Abstract
Cardiovascular disease is the leading cause of premature death worldwide, and atherosclerosis is the main contributor. Lipid-laden macrophages, known as foam cells, accumulate in the subendothelial space of the lesion area and contribute to consolidate a chronic inflammatory environment where oxygen and nitrogen derived oxidants are released. Oxidatively modified lipids and proteins are present both in plasma as well as atherosclerotic lesions. A relevant oxidative posttranslational protein modification is the addition of a nitro group to the hydroxyphenyl ring of tyrosine residues, mediated by nitric oxide derived oxidants. Nitrotyrosine modified proteins were found in the lesion and also in plasma from atherosclerotic patients. Despite the fact of the low yield of nitration, immunogenic, proatherogenic, and prothrombotic properties acquired by 3-nitrotyrosine modified proteins are in agreement with epidemiological studies showing a significant correlation between the level of nitration found in plasma proteins and the prevalence of cardiovascular disease, supporting the usefulness of this biomarker to predict the outcome and to take appropriate therapeutic decisions in atherosclerotic disease.
1. Introduction
A wide range of studies support the role of oxidative stress in the development of cardiovascular disease [1–6], and the evaluation of oxidant-mediated biomolecule modifications is able to predict clinical outcomes [7–9]. The atheromatous process is related to endothelial dysfunction, and the presence of atherosclerotic risk factors such as hypercholesterolemia and hypertension induces the expression of cell adhesion molecules such as VCAM-1, ICAM-1, E-selectin, and P-selectin [10], which promote the adhesion of monocytes and T cells to the vascular endothelium and its transmigration into the subendothelial space. Leukocytes migrating from the blood stream to the vascular wall play a fundamental role in atherosclerosis, acting as nucleating centers for modified biomolecules and also as the main source of oxidants inside the inflamed blood vessel. Uncontrolled uptake of LDL and altered cholesterol efflux are the main factors that contribute to macrophages lipid overload and foam cell formation [11]. In macrophages, the uptake of oxidized LDL is mediated by a group of receptors, including the scavenger receptors class A (SR-A) and CD36, a class B receptor, and the lectin-type oxidized LDL receptor 1 (LOX-1) [12, 13]. On the contrary, the scavenger receptor B1 (SR-B1) and the ATP-binding cassette transporters A1 (ABCA1) and G1 (ABCG1) are responsible for cholesterol efflux [14].
Activation of inflammatory cells into the subendothelial space is tightly associated with generation of reactive oxygen species (ROS) and nitrogen species (RNS), which can mediate protein and lipid modifications. Protein nitration is a posttranslational modification caused by nitric oxide (•NO) derived oxidants that frequently modifies the activity of the target molecule [15, 16]. The presence of proteins bearing the 3-nitrotyrosine modification was described in both plasma and atherosclerotic lesions from coronary artery disease patients and also from atherosclerotic prone mice [17, 18].
2. Mechanisms of Protein Nitration
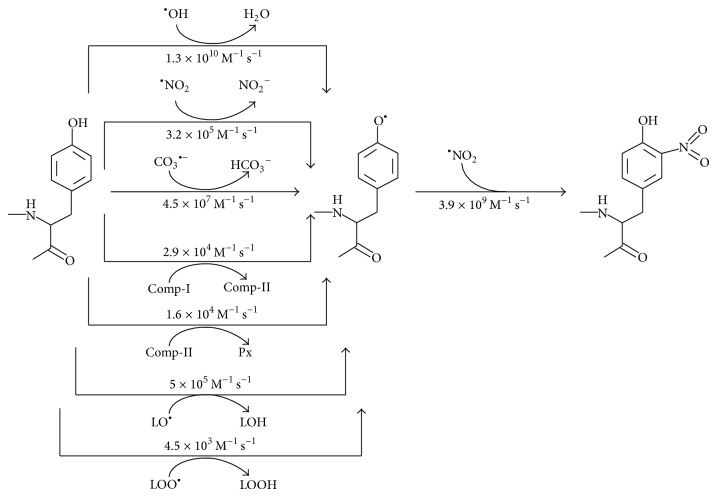
Protein nitration involves two steps (Figure 1); in the first one hydrogen atom is lost from the phenolic ring of tyrosine residues with the transient formation of a tyrosyl radical (Tyr•). This step is followed by the diffusion controlled reaction of Tyr• with nitrogen dioxide radical (•NO2) at diffusion controlled rate (k = 3.9 × 109 M−1 s−1) [19] (Figure 1). The initial oxidation of tyrosine can be achieved by a number of oxidants, including hydroxyl radical (•OH, k = 1.3 × 1010 M−1 s−1) [20] and •NO2 (k = 3.2 × 105 M−1 s−1) [21].
Figure 1.
Mechanism of protein 3-nitrotyrosine formation.
Peroxynitrite (ONOO−), the diffusion controlled reaction product between •NO and superoxide (O2 •−) (1), generates both radicals [16]:
| (1) |
Actually, the homolytic decomposition of the protonated form, peroxynitrous acid (pKa = 6.8), generates •OH and •NO2:
| (2) |
Carbonyl radicals (CO3 •−), produced by decomposition of nitrosoperoxycarboxylate (ONOOCO2), the product of the reaction between peroxynitrite and CO2(3), also react with tyrosine residues (k = 4.5 × 107 M−1 s−1) [22]:
| (3) |
Lipid-derived alkoxyl (LO•) (k = 5 × 105 M−1 s−1) [23] and peroxyl radicals (LOO•) (k = 4.5 × 103 M−1 s−1) [24] can also promote one-electron oxidations of tyrosine residues in proteins. Meanwhile, myeloperoxidase (MPO) is able to feed both steps. In the first one, MPO-derived compounds I (k = 2.9 × 104 M−1 s−1) [25] and II (k = 1.57 × 104 M−1 s−1) [26] react with tyrosine to yield Tyr•. In addition, both compounds generate •NO2 [27] ((4)–(6)), which is able to mediate the modification of tyrosine residues attained in both steps (Figure 1):
| (4) |
| (5) |
| (6) |
3. Vascular Sources of Reactive Species
Protein tyrosine nitration is localized within specific subcellular compartments in close proximity to the enzymes related to the production of the involved oxidants, as demonstrated by immunoelectron microscopy [28]. The formation of the main precursors of ROS is catalyzed by a group of specially committed enzymes present fundamentally in the plasma membrane and membrane surrounded organelles known as NADPH-oxidase (Nox, EC 1.6.3.1). The Nox family of enzymes is specifically dedicated to generate oxygen derived oxidants, in particular O2 •− and less frequently hydrogen peroxide (H2O2). Four Nox isoforms have been found in the vasculature, Nox1, Nox2, Nox4, and Nox5, and at the vascular level Nox enzymes have emerged as the major source of ROS [29]. The different Nox isoforms accomplish several biological functions, which are dependent not exclusively on the enzyme but also on the specific cell type. Nox1 is expressed in endothelium, smooth muscle cells, and adventitial fibroblasts [30, 31], while Nox2, Nox4, and Nox5 are found in all vascular wall cells [31, 32]. Generation of ROS by phagocytic cells, mostly mediated by Nox2, is activated by several stimuli through receptor-mediated protein kinase activation. In fact, cytokines and modified-LDL are able to trigger p47phox phosphorylation and its migration to the plasma membrane where it associates with the electron transferase (gp91phox) and p22phox, activating O2 •− production. During membrane migration p47phox is escorted by several cytosolic subunits, in particular p67phox and Rac2 [33]. While Nox1 is activated in an analogous way as Nox2, Nox4 activation requires the association of Poldip2 with p22phox [34], and Nox5 is activated by association of Ca2+ with its N-terminal calmodulin-like domain, which contains four Ca2+-binding EF-hand motifs. As other Nox enzymes, Nox5 is also regulated by protein kinase C (PKC) as well as the tyrosine kinase c-Abl [35].
Other putative sources of ROS at vascular level are xanthine oxidase (XO, EC 1.17.3.2) and the mitochondrial electron transport chain. Unlike its precursor xanthine dehydrogenase (XDH, EC 1.17.1.4), which uses NAD+, xanthine oxidase uses oxygen as electron acceptor [36–38]. Superoxide is generated as a mitochondria byproduct, by electron leakages predominantly at complexes I and III [39, 40].
Meanwhile •NO, the main precursor of reactive nitrogen species (RNS) is generated by the family of nitric oxide synthases (NOS, EC 1.14.13.39) from L-arginine. In the vasculature •NO produced by the endothelial isoform or NOS3 is responsible for the endothelial-mediated vascular relaxation. In the vascular wall the inducible form or NOS2 generates •NO after cell stimulation. Macrophage activation may lead to the simultaneous production of O2 •− and •NO and consequently to ONOO− formation. Actually, the presence of proinflammatory cytokines, as interleukin-1β, tumor necrosis factor α, and interferon γ, generated by inflammatory cells induces simultaneously the assembly of Nox2 and the expression of NOS2 [41–43].
Myeloperoxidase (EC 1.11.2.2) is a member of the mammalian heme peroxidase superfamily of enzymes and uses H2O2 to form more reactive oxidant species. In the presence of MPO and H2O2, hypochlorous acid (HOCl) and •NO2 are formed from Cl− and NO2 −, respectively [44, 45]. Circulating neutrophils, monocytes, and some tissue macrophages express MPO [46]. While MPO and its products are important defense factors against invading microorganisms, different evidences suggest that excessive activity of MPO can play a role in inflammatory tissue injury. In fact, plasma MPO independently predicted the early risk of myocardial infarction, as well as the risk of other major adverse cardiac events (MACE) in patients with chest pain [47, 48]. Additional evidences of the role of MPO in vascular pathology come from population studies, where elevated circulating levels of this enzyme in an initially healthy population predicted the risk of future coronary heart disease [9, 49, 50]. In patients being treated for coronary artery disease, increased MPO concentrations remained significantly associated with incident MACE over a follow-up of 3 years, even after adjusting for traditional cardiac risk factors, creatinine clearance, B-type natriuretic peptide, and high-sensitivity C-reactive protein [51]. In fact, the accumulation of leukocytes containing MPO in the subendothelial space in sites of erosion and breakdown of coronary plaque has been reported [52, 53], pointing to this enzyme as one of those responsible for the acute coronary syndrome.
4. Main Nitration Targets in Atherosclerosis
State-of-the-art technology has allowed precise evaluation of circulating nitrotyrosine modified proteins. Higher levels of 3-nitrotyrosine in plasma proteins have been reported in atherosclerotic patients and accurately measured by stable isotope dilution HPLC with on-line electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS) [54] and specific ELISA techniques developed to measure fibrinogen and 3-nitrotyrosine modified fibrinogen [55, 56]. An important increase of 3-nitrotyrosine modified apolipoprotein A-1 (apoA-1), apolipoprotein B-100 (apoB-100), and fibrinogen has been reported in plasma from individuals diagnosed with coronary artery disease (Table 1). In addition, using specific enrichment and mass spectrometric techniques the site of nitration was identified in several proteins isolated from human plasma (Table 2).
Table 1.
Quantitative estimation of 3-nitrotyrosine in CVD plasma.
| Control | CVD | Change (%) | Reference | |||
|---|---|---|---|---|---|---|
| NO2-Tyr/Tyr (μmol/mol)1 |
NO2-Tyr/protein molecule2 | NO2-Tyr/Tyr (μmol/mol)1 |
NO2-Tyr/protein molecule2 | |||
| Total serum proteins3 | 6.1 (3.9–7.8) | NA | 9.0 (5.7–12.9) | NA | 47 | [57] |
| ApoA-I3 | 438 (335–598) | 1/325 | 629 (431–876) | 1/227 | 44 | [57] |
| ApoB-1003 | 4.0 (1.3–6.9) | 1/1,644 | 8.7 (5.2–12.1) | 1/756 | 117 | [57] |
| Fibrinogen4 | 24.6 (23.4–25.9) | 1/303 | 31.8 (28.7–34.9) | 1/235 | 29 | [56] |
1Nitrotyrosine levels are reported as median (IQR).
2The number of Tyr modified residues per protein molecule (NO2-Tyr/protein molecule) was calculated using 153, 7, and 134 Tyr residues for apoB-100, apoA-1, and fibrinogen, respectively, from the PromParam tool (Expasy) [76].
3Data in reference [57] were obtained using stable isotope dilution LC/ESI/MS/MS.
4Plasma concentrations of total and nitrated fibrinogen in reference [56] were determined by ELISA and reported as mg/mL for fibrinogen and nM for nitrotyrosine; μmol NO2-Tyr/mol Tyr were calculated using a molecular weight for fibrinogen of 340 kDa and 134 Tyr residues.
Table 2.
Nitration sites in plasma proteins identified by mass spectrometry.
| Protein | Nitrated Tyr | Effect | References |
|---|---|---|---|
| ApoA-11 | Y166 and Y192 | Decreased activation of LCAT and ABCA1 | [59, 77–79] |
| ApoB-1002 | Y276, Y583, Y666, Y720, Y2524, Y3139, Y3295, Y3489, Y4141 | Increased affinity for LOX-1, CD36, SR-A | [69] |
| Fibrinogen β chain1 | Y292, Y422 | Accelerated clot formation | [55] |
| Ig gamma-1 chain C region1 | Y161, Y290 | Unknown | [18] |
| Ig kappa chain C region1 | Y32, Y84 | Unknown | [18] |
| Ig lambda chain C region1 | Y84 | Unknown | [18] |
| Ig mu chain C region1 | Y276 | Unknown | [18] |
| Ig heavy chain V-III1 | Y33, Y80, Y95 | Unknown | [18] |
| Zinc finger and BTB domain-containing protein 11 | Y83 | Unknown | [18] |
| Protein EFR3 homolog B1 | Y669 | Unknown | [18] |
1Data from proteins immunocaptured from individuals diagnosed with CVD.
2Data from an electronegative LDL fraction isolated from plasma from healthy humans.
Apolipoprotein A-1, the major protein in high density lipoprotein (HDL), was a preferential target for nitration in subjects with CVD. Experimental evidences support the role of MPO in circulating apoA-1 nitration [57]. In particular, coimmunoprecipitation experiments proved the presence of circulating apoA-1/MPO complexes in HDL isolated from human CVD plasma [58]. Colocalization of 3-nitrotyrosine modified HDL with MPO in human aortic atherosclerotic intima was also reported [59]. Moreover, MPO levels predicted accelerated progression of coronary atherosclerosis in diabetic patients [60]. Consequently, MPO appears to be responsible for the dramatic increase of nitrotyrosine and chlorotyrosine observed within apoA-1 in HDL recovered from serum and atherosclerotic lesions from individuals with CVD. In cholesterol-loaded murine macrophages, nitration and chlorination of apoA-1, both in vitro and in vivo, resulted in a less effective protein than the unmodified one to stimulate ABCA-1-dependent cholesterol efflux [57, 58, 61]. While in CVD patients, the site of union to lecithin cholesterol acyltransferase (LCAT), involving tyrosine 166, was the primary target for apoA-1 modification (Table 2). Nitration of this tyrosine residue decreased apoA-1-mediated LCAT activation and resulted in a dysfunctional HDL particle [62–64]. MPO-modified apoA-1 showed also a reduced capacity to stimulate endothelial cell proliferation and migration, through decreased Akt and ERK1/2 phosphorylation [65]. The modification of apoA-1 in vitro by MPO-derived hypochlorous acid, in protein residues different from tyrosine, switched the role of HDL in inflammation from anti- to proinflammatory. In fact, the association of this oxidized lipoprotein form to endothelial cells led to NF-κB activation and the appearance of VCAM on the cell surface. This gain of function was mediated by the saturable and specific binding of oxidized HDL to an unknown endothelial cell receptor, different from the scavenger receptors CD36 and SR-A [66].
Apolipoprotein B-100, the main LDL protein, has also been found nitrated in CVD plasma (Table 1). Several LDL nitration sites were identified by mass spectrometry after authentic peroxynitrite exposure [67, 68] and also by upregulation of Nox expression by bovine aortic endothelial cells exposed to oscillatory and pulsatile shear stress [67]. A similar pattern of tyrosine nitration was observed in circulating LDL isolated from healthy blood donors (Table 2) [69]; no data on CVD patients were reported. Nitrated apoB-100 showed profound conformational changes, which promoted increased LDL binding and uptake by endothelial cells. Internalization of this modified form of LDL was mediated by LOX-1, CD36, and SR-A [69]. Moreover, the in vitro exposure to nitrating agents derived from monocytes in the presence of exogenous NO2 − converted LDL into a form that was taken up and degraded by macrophages, leading to foam cell formation [70].
Fibrinogen is another important target of reactive species in CVD, and increased levels of nitrated fibrinogen were found in patients with coronary artery disease (Table 1) [56]. In otherwise healthy humans, an inflammatory challenge was able to induce fibrinogen nitration [71]. Moreover, in atherosclerosis-prone mice, knockout for the LDL receptor and apolipoprotein B mRNA editing enzyme (apobec), the lack of apoA-1 increased the level of nitrated fibrinogen in plasma, pointing to a subrogate role for the coagulation protein as a nitration target [17]. Besides, cigarette smoking, an important risk factor for both atherosclerosis and thrombosis, also induced an important increase in the level of 3-nitrotyrosine modified fibrinogen. In fact, nitrated fibrinogen was significantly higher in smokers (51.0 ± 5.5 μmol NO2-Tyr/mol Tyr) compared with nonsmokers (36.0 ± 3.2 μmol NO2-Tyr/mol Tyr) [55]. The presence of 3-nitrotyrosine in fibrinogen β chain significantly accelerated fibrin clot formation [17]. The fibrin clot architecture was altered, with increased stiffness, and the rate of clot lysis was reduced by nitration [55, 72]. This modified form of fibrinogen could favor fibrin deposition onto atherosclerotic plaques and explain the increased propensity for thrombotic events found in coronary artery disease subjects and atherosclerosis-prone mice.
To add to the pathogenic effects of protein nitration in atherosclerosis, the presence of increased levels of nitrotyrosine in circulating proteins and proteins isolated from atherosclerotic plaques was associated with the presence of circulating specific immunoglobulins against the nitrated epitope. Anti-3-nitrotyrosine antibodies were strongly associated with angiographic evidence of significant coronary artery disease [18]. The levels of immunoglobulins that recognize 3-nitrotyrosine were significantly high also in plasma of subjects with acute lung injury [73] and in atherosclerosis-prone mice [17]; however the functional repercussion of this immune response still remains unexplored. Passive immunization of experimentation animals would help to understand the role of anti-nitrotyrosine antibodies in atherosclerosis. In addition, the identification of protein(s) responsible for triggering the adaptive immune response against the nitrated epitope would help to bring some light onto the pathways linking inflammation, oxidative posttranslational protein modifications, and atherosclerosis.
5. Concluding Remarks
We and others have demonstrated the presence and structural and functional consequences of the modification by 3-nitrotyrosine on plasma as well as tissue proteins [16]. Despite the fact of significant increases observed on protein nitration in CVD, the inhibitory effect of this posttranslational protein modification on the activity of the entire population of protein molecules is minimal, since for each protein molecule modified by nitration there are several hundreds of them still unmodified and active [74]. For instance, apoA-1 nitration increased 47% in CVD patients (Table 1); this supposed a change in the number of tyrosine residues modified by nitration from one residue each 325 protein molecules to one residue each 227 protein molecules. The highly significant number of unmodified protein molecules would be enough to fulfill the protein function without any manifest biochemical effect. However, as already discussed, nitration of apoB-100 and fibrinogen promote new proatherogenic and prothrombotic functions, which together with the onset of an adaptive immune response triggered by the nitrated epitope agree with epidemiological results demonstrating a significant correlation between plasma 3-nitrotyrosine levels and higher cardiovascular risk and support the usefulness of this posttranslational protein modification as a risk marker [75].
Acknowledgment
The work was partially supported by PEDECIBA, Uruguay.
Abbreviations
- ROS:
Reactive oxygen species
- RNS:
Reactive nitrogen species
- NO2-Tyr:
3-Nitrotyrosine
- LDL:
Low-density lipoprotein
- HDL:
High density lipoprotein
- apoB-100:
Apolipoprotein B-100
- apoA-1:
Apolipoprotein A-1
- SR-A:
Scavenger receptor class A
- CD36:
Scavenger receptor CD36
- LOX-1:
Lectin-type oxidized LDL receptor 1
- SR-B1:
Scavenger receptor B1
- ABCA1:
ATP-binding cassette transporter A1
- ABCG1:
ATP-binding cassette transporters G1
- Nox:
NADPH-oxidase
- MPO:
Myeloperoxidase
- NOS:
Nitric oxide synthase
- LC/EIS/MS/MS:
Liquid chromatography electron ionization spray mass spectrometry.
Conflict of Interests
The author declares that there is no conflict of interests regarding the publication of this paper.
References
- 1.Niu X.-L., Madamanchi N. R., Vendrov A. E., et al. Nox activator 1: a potential target for modulation of vascular reactive oxygen species in atherosclerotic arteries. Circulation. 2010;121(4):549–559. doi: 10.1161/circulationaha.109.908319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minuz P., Patrignani P., Gaino S., et al. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease. Circulation. 2002;106(22):2800–2805. doi: 10.1161/01.CIR.0000039528.49161.E9. [DOI] [PubMed] [Google Scholar]
- 3.Hidaka T., Nakagawa K., Goto C., et al. Pioglitazone improves endothelium-dependent vasodilation in hypertensive patients with impaired glucose tolerance in part through a decrease in oxidative stress. Atherosclerosis. 2010;210(2):521–524. doi: 10.1016/j.atherosclerosis.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Heymes C., Bendall J. K., Ratajczak P., et al. Increased myocardial NADPH oxidase activity in human heart failure. Journal of the American College of Cardiology. 2003;41(12):2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 5.Barry-Lane P. A., Patterson C., van der Merwe M., et al. p47phox is required for atherosclerotic lesion progression in ApoE−/− mice. The Journal of Clinical Investigation. 2001;108(10):1513–1522. doi: 10.1172/jci200111927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballinger S. W., Patterson C., Knight-Lozano C. A., et al. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106(5):544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 7.Schwedhelm E., Bartling A., Lenzen H., et al. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 2004;109(7):843–848. doi: 10.1161/01.cir.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 8.Keaney J. F., Jr., Larson M. G., Vasan R. S., et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(3):434–439. doi: 10.1161/01.atv.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 9.Karakas M., Koenig W., Zierer A., et al. Myeloperoxidase is associated with incident coronary heart disease independently of traditional risk factors: results from the MONICA/KORA Augsburg study. Journal of Internal Medicine. 2012;271(1):43–50. doi: 10.1111/j.1365-2796.2011.02397.x. [DOI] [PubMed] [Google Scholar]
- 10.Madamanchi N. R., Vendrov A., Runge M. S. Oxidative stress and vascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(1):29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 11.Li A. C., Glass C. K. The macrophage foam cell as a target for therapeutic intervention. Nature Medicine. 2002;8(11):1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 12.Young M. P., Febbraio M., Silverstein R. L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. The Journal of Clinical Investigation. 2009;119(1):136–145. doi: 10.1172/jci35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greaves D. R., Gordon S. Recent insights into the biology of macrophage scavenger receptors. Journal of Lipid Research. 2005;46(1):11–20. doi: 10.1194/jlr.R400011-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.van Eck M., Pennings M., Hoekstra M., Out R., van Berkel T. J. C. Scavenger receptor BI and ATP-binding cassette transporter A1 in reverse cholesterol transport atherosclerosis. Current Opinion in Lipidology. 2005;16(3):307–315. doi: 10.1097/01.mol.0000169351.28019.04. [DOI] [PubMed] [Google Scholar]
- 15.Cassina A. M., Hodara R., Souza J. M., et al. Cytochrome c nitration by peroxynitrite. Journal of Biological Chemistry. 2000;275(28):21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 16.Trujillo M., Alvarez B., Souza J. M., et al. Mechanisms and biological consequences of peroxynitrite-dependent protein oxidation and nitration. In: Ignarro L., editor. Nitric Oxide. Biology and Pathobiology. San Diego, Calif, USA: Academic Press; 2010. pp. 1010–1050. [Google Scholar]
- 17.Parastatidis I., Thomson L., Fries D. M., et al. Increased protein nitration burden in the atherosclerotic lesions and plasma of apolipoprotein A-I-deficient mice. Circulation Research. 2007;101(4):368–376. doi: 10.1161/CIRCRESAHA.107.157537. [DOI] [PubMed] [Google Scholar]
- 18.Thomson L., Tenopoulou M., Lightfoot R., et al. Immunoglobulins against tyrosine-nitrated epitopes in coronary artery disease. Circulation. 2012;126(20):2392–2401. doi: 10.1161/CIRCULATIONAHA.112.103796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaz S. M., Augusto O. Inhibition of myeloperoxidase-mediated protein nitration by tempol: kinetics, mechanism, and implications. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(24):8191–8196. doi: 10.1073/pnas.0708211105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solar S., Solar W., Getoff N. Reactivity of hydroxyl with tyrosine in aqueous solution studied by pulse radiolysis. The Journal of Physical Chemistry. 1984;88(10):2091–2095. doi: 10.1021/j150654a030. [DOI] [Google Scholar]
- 21.Prütz W. A., Mönig H., Butler J., Land E. J. Reactions of nitrogen dioxide in aqueous model systems: oxidation of tyrosine units in peptides and proteins. Archives of Biochemistry and Biophysics. 1985;243(1):125–134. doi: 10.1016/0003-9861(85)90780-5. [DOI] [PubMed] [Google Scholar]
- 22.Augusto O., Bonini M. G., Amanso A. M., Linares E., Santos C. C. X., de Menezes S. L. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radical Biology and Medicine. 2002;32(9):841–859. doi: 10.1016/s0891-5849(02)00786-4. [DOI] [PubMed] [Google Scholar]
- 23.Folkes L. K., Bartesaghi S., Trujillo M., Radi R., Wardman P. Kinetics of oxidation of tyrosine by a model alkoxyl radical. Free Radical Research. 2012;46(9):1150–1156. doi: 10.3109/10715762.2012.695868. [DOI] [PubMed] [Google Scholar]
- 24.Bartesaghi S., Wenzel J., Trujillo M., et al. Lipid peroxyl radicals mediate tyrosine dimerization and nitration in membranes. Chemical Research in Toxicology. 2010;23(4):821–835. doi: 10.1021/tx900446r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tien M. Myeloperoxidase-catalyzed oxidation of tyrosine. Archives of Biochemistry and Biophysics. 1999;367(1):61–66. doi: 10.1006/abbi.1999.1226. [DOI] [PubMed] [Google Scholar]
- 26.Marquez L. A., Dunford H. B. Kinetics of oxidation of tyrosine and dityrosine by myeloperoxidase compounds I and II: implications for lipoprotein peroxidation studies. Journal of Biological Chemistry. 1995;270(51):30434–30440. doi: 10.1074/jbc.270.51.30434. [DOI] [PubMed] [Google Scholar]
- 27.van der Vliet A., Eiserich J. P., Halliwell B., Cross C. E. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite: a potential additional mechanism of nitric oxide- dependent toxicity. The Journal of Biological Chemistry. 1997;272(12):7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 28.Heijnen H. F. G., van Donselaar E., Slot J. W., et al. Subcellular localization of tyrosine-nitrated proteins is dictated by reactive oxygen species generating enzymes and by proximity to nitric oxide synthase. Free Radical Biology and Medicine. 2006;40(11):1903–1913. doi: 10.1016/j.freeradbiomed.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Lassègue B., Griendling K. K. NADPH oxidases: functions and pathologies in the vasculature. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(4):653–661. doi: 10.1161/atvbaha.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csányi G., Taylor W. R., Pagano P. J. NOX and inflammation in the vascular adventitia. Free Radical Biology and Medicine. 2009;47(9):1254–1266. doi: 10.1016/j.freeradbiomed.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lassègue B., Clempus R. E. Vascular NAD(P)H oxidases: specific features, expression, and regulation. The American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2003;285(2):R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 32.Chamseddine A. H., Miller F. J., Jr. gp91phox contributes to NADPH oxidase activity in aortic fibroblasts but not smooth muscle cells. The American Journal of Physiology—Heart and Circulatory Physiology. 2003;285(6):H2284–H2289. doi: 10.1152/ajpheart.00459.2003. [DOI] [PubMed] [Google Scholar]
- 33.Lassègue B., Martín A. S., Griendling K. K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circulation Research. 2012;110(10):1364–1390. doi: 10.1161/circresaha.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyle A. N., Deshpande N. N., Taniyama Y., et al. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circulation Research. 2009;105(3):249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Jamali A., Valente A. J., Lechleiter J. D., et al. Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radical Biology and Medicine. 2008;44(5):868–881. doi: 10.1016/j.freeradbiomed.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radi R., Rubbo H., Thomson L., Prodanov E. Luminol chemiluminescence using xanthine and hypoxanthine as xanthine oxidase substrates. Free Radical Biology and Medicine. 1990;8(2):121–126. doi: 10.1016/0891-5849(90)90084-V. [DOI] [PubMed] [Google Scholar]
- 37.Aslan M., Freeman B. A. Oxidases and oxygenases in regulation of vascular nitric oxide signaling and inflammatory responses. Immunologic Research. 2002;26(1–3):107–118. doi: 10.1385/ir:26:1-3:107. [DOI] [PubMed] [Google Scholar]
- 38.Houston M., Estevez A., Chumley P., et al. Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. Journal of Biological Chemistry. 1999;274(8):4985–4994. doi: 10.1074/jbc.274.8.4985. [DOI] [PubMed] [Google Scholar]
- 39.Kowaltowski A. J., de Souza-Pinto N. C., Castilho R. F., Vercesi A. E. Mitochondria and reactive oxygen species. Free Radical Biology and Medicine. 2009;47(4):333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Dröse S., Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Advances in Experimental Medicine and Biology. 2012;748:145–169. doi: 10.1007/978-1-4614-3573-0_6. [DOI] [PubMed] [Google Scholar]
- 41.Bedard K., Lardy B., Krause K.-H. NOX family NADPH oxidases: not just in mammals. Biochimie. 2007;89(9):1107–1112. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 42.Förstermann U., Sessa W. C. Nitric oxide synthases: regulation and function. European Heart Journal. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowenstein C. J., Padalko E. iNOS (NOS2) at a glance. Journal of Cell Science. 2004;117:2865–2867. doi: 10.1242/jcs.01166. [DOI] [PubMed] [Google Scholar]
- 44.Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Annals of Internal Medicine. 1980;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 45.Hazen S. L., Zhang R., Shen Z., et al. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation In vivo. Circulation Research. 1999;85(10):950–958. doi: 10.1161/01.res.85.10.950. [DOI] [PubMed] [Google Scholar]
- 46.Daugherty A., Dunn J. L., Rateri D. L., Heinecke J. W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. Journal of Clinical Investigation. 1994;94(1):437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brennan M.-L., Penn M. S., Van Lente F., et al. Prognostic value of myeloperoxidase in patients with chest pain. The New England Journal of Medicine. 2003;349(17):1595–1604. doi: 10.1056/nejmoa035003. [DOI] [PubMed] [Google Scholar]
- 48.Nicholls S. J., Wilson Tang W. H., Brennan D., et al. Risk prediction with serial myeloperoxidase monitoring in patients with acute chest pain. Clinical Chemistry. 2011;57(12):1762–1770. doi: 10.1373/clinchem.2011.166827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meuwese M. C., Stroes E. S. G., Hazen S. L., et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. Journal of the American College of Cardiology. 2007;50(2):159–165. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 50.Rana J. S., Arsenault B. J., Després J.-P., et al. Inflammatory biomarkers, physical activity, waist circumference, and risk of future coronary heart disease in healthy men and women. European Heart Journal. 2011;32(3):336–344. doi: 10.1093/eurheartj/ehp010. [DOI] [PubMed] [Google Scholar]
- 51.Tang W. H. W., Wu Y., Nicholls S. J., Hazen S. L. Plasma myeloperoxidase predicts incident cardiovascular risks in stable patients undergoing medical management for coronary artery disease. Clinical Chemistry. 2011;57(1):33–39. doi: 10.1373/clinchem.2010.152827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugiyama S., Kugiyama K., Aikawa M., Nakamura S., Ogawa H., Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(7):1309–1314. doi: 10.1161/01.atv.0000131784.50633.4f. [DOI] [PubMed] [Google Scholar]
- 53.Sugiyama S., Okada Y., Sukhova G. K., Virmani R., Heinecke J. W., Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. The American Journal of Pathology. 2001;158(3):879–891. doi: 10.1016/s0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shishehbor M. H., Aviles R. J., Brennan M.-L., et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. Journal of the American Medical Association. 2003;289(13):1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 55.Parastatidis I., Thomson L., Burke A., et al. Fibrinogen β-chain tyrosine nitration is a prothrombotic risk factor. The Journal of Biological Chemistry. 2008;283(49):33846–33853. doi: 10.1074/jbc.m805522200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez M., Cuker A., Mills A., et al. Nitrated fibrinogen is a biomarker of oxidative stress in venous thromboembolism. Free Radical Biology and Medicine. 2012;53(2):230–236. doi: 10.1016/j.freeradbiomed.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng L., Nukuna B., Brennan M. L., et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and function impairment in subjects with cardiovascular disease. Journal of Clinical Investigation. 2004;114(4):529–541. doi: 10.1172/jci200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicholls S. J., Hazen S. L. Myeloperoxidase, modified lipoproteins, and atherogenesis. The Journal of Lipid Research. 2009;50(supplement):S346–S351. doi: 10.1194/jlr.r800086-jlr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng L., Settle M., Brubaker G., et al. Localization of nitration and chlorination sites on apolipoprotein A-I catalysed by myeloperoxidase in human atheroma and associated oxidative impairment in ABCA1-dependent cholesterol efflux from macrophages. Journal of Biological Chemistry. 2005;280(1):38–47. doi: 10.1074/jbc.M407019200. [DOI] [PubMed] [Google Scholar]
- 60.Kataoka Y., Shao M., Wolski K., et al. Myeloperoxidase levels predict accelerated progression of coronary atherosclerosis in diabetic patients: insights from intravascular ultrasound. Atherosclerosis. 2014;232(2):377–383. doi: 10.1016/j.atherosclerosis.2013.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shao B., Tang C., Sinha A., et al. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circulation Research. 2014;114(11):1733–1742. doi: 10.1161/CIRCRESAHA.114.303454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Navab M., Reddy S. T., van Lenten B. J., Fogelman A. M. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nature Reviews Cardiology. 2011;8(4):222–232. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 63.Shao B., Cavigiolio G., Brot N., Oda M. N., Heinecke J. W. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(34):12224–12229. doi: 10.1073/pnas.0802025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Z., Wagner M. A., Zheng L., et al. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nature Structural and Molecular Biology. 2007;14:861–868. doi: 10.1038/nsmb1284. [DOI] [PubMed] [Google Scholar]
- 65.Pan B., Yu B., Ren H., et al. High-density lipoprotein nitration and chlorination catalyzed by myeloperoxidase impair its effect of promoting endothelial repair. Free Radical Biology and Medicine. 2013;60:272–281. doi: 10.1016/j.freeradbiomed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Urundhati A., Huang Y., Lupica J. A., Smith J. D., DiDonato J. A., Hazen S. L. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. Journal of Biological Chemistry. 2009;284(45):30825–30835. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsiai T. K., Hwang J., Barr M. L., et al. Hemodynamics influences vascular peroxynitrite formation: implication for low-density lipoprotein apo-B-100 nitration. Free Radical Biology and Medicine. 2007;42(4):519–529. doi: 10.1016/j.freeradbiomed.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chakraborty S., Cai Y., Tarr M. A. In vitro oxidative footprinting provides insight into apolipoprotein B-100 structure in low-density lipoprotein. Proteomics. 2014;14:2614–2622. doi: 10.1002/pmic.201300174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamilton R. T., Asatryan L., Nilsen J. T., et al. LDL protein nitration: implication for LDL protein unfolding. Archives of Biochemistry and Biophysics. 2008;479(1):1–14. doi: 10.1016/j.abb.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Podrez E. A., Schmitt D., Hoff H. F., Hazen S. L. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro . The Journal of Clinical Investigation. 1999;103(11):1547–1560. doi: 10.1172/jci5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heffron S. P., Parastatidis I., Cuchel M., et al. Inflammation induces fibrinogen nitration in experimental human endotoxemia. Free Radical Biology and Medicine. 2009;47(8):1140–1146. doi: 10.1016/j.freeradbiomed.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vadseth C., Souza J. M., Thomson L., et al. Pro-thrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. Journal of Biological Chemistry. 2004;279(10):8820–8826. doi: 10.1074/jbc.m306101200. [DOI] [PubMed] [Google Scholar]
- 73.Thomson L., Christie J., Vadseth C., et al. Identification of immunoglobulins that recognize 3-nitrotyrosine in patients with acute lung injury after major trauma. The American Journal of Respiratory Cell and Molecular Biology. 2007;36(2):152–157. doi: 10.1165/rcmb.2006-0288sm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peluffo G., Radi R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovascular Research. 2007;75(2):291–302. doi: 10.1016/j.cardiores.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 75.Vasan R. S. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113(19):2335–2362. doi: 10.1161/circulationaha.104.482570. [DOI] [PubMed] [Google Scholar]
- 76.Artimo P., Jonnalagedda M., Arnold K., et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Research. 2012;40(1):W597–W603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DiDonato J. A., Aulak K., Huang Y., et al. Site-specific nitration of apolipoprotein A-I at tyrosine 166 is both abundant within human atherosclerotic plaque and dysfunctional. The Journal of Biological Chemistry. 2014;289(15):10276–10292. doi: 10.1074/jbc.m114.556506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shao B., Oda M. N., Oram J. F., Heinecke J. W. Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein. Chemical Research in Toxicology. 2010;23(3):447–454. doi: 10.1021/tx9003775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shao B., Bergt C., Fu X., et al. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. The Journal of Biological Chemistry. 2005;280(7):5983–5993. doi: 10.1074/jbc.m411484200. [DOI] [PubMed] [Google Scholar]