Abstract
Neuropeptides are important mediators both within the nervous system and between neurons and other cell types. Neuropeptides such as substance P, calcitonin gene-related peptide and neuropeptide Y (NPY), vasoactive intestinal polypeptide, somatostatin and corticotropin-releasing factor are also likely to play a role in the bidirectional gut-brain communication. In this capacity they may influence the activity of the gastrointestinal microbiota and its interaction with the gut-brain axis. Current efforts in elucidating the implication of neuropeptides in the microbiota-gut-brain axis address 4 information carriers from the gut to the brain (vagal and spinal afferent neurons; immune mediators such as cytokines; gut hormones; gut microbiota-derived signalling molecules) and 4 information carriers from the central nervous system to the gut (sympathetic efferent neurons; parasympathetic efferent neurons; neuroendocrine factors involving the adrenal medulla; neuroendocrine factors involving the adrenal cortex). Apart from operating as neurotransmitters, many biologically active peptides also function as gut hormones. Given that neuropeptides and gut hormones target the same cell membrane receptors (typically G protein-coupled receptors), the two messenger roles often converge in the same or similar biological implications. This is exemplified by NPY and peptide YY (PYY), two members of the PP-fold peptide family. While PYY is almost exclusively expressed by enteroendocrine cells, NPY is found at all levels of the gut-brain and brain-gut axis. The function of PYY-releasing enteroendocrine cells is directly influenced by short chain fatty acids generated by the intestinal microbiota from indigestible fibre, while NPY may control the impact of the gut microbiota on inflammatory processes, pain, brain function and behaviour. Although the impact of neuropeptides on the interaction between the gut microbiota and brain awaits to be analysed, biologically active peptides are likely to emerge as neural and endocrine messengers in orchestrating the microbiota-gut-brain axis in health and disease.
Keywords: Anxiety, brain-gut axis, cytokines, depression, food intake, gut-brain axis, gut hormones, immune system, inflammation, microbial metabolites, microbiota, neuropeptide Y, pain, peptide YY, satiety, stress resilience, visceral hyperalgesia
Neuropeptides at the forefront of the brain-gut axis
Biologically active peptides have been instrumental in the formulation of the concept that brain and gut have much in common. When in the 1960s and 1970s several peptides were discovered to occur both in the brain and gastrointestinal tract, the term “gut-brain axis” was first coined, based on the prevailing concept that the brain would be essential for controlling gut function. The way to this concept was pathed by the so-called APUD (amine precursor uptake and decarboxylation) hypothesis which, owing to common histochemical characteristics, held that amine- and peptide-producing cells of the nervous system, the gut and other organs derive from a common origin in the neural crest [1,2]. While certain cells of the thyroid, adrenal medulla, carotid bodies and autonomic as well as enteric ganglia originate in fact from the neural crest, the peptide-secreting endocrine cells of the gut do not [2]. Although the APUD hypothesis has not stood the test of time, it was an important contribution to the current understanding of the coordinating function of neuropeptides in many organ systems. We now know that a vast number of neuropeptides is produced by central and peripheral neurons alongside with endocrine cells in the gastrointestinal tract and other endocrinologically active organs [2-4]. Biologically active peptides, particularly neuropeptides, play many diverse roles in the bidirectional data highway between the gut and brain and offer unforeseen opportunities for drug development. At the same time, the multiplicity of messengers (including neuropeptides) also represents a challenge in understanding the complex interactions between gut and brain. Although their precise role in the microbiota-gut-brain axis has not yet been defined, neuropeptides such as substance P, calcitonin gene-related peptide, neuropeptide Y (NPY), vasoactive intestinal polypeptide, somatostatin and corticotropin-releasing factor (CRF) are candidates to play an important role in this respect.
The gut-brain axis involves microbial, immune, endocrine and neural signalling pathways: neuropeptides may be involved in each pathway
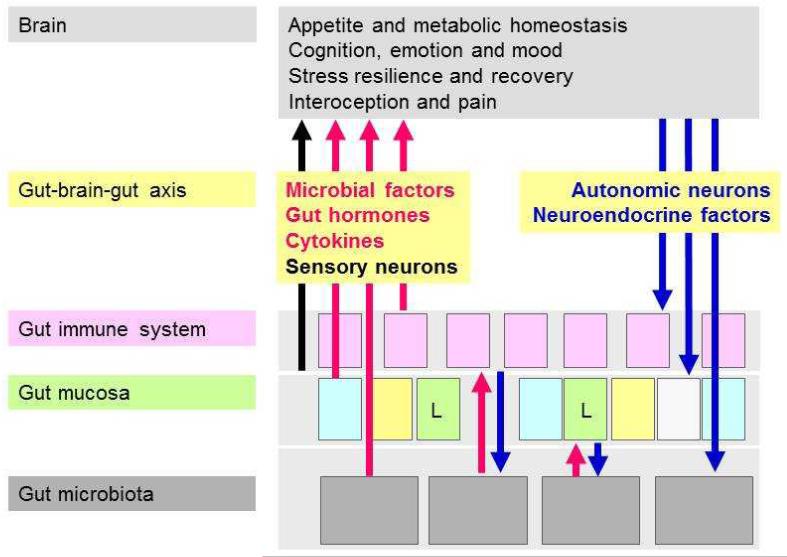
The term “gut-brain axis” refers to the bidirectional communication between the gut and the brain (Figure 1). Apart from the autonomic regulation of digestion by the central, parasympathetic, sympathetic and enteric nervous systems as well as by neuroendocrine factors (derived from the adrenal medulla and cortex), there is ongoing communication from the gut to the brain in health and disease [5,6]. Thus, visceral information is continuously fed into subcortical regions of the brain including the limbic system and the autonomic and neuroendocrine centres [5]. This information is integrated with other interoceptive information from the body and with contextual information from the environment [5]. Under pathological conditions, the interoceptive input from the periphery may reach the level of consciousness and give rise to the sensation of nausea, discomfort and/or pain [6]. In addition, the brain’s output to the gut via autonomic and neuroendocrine pathways may result in gastrointestinal dysfunction. The afferent part of this gut-brain-gut axis has recently been in the focus of investigation in order to understand why gastrointestinal disease such inflammatory bowel disease and irritable bowel syndrome is associated with pain and a number of psychiatric disturbances including anxiety, neuroticism and depression.
Figure 1.
The bidirectional microbiota-gut-brain axis. Four communication pathways (microbial factors, gut hormones, cytokines, sensory neurons) signal from the gut to the brain where they can modify cerebral function and behaviour. Two pathways (autonomic and neuroendocrine outputs) signal from the brain to the gut. L denotes endocrine L cells in the intestinal mucosa.
The gut-brain axis uses 4 major information carriers for the communication between the gut and the brain (Figure 1):
neural messages carried by vagal and spinal afferent neurons,
immune messages carried by cytokines,
endocrine messages carried by gut hormones and
microbial factors that may directly reach the brain via the blood stream but can also interact with the other 3 transmission pathways [6-8].
These communication systems are abundantly present in the gastrointestinal tract and, in an evolutionary perspective, are relevant for a number of vital functions:
The brain with its sensory systems needs to interact with the gut in finding appropriate food and assimilating it for the sake of metabolic survival.
The gut needs to distinguish between useful and useless as well as dangerous (antigenic, pathogenic, toxic) ingredients of food and sort them accordingly.
The gut needs to maintain homeostasis with the extensive community of microbes in the intestine, which are important in supporting nutrition, educating the immune system and communicating with other organ systems including the brain.
Each of the communication pathways between the gastrointestinal and central nervous system may involve neuropeptides and structurally related signalling molecules. Ever since their gradual discovery, biologically active peptides have been intimately related to the regulation of digestion and to the communication with the central nervous system. Regulation of food intake (appetite), metabolic homeostasis and pain have been areas that were addressed in particular detail. Neuropeptides comprise a class of evolutionarily well conserved molecules that, by definition, operate as transmitters in the enteric, peripheral and central nervous systems and share transduction mechanisms with other biologically active peptides such as gut hormones. Apart from their origin, it is frequently difficult to distinguish between their function as neuropeptides or gut hormones because they operate often via the same receptors and cellular transduction systems. Thus, neurons as well as endocrine, immune, interstitial, muscle, epithelial and microbial cells can respond to these signalling molecules by expressing the appropriate peptide receptors. The microbiota residing in the mucosa [9] is in immediate vicinity to the endocrine cells of the gastrointestal mucosa which produce more than 20 different gut hormones [10]. Apart from immune mediators, gut hormones may thus play an important role as communicators between the gut microbiota and host functions. Gut hormone signalling to the brain not only occurs by an endocrine route but may also involve activation of primary afferent neurons, especially in the vagus nerve (e.g., cholecystokinin and ghrelin). Furthermore, it is important to realize that the 4 communication pathways between the gut and the brain do not operate in isolation but are closely interrelated with each other.
Direct brain communication pathways used by the gut microbiota
With the emerging role of the microbiota a new gut-brain pathway has come to light. Thus, the gut microbiota communicate not only with gastrointestinal epithelial, immune and nerve cells in their immediate neighbourhood but also generate and release molecules that can signal to distant organs. This is true for molecules designated as pathogen-associated molecular patterns or, in a more benevolent vein, microbe-associated molecular patterns (MAMPs) as well as for many other microbial metabolites. There are experimental data to show that a significant part of the metabolites circulating in mammalian blood are derived from the intestinal microbial community [11-15]. Importantly, the presence or absence of the gut microbiota also influences the profile of metabolites (including peptides) present in the brain [16].
While the potential effects of the microbial metabolites on the host are still little understood, it is obvious that they could convey messages around the whole body. Some information in this respect can be derived from the actions of two MAMPs, lipopolysaccharide (LPS) and peptidoglycan components such as meso-diaminopimelic acid. These MAMPs are recognized by pattern recognition receptors of the innate immune system: LPS activates toll-like receptor 4 (TLR4) while the peptidoglycan structures stimulate nucleotide-binding oligomerization domain–containing protein-1 (Nod1) and/or Nod2. Importantly, translocation of peptidoglycan from the gut to the blood impacts on neutrophils in the bone marrow and primes their capacity to defend the body against bacterial infection via stimulating Nod1 [17].
In a similar manner, LPS translocated from the gut through a leaky mucosal barrier carries a microbial message to distant organs including the brain. The behavioural responses to systemic exposure of excess LPS are well characterized in animals and humans and comprise acute sickness [18,19] and delayed depression-like behaviour [20-24]. LPS originating from the gut microbiota may give rise to alterations in brain function via 3 different pathways. Following translocation across the intestinal mucosa it may, on the one hand, stimulate the intestinal immune system to produce cytokines which (i) can signal directly to the brain or (ii) sensitize/stimulate vagal and spinal afferent neurons [18,19,25,26]. On the other hand, (iii) the circulation may carry LPS itself to the central nervous system where it may modify brain function.
The latter possibility need be envisaged because – apart from the innate immune system – there is a widespread expression of TLR4 and other TLRs at several levels of the gut-brain axis. Thus, TLRs are present on gastrointestinal epithelial cells [27,28], neurons of the enteric nervous system [29,30], primary afferent neurons [29] and various cell types (neurons, microglial cells and astrocytes) in the brain [31-33]. By stimulating TLR4 and TLRs in the brain, LPS and other bacterial factors can stimulate the generation and release of proinflammatory cytokines and in this way give rise to neuroinflammatory processes. These effects are not only relevant to neurodegeneration and repair [31-33] but may also be involved in the manifestation of psychiatric disorders. Specifically, increased levels of IgA and IgM against LPS of commensal gut bacteria are found in the circulation of patients with depression or chronic fatigue syndrome, and the hypothesis has been put forward that increased translocation of LPS across a leaky gut may be a factor that contributes to these pathologies [34,35]. Taken all findings together, it would appear, therefore, that the physiological roles of the symbiotic gut microbiota relate not only to the regulation of digestion at the gastrointestinal level but also extend to systemic immunity and brain function.
Neuroactive factors released by the gut microbiota
There is increasing evidence that the gut microbiota sheds not only ligands for pattern recognition receptors, but also release factors that target specific neuronal systems involved in the gut-brain axis. Although it remains to be established whether the microbiota can produce neuropeptide-like compounds, they are capable of generating a number of neurotransmitters and neuromodulators [7,14]. Members of the genera Candida, Streptococcus, Escherichia and Enterococcus synthesize 5-hydroxytryptamine (5-HT), members of the genera Escherichia, Bacillus and Saccharomyces generate dopamine and/or noradrenaline, members of the genus Lactobacillus produce acetylcholine, and members of the genera Lactobacillus and Bifidobacterium manufacture gamma-aminobutyric acid (GABA) [7,14,36-39]. The release of microbiota-derived dopamine into the lumen of the intestine has been suggested to play a proabsorptive role in the colon [38]. Signalling via opioid and cannabinoid receptors may also be modified by the gut microbiota, a conclusion based on the ability of certain probiotics to alter the expression of opioid and cannabinoid receptors in the gut [7].
Moreover, the microbiota in the intestine is able to produce metabolites with benzodiazepine-like structures and effects [40-42]. Specifically, benzodiazepine receptor ligands originating from the gut microbiota have been proposed to contribute to the encephalopathy associated with fulminant hepatic failure [40]. Under these conditions, benzodiazepine-like molecules are likely to reach the brain at increased concentrations that will enhance neurotransmission via GABAA receptors and thus contribute to the disease process [40]. The pyrrolobenzodiazepines (e.g., anthramycin) synthesized by a number of gut microbes display not only benzodiazepine-like but also antibiotic and antineoplastic activities and may thus influence the biology of the microbiota and host alike in many respects. In addition, this circumstance indicates that the gut microbiota is a rich source of yet-to-be-identified compounds with therapeutic potential.
Apart from producing and releasing neuroactive factors, the microbiota modifies the levels of metabolites that are relevant to the synthesis of transmitters in the nervous system. For instance, the concentrations of tryptophan (the precursor of 5-HT), tyrosine (the precursor of dopamine and noradrenaline) and glutamine in the total brain of germ-free mice are lower than in mice that have been re-colonized by the gut microbiota [16]. In the hippocampus of germ-free mice, however, the concentrations of 5-HT and its main metabolite 5-hydroxyindoleacetic acid are higher than in conventionally colonized mice [43]. Colonization of the germ-free animals restores peripheral tryptophan levels to control values but fails to reverse the changes in hippocampal 5-HT levels [43]. The concentrations of tryptophan, 5-HT and tyrosine in the blood plasma are likewise increased in germ-free animals [11,43], the elevation of tryptophan being likely due to the absence of bacterial tryptophanase [11]. Another explanation could be that the gut microbiota re-directs the metabolism pathways of tryptophan which lead either to the production of 5-HT or kynurenine [7].
Interaction of the gut microbiota with gut peptides
Due to their spatial vicinity with the gastrointestinal mucosa, the gut microbiota is in a prime position to interact with the epithelial cells and to modify their activity. Among these cells, enteroendocrine cells are poised to govern the activity of cells in and outside the digestive system and in this way also to convey messages from the microbial community in the gut. The enteroendocrine L cells in the distal ileum and colon represent a distinct example of this interactive relationship. These cells are stimulated by particular nutrients and digestive products, which leads to the release of PYY, glucagon-like peptide-1 (GLP-1) and GLP-2 [6,44,45]. L cells are also stimulated by short chain fatty acids (e.g., acetate, butyrate, propionate), which particular microbes generate by fermentation of otherwise indigestible carbohydrate fibres. Short chain fatty acids stimulate L cells via activating G protein-coupled receptors such as Gpr41 [6,44,45]. The important role of this microbiota-host interaction is underscored by the finding that colonization of the mouse colon with a fermentative human microbial community increases the plasma level of PYY, an effect that is blunted by knockout of Gpr41 [45]. Gpr41 deficiency is associated with a reduced expression of PYY, an increase in intestinal transit rate and an attenuation of energy harvest [45].
Follwing their release from L cells, PYY and GLP-1 not only inhibit gastric motility and improve glucose homeostasis but also induce satiety and behavioural changes. Thus, butyrate is able to ameliorate aging-related memory decline in rats [46] but has inconsistent effects on anxiety and depression-like behaviour [47]. Propionate has been shown to evoke autism spectrum disorder-related behaviours in rodents [48,49].
The interaction between the gut microbiota and intestinal L cells can be modulated by the use of prebiotics (fermentable carbohydrates). Prebiotic supplementation in humans increases the plasma concentrations of GLP-1 and PYY, which is associated with satiety and a decrease of postprandial glucose levels [50]. Experiments in obese mice show that prebiotic treatment causes a change in the composition of the gut microbiota alongside with a decrease of inflammatory tone and an enforcement of mucosal barrier function [51]. The complex interactions between gut microbiota, mucosal function and metabolic homeostasis also involve the endocannabinoid system [52] and GLP-2 which improves intestinal function [51]. These interrelationships suggest that prebiotic supplementation has therapeutic potential as “pharmaco-nutritional” approach to reversing host metabolic alterations linked to intestinal dysbiosis in obesity and diabetes [53].
Given that nutritional status, dietary factors, physical activity and age have an important influence on the composition of the gut microbial community [54,55] it is not surprising that appetite-regulating hormones other than PYY, GLP-1 and GLP-2 will also interact with the gut microbiota in shaping appetite and metabolic status. Emerging evidence indicates that this applies to ghrelin [55,56], cholecystokinin [56] as well as leptin [56]. In addition, germ-free mice have a smaller number of enteroendocrine cells than conventionally colonized animals [56].
Interaction of the gut microbiota with brain function and behaviour: emerging neurochemical mediators
Accumulating evidence shows that the absence or disturbance of the gut microbiota has a significant impact on brain function and behaviour. There is also some information on the molecular factors that may play an important role in this interrelationship. In a first line of research, germ-free mice have been found to exhibit a number of neurochemical and functional alterations relative to conventionally colonized animals. For instance, the expression of the NMDA receptor subunit 2A (NR2A) in the cortex and hippocampus [57] and of the NR2B unit in the central amygdala [58] is decreased in germ-free mice, as is the expression of the 5-HT receptor 1A (5HT1A) in the dentate granule layer of the hippocampus [58]. In contrast, inconsistent changes in the levels of brain-derived neurotrophic factor (BDNF), a key neurotrophin involved in neuronal growth and survival, have been reported: two studies hold that BDNF in the hippocampus, amygdala and cortex of germ-free mice are decreased [57,59], while another study purports that the level of BDNF in the hippocampus of germ-free mice is increased [58].
At the behavioural level, germ-free animals exhibit reduced anxiety in three [43,58,59] but one study [60]. This outcome is somewhat surprising, since the hypothalamic-pituitary-adrenal axis in germ-free mice appears to be hyperactive rather than hypoactive [57]. Germ-free mice also show increased spontaneous motor activity, an observation that may be related to elevated dopamine, noradrenaline and 5-HT turnover in the striatum [59]. With regard to cognition, germ-free mice have deficits in simple non-spatial and working memory tasks [60]. It awaits to be examined whether the cognitive deficits are related to decreased synaptogenesis and a decrease in the expression of synaptic plasticity-related genes [59].
The impact of the gut microbiota on brain function has been confirmed by the impact of antibiotic-induced dysbiosis on the gut-brain axis and by the effects of selective probiotics on behaviour and brain chemistry. Disturbance of the gastrointestinal microbiota with a combination of nonabsorbable antibiotics (neomycin, bacitracin, and pimaricin) increases exploratory behaviour and enhances BDNF expression in the hippocampus [61]. Similar observations have been made with another combination of nonabsorbable antibiotics (neomycin, cefoperazone and ampicillin) which has an anxiolytic-like effect and impairs learning/memory in the object recognition test [62].
Chronic treatment of mice with the probiotic Lactobacillus rhamnosus has been found to cause region-dependent alterations of GABAAα2 and GABAB1b receptor mRNA in the brain, which are associated with a decrease in the stress-induced corticosterone response and a reduction of anxiety- and depression-related behaviour [63]. Importantly, these neurochemical and behavioural effects of probiotic treatment are prevented by bilateral subdiaphragmatic vagotomy. This role of vagal afferent neurons in communicating between gut bacteria and brain was confirmed by another study in which vagotomy abolished the anxiolytic effect of the probiotic Bifidobacterium longum NCC3001 in mice with experimentally induced colitis [64].
Interaction of the gut microbiota with brain function and behaviour: the direct involvement of neuropeptides awaits to be explored
Neuropeptides such as substance P, calcitonin gene-related peptide and NPY are expressed at all levels of the microbiota-gut-brain axis and are likely to play an important role in the bidirectional signalling between the gut and brain. Theoretically, neuropeptide-like molecules may also be produced by certain microbes, and the gut microbiota will respond to neuropeptides and gut hormones if they express the relevant receptors. However, direct evidence that these neuropeptides contribute to the communication between the gut microbial community and the central nervous system is sparse. It also remains to be investigated whether alterations in the microbial community within the gut impacts on neuropeptide systems in the central nervous system.
The information available is mostly restricted to peptide level changes associated with manipulation of the intestinal microbiota. For instance, the colonic content of substance P is enhanced following antibiotic-induced dysbiosis of the intestinal microbiota [65]. The expression of neuropeptides in primary afferent neurons (e.g., substance P, calcitonin gene-related peptide) has not yet been addressed in experimental studies, although such studies appear worthwhile in view of two lines of research relating the intestinal microbiota to pain. On the one hand, the establishment of inflammatory hyperalgesia is attenuated in germ-free mice [66]. On the other hand, treatment of rodents with the probiotic Lactobacillus reuteri attenuates sensory neuron excitability [67] and alleviates the pain-related response to gastric distension [68]. Lactobacillus acidophilus also reduces experimentally evoked visceral pain, an effect that is associated with enhanced expression of opioid and cannabinoid receptors in the intestinal mucosa [69]. Lactobacillus paracasei has been found to attenuate antibiotic-induced visceral hypersensitivity in mice [65], while Lactobacillus rhamnosus GG has a beneficial effect in abdominal pain-related functional gastrointestinal disorders in childhood [70].
Neuropeptide autoantibodies under the control of the intestinal microbiota
The gut microbiota is important in educating the immune system to recognize foreign antigens and to tolerate commensal microbes [71]. In this way, the gut microbes can modulate, tune and tame the host immune response [72]. Dysbiosis of the microbial community can lead to the development of autoimmunity [72,73], and experimental findings indicate that both autoimmune encephalomyelitis [74] and autoimmune demyelination [75] involve the gut microbiota. There is also evidence that the formation of autoantibodies against neuropeptides is governed by intestinal microbes [76-78].
Specifically, IgG and IgA autoantibodies against alpha-melanocyte-stimulating hormone, NPY, PYY, agouti-related protein (AgRP), ghrelin, leptin and some other neuropeptides/peptides involved in appetite control are present in the human blood [76-78]. Numerous intestinal microbes including Lactobacillus, Bacteroides, Helicobacter pylori, Escherichia coli and Candida species contain proteins that have amino acid sequences identical to these appetite-regulating peptides [78]. The circulating levels of autoantibodies against alpha-melanocyte-stimulating hormone, which are increased in anorexia nervosa and bulimia nervosa, correlate with the psychobehavioural abnormalities of these eating disorders [76]. Vice versa, germ-free rats have decreased levels of circulating IgA autoantibodies against several appetite-regulating peptides, while the levels of antighrelin IgG are increased [78]. A mechanistic analysis in rats has shown that alpha-melanocyte-stimulating hormone autoantibodies are involved in the regulation of feeding and anxiety [78]. It thus appears conceivable that the gut microbiota control appetite and emotional behaviour indirectly by inciting the formation of autoantibodies against neuropeptides/peptides involved in these processes.
Interaction of gut microbiota with brain function and behaviour: a potential role for NPY
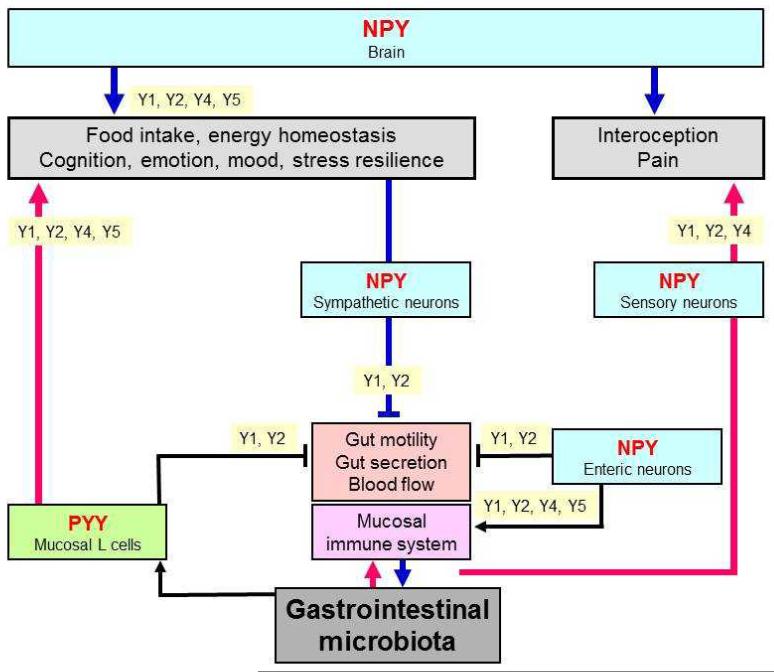
NPY is a neurotransmitter that in view of its multiple implications in brain function may play a particular role in the microbiota-gut-brain axis. This contention is based on this neuropeptide’s involvement in controlling inflammatory processes, pain, emotion, mood, cognition, stress resilience, ingestion and energy homeostasis [6]. Consisting of 36 amino acids, NPY exerts its biological actions via 5 NPY receptor types, termed Y1, Y2, Y4, Y5 and y6 (a human pseudogene), which are coupled to pertussis toxin-sensitive Gi/o protein transduction mechanisms [79]. Y receptors occur at all levels of the gut-brain and brain-gut axis [6,80-84], the major systems expressing this peptide being enteric neurons, primary afferent neurons, several neuronal pathways throughout the brain and sympathetic neurons (Figure 2).
Figure 2.
The NPY/Y receptor sytem in the microbiota-gut-brain axis. The graph shows the major sources of NPY and PYY along the gut-brain axis and the Y receptor subtypes which mediate the effects of these peptides at the different levels of the gut-brain axis. The symbol  denotes inhibition.
denotes inhibition.
Within the brain, NPY is one of the most abundant neuropeptides. In the context of the gut-brain axis it is particularly worth noting that NPY occurs in the nucleus of the solitary tract and ventrolateral medulla, periaqueductal grey and locus coeruleus, paraventricular nucleus of the thalamus, hypothalamus (arcuate nucleus, paraventricular nucleus and other regions), septum, hippocampus, amygdala, basal ganglia, nucleus accumbens and cerebral cortex [6,80-84]. Several important pathways utilizing NPY as a neurotransmitter have been identified. These include noradrenergic neurons originating in the locus coeruleus of the brainstem and issuing both ascending and descending projections in the central nervous system, neurons expressing both NPY and AgRP originating in the arcuate nucleus of the hypothalamus, and distinct pathways operating in the limbic system [80-84]. The major receptor subtypes which NPY acts on are the Y1 and Y2 receptors, which are widely distributed in the central nervous system, while the localization of Y4 and Y5 receptors is restricted to particular regions of the brain [82-85].
The NPY system may impact on the microbiota-gut-brain axis at distinct levels [6,81-84,86-91]. It may
influence the vitality of certain gut bacteria,
modify gut functions such as motility, secretion and blood flow,
regulate the activity of the immune system,
protect against behavioural disturbances caused by peripheral immune challenge,
inhibit nociceptive transmission in the spinal cord and brainstem,
protect from the impact of stress on the brain-gut axis,
regulate food intake and energy homeostasis, and
play a role in the interoceptive regulation of anxiety and mood.
Effect of NPY on gut bacteria
There is some evidence that the NPY system has an impact on the composition and function of the gut microbiota and its relevance to the gut-brain axis. Similarly to substance P, calcitonin gene-related peptide and vasoactive intestinal polypeptide, NPY has been found to exhibit a direct antimicrobial effect against various gut bacteria including Escherichia coli, Enterococcus faecalis, and Lactobacillus acidophilus [86].
Effects of NPY on the immune system
In the context of the microbiota-gut-brain axis it is important to mention that NPY has a distinct impact on immune function, within and outside the gastrointestinal tract [6,87-89]. NPY released from the sympathetic nerve fibres acts on Y receptors (notably of the Y1, Y2, Y4 and Y5 subtype) expressed by distinct classes of immune cells (e.g., dendritic cells, mononuclear cells, macrophages, granulocytes, T and B lymphocytes) to modify their activity [6,89-91]. In addition, NPY acts as a paracrine or autocrine immune mediator, because immune cells (e.g., B and T lymphocytes, macrophages) themselves can produce and release NPY [6,88,91]. The effects of NPY include modulation of immune cell trafficking, activation of antigen-presenting cell function, T helper cell differentiation, negative regulation of T cell function, cytokine secretion, phagocytosis and production of reactive oxygen species [6,87-91].
With this immunological activity profile, NPY regulates inflammatory processes in the gut, given that NPY-containing nerve fibres are in close contact with immune cells in the mouse ileum lamina propria [92]. Specifically, NPY is able to promote colonic inflammation, an effect that is supported by several lines of evidence: (i) NPY knockout mice are largely resistant to the induction of dextran sulfate sodium-induced colitis [93,94]. (ii) The result of NPY deletion is reproduced by treatment with a NPY antisense oligodeoxynucleotide [95] and by knockout or antagonism of Y1 receptors [96]. The antiinflammatory phenotype of Y1 receptor knockout mice results from a defect in antigen-presenting cell function, a reduction of TNF-alpha and IL-12 production by macrophages, and a decrease in the number of effector T cells [90]. Furthermore, experimentally induced colitis is associated with an increase in the colonic synthesis of NPY [93,95,97], a reduction of colonic Y1 receptor expression and a loss of the antisecretory action of NPY in the colon [98]. In contrast, the colonic levels of the related gut hormone PYY are decreased in rats with DSS-induced colitis [99]. These experimental data are in line with a decrease of colonic PYY levels in patients with inflammatory bowel disease [100-102], while circulating levels of PYY and NPY are enhanced [103,104]. The proinflammatory effect of NPY could in part be counterregulated by the vasoconstrictor effect of the peptide [105].
Effect of NPY to protect from immune challenge-evoked behavioural disturbances
Infection and inflammation are increasingly recognized to have an impact on the pathogenesis of mood disorders [18,19,106,107], and clinical evidence suggests that activation of the intestinal immune system by constituents of the intestinal microbiota can give rise to depression [34] and chronic fatigue syndrome [35]. Experimentally, the impact of peripheral immune challenge on brain function and behaviour can be modelled by systemic administration of LPS or Bacille Calmette-Guérin [18,19,106,108]. The signalling pathways whereby peripheral immune challenge alters brain mechanisms involve proinflammatory cytokines such as interleukin-6, tumour necrosis factor-alpha and interferon-gamma, which reach the brain via the circulation but also excite vagal afferent neurons and lead to the expression of cytokines by cerebral microglial cells and astrocytes [18,19,106-108].
The effect of peripheral immune challenge on brain function involves several brain areas that express NPY and various Y receptors [6,81-84]. NPY is involved in the regulation of emotional-affective behaviour [6,81-84], and there is indirect evidence that NPY-expressing neurons in the arcuate and paraventricular nuclei of the hypothalamus counteract the behavioural responses to immune stress and infection [109-111]. This implication has been confirmed by knockout experiments in which the NPY/Y receptor system has been found to protect against distinct functional disturbances in response to immune challenge. For instance, deletion of NPY as well as NPY plus PYY aggravates the Bacille Calmette-Guérin-induced loss of body weight and markedly delays recovery from this weight loss [112]. This finding attests to an important role of NPY and PYY in maintaining energy homeostasis in the face of immune stimulation [112].
Analogous observations have been made when the behavioural responses to LPS are analysed in Y2 and Y4 knockout mice [6]. Y2 receptor knockout mice are particularly susceptible to the acute action of LPS to attenuate locomotion and suppress social interaction [20]. In contrast, the LPS-induced rise of temperature and circulating corticosterone is suppressed by Y2 receptor knockout [20]. The short-term effect of LPS to enhance anxiety is enhanced in Y2 and Y4 receptor knockout mice [20,21]. In Y4 receptor knockout mice, the anxiogenic response to LPS persists at least for 4 weeks post-treatment by which time it has waned in WT mice [21]. Depression-related behaviour is enhanced 1 day post-LPS in control and Y2 receptor knockout mice, but not in Y4 receptor knockout mice. Four weeks post-treatment the depressogenic effect of LPS has waned in wildtype mice, but is maintained in Y2 receptor knockout mice and first observed in Y4 receptor knockout mice [21]. Thus, knockout of Y2 and/or Y4 receptors unmasks the ability of immune challenge with LPS to cause a delayed and prolonged increase in anxiety- and/or depression-like behaviour [6]. These findings suggest that NPY acting via Y2 and Y4 receptors prevents the development of long-term anxiety- and depression-like behaviour caused by immune challenge [6,21]. It awaits to be examined whether the behavioural disturbances associated with dysbiosis of the gut microbiota are likewise under the control of the NPY/Y receptor system.
Effect of NPY to inhibit nociceptive transmission
Spinal afferent neurons, which contain low amounts of NPY, terminate in the spinal cord where interneurons and descending noradrenergic neurons express appreciable amounts of NPY [6,113,114]. An abundant occurrence of Y1 and Y2 receptors in the spinal cord enables NPY to play an important role in the processing of incoming nociceptive information. Germ-line knockout of Y1 receptors or conditional knockdown of NPY is associated with thermal, chemical and mechanical hyperalgesia [6,113-116]. Peripheral inflammation leads to an upregulation of Y1 receptors in spinal afferent neurons and in the dorsal horn of the spinal cord [117]. While these studies have primarily focused on somatic pain, it remains to be investigated whether the NPY/Y receptor system also plays a role in the impact of the gut microbiota on visceral pain sensitivity [66-70]. Two major mechanisms whereby NPY controls pain transmission in the spinal cord have been envisaged: inhibition of transmitter release from the terminals of primary afferent neurons, mediated primarily by Y2 receptors, and inhibition of postsynaptic neurons in the dorsal horn, mediated primarily by Y1 receptors [113,114].
Apart from spinal sensory neurons, vagal afferent neurons terminating in the nucleus tractus solitarii (NTS) have also been established to play a role in visceral nociception, particularly in visceral chemonociception [118]. Y2 and Y4 receptors are the Y receptor subtypes prevailing in the NTS [6,119], and gene deletion experiments have revealed that endogenous NPY acting via Y2 and Y4 receptors attenuates the chemonociceptive input from the stomach to the brainstem [119].
Effect of NPY to protect from the impact of stress on the gut-brain axis
Inflammation and psychosocial stress have a marked impact on the bidirectional communication between the gut and brain [5]. Since NPY plays a role in stress coping [84], it may also be relevant to the impact of stress on the gut-brain axis. NPY as well as Y1, Y2 and Y5 receptors are widely expressed in cerebral areas critical to the regulation of stress resilience [81-84]. The expression of NPY in the human brain is related to polymorphisms in the NPY gene, and a low NPY expression genotype is associated with negative emotional processing, diminished stress resilience, a risk for major depression, and a reduced antidepressant treatment response [120-122]. If individuals with a low NPY expression genotype are exposed to negative stimuli, there is an exaggerated activation of the amygdala, medial prefrontal cortex and anterior cingulate cortex [120-122]. The concentration of NPY in the cerebrospinal fluid and plasma is reduced in patients with post-traumatic stress disorder, while trauma-exposed individuals who do not develop or have recovered from post-traumatic stress disorder have enhanced plasma levels of NPY [123-125]. It would appear, therefore, that the cerebrospinal and plasma concentration of NPY is a biological correlate of resilience to or recovery from the adverse effects of stress [124]. Animal experiments have confirmed that NPY is involved in the emotional processing of stress [6], and the question arises whether this role also relates to the impact of stress on the microbiota-gut-brain axis.
Since stress can alter the permeability of the gastrointestinal mucosa [126,127], it is very likely that stress will also alter the interaction between the gut microbiota and the mucosal immune system. CRF is a neuropeptide and gut hormone that is intimately related to stress, and there is considerable evidence that activation of peripheral CRF receptors contributes to stress-related alterations of gut physiology [126]. NPY may likewise be involved because it appears to mediate the effects of stress on many physiological systems including the gastrointestinal and immune systems [128,129]. For instance, deletion of NPY alters gastrointestinal, feeding and corticosterone responses to restraint stress, exaggerates stress-induced defaecation and reduces food intake [128]. Trinitrobenzene sulfonic acid-induced colitis increases the NPY concentration in brain and plasma [97], and gastrointestinal inflammation enhances anxiety- and depression-related behaviour, this effect being modified by deletion of NPY and/or PYY [94,130]. It follows that NPY and PYY participate in the effect of intestinal inflammation on the gut-brain axis. In addition, the depression-like phenotype of PYY knockout animals [94] suggests that alterations in the expression of this gut hormone modify mood and stress coping. This contention is in line with the finding that water avoidance stress lowers the plasma level of PYY, a change that is associated with an increase in gastrointestinal motility [131].
Effects of NPY and PYY to regulate food intake and energy homeostasis
The implications of NPY and PYY in gut-brain signalling are particularly well exemplified by their effects on hunger, food intake, satiety and energy balance. These roles have been extensively reviewed elsewhere [132-134] and may be of particular relevance to the impact of the gut microbiota on metabolic regulation, energy homeostasis and metabolic disorders. PYY is released postprandially from intestinal L cells and acts as a satiety factor, slowing gastrointestinal transit, inhibiting further intake of food and modifying the metabolic status of the organism [132]. In the circulation, PYY is truncated to PYY3-36 which is a relatively selective Y2 receptor agonist. Food intake is inhibited by PYY3-36 both via stimulation of Y2 receptors on vagal afferent neurons and an interaction with Y2 receptors in the hypothalamus [6,132,135,136]. Within the brain, PYY3-36 reduces food intake primarily via activation of Y2 receptors in the arcuate nucleus which is an important centre for integrating peripheral and central signals in the control of appetite and energy homeostasis [137]. NPY, on the other hand, is one of the most potent orexigenic peptides found in the brain [134,137]. Specifically, it occurs in neurons projecting from the arcuate nucleus to various areas of the hypothalamus in which the orexigenic effect of NPY is primarily mediated by Y1 receptors, although Y5 receptors also contribute [134,137]. Pathologies associated with a decrease in food intake such as experimental colitis lead to increased release of NPY from the paraventricular nucleus of the hypothalamus [138], again attesting to a role of NPY in gut-brain signalling.
NPY, PYY and other gut peptides in the interoceptive regulation of emotion and mood
Apart from regulating ingestion and energy homeostasis, gut hormones such as ghrelin, PYY, GLP-1 and GLP-2 have an impact on emotional-affective behaviour. In an evolutionary point of view, co-regulation of appetite and emotional state is an important strategy for survival, given that anxiety would be an adverse condition when there is a need to seek food [6]. Indeed, ghrelin which is released from the upper gastrointestinal tract under conditions of hunger reduces both anxiety-like and depression-related behaviour [139]. Under fed conditions, behaviour is changed to a hedonic state as observed when PYY3-36 is administered to reach postprandial plasma concentrations of the peptide [140]. The ability of PYY to promote hedonic behaviour is supported by the finding that knockout of PYY increases depression-like behaviour but does not alter anxiety [94]. Physiologically, however, emotion and mood under fed conditions will be determined by the presence of a variety of gut hormones such as PYY, GLP-1 and GLP-2 that are released postprandially. Thus, GLP-1 has been found to enhance anxiety-related behaviour [141-143], while GLP-2 attenuates depression-like behaviour [144]. Gut hormones whose release from the enteroendocrine cells is likely to be regulated by the gut microbiota thus provide a constant stream of interoceptive input from the gut to the brain.
Conclusion: the gut microbiota meets neuropeptides
The gut microbiota has proved as a novel factor relevant to health and disease. How the gut microbiota communicates with distant organs such as the brain is only beginning to emerge. It is very probable that the microbiota will take use of several information carriers from the gut to the brain including microbiota-derived signalling molecules, immune mediators, gut hormones as well as vagal and spinal afferent neurons. Biologically active gut peptides and neuropeptides play a role in several of these communication pathways. This is true for peptides produced by enteroendocrine cells which respond to metabolites generated with the help of the microbiota. PYY, which is one of these peptides, acts via Y receptor types that are also stimulated by the neuropeptide NPY. Neuropeptides are important transmitters in afferent, central and efferent pathways of the bidirectional gut-brain communication network. It remains to be shown whether the gut microbiota itself expresses neuropeptide receptors or releases metabolites that are ligands at neuropeptide receptors. Although a direct link between the gut microbiota and distinct neuropeptide systems has not yet been revealed, NPY, CRF and tachykinins are very likely to emerge as messengers in the microbiota-gut-brain axis.
Acknowledgements
This study was supported by the Zukunftsfonds Steiermark (grant 262), the Austrian Science Fund (FWF grants L25-B05, P23097-B18 and P25912-B23), and the Federal Ministry of Science and Research of the Republic of Austria (grant GZ 80.104/2-BrGT/2007).
References
- 1.Pearse AGE. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969;17(5):303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- 2.Strand FL. Neuropeptides: Regulators of Physiological Processes. MIT Press; Cambridge, MA: 1999. [Google Scholar]
- 3.Kastin AJ. Handbook of Biologically Active Peptides. 2nd Edition Academic Press; San Diego, CA: 2013. [Google Scholar]
- 4.Burbach JP. Neuropeptides from concept to online database. Eur J Pharmacol. 2010;626(1):27–48. doi: 10.1016/j.ejphar.2009.10.015. www.neuropeptides.nl. [DOI] [PubMed] [Google Scholar]
- 5.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381–396. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46(6):261–274. doi: 10.1016/j.npep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 8.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 9.Gorkiewicz G, Thallinger GG, Trajanoski S, Lackner S, Stocker G, Hinterleitner T, Gülly C, Högenauer C. Alterations in the colonic microbiota in response to osmotic diarrhea. PLoS One. 2013;8(2):e55817. doi: 10.1371/journal.pone.0055817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Field BC, Chaudhri OB, Bloom SR. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol. 2010 Aug;6(8):444–453. doi: 10.1038/nrendo.2010.93. [DOI] [PubMed] [Google Scholar]
- 11.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106(10):3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antunes LC, Han J, Ferreira RB, Lolić P, Borchers CH, Finlay BB. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob Agents Chemother. 2011;55(4):1494–1503. doi: 10.1128/AAC.01664-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 15.Marcobal A, Kashyap PC, Nelson TA, Aronov PA, Donia MS, Spormann A, Fischbach MA, Sonnenburg JL. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J. 2013 doi: 10.1038/ismej.2013.89. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto M, Kibe R, Ooga T, Aiba Y, Sawaki E, Koga Y, Benno Y. Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front Syst Neurosci. 2013;7:9. doi: 10.3389/fnsys.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16(2):228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCusker RH, Kelley KW. Immune-neural connections: how the immune system’s response to infectious agents influences behavior. J Exp Biol. 2013;216(Pt 1):84–98. doi: 10.1242/jeb.073411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Painsipp E, Herzog H, Holzer P. Implication of neuropeptide-Y Y2 receptors in the effects of immune stress on emotional, locomotor and social behavior of mice. Neuropharmacology. 2008;55(1):117–126. doi: 10.1016/j.neuropharm.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Painsipp E, Herzog H, Holzer P. Evidence from knockout mice that neuropeptide-Y Y2 and Y4 receptor signalling prevents long-term depression-like behavior caused by immune challenge. J Psychopharmacol. 2010;24(10):1551–1560. doi: 10.1177/0269881109348171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Painsipp E, Köfer MJ, Sinner F, Holzer P. Prolonged depression-like behavior caused by immune challenge: influence of mouse strain and social environment. PLoS One. 2011;6(6):e20719. doi: 10.1371/journal.pone.0020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubera M, Curzytek K, Duda W, Leskiewicz M, Basta-Kaim A, Budziszewska B, Roman A, Zajicova A, Holan V, Szczesny E, Lason W, Maes M. A new animal model of (chronic) depression induced by repeated and intermittent lipopolysaccharide administration for 4 months. Brain Behav Immun. 2013;31:96–104. doi: 10.1016/j.bbi.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Tarr AJ, Chen Q, Wang Y, Sheridan JF, Quan N. Neural and behavioral responses to low-grade inflammation. Behav Brain Res. 2012;235(2):334–334. doi: 10.1016/j.bbr.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holzer P, Danzer M, Schicho R, Samberger C, Painsipp E, Lippe IT. Vagal afferent input from the acid-challenged rat stomach to the brainstem: enhancement by interleukin-1beta. Neuroscience. 2004;129:439–445. doi: 10.1016/j.neuroscience.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 26.Hughes PA, Harrington AM, Castro J, Liebregts T, Adam B, Grasby DJ, Isaacs NJ, Maldeniya L, Martin CM, Persson J, Andrews JM, Holtmann G, Blackshaw LA, Brierley SM. Sensory neuro-immune interactions differ between Irritable Bowel Syndrome subtypes. Gut. 2013 doi: 10.1136/gutjnl-2011-301856. in press. [DOI] [PubMed] [Google Scholar]
- 27.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10(2):131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 28.Marques R, Boneca IG. Expression and functional importance of innate immune receptors by intestinal epithelial cells. Cell Mol Life Sci. 2011;68(22):3661–3673. doi: 10.1007/s00018-011-0829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A, Rumio C. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem. 2009;57(11):1013–1023. doi: 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology. 2012;143(4):1006–1016. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Noort JM, Bsibsi M. Toll-like receptors in the CNS: implications for neurodegeneration and repair. Prog Brain Res. 2009;175:139–148. doi: 10.1016/S0079-6123(09)17509-X. [DOI] [PubMed] [Google Scholar]
- 32.Arroyo DS, Soria JA, Gaviglio EA, Rodriguez-Galan MC, Iribarren P. Toll-like receptors are key players in neurodegeneration. Int Immunopharmacol. 2011;11(10):1415–1421. doi: 10.1016/j.intimp.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallard C. Innate immune regulation by toll-like receptors in the brain. ISRN Neurol. 2012;2012:701950. doi: 10.5402/2012/701950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord. 2012;141(1):55–62. doi: 10.1016/j.jad.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 35.Maes M, Twisk FN, Kubera M, Ringel K, Leunis JC, Geffard M. Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome. J Affect Disord. 2012;136(3):909–917. doi: 10.1016/j.jad.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574–581. doi: 10.1002/bies.201100024. [DOI] [PubMed] [Google Scholar]
- 37.Forsythe P, Kunze WA. Voices from within: gut microbes and the CNS. Cell Mol Life Sci. 2013;70(1):55–69. doi: 10.1007/s00018-012-1028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303(11):G1288–G295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- 39.Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C. gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113(2):411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 40.Yurdaydin C, Walsh TJ, Engler HD, Ha JH, Li Y, Jones EA, Basile AS. Gut bacteria provide precursors of benzodiazepine receptor ligands in a rat model of hepatic encephalopathy. Brain Res. 1995;679(1):42–48. doi: 10.1016/0006-8993(95)00241-h. [DOI] [PubMed] [Google Scholar]
- 41.Hu Y, Phelan V, Ntai I, Farnet CM, Zazopoulos E, Bachmann BO. Benzodiazepine biosynthesis in Streptomyces refuineus. Chem Biol. 2007;14(6):691–701. doi: 10.1016/j.chembiol.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Gerratana B. Biosynthesis, synthesis, and biological activities of pyrrolobenzodiazepines. Med Res Rev. 2012;32(2):254–293. doi: 10.1002/med.20212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early-life regulates the hippocampal serotonergic system in a gender-dependent manner. Mol Psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 44.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 45.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reolon GK, Maurmann N, Werenicz A, Garcia VA, Schröder N, Wood MA, Roesler R. Posttraining systemic administration of the histone deacetylase inhibitor sodium butyrate ameliorates aging-related memory decline in rats. Behav Brain Res. 2011;221(1):329–332. doi: 10.1016/j.bbr.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gundersen BB, Blendy JA. Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology. 2009;57(1):67–74. doi: 10.1016/j.neuropharm.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas RH, Meeking MM, Mepham JR, Tichenoff L, Possmayer F, Liu S, MacFabe DF. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J Neuroinflammation. 2012;9:153. doi: 10.1186/1742-2094-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacFabe DF, Cain NE, Boon F, Ossenkopp KP, Cain DP. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res. 2011;217(1):47–54. doi: 10.1016/j.bbr.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90(5):1236–1243. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- 51.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009 Aug;58(8):1091–103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, Cani PD. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacol Ther. 2011;130(2):202–212. doi: 10.1016/j.pharmthera.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 54.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 55.Queipo-Ortuño MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, Casanueva F, Tinahones FJ. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8(5):e65465. doi: 10.1371/journal.pone.0065465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duca FA, Swartz TD, Sakar Y, Covasa M. Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLoS One. 2012;7(6):e39748. doi: 10.1371/journal.pone.0039748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011;23(3):255–264. e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 59.Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, MacQueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 61.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotrophic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 62.Farzi A, Gorkiewicz G, Holzer P. Non-absorbable oral antibiotic treatment in mice affects multiple levels of the microbiota-gut-brain axis. Neurogastroenterol Motil. 2012;24(Suppl 2):78. [Google Scholar]
- 63.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 23(12):1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verdú EF, Bercik P, Verma-Gandhu M, Huang XX, Blennerhassett P, Jackson W, Mao Y, Wang L, Rochat F, Collins SM. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amaral FA, Sachs D, Costa VV, Fagundes CT, Cisalpino D, Cunha TM, Ferreira SH, Cunha FQ, Silva TA, Nicoli JR, Vieira LQ, Souza DG, Teixeira MM. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci USA. 2008;105(6):2193–2197. doi: 10.1073/pnas.0711891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma X, Mao YK, Wang B, Huizinga JD, Bienenstock J, Kunze W. Lactobacillus reuteri ingestion prevents hyperexcitability of colonic DRG neurons induced by noxious stimuli. Am J Physiol Gastrointest Liver Physiol. 2009;296(4):G868–G875. doi: 10.1152/ajpgi.90511.2008. [DOI] [PubMed] [Google Scholar]
- 68.Duncker SC, Kamiya T, Wang L, Yang P, Bienenstock J. Probiotic Lactobacillus reuteri alleviates the response to gastric distension in rats. J Nutr. 2011;141(10):1813–1818. doi: 10.3945/jn.110.136689. [DOI] [PubMed] [Google Scholar]
- 69.Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel JF, Ardid D, Desreumaux P. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13(1):35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 70.Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther. 2011;33(12):1302–1310. doi: 10.1111/j.1365-2036.2011.04665.x. [DOI] [PubMed] [Google Scholar]
- 71.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sathyabama S, Khan N, Agrewala JN. Friendly pathogens: prevent or provoke autoimmunity. Crit Rev Microbiol. 2013 doi: 10.3109/1040841X.2013.787043. in press. [DOI] [PubMed] [Google Scholar]
- 73.Chervonsky AV. Microbiota and autoimmunity. Cold Spring Harb Perspect Biol. 2013;5(3):a007294. doi: 10.1101/cshperspect.a007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479(7374):538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 76.Fetissov SO, Harro J, Jaanisk M, Järv A, Podar I, Allik J, Nilsson I, Sakthivel P, Lefvert AK, Hökfelt T. Autoantibodies against neuropeptides are associated with psychological traits in eating disorders. Proc Natl Acad Sci USA. 2005;102(41):14865–14870. doi: 10.1073/pnas.0507204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fetissov SO, Hamze Sinno M, Coëffier M, Bole-Feysot C, Ducrotté P, Hökfelt T, Déchelotte P. Autoantibodies against appetite-regulating peptide hormones and neuropeptides: putative modulation by gut microflora. Nutrition. 2008;24(4):348–359. doi: 10.1016/j.nut.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fetissov SO, Hamze Sinno M, Coquerel Q, Do Rego JC, Coëffier M, Gilbert D, Hökfelt T, Déchelotte P. Emerging role of autoantibodies against appetite-regulating neuropeptides in eating disorders. Nutrition. 2008;24(9):854–859. doi: 10.1016/j.nut.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 79.Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC) Br J Pharmacol. (5th edn.) 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wettstein JG, Earley B, Junien JL. Central nervous system pharmacology of neuropeptide Y. Pharmacol Ther. 1995;65(3):397–414. doi: 10.1016/0163-7258(95)98598-k. [DOI] [PubMed] [Google Scholar]
- 81.Kask A, Harro J, von Hörsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26(3):259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 82.Redrobe JP, Carvajal C, Kask A, Dumont Y, Quirion R. Neuropeptide Y and its receptor subtypes in the central nervous system: emphasis on their role in animal models of psychiatric disorders. Handb Exp Pharmacol. 2004;162:101–136. [Google Scholar]
- 83.Eaton K, Sallee FR, Sah R. Relevance of neuropeptide Y (NPY) in psychiatry. Curr Top Med Chem. 2007;7(17):1645–1659. doi: 10.2174/156802607782341037. 2007. [DOI] [PubMed] [Google Scholar]
- 84.Morales-Medina JC, Dumont Y, Quirion R. A possible role of neuropeptide Y in depression and stress. Brain Res. 2010;1314:194–205. doi: 10.1016/j.brainres.2009.09.077. [DOI] [PubMed] [Google Scholar]
- 85.Tasan RO, Lin S, Hetzenauer A, Singewald N, Herzog H, Sperk G. Increased novelty-induced motor activity and reduced depression-like behavior in neuropeptide Y (NPY)-Y4 receptor knockout mice. Neuroscience. 2009;158(4):1717–1730. doi: 10.1016/j.neuroscience.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El Karim IA, Linden GJ, Orr DF, Lundy FT. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J Neuroimmunol. 2008;200(1-2):11–16. doi: 10.1016/j.jneuroim.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 87.Bedoui S, von Hörsten S, Gebhardt T. A role for neuropeptide Y (NPY) in phagocytosis: implications for innate and adaptive immunity. Peptides. 2007;28(2):373–376. doi: 10.1016/j.peptides.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 88.Wheway J, Herzog H, Mackay F. NPY and receptors in immune and inflammatory diseases. Curr Top Med Chem. 2007;7(17):1743–1752. doi: 10.2174/156802607782341046. [DOI] [PubMed] [Google Scholar]
- 89.Dimitrijević M, Stanojević S. The intriguing mission of neuropeptide Y in the immune system. Amino Acids. 2013;45(1):41–53. doi: 10.1007/s00726-011-1185-7. [DOI] [PubMed] [Google Scholar]
- 90.Wheway J, Mackay CR, Newton RA, Sainsbury A, Boey D, Herzog H, Mackay F. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J Exp Med. 2005;202(11):1527–1538. doi: 10.1084/jem.20051971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wheway J, Herzog H, Mackay F. The Y1 receptor for NPY: a key modulator of the adaptive immune system. Peptides. 2007;28(2):453–458. doi: 10.1016/j.peptides.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 92.Shibata M, Hisajima T, Nakano M, Goris RC, Funakoshi K. Morphological relationships between peptidergic nerve fibers and immunoglobulin A-producing lymphocytes in the mouse intestine. Brain Behav Immun. 2008;22(2):158–166. doi: 10.1016/j.bbi.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 93.Chandrasekharan B, Bala V, Kolachala VL, Vijay-Kumar M, Jones D, Gewirtz AT, Sitaraman SV, Srinivasan S. Targeted deletion of neuropeptide Y (NPY) modulates experimental colitis. PLoS One. 2008;3(10):e3304. doi: 10.1371/journal.pone.0003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Painsipp E, Herzog H, Sperk G, Holzer P. Sex-dependent control of murine emotional-affective behaviour in health and colitis by peptide YY and neuropeptide Y. Br J Pharmacol. 2011;163(6):1302–1314. doi: 10.1111/j.1476-5381.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pang XH, Li TK, Xie Q, He FQ, Cui DJ, Chen YQ, Huang XL, Gan HT. Amelioration of dextran sulfate sodium-induced colitis by neuropeptide Y antisense oligodeoxynucleotide. Int J Colorectal Dis. 2010;25(9):1047–1053. doi: 10.1007/s00384-010-0964-z. [DOI] [PubMed] [Google Scholar]
- 96.Hassani H, Lucas G, Rozell B, Ernfors P. Attenuation of acute experimental colitis by preventing NPY Y1 receptor signaling. Am J Physiol Gastrointest Liver Physiol. 2005;288(3):G550–G556. doi: 10.1152/ajpgi.00182.2004. [DOI] [PubMed] [Google Scholar]
- 97.Baticic L, Detel D, Kucic N, Buljevic S, Pugel EP, Varljen J. Neuroimmunomodulative properties of dipeptidyl peptidase IV/CD26 in a TNBS-induced model of colitis in mice. J Cell Biochem. 2011;112(11):3322–3333. doi: 10.1002/jcb.23261. [DOI] [PubMed] [Google Scholar]
- 98.Klompus M, Ho W, Sharkey KA, McKay DM. Antisecretory effects of neuropeptide Y in the mouse colon are region-specific and are lost in DSS-induced colitis. Regul Pept. 2010;165(2-3):138–145. doi: 10.1016/j.regpep.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 99.Hirotani Y, Mikajiri K, Ikeda K, Myotoku M, Kurokawa N. Changes of the peptide YY levels in the intestinal tissue of rats with experimental colitis following oral administration of mesalazine and prednisolone. Yakugaku Zasshi. 2008;128(9):347–1353. doi: 10.1248/yakushi.128.1347. [DOI] [PubMed] [Google Scholar]
- 100.Tari A, Teshima H, Sumii K, Haruma K, Ohgoshi H, Yoshihara M, Kajiyama G, Miyachi Y. Peptide YY abnormalities in patients with ulcerative colitis. Jpn J Med. 1988;27(1):49–55. doi: 10.2169/internalmedicine1962.27.49. [DOI] [PubMed] [Google Scholar]
- 101.El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242(5):413–419. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- 102.Schmidt PT, Ljung T, Hartmann B, Hare KJ, Holst JJ, Hellström PM. Tissue levels and post-prandial secretion of the intestinal growth factor, glucagon-like peptide-2, in controls and inflammatory bowel disease: comparison with peptide YY. Eur J Gastroenterol Hepatol. 2005;17(2):207–212. doi: 10.1097/00042737-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 103.Adrian TE, Savage AP, Bacarese-Hamilton AJ, Wolfe K, Besterman HS, Bloom SR. Peptide YY abnormalities in gastrointestinal diseases. Gastroenterology. 1986;90(2):379–384. doi: 10.1016/0016-5085(86)90936-4. [DOI] [PubMed] [Google Scholar]
- 104.Straub RH, Herfarth H, Falk W, Andus T, Schölmerich J. Uncoupling of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis in inflammatory bowel disease? J Neuroimmunol. 2002;126(1-2):116–125. doi: 10.1016/s0165-5728(02)00047-4. [DOI] [PubMed] [Google Scholar]
- 105.Holzer P. Neural regulation of gastrointestinal blood flow. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 5th Edition Academic Press; Oxford: 2012. pp. 817–845. [Google Scholar]
- 106.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130(2):226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maes M, Song C, Yirmiya R. Targeting IL-1 in depression. Expert Opin Ther Targets. 2012;16(11):1097–1112. doi: 10.1517/14728222.2012.718331. [DOI] [PubMed] [Google Scholar]
- 108.Moreau M, André C, O’Connor JC, Dumich SA, Woods JA, Kelley KW, Dantzer R, Lestage J, Castanon N. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav Immun. 2008;22(7):1087–1095. doi: 10.1016/j.bbi.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McCarthy HD, Dryden S, Williams G. Interleukin-1beta-induced anorexia and pyrexia in rat: relationship to hypothalamic neuropeptide Y. Am J Physiol. 1995;269(5 Pt 1):E852–E857. doi: 10.1152/ajpendo.1995.269.5.E852. [DOI] [PubMed] [Google Scholar]
- 110.Sonti G, Ilyin SE, Plata-Salaman CR. Neuropeptide Y blocks and reverses interleukin-1beta-induced anorexia in rats. Peptides. 1996;17(3):517–520. doi: 10.1016/0196-9781(96)00016-2. [DOI] [PubMed] [Google Scholar]
- 111.McMahon CD, Buxton DF, Elsasser TH, Gunter DR, Sanders LG, Steele BP, Sartin JL. Neuropeptide Y restores appetite and alters concentrations of GH after central administration to endotoxic sheep. J Endocrinol. 1999;161(2):333–339. doi: 10.1677/joe.0.1610333. [DOI] [PubMed] [Google Scholar]
- 112.Painsipp E, Köfer MJ, Farzi A, Dischinger US, Sinner F, Sperk G, Herzog H, Holzer P. Neuropeptide Y and peptide YY protect from weight loss caused by Bacille Calmette-Guérin in mice. Br J Pharmacol. 2013 doi: 10.1111/bph.12354. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brumovsky P, Shi TS, Landry M, Villar MJ, Hökfelt T. Neuropeptide tyrosine and pain. Trends Pharmacol. Sci. 2007;28(2):93–102. doi: 10.1016/j.tips.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 114.Smith PA, Moran TD, Abdulla F, Tumber KK, Taylor BK. Spinal mechanisms of NPY analgesia. Peptides. 2007;28(2):464–474. doi: 10.1016/j.peptides.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 115.Painsipp E, Sperk G, Herzog H, Holzer P. Delayed stress-induced differences in locomotor and depression-related behaviour in female neuropeptide-Y Y1 receptor knockout mice. J Psychopharmacol. 2010;24(10):1541–1549. doi: 10.1177/0269881109104851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Solway B, Bose SC, Corder G, Donahue RR, Taylor BK. Tonic inhibition of chronic pain by neuropeptide Y. Proc Natl Acad Sci USA. 2011;108(17):7224–7229. doi: 10.1073/pnas.1017719108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ji RR, Zhang X, Wiesenfeld-Hallin Z, Hökfelt T. Expression of neuropeptide Y and neuropeptide Y (Y1) receptor mRNA in rat spinal cord and dorsal root ganglia following peripheral tissue inflammation. J Neurosci. 1994;14(11 Pt 1):6423–6434. doi: 10.1523/JNEUROSCI.14-11-06423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holzer P. The role of the vagus nerve in afferent signaling and homeostasis during visceral inflammation. In: Jancsó G, editor. Neurogenic Inflammation in Health and Disease. Neuroimmune Biology. Vol. 8. Elsevier; Amsterdam: 2008. pp. 321–338. [Google Scholar]
- 119.Wultsch T, Painsipp E, Thoeringer CK, Herzog H, Sperk G, Holzer P. Endogenous neuropeptide Y depresses the afferent signaling of gastric acid challenge to the mouse brainstem via neuropeptide Y type Y2 and Y4 receptors. Neuroscience. 2005;136(4):1097–1107. doi: 10.1016/j.neuroscience.2005.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou Z, Zhu G, Hariri AR, Enoch M, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452(7190):997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Domschke K, Dannlowski U, Hohoff C, Ohrmann P, Bauer J, Kugel H, Zwanzger P, Heindel W, Deckert J, Arolt V, Suslow T, Baune BT. Neuropeptide Y (NPY) gene: Impact on emotional processing and treatment response in anxious depression. Eur Neuropsychopharmacol. 2010;20(5):301–309. doi: 10.1016/j.euroneuro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 122.Mickey BJ, Zhou Z, Heitzeg MM, Heinz E, Hodgkinson CA, Hsu DT, Langenecker SA, Love TM, Peciña M, Shafir T, Stohler CS, Goldman D, Zubieta JK. Emotion processing, major depression, and functional genetic variation of neuropeptide Y. Arch Gen Psychiatry. 2011;68(2):158–166. doi: 10.1001/archgenpsychiatry.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morgan CA, Rasmusson AM, Winters B, Hauger RL, Morgan J, Hazlett G, Southwick S. Trauma exposure rather than posttraumatic stress disorder is associated with reduced baseline plasma neuropeptide-Y levels. Biol Psychiatry. 2003;54(10):1087–1091. doi: 10.1016/s0006-3223(03)00433-5. [DOI] [PubMed] [Google Scholar]
- 124.Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59(7):660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 125.Sah R, Ekhator NN, Strawn JR, Sallee FR, Baker DG, Horn PS, Geracioti TD. Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol Psychiatry. 2009;66(7):705–707. doi: 10.1016/j.biopsych.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kiank C, Taché Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain Behav Immun. 2010;24(1):41–48. doi: 10.1016/j.bbi.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37(9):1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 128.Forbes S, Herzog H, Cox H. A role for neuropeptide Y in the gender-specific gastrointestinal, corticosterone and feeding responses to stress. Br J Pharmacol. 2012;166(8):2307–2316. doi: 10.1111/j.1476-5381.2012.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hirsch D, Zukowska Z. NPY and stress 30 years later: the peripheral view. Cell Mol Neurobiol. 2012;32(5):645–659. doi: 10.1007/s10571-011-9793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Painsipp E, Wultsch T, Shahbazian A, Edelsbrunner M, Kreissl MC, Schirbel A, Bock E, Pabst MA, Thoeringer CK, Huber HP, Holzer P. Experimental gastritis in mice enhances anxiety in a gender-related manner. Neuroscience. 2007;150(3):522–536. doi: 10.1016/j.neuroscience.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liang C, Luo H, Liu Y, Cao J, Xia H. Plasma hormones facilitated the hypermotility of the colon in a chronic stress rat model. PLoS One. 2012;7(2):e31774. doi: 10.1371/journal.pone.0031774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Field BC, Chaudhri OB, Bloom SR. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol. 2010;6(8):444–453. doi: 10.1038/nrendo.2010.93. [DOI] [PubMed] [Google Scholar]
- 133.Kirchner H, Tong J, Tschöp MH, Pfluger PT. Ghrelin and PYY in the regulation of energy balance and metabolism: lessons from mouse mutants. Am J Physiol Endocrinol Metab. 2010;298(5):E909–E919. doi: 10.1152/ajpendo.00191.2009. [DOI] [PubMed] [Google Scholar]
- 134.Yulyaningsih E, Zhang L, Herzog H, Sainsbury A. NPY receptors as potential targets for anti-obesity drug development. Br J Pharmacol. 2011;163(6):1170–1202. doi: 10.1111/j.1476-5381.2011.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koda S, Date Y, Murakami N, Shimbara T, Hanada T, Toshinai K, Niijima A, Furuya M, Inomata N, Osuye K, Nakazato M. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146(5):2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 136.Ueno H, Yamaguchi H, Mizuta M, Nakazato M. The role of PYY in feeding regulation. Regul Pept. 2008;145(1-3):12–16. doi: 10.1016/j.regpep.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 137.Chee MJ, Colmers WF. Y eat? Nutrition. 2008;24(9):869–877. doi: 10.1016/j.nut.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 138.Ballinger AB, Williams G, Corder R, El-Haj T, Farthing MJ. Role of hypothalamic neuropeptide Y and orexigenic peptides in anorexia associated with experimental colitis in the rat. Clin Sci (Lond) 2001;100(2):221–229. [PubMed] [Google Scholar]
- 139.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11(7):752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450(7166):106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 141.Möller C, Sommer W, Thorsell A, Rimondini R, Heilig M. Anxiogenic-like action of centrally administered glucagon-like peptide-1 in a punished drinking test. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):119–122. doi: 10.1016/s0278-5846(01)00223-8. [DOI] [PubMed] [Google Scholar]
- 142.Kinzig KP, D’Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23(15):6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gulec G, Isbil-Buyukcoskun N, Kahveci N. Effects of centrally-injected glucagon-like peptide-1 on pilocarpine-induced seizures, anxiety and locomotor and exploratory activity in rat. Neuropeptides. 2010;44(4):285–291. doi: 10.1016/j.npep.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 144.Iwai T, Hayashi Y, Narita S, Kasuya Y, Jin K, Tsugane M, Oka J. Antidepressant-like effects of glucagon-like peptide-2 in mice occur via monoamine pathways. Behav Brain Res. 2009;204(1):235–240. doi: 10.1016/j.bbr.2009.06.020. [DOI] [PubMed] [Google Scholar]