Abstract
Neuropeptide-Y acting via Y1 receptors reduces anxiety and stress sensitivity in rodents. In Y1 receptor knockout (Y1−/−) mice, however, anxiety-related behaviour is altered only in a context-dependent manner. Here we investigated whether stress causes a delayed change in the emotional-affective behaviour of female Y1−/− mice. Locomotor and anxiety-related behaviour was assessed with the elevated plus-maze (EPM) test, and depression-like behaviour with the forced swim test (FST). These behavioural tests were also used as experimental stress paradigms. Locomotion and anxiety-like behaviour did not differ between naïve control and Y1−/− mice. One week after the FST, locomotion was reduced in control animals but unchanged in Y1−/− mice, whereas anxiety-like behaviour remained unaltered in both genotypes. Depression-like behaviour (immobility) was identical in naïve control and Y1−/− mice but, one week after the EPM test, was attenuated in Y1−/− mice relative to control animals. Our data show that naïve female Y1−/− mice do not grossly differ from female control animals in their locomotor and depression-like behaviour. Exposure to the stress associated with behavioural testing, however, leads to delayed genotype-dependent differences in locomotion and depression-like behaviour. These findings attest to a role of Y1 receptor signalling in the control of stress coping and/or adaptation.
Keywords: neuropeptide-Y, neuropeptide-Y Y1 receptors, locomotor activity, anxiety-like behaviour, depression-like behaviour, thermal nociception, stress sensitivity
INTRODUCTION
Neuropeptide-Y (NPY) is widely distributed in the central nervous system where it is involved in the regulation of energy balance, seizure activity, cognition, mood, anxiety and stress reactions (Vezzani et al., 1999; Kask et al., 2002; Fetissov et al., 2004; Lin et al., 2004; Eva et al., 2006; Karl and Herzog, 2007). Haplotype-driven expression of NPY in humans predicts brain responses to emotional and stress challenges and inversely correlates with trait anxiety (Zhou et al., 2008). The physiological actions of NPY are mediated by several classes of NPY receptors, five of which (Y1, Y2, Y4, Y5 and y6) have been elucidated at the gene and protein level (Michel et al., 1998; Redrobe et al., 2004). Coupled to Gi/o signalling pathways, these Y receptors mediate the functional implications of NPY in the brain.
Intracerebroventricular injection of NPY reduces both anxiety- and depression-related behaviour in several animal models, an action that is primarily mediated by Y1 receptors (Kask et al., 2002; Redrobe et al., 2002; Heilig, 2004; Primeaux et al., 2005; Karlsson et al., 2008). The implications of endogenous NPY acting via Y1 receptors in the control of emotionality, mood, stress reactions and nociception have been probed with Y1 receptor-selective antagonists, Y1 receptor antisense oligonucleotides and the use of Y1 receptor knockout (Y1−/−) mice (Eva et al., 2006). Intracerebroventricular injection of the Y1 receptor antagonist BIBP3226 to male rats enhances anxiety-like behaviour in the elevated plus-maze test (Kask et al., 1996), an effect that is reproduced by injection of BIBP3226 into the periaqueductal grey (Kask et al., 1998). Analysis of male Y1−/− mice has shown that their behavioural phenotypes either exhibit minimal changes or are at some variance with results of pharmacological studies (Lin et al., 2004; Wittmann et al., 2005; Eva et al., 2006). While male Y1−/− mice display thermal, chemical and mechanical hyperalgesia (Naveilhan et al., 2001; Shi et al., 2006), their locomotor, exploratory and anxiety-like behaviour is altered only in a context-dependent manner depending on diurnal cycle or acute stress (Karl et al., 2006).
Stress is known to reduce pain sensitivity and to affect emotional-affective behaviour (Mogil et al., 1997; Chotiwat and Harris, 2006; Adriaan Bouwknecht et al., 2007; Stam, 2007). While stress-induced analgesia does not seem to be altered in Y1−/− mice (Naveilhan et al., 2001), their exploratory behaviour is enhanced by acute stress whereas, paradoxically, anxiety-like behaviour is reduced in a context- and test-related manner (Karl et al., 2006). In view of the proposed role of NPY and Y1 receptors in stress coping and stress adaptation we hypothesized that, in Y1−/− mice, exposure to stress has a delayed influence on locomotor and emotional-affective behaviour. Locomotor and anxiety-related behaviour was assessed with the elevated plus-maze (EPM) test, and depression-like behaviour evaluated with the forced swim test (FST) and tail suspension test (TST). At the same time, the EPM test, the FST and electric foot-shocks were used as experimental stress conditions. The study was performed with female mice, because affective disorders are more prevalent in women than in men (Palanza, 2001; Simonds and Whiffen, 2003; Gorman, 2006) and because previous studies involving pharmacological Y1 blockade or Y1 receptor knockout were performed with male rather than female animals (Kask et al., 1996, 1998, 2000, 2001; Naveilhan et al., 2001; Redrobe et al., 2002; Primeaux et al., 2005; Wittmann et al., 2005; Karl et al., 2006; Shi et al., 2006).
METHODS AND MATERIALS
Experimental animals
This study was carried out with adult female mice which were housed in groups of 3 – 4 per cage under controlled temperature (21 °C) and a 12 h light/dark cycle (lights on at 6:00 h, lights off at 18:00 h). All experiments were approved by an ethical committee at the Federal Ministry of Science and Research of the Republic of Austria and conducted according to the Directive of the European Communities Council of 24 November 1986 (86/609/EEC). The experiments were designed in such a way that both the number of animals used and their suffering was minimized.
Specifically, the experiments were performed with germline Y1−/− mice and non-induced conditional Y1, Y2 and Y4 receptor knockout (FY1, FY2 and FY4) mice which were bred in the Department of Pharmacology of the Medical University of Innsbruck (Innsbruck, Austria), while all experiments were carried out at the Medical University of Graz. Germline Y1−/− mice were obtained by crossing chimeric mice carrying a Y1 floxed gene (Y1lox/lox) with oocyte-specific Cre recombinase-expressing C57BL/6 mice (Howell et al., 2003; Karl et al., 2004, 2006). Non-induced conditional FY1, FY2 and FY4 knockout mice were used as controls in all experiments and termed control mice throughout the paper (Sainsbury et al., 2002a, 2002b; Howell et al., 2003). Germline Y1−/− mice were generated from the same founders on the same mixed C57BL/6 : 129/SvJ (50 % : 50 %) background as the conditional FY1, FY2 and FY4 knockout mice. The deletion or presence of Y1 in the germline and non-induced conditional knockout mice was verified by Southern analysis (Howell et al., 2003; Karl et al., 2004, 2006).
Experimental protocols
Three studies with control and Y1−/− mice were performed. The general design of all studies was such that the behavioural tests in question were carried out with naïve animals and with animals that had been subjected to a behavioural test before. Each study was carried with separate groups of mice. In the first study, the locomotor and anxiety-like behaviour of control and Y1−/− mice was recorded with the EPM test, and the behaviour of naïve animals compared with that recorded one week after experience of the stress associated with the FST. In the second study, the depression-like behaviour of naïve control and Y1−/−mice was evaluated with the FST and TST. Forty-five minutes after the TST had begun the plasma levels of corticosterone were determined. In addition, the depression-like behaviour of control and Y1−/−mice in the FST was recorded one day or one week after experience of the stress associated with the EPM test.
In the third study, thermal nociception and stress-induced analgesia (Mogil et al., 1997; Naveilhan et al., 2001) in control and Y1−/− mice were checked with the plantar test. The paw withdrawal latency following exposure to radiant heat was recorded in naïve animals and in mice that had been exposed to the foot-shock paradigm (interval 2 min) or the stress associated with the FST (interval 2 or 60 min). The course of this experiment was such that naïve animals were allowed to acclimatize in the recording chambers for up to 60 min before the paw withdrawal latency was recorded. On the following day, they were subjected to the FST. Immediately afterwards, the mice were returned to their home cage, dried with infrared light and placed in the plantar test chamber to take measurements of the paw withdrawal latency 2 – 6 min (Mogil et al., 1997) and 60 – 64 min post-FST. In addition, the paw withdrawal latency was also measured 2 – 6 min after control and Y1−/− mice had been exposed to the foot-shock paradigm.
Throughout the experiments the animals were housed in groups of 3 – 4 animals per cage. After completion of each test, the animals were immediately returned to their cage mates in the home cage. Care was taken not to change the cage mates during the experiments.
Behavioural tests
Prior to all behavioural tests, the mice were allowed to adapt to the test room (22 °C, 50 % relative air humidity, lights on at 6:00 h, lights off at 18:00 h, maximal light intensity 100 lux) for two days.
Elevated plus-maze (EPM) test
The animals were placed in the center of a maze with 4 arms arranged in the shape of a plus (Pellow and File, 1986; Belzung and Griebel, 2001). The maze consisted of a central quadrangle (5 × 5 cm), two opposing open arms (30 cm long, 5 cm wide) and two opposing closed arms of the same size but equipped with 15 cm high walls at their sides and the far end. The device was made of opaque gray plastic and elevated 70 cm above the floor. The light intensity at the central quadrangle was 70 lux, on the open arms 80 lux and in the closed arms 40 lux.
At the beginning of each trial, the animals were placed on the central quadrangle facing an open arm. The movements of the animals during a 5 min test period were tracked by a video camera above the center of the maze and recorded with the software VideoMot2 (TSE Systems, Bad Homburg, Germany). This software was used to evaluate the animal tracks and to determine the number of entries into the open and closed arms, the time spent on the open and closed arms and the total distance traveled in the open and closed arms during the test session. Entry into an arm was defined as the instance when the mouse placed its four paws on that arm. The EPM test was carried out between 10:00 h and 12:00 h.
Locomotion was quantified by measuring the total distance traveled in the open and closed arms and the total number of entries into any arm during the 5 min test session. Anxiety-related behaviour was deduced from the time spent on the open arms and the number of entries into the open arms. The time spent on and the number of entries into the open arms is inversely related to the trait anxiety of the animals.
Forced swim test (FST)
When placed in an inescapable water container, mice first struggle to escape but sooner or later abandon this behaviour and become immobile (Cryan et al., 2002). Mice were individually placed in glass beakers (inner diameter 11.5 cm, height 24 cm, capacity 2 l) containing tap water at 25 °C. The water depth was 12 cm which prevented the mice from touching the bottom of the beaker with their paws or the tail. Mice were tested for 6 min and the time of immobility was scored by a trained observer. Mice were considered immobile when floating passively in the water, performing only those movements required to keep their heads above the water level (Cryan et al., 2002). The FST was carried out between 10:00 h and 12:00 h.
Tail suspension test (TST)
Following exposure to the inescapable stress of being suspended by their tail, mice first struggle to escape but sooner or later attain a posture of immobility (Steru et al. 1985; Liu & Gershenfeld 2001; Cryan et al. 2005). Mice were suspended by their tail with a 1.9 cm wide strapping tape (OmnitapeR, Paul Hartmann AG, Heidenheim, Germany) to a lever mounted to the top of a box (50 × 50 × 50 cm, length × width × height). Each trial took 6 min and was carried out at a light intensity of 20 lux. The time of immobility was scored by a trained observer. The TST was carried out between 10:00 h and 12:00 h.
Plantar test
Acute thermal nociception was assessed with a Plantar Test apparatus (model 7370, Ugo Basile, Comerio, Italy) as described by Montagne-Clavel and Oliveras (1996). The mice were placed in a clear plastic chamber (17 × 10.5 × 15 cm, length × width × height) with a glass floor and allowed to acclimatize before testing. During this time, the animals initially explored the chamber but subsequently stopped moving around and became quiet. In the test, a mobile radiant infrared heat source, located under the glass floor, was focused onto the plantar surface of one of the hindpaws. When the mice felt pain and withdrew their paw, the instrument automatically detected the withdrawal latency to the nearest 0.1 s. The intensity of the heat stimulus was adjusted so that the baseline latency in control mice was approximately 4 s. A cutoff time of 15 s was chosen to avoid tissue damage in the absence of a withdrawal response. The mean paw withdrawal latencies for both hindpaws were calculated from the average of 3 separate trials, taken at 2 min intervals. The plantar test was carried out between 10:00 h and 12:00 h.
Food-shock stress
Mice were exposed to inescapable foot-shocks after being placed in the light chamber of a TSE Passive Avoidance system (TSE Systems, Bad Homburg, Germany). The floor of this chamber (14 × 15.5 × 16 cm, length × width × height) was a stainless steel grid for delivering foot-shocks, the bars of which measured 4 mm in diameter and were spaced 8.9 mm apart. During a period of 5 min, the animals were exposed to a total of 5 foot-shocks, each of 0.4 mA intensity and 2 s duration (Stam, 2007). The shocks were delivered in a random order, the inter-shock interval varying from 30 to 120 s.
Circulating corticosterone
The plasma levels of corticosterone were determined between 10:45 h and 12:45 h, 45 min after the TST had begun. The animals were deeply anaesthetized with pentobarbital (150 mg/kg IP) before they were decapitated. Trunk blood was collected into vials coated with ethylenediamine tetraacetate (Greiner, Kremsmünster, Austria) kept on ice. Following centrifugation for 20 min at 4 °C and 1200 × g, blood plasma was collected and stored at −20 °C until assay. The plasma levels of corticosterone were determined with an enzyme immunoassay kit (Assay Designs, Ann Arbor, Michigan, USA). According to the manufacturer’s specifications, the sensitivity of the assay is 27 pg/ml, and the intra- and inter-assay coefficient of variation amounts to 7.7 and 9.7 %, respectively.
Statistics
Statistical evaluation of the results was performed on SPSS 14.0 (SPSS Inc., Chicago, IL, USA). Since differences in the emotional-affective behaviour between control and Y1−/− mice have previously been reported (Karl et al., 2006), all data were analyzed by planned comparisons (Kirk, 1995) and one- or two-way analysis of variance (ANOVA) to dissect statistical differences for the factors genotype and, if applicable, treatment (i.e., preceding exposure to a behavioural test). If planned comparisons and ANOVA yielded the same results, only those obtained by ANOVA are reported. Planned comparisons were made with the t-test or one-way ANOVA. The homogeneity of variances was assessed with the Levene test. In case of sphericity violations the Greenhouse-Geisser correction was applied. Post-ANOVA analysis of group differences was performed with the Tukey HSD (honestly significant difference) test, when the variances were homogeneous, and with the Games-Howell test, when the variances were unequal. Student’s t test was used when only two data groups were compared with each other. Probability values of P ≤ 0.05 were regarded as statistically significant. All data are presented as means ± SEM, n referring to the number of mice in each group.
RESULTS
General observations
As reported previously (Howell et al., 2003; Karl et al., 2004, 2006), Y1−/− mice did not have any gross abnormalities, did not exhibit any obvious signs of sensory deficits and appeared healthy. The body weight of the Y1−/− mice (31.6 ± 0.99 g, n = 30) was significantly (P < 0.001) higher than that of the control mice (25.9 ± 0.49 g, n = 36). The body weight did not change significantly during the course of the experiments.
Locomotor and anxiety-like behaviour
The locomotor and anxiety-related behaviour of control and Y1−/− mice was assessed with the EPM test. In order to examine any influence of a preceding stress experience, the locomotor and anxiety-related behaviour of naïve control and Y1−/−mice was compared with the behaviour recorded one week after exposure to the FST.
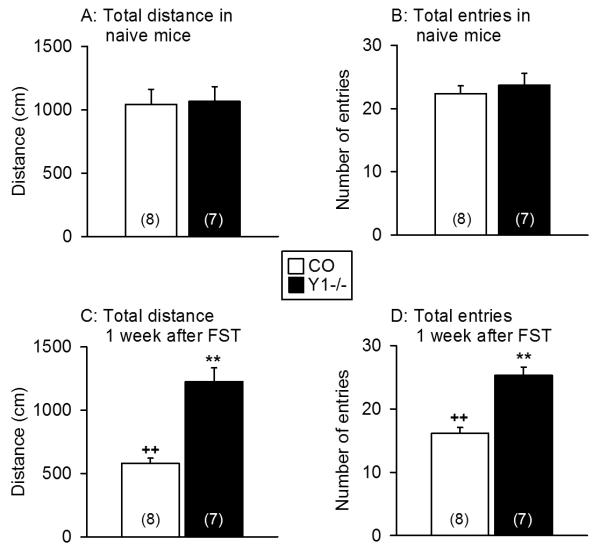
ANOVA demonstrated that the total distance traveled in the open and closed arms and the total number of entries into any arm differed with genotype (distance: F(1,26) = 11.10, P = 0.003; entries: F(1,26) = 15.31, P = 0.001) but not with treatment. There was a significant interaction between the two factors (distance: F(1,26) = 9.66, P = 0.05; entries: F(1,26) = 8.49, P = 0.007). Post-ANOVA analysis revealed that locomotion did not differ between naïve control and Y1−/− mice (Figure 1A,B). Exposure of control mice to stress one week before the EPM test significantly reduced the total distance traveled on the EPM arms and the total number of entries, whereas no significant change occurred in stressed Y1−/− mice (Figure 1A,B,C,D). As a result, the total distance traveled on the EPM arms and the total number of entries one week after exposure to stress were significantly smaller in control animals than in Y1−/− mice (Figure 1C,D).
Figure 1.
Locomotor behaviour on the EPM as measured in naïve control and Y1−/− mice and in mice that had been exposed to the FST one week before the EPM test. The graphs show the total distance traveled in the open and closed arms (A,C) and the total number of entries into any arm (B,D) during the 5 min test session. The values represent means ± SEM, n as indicated in parenthesis. ** P < 0.01 versus control mice under the same experimental conditions, ++ P < 0.01 versus naïve mice of the same genotype.
Anxiety-like behaviour as assessed by the time spent on the open arms and the number of entries into the open arms did not significantly differ with regard to treatment and genotype (Table 1). Thus, neither the open arm time nor the number of open arm entries of control and Y1−/− mice were significantly altered one week after exposure to stress (Table 1).
Table 1.
Anxiety-related behaviour on the EPM as measured in naïve control and Y1−/− mice and in mice that had been exposed to the FST one week before the EPM test
| Genotype and treatment | Time on open arms (s) | Number of open arm entries |
|---|---|---|
| Naïve control mice | 60.1 ± 10.3 (n = 7) | 10.3 ± 1.84 (n = 7) |
| Naïve Y1−/− mice | 76.2 ± 16.0 (n = 8) | 10.1 ± 1.47 (n = 8) |
| Control mice 1 week after FST | 57.9 ± 8.72 (n = 7) | 10.1 ± 1.56 (n = 7) |
| Y1−/− mice 1 week after FST | 36.5 ± 10.9 (n = 8) | 8.25 ± 0.88 (n = 8) |
The table shows the time spent on the open arms and the number of entries into the open arms during the 5 min test session. The values represent means ± SEM, n as indicated in parenthesis. There were no significant differences at the P ≤ 0.05 level.
Depression-like behaviour
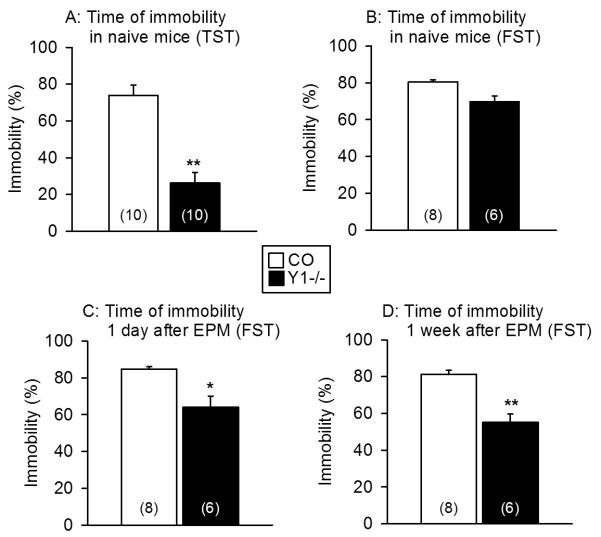
Considered to mirror depression-like behaviour, the time of immobility in the TST and FST was expressed as a percentage of the 6 min test duration. First, the behaviour of naïve control and Y1−/− mice in the two tests was explored. As shown in Figure 2A, the time of immobility of naïve Y1−/− mice in the TST was significantly shorter than in naïve control animals. In the FST, however, the time of immobility did not significantly differ between naïve control and Y1−/− mice (Figure 2B).
Figure 2.
Depression-related behaviour as measured with the TST in naïve control and Y1−/− mice (A) and with the FST in naïve control and Y1−/− mice (B) as well as in mice that had been exposed to the EPM test one day (C) and one week (D) before the FST. The graphs show the time of immobility expressed as a percentage of the 6 min test duration. The values represent means ± SEM, n as indicated in parenthesis. * P < 0.05, ** P < 0.01 versus control mice under the same experimental conditions.
There was no significant (P = 0.08) genotype-related difference in the plasma level of corticosterone measured 45 min after the TST had begun, the concentration in control mice being 241 ± 20.0 ng/ml (n = 10) and that in Y1−/− mice 179 ± 26.3 ng/ml (n = 10).
In examining any influence of a preceding stress experience, the behaviour of naïve control and Y1−/−mice was compared with that recorded one day and one week, respectively, after exposure to the EPM test (Figure 2B,C,D). Planned comparisons revealed that, one day and one week after exposure to stress, the time of immobility in Y1−/− mice was progressively shortened, as judged by the level of statistical significance, but left unaltered in control animals (Figure 2B,C,D).
Thermal nociception
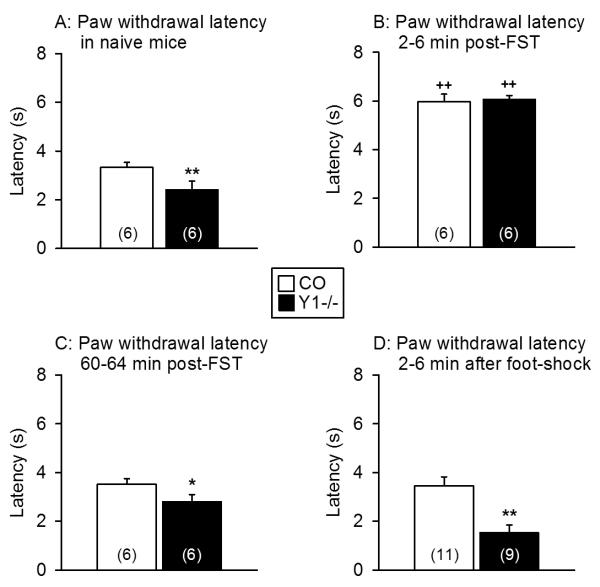
The paw withdrawal latency following exposure to radiant heat was determined as an index of acute thermal nociception. In order to examine any influence of a preceding stress experience, the behaviour of naïve control and Y1−/− mice was compared with that recorded 2 min after exposure to foot-shocks as well as 2 min and 60 min after completion of the FST. Planned comparisons showed that the paw withdrawal latency of naïve Y1−/− mice was significantly shorter than that of naïve control mice (Figure 3A). Two to six minutes after completion of the FST the paw withdrawal latency was significantly prolonged in both control and Y1−/− mice but did no longer differ between the two genotypes (Figure 3B). Sixty minutes post-FST the paw withdrawal latencies had returned to the values observed in naïve control and Y1−/− mice (Figure 3C). Exposure to foot-shocks failed to alter the paw withdrawal latencies and to change the genotype-related difference in the paw withdrawal latency between control and Y1−/− mice (Figure 3D).
Figure 3.
Thermal nociception in the plantar test as measured in naïve control and Y1−/− mice (A) and in mice that had been exposed to the FST 2 – 6 min (B) and 60 – 64 min (C) and to foot-shocks 2 – 6 min (D) before the plantar test. The graphs show the hindpaw withdrawal latencies. The values represent means ± SEM, n as indicated in parenthesis. * P < 0.05, ** P < 0.01 versus control mice under the same experimental conditions, ++ P < 0.01 versus naïve mice of the same genotype.
DISCUSSION
The aim of the current experiments was to explore whether stress has a delayed impact on the locomotor and emotional-affective behaviour of female Y1−/− mice relative to female control animals. We found that, one week after exposure to the stress of the FST, locomotion was reduced in control animals but remained unchanged in Y1−/− mice, whereas anxiety-like behaviour was left unaltered in both genotypes. After exposure to the EPM test, depression-related behaviour in the FST was progressively attenuated in Y1−/− but not control animals. Importantly, the stress associated with the EPM test and FST was sufficient to reveal this difference in stress sensitivity / stress adaptation in Y1−/− mice relative to control mice. In addition, our data indicate that the mechanism of stress-induced changes in depression-related behaviour of Y1−/− mice is different from that of stress-induced analgesia.
Since stress-related disorders have a higher prevalence in women than in men (Palanza, 2001; Simonds and Whiffen, 2003; Gorman, 2006) and female rodents are likewise more vulnerable to stress in terms of long-lasting changes in behavioural reactivity (Stam et al., 1999), we used female mice in the current study. Although the estrus cycle was not determined, we consider it unlikely that our data were significantly biased by this potentially confounding factor. First, the experiments were performed in an environment characterized by the strict absence of any male mice. Second, the coefficient of variation for the EPM test data obtained here with female mice was not greater than that for the respective data obtained with male mice of identical genetic background (Karl et al., 2006). Third, the behaviour of mice on the EPM does not vary significantly with the different phases of the estrus cycle which is synchronized not only among cage mates but also across cages (Painsipp et al., 2007).
As the exploratory and anxiety-related behaviour of Y1−/− animals varies with the diurnal cycle (Karl et al., 2006), the current experiments were carried out 4 – 6 h after the start of the photophase. At this time slot, female Y1−/− mice did not differ from control mice in their behaviour in the EPM test and FST. With regard to the EPM test, our observations are in overall accordance with previous findings that the locomotor and anxiety-related behaviour of male Y1−/− mice was altered 8 h, but not 2 h, after the lights had been switched on (Karl et al., 2006). Karlsson et al. (2008) likewise did not notice any gender difference in the behaviour of Y1−/− mice on the EPM and in other anxiety-related tests. Since pharmacological stimulation of Y1 receptors has anxiolytic-like and antidepressant-like effects in male rodents (Kask et al., 2002; Heilig, 2004; Lin et al., 2004; Eva et al., 2006), the context-dependent absence of an anxiety- and depression-related phenotype of naïve female Y1−/− mice may be the result of developmental compensations in germline knockout mice. Importantly, however, circadian rhythm and acute stress do affect exploratory and anxiety-related behaviour of male Y1−/− mice, which is consistent with a role of Y1 receptor signalling in stress coping of both rodents and humans (Heilig, 2004; Karl et al., 2006; Zhou et al., 2008).
As the current results reveal, the behavioural phenotype of female Y1−/− mice also depends on the preceding sequence of behavioural testing which has a differential influence in control and Y1−/− mice. While anxiety-related behaviour of control and Y1−/− mice did not significantly differ in the naïve state and one week after the FST, locomotor activity of Y1−/− mice one week post-stress was higher than in control animals. This observation is consistent with the increase in locomotion seen immediately after exposure to restraint stress (Karl et al., 2006). The present results show that this stress-induced stimulation of locomotion persists for one week, because control mice move less on repeated exposure to the stress of a behavioural test while Y1−/− mice stay as active as in the naïve state. This finding reinforces the notion that the behavioural phenotype of Y1−/− mice is highly context-dependent. There is also evidence that the interval between two runs of the open field test can have a strain-dependent influence on locomotor activity (Paylor et al., 2006).
Depression-like behaviour was assessed by the time of immobility in the TST and FST. The results obtained with naïve control and Y1−/− mice differed between the two tests inasmuch as in the TST Y1−/−mice spent significantly less time being immobile, while in the FST only a tendency towards a reduction of depression-like behaviour was noted. This observation attests to the view that different biological substrates may underlie the behaviour in the TST and FST (Cryan et al., 2005) and shows that the two tests can yield divergent results in animals whose behavioural phenotype is as context-dependent as that in Y1−/− mice. Particular interactions between task and context are likely to explain why Karlsson et al. (2008) reported enhanced immobility of male and female Y1−/− mice in the FST.
The influence of a preceding stress experience on depression-like behaviour was analyzed with the FST one day and one week after exposure to the EPM test. While the depression-like behaviour of control animals did not change, the immobility of Y1−/− mice decreased over time. This observation emphasizes that exposure even to the mildly aversive and controllable stress associated with the EPM test is sufficient to cause a delayed decrease in depression-like behaviour. The nature of this change is in contrast to what was expected from pharmacological studies in which an antidepressant action of NPY acting via Y1 receptors has been established in male rodents (Kask et al., 2002; Redrobe et al., 2002; Heilig, 2004). It is likely that the delayed antidepressant-like effect of stress in Y1−/− mice reflects a developmental overcompensation of the functional consequences of Y1 receptor deletion. Experiments involving transient Y1 receptor blockade or knockdown will be required to solve this discrepancy.
Using the plantar test, we found female Y1−/− mice to be hypersensitive to thermal pain, a finding that is in keeping with the thermal, chemical and mechanical hyperalgesia seen in male Y1−/− mice using other nociception assays (Naveilhan et al., 2001; Shi et al., 2006). As reported by Naveilhan et al. (2001), acute swim stress led to a similar degree of opioid-dependent and opioid-independent analgesia in wild-type and Y1−/− mice, an observation that was confirmed in the present study with the FST as stress paradigm. Importantly, the current results show that the stress-induced analgesia was of short duration, given that 60 min post-FST the paw withdrawal latencies in both control and Y1−/− mice had returned to pre-FST values. We thus conclude that the mechanism of stress-induced analgesia is unrelated to the mechanism that underlies the delayed stress-induced changes in locomotor and depression-related behaviour of Y1−/−mice relative to control animals. Following exposure of rodents to electrical foot shocks both analgesic and hyperalgesic effects have been observed (Yamada and Nabeshima, 1995; Imbe et al., 2006; Stam, 2007). Unlike the FST, electric foot-shocks failed to cause acute stress-induced analgesia in the current study, an observation that implies that the impact of this stress paradigm on the nociceptive system is different from that of the FST-associated stress condition. It was beyond the scope of this study to elucidate these Y1 receptor-independent issues.
NPY as well as Y1 receptors are widely distributed in the rodent brain, and there is considerable overlap between the density of Y1 receptors and NPY-like immunoreactivity (Dumont et al., 1998; Parker and Herzog, 1999; Kopp et al., 2002). The anxiolytic and antidepressant effect of Y1 receptor signalling in male rodents is thought to involve several brain nuclei including the amygdala, periaqueductal gray, septum and locus coeruleus (Kask et al., 1998, 2000, 2001, 2002; Saidyk et al., 1999; Heilig, 2004). While acute and repeated restraint stress have been found to cause a short-lasting down- and upregulation, respectively, of NPY expression in the amygdala of male rats (Thorsell et al., 1998, 1999), acute but not repeated restraint stress leads to a transient Y1 receptor upregulation in the amygdala and hypothalamic paraventricular nucleus of male mice (Mele et al., 2004). In addition, region-specific alterations in Y2 receptor expression have been described to occur in the brain of male Y1−/− mice (Wittmann et al., 2005). It will thus be a complex task to unravel the neurochemical basis that underlies the delayed impact of a stress experience on the emotional-affective behaviour in Y1−/− mice.
Y1 receptor signalling is able to stimulate the hypothalamic-pituitary-adrenal axis which is relevant to the expression of stress-, anxiety- and depression-like behaviour (Kask et al., 2002; Kopp et al., 2002; Heilig, 2004; Dimitrov et al., 2007). Thus, the circulating level of corticosterone in male rats is increased by Y1 receptor agonism and decreased by Y1 receptor antagonism (Ishihara et al., 2002; Dimitrov et al., 2007). Although no significant difference in the basal plasma level of corticosterone was observed in male Y1−/−mice (Raposinho et al., 2004), it should be noted that both the basal corticosterone levels (32.7 ng/ml in control and 21.5 ng/ml in Y1−/− mice; Raposinho et al., 2004) and the corticosterone levels measured after acute exposure to the TST (this study) tended to be lower in Y1−/− mice than in control animals. It would therefore seem worthwhile to examine whether alterations in the stress sensitivity of Y1−/− mice are related to subtle alterations in the activity of the hypothalamic-pituitary-adrenal axis.
In conclusion, our data show that deletion of Y1 receptors by itself has little influence on locomotor, anxiety-like and depression-related behaviour in female mice much as it has been reported for male mice. However, exposure even to the mild stress associated with tests of emotional-affective behaviour has a delayed genotype-related influence on the locomotor and depression-like behaviour, at least in female Y1−/− mice. It is worth noting that the behavioural modifications in response to stress develop over time or persist for one week, an issue that is important to consider if female Y1−/− mice are repeatedly subjected to behavioural tests. Taken all aspects together, our findings attest to an important role of NPY acting via Y1 receptors in the control of locomotion and stress coping or adaptation.
ACKNOWLEDGEMENTS
This study was supported by the Zukunftsfonds Steiermark (grant 262) and the Austrian Scientific Research Funds (FWF grants L25-B05). The support by the director and staff of the Center for Medical Research (ZMF I) of the Medical University of Graz is also greatly appreciated.
Footnotes
STATEMENT OF INTEREST The authors state no conflict of interest.
REFERENCES
- Adriaan Bouwknecht J, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Chotiwat C, Harris RB. Increased anxiety-like behavior during the post-stress period in mice exposed to repeated restraint stress. Horm Behav. 2006;50:489–495. doi: 10.1016/j.yhbeh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Dimitrov EL, DeJoseph MR, Brownfield MS, Urban JH. Involvement of neuropeptide Y Y1 receptors in the regulation of neuroendocrine corticotropin-releasing hormone neuronal activity. Endocrinology. 2007;148:3666–3673. doi: 10.1210/en.2006-1730. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Jacques D, Bouchard P, Quirion R. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J Comp Neurol. 1998;402:372–384. [PubMed] [Google Scholar]
- Eva C, Serra M, Mele P, Panzica G, Oberto A. Physiology and gene regulation of the brain NPY Y1 receptor. Front Neuroendocrinol. 2006;27:308–339. doi: 10.1016/j.yfrne.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Kopp J, Hökfelt T. Distribution of NPY receptors in the hypothalamus. Neuropeptides. 2004;38:175–188. doi: 10.1016/j.npep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Gorman JM. Gender differences in depression and response to psychotropic medication. Gender Med. 2006;3:93–109. doi: 10.1016/s1550-8579(06)80199-3. [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Howell OW, Scharfman HE, Herzog H, Sundstrom LE, Beck-Sickinger A, Gray WP. Neuropeptide Y is neuroproliferative for post-natal hippocampal precursor cells. J Neurochem. 2003;86:646–659. doi: 10.1046/j.1471-4159.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci. 2006;11:2179–2192. doi: 10.2741/1960. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Kanatani A, Okada M, Hidaka M, Tanaka T, Mashiko S, Gomori A, Kanno T, Hata M, Kanesaka M, Tominaga Y, Sato NA, Kobayashi M, Murai T, Watanabe K, Ishii Y, Fukuroda T, Fukami T, Ihara M. Blockade of body weight gain and plasma corticosterone levels in Zucker fatty rats using an orally active neuropeptide Y Y1 antagonist. Br J Pharmacol. 2002;136:341–346. doi: 10.1038/sj.bjp.0704696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl T, Herzog H. Behavioral profiling of NPY in aggression and neuropsychiatric diseases. Peptides. 2007;28:326–333. doi: 10.1016/j.peptides.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Karl T, Lin S, Schwarzer C, Sainsbury A, Couzens M, Wittmann W, Boey D, von Hörsten S, Herzog H. Y1 receptors regulate aggressive behavior by modulating serotonin pathways. Proc Natl Acad Sci USA. 2004;101:12742–12747. doi: 10.1073/pnas.0404085101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl T, Burne TH, Herzog H. Effect of Y1 receptor deficiency on motor activity, exploration, and anxiety. Behav Brain Res. 2006;167:87–93. doi: 10.1016/j.bbr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Choe JS, Cameron HA, Thorsell A, Crawley JN, Holmes A, Heilig M. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacology (Berlin) 2008;195:547–557. doi: 10.1007/s00213-007-0945-2. [DOI] [PubMed] [Google Scholar]
- Kask A, Rägo L, Harro J. Anxiogenic-like effect of the neuropeptide Y Y1 receptor antagonist BIBP3226: antagonism with diazepam. Eur J Pharmacol. 1996;317:R3–R4. doi: 10.1016/s0014-2999(96)00838-2. [DOI] [PubMed] [Google Scholar]
- Kask A, Rägo L, Harro J. Anxiogenic-like effect of the NPY Y1 receptor antagonist BIBP3226 administered into the dorsal periaqueductal gray matter in rats. Regul Pept. 1998;75-76:255–262. doi: 10.1016/s0167-0115(98)00076-7. [DOI] [PubMed] [Google Scholar]
- Kask A, Eller M, Oreland L, Harro J. Neuropeptide Y attenuates the effect of locus coeruleus denervation by DSP-4 treatment on social behaviour in the rat. Neuropeptides. 2000;34:58–61. doi: 10.1054/npep.1999.0788. [DOI] [PubMed] [Google Scholar]
- Kask A, Nguyen HP, Pabst R, von Hörsten S. Neuropeptide Y Y1 receptor-mediated anxiolysis in the dorsocaudal lateral septum: functional antagonism of corticotropin-releasing hormone-induced anxiety. Neuroscience. 2001;104:799–806. doi: 10.1016/s0306-4522(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Kask A, Harro J, von Hörsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design. Procedures for the Behavioral Sciences. 3rd edition Brooks/Cole; Pacific Grove, California: 1995. [Google Scholar]
- Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, Wong H, Walsh JH, Hökfelt T. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111:443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38:189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biol Psychiat. 2001;49:575–581. doi: 10.1016/s0006-3223(00)01028-3. [DOI] [PubMed] [Google Scholar]
- Mele P, Oberto A, Serra M, Pisu MG, Floris I, Biggio G, Eva C. Increased expression of the gene for the Y1 receptor of neuropeptide Y in the amygdala and paraventricular nucleus of Y1R/LacZ transgenic mice in response to restraint stress. J Neurochem. 2004;89:1471–1478. doi: 10.1111/j.1471-4159.2004.02444.x. [DOI] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- Mogil JS, Richards SP, O’Toole LA, Helms ML, Mitchell SR, Kest B, Belknap JK. Identification of a sex-specific quantitative trait locus mediating nonopioid stress-induced analgesia in female mice. J Neurosci. 1997;17:7995–8002. doi: 10.1523/JNEUROSCI.17-20-07995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne-Clavel J, Oliveras JL. The "plantar test" apparatus (Ugo Basile Biological Apparatus), a controlled infrared noxious radiant heat stimulus for precise withdrawal latency measurement in the rat, as a tool for humans? Somatosens Mot Res. 1996;13:215–223. doi: 10.3109/08990229609052577. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Hassani H, Lucas G, Blakeman KH, Hao JX, Xu XJ, Wiesenfeld-Hallin Z, Thorén P, Ernfors P. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature. 2001;409:513–517. doi: 10.1038/35054063. [DOI] [PubMed] [Google Scholar]
- Painsipp E, Wultsch T, Shahbazian A, Edelsbrunner M, Kreissl MC, Schirbel A, Bock E, Pabst MA, Thoeringer CK, Huber HP, Holzer P. Experimental gastritis in mice enhances anxiety in a gender-related manner. Neuroscience. 2007;150:522–536. doi: 10.1016/j.neuroscience.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav. 2006;87:95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, Cusick MC, York DA, Wilson MA. Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology. 2005;30:1589–1597. doi: 10.1038/sj.npp.1300705. [DOI] [PubMed] [Google Scholar]
- Raposinho PD, Pedrazzini T, White RB, Palmiter RD, Aubert ML. Chronic neuropeptide Y infusion into the lateral ventricle induces sustained feeding and obesity in mice lacking either Npy1r or Npy5r expression. Endocrinology. 2004;145:304–310. doi: 10.1210/en.2003-0914. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Fournier A, Quirion R. The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY-induced antidepressant-like activity in the mouse forced swimming test. Neuropsychopharmacology. 2002;26:615–624. doi: 10.1016/S0893-133X(01)00403-1. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Carvajal C, Kask A, Dumont Y, Quirion R. Neuropeptide Y and its receptor subtypes in the central nervous system: emphasis on their role in animal models of psychiatric disorders. In: Michel MC, editor. Neuropeptide Y and Related Peptides. Handbook of Experimental Pharmacology. Volume 162. Springer; Berlin: 2004. pp. 101–136. [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Fetissov S, Fürtinger S, Jenkins A, Cox HM, Sperk G, Hökfelt T, Herzog H. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc Natl Acad Sci USA. 2002a;99:8938–8943. doi: 10.1073/pnas.132043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, Herzog H. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Develop. 2002b;16:1077–1088. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999;368:143–147. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Li J, Dahlström A, Theodorsson E, Ceccatelli S, Decosterd I, Pedrazzini T, Hökfelt T. Deletion of the neuropeptide Y Y1 receptor affects pain sensitivity, neuropeptide transport and expression, and dorsal root ganglion neuron numbers. Neuroscience. 2006;140:293–304. doi: 10.1016/j.neuroscience.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Simonds VM, Whiffen VE. Are gender differences in depression explained by gender differences in co-morbid anxiety? J Affect Disord. 2003;77:197–202. doi: 10.1016/s0165-0327(02)00113-1. [DOI] [PubMed] [Google Scholar]
- Stam R. PTSD and stress sensitisation: a tale of brain and body. Part 2: animal models. Neurosci Biobehav Rev. 2007;31:558–584. doi: 10.1016/j.neubiorev.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Stam R, Croiset G, Bruijnzeel AW, Visser TJ, Akkermans LM, Wiegant VM. Sex differences in long-term stress-induced colonic, behavioural and hormonal disturbances. Life Sci. 1999;65:2837–2849. doi: 10.1016/s0024-3205(99)00553-6. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berlin) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Svensson P, Wiklund L, Sommer W, Ekman R, Heilig M. Suppressed neuropeptide Y (NPY) mRNA in rat amygdala following restraint stress. Regul Pept. 1998;75-76:247–254. doi: 10.1016/s0167-0115(98)00075-5. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Carlsson K, Ekman R, Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999;10:3003–3007. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- Wittmann W, Loacker S, Kapeller I, Herzog H, Schwarzer C. Y1-receptors regulate the expression of Y2-receptors in distinct mouse forebrain areas. Neuroscience. 2005;136:241–250. doi: 10.1016/j.neuroscience.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Stress-induced behavioral responses and multiple opioid systems in the brain. Behav Brain Res. 1995;67:133–145. doi: 10.1016/0166-4328(94)00150-e. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]