Abstract
Objectives
To characterize the dynamics of the pituitary-adrenal interaction during the course of coronary artery bypass grafting (CABG) both on and off pump. Since our data pointed to a major change in adrenal responsiveness to ACTH we used a reverse translation approach to investigate the molecular mechanisms underlying this change in a rat model of critical illness.
Design
Clinical studies: Prospective observational study
Animal studies: Controlled experimental study
Setting
Clinical studies: Cardiac surgery operating rooms and critical care units
Animal studies: University research laboratory
Subjects
Clinical studies: Twenty, male patients
Animal studies: Adult, male Sprague-Dawley rats.
Interventions
Clinical studies: Coronary artery bypass graft - both on and off pump
Animal studies: Injection of either lipopolysaccharide (LPS) or saline (controls) via a jugular vein cannula
Measurements and Results
Clinical studies: Blood samples were taken for 24 hours from placement of the first venous access. Cortisol and ACTH were measured every 10 and 60 minutes respectively, and corticosteroid binding globulin (CBG) was measured at the beginning and end of the 24 hour period and at the end of operation. There was an initial rise in both levels of ACTH and cortisol to supra-normal values at around the end of surgery. ACTH levels then returned towards pre-operative values. Ultradian pulsatility of both ACTH and cortisol was maintained throughout the peri-operative period in all individuals. The sensitivity of the adrenal gland to ACTH increased markedly at around 8 hours after surgery maintaining very high levels of cortisol in the face of ‘basal’ levels of ACTH. This sensitivity began to return towards pre-operative values at the end of the 24-hour sampling period.
Animal studies: Adult, male Sprague-Dawley rats were either given lipopolysaccharide (LPS) or sterile saline via a jugular vein cannula. Hourly blood samples were subsequently collected for ACTH and corticosterone measurement. Rats were sacrificed 6 hours after the injection and the adrenal glands were collected for measurement of StAR, SF-1 and DAX1 mRNA and protein using RTqPCR and Western immunoblotting, respectively. Adrenal levels of the ACTH receptor (MC2R) mRNA and its accessory protein (MRAP) were also measured by RTqPCR. In response to LPS, rats showed a pattern of ACTH and corticosterone that was similar to patients undergoing CABG. We were also able to demonstrate increased intra-adrenal corticosterone levels and an increase in StAR, SF-1 and MRAP mRNAs and StAR protein, and a reduction in DAX1 and MC2R mRNAs, 6h after LPS injection.
Conclusions
Severe inflammatory stimuli activate the HPA axis resulting in increased steroidogenic activity in the adrenal cortex and an elevation of cortisol levels in the blood. Following CABG there is a massive increase in both ACTH and cortisol secretion. Despite a subsequent fall of ACTH to basal levels, cortisol remains elevated and co-ordinated ACTH - cortisol pulsatility is maintained. This suggested that there is an increase in adrenal sensitivity to ACTH, which we confirmed in our animal model of immune activation of the HPA axis. Using this model we were able to show that this increased adrenal sensitivity results from changes in the regulation of both stimulatory and inhibitory intra-adrenal signaling pathways. Increased understanding of the dynamics of normal HPA responses to major surgery will provide us with a more rational approach to glucocorticoid therapy in critically ill patients.
Keywords: Glucocorticoid hormones, cortisol, ACTH, Cortisol binding globulin, Critical illness, Off pump surgery
Introduction
Glucocorticoid hormones are an essential part of the homeostatic response to major surgery and critical illness. Inappropriately low(1, 2) and excessively high(3, 4) levels can result in raised morbidity and mortality. Although the HPA axis itself is controlled by a feedback mechanism from circulating glucocorticoids, it is also regulated by external stimuli relaying information about the time of day and the presence of stressful stimuli(5). Circadian signals from the hypothalamic suprachiasmatic nucleus ensure cortisol is secreted in a diurnal rhythm, which is itself made up from an ultradian rhythm of discrete pulses(6-8). Although this pulsatile activity has long been considered to originate from a hypothalamic ‘pulse generator’(9), more recent work has shown that a negative feedback loop in the pituitary-adrenal system provides a mechanism for generating systems-level ultradian oscillations in ACTH and glucocorticoids (10, 11).
Glucocorticoid levels oscillate not only in the blood, but also within tissues(12-14). It is clear that glucocorticoid receptor-mediated signal transduction has adapted to read these pulsatile glucocorticoid signals. Pulses of corticosterone cause glucocorticoid responsive genes to ‘pulse’ in phase with the steroid and pulsatile and constant levels of glucocorticoids yield vastly different transcriptional responses(15).
The dynamics of HPA activity in major surgery and critical illness are poorly understood, and little is known about the ultradian pulsatility of endogenous ACTH and glucocorticoid secretion in the period following major surgery and in critical illness.
Our study was designed to characterize the ultradian rhythm of cortisol during and after major cardiac surgery, and discern how control of the HPA axis may alter during this time. Since our clinical data suggested a major change in adrenal sensitivity following CABG, we used a rat model of inflammatory stress to investigate the mechanism underlying these changes.
Materials and Methods
Clinical studies: participants, procedures and study design
The study was approved by a National Health Service (NHS) Research Ethics Committee (Ref: 11/H0107/9). After written, informed consent; twenty male patients admitted for elective CABG were included. Exclusion criteria were: emergency operations, redo surgery, etomidate use, myocardial infarction within the last month, concomitant procedures, left ventricular ejection fraction <30%, a past history of adrenal/pituitary disease and use of glucocorticoids within 6 weeks.
There is no valid methodology to perform power calculations on expected changes in ultradian patterns of HPA activity. However, in a study of patients with obstructive sleep apnea, our group was able to show highly significant changes in pulse characteristics before and after CPAP therapy in a sample size of ten patients(16).
Surgical, anesthetic and cardiopulmonary bypass (CPB) procedures followed established local protocols(17, 18). Blood was sampled for 24 hours from the in situ vascular catheters placed immediately before induction of anesthesia. To control for the impact of the circadian hormonal variation in cortisol levels between patients, only cases that were first on the days’ operating schedule were used in this study.
Coronary artery bypass grafting (CABG) can be performed with and without the use of CPB (“on-pump” and “off-pump” surgery respectively) according to preference of the operating surgeon. This study was designed to randomize patients to on- or off-pump surgery. However, restrictions imposed by the ethics committee made this impracticable. Therefore the study was continued as observational.
Blood samples were collected at 10-minute intervals for cortisol measurement and hourly for ACTH measurement. Blood samples for corticosteroid-binding globulin (CBG) measurement were collected at the start of the 24 hour sampling period, at skin closure and at the end of the 24 hour period.
Human cortisol samples were collected in BD vacutainer SST Advance tubes (Becton, Dickinson and Company, Oxford. UK) and were processed immediately after centrifugation. Samples for ACTH were collected in chilled 1ml EDTA tubes and stored on ice for a maximum of 30 minutes before centrifugation at 4°C and then stored at −80°C until assay. Human total cortisol and ACTH were measured by solid phase, chemo-luminescent enzyme linked immunoassay (ECLIA) using the Cobas e602 modular analyzer (Roche Diagnostics Ltd, West Sussex, UK). Measuring limits for the cortisol assay were 0.5 – 1750nmol/L (intra- and inter-assay co-efficients of variability (COV): 1.5 – 1.7% and 1.8 – 2.8% respectively) and for the ACTH assay were 1.0 – 2000 pg/ml (intra- and inter-assay COV: 0.6 – 2.7% and 3.5 – 5.4% respectively). CBG samples were collected in BD vacutainer SST Advance tubes (Becton, Dickinson and Company, Oxford. UK) and stored at −80°C after centrifugation until assay. CBG was assayed using a commercially available 125Iodine radioimmunoassay (RIA) kit (DiaSource, Louvain-La-Neuve, Belgium).
Experimental studies: animals, procedures and study design
All experiments were conducted on adult (250-300 g) male Sprague–Dawley rats (Harlan Laboratories, Inc., Blackthorn, UK). Animals were given a 1-week acclimatization period prior to the start of the experiments. During this period, animals were maintained under a 14 h light, 10 h dark schedule (lights on at 0500h) and they were housed four per cage with ad libitum access to food and water. All animal procedures were conducted in accordance with Home Office guidelines and the UK Animals (Scientific Procedures) Act, 1986.
Animals were anaesthetized with isoflurane and an indwelling cannula was inserted in the right jugular vein as described previously (19). All experiments started at 0900h and were performed 5-7 days after surgery.
To investigate the time course effect of lipopolysaccharide (LPS) on ACTH and corticosterone levels, rats were given either LPS (Escherichia coli; 055:B5; (Sigma, Dorset. UK) at a dose of 25μ/rat in 0.1 ml of sterile saline or sterile saline (0·1 ml/rat) injected via the jugular vein cannula (n=6/group). Serial blood samples were collected manually via the jugular vein cannula before and after LPS/saline injection at 1-hour intervals for 12 hours. After collection of each sample, an equivalent volume of sterile, heparinised saline was injected so that the total blood volume remained unchanged throughout the experiment. Immediately after collection, blood samples were stored on ice in eppendorf tubes containing EDTA (0·5M; pH 7·4) and Trasylol (Aprotinin, 500000KIU/ml, Roche). Plasma was separated by centrifugation and then stored at −80°C until processed for ACTH and corticosterone measurement.
To investigate the effect of LPS on corticosterone levels and steroidogenic protein expression in the adrenal cortex, rats were injected with either LPS (n=5) or saline (n=6) as described above and sacrificed 6 hours after injection (1500h); an untreated control group (n=5) was sacrificed at 0900h. Rats were overdosed with 0·2ml sodium pentobarbitone (Euthatal, 200mg/ml; Merial, Harlow, UK), the adrenal glands collected and the inner zones (comprising the zona fasciculata and zona reticularis of the cortex, and the adrenal medulla) were separated from the outer zone (containing the zona glomerulosa and the capsula). Individual inner zones were immediately frozen until processing for isolation of RNA for RT-qPCR (left adrenal), protein extraction for Western immunoblotting, and corticosterone measurement (right adrenal) as previously described(6, 20). Messenger RNA (mRNA) levels of StAR (steroidogenic acute regulatory protein), SF-1 (steroidogenic factor 1), DAX1 (dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1), MC2R (melanocortin type 2 receptor) and MRAP (MC2R accessory protein) were measured. In addition, protein levels of StAR, SF-1 and DAX1 were also measured.
To investigate the time-course effect of a high dose of ACTH on plasma corticosterone levels, rats were given 3 injections of ACTH (2 ug/kg, sc; Synachten Depot, Alliance Pharma plc, Cheltenam, UK ,) at 35-minute intervals via an implanted subcutaneous cannula (n=4/group). Serial blood samples were collected manually via the jugular vein cannula at 1-hour intervals for 6 hours before and after ACTH injections. Blood samples were processed for ACTH and corticosterone measurements as described above.
Data analysis: algorithm for selecting and analyzing concordant ACTH and cortisol pulses
A new algorithm was written to allow comparison of the ACTH and cortisol profiles that took into consideration the different sampling frequency of ACTH and cortisol. This allowed us to calculate the pulse amplitudes of corresponding ACTH and cortisol pulses. The ratio of the cortisol pulse height to ACTH pulse height was then calculated to give a marker of the sensitivity of the adrenals to ACTH.
Key assumptions for this algorithm are that: (i) a change in ACTH precedes a change in CORT; and (ii) increases in ACTH are followed by increases in CORT. The algorithm consists of the following steps:
- Select ACTH pulse and compute ACTH pulse amplitude
-
-Find two consecutive ACTH minima (ACTHmin1 and ACTHmin2), where an ACTH minimum, ACTHmin, is defined as follows:
- if ACTHt-1 > ACTHt < ACTHt+1 then ACTHt = ACTHmin.
-
-Find maximum value of ACTH between ACTHmin1 and ACTHmin2 (ACTHmax)
-
-ACTHpulse amp = ACTHmax – ACTHmin1
-
-
- Compute amplitude of associated CORT pulse
-
-Find minimum value of CORT (CORTmin) between ACTHmin1 and ACTHmax
-
-Find maximum value of CORT (CORTmax) between CORTmin and ACTHmin2
-
-CORTpulse amp = CORTmax - CORTmin
-
-
- Compute ratio of CORTpulse amplitude to ACTH pulse amplitude
-
-Ratio (CORT:ACTH) = CORTpulse amp / ACTHpulse amp
-
-
Statistical analysis: clinical studies
The area under the curve (AUC) of cortisol was analyzed using a bespoke program written in MatLab (Natick, Massachusetts, USA). Statistical analysis was carried out using PASW statistics 18 (IBM Inc. New York, USA) and all graphs were drawn in GraphPad Prism v5.0 (GraphPad Software Inc. La Jolla. CA)
Where multiple patients were used for analysis, times were converted to their corresponding sample number. Although the clock times differed slightly, this allowed us to compare similar stages in the peri-operative process.
The difference in mean AUC for cortisol between the two surgical techniques was analyzed using a t-test. Geometric means and asymmetric confidence intervals were calculated for Cortisol:ACTH ratios. No statistical test was used to analyze the change in ratio with time. CBG values were analyzed using repeated measures ANOVA (analysis of variance). The Bonferroni correction was applied post-hoc due to the multiple group comparison.
Statistical analysis: experimental studies
All statistical analyses were performed using SPSS version 19 (SPSS Inc., Chicago, IL). A repeated measures, two-way ANOVA was used to analyze the time course of plasma ACTH and corticosterone in response to LPS (time and LPS effect). A repeated measures, one-way ANOVA was used to analyze the time course of plasma ACTH and corticosterone in response to ACTH (time effect). When a significant effect of time, LPS, or their interaction (LPS injection experiment) or a significant effect of time (ACTH injection experiment) was observed, the Bonferroni post-hoc test was used to compare ACTH and corticosterone levels before and after LPS/saline or ACTH injection. Adrenal corticosterone, mRNA and protein data were analysed using one-way ANOVA, followed by Fisher’s least significant difference (LSD) post hoc test to compare differences between experimental groups. Statistical significance was set at p = 0.05.
Results
Clinical studies
Of the twenty patients recruited; 12 had “off-pump” surgery and 8 “on-pump” surgery. Demographic and operative data for all patients are shown in Table 1. All needle to skin times (i.e. the first sample in each case) were between 0800 and 0900h.
Table 1. Demographic and operative data for all patients.
| Mean | SD | |
|---|---|---|
| Age (years) | 64·6 | 7·26 |
| Operative Time (mins) | 184·5 | 48·7 |
| No. of Distal Anastamoses | 2·4 | 0·6 |
| Weight | 84·5 | 12·6 |
| Height | 174·8 | 4·7 |
| Haemoglobin (g/dl) | 14·3 | 1·2 |
| Creatinine (μmol/l) | 94·0 | 14·2 |
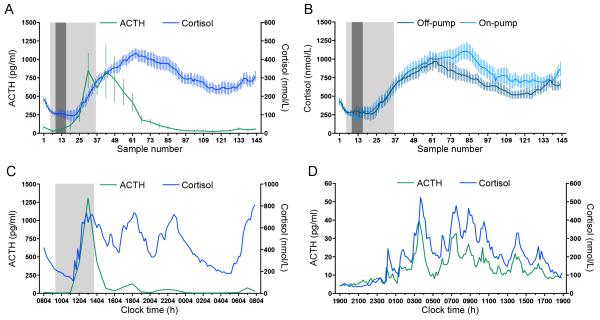
Across the 24-hour perioperative period, both the mean level of cortisol and ACTH for the whole group rose following surgery. While ACTH then began to return towards baseline values, cortisol remained elevated for the whole sampling period (Figure 1A). Throughout this time, individual ACTH and cortisol profiles remained pulsatile (Figure 1C).
Figure 1. Changes in cortisol and ACTH levels throughout the 24-hour perioperative period of cardiac surgery.
(A) Group mean±SEM cortisol and ACTH. All sampling (ie the first sample in every case) started between 0800 and 0900h. (B) Mean ±SEM 24-hour cortisol profile from patients undergoing CABG using the off-pump or the on-pump technique. All off-pump surgeries were performed between sample 5 and sample 35; all on-pump surgeries were performed between samples 5 and samples 36. In A and B: Light grey area, period during which some patients were undergoing surgery. Dark grey area: period during which all patients were undergoing surgery. (C) Individual 24-hour ACTH and cortisol profile of a patient undergoing off-pump CABG. After the initial surge of ACTH and cortisol, both ACTH and cortisol continue to pulse. However, while both the absolute values of ACTH and the pulse amplitude are reduced, the cortisol levels remains elevated. Light grey area: period during which the patient was undergoing surgery (0919-1349-h). (D) Individual 24-hour ACTH and cortisol profile of a healthy volunteer. ACTH and cortisol both display a tightly correlated ultradian rhythm.
The mean time course of cortisol levels for both on-pump and off-pump patients is shown in Figure 1B. Although there was a trend for a further late increase in cortisol in the on-pump patients, there was no statistically significant difference in total AUC of cortisol between on- and off-pump surgery during the 24 hour period (p=0·25).
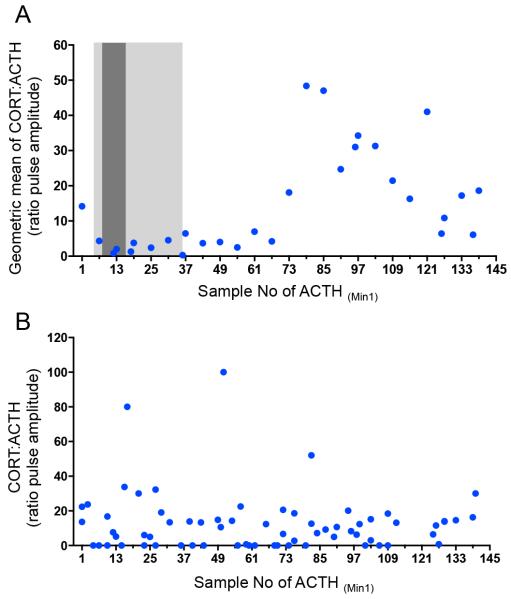
The relationship between ACTH and cortisol pulses was further investigated using a pulse-analysis algorithm (see Methods). This showed that the cortisol:ACTH pulse amplitude ratio increased approximately 6-8 hours post-operatively (12 hours into the 24-hour study), returning towards baseline by the end of the 24 hour period (Figure 2A).
Figure 2. Relationship between the ACTH and cortisol pulse amplitudes throughout the 24-hour sampling period.
(A) Geometric mean of the ratio of cortisol pulse amplitude to ACTH pulse amplitude throughout the 24-hour peri-operative period of CABG surgery. Light grey area: period during which some patients were undergoing surgery. Dark grey area: period during which all patients were undergoing surgery. The ratio of cortisol pulse amplitude to ACTH pulse amplitude changes around 6-8 hours after the end of surgery, such that there is a greater change in cortisol pulse amplitude compared to ACTH pulse amplitude. This begins to return towards intra- and early-postoperative values by the end of the 24-hour period. (B) Ratio of cortisol pulse amplitude to ACTH pulse amplitude in two healthy volunteers. No change in ratio is seen across the 24-hour period. Clock times are normalized to sample numbers with sample 1 being equivalent to 0800.
To ascertain whether this change in the ratio observed following surgery was due to the surgery itself and not the result of circadian variation in the relationship between ACTH and cortisol, we also computed the ratio over a 24-hour period in two age- and sex-matched healthy individuals under non-stressed conditions (Figure 1D). In contrast to our patients, we observed no change in their cortisol:ACTH ratios throughout the day.
Post-operative values of CBG were missing in two cases and were excluded from analysis. We found a significant effect of the surgery on CBG levels (ANOVA: F(2,53)=12·40 2; P<0·0001, Figure 3), with lower CBG levels both immediately post-operatively (P<0.0001) and at the end of the 24-hour sampling period(P=0.0016) compared to pre-operative levels. There was no difference between CBG levels at the two post-operative time points (P=0·883).
Figure 3. Mean ± SEM CBG levels in patients undergoing CABG surgery measured before the operation (Pre-op), immediately after the end of the operation (Post-op) and 24-hours after the start of the sampling (24h).
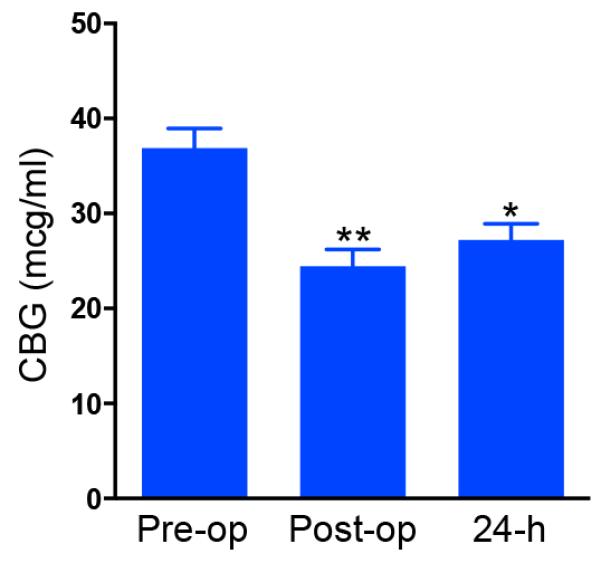
*P<0.005; **P<0.0001, compared to pre-op.
Experimental studies
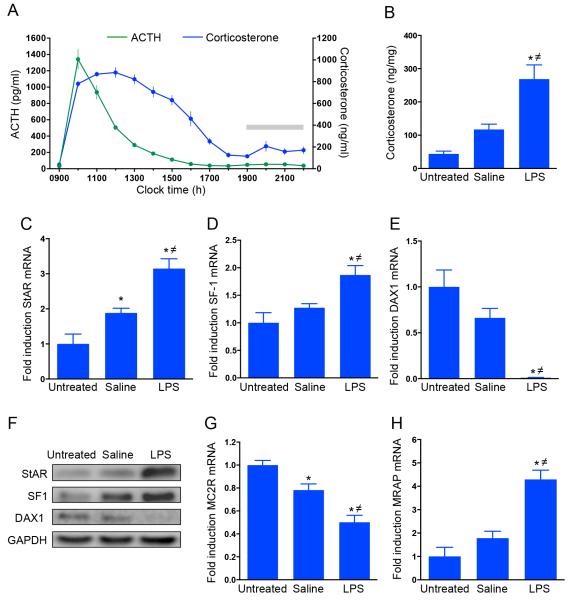
The time course of plasma ACTH and corticosterone levels in rats injected with LPS is shown in Figure 4A. Two-way ANOVA with repeated measures analysis showed that there was a significant effect of time, LPS and their interaction in both ACTH (time: F(1,13)=104.9 ;P<0.0001; LPS: F(1,13)=272.1; P<0.0001; time × LPS: F(1,13) = 105.1; P<0.0001) and corticosterone (time: F(1,13)=76.7; P<0.0001; LPS: F(1,13)=135.2; P<0.0001; time × LPS: F(1,13)=52.3; P<0.0001). The effect of LPS on ACTH was observed between 1000h and 1300h (1 and 4 hours after the injection, respectively; P<0·002), whereas a more prolonged effect of LPS on corticosterone was observed, with levels significantly higher than basal between 1000h and 1700h (1 and 8 hours after the injection, respectively; P<0.05). No significant changes in ACTH levels were observed in control rats injected with saline, while a significant variation in corticosterone was observed at 1700h and 1800h, and 2200h (P<0.05; data not shown). This effect is likely to be due to the normal circadian variation of corticosterone, as previously shown(20).
Figure 4. Effect of LPS on plasma ACTH and corticosterone, and on adrenal corticosterone and steroidogenic protein expression in the rat.
(A) Time course of effect of LPS on plasma ACTH and corticosterone. Rats were injected with LPS at 0900h and blood samples were manually collected prior to and after the injection via an indwelling cannula inserted in the jugular vein. Two-way ANOVA repeated measure showed a significant effect of LPS on both ACTH and corticosterone plasma levels. While ACTH returned to basal levels at 1400h, corticosterone levels remained elevated for a further 4 hours and returned to basal value at 1800h (see results for statistic). Grey bar indicates the dark period (1900- 0500h). (B) LPS injection induced an increase in intra-adrenal corticosterone levels, as measured 6 hours after the injection. This effect was paralleled by changes in steroidogenic gene expression, as measured using RTqPCR, including an increase in StAR mRNA (C), and SF-1 mRNA (D), and a decrease in DAX1 mRNA (E). LPS-induced increase in StAR mRNA was associated with an increase in StAR protein levels (F), as assessed by Western immunoblotting. Acute administration of LPS decreased MC2R mRNA levels (G), whereas increased MRAP mRNA levels (H). Grey bar indicates dark period (1900-0500h).
*P<0.05, significantly different from untreated control. ≠P<0.05, significantly different from saline.
The effect of LPS treatment on adrenal corticosterone levels and on steroidogenic gene expression and protein levels is shown in Figure 4B-H. There was a significant effect of LPS on adrenal corticosterone levels (ANOVA: F(2,13)=15·487; P=0·0003; Fig. 4B), with levels higher in LPS-treated rats compared to both untreated controls (P<0·0001) and saline-treated rats (P=0·0029).
The effect of LPS on corticosterone levels was associated with changes in steroidogenic gene expression in the adrenal cortex. There was a significant overall effect on StAR mRNA (F (2,13) =19·602; P=0·00012; Figure 4C), with higher levels in LPS-treated rats compared to untreated controls (P< 0·0001) and saline-treated rats (P=0·0029). Consistent with the observed increase in corticosterone levels, StAR mRNA was also increased in saline treated rats (P=0.03), in accordance to circadian changes in the steroidogenic pathway(20). The changes in StAR gene expression were paralleled by significant changes in SF-1 gene expression (F(2,13)=8·649; P=0·0041; Figure 4D), with SF-1 mRNA levels significantly higher in LPS-treated rats compared to both untreated controls (P=0·0014) and saline-treated rats (P=0·016). There were also changes in DAX-1 gene expression (F(2,13)=20·384; P=0·0001; Figure 4E). Low levels of DAX-1 mRNA were seen in LPS-treated rats compared to both untreated controls (P<0·0001) and saline-treated rats (P=0·0012).
The LPS-induced increase in StAR mRNA was also paralleled by changes in StAR protein levels (F(2,13)=17·17; P=0·00022) as confirmed by Western blot (Figure F). StAR levels were higher in LPS-treated rats compared to both untreated controls (P<0·0001) and saline-treated rats (P=0·002). Although a significant effect of LPS was observed on both SF-1 and DAX-1 mRRd, the translated changes in SF-1 and DAX-1 protein failed to reach significance (SF-1: F(2,13)=3·595; P=0·057; DAX1: F(2,13)=2·274; P=0·142).
Administration of LPS also induced significant changes in MC2R (F(2,13)=22·615; P<0·0001; Figure 4G) and MRAP (F(2,13)=22·135; P<0·0001; Figure 4H) gene expression. Specifically, lower MC2R mRNA levels were found in LPS-treated rats compared to untreated controls (P<0·0001) and saline-treated rats (P=0·0025). A decrease in MC2R mRNA was also observed in saline-treated rats (P=0,016), as a result of its circadian variation(20). In contrast, a significant increase in MRAP mRNA was found in LPS-treated rats compared to untreated controls (P<0·0001) and saline-treated rats (P<0·00034).
The time course of plasma ACTH and corticosterone levels in rats injected with ACTH is shown in Figure 5. One-way ANOVA with repeated measures analysis showed that there was a significant effect of time, in both ACTH (time: F (6,21)= 141.743; P<0.0001) and corticosterone (time: F (6,21)=33.805; P<0.0001). The effect of sc administration of ACTH depot on plasma ACTH and corticosterone was observed between 1000h and 1200h (1 and 3 hours after the injection, respectively; P<0.0001).
Figure 5. Time course of effects of sub cutaneous injections of ACTH depot on plasma ACTH and corticosterone.
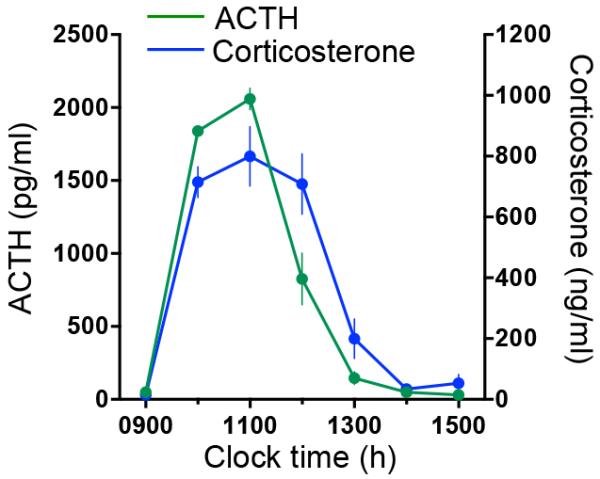
Rats were injected with ACTH at 0900h, 0935h and 1010h, and blood samples were manually collected via the jugular vein cannula at 1-hour intervals for 6 hours before and after ACTH injections. A repeated measures one-way ANOVA showed a significant effect of ACTH injection on both ACTH and corticosterone plasma levels. Both ACTH and corticosterone returned to basal levels at 1300h (see results for values).
Discussion
This study showed several changes in the pituitary-adrenal interaction in response to a major inflammatory stimulus in patients undergoing CABG. The large ACTH and consequent cortisol response occurred after, rather than during the surgical intervention. This suggests that the HPA response is secondary to inflammatory mediators rather than the anesthesia and operative activity directly. This time course fits well with the reported surge in circulating TNF-α, IL-1 and IL-6 that also remain raised for about 24 hours before returning to baseline(21-23).
Using high frequency blood sampling, we have shown not only that ACTH and cortisol pulsatility is maintained throughout the perioperative period, but that these pulses are concordant. Because ACTH and glucocorticoid oscillations emerge from the feedforward:feedback relationship between the pituitary and adrenal(10, 11) this implies that the pituitary-adrenal connection persists.
There was a marked sensitization of the adrenal response to each ACTH pulse after cardiac surgery. This was not seen in healthy individuals under non-stressed conditions. The subsequent fall in ACTH after the initial surge is consistent with the well-described glucocorticoid mediated feedback of endogenous glucocorticoids on ACTH secretion(24). This initial rise in ACTH is frequently seen after major surgery(25), but rarely seen in critical illness(26). This probably reflects the critically ill patients’ timing of presentation to healthcare services (and therefore blood sampling) after the initial stimulus.
We found changes in the cortisol:ACTH pulse amplitude ratio that when compared to other, non-surgical data analysed in the same way, suggest that adrenal sensitivity to ACTH is increased in the post-operative period. We have only sampled for the 24-hour peri-operative period and do not know how the pituitary-adrenal interaction changes with continued time after surgery. The alteration in the ratio of cortisol to ACTH suggests that other factors in addition to ACTH must play a role in regulating adrenal steroidogenesis in these conditions.
There are many mechanisms that may underlie the altered cortisol: ACTH pulse amplitude ratio. One possibility is that these patients have a reduced rate of peripheral conversion of circulating cortisol to cortisone as previously described in both critical illness(26) and cardiac surgery (27). Another possibility is that there is an increase in adrenal sensitivity to ACTH during the post-operative period. Potential candidates for this include the activity of the autonomic nervous system (28, 29). In addition, it is possible that the initial surge in ACTH increases the levels of steroidogenic proteins, thereby making the adrenal cortex more sensitive to subsequent ‘normal’ fluctuations in levels of ACTH.
The results from our clinical studies suggested that the major inflammatory stimulus associated with cardiac surgery resulted in a resetting of the gain of the adrenal cortical response to ACTH. To investigate the mechanism underlying the dissociation between the pituitary and adrenal observed in these patients, we used an animal model in which we could examine the adrenal steroidogenic response to a major systemic inflammatory stressor. We chose to use LPS stress in the rat. This results in the rapid release of cytokines (including Il-1β, IL-6 and TNF-α) (30), which are known to be potent secretagogues at multiple sites of the HPA axis(31-33). These changes show a similar time course to the increase in interleukins seen after cardiac surgery (34, 35). This model allowed us to study the effect of acute inflammatory stress not only on the dynamics of ACTH and corticosterone secretion, but also on the expression of the steroidogenic protein StAR (36, 37), which mediates the intra-mitochondrial transport of cholesterol (the rate limiting step in glucocorticoid synthesis) as well as other proteins mediating ACTH signaling within the adrenal, including MC2R (38, 39) and MRAP (40). LPS administration resulted in ACTH and corticosterone responses that are remarkably similar to our finding in the human patients; ACTH levels return to ‘normal’ soon after the stimulus, but corticosterone levels remain elevated further hours after this. Limitations on the amount of blood we could remove from the rats did not allow us to collect samples at sufficient frequency to assess whether pulsatile activity was maintained throughout this time.
To ascertain whether the increase of plasma corticosterone was predominantly due to increased synthesis or reduced clearance, we measured the intra-adrenal levels of corticosterone in the adrenals. They remained elevated 6 hours after the LPS administration; confirming that despite “normal” ACTH levels at this time, the adrenal was still synthesizing large amounts of corticosterone. This infers that there must be increased adrenal sensitivity to ACTH at this point and so to assess the mechanisms involved we measured the expression of steroidogenic proteins in the adrenal cortex including StAR, MC2R and MRAP, and the expression of proteins involved in their transcriptional regulation.
LPS markedly increased MRAP mRNA. Since MRAP regulates the surface expression and binding affinity of the MC2R for ACTH(39), this implies an LPS-mediated increase in ACTH signaling. Furthermore, LPS increased StAR mRNA and protein levels together indicating increased steroidogenic activity. The effects of LPS on StAR were paralleled by changes in the expression of genes encoding proteins that enhance (SF-1) and repress (DAX-1) StAR transcription, respectively(41, 42).
The mechanisms underlying this response are unclear. Interestingly, in contrast to rats injected with LPS, this increase in adrenal sensitivity was not observed in rats injected with a high dose of ACTH. This suggests that other factors, apart from ACTH must be responsible to these changes occurring at the level of adrenal steroidogenic pathway. Indeed, it is known that both circulating and intra-adrenal cytokines can affect glucocorticoid synthesis by both potentiating the effects of ACTH or by direct effects on steroidogenic protein synthesis(34, 35, 43, 44). In addition to the systemic effect of LPS-induced cytokines on adrenal sensitivity, cytokines and their receptors are expressed locally in the rat adrenal in response to LPS(45, 46). This suggests that intra-adrenal cytokines may be responsible for the observed increase in steroidogenic activity here. Although splanchnic nerve activity can also alter adrenal sensitivity to ACTH, this seems a less likely explanation for our findings because splanchnic nerve activity does not regulate adrenal StAR(47).
Another factor to be considered in respect of glucocorticoid signaling is CBG. In our study, CBG levels fell by around 30-50%. This has previously been shown to occur in both adult(48) and pediatric(49) cardiac surgery and is most likely due to haemodilution(48). This will result in elevated levels of free and therefore biologically active cortisol(50). In view of the persistently raised and pulsatile levels of total cortisol in our patients, the reduced CBG levels will be saturated at lower levels of total cortisol (saturation of CBG in normal subjects occurs at around 400-500 nmol/L cortisol), resulting in widely oscillating levels of free cortisol with potent effects on GR signaling. Core body temperature, pH and neutrophil activity also change throughout the peri-operative process, and fluctuations in any of these will also alter the tissue delivery of cortisol(51-53).
We have not reported free cortisol levels in our patients, as derived methods such as Coolens’(54) do not hold true in the dynamic conditions of the peri-operative period. The reasons for this are firstly, Coolens ignores saturation of CBG with cortisol (that occurs at ‘normal’ CBG levels of around 400nmol/L) - which will be an important aspect in patients with very high cortisol levels. Secondly, Coolens uses a population mean for albumin that will not apply to patients in the cardiac surgical period because albumin levels change rapidly across this timescale. Thirdly, due to the pulsatile nature of cortisol and changing CBG levels, we would have required large volumes of blood to accurately estimate free cortisol – operating surgeons were unhappy to accept this.
Although cardiac surgery with and without cardiopulmonary bypass has often been used as a model for the inflammatory response of critical illness, we must be careful not to extrapolate our findings too far. Critical illness, particularly sepsis is an ongoing stimulus as opposed to the point stimulus of cardiac surgery In the same way, cardiac surgery and LPS injection are both point insults causing severe inflammatory stress(21-23, 55).
Endeavors to classify who is relatively deficient in glucocorticoids at the time of major surgery and critical illness have stalled. Despite multiple attempts to identify these patients using static measures of adrenal function(56-61), none have been shown to be functionally useful at detecting this subset of the population(62). Our study would appear to confirm this; the dynamic, pulsatile nature of the HPA axis output after cardiac surgery shows that point measurements of cortisol after an acute inflammatory stimulus are not useful. The pulse amplitude of cortisol in this context may be as much as 600nmol/L within 2 hours and the point measurement may be either at the peak or nadir of this pulse. Therefore a single measurement of cortisol does not inform us about the overall levels of cortisol, or indeed what the cortisol level is one hour later. The fact that the adrenal sensitivity to ACTH is changing also means that the premises on which the synthetic ACTH test is based do not hold true in this context.
Hormone pulsatility in the chronic setting has significant clinical implications. Not only do patients who are taking non-pulsatile replacement glucocorticoids complain of weariness, fatigue and stress intolerance(63), but hypoadrenal patients who are receiving cortisol replacement have double the mortality of the general population of the same age group(64). We do not know what the implications of glucocorticoid pulsatility are in the acute setting. However, current international guidelines for glucocorticoid therapy in critical illness and sepsis(65) are that hydrocortisone should be given by continuous infusion. The pulsatility seen in the individual cortisol profiles throughout our study clearly demonstrate that this is not a normal physiological regimen and how this manifests in patient response is unknown.
In summary, this study highlights the marked changes in HPA activity that occur following a major inflammatory stimulus. In contrast to previous reports of a “disconnection” between the pituitary and adrenal glands(25, 48, 66), our rapid sampling technique in humans reveals maintenance of the interaction between ACTH and cortisol ultradian secretory activity. This was associated with an increase in adrenal sensitivity to ACTH. In a rodent model, we have shown that the initial high levels of glucocorticoid after a major inflammatory stimulus are predominately related to increased de novo synthesis rather than the reduced metabolism previously reported(27) and we suggest that the increased adrenal sensitivity to ACTH occurs due to a combination of increased expression of MRAP, the ACTH receptor accessory protein, and increased steroidogenic protein StAR.
Acknowledgements
We are grateful to Mr George Asimakopoulos (Consultant Cardiac Surgeon) for allowing us to use his patients in this study.
Funding: This work was supported by the British Heart Foundation (BG, GDA), the Medical Research Council (SLL, FS, GR, JJW, ZZ), the Wellcome Trust (SLL, YK,) and the National Institute for Health Research (NIHR) Biomedical Research Unit in Cardiovascular Disease at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol.
Role of the funding source: The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Data from this study were presented in part at the 34th International Symposium on Intensive Care and Emergency Medicine. Brussels, Belgium. March 2014.
Copyright form disclosures: Dr. Gibbison received support for article research from the Wellcome Trust. His institution received grant support from the British Heart Foundation and the National Institute of Health Research. Dr. Kershaw received support for article research from the Wellcome Trust. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Declaration of Interests: BG - None; FS - None; JJW - None; GMR - None; KS - None; YK - None; ZZ - None; DH - None; GDA - None; SLL - None.
References
- 1.Melby JC, Spink WW. Comparative studies on adrenal cortical function and cortisol metabolism in healthy adults and in patients with shock due to infection. J Clin Invest. 1958;37(12):1791–1798. doi: 10.1172/JCI103772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spink WW. ACTH and adrenocorticosteroids as therapeutic adjuncts in infectious diseases. N Engl J Med. 1957;257(20):979–983. doi: 10.1056/NEJM195711142572006. [DOI] [PubMed] [Google Scholar]
- 3.Bone RC, Fisher CJ, Jr., Clemmer TP, et al. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317(11):653–658. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- 4.Hinshaw LP, Young P, Sprung E, Shatney C, Sheagran C, Wilson J, Haakenson M, The Veterans Administration Systemic Sepsis Cooperative Study Group Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med. 1987;317(11):659–665. doi: 10.1056/NEJM198709103171102. [DOI] [PubMed] [Google Scholar]
- 5.Lightman SL, Conway-Campbell BL. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat Rev Neurosci. 2010;11(10):710–718. doi: 10.1038/nrn2914. [DOI] [PubMed] [Google Scholar]
- 6.Spiga F, Waite EJ, Liu Y, et al. ACTH-dependent ultradian rhythm of corticosterone secretion. Endocrinology. 2011;152(4):1448–1457. doi: 10.1210/en.2010-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veldhuis JD, Iranmanesh A, Lizarralde G, et al. Amplitude modulation of a burstlike mode of cortisol secretion subserves the circadian glucocorticoid rhythm. Am J Physiol. 1989;257(1 Pt 1):E6–14. doi: 10.1152/ajpendo.1989.257.1.E6. [DOI] [PubMed] [Google Scholar]
- 8.Waite EJ, McKenna M, Kershaw Y, et al. Ultradian corticosterone secretion is maintained in the absence of circadian cues. Eur J Neurosci. 2012 doi: 10.1111/j.1460-9568.2012.08213.x. [DOI] [PubMed] [Google Scholar]
- 9.Mershon JL, Sehlhorst CS, Rebar RW, et al. Evidence of a corticotropin-releasing hormone pulse generator in the macaque hypothalamus. Endocrinology. 1992;130(5):2991–2996. doi: 10.1210/endo.130.5.1572307. [DOI] [PubMed] [Google Scholar]
- 10.Walker JJ, Spiga F, Waite E, et al. The origin of glucocorticoid hormone oscillations. PLoS Biol. 2012;10(6):e1001341. doi: 10.1371/journal.pbio.1001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker JJ, Terry JR, Lightman SL. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc Biol Sci. 2010;277(1688):1627–1633. doi: 10.1098/rspb.2009.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhake RC, Leendertz JA, Linthorst AC, et al. Automated 24-hours sampling of subcutaneous tissue free cortisol in humans. J Med Eng Technol. 2013;37(3):180–184. doi: 10.3109/03091902.2013.773096. [DOI] [PubMed] [Google Scholar]
- 13.Droste SK, de Groote L, Atkinson HC, et al. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149(7):3244–3253. doi: 10.1210/en.2008-0103. [DOI] [PubMed] [Google Scholar]
- 14.Trifonova ST, Gantenbein M, Turner JD, et al. The use of saliva for assessment of cortisol pulsatile secretion by deconvolution analysis. Psychoneuroendocrinology. 2013;38(7):1090–1101. doi: 10.1016/j.psyneuen.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Stavreva DA, Wiench M, John S, et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009;11(9):1093–1102. doi: 10.1038/ncb1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henley DE, Russell GM, Douthwaite JA, et al. Hypothalamic-pituitary-adrenal axis activation in obstructive sleep apnea: the effect of continuous positive airway pressure therapy. J Clin Endocrinol Metab. 2009;94(11):4234–4242. doi: 10.1210/jc.2009-1174. [DOI] [PubMed] [Google Scholar]
- 17.Ascione R, Narayan P, Rogers CA, et al. Early and midterm clinical outcome in patients with severe left ventricular dysfunction undergoing coronary artery surgery. Ann Thorac Surg. 2003;76(3):793–799. doi: 10.1016/s0003-4975(03)00664-7. [DOI] [PubMed] [Google Scholar]
- 18.Pitsis AA, Angelini GD. Off pump coronary bypass grafting of the circumflex artery. Eur J Cardiothorac Surg. 1999;16(4):478–479. doi: 10.1016/s1010-7940(99)00292-4. [DOI] [PubMed] [Google Scholar]
- 19.Spiga F, Harrison LR, Wood SA, et al. Effect of the glucocorticoid receptor antagonist Org 34850 on basal and stress-induced corticosterone secretion. J Neuroendocrinol. 2007;19(11):891–900. doi: 10.1111/j.1365-2826.2007.01605.x. [DOI] [PubMed] [Google Scholar]
- 20.Park SY, Walker JJ, Johnson NW, et al. Constant light disrupts the circadian rhythm of steroidogenic proteins in the rat adrenal gland. Mol Cell Endocrinol. 2013;371(1-2):114–123. doi: 10.1016/j.mce.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 21.de Mendonca-Filho HT, Pereira KC, Fontes M, et al. Circulating inflammatory mediators and organ dysfunction after cardiovascular surgery with cardiopulmonary bypass: a prospective observational study. Crit Care. 2006;10(2):R46. doi: 10.1186/cc4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahat N, Zlotnick AY, Shtiller R, et al. Serum levels of IL-1, IL-6 and tumour necrosis factors in patients undergoing coronary artery bypass grafts or cholecystectomy. Clin Exp Immunol. 1992;89(2):255–260. doi: 10.1111/j.1365-2249.1992.tb06941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth-Isigkeit A, Borstel TV, Seyfarth M, et al. Perioperative serum levels of tumour-necrosis-factor alpha (TNF-alpha), IL-1 beta, IL-6, IL-10 and soluble IL-2 receptor in patients undergoing cardiac surgery with cardiopulmonary bypass without and with correction for haemodilution. Clin Exp Immunol. 1999;118(2):242–246. doi: 10.1046/j.1365-2249.1999.01050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dallman MF, Jones MT, Vernikos-Danellis J, et al. Corticosteroid feedback control of ACTH secretion: rapid effects of bilateral adrenalectomy on plasma ACTH in the rat. Endocrinology. 1972;91(4):961–968. doi: 10.1210/endo-91-4-961. [DOI] [PubMed] [Google Scholar]
- 25.Roth-Isigkeit AK, Schmucker P. Postoperative dissociation of blood levels of cortisol and adrenocorticotropin after coronary artery bypass grafting surgery. Steroids. 1997;62(11):695–699. doi: 10.1016/s0039-128x(97)00069-x. [DOI] [PubMed] [Google Scholar]
- 26.Boonen E, Vervenne H, Meersseman P, et al. Reduced Cortisol Metabolism during Critical Illness. N Engl J Med. 2013 doi: 10.1056/NEJMoa1214969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogeser M, Felbinger TW, Roll W, et al. Cortisol metabolism in the postoperative period after cardiac surgery. Exp Clin Endocrinol Diabetes. 1999;107(8):539–546. doi: 10.1055/s-0029-1232563. [DOI] [PubMed] [Google Scholar]
- 28.Edwards AV, Jones CT. The effect of splanchnic nerve section on the sensitivity of the adrenal cortex to adrenocorticotrophin in the calf. J Physiol. 1987;390:23–31. doi: 10.1113/jphysiol.1987.sp016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engeland WC, Gann DS. Splanchnic nerve stimulation modulates steroid secretion in hypophysectomized dogs. Neuroendocrinology. 1989;50(2):124–131. doi: 10.1159/000125211. [DOI] [PubMed] [Google Scholar]
- 30.Givalois L, Dornand J, Mekaouche M, et al. Temporal cascade of plasma level surges in ACTH, corticosterone, and cytokines in endotoxin-challenged rats. Am J Physiol. 1994;267(1 Pt 2):R164–170. doi: 10.1152/ajpregu.1994.267.1.R164. [DOI] [PubMed] [Google Scholar]
- 31.Perlstein RS, Whitnall MH, Abrams JS, et al. Synergistic roles of interleukin-6, interleukin-1, and tumor necrosis factor in the adrenocorticotropin response to bacterial lipopolysaccharide in vivo. Endocrinology. 1993;132(3):946–952. doi: 10.1210/endo.132.3.8382602. [DOI] [PubMed] [Google Scholar]
- 32.Rivier C, Chizzonite R, Vale W. In the mouse, the activation of the hypothalamic-pituitary-adrenal axis by a lipopolysaccharide (endotoxin) is mediated through interleukin-1. Endocrinology. 1989;125(6):2800–2805. doi: 10.1210/endo-125-6-2800. [DOI] [PubMed] [Google Scholar]
- 33.Sapolsky R, Rivier C, Yamamoto G, et al. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238(4826):522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 34.Judd AM, Call GB, Barney M, et al. Possible function of IL-6 and TNF as intraadrenal factors in the regulation of adrenal steroid secretion. Ann N Y Acad Sci. 2000;917:628–637. doi: 10.1111/j.1749-6632.2000.tb05428.x. [DOI] [PubMed] [Google Scholar]
- 35.Salas MA, Evans SW, Levell MJ, et al. Interleukin-6 and ACTH act synergistically to stimulate the release of corticosterone from adrenal gland cells. Clin Exp Immunol. 1990;79(3):470–473. doi: 10.1111/j.1365-2249.1990.tb08114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin D, Sugawara T, Strauss JF, 3rd, et al. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267(5205):1828–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- 37.Stocco DM, Clark BJ. Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem Pharmacol. 1996;51(3):197–205. doi: 10.1016/0006-2952(95)02093-4. [DOI] [PubMed] [Google Scholar]
- 38.Mountjoy KG, Robbins LS, Mortrud MT, et al. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257(5074):1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 39.Metherell LA, Chapple JP, Cooray S, et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005;37(2):166–170. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- 40.Hofland J, Delhanty PJ, Steenbergen J, et al. Melanocortin 2 Receptor-Associated Protein (MRAP) and MRAP2 in Human Adrenocortical Tissues: Regulation of Expression and Association with ACTH Responsiveness. Journal of Clinical Endocrinology and Metabolism. 2012;97(5):E747–E754. doi: 10.1210/jc.2011-2328. [DOI] [PubMed] [Google Scholar]
- 41.Sugawara T, Holt JA, Kiriakidou M, et al. Steroidogenic factor 1-dependent promoter activity of the human steroidogenic acute regulatory protein (StAR) gene. Biochemistry. 1996;35(28):9052–9059. doi: 10.1021/bi960057r. [DOI] [PubMed] [Google Scholar]
- 42.Zazopoulos E, Lalli E, Stocco DM, et al. DNA binding and transcriptional repression by DAX-1 blocks steroidogenesis. Nature. 1997;390(6657):311–315. doi: 10.1038/36899. [DOI] [PubMed] [Google Scholar]
- 43.Ma Y, Ren S, Pandak WM, et al. The effects of inflammatory cytokines on steroidogenic acute regulatory protein expression in macrophages. Inflammation research. 2007;56(12):495–501. doi: 10.1007/s00011-007-6133-3. [DOI] [PubMed] [Google Scholar]
- 44.Tkachenko IV, Jaaskelainen T, Jaaskelainen J, et al. Interleukins lalpha and lbeta as regulators of steroidogenesis in human NCI-H295R adrenocortical cells. Steroids. 2011;76(10-11):1103–1115. doi: 10.1016/j.steroids.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Bornstein SR, Vaudry H. Paracrine and neuroendocrine regulation of the adrenal gland--basic and clinical aspects. Horm Metab Res. 1998;30(6-7):292–296. doi: 10.1055/s-2007-978887. [DOI] [PubMed] [Google Scholar]
- 46.Ehrhart-Bornstein M, Hinson JP, Bornstein SR, et al. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19(2):101–143. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- 47.Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R1128–1135. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- 48.Roth-Isigkeit AK, Dibbelt L, Schmucker P. Blood levels of corticosteroid-binding globulin, total cortisol and unbound cortisol in patients undergoing coronary artery bypass grafting surgery with cardiopulmonary bypass. Steroids. 2000;65(9):513–520. doi: 10.1016/s0039-128x(00)00133-1. [DOI] [PubMed] [Google Scholar]
- 49.Wald EL, Preze E, Eickhoff JC, et al. The effect of cardiopulmonary bypass on the hypothalamic-pituitary-adrenal axis in children. Pediatr Crit Care Med. 2011;12(2):190–196. doi: 10.1097/PCC.0b013e3181f36d17. [DOI] [PubMed] [Google Scholar]
- 50.Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10(3):232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- 51.Cameron A, Henley D, Carrell R, et al. Temperature-responsive release of cortisol from its binding globulin: a protein thermocouple. J Clin Endocrinol Metab. 2010;95(10):4689–4695. doi: 10.1210/jc.2010-0942. [DOI] [PubMed] [Google Scholar]
- 52.Hammond GL, Smith CL, Paterson NA, et al. A role for corticosteroid-binding globulin in delivery of cortisol to activated neutrophils. J Clin Endocrinol Metab. 1990;71(1):34–39. doi: 10.1210/jcem-71-1-34. [DOI] [PubMed] [Google Scholar]
- 53.Henley DE, Lightman SL. New insights into corticosteroid-binding globulin and glucocorticoid delivery. Neuroscience. 2011;180:1–8. doi: 10.1016/j.neuroscience.2011.02.053. [DOI] [PubMed] [Google Scholar]
- 54.Coolens JL, Van Baelen H, Heyns W. Clinical use of unbound plasma cortisol as calculated from total cortisol and corticosteroid-binding globulin. J Steroid Biochem. 1987;26(2):197–202. doi: 10.1016/0022-4731(87)90071-9. [DOI] [PubMed] [Google Scholar]
- 55.Tytherleigh MY, Vedhara K, Lightman SL. Mineralocorticoid and glucocorticoid receptors and their differential effects on memory performance in people with Addison’s disease. Psychoneuroendocrinology. 2004;29(6):712–723. doi: 10.1016/S0306-4530(03)00103-3. [DOI] [PubMed] [Google Scholar]
- 56.Annane D, Sebille V, Troche G, et al. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. Jama. 2000;283(8):1038–1045. doi: 10.1001/jama.283.8.1038. [DOI] [PubMed] [Google Scholar]
- 57.Arafah BM, Nishiyama FJ, Tlaygeh H, et al. Measurement of salivary cortisol concentration in the assessment of adrenal function in critically ill subjects: a surrogate marker of the circulating free cortisol. J Clin Endocrinol Metab. 2007;92(8):2965–2971. doi: 10.1210/jc.2007-0181. [DOI] [PubMed] [Google Scholar]
- 58.Beishuizen A, Thijs LG. Relative adrenal failure in intensive care: an identifiable problem requiring treatment? Best Pract Res Clin Endocrinol Metab. 2001;15(4):513–531. doi: 10.1053/beem.2001.0167. [DOI] [PubMed] [Google Scholar]
- 59.Cohen J, Smith ML, Deans RV, et al. Serial changes in plasma total cortisol, plasma free cortisol, and tissue cortisol activity in patients with septic shock: an observational study. Shock. 2012;37(1):28–33. doi: 10.1097/SHK.0b013e318239b809. [DOI] [PubMed] [Google Scholar]
- 60.Molenaar N, Johan Groeneveld AB, Dijstelbloem HM, et al. Assessing adrenal insufficiency of corticosteroid secretion using free versus total cortisol levels in critical illness. Intensive Care Med. 2011;37(12):1986–1993. doi: 10.1007/s00134-011-2342-x. [DOI] [PubMed] [Google Scholar]
- 61.Venkatesh B, Mortimer RH, Couchman B, et al. Evaluation of random plasma cortisol and the low dose corticotropin test as indicators of adrenal secretory capacity in critically ill patients: a prospective study. Anaesth Intensive Care. 2005;33(2):201–209. doi: 10.1177/0310057X0503300208. [DOI] [PubMed] [Google Scholar]
- 62.Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36(6):1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 63.Hahner S, Loeffler M, Fassnacht M, et al. Impaired subjective health status in 256 patients with adrenal insufficiency on standard therapy based on cross-sectional analysis. J Clin Endocrinol Metab. 2007;92(10):3912–3922. doi: 10.1210/jc.2007-0685. [DOI] [PubMed] [Google Scholar]
- 64.Bergthorsdottir R, Leonsson-Zachrisson M, Oden A, et al. Premature mortality in patients with Addison’s disease: a population-based study. J Clin Endocrinol Metab. 2006;91(12):4849–4853. doi: 10.1210/jc.2006-0076. [DOI] [PubMed] [Google Scholar]
- 65.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. 10.1097/CCM.1090b1013e31827e31883af. [DOI] [PubMed] [Google Scholar]
- 66.Vermes I, Beishuizen A, Hampsink RM, et al. Dissociation of plasma adrenocorticotropin and cortisol levels in critically ill patients: possible role of endothelin and atrial natriuretic hormone. J Clin Endocrinol Metab. 1995;80(4):1238–1242. doi: 10.1210/jcem.80.4.7714094. [DOI] [PubMed] [Google Scholar]