Abstract
Acidosis is a noxious condition associated with inflammation, ischaemia or defective acid containment. As a consequence, acid sensing has evolved as an important property of afferent neurons with unmyelinated and thinly myelinated nerve fibres. Protons evoke multiple currents in primary afferent neurons, which are carried by several acid-sensitive ion channels. Among these, acid-sensing ion channels (ASICs) and transient receptor potential (TRP) vanilloid-1 (TRPV1) ion channels have been most thoroughly studied. ASICs survey moderate decreases in extracellular pH whereas TRPV1 is activated only by severe acidosis resulting in pH values below 6. Two-pore domain K+ (K2P) channels are differentially regulated by small deviations of extra- or intracellular pH from physiological levels. Other acid-sensitive channels comprise TRPV4, TRPC4, TRPC5, TRPP2 (PKD2L1), ionotropic purinoceptors (P2X), inward rectifier K+ channels, voltage-activated K+ channels, L-type Ca2+ channels, hyperpolarization-activated cyclic nucleotide-gated channels, gap junction channels, and Cl− channels. In addition, acid-sensitive G protein-coupled receptors have also been identified. Most of these molecular acid sensors are expressed by primary sensory neurons, although to different degrees and in various combinations. Emerging evidence indicates that many of the acid-sensitive ion channels and receptors play a role in acid sensing, acid-induced pain and acid-evoked feedback regulation of homeostatic reactions. The existence and apparent redundancy of multiple pH surveillance systems attests to the concept that acid-base regulation is a vital issue for cell and tissue homeostasis. Since upregulation and overactivity of acid sensors appear to contribute to various forms of chronic pain, acid-sensitive ion channels and receptors are considered as targets for novel analgesic drugs. This approach will only be successful if the pathological implications of acid sensors can be differentiated pharmacologically from their physiological function.
1. ACID SENSING BY SENSORY NEURONS
1.1 Acid as a noxious stimulus
Regulation of the acid-base balance and maintenance of pH at a narrow range around 7.4 is one of the basic principles of cellular homeostasis. This balance can be put into danger by many circumstances including, for instance, excess intake of acid, excess gastric acid secretion, defective acid containment in the gastrointestinal and urogenital tracts, metabolic acidosis and acidosis due to ischaemia (hypoxia) or inflammation. To meet with these challenges, there are not only cellular mechanisms of acid-base regulation but also systemic monitoring systems to detect harmful acidosis, to initiate appropriate emergency reactions, and thereby to limit any tissue damage that may arise. The most important systemic acid sensors are primary afferent neurons and the taste receptor cells mediating the sour taste.
It has long been known that acid can elicit pain (Steen and Reeh 1993; Steen et al. 1995), and there is plausible evidence that acidosis contributes to the pain associated with inflammation and ischaemia. Several reports summarized by Steen et al. (1992), Kress and Waldmann (2006) and Wemmie et al. (2006) indicate that interstitial pH values can fall to 4.7 in fracture-related haematomas, to 5.4 in inflammation, to 5.7 in cardiac ischaemia, and to 6.2 during exhausting skeletal muscle contractions. In the lumen of the stomach, gastric acid secretion causes the pH to drop down to 1, and this acid load can only be managed by compartmentalization and a strong mucosal acid barrier in the foregut (Holzer 2007). Intrusion of acid into the mucosa of the oesophagus, stomach or duodenum contributes not only to mucosal injury but also to the pain associated with gastro-oesophageal reflux and peptic ulcer disease (Kang and Yap 1991). Acid may likewise be a factor in the pain accompanying cystitis, in which a breakdown of the uroethelial barrier exposes sensory nerve endings to the acidic and hyperosmotic urine (Chuang et al. 2003). There is now ample evidence that the pain associated with angina pectoris is due to ischaemia-induced acidosis (Sutherland et al. 2001; Yagi et al. 2006), that pulmonary acidosis is associated with asthma (Ricciardolo et al. 2004; Hunt 2006), and that acid is a stimulus to elicit the cough reflex (Kollarik et al. 2007). Acidosis also occurs in and around malignant tumours (Vaupel et al. 1989; Newell et al. 1993). Metastases in bone are particularly painful, an instance that is related to enhanced activity of osteoclasts which resorb bone by decreasing interstitial pH below 5 (Honore et al. 2000; Luger et al. 2001; Ghilardi et al. 2005; Nagae et al. 2007).
1.2 Proton-gated currents in sensory neurons
Consistent with the ability of acidosis to induce pain is its capacity to excite primary sensory neurons and to sensitize them to other noxious stimuli (Clarke and Davison 1978; Krishtal and Pidoplichko 1981; Bevan and Yeats 1991; Steen et al. 1992; Bevan and Geppetti 1994; Reeh and Kress 2001; Krishtal 2003; Kress and Waldmann 2006). The molecular basis of acid sensing was discovered when proton-activated cationic currents were described in dorsal root ganglion (DRG) neurons (Krishtal and Pidoplichko 1981; Bevan and Yeats 1991). As reviewed by Kress and Waldmann (2006), two principal types of proton-gated inward currents are observed. The first type is characterized by a fast and rapidly inactivating inward current carried by Na+ and by a high sensitivity to H+, threshold activation occurring at a pH of 7 and maximum activation taking place at a pH around 6 (Krishtal and Pidoplichko 1981; Konnerth et al. 1987; Davies et al. 1988). While this type of proton-gated current is seen in most DRG neurons, the second type is observed only in DRG neurons that are also excited by capsaicin (Bevan and Yeats 1991; Bevan and Geppetti 1994). Unlike the first type, this current is less sensitive to acidosis, activated only at pH levels below 6.2, sustained, slowly inactivating and developing tachyphylaxis on repeated activation (Bevan and Yeats 1991; Petersen and LaMotte 1993). Further studies showed that the sustained currrent is due to an increase in cation conductance that allows Na+, K+ and Ca2+ to pass (Bevan and Yeats 1991; Zeilhofer et al. 1996; Zeilhofer et al. 1997).
Molecular analysis has shown that several acid-sensitive ion channels contribute to the capacity of afferent neurons to monitor acidosis (Figure 1). In some cases, accessory cells (such as the chemoreceptor cells in the taste buds or carotid bodies) survey the pH of their environment and transmit any aberration to adjacent sensory neurons. Among the molecular acid sensors, acid-sensing ion channels (ASICs) and transient receptor potential (TRP) vanilloid-1 (TRPV1) ion channels have been most thoroughly studied (Caterina and Julius 2001; Kress and Waldmann 2006; Wemmie et al. 2006; Diochot et al. 2007; Lingueglia 2007; Szallasi et al. 2007). While the slow proton-activated conductance in DRG neurons shares many similarities with the acidosis-evoked current through TRPV1, the fast acid-induced current resembles currents carried by ASICs (Kress and Waldmann 2006). However, the characteristics of proton-gated currents in sensory neurons are complex and subject to regional and species differences (Leffler et al. 2006; Smith et al. 2007; Sugiura et al. 2007). Accordingly, there is increasing evidence that further acid-sensitive ion channels are involved in monitoring acidosis (Figure 1). These include TRPV4, TRPC4, TRPC5, and PKD2L1, another member of the TRP ion channel that is relevant to the perception of the sour taste (Chandrashekar et al. 2006). Other candidates comprise members of the two-pore domain K+ (K2P) channel family, inward rectifier K+ channels, voltage-gated K+ channels, ionotropic purinoceptors (P2X) containing the P2X2 subunit (Reeh and Kress 2001; Holzer 2003; Duprat et al. 2007) and proton-sensing G protein-coupled receptors (Ludwig et al. 2003; Tomura et al. 2005).
Figure 1.
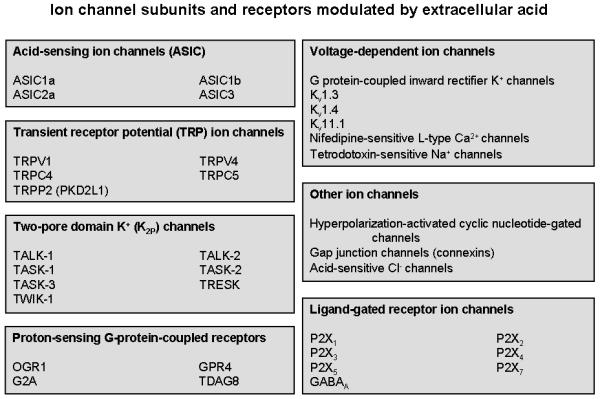
Overview of ion channel subunits and receptors that are modulated by changes in the extracellular pH (acidification) and expressed by primary afferent neurons or their associated cells. For details see text.
The objective of this article is to give an overview of the ability of primary sensory neurons to monitor acidosis, to describe the molecular acid sensors involved (Figure 1), and to address the physiological and pathophysiological implications of acid-sensitive ion channels and receptors in nociception with a pharmacological perspective.
2. ACID SENSORS ON SENSORY NEURONS
2.1 Acid-sensing ion channels (ASICs)
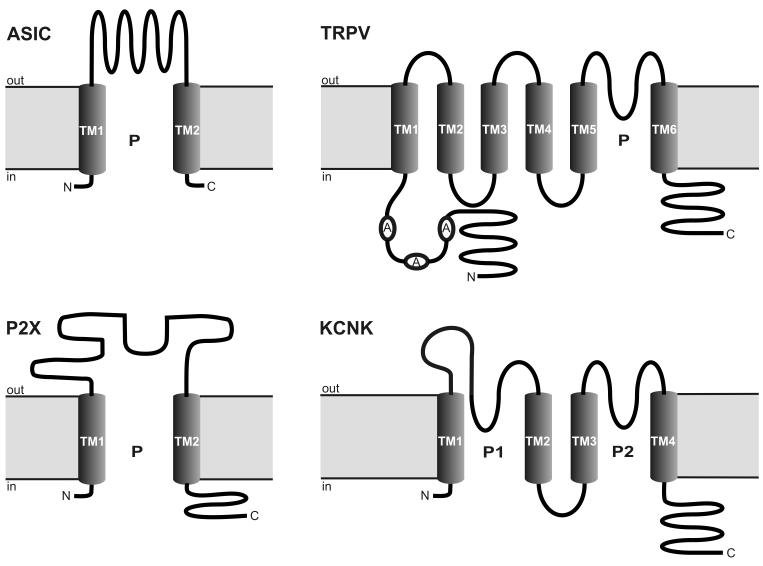
ASICs belong to the voltage-insensitive, amiloride-sensitive epithelial Na+ channel/degenerin family of cation channels (Waldmann and Lazdunski 1998; Kellenberger and Schild 2002; Waldmann 2001; Welsh et al. 2002). The proton-sensitive members of this family expressed in mammals are encoded by 3 different genes (ACCN1, ACCN2 and ACCN3) which are alternatively spliced to produce 5 subunits: ASIC1a, ASIC1b, ASIC2a, ASIC2b and ASIC3 (Kress and Waldmann 2006; Wemmie et al. 2006; Lingueglia 2007). These subunits are characterized by two membrane-spanning α-helical sequences (transmembrane domains 1 and 2), a large cysteine-rich extracellular loop and short intracellular N- and C-termini (Waldmann and Lazdunski 1998; Kellenberger and Schild 2002; Welsh et al. 2002; Lingueglia 2007). With this transmembrane topology (Figure 2), ASICs have the same structure as the ionotropic purinoceptors (P2X) but, since they lack significant sequence homology, do not seem to share a common ancestor (Kress and Waldmann 2006). The different subunits form distinct homomultimeric and heteromultimeric complexes which differ in their kinetics, external pH sensitivity, tissue distribution and pharmacological properties (Waldmann et al. 1999; Alvarez de la Rosa et al. 2002; Benson et al. 2002; Welsh et al. 2002; Kress and Waldmann 2006; Wemmie et al. 2006). Although it is not known precisely how many subunits are required to form a functional channel, there is increasing evidence that ASICs are arranged as homo- or heterotetramers (Gao Y et al. 2007; Lingueglia 2007). When activated, ASICs are preferentially permeable to Na+ but some of them can also carry other cations such as Ca2+ (ASIC1a) and K+ (ASIC1b) (Kress and Waldmann 2006; Wemmie et al. 2006; Lingueglia 2007).
Figure 2.
Membrane topology of 4 classes of acid-sensitive ion channel subunits: ASIC (acid-sensing ion channel), TRPV (transient receptor potential ion channel of the vanilloid subtype), P2X (ionotropic purinoceptor) and KCNK (K2P ion channel). A, ankyrin; C, COOH terminal; N, NH2 terminal; P, pore; TM, transmembrane domain.
The functional properties of the different ASIC subunits have been characterized following heterologous expression in Xenopous laevis oocytes and mammalian cell lines. Mutational analyses indicate that their pH sensitivity resides in several regions of the ASIC protein, particularly with His-72 and Gly-430 in the extracellular loop (Waldmann 2001; Diochot et al. 2007). ASIC1a, ASIC1b, ASIC2a and ASIC3 are directly gated by protons, whereas ASIC2b does not respond to acidosis when expressed as a homomultimer but can form functional heteromultimers with other ASIC subunits, particularly ASIC3 (Kress and Waldmann 2006; Wemmie et al. 2006). ASICS are activated by changes in pH only if they occur extracellularly, the threshold for activation of ASIC3 being as low as a fall of pH to 7.2 (Kress and Waldmann 2006; Wemmie et al. 2006). The pH values required for half-maximal activation are 6.2 - 6.8 for ASIC1a, 5.9 - 6.2 for ASIC1b, around 4.9 for ASIC2a and 6.5 - 6.7 for ASIC3 (Benson et al. 2002; Kress and Waldmann 2006). Under physiological pH, ASIC3 is blocked by Ca2+ bound to a high-affinity binding site on the extracellular side of the channel pore, and protons open ASIC3 by relieving this Ca2+ block (Immke and McCleskey 2003).
The proton-gated ASICs are highly sensitive acid sensors as deduced from the steepness of the their stimulus–response relationship. Although ASIC currents are in general fast and rapidly inactivating, there is evidence that they can also monitor prolonged acidosis. ASIC3 homomultimers and ASIC2a/ASIC3 as well as ASIC2b/ASIC3 heteromultimers produce two types of a sustained current: (i) a current that occurs at low acidic or even neutral pH and is thought to result from an overlap of activation and desensitization kinetics (Benson et al. 1999; Kress and Waldmann 2006; Wemmie et al. 2006; Yagi et al. 2006), and (ii) a current that is seen only at pH values below 5 (Kellenberger and Schild 2002; Kress and Waldmann 2006). In addition, persistent currents are observed when ASIC1 and ASIC3 channels are activated by protons in the presence of the neuropeptides NPFF or FMRFamide (Askwith et al. 2000; Catarsi et al. 2001; Deval et al. 2003).
The properties of ASIC channel currents delineated above resemble the fast and rapidly inactivating inward Na+ current that is evoked by minor acidosis in native DRG neurons (Krishtal and Pidoplichko 1981; Konnerth et al. 1987; Davies et al. 1988; Kellenberger and Schild 2002; Leffler et al. 2006; Poirot et al. 2006). The question as to which ASIC subunits contribute to the native ASIC-like current in sensory neurons has been addressed by comparing the currents carried by ASIC homo- and heteromultimers with the native currents and by analyzing the expression of ASICs in sensory neurons. The pertinent studies revealed distinct regional differences, proton-gated currents being mediated by ASIC1a or ASIC3 homomultimers in some instances (Escoubas et al. 2000; Sutherland et al. 2001) and by ASIC2a/ASIC3 as well as ASIC2b/ASIC3 heteromultimers in other instances (Waldmann et al. 1999; Xie et al. 2002; Diochot et al. 2004; Yagi et al. 2006).
This heterogeneity in function is paralleled by a heterogeneity in the expression of ASIC1a, ASIC1b, ASIC2a, ASIC2b and ASIC3 by different populations of afferent neurons (Waldmann et al. 1999; Voilley et al. 2001; Alvarez de la Rosa et al. 2002; Benson et al. 2002; Mamet et al. 2002; Schicho et al. 2004; Kress and Waldmann 2006). While ASIC1a is present in both sensory neurons and neurons of the central nervous system (CNS), ASIC1b is largely confined to primary afferent neurons (Chen et al. 1998; Alvarez de la Rosa et al. 2002). In contrast, the levels of ASIC2a in sensory neurons are quite low, whereas ASIC2b occurs in both sensory and CNS neurons, and ASIC3 is almost exclusively expressed by sensory neurons (Lingueglia et al. 1997; Waldmann et al. 1999; Price et al. 2001; Voilley et al. 2001; Alvarez de la Rosa et al. 2002; Chen et al. 2002; Xie et al. 2002). The various ASIC subunits have been localized to primary afferent neurons of small, medium and large diameter innervating skin, eye, ear, taste buds, heart, gut, skeletal muscle and bone (Kress and Waldmann 2006; Wemmie et al. 2006; Holzer 2007). Their expression has been analyzed in greatest detail in the sensory innervation of the skin in which ASIC-like immunoreactivity occurs not only in free nerve endings but, as is particularly true for ASIC2, also in specialized mechanosensitive nerve endings (Jiang et al. 2006; Kress and Waldmann 2006; Wemmie et al. 2006). ASIC1, ASIC 2 and ASIC3 have also been localized to glossopharyngeal, vagal and spinal afferent neurons innervating the gut and other visceral organs (Page et al. 2005; Fukuda et al. 2006; Holzer 2007; Hughes et al. 2007), and retrograde tracing has revealed that 75 % of the nodose ganglion neurons and 82 % of the DRG neurons projecting to the rat stomach express ASIC3-like immunoreactivity (Schicho et al. 2004). Analysis of mouse thoracolumbar DRG has revealed that ASIC3 is expressed in 73 %, ASIC2 in 47 % and ASIC1 in 30 % of the somata projecting to the mouse colon (Hughes et al. 2007).
2.2 Transient receptor potential (TRP) ion channels
The transient receptor potential (TRP) ion channels are named after the role these channels have in Drosophila phototransduction. At least 28 different TRP subunit genes have been identified in mammals (Clapham et al. 2005), comprising 6 subfamilies of the mammalian TRP superfamily (Figure 3). The primary structure of the TRP channels consists of 6 transmembrane domains with a pore domain between transmembrane domains 5 and 6 and with both the C- and N-termini located intracellularly (Clapham et al. 2005). This architecture (Figure 2) is common to hundreds of ion channels but, despite the topographic similarities between the TRPs and the voltage-gated K+ channels, the TRPs are only distantly related to these channels (Clapham et al. 2005). Since TRP channels are the subject of another chapter in this book, only TRP channels that are sensitive to pH changes (Figure 3) are considered here.
Figure 3.
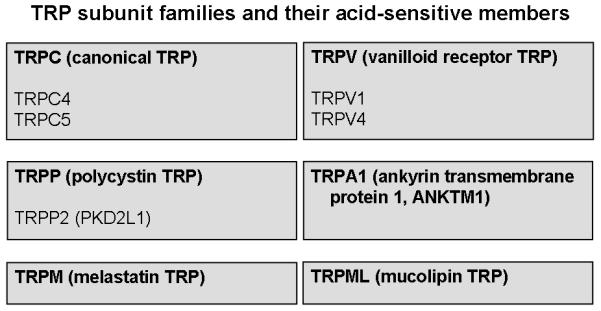
Overview of the TRP channel subfamilies and of the acid-sensitive subunit members among the TRPC, TRPP and TRPV subfamilies. For details see text.
2.2.1 TRPV1
The existence of TRPV1 (initially termed vanilloid receptor 1, VR1), also known as the capsaicin receptor, has long been envisaged from the specific action of capsaicin on nociceptive afferent neurons (Jancsó 1960; Holzer 1991; Szallasi and Blumberg 1999). It is now known as a polymodal nocisensor par excellence, being receptive to noxious heat (above 43 °C), capsaicin, endovanilloids, and acid (Caterina et al. 1997; Tominaga et al. 1998; Jordt et al. 2000; Caterina and Julius 2001; Patapoutian et al. 2003). Assembled most likely as a homotetramer, TRPV1 is a non-selective cation channel with high permeability for Ca2+ (Caterina and Julius 2001; Gunthorpe et al. 2002; Patapoutian et al. 2003; García-Sanz et al. 2004). In addition, TRPV1 has also been recognized as a channel that allows protons to enter the cell in an acidic environment (Hellwig et al. 2004; Vulcu et al. 2004). The conductance of H+ through TRPV1 results in intracellular acidification (Hellwig et al. 2004), which in turn may act on membrane channels that are sensitive to changes in intracellular pH, e.g., certain two-pore domain K+ channels. How TRPV1 regulates an acid-sensitive Cl− channel in Sertoli cells (Auzanneau et al. 2008) has not yet been elucidated.
The pH sensitivity of TRPV1 is fundamentally different from that of ASICs, because TRPV1 is gated open only if the extracellular pH is reduced below 6, in which case a sustained channel current is generated (Caterina et al. 1997; Tominaga et al. 1998; Jordt et al. 2000). However, mild acidosis in the range of pH 7 - 6 can sensitize TRPV1 to other stimuli such as capsaicin and heat (Tominaga et al. 1998; McLatchie and Bevan 2001; Ryu et al. 2003; Neelands et al. 2005). As a result, the temperature threshold for TRPV1 activation is lowered under acidotic circumstances so that this cation channel becomes active at normal body temperature (Tominaga et al. 1998). Proton-induced sensitization involves both an increase in current activation rate and a decrease in current deactivation rate (Ryu et al. 2003; Neelands et al. 2005). Besides mild acidosis, many other signalling pathways (stimulated, e.g., by inflammatory mediators such as prostaglandins, bradykinin, adenosine triphosphate, 5-hydroxytryptamine and nerve growth factor) converge on TRPV1 and enhance the probability of channel gating by protons, capsaicin and heat (Caterina and Julius 2001; Vellani et al. 2001; Gunthorpe et al. 2002; Szallasi et al. 2007).
The ability of protons to sensitize TRPV1 to heat and other stimuli, on the one hand, and to activate TRPV1 per se, on the other hand, is mediated by different amino acid residues of the channel protein. Glu-600 on the extracellular side of transmembrane segment 5 is crucial for proton-induced sensitization of TRPV1, while Val-538 in the extracellular linker between transmembrane segments 3 and 4, Thr-633 in the pore helix and Glu-648 in the linker between the selectivity filter of the pore and transmembrane segment 6 are essential for proton-induced gating of TRPV1 (Jordt et al. 2000; Ryu et al. 2007). Mutation of these amino acid residues selectively abrogates proton-evoked currents but preserves the current responses to capsaicin and heat and their potentiation by mildly acidic pH (Jordt et al. 2000; Ryu et al. 2007). Thus, the sites in the TRPV1 protein targeted by protons differ from those targeted by other stimuli (Jordt et al. 2000; Welch et al. 2000; McLatchie and Bevan 2001; Gavva et al. 2004; Ryu et al. 2007). This instance allows for the development of TRPV1 blockers that inhibit TRPV1 activation by capsaicin but not acid (Gavva et al. 2005a).
DRG neurons of TRPV1 null mice lack the slow and non-desensitizing proton-gated currents that are seen in DRG neurons of wild-type animals, whereas the fast and rapidly inactivating proton-gated currents mediated by ASICs are maintained (Caterina et al. 2000; Davis et al. 2000). The TRPV1-mediated currents due to acidification are largely confined to DRG neurons with unmyelinated fibres whereas the ASIC-mediated currents are also found on DRG neurons with thinly myelinated axons (Leffler et al. 2006). This is consistent with the predominant expression of TRPV1 in unmyelinated primary afferent nerve fibres originating from the trigeminal, nodose and DRG ganglia although some thinly myelinated fibres also stain for TRPV1 (Caterina et al. 1997; Guo et al. 1999; Michael and Priestley 1999; Patterson et al. 2003; Schicho et al. 2004; Szallasi et al. 2007). Of the nodose ganglion neurons that innervate the rat stomach, 42 - 80 % stain for TRPV1, whereas 71 - 82 % of the DRG neurons projecting to the rat stomach and mouse colon express TRPV1 (Patterson et al. 2003; Robinson et al. 2004; Schicho et al. 2004). In addition, TRPV1 is present on afferent neuron-associated cells such as epithelial cells in the urinary bladder (Birder et al. 2001).
2.2.2 TRPV4
Much like TRPV1, TRPV4 is gated by a drop of pH below 6 and the channel current reaches a maximum at a pH of about 4 (Suzuki et al. 2003a). TRPV4 is also activated by citrate, but not lactate (Suzuki et al. 2003a), and has turned out to play a role in mechano- and osmosensation (Güler et al. 2002; Mizuno et al. 2003; Suzuki et al. 2003a). As TRPV4 has been localized to DRG neurons with both low- and high-threshold mechanosensitive afferent nerve fibres in the skin (Suzuki et al. 2003b), a role of this TRP channel in acid sensing warrants further exploration.
2.2.3 TRPC4 and TRPC5
The TRPC subfamily, specified as canonical or classical because TRPC1 has been the first member of the mammalian TRP family known to form an ion channel, can be divided into three subgroups by sequence homology and functional similarities: C1/C4/C5, C3/C6/C7, and C2 (Clapham et al. 2005). Accordingly, TRPC4 and TRPC5 are most closely related to TRPC1 which is a component of different heteromeric TRP complexes (Clapham et al. 2005). As other TRP channels, TRPC4 and TRPC5 form Ca2+-permeable cation channels that are involved in receptor-mediated increases in intracellular Ca2+. Their mode of activation has remained somewhat elusive, as TRPC4 and TRPC5 are activated in a phospholipase C-dependent manner by an unidentified messenger.
It has recently been reported that TRPC4 and TRPC5 respond to changes in extracellular pH, given that small decreases in pH (from 7.4 to 7.0) increase both G protein-activated and spontaneous TRPC5 currents (Semtner et al. 2007). TRPC4 channel activity is likewise potentiated by decreases in pH. The effects of a pH decrease on TRPC4 and TRPC5 activity are biphasic, the currents being increased by a reduction of pH down to about 6.5 but inhibited when pH is decreased further (Semtner et al. 2007). H+ modifies TRPC5 currents by interacting with the Gd3+ binding site typical of TRPC4 and TRPC5 (Semtner et al. 2007). These findings clearly indicate that TRPC4 and TRPC5 can act as acid sensors that link decreases in extracellular pH to Ca2+ entry and depolarization. The expression of these TRP subunits in acid-sensitive cells and their functional implications in acid monitoring await to be shown.
2.2.4 TRPP2 (PKD2L1, polycystic kidney disease-like ion channel)
PKD2L1 is a member of the polycystin (TRPP) subfamily of TRP channels. Apart from PKD2L1 (TRPP2), the polycystic kidney disease (PKD) proteins or polycystins also comprise PKD2 (TRPP1) and PKD2L2 (TRPP3) (Clapham et al. 2005). The term polycystin is derived from the association of a PKD2 gene mutation with autosomal dominant polycystic kidney disease (Delmas 2005). Accordingly, the mouse ortholog of TRPP2 is deleted in krd mice which suffer from defects in the kidney and retina (Nomura et al. 1998).
PKD2L1 has recently joined the network of acid-sensitive ion channels after it has been discovered to play a major role in sour taste sensing (Huang et al. 2006; Ishimaru et al. 2006; LopezJimenez et al. 2006). In order to form functional channels, PKD2L1 needs to associate as heteromer with related proteins of the PKD1 family (Delmas 2005; Ishimaru et al. 2006). The PKD1 polycystins (PKD1, PKD1L1, PKD1L2, PKD1L3 and PKDREJ) are not included in the TRP channel family (Clapham et al. 2005) because they are large proteins with a very long N-terminal extracellular domain and 11 transmembrane domains that include a 6-transmembrane TRP-like channel domain at the C terminus. The expression of PKD2L1 in a select class of taste chemoreceptor cells and the deleterious effect of PKD2L1 deletion on the sour taste have led to the concept that PKD2L1is the molecular sour sensor (Huang et al. 2006; Ishimaru et al. 2006; LopezJimenez et al. 2006).
2.3 Two-pore domain K+ (K2P) channels
Two-pore (or tandem-pore) domain potassium (K2P) channels, encoded by the KCNK genes, represent one of the subfamilies of the large superfamily of K+ channels. Defined by their membrane topology (Figure 2), these channels possess four transmembrane domains, two pore-forming loops between transmembrane domains 1 and 2 as well as 3 and 4, and a large extracellular linker region between transmembrane domain 1 and the first pore-forming loop, which forms the K+ selectivity filter (Lesage and Lazdunski 2000; Goldstein et al. 2001; Patel and Honoré 2001; Goldstein et al. 2005; Duprat et al. 2007). Functional K2P channels are made up as homo- or heterodimers (Duprat et al. 2007). Thus far, 15 human K2P channel subunits (Figure 4) have been identified and grouped into 6 structurally and functionally different subclasses (Goldstein et al. 2005; Duprat et al. 2007). Many of these channels are background channels that are independent of membrane voltage, constitutively active and non-inactivating. With these properties, K2P channels play a key role in setting the resting membrane potential as well as membrane input resistance and, consequently, the excitability of neurons (Lesage and Lazdunski 2000; Goldstein et al. 2001; Patel and Honoré 2001; Duprat et al. 2007). In addition, many K2P channels possess receptor properties, given that they are responsive to mechanical and chemical stimuli and are increasingly considered to be sensors for hypoxia, hypercapnia, glucose and modifications of intra- and extracellular pH (Duprat et al. 2007). As summarized in Figure 4, acidification or alkalinization modulates the activity of most K2P subunits (Holzer 2003; Goldstein et al. 2005).
Figure 4.
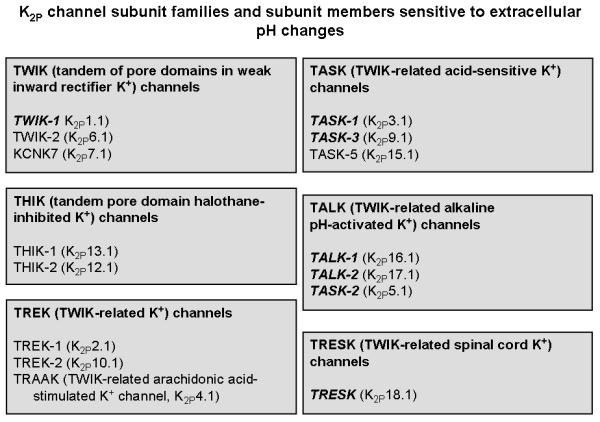
Overview of the K2P channel subunit families and of the subunit members (indicated in bold and italics) that are modulated by changes in the extracellular pH. For details see text.
As their abbreviation for “TWIK-related acid-sensitive K+ channels” implies, TASK channels are remarkably sensitive to variations in extracellular pH (Figure 4). TASK-1, TASK-2 and TASK-3 homo- and heteromers are inhibited by extracellular acidification but left unaffected by intracellular pH changes, while TASK-5 is inactive when expressed as homomer (Duprat et al. 1997; Reyes et al. 1998; Chapman et al. 2000; Kim et al. 2000; Rajan et al. 2000; Meadows and Randall 2001; Kang and Kim 2004; Goldstein et al. 2005; Duprat et al. 2007). TASK-1 is particularly sensitive, given that only 10 % of the maximal current is recorded at pH 6.7, 50 % at pH 7.3 and 90 % at pH 7.7 (Duprat et al. 1997). The pH sensitivity of TASK-3 is critically dependent on His-98 in the first pore-forming loop, an amino acid residue that is also present in TASK-1 but absent in TASK-2 (Kim et al. 2000; Rajan et al. 2000). In addition, His-72, Lys-73, Ile-94, Gly-95, Asp-204 and Lys-210 contribute to the acid sensing capacity of TASK-1 and TASK-3 subunits (Kim et al. 2000; Morton et al. 2003; Yuill et al. 2004; Yuill et al. 2007). TASK-2 differs from TASK-1 and TASK-3 not only by the amino acid residues critical to its pH sensitivity (Glu28, Lys-32, Lys-35, Lys-47 and Arg-224), but also by its property of being an alkaline-activated K2P channel (Morton et al. 2005; Duprat et al. 2007; Niemeyer et al. 2007).
Acid-induced inhibition of TASK channel activity will enhance nerve excitability and hence indirectly encode the presence of acid. The high proton sensitivity of TASK subunits points to a role in surveillance of tissue acidification by ischaemia, inflammation or backdiffusion of luminal acid into the mucosa of the foregut (Lesage and Lazdunski 2000; Holzer 2003: Duprat et al. 2007). This possibility is strongly envisaged from the expression of TASK-1, TASK-2 and TASK-3 mRNA and protein in rat and human DRG neurons with nociceptive properties and in areas of the spinal cord (dorsal horn) and brainstem that receive afferent input from the periphery (Duprat et al. 1997; Bayliss et al. 2001; Medhurst et al. 2001; Talley et al. 2001; Gabriel et al. 2002; Baumann et al. 2004; Cooper et al. 2004; Rau et al. 2006).
TRESK channels are blocked by extra- and intracellular acidification and activated by extra- and intracellular alkalinization (Sano et al. 2003). TRESK is a major background K2P channel in DRG neurons, and disruption of the TRESK gene has revealed that this channel plays a role in the regulation of DRG neuron excitability (Kang and Kim 2006; Dobler et al. 2007).
TREK-1 and TREK-2 do not respond to changes in extracellular pH but are inhibited by intracellular alkalinization and activated by intracellular acidification such that they become constitutively active (Maingret et al. 1999; Bang et al. 2000; Lesage et al. 2000; Kim et al. 2001a; Patel and Honoré 2001; Honoré et al. 2002; Miller et al. 2004). Activation of TREK-1 by intracellular acidification depends critically on protonation of Glu-306 (Honoré et al. 2002). TREK channels are also activated by a number of extracellular stimuli (including arachidonic acid, other unsaturated fatty acids, heat, stretch, negative pressure and heat) and inhibited by intracellular signalling cascades involving protein kinases A and C. A sensory role of TREK channels may be deduced from the expression of TREK-1 and TREK-2 in human DRG neurons as well as in neurons of the spinal cord and brainstem (Bearzatto et al. 2000; Maingret et al. 2000; Hervieu et al. 2001; Medhurst et al. 2001; Talley et al. 2001; Gu et al. 2002; Kang and Kim 2006). In the mouse DRG, TREK-1 is present in small to medium-sized primary afferent neurons (Maingret et al. 2000).
The activity of TWIK-1, but not TWIK-2, is depressed by extracellular acidification (Goldstein et al. 2005; Rajan et al. 2005), whereas intracellular acidification inhibits both TWIK-1 and TWIK-2 (Chavez et al. 1999; Patel et al. 2000). A possible role in sensory mechanisms can be envisaged from the expression of TWIK-1 and TWIK-2 mRNA in human and rat DRG neurons as well as in neurons of the spinal cord (Medhurst et al. 2001; Talley et al. 2001).
TRAAK channels are activated by intracellular alkalinization but not acidification (Lesage and Lazdunski 2000; Kim et al. 2001b; Patel and Honoré 2001). TRAAK mRNA and protein are appreciably expressed in human and rat DRG neurons as well as in the human, mouse and rat spinal cord (Bearzatto et al. 2000; Reyes et al. 2000; Medhurst et al. 2001; Talley et al. 2001; Kang and Kim 2006).
TALK-1, TALK-2 and their splice variants are blocked by extracellular acidification but gated open by extracellular alkalinization, with Lys-224 playing a critical role in their pH sensitivity (Decher et al. 2001; Girard et al. 2001; Han et al. 2003; Duprat et al. 2005; Goldstein et al. 2005; Niemeyer et al. 2007).
Taken together, the activity of many K2P channels (TASK-1, TASK-2, TASK-3, TRESK, TWIK-1, TALK-1 and TALK-2) is modified by changes in extracellular pH. Although their functional implications in acid sensing await to be explored, K2P channels could play a multimodal sensory role in the peripheral and central nervous system as has been envisaged for the TASK subfamily (Duprat et al. 2007).
2.4 Proton-sensing G-protein-coupled receptors (GPCRs)
Proton-sensitive G-protein-coupled receptors (GPCRs) are emerging as a new class of acid sensors on nociceptive afferent neurons (Figure 1). These receptors comprise the ovarian cancer G-protein-coupled receptor 1 (OGR1), G-protein-coupled receptor 4 (GPR4), the G2 accumulation (G2A) receptor, and the T-cell death-associated gene 8 (TDAG8) receptor. Initially described as receptors for lipid molecules such as sphingosylphosphorylcholine, lysophosphatidylcholine, and psychosine, some of these GPCRs have turned out to be sensors for extracellular acidosis (Ludwig et al. 2003; Tomura et al. 2005). As other GPCRs, these acid-sentitive receptors are composed of 7 transmembrane domains, their signalling involving Gs, Gi, Gq, and G12/13 pathways. The sensitivity of OGR1 to extracellular pH changes resides with several histidine residues and is extremely high, given that half-maximum activation occurs at pH 7.2 - 7.5 and full activation at pH 6.4 - 6.8 (Ludwig et al. 2003; Tomura et al. 2005). The transcripts of proton-sensing GPCRs are widely distributed and, importantly, also expressed by DRG neurons, particularly by small-diameter afferent neurons that are involved in nociception (Huang et al. 2007). Although the physiological and pathophysiological roles of proton-sensitive GPCRs are - for the time being - speculative, they could add significantly to the network of acid sensors of afferent neurons and their associated cells.
2.5 Ionotropic purinoceptors (P2X)
P2X purinoceptors are ligand-gated membrane cation channels that open when extracellular adenosine triphosphate (ATP) is bound. They are assembled as homo- or heteromultimers (trimers or hexamers) of P2X subunits, seven of which (P2X1 - P2X7) have been identified at the gene and protein level (Chizh and Illes 2001; Dunn et al. 2001; North 2002; Burnstock 2007). Their membrane topology (Figure 2) is characterized by a very long extracellular polypeptide loop, which consists of about 280 amino acids and is rich in cystein, between two transmembrane domains, with both the N- and C-termini located intracellularly (Dunn et al. 2001; North 2002). Since the role of purinoceptors in sensory neuron physiology and pharmacology is the topic of another chapter in this volume, only aspects relating to the acid sensitivity of P2X purinoceptors are discussed here.
Of the various P2X subunits, P2X1, P2X2, P2X3, P2X4, P2X5 and P2X7 (Figure 1) are modulated by alterations in the extracellular pH (Holzer 2003). Thus, acidification reduces the potency of ATP to gate homomultimeric P2X1, P2X3, P2X4 and P2X7 receptors usually without a change in the maximal response, while alkalinization has no effect on agonist potency and efficacy (Stoop et al. 1997; Dunn et al. 2001; Liu et al. 2001; Gerevich et al. 2007). In P2X5 homomultimers, however, protons reduce both the potency and efficacy of ATP to gate the channel (Wildman et al. 2002). In contrast, acidification sensitizes homomultimeric P2X2 receptors to the excitatory effect of ATP, whereas agonist potency at homomultimeric P2X2 receptors is decreased at alkaline pH levels above 7.5 (Stoop et al. 1997; Ding and Sachs 1999; North 2002; Burnstock 2007). The maximal response of P2X2 receptors to ATP is not altered by alkalinization or acidification. His-319 is particularly important for the effect of protons to potentiate the agonist effect of ATP on P2X2 (Clyne et al. 2002), while protonation of His-206 and His-286 accounts for the inhibition of agonist-induced currents in P2X3 and P2X4, respectively (Clarke et al. 2000; Gerevich et al. 2007).
Acidification has a dual effect on P2X3 channels in response to agonist application. While at low agonist concentrations the current amplitude is reduced due to a decrease in the activation rate, it is enhanced at high agonist concentrations due to a decrease in the desensitization rate (Gerevich et al. 2007). It has therefore been proposed that the effect of low ATP concentrations on P2X3 channels may be attenuated during inflammatory acidosis, whereas the effects of a massive release of ATP by tissue damage may be potentiated by acidosis (Gerevich et al. 2007).
When P2X1, P2X2 or P2X3 subunits are coexpressed with each other, the resultant heteromultimers show a pH sensitivity that is different from that of P2X homomers (Surprenant et al. 2000; Dunn et al. 2001; Liu et al. 2001; Brown et al. 2002). For instance, the potency and efficacy of ATP to gate heteromeric P2X1/2 receptors expressed in Xenopous oocytes is increased under both acidic and alkaline conditions (Brown et al. 2002). While the agonist-induced currents in P2X2/3 heteromers are less enhanced by protons than the equivalent responses in P2X2 homomers (Liu et al. 2001), the ligand-induced currents in P2X2/6 heteromers are potentiated by pH levels down to 6.5, but are inhibited by pH levels lower than 6.3 (King et al. 2000). The ATP-evoked currents in P2X4/6 heteromers are inhibited in the presence of protons (Dunn et al. 2001), and the ligand-evoked stimulation of P2X1/5 heteromers is inhibited by either an increase or a decrease of the extracellular pH, both in terms of potency and efficacy (Surprenant et al. 2000).
P2X receptors are expressed by many cells including primary afferent neurons. The P2X receptors on nodose ganglion neurons comprise predominantly homomultimeric P2X2 and some heteromultimeric P2X2/3 receptors whereas on DRG neurons homomultimeric P2X3 prevail over heteromultimeric P2X2/3 receptors (Cockayne et al. 2000; Dunn et al. 2001; Burnstock 2007). The different P2X subunit distribution in spinal and vagal sensory neurons explains why the ATP-evoked inward currents in nodose ganglion neurons are persistent whereas those in DRG neurons exhibit transient, persistent or biphasic components (Dunn et al. 2001). Since only P2X2 homomultimers and heteromultimers involving P2X2, i.e., P2X1/2, P2X2/3 and P2X2/6 receptors, are sensitized by acid, it is primarily P2X2-containing purinoceptors that function as indirect acid sensors. The monitoring of acidification depends on the concomitant release and/or presence of ATP or related purines whose agonist action is enhanced by a decrease of the extracellular pH. This scenario may be of functional significance, given that ATP is liberated from a number of cellular sources in response to both physiological and pathological stimuli. As a result, P2X receptors have been envisaged as potential targets in pain research because, firstly, P2X3 receptors are preferentially expressed by a group of primary afferent neurons that subserve a nociceptor function and, secondly, P2X receptors on these neurons are upregulated by inflammation and nerve injury (Cockayne et al. 2000; Hamilton et al. 2001; Yiangou et al. 2001a; Xu and Huang 2002; Burnstock 2007).
2.6 Other acid-sensitive ion channels
There is an increasing network of ion channels, receptors and other membrane proteins whose activity is modified by changes in the extra- and/or intracellular pH (Figure 1). To review these many principles is beyond the scope of the current article which focuses on acid sensing as an important property of sensory neurons and associated cells. Mention needs to be made, however, of a number of mechanisms that have been discussed as contributing to the acid-monitoring capacity of chemosensory neurons both in the periphery and brain.
Several members of the inward rectifier K+ channel (Kir) family, such as Kir1.1, Kir4.1, Kir5.1 and Kir6.1 are highly sensitive to changes in the intra- or extracellular pH at near physiological levels. While the activity of Kir1.1, Kir4.1 and Kir5.1 channels is inhibited by a decrease in intracellular pH (Jiang et al. 1999; Claydon et al. 2000; Putnam et al. 2004; Jiang et al. 2005; Liu et al. 2005; Lopez-Lopez and Perez-Garcia 2007), Kir6.1 (KATP) channels are activated by intracellular acidosis (Xu et al. 2001; Wang et al. 2003). Similarly, G-protein-coupled inward rectifier K+ channels are activated by extracellular acidification (Mao et al. 2002). The inactivation of the voltage-activated K+ channel Kv1.3 is delayed when extracellular pH is lowered (Somodi et al. 2004), whereas the inactivation of Kv1.4 and and Kv11.1 channels is facilitated by extracellular acidosis (Jiang et al. 1999; Claydon et al. 2000; Somodi et al. 2004; Chandrashekar et al. 2006).
Nifedipine-sensitive L-type Ca2+ channels can be activated by extracellular acidification (Filosa and Putnam 2003), although there are also reports that high voltage-gated Ca2+ channels and tetrodotoxin-sensitive Na+ channels are blocked by a drop of extracellular pH (Reeh and Kress 2001). Hyperpolarization-activated cyclic nucleotide-gated channels (Stevens et al. 2001; Zong et al. 2001; Mistrík and Torre 2004) and gap junction channels (connexins) (Dean et al. 2002) are inhibited by intracellular acidosis. Sertoli cells express an acid-sensitive Cl− channel which is outwardly rectifying and activated only at acidic pH values in the extracellular space (Auzanneau et al. 2003; Auzaneeau et al. 2008). Neurotransmitter receptors other than P2X purinoceptors are likewise modulated by pH changes. This is true for GABAA receptors which can be modified by extracellular pH changes, the effect depending on the stage of development and the receptor subunit composition (Krishek and Smart 2001). Given that intracellular pH is regulated by ion pumps, it needs to be envisaged that many transporters including Na+/H+ exchangers can modify the acid sensitivity of chemosensory cells (Lyall et al. 2004; Shimokawa et al. 2005; Montrose et al. 2006).
3. PHYSIOLOGICAL AND PATHOPHYSIOLOGICAL IMPLICATIONS OF ACID SENSORS
In most tissues, stimulation of sensory neurons by acidosis and other noxious stimuli has two different effects: local release of neuropeptides from the peripheral nerve fibres in the tissue and induction of autonomic reflexes, sensation and pain (Holzer 1988; Holzer and Maggi 1998). By releasing peptide transmitters in the periphery, sensory nerve fibres can regulate vascular and other tissue activities embodied in the term neurogenic inflammation. This efferent-like mode of operation may take place independently of nociception, and it has been hypothesized that some DRG neurons are specialized in controlling peripheral effector mechanisms only, while other DRG neurons may be specialized in the afferent mode of action or both (Holzer and Maggi 1998). The neuropeptides involved in the efferent-like mode of operation include calcitonin gene-related peptide (CGRP) and the tachykinins substance P and neurokinin A. Acidosis-evoked release of CGRP, one of the most potent vasodilator peptides, has been demonstrated in a variety of tissues including the dental pulp (Goodis et al. 2006), the gastric mucosa (Geppetti et al. 1991; Manela et al. 2005) and the myocardium (Strecker et al. 2005). Pharmacological antagonist and gene disruption studies provide increasing evidence that acid-sensitive ion channels and receptors are involved in a number of physiological and pathophysiological reactions to acidosis (Figure 5).
Figure 5.
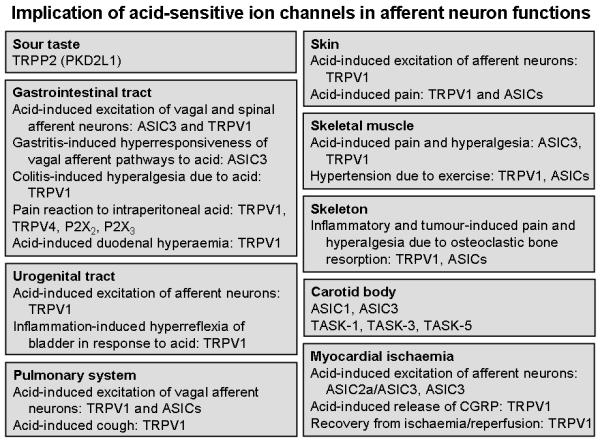
Overview of the pathophysiological implications of acid-sensitive ion channels in afferent neuron function, based primarily on pharmacological antagonist and gene disruption studies. For details see text.
3.1 Sour taste
The detection of sour taste plays an important role in warning against ingestion of acidic (e.g., spoiled or unripe) food sources (Huang et al. 2006). Taste reception occurs at the apical tip of taste receptor cells that form taste buds composed of 50 - 100 taste receptor cells. After transduction of one of the 5 distinct taste modalities (bitter, sweet, umami, salty, and sour), the taste receptor cells transmit their information to afferent neurons. Several receptors and receptor mechanisms have been proposed to mediate the sour taste (Chandrashekar et al. 2006). These comprise ASIC2a and ASIC2b (Ugawa et al. 2003; Shimada et al. 2006), K2P channels (Lin et al. 2004; Richter et al. 2004a), inward rectifier K+ channels (Liu et al. 2005), proton-activated voltage-dependent Ca2+ channels (Chandrashekar et al. 2006), hyperpolarization-activated cyclic nucleotide-gated channels (Stevens et al. 2001), Na+/H+ exchangers (Lyall et al. 2004) and acid-induced inactivation of K+ channels (Chandrashekar et al. 2006). However, ASIC2 knockout mice respond normally to sour taste stimuli (Richter et al. 2004b), and there is also a lack of conclusive evidence for the other receptor mechanisms.
Histological, genetic and functional studies demonstrate that the TRP channel PKD2L1 (TRPP2) is probably the most important sour taste receptor (Huang et al. 2006; Ishimaru et al. 2006; LopezJimenez et al. 2006; Kataoka et al. 2008). PKD2L1 and the related PKD1L3 are selectively coexpressed in a population of taste receptor cells distinct from those mediating sweet, umami and bitter tastes (Huang et al. 2006; Ishimaru et al. 2006; LopezJimenez et al. 2006). PKD2L1 is accumulated at the taste pore region where taste chemicals are detected, and coexpression of PKD2L1 and PKD1L3 is necessary for their functional cell surface expression (Ishimaru et al. 2006). In addition, PKD2L1 and PKD1L3 are activated by various acids when coexpressed in heterologous cells but not by other classes of tastants (Ishimaru et al. 2006). Targeted ablation of PKD2L1-expressing taste receptor cells results in a specific and total loss of the sour taste, whereas responses to sweet, umami, bitter or salty tastants remain indistuinguishable from those in wild-type animals (Huang et al. 2006). These findings firmly establish PKD2L1-expressing cells as specific sour taste receptors. Since PKD2L1 is expressed in neurons surrounding the central canal of the spinal cord, this channel has also been suggested to serve as a chemoreceptor monitoring the acidity of the cerebrospinal fluid (Huang et al. 2006).
3.2 Acidosis in the gastrointestinal tract
3.2.1 Acidity and acidosis in the gastrointestinal tract
The stomach is the most productive source of acid in the body. The gastric parietal cells can secrete hydrochloric acid (HCl) to yield a H+ concentration in the gastric lumen that - with an average diurnal pH of 1.5 - is 6 orders of magnitude higher than in the interstitial space of the gastric lamina propria (Holzer 2007). Most tissues would rapidly disintegrate if exposed to this pH, yet gastric acid is essential for the digestive breakdown of food and elimination of ingested pathogens. The autoaggressive potential of HCl is kept in check by an elaborate network of mucosal defence mechanisms and by the functional compartmentalization of the oesophago-gastro-duodenal region (Holzer 2007). Both strategies require an acid surveillance system among which acid-sensitive afferent neurons play a important role. If the pathophysiological impact of gastric acid gets out of control, acid-related diseases including gastritis, gastroduodenal ulceration, dyspepsia and gastro-oesophageal reflux disease may ensue.
Acid sensors are not only relevant to control the secretion and actions of gastric acid but also to detect tissue acidosis resulting from ischaemia, inflammation, microbial activity, malignant tumour growth and gastrointestinal motor stasis. The pH profile in the gastrointestinal lumen of healthy subjects shows a distinct shape (Fallingborg 1999; Nugent et al. 2001), with peaks of acidity in the stomach and proximal large bowel. While HCl and bicarbonate (HCO3−) secretion are the major determinants of luminal pH in the foregut, luminal pH in the colon depends on mucosal HCO3− and lactate production as well as on microbial transformation of carbohydrates to short chain fatty acids and formation of ammonia. This pH profile can be changed by surgical interventions and in inflammatory bowel disease (Nugent et al. 2001; Holzer 2007).
3.2.2 Acid sensing as a feedback in the control of foregut homeostasis
The secretion of gastric acid at highly toxic concentrations requires a tight control of its production according to need. The major inhibitory regulator is an increase in intragastric acidity, given that a decrease of luminal pH below 3 has a concentration-dependent inhibitory influence on HCl and gastrin secretion, and at pH 1 further acid output is abolished (Shulkes et al. 2006). The major mediator of this feedback inhibition is somatostatin which via paracrine and endocrine pathways inhibits parietal cell function both directly and indirectly via reduction of gastrin secretion. The activity of D cells is in part regulated by acid-sensitive primary afferent neurons in the gastric mucosa which following luminal acidification release CGRP to stimulate D cells (Manela et al. 1995; Holzer 1998). The acid sensors of the somatostatin-releasing D cells and of other endocrine cells in the gastrointestinal mucosa await to be explored. Excess acid causes release of 5-hydroxytryptamine from enterochromaffin cells in the rat gastric mucosa (Wachter et al. 1998). Enterochromaffin cells are often called the “taste buds” of the gut, but whether they monitor intraluminal pH is not known.
Exposure of the oesophageal, gastric and duodenal mucosa to excess acid elicits protective mechanisms including an increase in mucus gel thickness, HCO3− secretion and mucosal blood flow (Holzer 1998; Aihara et al. 2005; Akiba et al. 2006b; Montrose et al. 2006). These reactions are initiated in part by epithelial cells and their acid sensing mechanisms and in part by capsaicin-sensitive afferent nerve fibres. Since these nerve fibres reside in the lamina propria behind the epithelium, the mucosal acid signal must be transduced across the epithelium. In the duodenum, this seems to be achieved by diffusion of CO2 into the epithelial cells, hydration to H+ and HCO3−, intracellular acidification and exit of H+ via the basolateral sodium-proton exchanger of type 1 (Akiba et al. 2006b; Montrose et al. 2006). As a result, interstitial pH is lowered, which activates sensory nerve terminals that release the vasodilator peptide CGRP (Akiba et al. 2006b). TRPV1 is involved in the duodenal hyperaemia due to luminal acid exposure, since it is attenuated by the TRPV1 blocker capsazepine (Akiba et al. 2006b), whereas the gastric hyperaemia is left unaltered by capsazepine (Tashima et al. 2002). The acid-evoked secretion of gastric and duodenal HCO3− also remains unchanged by capsazepine (Kagawa et al. 2003; Aihara et al. 2005).
The injurious potential of gastric acid is, in addition, kept in check by compartmentalization of the oesophago-gastro-duodenal region. This strategy is to restrict the presence of high acid concentrations to the stomach, the mucosa of which is most resistant to intrusion by H+, and to precisely control H+ passage from the stomach to the duodenum through coordinated activity of the lower oesophageal and pyloric sphincters. Both sphincters are under the control of neural reflexes involving acid-sensitive neurons which adjust the tone of these sphincters to balance the levels of acid present in the oesophagus, stomach and duodenum with the mucosal defence mechanisms in these compartments (Forster et al. 1990; Lu and Owyang 1999; Holzer et al. 2003; Holzer 2007). The molecular acid sensors and sensory neurons involved in the control of oesophago-gastro-duodenal motor activity await full exploration (Holzer 2007). Apart from extrinsic sensory neurons, it is likely that intrinsic primary afferent neurons of the enteric nervous system are involved, given that they have been found to respond to acidosis (Bertrand et al. 1997; Schicho et al. 2003).
Paradoxically, knockout of TRPV1 has been reported to ameliorate acid-induced injury in the oesophagus and stomach (Akiba et al. 2006a; Fujino et al. 2006). Analysis of this unexpected observation in the stomach has revealed that disruption of the TRPV1 gene causes a compensatory upregulation of other protective mechanisms in the gastric mucosa (Akiba et al. 2006a). Thus, experiments with selective TRPV1 blockers are needed to unveil the precise role of TRPV1 in acid-induced mucosal injury in the foregut.
3.2.3 Acid as a factor in abdominal pain
Acid is not only a factor in gastrointestinal tissue injury but also in gastrointestinal pain, contributing to the symptoms of gastro-oesophageal reflux disease and peptic ulcer (Kang and Yap 1991). Whether acid also plays a role in the pain associated with functional gastrointestinal disorders such as non-cardiac chest pain, functional dyspepsia, irritable bowel syndrome and functional abdominal pain syndrome is less well understood (Holzer 2007). Whole-cell voltage-clamp recordings from DRG and nodose ganglion neurons innervating the rat stomach and mouse colon have shown that acidosis induces currents that can to a variable degree be attributed to the gating of ASICs and TRPV1 (Sugiura et al. 2005; Sugiura et al. 2007). The pH sensitivity and kinetics of these currents are distinctly altered after experimental induction of gastric ulcers (Sugiura et al. 2005).
Intramucosal acidosis induced by exposure of the rat or mouse gastric lumen to supraphysiological HCl concentrations (> 0.15 M) elicits a visceromotor response indicative of pain (Lamb et al. 2003) and causes many neurons in the nucleus of the solitary tract in the brainstem to express c-Fos, a marker of neuronal excitation (Schuligoi et al. 1998; Danzer et al. 2004; Wultsch et al. 2008). The gastric HCl-evoked visceromotor reaction and medullary c-Fos response are suppressed by vagotomy, but not transection of the sympathetic nerve supply to the stomach, which indicates that gastric HCl-evoked nociception depends critically on the integrity of the vagal afferent innervation (Schuligoi et al. 1998; Lamb et al. 2003). Apart from eliciting pain, acid causes sensitization of mechanosensitive afferent pathways from the oesophagus, stomach and colon (Coffin et al. 2001; Medda et al. 2005). Experimentally induced gastritis and gastric ulceration enhance the gastric HCl-evoked visceromotor reaction and medullary c-Fos response (Lamb et al. 2003; Holzer et al. 2007; Wultsch et al. 2008).
The gastric HCl-evoked visceromotor reaction is inhibited by pretreatment of rats with a neurotoxic dose of capsaicin (Lamb et al. 2003). In contrast, the medullary c-Fos response to gastric acid challenge is neither altered by pretreatment with capsaicin (Schuligoi et al. 1998) nor by deletion of the TRPV1 gene (Peter Holzer, Thomas Wultsch and Peter W. Reeh, unpublished observation). While afferent acid signalling from the normal stomach to the brainstem is preserved in ASIC3 knockout mice, the effect of gastritis to enhance the gastric acid-evoked expression of c-Fos in the brainstem is abolished by disruption of the ASIC3 gene (Wultsch et al. 2008). ASIC3 thus seems to play a major role in the inflammatory hyperresponsiveness of the vagal afferent - brainstem axis to gastric acid. Conversely, ASIC2 gene knockout does not alter inflammatory hyperresponsiveness but enhances the medullary c-Fos response to gastric acid challenge of the normal stomach (Wultsch et al. 2008). Although this finding suggests that ASIC2 may normally dampen acid-induced afferent input, it must not be forgotten that compensatory changes in germline knockout mice may obscure the functional implication of the disrupted gene.
Activation of TRPV1 on abdominal afferent neurons by capsaicin elicits visceral pain in animals and humans (Drewes et al. 2003; Holzer 2004a; Schmidt et al. 2004). The stimulant effect of luminal acidification on gastric and oesophageal vagal afferent nerve fibres is ablated in both TRPV1 null and ASIC3 null mice (Bielefeldt and Davis 2007). Similarly, disruption of the TRPV1 gene and blockade of TRPV1 by capsazepine depress the acid-evoked stimulation of afferent nerve fibres supplying the mouse jejunum (Rong et al. 2004) and the acid-evoked currents in thoracolumbar and lumbosacral DRG neurons innervating the mouse colon (Sugiura et al. 2007). Experimental colitis induced by trinitrobenzene sulfonic acid is associated with an increase in TRPV1 expression in thoracolumbar and lumbosacral DRG neurons and in the visceromotor response to intracolonic acid administration (Miranda et al. 2007). The effects of trinitrobenzene sulfonic acid to induce colitis, TRPV1 overexpression and hyperalgesia in response to acid challenge are counteracted by the TRPV1 blocker JYL1421 (Miranda et al. 2007).
Pharmacological blockade of TRPV1 with SDZ 249-665, a vanilloid compound causing desensitization of sensory neurons to capsaicin, attenuates the behavioural pain response to intraperitoneal administration of acetic acid in rats (Urban et al. 2000). This pain reaction may indeed reflect a response to acidosis because the writhing response to intraperitoneal injection of acetic, lactic and propionic acid is attenuated by capsazepine, whereas that to phenylbenzoquinone is not (Ikeda et al. 2001). An involvement of TRPV1 in acetic acid-induced writhing is further corroborated by the ability of various TRPV1 blockers to reduce the abdominal contractions caused by intraperitoneal injection of acetic acid (Rigoni et al. 2003; Tang et al. 2007). Likewise, mice lacking TRPV4 are hyporesponsive to intraperitoneal injection of acetic acid (Suzuki et al. 2003a). Overexpression of a dominant-negative ASIC3 subunit has been found to increase the writhing response to intraperitoneal acetic acid (Mogil et al. 2005), a change that paradoxically is also seen in ASIC3 null mice (Chen et al. 2002). Whether this finding is the result of a change in the kinetics of ASIC1 and ASIC2 after knockout of ASIC3 (Kress and Waldmann 2006) or of a compensatory upregulation of acid sensors other than ASIC3 is not known.
The available information points to a role of TRPV1 and ASIC3 in acid sensing within the gastrointestinal tract as well as in ulceration- and inflammation-evoked sensitization of afferent neurons (Figure 5). This inference is consistent with a number of findings that show that abdominal hyperalgesia is associated with an upregulation in acid sensor expression and/or function. For instance, acute exposure of the rat gastric mucosa to a noxious HCl concentration leads to a rise of TRPV1 immunoreactivity, but not TRPV1 mRNA, in DRG neurons innervating the stomach (Schicho et al. 2004). TRPV1 in vagal and spinal afferent neurons is upregulated in acid-evoked oesophagitis as well as in trinitrobenzene sulfonic acid-induced pancreatitis and colitis (Banerjee et al. 2007; Miranda et al. 2007; Xu et al. 2007). Similarly, the expression of TRPV1 and ASIC3, but not ASIC1 and ASIC2, is enhanced in the colonic mucosa of patients with inflammatory bowel disease (Yiangou et al. 2001b; Yiangou et al. 2001c). TRPV1-like immunoreactivity is likewise increased in oesophagitis (Matthews et al. 2004), non-erosive reflux disease (Bhat and Bielefeldt 2006), rectal hypersensitivity and faecal urgency (Chan et al. 2003).
There is some evidence that P2X receptors are involved in gastrointestinal sensation and pain related to acidosis. Protons potentiate the ATP-evoked stimulation of nodose and DRG ganglion cells (Li et al. 1996; Li et al. 1997; Dunn et al. 2001; Zhong et al. 2001). The writhing behaviour elicited by intraperitoneal injection of acetic acid is inhibited by trinitrophenyl-ATP (a P2X1, P2X3 and P2X2/3 receptor blocker) and A-317491 (a P2X3 and P2X2/3 receptor antagonist), whereas the P2X1 channel blocker diinosine pentaphosphate is ineffective (Honore et al. 2002; Jarvis et al. 2002). Distension of the gut is thought to release ATP from the intestinal mucosa which subsequently excites afferent neurons expressing P2X receptors. This mode of mechanosensory transduction via P2X3 receptors is enhanced by experimental colitis in the rat (Wynn et al. 2004) and may, conceivably, involve inflammation-associated acidosis and/or upregulation of P2X3 purinoceptors as has been observed in the colonic mucosa of patients with inflammatory bowel disease (Yiangou et al. 2001a).
3.3 Acidosis in the urogenital tract
The sensory innervation of the urogenital tract is of paramount relevance to the regulation of urine storage and voiding. Given that the urine is usually acidic and hyperosmotic, nerve endings behind the urotheliun are likely to be exposed to excess acid if the urothelial barrier is disrupted (Chuang et al. 2003). This scenario is likely to occur under the conditions of irritable bladder and cystitis in which there is evidence for sensitization of afferent neurons, at least to mechanical stimuli. As a result, bladder hyperactivity, bladder hyperreflexia and pain may occur. The plausibility of this concept receives support from the finding that both populations of afferent neurons innervating the bladder, thoracolumbar and lumbosacral DRG neurons, are excited by acidification and exhibit proton-evoked currents with different inactivation kinetics (Dang et al. 2005; Daly et al. 2007). Furthermore, there is emerging evidence that acid sensors on afferent neurons such as TRPV1 play an appreciable role in pathologies of the urogenital tract (Figure 5).
An implication of TRPV1 in bladder overactivity and pain has been proved both by experimental and clinical evidence. Thus, chronic intravesical administration of capsaicin or resiniferatoxin to desensitize the sensory innervation of the bladder is beneficial in patients with urinary bladder pain and hyperreflexia (Bley 2004; Brady et al. 2004; Avelino and Cruz 2006; Cruz and Dinis 2007). This finding is consistent with the finding that TRPV1-like immunoreactivity is upregulated in neurogenic bladder overactivity (Brady et al. 2004). The symptoms of women with sensory urgency, but not idiopathic detrusor overactivity, have been associated with increased expression of TRPV1 mRNA in the trigonal mucosa (Liu et al. 2007). A contribution of TRPV1 to bladder function has been confirmed experimentally by genetic and pharmacological studies. Knockout of the TRPV1 gene blunts the responsiveness of bladder afferent neurons to intravesical administration of hydrochloric acid and capsaicin as well as to bladder distension and impairs the function of low-threshold afferents (Birder et al. 2002; Daly et al. 2007).
There is ample evidence that P2X2 and P2X3 purinoceptors are involved in the physiology and pathophysiology of urinary bladder voiding (Cockayne et al. 2000; Cockayne et al. 2005; Burnstock 2007), but it is not known whether they contribute to acid sensing in the urogenital tract.
3.4 Acidosis in the pulmonary system
DRG and nodose ganglion neurons projecting to the lung and pleura express TRPV1 and ASICs (Groth et al. 2006; Jia and Lee 2007; Kollarik et al. 2007). Accordingly, vagal afferent neurons supplying the rat and guinea-pig lung display proton-induced currents with rapid and slow inactivation currents that appear to be carried by ASICs and TRPV1 as they are inhibited by amiloride and capsazepine, respectively (Kollarik and Undem 2002; Gu and Lee 2006). Acidosis in the aiways can be the result of several processes including inflammation, ischaemia or aspiration of refluxing gastric contents, and exhaled breath condensate studies indicate that acidosis is associated with obstructive airway diseases such as asthma (Ricciardolo et al. 2004; Hunt 2006). The functional implications of acidosis in the airways are manyfold and comprise local effects on airway muscle tone and inflammatory processes as well as effects involving the central nervous system: cough, discomfort and pain (Jia and Lee 2007). In the guinea-pig isolated trachea acidosis has a differential effect on basal airway muscle tone and muscle responsiveness to contractile stimuli (Faisy et al. 2007). The acid-induced airway relaxation seems to be independent of sensory neurons and mediated by ASICs on smooth muscle cells, whereas the acid-evoked muscle hyperresponsiveness to acetylcholine involves sensory neurons expressing ASICs and TRPV1 (Faisy et al. 2007).
There is increasing evidence that acid is an important mediator in the pathogenesis of cough, given that inhalation of exogenous acid triggers cough, and acidosis accompanies a variety of respiratory diseases (Jia and Lee 2007; Kollarik et al. 2007). Following local generation, inhalation or aspiration, acid can directly stimulate vagal bronchopulmonary sensory nerve fibres involved in the cough reflex, Aα-fibre nociceptors in the large airways being most efficiently stimulated by rapid acidification (Kollarik et al. 2007). In contrast, C-fibre nociceptors expressing TRPV1 are able to continuously monitor the pH in the tracheopulmonary tissue and thus to react to persistent acidosis as it occurs in inflammation. In addition, acid is the single most important mediator of cough due to gastro-oesophageal reflux, given that acid-sensitive oesophageal afferent neurons sensitize the neural pathways underlying the cough reflex (Kollarik et al. 2007).
The sensors of vagal afferent neurons involved in acid surveillance and the cough reflex include TRPV1 and other probes (Figure 5). Accordingly, the TRPV1 blockers iodo-resiniferatoxin, JNJ17203212 and V112220 are able to attenuate cough induced by citric acid inhalation in guinea-pigs (Trevisani et al. 2004; Bhattacharya et al. 2007; Leung et al. 2007). It is not yet known whether ASICs account in full for the TRPV1-independent mechanisms of acid sensing in the airways (Canning et al. 2006; Kollarik et al. 2007; Leung et al. 2007). There is evidence that a TREK-like K2P channel, which is sensitive to intracellular pH changes, could contribute to the function of intrapulmonary chemoreceptors (Bina and Hempleman 2007).
3.5 Acidosis in the skin
DRG neurons innervating the skin express many of the known acid-sensitive ion channels including TRPV1, ASICs and P2X. ASICs, for instance, are expressed by nociceptive and non-nociceptive afferent neurons innervating glabrous and hairy skin (Jiang et al. 2006). While intracutaneous perfusion of acid in human volunteers induces long-lasting non-adapting pain (Steen and Reeh 1993; Steen et al. 1995), transdermal iontophoresis of protons elicits transient pain that subsides within 5 min despite extended acid application (Jones et al. 2004; Cadiou et al. 2007). Intracutaneous injection of protons evokes an even shorter pain sensation that declines within 100 s (Rukwied et al. 2007). The route-dependent time course of proton-evoked cutaneous pain is probably due to differences in the kinetics of acid exposure as well as in the activation/inactivation kinetics of the acid-sensitive ion channels involved (Kress and Waldmann 2006).
Intracutaneous co-injection of prostaglandin E2 and acid has enabled Rukwied et al. (2007) to differentiate an early and late phase of proton-evoked pain. The late phase is thought to be mediated by TRPV1, because it is selectively potentiated by prostaglandin E2 (Rukwied et al. 2007). Indeed, disruption of the TRPV1 gene prevents acid from stimulating unmyelinated afferent nerve fibres in a mouse skin-nerve preparation, whereas the proton-induced excitation of thinly myelinated nerve fibres persists (Caterina et al. 2000). Similarly, the effect of strong acidosis (pH 5.2) to cause release of CGRP from rat sciatic nerve axons is attenuated by capsazepine, whereas the peptide release evoked by mild acidosis (pH 6.1) is left unaltered (Fischer et al. 2003). Inflammation leads to upregulation of TRPV1 expression and function in afferent neurons supplying the skin and to an increase in the proton sensitivity of isolectin B4-positive C-fibres (Carlton and Coggeshall 2001; Ji et al. 2002; Breese et al. 2005).
Other studies attribute ASICs an important role (Figure 5). Nitric oxide donors potentiate acid-induced currents in ASIC1, ASIC2 and ASIC3 homomeric channels and increase acid-evoked pain in the human skin (Cadiou et al. 2007). Acid-evoked pain is attenuated by nonsteroidal anti-inflammatory drugs (NSAIDs), which are known to interfere with ASIC expression and function, but left unaffected by the TRPV1 blocker capsazepine or desensitization to capsaicin (Steen et al. 1995; Voilley et al. 2001; Ugawa et al. 2002; Jones et al. 2004). These findings suggest that part of the analgesic effect of NSAIDs could be due to interference with ASICs, a conjecture that is consistent with the upregulation of ASICs in DRG neurons by cutaneous inflammation due to Freund’s adjuvant (Voilley et al. 2001). An implication of ASICs is further supported by the ability of amiloride to reduce acid-induced pain in the skin but to spare capsaicin-induced pain (Ugawa et al. 2002; Jones et al. 2004). Post-operative pain due to skin incision in rats is attenuated by A-317567, a blocker of ASIC1, ASIC2 and ASIC3 subunits (Dubé et al. 2005).
Studies with ASIC knockout mice have yielded ambiguous results, which is not totally unexpected in view of the redundancy of acid sensors present on sensory neurons and the likelihood of compensatory changes that occur during the development of ASIC null mice. In the isolated skin-nerve preparation, the response of mechanoheat-sensitive C-fibres to acid is reduced in ASIC3 null mice (Price et al. 2001). Paradoxically, however, the behavioural licking response to acetic acid injection into the paw is preserved in ASIC3 knockout animals (Price et al. 2001). It awaits to be explored whether other acid sensors, e.g. K2P channels and P2X purinoceptors, contribute to acidosis-evoked pain in the skin.
3.6 Acid sensors in the carotid body
Detection of blood pH and systemic acidosis is important for feedback regulation of respiration and cardiovascular function. To this end, O2-, CO2- and pH-sensitive chemoreceptors are present both in the carotid bodies and in the brain (López-López and Pérez-Garcia 2007). The acid sensors operating in the chemoreceptor (glomus type I) cells of the carotid body (Figure 5) comprise inward rectifier K+ channels (Putnam et al. 2004), K2P channels containing the TASK-1, TASK-3 and/or TASK-5 subunits (Buckler 2007; Duprat et al. 2007), inwardly rectifying Cl− channels (Petheo et al. 2001) and ASIC channels made up of ASIC1 and ASIC 3 (Tan et al. 2007). It is thus emerging that there is a parallel processing of O2, CO2 and pH signals in carotid body chemoreceptor cells, the resulting depolarization causing release of transmitters (ATP, acetylcholine and dopamine) from the chemoreceptor cells and subsequent excitation of afferent nerve endings (Rong et al. 2003; Zhang and Nurse 2004; López-López and Pérez-Garcia 2007; Tan et al. 2007).
Apart from acting on glomus type I cells, acidosis could also directly modify sensory neurons in the glossopharyngeal nerve, given that these neurons display a background K+ conductance resembling THIK-1 which is weakly inhibited by extracellular acidosis (Campanucci et al. 2003). Acidosis enhances the ATP-induced whole cell current of petrosal ganglion afferents, which suggests that increased sensitivity of P2X receptors on afferent nerve fibres contributes to the transmission of acidosis in the carotid body (Zhang and Nurse 2004). Genetic disruption of the P2X2 gene blunts the hyperventilatory response to hypoxia, which is consistent with the release of ATP from the glomus cells and the expression of P2X purinoceptors containing the acid-sensitive P2X2 subunit by afferent neurons in the carotid sinus nerves (Rong et al. 2003). The ability of compounds such as halothane and isoflurane to activate TASK-3 channels in the carotid body is likely to have a direct bearing on their property to depress the hypoxic ventilatory drive when used for anaesthesia (Buckler 2007; Duprat et al. 2007).
3.7 Acidosis due to myocardial ischaemia
There is emerging evidence that ASICs account for the anginal pain associated with myocardial ischaemia, with lactate as a factor involved in channel activation. ASIC3 is highly expressed by sensory neurons innervating the rat heart (Benson et al. 1999; Sutherland et al. 2001; Yagi et al. 2006). Patch-clamp analysis of rat ASIC3 homomers and ASIC2a/ASIC3 heteromers shows that both channel complexes generate persistent inward currents at the modest extracellular pH changes typical of muscle ischaemia, the currents produced by the ASIC2a/ASIC3 heteromers being much larger than those produced by ASIC3 homomers (Yagi et al. 2006). The sustained current is caused by a region of pH where there is overlap between inactivation and activation of the channel (Yagi et al. 2006). Lactate causes the current to activate at slightly more basic pH values. The currents produced by the heterologously expressed ASIC3 and ASIC2a/ASIC3 complexes are in keeping with the sustained currents that changes of pH from 7.4 to 7.0 produce in somata of DRG neurons innervating the rat heart (Yagi et al. 2006). These observations indicate that ASIC3 homo- and heteromers may play a major role in detecting sustained myocardial ischaemia and in mediating prolonged anginal pain (Figure 5).
In contrast, the acidosis-evoked release of CGRP from sensory nerve fibres within the mouse heart appears to be mediated by TRPV1, since the response is absent in TRPV1 null mice but left unaltered in ASIC3 knockout animals (Strecker et al. 2005). Given that the neuropeptides (CGRP and substance P) released from afferent nerve fibres in the tissue are potent vasodilators, the TRPV1-mediated release of these peptides in myocardial ischaemia is likely to have a local cardioprotective effect (Wang and Wang 2005; Zhong and Wang 2007). This concept is supported by the finding that the recovery of cardiac function after exposure to ischaemia/reperfusion is impaired by capsazepine and disruption of the TRPV1 gene (Wang and Wang 2005). The beneficial effect of preconditioning against cardiac injury induced by ischaemia/reperfusion is likewise attenuated in TRPV1 null mice (Zhong and Wang 2007).
3.8. Acidosis in the skeletal muscle
Unmyelinated afferent neurons innervating the skeletal muscle are excited by acidosis (Hoheisel et al. 2004), and injection of acid into the anterior tibial muscle of humans elicits a biphasic but short-lasting pain response (Rukwied et al. 2007). The second phase is suggested to involve TRPV1, because it is selectively potentiated by prostaglandin E2 (Rukwied et al. 2007). Other studies provide evidence that ASIC3 is relevant to sensing acidosis in skeletal muscle. Thus, repeated intramuscular injection of acidic saline induces mechanical hyperalgesia, a response that is blunted by amiloride and knockout of the ASIC3 gene (Figure 5), but not by deletion of ASIC1a (Price et al. 2001; Sluka et al. 2003). Furthermore, ASIC3 is involved in the mechanical hyperalgesia that is associated with muscle inflammation (Sluka et al. 2007). Thus, ASIC3-deficient mice fail to develop mechanical hyperalgesia following experimental inflammation by injection of carrageenan into the muscle, whereas injection of a recombinant herpes virus vector to express ASIC3 in the muscle of ASIC3 knockout mice rescues the hyperalgesia phenotype (Sluka et al. 2007).
Analogously to the situation in the myocardium (Benson et al. 1999; Sutherland et al. 2001; Yagi et al. 2006) there is good reason to assume that lactic acid is a factor relevant to acidosis-evoked muscle pain. Lactate is formed under limited oxygen availability in the muscle and causes acidification of the extracellular space. In addition, lactate is able to sensitize ASIC3 to acid by decreasing the extracellular concentration of Ca2+ (Immke and McCleskey 2001). Lactic acid accumulation and activation of acid-sensitive afferent neurons in skeletal muscle contribute to the reflex hypertension and tachycardia that is evoked by exercise. A participation of ASICs is deduced from the ability of a low dose of amiloride (0.5.00g/kg) to selectively block the hypertensive response to muscle exercise (Figure 5), whereas the response to capsaicin or muscle stretch is spared (Li et al. 2004; Hayes et al. 2007). In addition, P2X receptors and - at more pronounced acidity - TRPV1 also come into play (Gao Z et al. 2007). Another factor contributing to the muscle pressor reflex is thought to be diprotonated phosphate (H2PO4−) whose excitatory action on afferent neurons involves both TRPV1 and ASICs as deduced from the inhibitory effects of capsazepine and amiloride, respectively (Gao et al. 2006).
3.9 Acidosis in the skeleton
ASICs are expressed not only by sensory neurons innervating bone but also by cells of the skeleton. Thus, ASIC1, ASIC2 and ASIC3 are found in the skeleton, with ASIC1 prevailing in chondrocytes, and ASIC2 and ASIC3 predominating in osteoclasts and osteoblasts (Jahr et al. 2005). These findings carry two important perspectives, because osteoclasts actively secrete acid when they resorb bone and because bone disorders with increased bone resorption by osteoclasts are frequently associated with pain. Osteoclasts degrade bone by secreting protons through a vacuolar H+-ATPase, thereby creating an acidic microenvironment. Inflammation-induced mineral resorption in metatarsal bones of the rat is associated with hyperalgesia, increased expression of ASIC1a, ASIC1b and ASIC3 in DRG neurons and enhanced c-Fos expression in the dorsal horn of the spinal cord (Nagae et al. 2006). The inflammation-induced hyperalgesia and upregulation of ASICs are reversed by the bisphosphonate zoledronic acid, which inhibits osteoclastic bone resoption, and by bafilomycin A1, an inhibitor of vacuolar H+-ATPase (Nagae et al. 2006). Since amiloride is also beneficial (Nagae et al. 2006), it would seem that ASICs play a role in bone pain associated with osteoclastic mineral resorption (Figure 5).
Bone pain is one of the most common complications in cancer patients with bone metastases. Experimental evidence indicates that this type of bone pain is also related to bone degradation by osteoclasts and the acidic microenvironment that afferent neurons in the bone matrix are exposed to (Honore et al. 2000; Luger et al. 2001). Afferent neurons expressing TRPV1 innervate the mouse femur, and both genetic and pharmacological studies indicate that TRPV1 contributes to the hyperalgesia associated with an in vivo bone cancer model (Figure 5), given that disruption of the TRPV1 gene or treatment with the TRPV1 blocker capsazepine attenuates both ongoing and movement-evoked nocifensive behaviours (Ghilardi et al. 2005). In a model of bone cancer pain in the rat tibia, hyperalgesia is associated with an upregulation of mRNA for ASIC1a and ASIC1b, but not ASIC3 and TRPV1, in DRG neurons (Nagae et al. 2007). Although the findings in the rat and mouse have not yet been reconciled with each other, it is envisaged that both TRPV1 and ASICs participate in bone cancer pain.
Another line of investigation ascribes ASICs a role in the pain arising from vertebral disc herniation. The vertebral discs are innervated by primary afferent neurons (Ohtori et al. 2007), and an experimental model of lumbar disc herniation produced by application of nucleus pulposus to, and pinching of, L5 nerve roots is associated with mechanical allodynia and an upregulation of ASIC3 in the respective DRG (Ohtori et al. 2006). Local administration of lidocaine has been found to reverse both mechanical allodynia and ASIC3 upregulation (Ohtori et al. 2006), which makes a case for ASIC3 being involved in radicular pain. In this context it need be considered that ASIC3 is present on nucleus pulposus cells of the intervertebral discs and that ASIC3 expression by these cells is under the control of nerve growth factor (Uchiyama et al. 2007). In a functional perspective it would seem that ASIC3 is required for adaptation of nucleus pulposus cells to the acidic and hyperosmotic microenvironment of the intervertebral disc (Uchiyama et al. 2007).
4. PHARMACOLOGICAL INTERFERENCE WITH ACID SENSORS
From the pertinent studies it is clear that no single molecular sensor alone accounts for the acid sensitivity of afferent neurons. Neuroanatomical analyses and recordings from DRG and nodose ganglion neurons have shown that acidosis induces currents that can to a variable degree be attributed to the gating of ASICs, TRPV1 and other acid sensors, because these probes are differentially expressed by different subpopulations of sensory neurons (Liu et al. 2004; Dang et al. 2005; Sugiura et al. 2005; Ugawa et al. 2005; Leffler et al. 2006; Poirot et al. 2006; Smith et al. 2007; Sugiura et al. 2007). Although ASICs and TRPV1 survey different spectra of acidic pH, it is obvious that there is a redundancy of molecular acid sensors, which signifies that early detection of acidosis is physiologically so important that multiple mechanisms of acid sensing have evolved.
Acid sensors can be considered as drug targets in more than one respect. Many of the acid-sensitive ion channels are highly regulated by a number of endogenous factors as well as pharmacological agents. This is true, e.g., for NSAIDs regulating the expression and function of ASICs, pro-inflammatory mediators causing sensitization of TRPV1, and anaesthetics and other neuroactive drugs controlling the activity of K2P channels. A number of acid-sensitive ion channels and receptors including ASICs, TRPV1 and P2X is upregulated by inflammation and nerve injury and appears to contribute to the hyperalgesia associated with these conditions. If so, drugs targeting peripheral acid sensors may evolve as novel therapies of chronic inflammation and pain. This concept is attractive for a number of reasons, particularly because it offers the opportunity to develop antinociceptive drugs with a peripherally restricted site of action, avoiding unwanted effects on the central nervous system. However, interfering with molecular probes that are physiologically so important poses a serious threat to homeostasis unless selective inhibition of “excess” acid sensors can be achieved while their physiological function is preserved.
With these considerations in mind, some of the attempts and developments to pharmacologically modulate acid sensors are discussed in the following sections, along with the rationale behind their design and emerging information on their utility and limitations.
4.1 ASICs
There is emerging evidence that ASIC expression is altered following inflammation and nerve injury (Voilley et al. 2001; Yiangou et al. 2001c; Mamet et al. 2002; Ohtori et al. 2006; Poirot et al. 2006; Nagae et al. 2007). These pathological implications of ASICs in pain and hyperalgesia suggest that ASIC blockade could be a novel avenue in pain therapy.
The pharmacology of specific ASIC-targeting drugs is just emerging. Most ASIC currents are blocked by the diuretic drug amiloride and its derivatives (e.g., benzamil) which, at the concentrations needed to inhibit ASIC activity, also suppress other ion channels and ion exchangers (Kress and Waldmann 2006; Page et al. 2007). As a consequence, the use of amiloride as a probe for the functional implications of ASICs is a pharmacological approach with limited specificity. In contrast, the peptide psalmotoxin 1 (PcTx1) isolated from the venom of the South American tarantula Psalmopoeus cambridgei is a highly selective ASIC blocker as it inhibits ASIC1a homomers with an affinity of 0.7 nM (IC50) but does not affect ASIC1a-containing heteromers (Escoubas et al. 2000). It modifies ASIC1a gating by shifting the channel from the resting to an inactivated state (Escoubas et al. 2000; Diochot et al. 2007; Mazzuca et al. 2007). Another selective ASIC blocker, APETx2, has been isolated from the venom of the sea anemone Anthopleura elegantissima. This peptide toxin inhibits several ASIC3-containing channels with IC50 values between 63 nM and 2.00M, while its mode of action awaits to be elucidated (Diochot et al. 2004; Diochot et al. 2007). The first synthetic ASIC inhibitor different from amiloride is A-317567, a compound that blocks proton-evoked currents through ASIC1, ASIC2 and ASIC3 channels as well as the sustained phase of the ASIC3-like current in DRG neurons (Dubé et al. 2005).
A number of drugs interferes with ASIC function in a nonselective manner. This is in particular true for NSAIDs which counteract the upregulation of ASICs caused by experimental inflammation and inhibit proton-evoked ASIC currents in afferent neurons (Voilley et al. 2001; Mamet et al. 2002). Ibuprofen and flurbiprofen inhibit homomultimeric ASIC1a channels, whereas salicylic acid, aspirin and diclofenac block the sustained currents of ASIC3 and ASIC2b/ASIC3 channels (Voilley et al. 2001). Although it remains to be established whether the effects of NSAIDs on ASICs are relevant to their anti-inflammatory and analgesic action, it appears conceivable that ASICs participate in inflammatory hyperalgesia. This inference is supported by the ability of proinflammatory mediators such as nerve growth factor, 5-hydroxytryptamine, interleukin-1 and bradykinin to promote the transcription of ASIC3 in sensory neurons, 5-HT and NGF interacting directly with the promoter region of the ASIC3 gene (Mamet et al. 2002). Another proinflammatory mediator, arachidonic acid, is able to potentiate proton-evoked currents in heterologously expressed ASIC1a, ASIC2a and ASIC3 by a direct action on the channel protein (Smith et al. 2007).
Peripheral inflammation causes upregulation of FMRFamide-like peptides including neuropeptide FF and NPFF-R2 in DRG neurons and the spinal cord, and both neuropeptide FF and FMRFamide are able to potentiate H+-gated currents in cultured sensory neurons and heterologously expressed ASIC1 and ASIC3 channels (Askwith et al. 2000; Catarsi et al. 2001; Deval et al. 2003; Yang et al. 2008). This action appears to result from a delay in current inactivation or from enhancement of a sustained and slowly inactivating current. ASICs are modulated by a number of other factors, some of which may be generated or released during inflammation and acidosis. Nitric oxide, for instance, is able to potentiate acid-induced currents in ASIC1, ASIC2 and ASIC3 homomeric channels (Cadiou et al. 2007). Serine proteases modulate the pH sensitivity of ASIC1a and ASIC1b, probably by proteolytic cleavage of the long extracellular loop (Poirot et al. 2004).
An approach to reduce excess ASIC expression and function could be the interference with ASIC translocation to the cell membrane, integration in the cell membrane, and phosphorylation. As other membrane proteins, ASICs appear to form macromolecular complexes with other proteins that may control the trafficking, function and turnover of ASIC subunits (Kress and Waldmann 2006; Wemmie et al. 2006) and be future targets for pharmacological intervention.
ASICs do not only play a role in pain owing to their peripheral nocisensor function but also in the spinal transmission of pain impulses. ASIC1a is prominently expressed by neurons in the dorsal horn of the spinal cord, and downregulation of these ASIC1a channels by antisense oligonucleotides attenuates thermal and mechanical hypersensitivity induced by peripheral administration of complete Freund’s adjuvant (Duan et al. 2007). ASIC1a participates in two forms of central sensitization in the spinal cord: C-fibre-induced “wind-up” and inflammation-evoked hypersensitivity of dorsal horn neurons (Duan et al. 1997). Based on these observations it has been proposed that specific blockade of Ca2+-permeable ASIC1a channels may have an antinociceptive effect by reducing or preventing the development of central sensitization due to inflammation (Duan et al. 2007). ASIC1a in the brain emerges as being involved in cell damage after ischaemic stroke (Wemmie et al. 2006; Friese et al. 2007), and blockade of this Ca2+-permeable channel has been proposed to offer a 5 h therapeutic time window following a cerebral vascular insult (Xiong et al. 2006).
4.2 TRPV channels
Recognition of TRPV1 as a multimodal nocisensor, its sensitization by a number of proalgesic pathways and its upregulation under conditions of hyperalgesia have made this ion channel an attractive target for novel antinociceptive drugs. TRPV1 function can be counteracted both by desensitizing TRPV1 agonists as well as TRPV1 antagonists, and the current patent literature discloses more than 1,000 natural and synthetic compounds as TRPV1 activators or blockers (Gharat and Szallasi, 2008). It is important to consider that TRPV1 agonists and antagonists are not equivalent approaches (Holzer 1991; Szallasi et al. 2007). Capsaicin-sensitive afferent neurons express a plethora of different nocisensors, and desensitizing TRPV1 agonists do not only inactivate this nocisensor alone but defunctionalize the whole afferent neuron expressing TRPV1. In contrast, TRPV1 blockers selectively target this nocisensor and prevent its function. Resiniferatoxin and other compounds such as SDZ 249-665 are examples of TRPV1 agonists whose action manifests itself primarily in a defunctionalization of nociceptive neurons. Intravesical resiniferatoxin has been shown to be beneficial in patients with neurogenic bladder disorders (Avelino and Cruz 2006; Cruz and Dinis 2007) in which activation of afferent neurons by acidic urine penetrating through a leaky urothelium may play a role. SDZ 249-665 is able to attenuate acid-induced nociception arising from the peritoneal cavity of rats (Urban et al. 2000).
Most efforts have been directed at developing compounds that block TRPV1 activation in a competitive or noncompetitive manner. The first of this kind, capsazepine, has been extensively used in the exploration of the pathophysiological implications of TRPV1. However, the results obtained with this compound need to be judged with caution because the selectivity of capsazepine as a TRPV1 blocker is limited by its inhibitory action on nicotinic acetylcholine receptors, voltage-activated Ca2+ channels and other TRP channels such as TRPM8 (Docherty et al. 1997; Liu and Simon 1997; Behrendt et al. 2004). Since TRPV1 is activated by multiple stimuli that interact with different domains of the channel protein, not all TRPV1 blockers prevent acid from gating TRPV1 (Gavva et al. 2005a). For instance, AMG-0610 and SB-366791 inhibit the activation of rat TRPV1 by capsaicin but not acid, whereas iodo-resiniferatoxin, BCTC, AMG-6880, AMG-7472, AMG-9810 and A-425619 are TRPV1 antagonists that do not differentiate between capsaicin and protons (Seabrook et al. 2002; Gavva et al. 2004; Gavva et al. 2005a; Gavva et al. 2005b; Neelands et al. 2005). In addition, there are species differences in the stimulus selectivity of TRPV1 blockers as, e.g., capsazepine and SB-366791 are more effective in blocking proton-induced gating of human TRPV1 than of rat TRPV1 (Gunthorpe et al. 2004; Gavva et al. 2005a).
While the vast list of emerging TRPV1 blockers attests to the antinociceptive potential that is attributed to this class of pharmacological agent, it is important to be aware of the likely drawbacks these compounds may have. It has repeatedly been argued that TRPV1 subserves important homeostatic functions, and that the challenge for an effective and safe therapy with TRPV1 blockers will be to suppress the pathological contribution of “excess” TRPV1 while preserving its physiological role (Holzer 2004b; Hicks 2006; Szallasi et al. 2007). TRPV1 is emerging as an important heat sensor involved in thermoregulation, given that most TRPV1 blockers cause hyperthermia in rats, dogs, monkeys and humans by a peripheral site of action (Gavva et al. 2007; Gava et al. 2008; Lehto et al. 2008). This hyperthermic action is unrelated to the ability of TRPV1 antagonists to block proton-induced activation of TRPV1 (Gavva et al. 2007). Hyperthermia is a serious adverse effect of TRPV1 blockade that went unnoticed after disruption of the TRPV1 gene (Szelényi et al. 2004; Woodbury et al. 2004), most probably because of developmental compensations in heat sensing.
One possible approach to differentiate between the pathological and physiological implications of TRPV1 is the development of uncompetitive blockers that preferentially bind to the active, open state of the channel and therefore will predominantly silence overactive TRPV1 (García-Martínez et al. 2006). Another approach that appears increasingly feasible is interference with the intracellular trafficking of TRPV1 to the cell membrane, which will result in a reduction of TRPV1 channels on the cell surface (Morenilla-Palao et al. 2004; Planells-Cases et al. 2005). Sensitization of TRPV1 is due not only to an enhancement of channel currents but also to a rapid translocation of TRPV1 from the cytosol to the plasma membrane (Morenilla-Palao et al. 2004; Van Buren et al. 2005; Zhang et al. 2005). The trafficking of TRPV1 (and other channels) to the cell surface is blocked by botulinum neurotoxin A (Morenilla-Palao et al. 2004), which may explain why intra-detrusor injection of botulinum neurotoxin A in patients with urinary bladder overactivity reduces TRPV1- and P2X3-like immunoreactivity in the detrusor muscle and causes improvement of clinical and urodynamic parameters (Apostolidis et al. 2005). Intravesical administration of botulinum toxin likewise counteracts acetic acid-evoked bladder overactivity in rats (Chuang et al. 2004).
4.3 K2P channels
Blockade of K2P channels contributes to proton-evoked depolarization of DRG neurons from humans with neuropathic pain (Baumann et al. 2004), which makes it worthwile to elucidate the role of these background channels in health and disease. Apart from the targeted design of drugs specifically acting at K2P channels, it is important to realize that many endogenous and exogenous factors modify and regulate the activity of these channels (Duprat et al. 2007; Mathie 2007). For instance, K2P channels of the TASK subfamily are regulated by a number of different G protein-coupled receptor pathways (Duprat et al. 2007; Mathie 2007). Along these lines, TASK-1 and TASK-3 are inhibited by the endocannabinoid anandamide (Maingret et al. 2001; Duprat et al. 2007). Another interaction that is of clinical relevance relates to the effect of anaesthetics on K2P channels. TASK-1 activity is stimulated by halothane and partially inhibited by isoflurane, whereas TASK-3 channel activity is enhanced by halothane as well as isoflurane (Duprat et al. 2007). In addition, the activity of both TASK-1 and TASK-3 is inhibited by bupivacaine (Duprat et al. 2007).
5. CONCLUSIONS
Acidosis is a noxious condition associated with many pathological changes such as inflammation, ischaemia or mucosal defects in the upper gastrointestinal tract. To monitor these challenges of homeostasis, chemonociceptive afferent neurons express several acid sensors with distinct and overlapping properties. ASICs and TRPV1 have been most thoroughly studied, given that their proton sensitivities cover a complementary range of pH aberrations. While ASICs survey moderate decreases in extracellular pH, TRPV1 is activated only by severe acidosis resulting in pH values below 6. Acidosis, however, can sensitize TRPV1 to stimuli that are known to participate in inflammatory hyperalgesia. Emerging evidence indicates that other TRP channels such asTRPV4, TRPC4, TRPC5 and TRPP2, P2X purinoceptors, K2P channels, inward rectifier K+ channels, voltage-activated K+ channels, L-type Ca2+ channels, hyperpolarization-activated cyclic nucleotide-gated channels, gap junction channels, Cl− channels and acid-sensitive G protein-coupled receptors also contribute to the acid sensitivity of afferent neurons. This redundancy of pH surveillance systems testifies that the maintenance of interstitial and intracellular pH within a narrow range is of paramount physiological importance. Since acid-sensitive ion channels and receptors have been found to be overactive and/or upregulated in chronic pain conditions and to play a role in chemonociception, they represent worthwile targets for novel analgesic drugs. The pharmacological challenge in pursuing this goal is to differentiate between the pathological implications of acid sensors, which should be suppressed, and their physiological functions which should be preserved.
Acknowledgements
Work performed in the author’s laboratory was supported by the Zukunftsfonds Steiermark (grant 262), the Austrian Scientific Research Funds (FWF grant L25-B05), the Jubilee Foundation of the Austrian National Bank (grant 9858) and the Austrian Federal Ministry of Science and Research. Evelin Painsipp, M.Sc., is acknowledged for drawing Figure 2.
References
- Aihara E, Hayashi M, Sasaki Y, Kobata A, Takeuchi K. Mechanisms underlying capsaicin-stimulated secretion in the stomach: comparison with mucosal acidification. J Pharmacol Exp Ther. 2005;315:423–432. doi: 10.1124/jpet.105.087619. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Takeuchi T, Mizumori M, Guth PH, Engel E, Kaunitz JD. TRPV-1 knockout paradoxically protects mouse gastric mucosa from acid/ethanol-induced injury by upregulating compensatory protective mechanisms. Gastroenterology. 2006a;130(Suppl 2):A–106. [Google Scholar]
- Akiba Y, Ghayouri S, Takeuchi T, Mizumori M, Guth PH, Engel E, Swenson ER, Kaunitz JD. Carbonic anhydrases and mucosal vanilloid receptors help mediate the hyperemic response to luminal CO2 in rat duodenum. Gastroenterology. 2006b;131:142–152. doi: 10.1053/j.gastro.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Alvarez de la Rosa D, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci USA. 2002;99:2326–2331. doi: 10.1073/pnas.042688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, Dasgupta P, Fowler CJ, Anand P. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005;174:977–982. doi: 10.1097/01.ju.0000169481.42259.54. [DOI] [PubMed] [Google Scholar]
- Askwith CC, Cheng C, Ikuma M, Benson C, Price MP, Welsh MJ. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron. 2000;26:133–141. doi: 10.1016/s0896-6273(00)81144-7. [DOI] [PubMed] [Google Scholar]
- Auzanneau C, Thoreau V, Kitzis A, Becq F. A novel voltage-dependent chloride current activated by extracellular acidic pH in cultured rat Sertoli cells. J Biol Chem. 2003;278:19230–19236. doi: 10.1074/jbc.M301096200. [DOI] [PubMed] [Google Scholar]
- Auzanneau C, Norez C, Antigny F, Thoreau V, Jougla C, Cantereau A, Becq F, Vandebrouck C. Transient receptor potential vanilloid 1 (TRPV1) channels in cultured rat Sertoli cells regulate an acid sensing chloride channel. Biochem Pharmacol. 2008;75:476–483. doi: 10.1016/j.bcp.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Avelino A, Cruz F. TRPV1 (vanilloid receptor) in the urinary tract: expression, function and clinical applications. Naunyn Schmiedeberg’s Arch Pharmacol. 2006;373:287–299. doi: 10.1007/s00210-006-0073-2. [DOI] [PubMed] [Google Scholar]
- Banerjee B, Medda BK, Lazarova Z, Bansal N, Shaker R, Sengupta JN. Effect of reflux-induced inflammation on transient receptor potential vanilloid one (TRPV1) expression in primary sensory neurons innervating the oesophagus of rats. Neurogastroenterol Motil. 2007;19:681–691. doi: 10.1111/j.1365-2982.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- Baumann TK, Chaudhary P, Martenson ME. Background potassium channel block and TRPV1 activation contribute to proton depolarization of sensory neurons from humans with neuropathic pain. Eur J Neurosci. 2004;19:1343–1351. doi: 10.1111/j.1460-9568.2004.03097.x. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive ‘leak’ K+ channel expressed in brainstem respiratory neurons. Respir Physiol. 2001;129:159–174. doi: 10.1016/s0034-5687(01)00288-2. [DOI] [PubMed] [Google Scholar]
- Bearzatto B, Lesage F, Reyes R, Lazdunski M, Laduron PM. Axonal transport of TREK and TRAAK potassium channels in rat sciatic nerves. NeuroReport. 2000;11:927–930. doi: 10.1097/00001756-200004070-00006. [DOI] [PubMed] [Google Scholar]
- Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: a possible mediator of myocardial ischemic sensation. Circ Res. 1999;84:921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol. 1997;273:G422–G435. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994;17:509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Bevan S, Yeats J. Protons activate a cation conductance in a sub-population of rat dorsal root ganglion neurones. J Physiol. 1991;433:145–161. doi: 10.1113/jphysiol.1991.sp018419. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat YM, Bielefeldt K. Capsaicin receptor (TRPV1) and non-erosive reflux disease. Eur J Gastroenterol Hepatol. 2006;18:263–270. doi: 10.1097/00042737-200603000-00006. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Scott BP, Nasser N, Ao H, Maher MP, Dubin AE, Swanson DM, Shankley NP, Wickenden AD, Chaplan SR. Pharmacology and antitussive efficacy of 4-(3-trifluoromethyl-pyridin-2-yl)-piperazine-1-carboxylic acid (5-trifluoromethyl-pyridin-2-yl)-amide (JNJ17203212), a transient receptor potential vanilloid 1 antagonist in guinea pigs. J Pharmacol Exp Ther. 2007;323:665–674. doi: 10.1124/jpet.107.127258. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Davis BM. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am J Physiol. 2008;294:G130–G138. doi: 10.1152/ajpgi.00388.2007. [DOI] [PubMed] [Google Scholar]
- Bina RW, Hempleman SC. Evidence for TREK-like tandem-pore domain channels in intrapulmonary chemoreceptor chemotransduction. Respir Physiol Neurobiol. 2007;156:120–131. doi: 10.1016/j.resp.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Bley KR. Recent developments in transient receptor potential vanilloid receptor 1 agonist-based therapies. Expert Opin Investig Drugs. 2004;13:1445–1456. doi: 10.1517/13543784.13.11.1445. [DOI] [PubMed] [Google Scholar]
- Brady CM, Apostolidis AN, Harper M, Yiangou Y, Beckett A, Jacques TS, Freeman A, Scaravilli F, Fowler CJ, Anand P. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004;93:770–776. doi: 10.1111/j.1464-410X.2003.04722.x. [DOI] [PubMed] [Google Scholar]
- Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain. 2005;115:37–49. doi: 10.1016/j.pain.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Brown SG, Townsend-Nicholson A, Jacobson KA, Burnstock G, King BF. Heteromultimeric P2X1/2 receptors show a novel sensitivity to extracellular pH. J Pharmacol Exp Ther. 2002;300:673–680. doi: 10.1124/jpet.300.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ. TASK-like potassium channels and oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2007;157:55–64. doi: 10.1016/j.resp.2007.02.013. 2007. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Cadiou H, Studer M, Jones NG, Smith ES, Ballard A, McMahon SB, McNaughton PA. Modulation of acid-sensing ion channel activity by nitric oxide. J Neurosci. 2007;27:13251–13260. doi: 10.1523/JNEUROSCI.2135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanucci VA, Fearon IM, Nurse CA. A novel O2-sensing mechanism in rat glossopharyngeal neurones mediated by a halothane-inhibitable background K+ conductance. J Physiol. 2003;548:731–743. doi: 10.1113/jphysiol.2002.035998. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Farmer DG, Mori N. Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am J Physiol. 2006;291:R454–R463. doi: 10.1152/ajpregu.00862.2005. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Coggeshall RE. Peripheral capsaicin receptors increase in the inflamed rat hindpaw: a possible mechanism for peripheral sensitization. Neurosci Lett. 2001;310:53–56. doi: 10.1016/s0304-3940(01)02093-6. [DOI] [PubMed] [Google Scholar]
- Catarsi S, Babinski K, Séguéla P. Selective modulation of heteromeric ASIC proton-gated channels by neuropeptide FF. Neuropharmacology. 2001;41:592–600. doi: 10.1016/s0028-3908(01)00107-1. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chan CL, Facer P, Davis JB, Smith GD, Egerton J, Bountra C, Williams NS, Anand P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet. 2003;361:385–391. doi: 10.1016/s0140-6736(03)12392-6. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Chapman CG, Meadows HJ, Godden RJ, Campbell DA, Duckworth M, Kelsell RE, Murdock PR, Randall AD, Rennie GI, Gloger IS. Cloning, localisation and functional expression of a novel human, cerebellum specific, two pore domain potassium channel. Mol Brain Res. 2000;82:74–83. doi: 10.1016/s0169-328x(00)00183-2. [DOI] [PubMed] [Google Scholar]
- Chavez RA, Gray AT, Zhao BB, Kindler CH, Mazurek MJ, Mehta Y, Forsayeth JR, Yost CS. TWIK-2, a new weak inward rectifying member of the tandem pore domain potassium channel family. J Biol Chem. 1999;274:7887–7892. doi: 10.1074/jbc.274.12.7887. [DOI] [PubMed] [Google Scholar]
- Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci USA. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci USA. 2002;99:8992–8997. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizh BA, Illes P. P2X receptors and nociception. Pharmacol Rev. 2001;53:553–568. [PubMed] [Google Scholar]
- Chuang YC, Chancellor MB, Seki S, Yoshimura N, Tyagi P, Huang L, Lavelle JP, De Groat WC, Fraser MO. Intravesical protamine sulfate and potassium chloride as a model for bladder hyperactivity. Urology. 2003;61:664–670. doi: 10.1016/s0090-4295(02)02280-x. [DOI] [PubMed] [Google Scholar]
- Chuang YC, Yoshimura N, Huang CC, Chiang PH, Chancellor MB. Intravesical botulinum toxin a administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol. 2004;172:1529–1532. doi: 10.1097/01.ju.0000137844.77524.97. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- Clarke GD, Davison JS. Mucosal receptors in the gastric antrum and small intestine of the rat with afferent fibres in the cervical vagus. J Physiol. 1978;284:55–67. doi: 10.1113/jphysiol.1978.sp012527. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CE, Benham CD, Bridges A, George AR, Meadows HJ. Mutation of histidine 286 of the human P2X4 purinoceptor removes extracellular pH sensitivity. J Physiol. 2000;523:697–703. doi: 10.1111/j.1469-7793.2000.00697.x. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claydon TW, Boyett MR, Sivaprasadarao A, Orchard CH. Two pore residues mediate acidosis-induced enhancement of C-type inactivation of the Kv1.4 K+ channel. Am J Physiol. 2000;283:C1114–C1121. doi: 10.1152/ajpcell.00542.2001. [DOI] [PubMed] [Google Scholar]
- Clyne JD, LaPointe LD, Hume RI. The role of histidine residues in modulation of the rat P2X2 purinoceptor by zinc and pH. J Physiol. 2002;539:347–359. doi: 10.1113/jphysiol.2001.013244. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford AP. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–639. doi: 10.1113/jphysiol.2005.088435. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin B, Chollet R, Flourie B, Lemann M, Franchisseur C, Rambaud JC, Jian R. Intraluminal modulation of gastric sensitivity to distension: effects of hydrochloric acid and meal. Am J Physiol. 2001;280:G904–G909. doi: 10.1152/ajpgi.2001.280.5.G904. [DOI] [PubMed] [Google Scholar]
- Cooper BY, Johnson RD, Rau KK. Characterization and function of TWIK-related acid sensing K+ channels in a rat nociceptive cell. Neuroscience. 2004;129:209–224. doi: 10.1016/j.neuroscience.2004.06.066. [DOI] [PubMed] [Google Scholar]
- Cruz F, Dinis P. Resiniferatoxin and botulinum toxin type A for treatment of lower urinary tract symptoms. Neurourol Urodyn. 2007;26(6 Suppl):920–927. doi: 10.1002/nau.20479. [DOI] [PubMed] [Google Scholar]
- Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2007;583:663–674. doi: 10.1113/jphysiol.2007.139147. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci. 2005;25:3973–3984. doi: 10.1523/JNEUROSCI.5239-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer M, Jocic M, Samberger C, Painsipp E, Bock E, Pabst MA, Crailsheim K, Schicho R, Lippe IT, Holzer P. Stomach-brain communication by vagal afferents in response to luminal acid backdiffusion, gastrin, and gastric acid secretion. Am J Physiol. 2004;286:G403–G411. doi: 10.1152/ajpgi.00308.2003. [DOI] [PubMed] [Google Scholar]
- Davies NW, Lux HD, Morad M. Site and mechanism of activation of proton-induced sodium current in chick dorsal root ganglion neurones. J Physiol. 1988;400:159–187. doi: 10.1113/jphysiol.1988.sp017116. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Delmas P. Polycystins: polymodal receptor/ion-channel cellular sensors. Pflügers Arch. 2005;451:264–276. doi: 10.1007/s00424-005-1431-5. [DOI] [PubMed] [Google Scholar]
- Dean JB, Ballantyne D, Cardone DL, Erlichman JS, Solomon IC. Role of gap junctions in CO2 chemoreception and respiratory control. Am J Physiol. 2002;283:L665–L670. doi: 10.1152/ajplung.00142.2002. [DOI] [PubMed] [Google Scholar]
- Decher N, Maier M, Dittrich W, Gassenhuber J, Bruggemann A, Busch AE, Steinmeyer K. Characterization of TASK-4, a novel member of the pH-sensitive, two-pore domain potassium channel family. FEBS Lett. 2001;492:84–89. doi: 10.1016/s0014-5793(01)02222-0. [DOI] [PubMed] [Google Scholar]
- Deval E, Baron A, Lingueglia E, Mazarguil H, Zajac JM, Lazdunski M. Effects of neuropeptide SF and related peptides on acid sensing ion channel 3 and sensory neuron excitability. Neuropharmacology. 2003;44:662–671. doi: 10.1016/s0028-3908(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Ding S, Sachs F. Single channel properties of P2X2 purinoceptors. J Gen Physiol. 1999;113:695–720. doi: 10.1085/jgp.113.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, Salinas M, Lazdunski M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004;23:1516–1525. doi: 10.1038/sj.emboj.7600177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Salinas M, Baron A, Escoubas P, Lazdunski M. Peptides inhibitors of acid-sensing ion channels. Toxicon. 2007;49:271–284. doi: 10.1016/j.toxicon.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Dobler T, Springauf A, Tovornik S, Weber M, Schmitt A, Sedlmeier R, Wischmeyer E, Döring F. TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine dorsal root ganglion neurones. J Physiol. 2007;585:867–879. doi: 10.1113/jphysiol.2007.145649. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Piper AS. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br J Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes AM, Schipper KP, Dimcevski G, Petersen P, Gregersen H, Funch-Jensen P, Arendt-Nielsen L. Gut pain and hyperalgesia induced by capsaicin: a human experimental model. Pain. 2003;104:333–341. doi: 10.1016/s0304-3959(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Duan B, Wu LJ, Yu YQ, Ding Y, Jing L, Xu L, Chen J, Xu TL. Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J Neurosci. 2007;27:11139–11148. doi: 10.1523/JNEUROSCI.3364-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honoré P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain. 2005;117:88–96. doi: 10.1016/j.pain.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Girard C, Jarretou G, Lazdunski M. Pancreatic two P domain K+ channels TALK-1 and TALK-2 are activated by nitric oxide and reactive oxygen species. J Physiol. 2005;562:235–244. doi: 10.1113/jphysiol.2004.071266. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Lauritzen I, Patel A, Honoré E. The TASK background K2P channels: chemo- and nutrient sensors. Trends Neurosci. 2007;30:573–580. doi: 10.1016/j.tins.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem. 2000;275:25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- Faisy C, Planquette B, Naline E, Risse PA, Frossard N, Fagon JY, Advenier C, Devillier P. Acid-induced modulation of airway basal tone and contractility: role of acid-sensing ion channels (ASICs) and TRPV1 receptor. Life Sci. 2007;81:1094–1102. doi: 10.1016/j.lfs.2007.08.026. [DOI] [PubMed] [Google Scholar]
- Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan Med Bull. 1999;46:183–196. [PubMed] [Google Scholar]
- Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am J Physiol. 2003;284:C145–C155. doi: 10.1152/ajpcell.00346.2002. [DOI] [PubMed] [Google Scholar]
- Fischer MJ, Reeh PW, Sauer SK. Proton-induced calcitonin gene-related peptide release from rat sciatic nerve axons, in vitro, involving TRPV1. Eur J Neurosci. 2003;18:803–810. doi: 10.1046/j.1460-9568.2003.02811.x. [DOI] [PubMed] [Google Scholar]
- Forster ER, Green T, Elliot M, Bremner A, Dockray GJ. Gastric emptying in rats: role of afferent neurons and cholecystokinin. Am J Physiol. 1990;258:G552–G556. doi: 10.1152/ajpgi.1990.258.4.G552. [DOI] [PubMed] [Google Scholar]
- Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- Fujino K, de la Fuente SG, Takami Y, Takahashi T, Mantyh CR. Attenuation of acid induced oesophagitis in VR-1 deficient mice. Gut. 2006;55:34–40. doi: 10.1136/gut.2005.066795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Ichikawa H, Terayama R, Yamaai T, Kuboki T, Sugimoto T. ASIC3-immunoreactive neurons in the rat vagal and glossopharyngeal sensory ganglia. Brain Res. 2006;1081:150–155. doi: 10.1016/j.brainres.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Gabriel A, Abdallah M, Yost CS, Winegar BD, Kindler CH. Localization of the tandem pore domain K+ channel KCNK5 (TASK-2) in the rat central nervous system. Mol Brain Res. 2002;98:153–163. doi: 10.1016/s0169-328x(01)00330-8. [DOI] [PubMed] [Google Scholar]
- Gao Y, Liu SS, Qiu S, Cheng W, Zheng J, Luo JH. Fluorescence resonance energy transfer analysis of subunit assembly of the ASIC channel. Biochem Biophys Res Commun. 2007;359:143–150. doi: 10.1016/j.bbrc.2007.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Henig O, Kehoe V, Sinoway LI, Li J. Vanilloid type 1 receptor and the acid-sensing ion channel mediate acid phosphate activation of muscle afferent nerves in rats. J Appl Physiol. 2006;100:421–426. doi: 10.1152/japplphysiol.00659.2005. [DOI] [PubMed] [Google Scholar]
- Gao Z, Li JD, Sinoway LI, Li J. Effect of muscle interstitial pH on P2X and TRPV1 receptor-mediated pressor response. J Appl Physiol. 2007;102:2288–2293. doi: 10.1152/japplphysiol.00161.2007. [DOI] [PubMed] [Google Scholar]
- García-Martínez C, Fernández-Carvajal A, Valenzuela B, Gomis A, Van Den Nest W, Ferroni S, Carreño C, Belmonte C, Ferrer-Montiel A. Design and characterization of a noncompetitive antagonist of the transient receptor potential vanilloid subunit 1 channel with in vivo analgesic and anti-inflammatory activity. J Pain. 2006;7:735–746. doi: 10.1016/j.jpain.2006.03.008. [DOI] [PubMed] [Google Scholar]
- García-Sanz N, Fernández-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sánchez E, Fernández-Ballester G, Ferrer-Montiel A. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J Neurosci. 2004;24:5307–5314. doi: 10.1523/JNEUROSCI.0202-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, Zhang TJ, Viswanadhan VN, Toth A, Pearce LV, Vanderah TW, Porreca F, Blumberg PM, Lile J, Sun Y, Wild K, Louis JC, Treanor JJ. Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Tamir R, Klionsky L, Norman MH, Louis JC, Wild KD, Treanor JJ. Proton activation does not alter antagonist interaction with the capsaicin-binding pocket of TRPV1. Mol Pharmacol. 2005a;68:1524–1533. doi: 10.1124/mol.105.015727. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, Doherty EM, Norman MH, Wild KD, Bannon AW, Louis JC, Treanor JJ. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther. 2005b;313:474–484. doi: 10.1124/jpet.104.079855. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, Kuang R, Le A, Tamir R, Wang J, Youngblood B, Zhu D, Norman MH, Magal E, Treanor JJ, Louis JC. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, Jang GR, Kesslak JP, Ni L, Norman MH, Palluconi G, Rose MJ, Salfi M, Tan E, Romanovsky AA, Banfield C, Davar G. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Gerevich Z, Zadori ZS, Köles L, Kopp L, Milius D, Wirkner K, Gyires K, Illes P. Dual effect of acid pH on purinergic P2X3 receptors depends on the histidine 206 residue. J Biol Chem. 2007;282:33949–33957. doi: 10.1074/jbc.M705840200. [DOI] [PubMed] [Google Scholar]
- Gharat LA, Szallasi A. Advances in the design and therapeutic use of capsaicin receptor TRPV1 agonists and antagonists. Expert Opin Ther Patents. 2008;18:159–209. [Google Scholar]
- Ghilardi JR, Röhrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, Carruthers NI, Swanson D, Kuskowski M, Flores CM, Julius D, Mantyh PW. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25:3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppetti P, Tramontana M, Evangelista S, Renzi D, Maggi CA, Fusco BM, Del Bianco E. Differential effect on neuropeptide release of different concentrations of hydrogen ions on afferent and intrinsic neurons of the rat stomach. Gastroenterology. 1991;101:1505–1511. doi: 10.1016/0016-5085(91)90385-x. [DOI] [PubMed] [Google Scholar]
- Girard C, Duprat F, Terrenoire C, Tinel N, Fosset M, Romey G, Lazdunski M, Lesage F. Genomic and functional characteristics of novel human pancreatic 2P domain K+ channels. Biochem Biophys Res Commun. 2001;282:249–256. doi: 10.1006/bbrc.2001.4562. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O’Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Goldstein SA, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol Rev. 2005;57:527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- Goodis HE, Poon A, Hargreaves KM. Tissue pH and temperature regulate pulpal nociceptors. J Dent Res. 2006;85:1046–1049. doi: 10.1177/154405910608501114. [DOI] [PubMed] [Google Scholar]
- Groth M, Helbig T, Grau V, Kummer W, Haberberger RV. Spinal afferent neurons projecting to the rat lung and pleura express acid sensitive channels. Respir Res. 2006;7:96. doi: 10.1186/1465-9921-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Lee LY. Characterization of acid signaling in rat vagal pulmonary sensory neurons. Am J Physiol. 2006;291:L58–L65. doi: 10.1152/ajplung.00517.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Schlichthörl G, Hirsch JR, Engels H, Karschin C, Karschin A, Derst C, Steinlein OK, Daut J. Expression pattern and functional characteristics of two novel splice variants of the two-pore-domain potassium channel TREK-2. J Physiol. 2002;539:657–668. doi: 10.1113/jphysiol.2001.013432. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Rami HK, Jerman JC, Smart D, Gill CH, Soffin EM, Luis Hannan S, Lappin SC, Egerton J, Smith GD, Worby A, Howett L, Owen D, Nasir S, Davies CH, Thompson M, Wyman PA, Randall AD, Davis JB. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology. 2004;46:133–149. doi: 10.1016/s0028-3908(03)00305-8. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hamilton SG, McMahon SB, Lewin GR. Selective activation of nociceptors by P2X receptor agonists in normal and inflamed rat skin. J Physiol. 2001;534:437–445. doi: 10.1111/j.1469-7793.2001.00437.x. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Kang D, Kim D. Functional properties of four splice variants of a human pancreatic tandem-pore K+ channel, TALK-1. Am J Physiol. 2003;285:C529–C538. doi: 10.1152/ajpcell.00601.2002. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol. 2007;581:1271–1282. doi: 10.1113/jphysiol.2007.129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig N, Plant TD, Janson W, Schäfer M, Schultz G, Schaefer M. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J Biol Chem. 2004;279:34553–34561. doi: 10.1074/jbc.M402966200. [DOI] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Gray CW, Green PJ, Ranson JL, Randall AD, Meadows HJ. Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience. 2001;103:899–919. doi: 10.1016/s0306-4522(01)00030-6. [DOI] [PubMed] [Google Scholar]
- Hicks GA. TRP channels as therapeutic targets: hot property, or time to cool down? Neurogastroenterol Motil. 2006;18:590–594. doi: 10.1111/j.1365-2982.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Reinöhl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain. 2004;110:149–157. doi: 10.1016/j.pain.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823–839. doi: 10.1016/s0016-5085(98)70597-9. [DOI] [PubMed] [Google Scholar]
- Holzer P. Acid-sensitive ion channels in gastrointestinal function. Curr Opin Pharmacol. 2003;3:618–625. doi: 10.1016/j.coph.2003.06.008. [DOI] [PubMed] [Google Scholar]
- Holzer P. TRPV1 and the gut: from a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur J Pharmacol. 2004a;500:231–241. doi: 10.1016/j.ejphar.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Holzer P. Vanilloid receptor TRPV1: hot on the tongue and inflaming the colon. Neurogastroenterol Motil. 2004b;16:697–699. doi: 10.1111/j.1365-2982.2004.00598.x. [DOI] [PubMed] [Google Scholar]
- Holzer P. Taste receptors in the gastrointestinal tract. V. Acid sensing in the gastrointestinal tract. Am J Physiol. 2007;292:G699–G705. doi: 10.1152/ajpgi.00517.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Maggi CA. Dissociation of dorsal root ganglion neurons into afferent and efferent-like neurons. Neuroscience. 1998;86:389–398. doi: 10.1016/s0306-4522(98)00047-5. [DOI] [PubMed] [Google Scholar]
- Holzer P, Painsipp E, Jocic M, Heinemann A. Acid challenge delays gastric pressure adaptation, blocks gastric emptying and stimulates gastric fluid secretion in the rat. Neurogastroenterol Motil. 2003;15:45–55. doi: 10.1046/j.1365-2982.2003.00382.x. [DOI] [PubMed] [Google Scholar]
- Holzer P, Wultsch T, Edelsbrunner M, Mitrovic M, Shahbazian A, Painsipp E, Bock E, Pabst MA. Increase in gastric acid-induced afferent input to the brainstem in mice with gastritis. Neuroscience. 2007;145:1108–1119. doi: 10.1016/j.neuroscience.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Luger NM, Sabino MA, Schwei MJ, Rogers SD, Mach DB, O’Keefe PF, Ramnaraine ML, Clohisy DR, Mantyh PW. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med. 2000;6:521–528. doi: 10.1038/74999. [DOI] [PubMed] [Google Scholar]
- Honore P, Mikusa J, Bianchi B, McDonald H, Cartmell J, Faltynek C, Jarvis MF. TNP-ATP, a potent P2X3 receptor antagonist, blocks acetic acid-induced abdominal constriction in mice: comparison with reference analgesics. Pain. 2002;96:99–105. doi: 10.1016/s0304-3959(01)00434-1. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CW, Tzeng JN, Chen YJ, Tsai WF, Chen CC, Sun WH. Nociceptors of dorsal root ganglion express proton-sensing G-protein-coupled receptors. Mol Cell Neurosci. 2007;36:195–210. doi: 10.1016/j.mcn.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Hughes PA, Brierley SM, Young RL, Blackshaw LA. Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J Comp Neurol. 2007;500:863–875. doi: 10.1002/cne.21204. [DOI] [PubMed] [Google Scholar]
- Hunt J. Exhaled breath condensate pH: reflecting acidification of the airway at all levels. Am J Respir Crit Care Med. 2006;173:366–367. doi: 10.1164/rccm.2512001. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Ueno A, Naraba H, Oh-ishi S. Involvement of vanilloid receptor VR1 and prostanoids in the acid-induced writhing responses of mice. Life Sci. 2001;69:2911–2919. doi: 10.1016/s0024-3205(01)01374-1. [DOI] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 2003;37:75–84. doi: 10.1016/s0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci USA. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr H, van Driel M, van Osch GJ, Weinans H, van Leeuwen JP. Identification of acid-sensing ion channels in bone. Biochem Biophys Res Commun. 2005;337:349–354. doi: 10.1016/j.bbrc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- Jancsó N. Role of the nerve terminals in the mechanism of inflammatory reactions. Bull Millard Fillmore Hosp. 1960;7:53–77. [Google Scholar]
- Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci USA. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Jia Y, Lee LY. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta. 2007;1772:915–927. doi: 10.1016/j.bbadis.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Jiang C, Rojas A, Wang R, Wang X. CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol. 2005;145:115–126. doi: 10.1016/j.resp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Jiang M, Dun W, Tseng GN. Mechanism for the effects of extracellular acidification on HERG-channel function. Am J Physiol. 1999;277:H1283–H1292. doi: 10.1152/ajpheart.1999.277.4.H1283. [DOI] [PubMed] [Google Scholar]
- Jiang N, Rau KK, Johnson RD, Cooper BY. Proton sensitivity Ca2+ permeability and molecular basis of acid-sensing ion channels expressed in glabrous and hairy skin afferents. J Neurophysiol. 2006;95:2466–2478. doi: 10.1152/jn.00861.2005. [DOI] [PubMed] [Google Scholar]
- Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB. Acid-induced pain and its modulation in humans. J Neurosci. 2004;24:10974–10979. doi: 10.1523/JNEUROSCI.2619-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci USA. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa S, Aoi M, Kubo Y, Kotani T, Takeuchi K. Stimulation by capsaicin of duodenal HCO3− secretion via afferent neurons and vanilloid receptors in rats: comparison with acid-induced HCO3− response. Dig Dis Sci. 2003;48:1850–1856. doi: 10.1023/a:1025480003388. [DOI] [PubMed] [Google Scholar]
- Kang D, Kim D. Single-channel properties and pH sensitivity of two-pore domain K+ channels of the TALK family. Biochem Biophys Res Commun. 2004;315:836–844. doi: 10.1016/j.bbrc.2004.01.137. [DOI] [PubMed] [Google Scholar]
- Kang D, Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol. 2006;291:C138–C146. doi: 10.1152/ajpcell.00629.2005. [DOI] [PubMed] [Google Scholar]
- Kang JY, Yap I. Acid and gastric ulcer pain. J Clin Gastroenterol. 1991;13:514–516. doi: 10.1097/00004836-199110000-00007. [DOI] [PubMed] [Google Scholar]
- Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sévigny J, Kinnamon JC, Finger TE. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses. 2008;33:243–254. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K+ channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Kim Y, Gnatenco C, Bang H, Kim D. Localization of TREK-2 K+ channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pHi. Pflügers Arch. 2001a;442:952–960. doi: 10.1007/s004240100626. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Gnatenco C, Kim D. Synergistic interaction and the role of C-terminus in the activation of TRAAK K+ channels by pressure, free fatty acids and alkali. Pflügers Arch. 2001b;442:64–72. doi: 10.1007/s004240000496. [DOI] [PubMed] [Google Scholar]
- King BF, Townsend-Nicholson A, Wildman SS, Thomas T, Spyer KM, Burnstock G. Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. J Neurosci. 2000;20:4871–4877. doi: 10.1523/JNEUROSCI.20-13-04871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol. 2002;543:591–600. doi: 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik M, Fei Ru F, Undem BJ. Acid-sensitive vagal sensory pathways and cough. Pulm Pharmacol Ther. 2007;20:402–411. doi: 10.1016/j.pupt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A, Lux HD, Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol. 1987;386:603–633. doi: 10.1113/jphysiol.1987.sp016553. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress M, Waldmann R. Acid sensing ionic channels. Curr Top Membranes. 2006;57:241–276. [Google Scholar]
- Krishek BJ, Smart TG. Proton sensitivity of rat cerebellar granule cell GABAA receptors: dependence on neuronal development. J Physiol. 2001;530:219–233. doi: 10.1111/j.1469-7793.2001.0219l.x. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- Krishtal OA, Pidoplichko VI. A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience. 1981;6:2599–2601. doi: 10.1016/0306-4522(81)90105-6. [DOI] [PubMed] [Google Scholar]
- Lamb K, Kang YM, Gebhart GF, Bielefeldt K. Gastric inflammation triggers hypersensitivity to acid in awake rats. Gastroenterology. 2003;125:1410–1418. doi: 10.1016/j.gastro.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Leffler A, Mönter B, Koltzenburg M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience. 2006;139:699–709. doi: 10.1016/j.neuroscience.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Lehto SG, Tamir R, Deng H, Klionsky L, Kuang R, Le A, Lee D, Louis JC, Magal E, Manning BH, Rubino J, Surapaneni S, Tamayo N, Wang T, Wang J, Wang J, Wang W, Youngblood B, Zhang M, Zhu D, Norman MH, Gavva NR. Antihyperalgesic effects of AMG8562, a novel vanilloid receptor TRPV1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther. 2008 doi: 10.1124/jpet.107.132233. in press. [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J Biol Chem. 2000;275:28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- Leung SY, Niimi A, Williams AS, Nath P, Blanc FX, Dinh QT, Chung KF. Inhibition of citric acid- and capsaicin-induced cough by novel TRPV-1 antagonist, V112220, in guinea-pig. Cough. 2007;3:10. doi: 10.1186/1745-9974-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Proton potentiation of ATP-gated ion channel responses to ATP and Zn2+ in rat nodose ganglion neurons. J Neurophysiol. 1996;76:3048–3058. doi: 10.1152/jn.1996.76.5.3048. [DOI] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Enhancement of ATP-activated current by protons in dorsal root ganglion neurons. Pflügers Arch. 1997;433:446–454. doi: 10.1007/s004240050299. [DOI] [PubMed] [Google Scholar]
- Li J, Maile MD, Sinoway AN, Sinoway LI. Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol. 2004;97:1709–1714. doi: 10.1152/japplphysiol.00389.2004. [DOI] [PubMed] [Google Scholar]
- Lin W, Burks CA, Hansen DR, Kinnamon SC, Gilbertson TA. Taste receptor cells express pH-sensitive leak K+ channels. J Neurophysiol. 2004;92:2909–2919. doi: 10.1152/jn.01198.2003. [DOI] [PubMed] [Google Scholar]
- Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007;282:17325–17329. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- Liu L, Simon SA. Capsazepine, a vanilloid receptor antagonist, inhibits nicotinic acetylcholine receptors in rat trigeminal ganglia. Neurosci Lett. 1997;228:29–32. doi: 10.1016/s0304-3940(97)00358-3. [DOI] [PubMed] [Google Scholar]
- Liu L, Hansen DR, Kim I, Gilbertson TA. Expression and characterization of delayed rectifying K+ channels in anterior rat taste buds. Am J Physiol. 2005;289:C868–C880. doi: 10.1152/ajpcell.00115.2005. [DOI] [PubMed] [Google Scholar]
- Liu L, Mansfield KJ, Kristiana I, Vaux KJ, Millard RJ, Burcher E. The molecular basis of urgency: regional difference of vanilloid receptor expression in the human urinary bladder. Neurourol Urodyn. 2007;26:433–438. doi: 10.1002/nau.20326. [DOI] [PubMed] [Google Scholar]
- Liu M, King BF, Dunn PM, Rong W, Townsend-Nicholson A, Burnstock G. Coexpression of P2X3 and P2X2 receptor subunits in varying amounts generates heterogeneous populations of P2X receptors that evoke a spectrum of agonist responses comparable to that seen in sensory neurons. J Pharmacol Exp Ther. 2001;296:1043–1050. [PubMed] [Google Scholar]
- Liu M, Willmott NJ, Michael GJ, Priestley JV. Differential pH and capsaicin responses of Griffonia simplicifolia IB4 (IB4)-positive and IB4-negative small sensory neurons. Neuroscience. 2004;127:659–672. doi: 10.1016/j.neuroscience.2004.05.041. [DOI] [PubMed] [Google Scholar]
- LopezJimenez ND, Cavenagh MM, Sainz E, Cruz-Ithier MA, Battey JF, Sullivan SL. Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J Neurochem. 2006;98:68–77. doi: 10.1111/j.1471-4159.2006.03842.x. [DOI] [PubMed] [Google Scholar]
- López-López JR, Pérez-García MT. An ASIC channel for acid chemotransduction. Circ Res. 2007;101:965–967. doi: 10.1161/CIRCRESAHA.107.164442. [DOI] [PubMed] [Google Scholar]
- Lu YX, Owyang C. Duodenal acid-induced gastric relaxation is mediated by multiple pathways. Am J Physiol. 1999;276:G1501–G1506. doi: 10.1152/ajpgi.1999.276.6.G1501. [DOI] [PubMed] [Google Scholar]
- Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- Luger NM, Honore P, Sabino MA, Schwei MJ, Rogers SD, Mach DB, Clohisy DR, Mantyh PW. Osteoprotegerin diminishes advanced bone cancer pain. Cancer Res. 2001;61:4038–4047. [PubMed] [Google Scholar]
- Lyall V, Alam RI, Malik SA, Phan TH, Vinnikova AK, Heck GL, DeSimone JA. Basolateral Na+-H+ exchanger-1 in rat taste receptor cells is involved in neural adaptation to acidic stimuli. J Physiol. 2004;556:159–173. doi: 10.1113/jphysiol.2003.057745. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honoré E. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honoré E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22:10662–10670. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manela FD, Ren J, Gao J, McGuigan JE, Harty RF. Calcitonin gene-related peptide modulates acid-mediated regulation of somatostatin and gastrin release from rat antrum. Gastroenterology. 1995;109:701–706. doi: 10.1016/0016-5085(95)90376-3. [DOI] [PubMed] [Google Scholar]
- Mao J, Li L, McManus M, Wu J, Cui N, Jiang C. Molecular determinants for activation of G-protein-coupled inward rectifier K+ (GIRK) channels by extracellular acidosis. J Biol Chem. 2002;277:46166–46171. doi: 10.1074/jbc.M205438200. [DOI] [PubMed] [Google Scholar]
- Mathie A. Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol. 2007;578:377–385. doi: 10.1113/jphysiol.2006.121582. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PJ, Aziz Q, Facer P, Davis JB, Thompson DG, Anand P. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepatol. 2004;16:897–902. doi: 10.1097/00042737-200409000-00014. [DOI] [PubMed] [Google Scholar]
- Mazzuca M, Heurteaux C, Alloui A, Diochot S, Baron A, Voilley N, Blondeau N, Escoubas P, Gélot A, Cupo A, Zimmer A, Zimmer AM, Eschalier A, Lazdunski M. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat Neurosci. 2007;10:943–945. doi: 10.1038/nn1940. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Bevan S. The effects of pH on the interaction between capsaicin and the vanilloid receptor in rat dorsal root ganglia neurons. Br J Pharmacol. 2001;132:899–908. doi: 10.1038/sj.bjp.0703900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows HJ, Randall AD. Functional characterisation of human TASK-3, an acid-sensitive two-pore domain potassium channel. Neuropharmacology. 2001;40:551–559. doi: 10.1016/s0028-3908(00)00189-1. [DOI] [PubMed] [Google Scholar]
- Medda BK, Sengupta JN, Lang IM, Shaker R. Response properties of the brainstem neurons of the cat following intra-esophageal acid-pepsin infusion. Neuroscience. 2005;135:1285–1294. doi: 10.1016/j.neuroscience.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, Gloger II, Pangalos MN. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Mol Brain Res. 2001;86:101–114. doi: 10.1016/s0169-328x(00)00263-1. [DOI] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P, Peers C, Kemp PJ. Polymodal regulation of hTREK1 by pH, arachidonic acid, and hypoxia: physiological impact in acidosis and alkalosis. Am J Physiol. 2004;286:C272–C282. doi: 10.1152/ajpcell.00334.2003. [DOI] [PubMed] [Google Scholar]
- Miranda A, Nordstrom E, Mannem A, Smith C, Banerjee B, Sengupta JN. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience. 2007;148:1021–1032. doi: 10.1016/j.neuroscience.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistrík P, Torre V. Histidine 518 in the S6-CNBD linker controls pH dependence and gating of HCN channel from sea-urchin sperm. Pflügers Arch. 2004;448:76–84. doi: 10.1007/s00424-003-1228-3. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Matsumoto N, Imai M, Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol. 2003;285:C96–C101. doi: 10.1152/ajpcell.00559.2002. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Breese NM, Witty MF, Ritchie J, Rainville ML, Ase A, Abbadi N, Stucky CL, Seguela P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci. 2005;25:9893–9901. doi: 10.1523/JNEUROSCI.2019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrose MH, Akiba Y, Takeuchi K, Kaunitz JD. Gastroduodenal mucosal defense. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Fourth Edition Academic Press; San Diego: 2006. pp. 1259–1291. [Google Scholar]
- Morenilla-Palao C, Planells-Cases R, García-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279:25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- Morton MJ, O’Connell AD, Sivaprasadarao A, Hunter M. Determinants of pH sensing in the two-pore domain K+ channels TASK-1 and -2. Pflügers Arch. 2003;445:577–583. doi: 10.1007/s00424-002-0901-2. [DOI] [PubMed] [Google Scholar]
- Morton MJ, Abohamed A, Sivaprasadarao A, Hunter M. pH sensing in the two-pore domain K+ channel, TASK2. Proc Natl Acad Sci USA. 2005;102:16102–16106. doi: 10.1073/pnas.0506870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae M, Hiraga T, Wakabayashi H, Wang L, Iwata K, Yoneda T. Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone. 2006;39:1107–1115. doi: 10.1016/j.bone.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Nagae M, Hiraga T, Yoneda T. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab. 2007;25:99–104. doi: 10.1007/s00774-006-0734-8. [DOI] [PubMed] [Google Scholar]
- Neelands TR, Jarvis MF, Han P, Faltynek CR, Surowy CS. Acidification of rat TRPV1 alters the kinetics of capsaicin responses. Mol Pain. 2005;1:28. doi: 10.1186/1744-8069-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell K, Franchi A, Pouysségur J, Tannock I. Studies with glycolysis-deficient cells suggest that production of lactic acid is not the only cause of tumor acidity. Proc Natl Acad Sci USA. 1993;90:1127–1131. doi: 10.1073/pnas.90.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer MI, González-Nilo FD, Zúñiga L, González W, Cid LP, Sepúlveda FV. Neutralization of a single arginine residue gates open a two-pore domain, alkali-activated K+ channel. Proc Natl Acad Sci USA. 2007;104:666–671. doi: 10.1073/pnas.0606173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H, Turco AE, Pei Y, Kalaydjieva L, Schiavello T, Weremowicz S, Ji W, Morton CC, Meisler M, Reeders ST, Zhou J. Identification of PKDL, a novel polycystic kidney disease 2-like gene whose murine homologue is deleted in mice with kidney and retinal defects. J Biol Chem. 1998;273:25967–25973. doi: 10.1074/jbc.273.40.25967. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Nugent SG, Kumar D, Rampton DS, Evans DF. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48:71–577. doi: 10.1136/gut.48.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtori S, Inoue G, Koshi T, Ito T, Doya H, Saito T, Moriya H, Takahashi K. Up-regulation of acid-sensing ion channel 3 in dorsal root ganglion neurons following application of nucleus pulposus on nerve root in rats. Spine. 2006;31:2048–2052. doi: 10.1097/01.brs.0000231756.56230.13. [DOI] [PubMed] [Google Scholar]
- Ohtori S, Inoue G, Koshi T, Ito T, Watanabe T, Yamashita M, Yamauchi K, Suzuki M, Doya H, Moriya H, Takahashi Y, Takahashi K. Sensory innervation of lumbar vertebral bodies in rats. Spine. 2007;32:1498–1502. doi: 10.1097/BRS.0b013e318067dbf8. [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Hughes PA, Blackshaw LA. Acid sensing ion channels 2 and 3 are required for inhibition of visceral nociceptors by benzamil. Pain. 2007;133:150–160. doi: 10.1016/j.pain.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honoré E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Maingret F, Magnone V, Fosset M, Lazdunski M, Honoré E. TWIK-2, an inactivating 2P domain K+ channel. J Biol Chem. 2000;275:28722–28730. doi: 10.1074/jbc.M003755200. [DOI] [PubMed] [Google Scholar]
- Patterson LM, Zheng H, Ward SM, Berthoud HR. Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res. 2003;311:277–287. doi: 10.1007/s00441-002-0682-0. [DOI] [PubMed] [Google Scholar]
- Petersen M, LaMotte RH. Effect of protons on the inward current evoked by capsaicin in isolated dorsal root ganglion cells. Pain. 1993;54:37–42. doi: 10.1016/0304-3959(93)90097-9. [DOI] [PubMed] [Google Scholar]
- Petheo GL, Molnár Z, Róka A, Makara JK, Spät A. A pH-sensitive chloride current in the chemoreceptor cell of rat carotid body. J Physiol. 2001;535:95–106. doi: 10.1111/j.1469-7793.2001.00095.x. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planells-Cases R, Garcìa-Sanz N, Morenilla-Palao C, Ferrer-Montiel A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflügers Arch. 2005;451:151–159. doi: 10.1007/s00424-005-1423-5. [DOI] [PubMed] [Google Scholar]
- Poirot O, Vukicevic M, Boesch A, Kellenberger S. Selective regulation of acid-sensing ion channel 1 by serine proteases. J Biol Chem. 2004;279:38448–38457. doi: 10.1074/jbc.M407381200. [DOI] [PubMed] [Google Scholar]
- Poirot O, Berta T, Decosterd I, Kellenberger S. Distinct ASIC currents are expressed in rat putative nociceptors and are modulated by nerve injury. J Physiol. 2006;576:215–234. doi: 10.1113/jphysiol.2006.113035. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Xin Liu G, Preisig-Müller R, Daut J, Karschin A, Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histidine as pH sensor. J Biol Chem. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA. Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell. 2005;121:37–47. doi: 10.1016/j.cell.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Rau KK, Cooper BY, Johnson RD. Expression of TWIK-related acid sensitive K+ channels in capsaicin sensitive and insensitive cells of rat dorsal root ganglia. Neuroscience. 2006;141:955–963. doi: 10.1016/j.neuroscience.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Reeh PW, Kress M. Molecular physiology of proton transduction in nociceptors. Curr Opin Pharmacol. 2001;1:45–51. doi: 10.1016/s1471-4892(01)00014-5. [DOI] [PubMed] [Google Scholar]
- Reyes R, Duprat F, Lesage F, Fink M, Salinas M, Farman N, Lazdunski M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J Biol Chem. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- Reyes R, Lauritzen I, Lesage F, Ettaiche M, Fosset M, Lazdunski M. Immunolocalization of the arachidonic acid and mechanosensitive baseline TRAAK potassium channel in the nervous system. Neuroscience. 2000;95:893–901. doi: 10.1016/s0306-4522(99)00484-4. [DOI] [PubMed] [Google Scholar]
- Ricciardolo FL, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol. 2004;113:610–619. doi: 10.1016/j.jaci.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Richter TA, Dvoryanchikov GA, Chaudhari N, Roper SD. Acid-sensitive two-pore domain potassium (K2P) channels in mouse taste buds. J Neurophysiol. 2004a;92:1928–1936. doi: 10.1152/jn.00273.2004. [DOI] [PubMed] [Google Scholar]
- Richter TA, Dvoryanchikov GA, Roper SD, Chaudhari N. Acid-sensing ion channel-2 is not necessary for sour taste in mice. J Neurosci. 2004b;24:4088–4091. doi: 10.1523/JNEUROSCI.0653-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoni M, Trevisani M, Gazzieri D, Nadaletto R, Tognetto M, Creminon C, Davis JB, Campi B, Amadesi S, Geppetti P, Harrison S. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br J Pharmacol. 2003;138:977–985. doi: 10.1038/sj.bjp.0705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci. 2003;23:11315–11321. doi: 10.1523/JNEUROSCI.23-36-11315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2004;560:867–881. doi: 10.1113/jphysiol.2004.071746. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukwied R, Chizh BA, Lorenz U, Obreja O, Margarit S, Schley M, Schmelz M. Potentiation of nociceptive responses to low pH injections in humans by prostaglandin E2. J Pain. 2007;8:443–451. doi: 10.1016/j.jpain.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Ryu S, Liu B, Qin F. Low pH potentiates both capsaicin binding and channel gating of VR1 receptors. J Gen Physiol. 2003;122:45–61. doi: 10.1085/jgp.200308847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Liu B, Yao J, Fu Q, Qin F. Uncoupling proton activation of vanilloid receptor TRPV1. J Neurosci. 2007;27:12797–12807. doi: 10.1523/JNEUROSCI.2324-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y, Inamura K, Miyake A, Mochizuki S, Kitada C, Yokoi H, Nozawa K, Okada H, Matsushime H, Furuichi K. A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J Biol Chem. 2003;278:27406–27412. doi: 10.1074/jbc.M206810200. [DOI] [PubMed] [Google Scholar]
- Schicho R, Schemann M, Pabst MA, Holzer P, Lippe IT. Capsaicin-sensitive extrinsic afferents are involved in acid-induced activation of distinct myenteric neurons in the rat stomach. Neurogastroenterol Motil. 2003;15:33–44. doi: 10.1046/j.1365-2982.2003.00384.x. [DOI] [PubMed] [Google Scholar]
- Schicho R, Florian W, Liebmann I, Holzer P, Lippe IT. Increased expression of TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur J Neurosci. 2004;19:1811–1818. doi: 10.1111/j.1460-9568.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Hammer J, Holzer P, Hammer HF. Chemical nociception in the jejunum induced by capsaicin. Gut. 2004;53:1109–1116. doi: 10.1136/gut.2003.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuligoi R, Jocic M, Heinemann A, Schöninkle E, Pabst MA, Holzer P. Gastric acid-evoked c-fos messenger RNA expression in rat brainstem is signaled by capsaicin-resistant vagal afferents. Gastroenterology. 1998;115:649–660. doi: 10.1016/s0016-5085(98)70144-1. [DOI] [PubMed] [Google Scholar]
- Seabrook GR, Sutton KG, Jarolimek W, Hollingworth GJ, Teague S, Webb J, Clark N, Boyce S, Kerby J, Ali Z, Chou M, Middleton R, Kaczorowski G, Jones AB. Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxy-5-iodo-3-methoxyphenylacetate ester) iodo-resiniferatoxin. J Pharmacol Exp Ther. 2002;303:1052–1060. doi: 10.1124/jpet.102.040394. [DOI] [PubMed] [Google Scholar]
- Semtner M, Schaefer M, Pinkenburg O, Plant TD. Potentiation of TRPC5 by protons. J Biol Chem. 2007;282:33868–33878. doi: 10.1074/jbc.M702577200. [DOI] [PubMed] [Google Scholar]
- Shimada S, Ueda T, Ishida Y, Yamamoto T, Ugawa S. Acid-sensing ion channels in taste buds. Arch Histol Cytol. 2006;69:227–231. doi: 10.1679/aohc.69.227. [DOI] [PubMed] [Google Scholar]
- Shimokawa N, Dikic I, Sugama S, Koibuchi N. Molecular responses to acidosis of central chemosensitive neurons in brain. Cell Signal. 2005;17:799–808. doi: 10.1016/j.cellsig.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Shulkes A, Baldwin GS, Giraud AS. Regulation of gastric acid secretion. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Fourth Edition Academic Press; San Diego: 2006. pp. 1223–1258. [Google Scholar]
- Sluka KA, Price MP, Breese NA, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ES, Cadiou H, McNaughton PA. Arachidonic acid potentiates acid-sensing ion channels in rat sensory neurons by a direct action. Neuroscience. 2007;145:686–698. doi: 10.1016/j.neuroscience.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Somodi S, Varga Z, Hajdu P, Starkus JG, Levy DI, Gáspár R, Panyi G. pH-dependent modulation of Kv1.3 inactivation: role of His399. Am J Physiol. 2004;287:C1067–C1076. doi: 10.1152/ajpcell.00438.2003. [DOI] [PubMed] [Google Scholar]
- Steen KH, Reeh PW. Sustained graded pain and hyperalgesia from harmless experimental tissue acidosis in human skin. Neurosci Lett. 1993;154:113–116. doi: 10.1016/0304-3940(93)90184-m. [DOI] [PubMed] [Google Scholar]
- Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen KH, Reeh PW, Kreysel HW. Topical acetylsalicylic, salicylic acid and indomethacin suppress pain from experimental tissue acidosis in human skin. Pain. 1995;62:339–347. doi: 10.1016/0304-3959(95)00011-G. [DOI] [PubMed] [Google Scholar]
- Stevens DR, Seifert R, Bufe B, Müller F, Kremmer E, Gauss R, Meyerhof W, Kaupp UB, Lindemann B. Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature. 2001;413:631–635. doi: 10.1038/35098087. [DOI] [PubMed] [Google Scholar]
- Stoop R, Surprenant A, North RA. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. J Neurophysiol. 1997;78:1837–1840. doi: 10.1152/jn.1997.78.4.1837. [DOI] [PubMed] [Google Scholar]
- Strecker T, Messlinger K, Weyand M, Reeh PW. Role of different proton-sensitive channels in releasing calcitonin gene-related peptide from isolated hearts of mutant mice. Cardiovasc Res. 2005;65:405–410. doi: 10.1016/j.cardiores.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci. 2005;25:2617–2627. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Bielefeldt K, Gebhart GF. Mouse colon sensory neurons detect extracellular acidosis via TRPV1. Am J Physiol. 2007;292:C1768–C1774. doi: 10.1152/ajpcell.00440.2006. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Schneider DA, Wilson HL, Galligan JJ, North RA. Functional properties of heteromeric P2X1/5 receptors expressed in HEK cells and excitatory junction potentials in guinea-pig submucosal arterioles. J Auton Nerv Syst. 2000;81:249–263. doi: 10.1016/s0165-1838(00)00123-5. [DOI] [PubMed] [Google Scholar]
- Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Aci USA. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003a;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Watanabe Y, Oyama Y, Mizuno A, Kusano E, Hirao A, Ookawara S. Localization of mechanosensitive channel TRPV4 in mouse skin. Neurosci Lett. 2003b;353:189–192. doi: 10.1016/j.neulet.2003.09.041. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Szelényi Z, Hummel Z, Szolcsányi J, Davis JB. Daily body temperature rhythm and heat tolerance in TRPV1 knockout and capsaicin pretreated mice. Eur J Neurosci. 2004;19:1421–1424. doi: 10.1111/j.1460-9568.2004.03221.x. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ZY, Lu Y, Whiteis CA, Benson CJ, Chapleau MW, Abboud FM. Acid-sensing ion channels contribute to transduction of extracellular acidosis in rat carotid body glomus cells. Circ Res. 2007;101:1009–1019. doi: 10.1161/CIRCRESAHA.107.154377. [DOI] [PubMed] [Google Scholar]
- Tang L, Chen Y, Chen Z, Blumberg PM, Kozikowski AP, Wang ZJ. Antinociceptive pharmacology of N-(4-chlorobenzyl)-N’-(4-hydroxy-3-iodo-5-methoxybenzyl) thiourea, a high-affinity competitive antagonist of the transient receptor potential vanilloid 1 receptor. J Pharmacol Exp Ther. 2007;321:791–798. doi: 10.1124/jpet.106.117572. [DOI] [PubMed] [Google Scholar]
- Tashima K, Nakashima M, Kagawa S, Kato S, Takeuchi K. Gastric hyperemic response induced by acid back-diffusion in rat stomachs following barrier disruption - relation to vanilloid type-1 receptors. Med Sci Monit. 2002;8:BR157–BR163. 2002. [PubMed] [Google Scholar]
- Tominaga M, Caterina M, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tomura H, Mogi C, Sato K, Okajima F. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: a novel type of multi-functional receptors. Cell Signal. 2005;17:1466–1476. doi: 10.1016/j.cellsig.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Milan A, Gatti R, Zanasi A, Harrison S, Fontana G, Morice AH, Geppetti P. Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax. 2004;59:769–772. doi: 10.1136/thx.2003.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama Y, Cheng CC, Danielson KG, Mochida J, Albert TJ, Shapiro IM, Risbud MV. Expression of acid-sensing ion channel 3 (ASIC3) in nucleus pulposus cells of the intervertebral disc is regulated by p75NTR and ERK signaling. J Bone Miner Res. 2007;22:1996–2006. doi: 10.1359/jbmr.070805. [DOI] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110:1185–1190. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa S, Yamamoto T, Ueda T, Ishida Y, Inagaki A, Nishigaki M, Shimada S. Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel. J Neurosci. 2003;23:3616–3622. doi: 10.1523/JNEUROSCI.23-09-03616.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Yamamura H, Shimada S. In situ hybridization evidence for the coexistence of ASIC and TRPV1 within rat single sensory neurons. Mol Brain Res. 2005;136:125–133. doi: 10.1016/j.molbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Urban L, Campbell EA, Panesar M, Patel S, Chaudhry N, Kane S, Buchheit K, Sandells B, James IF. In vivo pharmacology of SDZ 249-665, a novel, non-pungent capsaicin analogue. Pain. 2000;89:65–74. doi: 10.1016/S0304-3959(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005;1:17. doi: 10.1186/1744-8069-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulcu SD, Liewald JF, Gillen C, Rupp J, Nawrath H. Proton conductance of human transient receptor potential-vanilloid type-1 expressed in oocytes of Xenopus laevis and in Chinese hamster ovary cells. Neuroscience. 2004;125:861–866. doi: 10.1016/j.neuroscience.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Wachter CH, Heinemann A, Donnerer J, Pabst MA, Holzer P. Mediation by 5-hydroxytryptamine of the femoral vasoconstriction induced by acid challenge of the rat gastric mucosa. J Physiol. 1998;509:541–550. doi: 10.1111/j.1469-7793.1998.541bn.x. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R. Proton-gated cation channels - neuronal acid sensors in the central and peripheral nervous system. Adv Exp Med Biol. 2001;502:293–304. doi: 10.1007/978-1-4757-3401-0_19. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H+-gated cation channels. Ann New York Acad Sci. 1999;868:67–76. doi: 10.1111/j.1749-6632.1999.tb11274.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang DH. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation. 2005;112:3617–3623. doi: 10.1161/CIRCULATIONAHA.105.556274. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu J, Li L, Chen F, Wang R, Jiang C. Hypercapnic acidosis activates KATP channels in vascular smooth muscles. Circ Res. 2003;92:1225–1232. doi: 10.1161/01.RES.0000075601.95738.6D. [DOI] [PubMed] [Google Scholar]
- Welch JM, Simon SA, Reinhart PH. The activation mechanism of rat vanilloid receptor 1 by capsaicin involves the pore domain and differs from the activation by either acid or heat. Proc Natl Acad Sci USA. 2000;97:13889–13894. doi: 10.1073/pnas.230146497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MJ, Price MP, Xie J. Biochemical basis of touch perception: mechanosensory function of degenerin/epithelial Na+ channels. J Biol Chem. 2002;277:2369–2372. doi: 10.1074/jbc.R100060200. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Wildman SS, Brown SG, Rahman M, Noel CA, Churchill L, Burnstock G, Unwin RJ, King BF. Sensitization by extracellular Ca2+ of rat P2X5 receptor and its pharmacological properties compared with rat P2X1. Mol Pharmacol. 2002;62:957–966. doi: 10.1124/mol.62.4.957. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wultsch T, Painsipp E, Shahbazian A, Mitrovic M, Edelsbrunner M, Waldmann R, Lazdunski M, Holzer P. Deletion of the acid-sensing ion channel ASIC3 prevents gastritis-induced acid hyperresponsiveness of the stomach-brainstem axis. Pain. 2008;134:245–253. doi: 10.1016/j.pain.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn G, Ma B, Ruan HZ, Burnstock G. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol. 2004;287:G647–G657. doi: 10.1152/ajpgi.00020.2004. [DOI] [PubMed] [Google Scholar]
- Xie J, Price MP, Berger AL, Welsh MJ. DRASIC contributes to pH-gated currents in large dorsal root ganglion sensory neurons by forming heteromultimeric channels. J Neurophysiol. 2002;87:2835–2843. doi: 10.1152/jn.2002.87.6.2835. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Chu XP, Simon RP. Ca2+-permeable acid-sensing ion channels and ischemic brain injury. J Membr Biol. 2006;209:59–68. doi: 10.1007/s00232-005-0840-x. [DOI] [PubMed] [Google Scholar]
- Xu GY, Huang LY. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci. 2002;22:93–102. doi: 10.1523/JNEUROSCI.22-01-00093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Winston JH, Shenoy M, Yin H, Pendyala S, Pasricha PJ. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology. 2007;133:1282–1292. doi: 10.1053/j.gastro.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Xu H, Cui N, Yang Z, Wu J, Giwa LR, Abdulkadir L, Sharma P, Jiang C. Direct activation of cloned KATP channels by intracellular acidosis. J Biol Chem. 2001;276:12898–12902. doi: 10.1074/jbc.M009631200. [DOI] [PubMed] [Google Scholar]
- Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99:501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- Yang HYT, Tao T, Iadarola MJ. Modulatory role of neuropeptide FF system in nociception and opiate analgesia. Neuropeptides. 2008;42:1–18. doi: 10.1016/j.npep.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Baecker PA, Ford AP, Knowles CH, Chan CL, Williams NS, Anand P. ATP-gated ion channel P2X3 is increased in human inflammatory bowel disease. Neurogastroenterol Motil. 2001a;13:365–369. doi: 10.1046/j.1365-2982.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Dyer NH, Chan CL, Knowles C, Williams NS, Anand P. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001b;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Smith JA, Sangameswaran L, Eglen R, Birch R, Knowles C, Williams N, Anand P. Increased acid-sensing ion channel ASIC-3 in inflamed human intestine. Eur J Gastroenterol Hepatol. 2001c;13:891–896. doi: 10.1097/00042737-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Yuill K, Ashmole I, Stanfield PR. The selectivity filter of the tandem pore potassium channel TASK-1 and its pH-sensitivity and ionic selectivity. Pflügers Arch. 2004;448:63–69. doi: 10.1007/s00424-003-1218-5. [DOI] [PubMed] [Google Scholar]
- Yuill KH, Stansfeld PJ, Ashmole I, Sutcliffe MJ, Stanfield PR. The selectivity, voltage-dependence and acid sensitivity of the tandem pore potassium channel TASK-1: contributions of the pore domains. Pflügers Arch. 2007;455:333–348. doi: 10.1007/s00424-007-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Swandulla D, Reeh PW, Kress M. Ca2+ permeability of the sustained proton-induced cation current in adult rat dorsal root ganglion neurons. J Neurophysiol. 1996;76:2834–2840. doi: 10.1152/jn.1996.76.5.2834. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Kress M, Swandulla D. Fractional Ca2+ currents through capsaicin- and proton-activated ion channels in rat dorsal root ganglion neurones. J Physiol. 1997;503:67–78. doi: 10.1111/j.1469-7793.1997.067bi.x. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Nurse CA. CO2/pH chemosensory signaling in co-cultures of rat carotid body receptors and petrosal neurons: role of ATP and Ach. J Neurophysiol. 2004;92:3433–3445. doi: 10.1152/jn.01099.2003. [DOI] [PubMed] [Google Scholar]
- Zhong B, Wang DH. TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am J Physiol. 2007;293:H1791–H1798. doi: 10.1152/ajpheart.00169.2007. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Dunn PM, Bardini M, Ford APDW, Cockayne DA, Burnstock G. Changes in P2X receptor responses of sensory neurons from P2X3-deficient mice. Eur J Neurosci. 2001;14:1784–1792. doi: 10.1046/j.0953-816x.2001.01805.x. [DOI] [PubMed] [Google Scholar]
- Zong X, Stieber J, Ludwig A, Hofmann F, Biel M. A single histidine residue determines the pH sensitivity of the pacemaker channel HCN2. J Biol Chem. 2001;276:6313–6319. doi: 10.1074/jbc.M010326200. [DOI] [PubMed] [Google Scholar]