101/gr.114595.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D., Ahringer J. (2010). Cell polarity in eggs and epithelia: parallels and diversity. Cell 141, 757–774 10.1016/j.cell.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Tepass U. (2012). The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu. Rev. Cell Dev. Biol. 28, 655–685 10.1146/annurev-cellbio-092910-154033 [DOI] [PubMed] [Google Scholar]
- Tursun B., Cochella L., Carrera I., Hobert O. (2009). A toolkit and robust pipeline for the generation of fosmid-based reporter genes in C. elegans. PLoS ONE 4, e4625 10.1371/journal.pone.0004625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pavert S. A., Kantardzhieva A., Malysheva A., Meuleman J., Versteeg I., Levelt C., Klooster J., Geiger S., Seeliger M. W., Rashbass P. et al. (2004). Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J. Cell Sci. 117, 4169–4177 10.1242/jcs.01301 [DOI] [PubMed] [Google Scholar]
- van den Hurk J. A. J. M., Rashbass P., Roepman R., Davis J., Voesenek K. E. J., Arends M. L., Zonneveld M. N., van Roekel M. H. G., Cameron K., Rohrschneider K. et al. (2005). Characterization of the Crumbs homolog 2 (CRB2) gene and analysis of its role in retinitis pigmentosa and Leber congenital amaurosis. Mol. Vis. 11, 263–273. [PubMed] [Google Scholar]
- van Rossum A. G. S. H., Aartsen W. M., Meuleman J., Klooster J., Malysheva A., Versteeg I., Arsanto J.-P., Le Bivic A., Wijnholds J. (2006). Pals1/Mpp5 is required for correct localization of Crb1 at the subapical region in polarized Muller glia cells. Hum. Mol. Genet. 15, 2659–2672 10.1093/hmg/ddl194 [DOI] [PubMed] [Google Scholar]
- Waaijers S., Portegijs V., Kerver J., Lemmens B. B. L. G., Tijsterman M., van den Heuvel S., Boxem M. (2013). CRISPR/Cas9-targeted mutagenesis in Caenorhabditis elegans. Genetics 195, 1187–1191 10.1534/genetics.113.156299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther R. F., Pichaud F. (2010). Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr. Biol. 20, 1065–1074 10.1016/j.cub.2010.04.049 [DOI] [PubMed] [Google Scholar]
- Whiteman E. L., Fan S., Harder J. L., Walton K. D., Liu C.-J., Soofi A., Fogg V. C., Hershenson M. B., Dressler G. R., Deutsch G. H. et al. (2014). Crumbs3 is essential for proper epithelial development and viability. Mol. Cell. Biol. 34, 43–56 10.1128/MCB.00999-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A., Hinz U., Engelbert M., Knust E. (1995). Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell 82, 67–76 10.1016/0092-8674(95)90053-5 [DOI] [PubMed] [Google Scholar]
- Xiao H., Hapiak V. M., Smith K. A., Lin L., Hobson R. J., Plenefisch J., Komuniecki R. (2006). SER-1, a Caenorhabditis elegans 5-HT2-like receptor, and a multi-PDZ domain containing protein (MPZ-1) interact in vulval muscle to facilitate serotonin-stimulated egg-laying. Dev. Biol. 298, 379–391 10.1016/j.ydbio.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Xiao Z., Patrakka J., Nukui M., Chi L., Niu D., Betsholtz C., Pikkarainen T., Vainio S., Tryggvason K. (2011). Deficiency in Crumbs homolog 2 (Crb2) affects gastrulation and results in embryonic lethality in mice. Dev. Dyn. 240, 2646–2656 10.1002/dvdy.22778 [DOI] [PubMed] [Google Scholar]
- Yin Y., Sheng J., Hu R., Yang Y., Qing S. (2014). The expression and localization of Crb3 in developmental stages of the mice embryos and in different organs of 1-week-old female mice. Reprod. Domest. Anim. 49, 824–830 10.1111/rda.12374 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.