Abstract
Steroid hormones synthesized in and secreted from peripheral endocrine glands pass through the blood-brain barrier and play a role in the central nervous system. In addition, the brain possesses an inherent endocrine system and synthesizes steroid hormones known as neurosteroids. Increasing evidence shows that neuroactive steroids protect the central nervous system from various harmful stimuli. Reports show that the neuroprotective actions of steroid hormones attenuate oxidative stress. In this review, we summarize the antioxidative effects of neuroactive steroids, especially 17β-estradiol and progesterone, on neuronal injury in the central nervous system under various pathological conditions, and then describe our recent findings concerning the neuroprotective actions of 17β-estradiol and progesterone on oxidative neuronal injury induced by organometallic compounds, tributyltin, and methylmercury.
1. Introduction
Reactive oxygen species (ROS) are the general term for reactive molecules derived from oxygen, including superoxide, hydrogen peroxide, and hydroxyl radicals. ROS are generated constitutively from cellular organelles, especially mitochondria, and a variety of xenobiotics such as quinones induce ROS production. Cells have defense systems to cope with routinely generated ROS. Superoxide is dismutated to hydrogen peroxide and molecular oxygen by superoxide dismutase (SOD). SOD1 (Cu/Zn-SOD) is present in the cytoplasm, nucleus, mitochondria, and peroxisomes of all mammalian cells, where it scavenges superoxide [1, 2]. SOD2 (mitochondrial Mn-SOD) efficiently eliminates the superoxide that arises from molecular oxygen in the respiratory chain. Hydrogen peroxide produced by the dismutation of superoxide is degraded to molecular oxygen and water by catalase, glutathione peroxidase (GPx), and peroxiredoxin. Catalases are present ubiquitously in aerobic organisms, and the highest level of catalase activity is found in the liver and erythrocytes. Within cells, catalases are located mostly in the peroxisomes because of the presence of many hydrogen peroxide-producing enzymes. There are 4 types of GPx: classical GPx (GPx1), gastrointestinal GPx (GPx2), plasma GPx (GPx3), and phospholipid hydroperoxide GPx (GPx4) [3]. GPx1, GPx2, and GPx3 reduce hydrogen peroxide and organic alkyl hydroperoxides to water and corresponding alcohols at the expense of oxidation of GSH to GSSG. The last type of peroxidase, GPx4, is different from the other 3 GPx enzymes with regard to its substrate specificity and localization. GPx4 has the unique ability to reduce membrane lipid hydroperoxides such as phospholipids and cholesterol hydroperoxides directly, and it is located mostly in the testis [4]. Peroxiredoxins are hydrogen peroxide-scavenging enzymes that were discovered more recently than the above-mentioned catalase and GPx enzymes. Peroxiredoxin enzymes contain conserved cysteine residues that undergo peroxide-dependent oxidation and thiol- (thioredoxin-) dependent reduction during a catalytic cycle. Mammalian cells express 6 isoforms of peroxiredoxin (peroxiredoxins I to VI), which are classified into 3 subgroups (2-Cys, atypical 2-Cys, and 1-Cys) on the basis of the number and position of cysteine residues in the active sites. The relatively high abundance of peroxiredoxin enzymes in mammalian cells appears to be a result of the role these proteins play in removing the low levels of peroxides produced during normal cellular metabolism [5]. Besides the antioxidative enzymes described above, cells have small molecules with antioxidative capacity, such as ascorbic acid and α-tocopherol [6], which are able to scavenge ROS efficiently and specifically at relatively low concentrations. When ROS are not eliminated sufficiently by antioxidative enzymes or small molecules, cells are damaged by oxidative insults, leading to cell death.
Steroid hormones synthesized in and secreted from peripheral endocrine glands pass through the blood-brain barrier and perform functions in the central nervous system (CNS). In addition, the brain possesses an inherent endocrine system and synthesizes steroid hormones known as neurosteroids [7]. Several reports have described neurotrophic and neuroprotective properties of steroid hormones, including pregnenolone, pregnenolone sulfate, progesterone, allopregnanolone, dehydroepiandrosterone, dehydroepiandrosterone sulfate, deoxycorticosterone, allotetrahydrodeoxycorticosterone, testosterone, and 17β-estradiol [8–14]. The biological functions of the neurosteroids and steroids from circulation are exerted either through a conventional genomic process via estrogen receptors (ERs), androgen receptors, progesterone receptors (PRs), mineral corticoid receptors, and glucocorticoid receptors or through interaction with membrane receptors as allosteric modulators of the gamma-aminobutyric acid (GABA)-A/central-type benzodiazepine receptor complex, N-methyl-d-aspartate receptors, kainate receptors, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors, sigma receptors, glycine receptors, serotonin receptors, nicotinic receptors, and muscarinic receptors. Neurosteroids may directly activate G protein coupled transmembrane receptors or indirectly modulate the binding of neuropeptides to their receptors [15]. Detailed protective mechanisms of steroid hormones are now emerging, and antioxidative action is considered to be a key part of the neuroprotective effects of steroids.
While the protective actions of steroid hormones in the brain under pathophysiological conditions such as Alzheimer's disease and stroke are well studied, the effects of steroids on exogenous neurotoxicants such as environmental chemicals and pesticides are unclear. We have investigated the antioxidative action of neuroactive steroids on organometal-induced toxicity in the brain. In this review, we summarize the antioxidative effects of neuroactive steroids, especially 17β-estradiol and progesterone, on neuronal injury in the CNS during various pathological conditions and then describe our recent findings on the neuroprotective effects of 17β-estradiol and progesterone on oxidative neuronal injury induced by organometallic compounds, tributyltin, and methylmercury.
2. 17β-Estradiol Protection against Oxidative Injury in the CNS
17β-Estradiol is a female sex steroid that has been shown to have several beneficial effects, such as preventing bone loss [16] and decreasing risk of coronary disease [17], as well as essential functions on female reproduction. 17β-Estradiol produces neuroprotective effects during ischemic brain injury [18] and Alzheimer's disease [19]. In addition, 17β-estradiol is known to attenuate oxidative stress in a manner that protects the CNS from several harmful stimuli. Treatment of ozone-exposed rats with 17β-estradiol suppressed lipid peroxidation and subsequent decreases in cells in the olfactory bulb [20]. 17β-Estradiol attenuated ROS levels and behavior disorder induced by irradiation of X-rays in neonatal rats [21]. Oxidative stress and subsequent neurotoxicity induced by ethanol [22], 6-hydroxydopamine [23], and oxygen-glucose deprivation [24] were suppressed by treatment with 17β-estradiol. Some antioxidative mechanisms of 17β-estradiol have been proposed.
2.1. Genomic Pathway of ERs
The intracellular targets of 17β-estradiol are ERα and ERβ, which are dominantly located in the nuclei. ERs are classified as a nuclear receptor superfamily and form the transcription initiation complex on the estrogen response element, followed by transcription of target genes, regulating several cellular functions (the genomic pathway of ERs). Regulation of gene expression by 17β-estradiol is closely related to its antioxidative action. Upregulation of antioxidative enzymes is the simplest of the antioxidative mechanisms of 17β-estradiol that are mediated via the genomic ER pathway. SK-N-MC human neuroblastoma cells with ERα overexpression showed increased expression of GPx and catalase [25]. Therefore, ERα is thought to confer cellular tolerance against oxidative stress. In contrast, overexpression of ERβ did not affect the expression of GPx and catalase [25]. In addition, 17β-estradiol pretreatment decreases infarct volume in ERβ null mice, but not in ERα null mice, that have been subjected to middle cerebral artery occlusion, suggesting that ERα, rather than ERβ, is centrally involved in 17β-estradiol-mediated neuroprotection against oxidative stress [26, 27]. Administration of 17β-estradiol increased SOD1 and SOD2 immunoreactivity in nigral neurons of mice subjected to the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment method of Parkinson's disease induction [28]. 17β-Estradiol treatment for 11 days also reduced nitrotyrosine immunoreactivity in nigral neurons, suggesting that 17β-estradiol suppressed oxidative stress via SOD1 and SOD2 upregulation. Oxidative stress and neuronal cell death induced by oxygen-glucose deprivation in mouse brain slices were attenuated by 17β-estradiol treatment via upregulation of SOD1 [24]. Thus, SOD seems to be a major target of 17β-estradiol to attenuate oxidative neuronal injury via the genomic ER pathway. Superoxide is initially generated in organisms, and it is converted to hydrogen peroxide, hydroxyl radicals, and other reactive metabolites [29]. Because SOD also suppresses the production of hydrogen peroxide and hydroxyl radicals by eliminating superoxide, upregulation of SOD by 17β-estradiol might produce antioxidative responses in living animals. Phase II antioxidative enzymes, glutathione S-transferase (GST) and NADPH:quinone oxidoreductase, are reported to be induced in the rat brain by chronic exposure to 17β-estradiol [30], indicating that several antioxidative enzymes other than SOD also might be targets of genomic ER signals.
SK-N-MC cells with ERα overexpression also showed reduced expression of neuronal nitric oxide synthase and inducible nitric oxide synthase [25]. Superoxide and nitric oxide are readily converted by nonenzymic chemical reactions into reactive nonradical species peroxynitrite (ONOO−), which can in turn give rise to new radicals, leading to cellular injury. Therefore, decreases in nitric oxide synthase might contribute to suppress oxidative stress via elimination of reactive radical species.
Factors with protective effects in the CNS in addition to antioxidative enzymes induced by 17β-estradiol have been reported. Seladin-1 was found to be downregulated in brain regions affected by Alzheimer's disease [31]. Thereafter, seladin-1 was demonstrated to encode the gene 3β-hydroxysterol Δ24-reductase, which catalyzes the reduction of the Δ24 double bond of desmosterol to generate cholesterol [32]. 17β-Estradiol upregulates seladin-1 in long-term neuroblast cell cultures from human fetal olfactory epithelium [33], and increased seladin-1 via ERα-dependent transcription protects neurons from β-amyloid and hydrogen peroxide toxicity by suppressing caspase-3 activity and oxidative stress [33, 34]. The promoter region of seladin-1 includes the estrogen responsive element [34]. Neuroglobin is also a target of 17β-estradiol in the brain. Neuroglobin was initially discovered in neurons as a 150 amino acid-long heme-protein displaying <25% sequence identity to conventional vertebrate hemoglobin or myoglobin [35, 36], and neuroglobin was shown to protect neuron from several neurotoxic conditions such as ischemia in vivo and in vitro [37–39]. Recently, 17β-estradiol was reported to upregulate neuroglobin via ERβ in the SK-N-BE human neuroblastoma cell line, and this effect inhibited apoptosis induced by hydrogen peroxide [40]. In addition, neuroglobin elicited by 17β-estradiol decreased inflammatory markers interleukin 6 and interferon γ-inducible protein 10 in primary astrocytes [41]. Moreover, proapoptotic genes are suppressed by treatment with 17β-estradiol. Hydrogen peroxide induced apoptosis in the C6 rat glioma cell line, with upregulation of proapoptotic Bcl family protein Bax [42], and 17β-estradiol attenuated Bax upregulation and hydrogen peroxide-induced apoptosis [42]. Because C6 cells express ERβ, but not ERα, this protective action of 17β-estradiol might be due to ERβ-dependent signaling [42]. 17β-Estradiol regulates numerous genes directly involved in or likely related to the modulation of oxidative stress via the genomic ER pathway. Multiple factors, such as cell species, stimuli harmful to the CNS, ER expression levels, and cofactors binding to the ER, might interfere with the antioxidative effects caused by transcriptional regulation by 17β-estradiol. Genomic actions of 17β-estradiol are listed in Table 1.
Table 1.
Genes involved in antioxidative neuroprotection mediated by 17β-estradiol in the CNS.
| Target gene (up or down) | Proposed effects | Reference(s) |
|---|---|---|
| SOD1 (↑) | ROS elimination | [24, 28] |
| SOD2 (↑) | ROS elimination | [28] |
| GPx (↑) | ROS elimination | [25] |
| Catalase (↑) | ROS elimination | [25] |
| iNOS (↓) | Decreases in reactive radicals | [25, 69, 70] |
| nNOS (↓) | Decreases in reactive radicals | [25] |
| GST (↑) | Elimination of ROS-derived reactive metabolites | [30] |
| NQO1 (↑) | Elimination of ROS-derived reactive metabolites | [30] |
| Seladin-1 (↑) | Antiapoptosis | [33, 34] |
| Neuroglobin (↑) | Antiapoptosis, anti-inflammation | [40, 41] |
| IL-6 (↓) | Anti-inflammation | [41] |
| IP-10 (↓) | Anti-inflammation | [41] |
| MMP-9 (↓) | Anti-inflammation | [25, 69, 70] |
| Cytochrome c oxidase (↑) | Increment of mitochondrial efficiency | [50] |
| Bax (↓) | Antiapoptosis | [42] |
IL-6: interleukin-6; IP-10: IFN-gamma-inducible protein 10; MMP-9: matrix metalloproteinase-9; NOS: nitric oxide synthase; NQO1, NADPH: quinone oxidoreductase 1.
2.2. Nongenomic Pathway of ERs
ERs are also located in the cytosol, and stimulation of cytosolic ERs elicits intracellular signaling events such as kinase activation (the nongenomic pathway of ERs). These signals protect neurons from toxic stimuli, similar to the regulation of gene expression mediated by ERs. It is well established that 17β-estradiol activates extracellular signal-regulated kinase (ERK), a mitogen-activated protein kinase (MAPK). Dorsa and colleagues demonstrated that 17β-estradiol induced ERK phosphorylation via an ERα- and ERβ-dependent nongenomic pathway using the HT22 hippocampus-derived cell line, which overexpresses ERα and ERβ [43, 44]. The protective effects of 17β-estradiol against oxidative cell death induced by amyloid β or glutamate were largely attenuated by pretreatment with the MEK inhibitor PD98059 [43, 44], clearly indicating that ERK activation by 17β-estradiol via the nongenomic ER pathway is involved in neuroprotection against oxidative injury. Akt is also activated downstream of ERs. Zhang et al. reported that extranuclear ERα stimulated by 17β-estradiol induced Akt phosphorylation and subsequent phosphorylation/inactivation of Rac1, a factor critical for activation of NOX2 NADPH oxidase, decreasing superoxide generation from NOX2 and attenuating oxidative neuronal damage elicited by ischemia in the hippocampal CA1 region [45]. In addition, neuroprotective effects against amyloid β toxicity are thought to be involved in the formation of the ERα/GSK-3β/β-catenin complex, resulting in modulation of Wnt signaling [46]. According to this hypothesis, ERα can regulate intracellular Ca2+ levels independent of its transcriptional activity.
2.3. Mitochondrial Efficiency
Several lines of evidence support the possibility that 17β-estradiol exerts its potent neuroprotective effects through a mitochondrial mechanism. Mitochondria are major sites of ROS generation inside cells. Approximately 1 to 5% of the total oxygen consumed in the mitochondrial respiratory chain is incompletely reduced and thus leads to ROS production [47, 48]. It is generally accepted that superoxide generated from the respiratory complex in the inner mitochondrial membrane is vectorially released into the mitochondrial matrix and subsequently dismutated to hydrogen peroxide by SOD2, although the direct release of superoxide into the intermembrane space has been proposed [49]. Brain mitochondria isolated from rats treated with 17β-estradiol showed increased expression and activity of the electron transport chain complex IV (cytochrome c oxidase) [50], and this change was coupled with a decreased rate of ROS leakage and reduced lipid peroxidation, representing a systematic enhancement of brain mitochondrial efficiency. Mitochondria isolated from cerebral blood vessels obtained from ovariectomized rats with 17β-estradiol replacement also showed increased complex IV activity and decreased hydrogen peroxide production from mitochondria [51]. 17β-Estradiol increased levels of cardiolipin, and thus restored mitochondrial integrity [52]. In brain endothelial cells, 17β-estradiol treatment reduced ROS derived from mitochondria due to increased cytochrome c, but not ROS elimination by SOD2 [53]. In rat primary astrocytes, 17β-estradiol attenuated ROS generation, ATP depletion, and mitochondrial membrane potential decreases accompanied by oxygen-glucose deprivation and subsequent reoxygenation [54].
ERβ is reported to be localized to mitochondria in rat primary neurons [55]. ERβ knockdown results in a lower resting mitochondrial membrane potential, and these cells show the resistance to oxidative stress-induced depolarization of mitochondrial membrane potential, ATP depletion, and ROS generation [56], suggesting ERβ in mitochondria could function as a mitochondrial vulnerability factor that is involved in mitochondrial membrane potential maintenance and mitochondrial vulnerability. In addition, mitochondrial ERβ is reduced in female Alzheimer's disease patients [57]. Higher ROS generation in brain mitochondria is observed in ERβ-knockout mice treated with amyloid beta-peptides compared with wild-type mice. Taken together, these results show that 17β-estradiol could induce mitochondrial alterations in the CNS in a manner that reduces oxidative stress.
2.4. Direct Elimination of ROS
Antioxidant effects have been proposed as a mechanism through which the neuroprotective action of 17β-estradiol is mediated. Behl et al. reported that high concentrations (micromolar) of 17β-estradiol or 17β-estradiol, an isomer of 17β-estradiol that is at least 200-fold less active as a hormone [58], showed neuroprotective effects against oxidative stress. Estrogen and estrogen derivatives with a hydroxyl group at the C3 position on the A-ring can act as powerful neuroprotectants in a short-term, ER-independent manner because of their antioxidant capacity [58]. The concentrations of 17β-estradiol required for significant antioxidative neuroprotection by direct radical scavenging activity tend to be higher than the estrogen levels that occur naturally in vivo [59, 60]. However, remarkably, a mechanism through which relatively low concentrations of 17β-estradiol effectively and directly scavenge radicals was proposed using a synthetic derivative of 17β-estradiol, 17β-butoxy-1,3,5(10)-estratrien-3-ol, via a radical-scavenging antioxidant cycle catalyzed by reductases [61]. The phenolic A-ring is transformed into 10β-butoxy-17β-butoxy-1,3,5(10)-estratrien-3-one, a nonaromatic paraquinol, upon capturing hydroxyl radicals, which results in the complete loss of ER affinity and antioxidant activity, after which the parent compound is recovered from paraquinol via enzyme-catalyzed NADPH-dependent reductive aromatization without causing oxidative stress. In this reaction, 17β-estradiol could enzymatically eliminate hydroxyl radicals with NADPH consumption. This process may explain how 17β-estradiol acts as an antioxidant and attenuates oxidative stress at low concentrations. However, a quinone-quinol redox cycle, which is also catalyzed by NADPH-dependent reductases, is known to generate superoxide, leading to oxidative cell injury [62]. Because there are numerous endogenous quinone compounds, such as dopamine quinones, in the brain, the efficiency of the 17β-estradiol antioxidant cycle might depend on reductase activity, as well as reductase specificity for 17β-estradiol-derived paraquinol.
2.5. Microglial Inactivation
Microglia, a type of glial cell in the CNS, are target cells through which 17β-estradiol protects neurons from excess inflammation. Microglia are the primary immune cells of the CNS and are activated quickly in response to external pathogens or cell debris, after which they act by releasing inflammatory factors or engulfing foreign bodies to mediate the inflammatory response. However, excessive activation of microglia may be harmful for host cells; microglia can promote the development of some neuronal diseases by producing large amounts of cytokines and other inflammatory molecules such as tumor necrosis factor-α, interleukin-1β, nitric oxide, and ROS. Activated microglia are associated with the pathogenesis of Parkinson's disease, Alzheimer's disease, ischemia-reperfusion injury, trauma, epilepsy, depression, and schizophrenia [63–67].
Recently, increasing evidence has shown that 17β-estradiol protects neurons from excess or prolonged inflammation in the brain. We demonstrated that rat primary microglia express both ERα and ERβ predominantly in the nucleus [68]; therefore, microglia can respond to 17β-estradiol stimulation. Treatment with 17β-estradiol suppresses inflammatory cytokine expression and nitric oxide production induced by lipopolysaccharide in microglia [69, 70]. These suppressive effects of 17β-estradiol are mediated via the ERs and act by blocking DNA binding and transcriptional activation by nuclear factor-kappa B p65 by preventing its nuclear translocation [71]. 17β-Estradiol has also been reported to inhibit neuroinflammation in an ER-dependent manner in studies using in vivo models of CNS diseases [72, 73]. Therefore, the antioxidative activity of 17β-estradiol could be mediated by suppressive effects on activated microglia.
2.6. Other Mechanisms
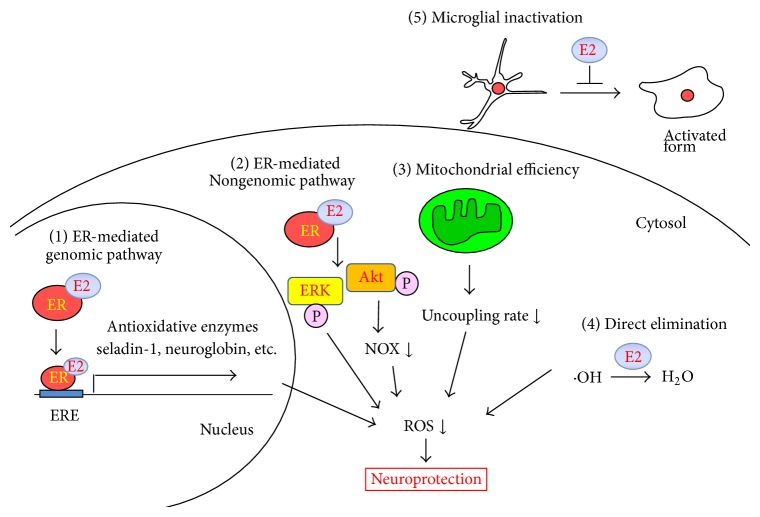
Because the antioxidative effects of 17β-estradiol are mediated by the diverse mechanisms described above, they may be the sum of both ER-dependent and ER-independent actions. In addition, Simpkins and colleagues demonstrated that 17β-estradiol attenuated decreases in the activity of serine/threonine protein phosphatases PP1, PP2A, and calcineurin induced by glutamate excitotoxicity in rat primary neurons [74] and middle cerebral artery occlusion in rats [75] and thus suppressed oxidative neuronal injury. ERs are not involved in these protective effects of 17β-estradiol. 17β-Estradiol likely protects cells by blocking ubiquitination and/or degradation of protein phosphatases caused by oxidative or excitotoxic stress. Therefore, novel targets might be also fundamental for 17β-estradiol-mediated neuroprotection against oxidative stress. The antioxidative effects of 17β-estradiol are presented schematically in Figure 1.
Figure 1.
Antioxidative mechanisms of 17β-estradiol in the brain. The proposed antioxidative mechanisms of 17β-estradiol in the CNS are as follows: (1) Antioxidative enzymes or other functional proteins are transcriptionally activated by ER signaling. (2) Intracellular survival signaling is activated by ER independent of transcriptional regulation. (3) 17β-Estradiol affects mitochondrial antioxidative enzymes or respiratory complexes to decrease ROS by enhancing mitochondrial efficiency. (4) 17β-Estradiol directly scavenges ROS or other reactive radicals. (5) 17β-Estradiol suppresses ROS generation from microglia by inhibiting their activation. E2, 17β-estradiol; ER, estrogen receptor; ERE, estrogen response element; ERK, extracellular signal-regulated kinase; NOX, NADPH oxidase; ROS, reactive oxygen species.
The expression of cytochrome P450 aromatase (CYP19), which synthesizes 17β-estradiol from testosterone, is increased by oxidative stress in neurons and astrocytes [76, 77], indicating that oxidative stress could potentiate 17β-estradiol production. Cultured astrocytes isolated from female rats with high expression levels of cytochrome P450 aromatase showed better tolerance to cellular injury induced by oxygen-glucose deprivation than male rats with low cytochrome P450 aromatase expression [78]. Moreover, sex differences in oxygen-glucose deprivation-induced cell death were diminished by inhibition of cytochrome P450 aromatase in astrocytes prepared from female rats. These data indicate that expression of cytochrome P450 aromatase is a determinant for the severity of neuronal injury, and 17β-estradiol might play a role in adaptive responses against oxidative stress in the brain.
Several negative effects of 17β-estradiol on oxidative stress have been reported. 17β-Estradiol did not suppress glutamate excitotoxicity and apoptosis mediated by potassium-deprivation or ceramide-mediated apoptosis in cerebellar granule cells [79]. Because 17β-estradiol activated ERK in these studies, the authors concluded that ERK activation induced by 17β-estradiol treatment was not involved in its neuroprotective effects. Furthermore, Gordon et al. reported that administration of 17β-estradiol exacerbated infarct formation and lipid peroxidation induced by middle cerebral artery occlusion [80]. Chronic 17β-estradiol treatment reportedly enhanced expression of glial fibrillary acidic protein and interleukin-1β in the arcuate nucleus, followed by potentiation of oxidative stress [81]. In this regard, the effects of 17β-estradiol on oxidative stress are suggested to be tissue-specific and dependent on the timing of the treatment. Circulating estrogens are negatively correlated with ERα expression in the inner ear [82], indicating that 17β-estradiol could downregulate ERs in certain situations. Careful consideration of ER dynamics might be required for effective evaluation of the effects of 17β-estradiol on oxidative stress.
3. Progesterone Protection against Oxidative Neuronal Injury
Similar to 17β-estradiol, progesterone is a female sex steroid that is present in the brain; however, there are far fewer reports on the antioxidative action of progesterone. Progesterone suppresses impairment of memory and oxidative stress, lipid peroxidation, and protein oxidation, induced by phosphamidon, an organophosphorus pesticide, in rats [83]. Progesterone suppressed lipid peroxidation and decreases in GSH levels caused by brain ischemia-reperfusion injury in rats [84]. Progesterone binds to the PR in the nucleus, activating gene transcription. SOD, GPx, catalase, and glutathione reductase are target genes of progesterone involved in oxidative stress tolerance [85–87]. Therefore, progesterone protects neuronal cells from oxidative stress by upregulating antioxidative enzymes via a PR-dependent genomic pathway. In addition, progesterone reportedly decreased mitochondrial ROS production by upregulating complex IV and SOD2 expression, followed by increased respiratory activity [50]. Thus, the antioxidative effects of progesterone are similar, at least in part, to those of 17β-estradiol.
Negative effects of progesterone on oxidative stress are also reported. Progesterone does not alter ischemic oxidative stress in rats subjected to a decapitation ischemia model [88]. Furthermore, the clinical progestin medroxyprogesterone is reported to counteract the antioxidative effects of 17β-estradiol, such as increased expressions of SOD2 and peroxiredoxin V, decreases in lipid peroxides, and enhancement of mitochondrial respiration in primary cultures of hippocampal neurons and glial cells [89]. Further study is needed to reveal the overall antioxidative actions of progesterone, and the manner in which it might antagonize the effects of 17β-estradiol.
4. Oxidative Stress and Neurotoxicity Induced by Tributyltin (TBT)
Organotin compounds have long been used as thermal stabilizers, catalytic agents, and biocidal compounds for preserving wood, textiles, cordage fibers, and electronic equipment [90]. Among organotin compounds, TBT has been most widely used in paint formulations to prevent marine fouling on ships, boats, and fish nets. However, concerns about toxic effects such as imposex in sea snails and other malformations in marine organisms led to a ban on the use of TBT in antifouling compounds [91]. Nonetheless, the pollution of coastal waters continues. Environmental surveying and monitoring of TBT are conducted in order to prevent the consumption of bioaccumulated TBT by humans. The average intake of TBT by humans from market-bought seafood has been estimated to vary worldwide between 0.18 and 2.6 μg per day per person [92], and butyltin compounds, including TBT, have been reported at concentrations between 50 nM and 400 nM in human blood [93]. TBT is thought to be metabolized successively to dibutyltin (DBT) and monobutyltin (MBT). However, an examination of butyltin compounds in human blood collected from subjects in central Michigan revealed that the concentration of TBT in blood was almost the same as that of DBT and MBT [94]. Therefore, a certain quantity of TBT remains in blood without metabolism. TBT are highly lipophilic and readily penetrate the blood-brain barrier to enter the brain. Indeed, the administration of TBT elicited abnormal behavior and reduced brain weight within the cerebellum and synaptogenesis in rats [95–98]. Therefore, the effects of TBT on the CNS are of great concern.
Neural cell death induced by TBT in rat hippocampal slices was reportedly suppressed by pretreatment with antioxidants, including SOD, catalase, trolox, and tocopherol [99]. Furthermore, Kurita et al. demonstrated that hydrogen peroxide was generated by treatment of rat cortical neurons with TBT [100]. Therefore, induction of oxidative stress is considered to be one of the earlier events in the process of TBT-induced neuronal cell death. We also demonstrated that TBT induced ROS production and lipid peroxidation in rat hippocampal slices [101]. However, few reports have examined oxidative injury induced by TBT in detail, and there is little evidence of the mechanism through which TBT elicits ROS production. Recently, we reported that TBT strongly inhibited GST in the hippocampus, but we did not find effects on other GSH-related enzymes, including GPx and glutathione reductase [101]. While pretreatment with ethacrynic acid, a potent GST inhibitor, potentiates ROS production, lipid peroxidation, and neuronal cell death induced by TBT, pretreatment with sulforaphane, which induces GST expression in hippocampal slices, largely suppresses TBT-elicited oxidative stress and cell death [101]. These results indicate that GST inhibition by TBT is a potent mechanism through which it generates ROS and oxidative neuronal injury. GST is a phase II-detoxifying enzyme that conjugates GSH to xenobiotic and endogenous toxins to facilitate their excretion from the organism [102]. Substrates for GST inside cells are organic hydroperoxides and 4-hydroxyalkenals [103], which can induce oxidative stress [104–107]. Therefore, TBT may induce oxidative stress by suppressing degradation of oxidative metabolites, leading to GST inhibition. Furthermore, GST metabolizes cyclized o-quinones, which are oxidized products of catecholamines, via GSH conjugation [108, 109]. When intracellular o-quinones are not metabolized, they undergo one-electron reduction by intracellular reductases to yield semiquinone radicals, which are then reoxidized and can enter redox cycles in cooperation with molecular oxygen to form superoxide anions [62]. Therefore, TBT might induce oxidative stress via potentiation of the quinone redox cycle.
5. Protective Effects of 17β-Estradiol and Progesterone on TBT-Induced Neuronal Injury
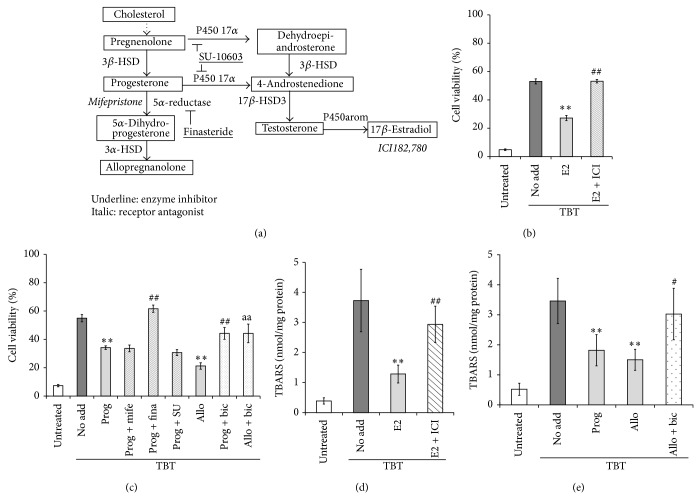
Steroid hormones synthesized in and secreted from peripheral endocrine glands pass through the blood-brain barrier and perform functions in the CNS. In addition, the brain possesses an inherent endocrine system and synthesizes some steroid hormones [110]. The hippocampus can actively synthesize steroid hormones because steroid hormone synthesizing enzymes are highly expressed (Figure 2(a)). Therefore, we investigated whether two female sex steroids, 17β-estradiol and progesterone, prevent neuronal oxidative injury induced by TBT using rat organotypic hippocampal slice cultures.
Figure 2.
Effects of 17β-estradiol and progesterone on TBT-induced oxidative stress and cell death in rat hippocampal slices. (a) Pathway of steroid hormone metabolism in the rat hippocampus. HSD, hydroxysteroid dehydrogenase. (b, d) Rat hippocampal slices were pretreated with ER antagonist ICI182,780 (ICI, 100 μM) for 20 min, after which 1 μM of 17β-estradiol (E2) was added to the culture. After 6 h of incubation, the slices were treated with 3 μM of TBT for 24 h. Cell viability of the CA1, CA3, and dentate gyrus regions was measured by propidium iodide staining (b), and lipid peroxidation was evaluated by determination of thiobarbituric acid reactive substance (TBARS) content (d). The reported values are the mean ± S.E. of 5 separate experiments. ** P < 0.01 versus 3 μM TBT-treated group. ## P < 0.01 versus 3 μM TBT and 1 μM 17β-estradiol-treated group. (c, e) Rat hippocampal slices were pretreated with progesterone receptor antagonist mifepristone (Mife, 10 μM), 5α-reductase inhibitor finasteride (Fina, 100 μM), cytochrome P450 17α inhibitor SU-10603 (SU, 20 μM), or GABAA receptor antagonist bicuculline (Bic, 100 μM), after which progesterone (Prog, 1 μM) or allopregnanolone (Allo, 1 μM) was added to the culture. After a 2 h incubation, the slices were exposed to 3 μM TBT for 24 h. Cell viability (c) and lipid peroxidation (e) were evaluated. The reported values are the mean ± S.E. of 5 separate experiments. ** P < 0.01 versus 3 μM TBT-treated group. # P < 0.05, ## P < 0.01 versus 3 μM TBT and 1 μM progesterone-treated group. aa P < 0.01 versus 3 μM TBT and 1 μM allopregnanolone-treated group.
In our study, pretreatment with 17β-estradiol dose-dependently suppressed ROS production, lipid peroxidation, and neuronal cell death elicited by TBT (Figures 2(b) and 2(d), and [111]). ICI182,780, an ER antagonist, abolished neuroprotection mediated by 17β-estradiol, but actinomycin D and cycloheximide, an mRNA synthesis inhibitor and a protein synthesis inhibitor, respectively, did not show any effects on decreases in cell viability induced by TBT [111], clearly indicating that neuroprotection by 17β-estradiol against TBT-induced neuronal injury is mediated by the nongenomic ER-dependent signaling pathway. Because Akt was activated by treatment with 17β-estradiol and the protective effects of 17β-estradiol on TBT-induced oxidative stress and subsequent cell death were attenuated by the Akt inhibitor triciribine [111], Akt might be responsible for suppressing TBT-mediated neuronal oxidative injury downstream of the ER in a manner independent of gene transcription. Once generated, ROS present anywhere inside cells trigger ROS release by mitochondria (ROS-induced ROS release), amplifying oxidative stress [112]; thus, mitochondria are the major source and target of ROS. 17β-Estradiol suppresses hydrogen peroxide-induced apoptosis and protects mitochondria from ROS by phosphorylating Akt, and subsequently Bad, via the ERα and ERβ-dependent pathway [113]. Therefore, the protective effect of Akt activation on mitochondria may contribute to the attenuation of TBT-induced neuronal injury by 17β-estradiol. Akt attenuates ROS production by decreasing NADPH oxidase activity following Rac1 phosphorylation [45]. In addition, a GST inhibitor was reported to decrease Akt activity [114]. We also reported that TBT induces oxidative stress via GST inhibition [101]. Considering these findings, a reduction in NADPH oxidase activity may be involved in the suppression of oxidative stress mediated by 17β-estradiol.
Progesterone also showed protective effects against TBT-induced neuronal injury. Pretreatment of rat hippocampal slices with progesterone significantly attenuated lipid peroxidation and cell death induced by TBT (Figures 2(c) and 2(e), and [115]). Interestingly, mifepristone, a progesterone receptor antagonist, did not affect neuronal cell death induced by TBT, indicating that the protective effects of progesterone were not mediated by activation of the progesterone receptor [115]. Alternatively, allopregnanolone, a reactive metabolite of progesterone, is thought to mediate neuroprotection against TBT-elicited oxidative neuronal injury for the following 3 reasons: (i) progesterone added in the culture is converted to allopregnanolone, (ii) inhibition of the metabolism of progesterone to allopregnanolone by the 5α-reductase inhibitor finasteride abolished the neuroprotective action of progesterone, and (iii) pretreatment of hippocampal slices with allopregnanolone largely suppressed lipid peroxidation and cell death induced by TBT (Figures 2(c) and 2(e), and [115]). Increasing evidence shows that allopregnanolone acts as an agonist of GABAA receptors [116], and the neuroprotective effect of allopregnanolone was revealed to be dependent on GABAA receptor activity [117]. In our study, pretreatment with bicuculline, a potent GABAA receptor antagonist, significantly abrogated the neuroprotective actions of progesterone and allopregnanolone (Figures 2(c) and 2(e), and [115]), indicating that the GABAA receptor is involved in the protective effects of progesterone on neuronal injury induced by TBT. Nakatsu et al. showed that TBT-stimulated potentiation of glutamate release in rat cortical neurons occurred upstream of ROS generation [118]. Furthermore, the GABAA receptor agonist muscimol attenuated glutamate release and subsequent ROS production and thus suppressed cell death induced by amyloid β proteins (25–35) in rat cortical neurons [119]. Therefore, in our experimental system, the neuroprotective effects of progesterone, for example, attenuation of TBT-induced excitotoxicity and subsequent oxidative injury, could be mediated by GABAA receptor activation. However, the protective effects of progesterone and allopregnanolone against TBT in hippocampal neurons were only partial, indicating the diverse mechanisms of cell death induced by TBT.
TBT can induce oxidative stress through various mechanisms such as GST inhibition and glutamate excitotoxicity. However, interestingly, multiple neuroactive steroids partly transconverted in hippocampal slices can suppress oxidative stress via several pathways (Figure 3). Therefore, neuroactive steroids might protect against xenobiotics and, considering the constant synthesis of steroid hormones in the brain, might be involved in adaptive reactions to xenobiotics. In the next section, we discuss the action of de novo synthesized steroid hormones on oxidative neuronal injury in the hippocampus.
Figure 3.
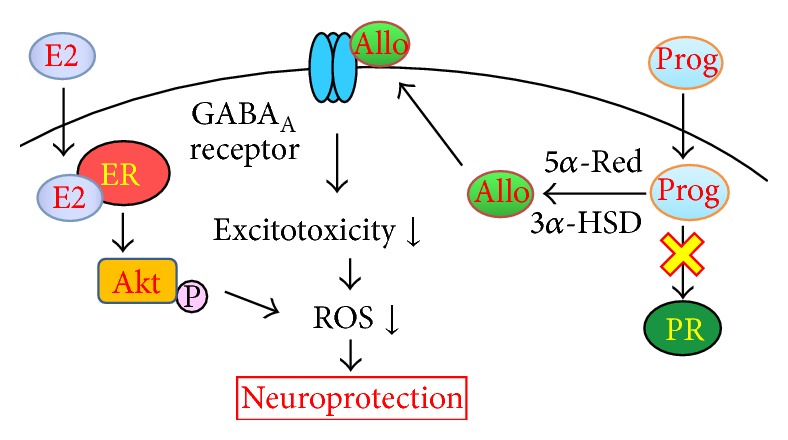
Putative antioxidative mechanisms of 17β-estradiol and progesterone in TBT-induced oxidative neuronal injury. 17β-Estradiol (E2) suppresses TBT-induced neuronal injury via an ER-dependent nongenomic pathway. The attenuation of oxidative stress downstream of Akt activation is considered to be involved in the neuroprotection mediated by 17β-estradiol. Progesterone is readily converted to allopregnanolone, and the neuroprotective activity of allopregnanolone is attributed to modulation of GABAA receptor activity. TBT-induced oxidative stress could be suppressed by multiple pathways stimulated by neuroactive steroids. Allo, allopregnanolone; E2, 17β-estradiol; ER, estrogen receptor; 3α-HSD, 3α-hydroxysteroid dehydrogenase; Prog, progesterone; PR, progesterone receptor; 5α-red, 5α-reductase; ROS, reactive oxygen species.
6. Oxidative Stress Induced by Methylmercury (MeHg)
MeHg is a hazardous pollutant to which humans are exposed mainly through consumption of fish [120, 121]. Minamata disease, anthropogenic exposure to MeHg in Japan, and MeHg poisoning in Iraq have established the toxicity of MeHg in the nervous system [122–124]. Once MeHg is taken into the blood via food intake, it easily passes through the blood-brain barrier as a cysteine conjugate by using the neutral amino acid transport system and thus quickly reaches the brain [125, 126]. Thus, the primary target of MeHg is the CNS, and MeHg produces abnormal behaviors via CNS disruption [123]. Indeed, MeHg elicits sensory and auditory impairment in humans [127], visual disturbances and tremors in cats and monkeys [128, 129], and hind limb bending in rats [130].
Oxidative stress is a major toxic mechanism of MeHg in the CNS (reviewed in [131]). MeHg can interact with nucleophilic groups, mainly thiol and selenol, because of its electrophilicity. Indeed, MeHg reacts with R-SH and R-SeH to form the very stable complexes R-SHgCH3 and R-SeHgCH3. Considering that many metabolic enzymes and antioxidative enzymes include thiol and/or selenol groups through which their catalytic activity can be regulated and that MeHg affects the activity of such enzymes [132–136], these interactions could induce imbalances between ROS and cellular antioxidative capacity, leading to oxidative cell damage.
GPx, which includes selenol in its active site, is an intracellular target of MeHg. Due to the high affinity of MeHg for selenol groups, decreased GPx activity after MeHg exposure has been attributed to direct inhibition of the enzyme [137]. Furthermore, MeHg has been reported to induce a selenium-deficient-like condition [138]. Thus, MeHg can be considered to cause decreases in GPx synthesis. Reduction of GPx activity inside cells could increase levels of ROS or related reactive compounds such as hydrogen peroxide and lipid peroxide, leading to neuronal oxidative injury. MeHg also acts on mitochondria. MeHg stimulates superoxide production independent of the effects against SOD2 [139, 140]. Complexes II and III in the respiratory chain are reported to be targets of MeHg, and impairment of these complexes elicits excess production of hydrogen peroxide [141]. In addition, MeHg induces glutamate dyshomeostasis. MeHg has been shown to inhibit glutamate uptake into cultured astrocytes [142, 143], rat synaptic vesicles [144], and cerebral cortical slices [145] and to increase spontaneous release of glutamate from mouse cerebellar slices [146] and cultured neuronal cells [147], indicating that MeHg may induce increases in extracellular glutamate levels. These in vitro findings have been confirmed in vivo with microdialysis probes implanted in the frontal cortex of adult rats [148]. Therefore, MeHg can elicit oxidative stress via glutamate excitotoxicity.
Some endogenous molecules reportedly attenuate MeHg toxicity. In MeHg-treated rodents, treatment with vitamin E and selenium suppresses decreases in body weight and neurological symptoms such as auditory responses [149–151]. Vitamin K, pyrroloquinoline quinone, and metallothionein also attenuate cytotoxicity induced by MeHg [152–155]. In addition, MeHg activates the transcription factor Nrf-2 in a manner coupled to S-mercuration of its negative regulator Keap1, and the Keap1/Nrf-2 pathway protects neurons from MeHg-induced oxidative injury [156]. Together, the balance between ROS production induced by MeHg and endogenous and/or inducible protective mechanisms that act against MeHg might determine levels of oxidative stress and cellular damage.
7. Protective Effects of De Novo Synthesized 17β-Estradiol on MeHg-Induced Neuronal Injury
The toxic effect of MeHg has been shown to be attenuated by exogenous 17β-estradiol in primary cultured rat cerebellar granule cells. In these cultured cells, 17β-estradiol protected against MeHg toxicity by acting as an antioxidant without stimulating ERs [157]. In male mice, 17β-estradiol administration partially prevented MeHg-induced motor activity deficits and modification of cerebellar glutathione metabolism [158]. These data indicate that 17β-estradiol suppresses oxidative stress and subsequent cellular injury induced by MeHg. As described in the Introduction, the brain synthesizes some steroid hormones, including 17β-estradiol, and we have previously shown that cultured hippocampal slices also produce 17β-estradiol [159]. Thus, we examined the protective action of de novo synthesized 17β-estradiol against MeHg-induced cell death.
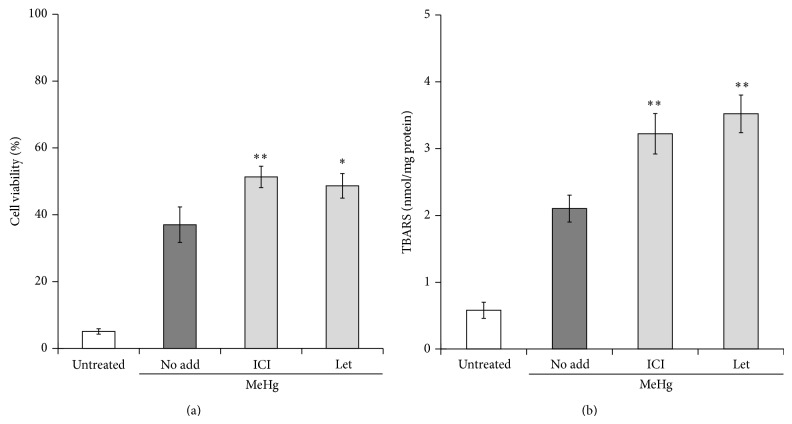
MeHg exposure injured hippocampal neurons, especially in the dentate gyrus region [160]. Exogenous treatment with 17β-estradiol clearly suppressed MeHg-induced neuronal cell death, and this protective action was abolished by the ER antagonist ICI182,780 [160], showing that 17β-estradiol protects neurons from MeHg-toxicity in an ER-dependent manner. Interestingly, MeHg neurotoxicity was enhanced by pretreatment with ICI182,780 (Figure 4(a) and [160]). Lipid peroxidation induced by MeHg was also potentiated by ICI182,780 (Figure 4(b)). These results suggest that ER activation attenuated MeHg-mediated oxidative neuronal injury in the absence of added 17β-estradiol. Treatment of hippocampal slices with letrozole, which inhibits cytochrome P450 aromatase and thus suppresses 17β-estradiol synthesis, significantly enhances lipid peroxidation and cell death elicited by MeHg (Figures 4(a) and 4(b) and [160]). Therefore, considering that cultured hippocampal slices synthesized 17β-estradiol [159], de novo synthesized 17β-estradiol can protect neurons from MeHg-induced oxidative neuronal injury. We have observed that progesterone and allopregnanolone also suppress neuronal injury induced by MeHg in rat hippocampal slices (unpublished data). Thus, various types of neurosteroids might show neuroprotective action against environmental chemicals, including MeHg.
Figure 4.
Suppressive effects of de novo synthesized 17β-estradiol on MeHg-induced oxidative stress and cell death in rat hippocampal slices. Rat hippocampal slices were pretreated with ER antagonist ICI182,780 (ICI, 100 μM) or cytochrome P450 aromatase inhibitor letrozole (Let, 10 μM) for 20 min and then the slices were treated with 1 μM of MeHg for 24 h. Cell viability of the dentate gyrus region was measured by propidium iodide staining (a), and lipid peroxidation was evaluated by determination of thiobarbituric acid reactive substance (TBARS) contents (b). The reported values are the mean ± S.E. of 5 separate experiments. * P < 0.05, ** P < 0.01 versus 1 μM MeHg-treated group.
8. Conclusion
Few studies have reported the protective effects of 17β-estradiol and progesterone on neurotoxicity induced by environmental chemicals. We have demonstrated the protective actions of 17β-estradiol and progesterone on organometallic compound-induced oxidative neuronal injury, and other groups have reported that 17β-estradiol attenuated oxidative stress and cell death induced by lead [161], suggesting that 17β-estradiol and progesterone can act as endogenous protective factors against environmental chemicals. This hypothesis is supported by the finding that steroid hormone levels in the brain at birth, when the blood-brain barrier is immature and thus the brain is exposed to various xenobiotics, are much higher than those in the brain of an adult [162, 163].
The brain is supplied with steroid hormones from the blood and synthesizes steroid hormones (neurosteroids) to be able to respond to stresses. Many environmental chemicals are known to induce oxidative stress, and the brain could increase production of 17β-estradiol when it detects oxidative stress. In this regard, neuroactive steroids might mediate adaptive responses in the CNS to environmental chemicals. As described in this review, 17β-estradiol and progesterone induce divergent intracellular events in the CNS under oxidative stress. The sum of these events determines the response of cells and/or individuals. To reveal the interactions between neuroactive steroids and environmental chemicals (or other harmful stimuli), the protective mechanisms of steroids must be identified.
Acknowledgments
This work was supported by grants (KAKENHI) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Yasuhiro Ishihara and Takeshi Yamazaki) and a grant for the Study (Group) of the Health Effects of Heavy Metals Organized by the Ministry of the Environment, Japan (Takeshi Yamazaki).
Abbreviations
- CNS:
Central nervous system
- ER:
Estrogen receptor
- ERK:
Extracellular signal-regulated kinase
- GABA:
Gamma-aminobutyric acid
- GPx:
Glutathione peroxidase
- GST:
Glutathione S-transferase
- MeHg:
Methylmercury
- PR:
Progesterone receptor
- ROS:
Reactive oxygen species
- SOD:
Superoxide dismutase
- TBT:
Tributyltin.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Crapo J. D., Oury T., Rabouille C., Slot J. W., Chang L.-Y. Copper, zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(21):10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kira Y., Sato E. F., Inoue M. Association of Cu,Zn-type superoxide dismutase with mitochondria and peroxisomes. Archives of Biochemistry and Biophysics. 2002;399(1):96–102. doi: 10.1006/abbi.2001.2738. [DOI] [PubMed] [Google Scholar]
- 3.Imai H., Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radical Biology and Medicine. 2003;34(2):145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 4.Thomas J. P., Maiorino M., Ursini F., Girotti A. W. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation: in situ reduction of phospholipid and cholesterol hydroperoxides. The Journal of Biological Chemistry. 1990;265(1):454–461. [PubMed] [Google Scholar]
- 5.Sue G. R., Ho Z. C., Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radical Biology and Medicine. 2005;38(12):1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Matés J. M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153(1–3):83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- 7.Baulieu E. E. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Progress in Hormone Research. 1997;52:1–32. [PubMed] [Google Scholar]
- 8.Wojtal K., Trojnar M. K., Czuczwar S. J. Endogenous neuroprotective factors: neurosteroids. Pharmacological Reports. 2006;58(3):335–340. [PubMed] [Google Scholar]
- 9.Liu M., Kelley M. H., Herson P. S., Hurn P. D. Neuroprotection of sex steroids. Minerva Endocrinologica. 2010;35(2):127–143. [PMC free article] [PubMed] [Google Scholar]
- 10.Faroni A., Magnaghi V. The neurosteroid allopregnanolone modulates specific functions in central and peripheral glial cells. Frontiers in Endocrinology. 2011;2, article 103 doi: 10.3389/fendo.2011.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melcangi R. C., Panzica G., Garcia-Segura L. M. Neuroactive steroids: focus on human brain. Neuroscience. 2011;191:1–5. doi: 10.1016/j.neuroscience.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Traish A. M., Kang H. P., Saad F., Guay A. T. Dehydroepiandrosterone (DHEA)—a precursor steroid or an active hormone in human physiology (CME) Journal of Sexual Medicine. 2011;8(11):2960–2982. doi: 10.1111/j.1743-6109.2011.02523.x. [DOI] [PubMed] [Google Scholar]
- 13.Scott E., Zhang Q. G., Wang R., Vadlamudi R., Brann D. Estrogen neuroprotection and the critical period hypothesis. Frontiers in Neuroendocrinology. 2012;33(1):85–104. doi: 10.1016/j.yfrne.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh M., Su C. Progesterone and neuroprotection. Hormones and Behavior. 2013;63(2):284–290. doi: 10.1016/j.yhbeh.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Do Rego J. L., Seong J. Y., Burel D., et al. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Frontiers in Neuroendocrinology. 2009;30(3):259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Meema S., Bunker M. L., Meema H. E. Preventive effect of estrogen on postmenopausal bone loss. Archives of Internal Medicine. 1975;135(11):1436–1440. doi: 10.1001/archinte.135.11.1436. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S., Anand S. Hormone replacement therapy: a time for pause. Canadian Medical Association Journal. 2002;167(4):357–359. [PMC free article] [PubMed] [Google Scholar]
- 18.Dubal D. B., Kashon M. L., Pettigrew L. C., et al. Estradiol protects against ischemic injury. Journal of Cerebral Blood Flow and Metabolism. 1998;18(11):1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Simpkins J. W., Green P. S., Gridley K. E., Singh M., De Fiebre N. C., Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer's disease. American Journal of Medicine. 1997;103(3):19S–25S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- 20.Guevara-Guzmán R., Arriaga V., Kendrick K. M., et al. Estradiol prevents ozone-induced increases in brain lipid peroxidation and impaired social recognition memory in female rats. Neuroscience. 2009;159(3):940–950. doi: 10.1016/j.neuroscience.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 21.Caceres L. G., Uran S. L., Zubilete M. A. Z., Romero J. I., Capani F., Guelman L. R. An early treatment with 17-β-estradiol is neuroprotective against the long-term effects of neonatal ionizing radiation exposure. Journal of Neurochemistry. 2011;118(4):626–635. doi: 10.1111/j.1471-4159.2011.07334.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramezani A., Goudarzi I., Lashkarbolouki T., Ghorbanian M. T., Elahdadi Salmani M., Abrari K. Neuroprotective effects of the 17β-estradiol against ethanol-induced neurotoxicity and oxidative stress in the developing male rat cerebellum: biochemical, histological and behavioral changes. Pharmacology Biochemistry and Behavior. 2011;100(1):144–151. doi: 10.1016/j.pbb.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Baraka A. M., Korish A. A., Soliman G. A., Kamal H. The possible role of estrogen and selective estrogen receptor modulators in a rat model of Parkinson's disease. Life Sciences. 2011;88(19-20):879–885. doi: 10.1016/j.lfs.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Rao A. K., Dietrich A. K., Ziegler Y. S., Nardulli A. M. 17β-Estradiol-mediated increase in Cu/Zn superoxide dismutase expression in the brain: a mechanism to protect neurons from ischemia. The Journal of Steroid Biochemistry and Molecular Biology. 2011;127(3–5):382–389. doi: 10.1016/j.jsbmb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manthey D., Behl C. From structural biochemistry to expression profiling: neuroprotective activities of estrogen. Neuroscience. 2006;138(3):845–850. doi: 10.1016/j.neuroscience.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 26.Dubal D. B., Zhu H., Yu J., et al. Estrogen receptor α, not β, is a critical link in estradiol-mediated protection against brain injury. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(4):1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubal D. B., Rau S. W., Shughrue P. J., et al. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147(6):3076–3084. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- 28.Tripanichkul W., Sripanichkulchai K., Duce J. A., Finkelstein D. I. 17Beta-Estradiol reduces nitrotyrosine immunoreactivity and increases SOD1 and SOD2 immunoreactivity in nigral neurons in male mice following MPTP insult. Brain Research. 2007;1164(1):24–31. doi: 10.1016/j.brainres.2007.05.076. [DOI] [PubMed] [Google Scholar]
- 29.Dröge W. Free radicals in the physiological control of cell function. Physiological Reviews. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 30.Stakhiv T. M., Mesia-Vela S., Kauffman F. C. Phase II antioxidant enzyme activities in brain of male and female ACI rats treated chronically with estradiol. Brain Research. 2006;1104(1):80–91. doi: 10.1016/j.brainres.2006.05.093. [DOI] [PubMed] [Google Scholar]
- 31.Greeve I., Hermans-Borgmeyer I., Brellinger C., et al. The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer's disease-associated neurodegeneration and oxidative stress. The Journal of Neuroscience. 2000;20(19):7345–7352. doi: 10.1523/JNEUROSCI.20-19-07345.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waterham H. R., Koster J., Romeijn G. J., et al. Mutations in the 3beta-hydroxysterol Delta24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. The American Journal of Human Genetics. 2001;69(4):685–694. doi: 10.1086/323473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benvenuti S., Luciani P., Vannelli G. B., et al. Estrogen and selective estrogen receptor modulators exert neuroprotective effects and stimulate the expression of selective Alzheimer's disease indicator-1, a recently discovered antiapoptotic gene, in human neuroblast long-term cell cultures. The Journal of Clinical Endocrinology & Metabolism. 2005;90(3):1775–1782. doi: 10.1210/jc.2004-0066. [DOI] [PubMed] [Google Scholar]
- 34.Luciani P., Deledda C., Rosati F., et al. Seladin-1 is a fundamental mediator of the neuroprotective effects of estrogen in human neuroblast long-term cell cultures. Endocrinology. 2008;149(9):4256–4266. doi: 10.1210/en.2007-1795. [DOI] [PubMed] [Google Scholar]
- 35.Burmester T., Welch B., Reinhardt S., Hankeln T. A verteblrate globin expressed in the brain. Nature. 2000;407(6803):520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 36.Pesce A., Dewilde S., Nardini M., et al. Human brain neuroglobin structure reveals a distinct mode of controlling oxygen affinity. Structure. 2003;11(9):1087–1095. doi: 10.1016/S0969-2126(03)00166-7. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y., Jin K., Peel A., Ou Mao X., Xie L., Greenberg D. A. Neuroglobin protects the brain from experimental stroke in vivo . Proceedings of the National Academy of Sciences of the United States of America. 2003;100(6):3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fordel E., Thijs L., Martinet W., Schrijvers D., Moens L., Dewilde S. Anoxia or oxygen and glucose deprivation in SH-SY5Y cells: a step closer to the unraveling of neuroglobin and cytoglobin functions. Gene. 2007;398(1-2):114–122. doi: 10.1016/j.gene.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 39.Khan A. A., Wang Y., Sun Y., et al. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(47):17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Marinis E., Ascenzi P., Pellegrini M., et al. 17β-Estradiol—a new modulator of neuroglobin levels in neurons: role in neuroprotection against H2O2-induced toxicity. Neuro-Signals. 2010;18(4):223–235. doi: 10.1159/000323906. [DOI] [PubMed] [Google Scholar]
- 41.De Marinis E., Acaz-Fonseca E., Arevalo M. A., et al. 17β-Oestradiol anti-inflammatory effects in primary astrocytes require oestrogen receptor β-mediated neuroglobin up-regulation. Journal of Neuroendocrinology. 2013;25(3):260–270. doi: 10.1111/jne.12007. [DOI] [PubMed] [Google Scholar]
- 42.Sur P., Sribnick E. A., Wingrave J. M., Nowak M. W., Ray S. K., Banik N. L. Estrogen attenuates oxidative stress-induced apoptosis in C6 glial cells. Brain Research. 2003;971(2):178–188. doi: 10.1016/S0006-8993(03)02349-7. [DOI] [PubMed] [Google Scholar]
- 43.Mize A. L., Shapiro R. A., Dorsa D. M. Estrogen receptor-mediated neuroprotection from oxidative stress requires activation of the mitogen-activated protein kinase pathway. Endocrinology. 2003;144(1):306–312. doi: 10.1210/en.2002-220698. [DOI] [PubMed] [Google Scholar]
- 44.Fitzpatrick J. L., Mize A. L., Wade C. B., Harris J. A., Shapiro R. A., Dorsa D. M. Estrogen-mediated neuroprotection against beta-amyloid toxicity requires expression of estrogen receptor alpha or beta and activation of the MAPK pathway. Journal of Neurochemistry. 2002;82(3):674–682. doi: 10.1046/j.1471-4159.2002.01000.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q.-G., Raz L., Wang R., et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor α-mediated inhibition of NADPH oxidase activation. The Journal of Neuroscience. 2009;29(44):13823–13836. doi: 10.1523/jneurosci.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quintanilla R. A., Muñoz F. J., Metcalfe M. J., et al. Trolox and 17β-estradiol protect against amyloid β-peptide neurotoxicity by a mechanism that involves modulation of the Wnt signaling pathway. The Journal of Biological Chemistry. 2005;280(12):11615–11625. doi: 10.1074/jbc.m411936200. [DOI] [PubMed] [Google Scholar]
- 47.Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochemical Journal. 1972;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochemical Journal. 1973;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han D., Williams E., Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. The Biochemical Journal. 2001;353, part 2:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Irwin R. W., Yao J., Hamilton R. T., Cadenas E., Brinton R. D., Nilsen J. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology. 2008;149(6):3167–3175. doi: 10.1210/en.2007-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stirone C., Duckles S. P., Krause D. N., Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Molecular Pharmacology. 2005;68(4):959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- 52.Jones T. T., Brewer G. J. Critical age-related loss of cofactors of neuron cytochrome C oxidase reversed by estrogen. Experimental Neurology. 2009;215(2):212–219. doi: 10.1016/j.expneurol.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Razmara A., Sunday L., Stirone C., et al. Mitochondrial effects of estrogen are mediated by estrogen receptor α in brain endothelial cells. Journal of Pharmacology and Experimental Therapeutics. 2008;325(3):782–790. doi: 10.1124/jpet.107.134072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo J., Duckles S. P., Weiss J. H., Li X., Krause D. N. 17β-Estradiol prevents cell death and mitochondrial dysfunction by an estrogen receptor-dependent mechanism in astrocytes after oxygen-glucose deprivation/reperfusion. Free Radical Biology & Medicine. 2012;52(11-12):2151–2160. doi: 10.1016/j.freeradbiomed.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang S.-H., Liu R., Perez E. J., et al. Mitochondrial localization of estrogen receptor β . Proceedings of the National Academy of Sciences of the United States of America. 2004;101(12):4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang S.-H., Sarkar S. N., Liu R., et al. Estrogen receptor beta as a mitochondrial vulnerability factor. The Journal of Biological Chemistry. 2009;284(14):9540–9548. doi: 10.1074/jbc.m808246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Long J., He P., Shen Y., Li R. New evidence of mitochondria dysfunction in the female alzheimer's disease brain: deficiency of estrogen receptor-β . Journal of Alzheimer's Disease. 2012;30(3):545–558. doi: 10.3233/jad-2012-120283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Behl C., Skutella T., Lezoualc'h F., et al. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Molecular Pharmacology. 1997;51(4):535–541. [PubMed] [Google Scholar]
- 59.Moosmann B., Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(16):8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugioka K., Shimosegawa Y., Nakano M. Estrogens as natural antioxidants of membrane phospholipid peroxidation. FEBS Letters. 1987;210(1):37–39. doi: 10.1016/0014-5793(87)81293-0. [DOI] [PubMed] [Google Scholar]
- 61.Prokai-Tatrai K., Perjesi P., Rivera-Portalatin N. M., Simpkins J. W., Prokai L. Mechanistic investigations on the antioxidant action of a neuroprotective estrogen derivative. Steroids. 2008;73(3):280–288. doi: 10.1016/j.steroids.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishihara Y., Shiba D., Shimamoto N. Enhancement of DMNQ-induced hepatocyte toxicity by cytochrome P450 inhibition. Toxicology and Applied Pharmacology. 2006;214(2):109–117. doi: 10.1016/j.taap.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Hickman S. E., Allison E. K., El Khoury J. Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer's disease mice. The Journal of Neuroscience. 2008;28(33):8354–8360. doi: 10.1523/jneurosci.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marinova-Mutafchieva L., Sadeghian M., Broom L., Davis J. B., Medhurst A. D., Dexter D. T. Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: a time course study in a 6-hydroxydopamine model of Parkinson's disease. Journal of Neurochemistry. 2009;110(3):966–975. doi: 10.1111/j.1471-4159.2009.06189.x. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y.-C., Lin S., Yang Q.-W. Toll-like receptors in cerebral ischemic inflammatory injury. Journal of Neuroinflammation. 2011;8, article 134 doi: 10.1186/1742-2094-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Najjar S., Pearlman D. M., Alper K., Najjar A., Devinsky O. Neuroinflammation and psychiatric illness. Journal of Neuroinflammation. 2013;10, article 43 doi: 10.1186/1742-2094-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xanthos D. N., Sandkühler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nature Reviews Neuroscience. 2014;15(1):43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- 68.Ishihara Y., Itoh K., Ishida A., Yamazaki T. Selective estrogen-receptor modulators suppress microglial activation and neuronal cell death via an estrogen receptor-dependent pathway. The Journal of Steroid Biochemistry and Molecular Biology. 2015;145:85–93. doi: 10.1016/j.jsbmb.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Vegeto E., Bonincontro C., Pollio G., et al. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. The Journal of Neuroscience. 2001;21(6):1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bruce-Keller A. J., Keeling J. L., Keller J. N., Huang F. F., Camondola S., Mattson M. P. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141(10):3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- 71.Ghisletti S., Meda C., Maggi A., Vegeto E. 17β-estradiol inhibits inflammatory gene expression by controlling NF-κB intracellular localization. Molecular and Cellular Biology. 2005;25(8):2957–2968. doi: 10.1128/mcb.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vegeto E., Belcredito S., Etteri S., et al. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(16):9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brown C. M., Mulcahey T. A., Filipek N. C., Wise P. M. Production of proinflammatory cytokines and chemokines during neuroinflammation: novel roles for estrogen receptors α and β . Endocrinology. 2010;151(10):4916–4925. doi: 10.1210/en.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yi K. D., Cai Z. Y., Covey D. F., Simpkins J. W. Estrogen receptor-independent neuroprotection via protein phosphatase preservation and attenuation of persistent extracellular signal-regulated kinase 1/2 activation. Journal of Pharmacology and Experimental Therapeutics. 2008;324(3):1188–1195. doi: 10.1124/jpet.107.132308. [DOI] [PubMed] [Google Scholar]
- 75.Yi K. D., Simpkins J. W. Protein phosphatase 1, protein phosphatase 2A, and calcineurin play a role in estrogen-mediated neuroprotection. Endocrinology. 2008;149(10):5235–5243. doi: 10.1210/en.2008-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lepore G., Gadau S., Mura A., Zedda M., Farina V. Aromatase immunoreactivity in fetal ovine neuronal cell cultures exposed to oxidative injury. European Journal of Histochemistry. 2009;53(4):233–238. doi: 10.4081/ejh.2009.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lepore G., Gadau S., Peruffo A., et al. Aromatase expression in cultured fetal sheep astrocytes after nitrosative/oxidative damage. Cell and Tissue Research. 2011;344(3):407–413. doi: 10.1007/s00441-011-1160-3. [DOI] [PubMed] [Google Scholar]
- 78.Liu M., Hurn P. D., Roselli C. E., Alkayed N. J. Role of P450 aromatase in sex-specific astrocytic cell death. Journal of Cerebral Blood Flow and Metabolism. 2007;27(1):135–141. doi: 10.1038/sj.jcbfm.9600331. [DOI] [PubMed] [Google Scholar]
- 79.Miñano A., Cerbón M. A., Xifró X., Malagelada C., Aguilera J., Rodríguez-Alvarez J. 17β-estradiol does not protect cerebellar granule cells from excitotoxicity or apoptosis. Journal of Neurochemistry. 2007;102(2):354–364. doi: 10.1111/j.1471-4159.2007.04475.x. [DOI] [PubMed] [Google Scholar]
- 80.Gordon K. B., Macrae I. M., Carswell H. V. O. Effects of 17beta-oestradiol on cerebral ischaemic damage and lipid peroxidation. Brain Research. 2005;1036(1-2):155–162. doi: 10.1016/j.brainres.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 81.MohanKumar S. M. J., Kasturi B. S., Shin A. C., et al. Chronic estradiol exposure induces oxidative stress in the hypothalamus to decrease hypothalamic dopamine and cause hyperprolactinemia. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology. 2011;300(3):R693–R699. doi: 10.1152/ajpregu.00481.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Charitidi K., Meltser I., Canlon B. Estradiol treatment and hormonal fluctuations during the estrous cycle modulate the expression of estrogen receptors in the auditory system and the prepulse inhibition of acoustic startle response. Endocrinology. 2012;153(9):4412–4421. doi: 10.1210/en.2012-1416. [DOI] [PubMed] [Google Scholar]
- 83.Sharma A. K., Bhattacharya S. K., Khanna N., et al. Effect of progesterone on phosphamidon-induced impairment of memory and oxidative stress in rats. Human & Experimental Toxicology. 2011;30(10):1626–1634. doi: 10.1177/0960327110396522. [DOI] [PubMed] [Google Scholar]
- 84.Ozacmak V. H., Sayan H. The effects of 17β estradiol, 17α estradiol and progesterone on oxidative stress biomarkers in ovariectomized female rat brain subjected to global cerebral ischemia. Physiological Research. 2009;58(6):909–912. doi: 10.33549/physiolres.931647. [DOI] [PubMed] [Google Scholar]
- 85.Aggarwal R., Medhi B., Pathak A., Dhawan V., Chakrabarti A. Neuroprotective effect of progesterone on acute phase changes induced by partial global cerebral ischaemia in mice. The Journal of Pharmacy and Pharmacology. 2008;60(6):731–737. doi: 10.1211/jpp.60.6.0008. [DOI] [PubMed] [Google Scholar]
- 86.Moorthy K., Sharma D., Basir S. F., Baquer N. Z. Administration of estradiol and progesterone modulate the activities of antioxidant enzyme and aminotransferases in naturally menopausal rats. Experimental Gerontology. 2005;40(4):295–302. doi: 10.1016/j.exger.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Pajović S. B., Saičić Z. S., Spasić M. B., Petrović V. M., Martinović J. V. Effects of progesterone and estradiol benzoate on glutathione dependent antioxidant enzyme activities in the brain of female rats. General Physiology and Biophysics. 1999;18(1):35–44. [PubMed] [Google Scholar]
- 88.Kume-Kick J., Rice M. E. Estrogen-dependent modulation of rat brain ascorbate levels and ischemia-induced ascorbate loss. Brain Research. 1998;803(1-2):105–113. doi: 10.1016/s0006-8993(98)00628-3. [DOI] [PubMed] [Google Scholar]
- 89.Irwin R. W., Yao J., Ahmed S. S., Hamilton R. T., Cadenas E., Brinton R. D. Medroxyprogesterone acetate antagonizes estrogen up-regulation of brain mitochondrial function. Endocrinology. 2011;152(2):556–567. doi: 10.1210/en.2010-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fent K. Ecotoxicology of organotin compounds. Critical Reviews in Toxicology. 1996;26(1):1–117. [PubMed] [Google Scholar]
- 91.Antizar-Ladislao B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. A review. Environment International. 2008;34(2):292–308. doi: 10.1016/j.envint.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 92.Tsuda T., Inoue T., Kojima M., Aoki S. Daily intakes of tributyltin and triphenyltin compounds from meals. Journal of AOAC International. 1995;78(4):941–943. [PubMed] [Google Scholar]
- 93.Whalen M. M., Loganathan B. G., Kannan K. Immunotoxicity of environmentally relevant concentrations of butyltins on human natural killer cells in vitro. Environmental Research. 1999;81(2):108–116. doi: 10.1006/enrs.1999.3968. [DOI] [PubMed] [Google Scholar]
- 94.Kannan K., Senthilkumar K., Giesy J. P. Occurrence of butyltin compounds in human blood. Environmental Science and Technology. 1999;33(10):1776–1779. doi: 10.1021/es990011w. [DOI] [Google Scholar]
- 95.Ema M., Itami T., Kawasaki H. Behavioral effects of acute exposure to tributyltin chloride in rats. Neurotoxicology and Teratology. 1991;13(5):489–493. doi: 10.1016/0892-0362(91)90054-Z. [DOI] [PubMed] [Google Scholar]
- 96.Ema M., Itami T., Kawasaki H. Changes of spontaneous motor activity of rats after acute exposure to tributyltin chloride. Drug and Chemical Toxicology. 1991;14(1-2):161–171. doi: 10.3109/01480549109017874. [DOI] [PubMed] [Google Scholar]
- 97.O'Callaghan J. P., Miller D. B. Acute exposure of the neonatal rat to tributyltin results in decreases in biochemical indicators of synaptogenesis and myelinogenesis. Journal of Pharmacology and Experimental Therapeutics. 1988;246(1):394–402. [PubMed] [Google Scholar]
- 98.Yamada J., Inoue K., Furukawa T., Fukuda A. Low-concentration tributyltin perturbs inhibitory synaptogenesis and induces neuronal death in immature but not mature neurons. Toxicology Letters. 2010;198(2):282–288. doi: 10.1016/j.toxlet.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 99.Mizuhashi S., Ikegaya Y., Matsuki N. Cytotoxicity of tributyltin in rat hippocampal slice cultures. Neuroscience Research. 2000;38(1):35–42. doi: 10.1016/S0168-0102(00)00137-1. [DOI] [PubMed] [Google Scholar]
- 100.Kurita R., Hayashi K., Torimitsu K., Niwa O. Continuous measurement of glutamate and hydrogen peroxide using a microfabricated biosensor for studying the neurotoxicity of tributyltin. Analytical Sciences. 2003;19(12):1581–1585. doi: 10.2116/analsci.19.1581. [DOI] [PubMed] [Google Scholar]
- 101.Ishihara Y., Kawami T., Ishida A., Yamazaki T. Tributyltin induces oxidative stress and neuronal injury by inhibiting glutathione S-transferase in rat organotypic hippocampal slice cultures. Neurochemistry International. 2012;60(8):782–790. doi: 10.1016/j.neuint.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 102.Oakley A. Glutathione transferases: a structural perspective. Drug Metabolism Reviews. 2011;43(2):138–151. doi: 10.3109/03602532.2011.558093. [DOI] [PubMed] [Google Scholar]
- 103.Ålin P., Danielson U. H., Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Letters. 1985;179(2):267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- 104.Guo Q., Qian S. Y., Mason R. P. Separation and identification of DMPO adducts of oxygen-centered radicals formed from organic hydroperoxides by HPLC-ESR, ESI-MS and MS/MS. Journal of the American Society for Mass Spectrometry. 2003;14(8):862–871. doi: 10.1016/S1044-0305(03)00336-2. [DOI] [PubMed] [Google Scholar]
- 105.Klimek J., Woźniak M., Szymańska G., Zelewski L. Inhibitory effect of free radicals derived from organic hydroperoxide on progesterone synthesis in human term placental mitochondria. Free Radical Biology and Medicine. 1998;24(7-8):1168–1175. doi: 10.1016/S0891-5849(97)00442-5. [DOI] [PubMed] [Google Scholar]
- 106.Kostyuk V. A., Potapovich A. I., Cesareo E., et al. Dysfunction of glutathione s-transferase leads to excess 4-hydroxy-2-nonenal and H2O2 and impaired cytokine pattern in cultured keratinocytes and blood of vitiligo patients. Antioxidants and Redox Signaling. 2010;13(5):607–620. doi: 10.1089/ars.2009.2976. [DOI] [PubMed] [Google Scholar]
- 107.Uchida K., Shiraishi M., Naito Y., Torii Y., Nakamura Y., Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation: 4-Hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. The Journal of Biological Chemistry. 1999;274(4):2234–2242. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- 108.Baez S., Segura-Aguilar J., Widersten M., Johansson A.-S., Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. The Biochemical Journal. 1997;324(1):25–28. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dagnino-Subiabre A., Cassels B. K., Baez S., Johansson A.-S., Mannervik B., Segura-Aguilar J. Glutathione transferase M2-2 catalyzes conjugation of dopamine and dopa o-quinones. Biochemical and Biophysical Research Communications. 2000;274(1):32–36. doi: 10.1006/bbrc.2000.3087. [DOI] [PubMed] [Google Scholar]
- 110.Kawato S., Yamada M., Kimoto T. Brain neurosteroids are 4th generation neuromessengers in the brain: cell biophysical analysis of steroid signal transduction. Advances in Biophysics. 2003;37:1–48. doi: 10.1016/s0065-227x(03)80002-3. [DOI] [PubMed] [Google Scholar]
- 111.Ishihara Y., Fujitani N., Kawami T., Adachi C., Ishida A., Yamazaki T. Suppressive effects of 17β-estradiol on tributyltin-induced neuronal injury via Akt activation and subsequent attenuation of oxidative stress. Life Sciences. 2014;99(1-2):24–30. doi: 10.1016/j.lfs.2014.01.061. [DOI] [PubMed] [Google Scholar]
- 112.Zorov D. B., Juhaszova M., Sollott S. J. Mitochondrial ROS-induced ROS release: an update and review. Biochimica et Biophysica Acta: Bioenergetics. 2006;1757(5-6):509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 113.Vasconsuelo A., Milanesi L., Boland R. 17β-estradiol abrogates apoptosis in murine skeletal muscle cells through estrogen receptors: role of the phosphatidylinositol 3-kinase/Akt pathway. Journal of Endocrinology. 2008;196(2):385–397. doi: 10.1677/joe-07-0250. [DOI] [PubMed] [Google Scholar]
- 114.Röth E., Marczin N., Balatonyi B., et al. Effect of a glutathione S-transferase inhibitor on oxidative stress and ischemia-reperfusion-induced apoptotic signalling of cultured cardiomyocytes. Experimental and Clinical Cardiology. 2011;16(3):92–96. [PMC free article] [PubMed] [Google Scholar]
- 115.Ishihara Y., Kawami T., Ishida A., Yamazaki T. Allopregnanolone-mediated protective effects of progesterone on tributyltin-induced neuronal injury in rat hippocampal slices. The Journal of Steroid Biochemistry and Molecular Biology. 2013;135(1):1–6. doi: 10.1016/j.jsbmb.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 116.Belelli D., Lambert J. J. Neurosteroids: endogenous regulators of the GABAA receptor. Nature Reviews Neuroscience. 2005;6(7):565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 117.Ardeshiri A., Kelley M. H., Korner I. P., Hurn P. D., Herson P. S. Mechanism of progesterone neuroprotection of rat cerebellar Purkinje cells following oxygen-glucose deprivation. The European Journal of Neuroscience. 2006;24(9):2567–2574. doi: 10.1111/j.1460-9568.2006.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakatsu Y., Kotake Y., Komasaka K., et al. Glutamate excitotoxicity is involved in cell death caused by tributyltin in cultured rat cortical neurons. Toxicological Sciences. 2006;89(1):235–242. doi: 10.1093/toxsci/kfj007. [DOI] [PubMed] [Google Scholar]
- 119.Lee B. Y., Ban J. Y., Seong Y. H. Chronic stimulation of GABAA receptor with muscimol reduces amyloid β protein (25–35)-induced neurotoxicity in cultured rat cortical cells. Neuroscience Research. 2005;52(4):347–356. doi: 10.1016/j.neures.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 120.Aschner M., Syversen T. Methylmercury: recent advances in the understanding of its neurotoxicity. Therapeutic Drug Monitoring. 2005;27(3):278–283. doi: 10.1097/01.ftd.0000160275.85450.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clarkson T. W. The toxicology of mercury. Critical Reviews in Clinical Laboratory Sciences. 1997;34(4):369–403. doi: 10.3109/10408369708998098. [DOI] [PubMed] [Google Scholar]
- 122.Eto K. Minamata disease. Neuropathology. 2000;20(supplement):S14–S19. doi: 10.1046/j.1440-1789.2000.00295.x. [DOI] [PubMed] [Google Scholar]
- 123.Takeuchi T. Pathology of Minamata disease. With special reference to its pathogenesis. Acta Pathologica Japonica. 1982;32(supplement 1):73–99. [PubMed] [Google Scholar]
- 124.Bakir F., Damluji S. F., Amin-Zaki I., et al. Methylmercury poisoning in Iraq. Science. 1973;181(4096):230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- 125.Aschner M., Clarkson T. W. Uptake of methylmercury in the rat brain: effects of amino acids. Brain Research. 1988;462(1):31–39. doi: 10.1016/0006-8993(88)90581-1. [DOI] [PubMed] [Google Scholar]
- 126.Aschner M., Clarkson T. W. Methyl mercury uptake across bovine brain capillary endothelial cells in vitro: the role of amino acids. Pharmacology and Toxicology. 1989;64(3):293–297. doi: 10.1111/j.1600-0773.1989.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 127.Uchino M., Okajima T., Eto K., Kumamoto T., Mishima I., Ando M. Neurologic features of chronic Minamata disease (organic mercury poisoning) certified at autopsy. Internal Medicine. 1995;34(8):744–747. doi: 10.2169/internalmedicine.34.744. [DOI] [PubMed] [Google Scholar]
- 128.Charbonneau S. M., Munro I. C., Nera E. A., et al. Subacute toxicity of methylmercury in the adult cat. Toxicology and Applied Pharmacology. 1974;27(3):569–581. doi: 10.1016/0041-008X(74)90036-2. [DOI] [PubMed] [Google Scholar]
- 129.Ikeda Y., Tobe M., Kobayashi K., Suzuki S., Kawasaka Y., Yonemaru H. Long-term toxicity study of methylmercuric chloride in monkeys (first report) Toxicology. 1973;1(4):361–375. doi: 10.1016/0300-483x(73)90043-7. [DOI] [PubMed] [Google Scholar]
- 130.Klein R., Herman S. P., Brubaker P. E., Lucier G. W., Krigman M. R. A model of acute methyl mercury intoxication in rats. Archives of Pathology. 1972;93(5):408–418. [PubMed] [Google Scholar]
- 131.Farina M., Aschner M., Rocha J. B. T. Oxidative stress in MeHg-induced neurotoxicity. Toxicology and Applied Pharmacology. 2011;256(3):405–417. doi: 10.1016/j.taap.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tsuzuki Y., Yamada T. Inhibitory actions of mercury compounds against glucose-6-phosphate dehydrogenase from yeast. Journal of Toxicological Sciences. 1979;4(2):105–113. doi: 10.2131/jts.4.105. [DOI] [PubMed] [Google Scholar]
- 133.Glaser V., Leipnitz G., Straliotto M. R., et al. Oxidative stress-mediated inhibition of brain creatine kinase activity by methylmercury. NeuroToxicology. 2010;31(5):454–460. doi: 10.1016/j.neuro.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 134.Stringari J., Nunes A. K. C., Franco J. L., et al. Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicology and Applied Pharmacology. 2008;227(1):147–154. doi: 10.1016/j.taap.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Franco J. L., Posser T., Dunkley P. R., et al. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radical Biology & Medicine. 2009;47(4):449–457. doi: 10.1016/j.freeradbiomed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 136.Branco V., Canário J., Holmgren A., Carvalho C. Inhibition of the thioredoxin system in the brain and liver of zebra-seabreams exposed to waterborne methylmercury. Toxicology and Applied Pharmacology. 2011;251(2):95–103. doi: 10.1016/j.taap.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 137.Farina M., Campos F., Vendrell I., et al. Probucol increases glutathione peroxidase-1 activity and displays long-lasting protection against methylmercury toxicity in cerebellar granule cells. Toxicological Sciences. 2009;112(2):416–426. doi: 10.1093/toxsci/kfp219. [DOI] [PubMed] [Google Scholar]
- 138.Usuki F., Yamashita A., Fujimura M. Post-transcriptional defects of antioxidant selenoenzymes cause oxidative stress under methylmercury exposure. Journal of Biological Chemistry. 2011;286(8):6641–6649. doi: 10.1074/jbc.M110.168872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Grotto D., de Castro M. M., Barcelos G. R. M., Garcia S. C., Barbosa F., Jr. Low level and sub-chronic exposure to methylmercury induces hypertension in rats: nitric oxide depletion and oxidative damage as possible mechanisms. Archives of Toxicology. 2009;83(7):653–662. doi: 10.1007/s00204-009-0437-8. [DOI] [PubMed] [Google Scholar]
- 140.de Freitas A. S., Funck V. R., Rotta M. D. S., et al. Diphenyl diselenide, a simple organoselenium compound, decreases methylmercury-induced cerebral, hepatic and renal oxidative stress and mercury deposition in adult mice. Brain Research Bulletin. 2009;79(1):77–84. doi: 10.1016/j.brainresbull.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 141.Mori N., Yasutake A., Hirayama K. Comparative study of activities in reactive oxygen species production/defense system in mitochondria of rat brain and liver, and their susceptibility to methylmercury toxicity. Archives of Toxicology. 2007;81(11):769–776. doi: 10.1007/s00204-007-0209-2. [DOI] [PubMed] [Google Scholar]
- 142.Brookes N., Kristt D. A. Inhibition of amino acid transport and protein synthesis by HgCl2 and methylmercury in astrocytes: selectivity and reversibility. Journal of Neurochemistry. 1989;53(4):1228–1237. doi: 10.1111/j.1471-4159.1989.tb07419.x. [DOI] [PubMed] [Google Scholar]
- 143.Aschner M., Yao C. P., Allen J. W., Tan K. H. Methylmercury alters glutamate transport in astrocytes. Neurochemistry International. 2000;37(2-3):199–206. doi: 10.1016/S0197-0186(00)00023-1. [DOI] [PubMed] [Google Scholar]
- 144.Porciúncula L. O., Rocha J. B. T., Tavares R. G., Ghisleni G., Reis M., Souza D. O. Methylmercury inhibits glutamate uptake by synaptic vesicles from rat brain. NeuroReport. 2003;14(4):577–580. doi: 10.1097/00001756-200303240-00010. [DOI] [PubMed] [Google Scholar]
- 145.Moretto M. B., Funchal C., Santos A. Q., et al. Ebselen protects glutamate uptake inhibition caused by methyl mercury but does not by Hg2+ . Toxicology. 2005;214(1-2):57–66. doi: 10.1016/j.tox.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 146.Reynolds J. N., Racz W. J. Effects of methylmercury on the spontaneous and potassium-evoked release of endogenous amino acids from mouse cerebellar slices. Canadian Journal of Physiology and Pharmacology. 1987;65(5):791–798. doi: 10.1139/y87-127. [DOI] [PubMed] [Google Scholar]
- 147.Vendrell I., Carrascal M., Vilaró M.-T., Abián J., Rodríguez-Farré E., Suñol C. Cell viability and proteomic analysis in cultured neurons exposed to methylmercury. Human & Experimental Toxicology. 2007;26(4):263–272. doi: 10.1177/0960327106070455. [DOI] [PubMed] [Google Scholar]
- 148.Juárez B. I., Martínez M. L., Montante M., Dufour L., García E., Jiménez-Capdeville M. E. Methylmercury increases glutamate extracellular levels in frontal cortex of awake rats. Neurotoxicology and Teratology. 2002;24(6):767–771. doi: 10.1016/s0892-0362(02)00270-2. [DOI] [PubMed] [Google Scholar]
- 149.Ganther H. E. Modification of methylmercury toxicity and metabolism by selenium and vitamin E: possible mechanisms. Environmental Health Perspectives. 1978;25:71–76. doi: 10.1289/ehp.782571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chang L. W., Gilbert M., Sprecher J. Modification of methylmercury neurotoxicity by vitamin E. Environmental Research. 1978;17(3):356–366. doi: 10.1016/0013-9351(78)90040-3. [DOI] [PubMed] [Google Scholar]
- 151.Beyrouty P., Chan H. M. Co-consumption of selenium and vitamin E altered the reproductive and developmental toxicity of methylmercury in rats. Neurotoxicology and Teratology. 2006;28(1):49–58. doi: 10.1016/j.ntt.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 152.Prasad K. N., Ramanujam S. Vitamin E and vitamin C alter the effect of methylmercuric chloride on neuroblastoma and glioma cells in culture. Environmental Research. 1980;21(2):343–349. doi: 10.1016/0013-9351(80)90036-5. [DOI] [PubMed] [Google Scholar]
- 153.West A. K., Hidalgo J., Eddins D., Levin E. D., Aschner M. Metallothionein in the central nervous system: roles in protection, regeneration and cognition. Neurotoxicology. 2008;29(3):489–503. doi: 10.1016/j.neuro.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang P., Xu Y., Li L., Jiang Q., Wang M., Jin L. In vitro protective effects of pyrroloquinoline quinone on methylmercury-induced neurotoxicity. Environmental Toxicology and Pharmacology. 2009;27(1):103–110. doi: 10.1016/j.etap.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 155.Sakaue M., Mori N., Okazaki M., et al. Vitamin K has the potential to protect neurons from methylmercury-induced cell death in vitro. Journal of Neuroscience Research. 2011;89(7):1052–1058. doi: 10.1002/jnr.22630. [DOI] [PubMed] [Google Scholar]
- 156.Kumagai Y., Kanda H., Shinkai Y., Toyama T. The role of the Keap1/Nrf2 pathway in the cellular response to methylmercury. Oxidative Medicine and Cellular Longevity. 2013;2013:8. doi: 10.1155/2013/848279.848279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Daré E., Götz M. E., Zhivotovsky B., Manzo L., Ceccatelli S. Antioxidants J811 and 17β-estradiol protect cerebellar granule cells from methylmercury-induced apoptotic cell death. Journal of Neuroscience Research. 2000;62(4):557–565. doi: 10.1002/1097-4547(20001115)62:4x003C;557::aid-jnr10x0003e;3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 158.Malagutti K. S., da Silva A. P., Braga H. C., et al. 17β-estradiol decreases methylmercury-induced neurotoxicity in male mice. Environmental Toxicology and Pharmacology. 2009;27(2):293–297. doi: 10.1016/j.etap.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 159.Munetsuna E., Hojo Y., Hattori M., et al. Retinoic acid stimulates 17β-estradiol and testosterone synthesis in rat hippocampal slice cultures. Endocrinology. 2009;150(9):4260–4269. doi: 10.1210/en.2008-1644. [DOI] [PubMed] [Google Scholar]
- 160.Yamazaki T., Yamamoto M., Ishihara Y., et al. De novo synthesized estradiol protects against methylmercury-induced neurotoxicity in cultured rat hippocampal slices. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0055559.e55559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chetty C. S., Vemuri M. C., Reddy G. R., Suresh C. Protective effect of 17-β-estradiol in human neurocellular models of lead exposure. NeuroToxicology. 2007;28(2):396–401. doi: 10.1016/j.neuro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 162.Konkle A. T. M., McCarthy M. M. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152(1):223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Amateau S. K., Alt J. J., Stamps C. L., McCarthy M. M. Brain estradiol content in newborn rats: Sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145(6):2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]