Abstract
Neuropeptide Y (NPY) acting via Y1 receptors reduces anxiety- and depression-like behavior in rodents, whereas Y2 receptor stimulation has the opposite effect. This study addressed the implication of Y4 receptors in emotional behavior by comparing female germline Y4 knockout (Y4−/−) mice with control and germline Y2−/− animals. Anxiety-like and depression-like behavior was assessed with the open field (OF), elevated plus-maze (EPM), stress-induced hyperthermia (SIH) and tail suspension test (TST), respectively. Learning and memory was evaluated with the object recognition test (ORT). In the OF and EPM, both Y4−/− and Y2−/−mice exhibited reduced anxiety-related behavior and enhanced locomotor activity relative to control animals. Locomotor activity in a familiar environment was unchanged in Y4−/− but reduced in Y2−/−mice. The basal rectal temperature exhibited diurnal and genotype-related alterations. Control mice had temperature minima at noon and midnight, whereas Y4−/− and Y2−/− mice displayed only one temperature minimum at noon. The magnitude of SIH was related to time of the day and genotype in a complex manner. In the TST, the duration of immobility was significantly shorter in Y4−/− and Y2−/− mice than in controls. Object memory 6 h after initial exposure to the ORT was impaired in Y2−/− but not Y4−/− mice, relative to control mice. These results demonstrate that genetic deletion of Y4 receptors, like that of Y2 receptors, reduces anxiety-like and depression-related behavior. Unlike Y2 receptor knockout, Y4 receptor knockout does not impair object memory. We propose that Y4 receptors play an important role in the regulation of behavioral homeostasis.
Keywords: neuropeptide Y, Y2 receptors, Y4 receptors, anxiety-related behavior, elevated plus maze test, open field test, depression-related behavior, tail-suspension test, home cage activity, object recognition
INTRODUCTION
Neuropeptide Y (NPY) is widely distributed in the central nervous system where it is involved, among others, in the homeostatic regulation of mood, anxiety, stress sensitivity and cognition (Kask et al. 2002; Heilig 2004; Lin et al. 2004; Harro 2006; Karl & Herzog 2007). Its physiological actions are mediated by several classes of NPY receptors, five of which (Y1,Y2,Y4,Y5,Y6) have been elucidated at the gene and protein level (Michel et al. 1998; Redrobe et al. 2004a). Coupled to Gi/o signaling pathways, these Y receptors mediate the functional implications of NPY in the brain.
There is evidence that both Y1 and Y2 receptors are relevant to emotional behavior. Intracerebroventricular injection of NPY reduces anxiety- and depression-related behavior in several animal models, this action being primarily mediated by Y1 receptors (Kask et al. 2002; Redrobe et al. 2002; Heilig 2004; Primeaux et al. 2005). NPY acting via Y2 receptors enhances anxiety- and depression-like behavior as deduced from the behavioral characterization of Y2 receptor knockout (Y2−/−) mice (Redrobe et al. 2003; Tschenett et al. 2003). In addition, Y2 receptors are relevant to cognitive functions, given that Y2−/− mice exhibit impaired performance in the Morris water maze and object recognition tests (Redrobe et al. 2004b).
The possible role of Y4 receptors in the control of affective behavior has not yet been examined. Albeit less widely distributed in the brain than Y1 and Y2 receptors, the presence of Y4 receptors in hypothalamus, limbic system and medullary brainstem (Dumont et al. 1998; Parker & Herzog, 1999; Kask et al. 2002; Fetissov et al. 2004; Heilig 2004; Stanic et al. 2006) is consistent with a putative role of Y4 receptors in emotional and stress-related behavior. Since Y4 receptor-selective antagonists are not yet available, the first and major aim of the present study was to evaluate anxiety-like and depression-related behavior in Y4 receptor knockout (Y4−/−) mice. Anxiety-related behavior was assessed with the open field (OF), elevated plus-maze (EPM) and stress-induced hyperthermia (SIH) tests, while depression-related behavior was evaluated with the tail suspension test (TST). Locomotor activity in the novel and familiar environment of the homecage was also evaluated.
Since Y2−/− mice have a deficit in learning and memory (Redrobe et al. 2004b), the second aim was to test Y4−/− mice for their performance in the object recognition test (ORT) and to compare them with Y2−/− and control mice.
The presence of NPY and Y4 receptors in the hypothalamus led us to ask whether NPY acting via Y4 receptors has an impact on the hypothalamic-pituitary-adrenal (HPA) axis which is involved in the regulation of depression-related behavior (Holsboer 2000). The third study aim was hence to determine the plasma levels of corticosterone at baseline and following exposure to restraint stress in order to obtain an index of HPA axis activity in control and Y4−/− mice.
Since SIH test, TST and the corticosterone response test have not yet been performed with Y2−/− mice, the fourth study aim was to compare control, Y2−/− and Y4−/− mice in their performance in these tests.
METHODS
Experimental animals
This study was carried out with adult female mice, weighing 21 – 33 g, which were housed in groups of 3 – 4 per cage under controlled temperature (21 °C) and a 12 h light/dark cycle (lights on at 6:00 h, lights off at 18:00 h). All experiments were approved by an ethical committee at the Federal Ministry of Science and Research of the Republic of Austria and conducted according to the Directive of the European Communities Council of 24 November 1986 (86/609/EEC). The experiments were designed in such a way that the number of animals used and their suffering was minimized.
Specifically, the experiments were performed with germline Y2−/− and Y4−/− mice and non-induced conditional Y2 and Y4 receptor knockout (FY2 and FY4) mice which were bred in the Department of Pharmacology of the Medical University of Innsbruck (Innsbruck, Austria), while all experiments were carried out at the Medical University of Graz. The generation of Y2−/−, Y4−/−, FY2 and FY4 mice has been described previously (Sainsbury et al. 2002a, 2002c). Germline Y2−/− and Y4−/− mice were generated from the same founders on the same mixed C57BL/6 : 129/SvJ (50 % : 50 %) background as the conditional FY2 and FY4 knockout mice. Germline Y2−/− and Y4−/− mice were obtained by crossing chimeric mice carrying a Y2 floxed gene (Y2lox/lox) or a Y4 floxed gene (Y4lox/lox), respectively, with oocyte-specific Cre recombinase-expressing C57BL/6 mice (Sainsbury et al. 2002a, 2002c). Non-induced conditional FY2 and FY4 knockout mice were used as controls in all experiments and termed control mice throughout the paper. As demonstrated before, these non-induced conditional Y2lox/lox and Y4lox/lox mice do not differ from wild-type mice, as the level of expression of Y2 and Y4 receptors is not influenced by the introduction of the loxP sites (Sainsbury et al. 2002a, 2002c). The deletion or presence of Y2 and Y4 receptors in the germline and non-induced conditional knockout mice was verified by receptor autoradiography using [125I]PYY3-36 and [125I]PP, respectively, in situ hybridization (data not shown) as well as by polymerase chain reaction using oligonucleotide primers recognizing DNA sequences adjacent to the loxP sites flanking the deleted or residing Y2 and Y4 receptor gene (Sainsbury et al. 2002a, 2002c).
As reported previously (Sainsbury et al. 2002a, 2002c; Redrobe et al. 2003; Tschenett et al. 2003), the knockout animals did not have any gross abnormalities, did not exhibit any obvious signs of sensory deficits and appeared healthy. There was no significant difference in the body weight between the different genotypes used in this study.
Experimental protocols
Four studies with three different cohorts of animals of each genotype were performed. In the first study the mice were subjected to a sequence of three behavioral tests spaced apart for at least one week. The series of behavioral tests was started with the EPM test, continued with the TST and completed with the SIH test. This series of behavioral tests was replicated with a second group of animals and, since the results of the two test series were very similar, the data were pooled and are presented as one data set. In the second study the effect of stress on the levels of circulating corticosterone was examined in a separate group of mice of each genotype under study. To this end, the levels of circulating corticosterone were measured in the absence of stress and following a 30 min exposure to moderate restraint stress. In the third study the mice were subjected to the OF test followed by the ORT one week later. The fourth study was carried out to measure locomotor activity in the homecage at the time window during which the TST as well as the EPM and OF tests were performed.
Behavioral tests
Prior to all behavioral tests, the mice were allowed to adapt to the test room (22 ± 1 °C, 50 ± 15 % relative air humidity, lights on at 6:00 h, lights off at 18:00 h, maximal light intensity 100 lux) for at least two days.
Homecage activity
The locomotor activity of mice in the homecage was recorded with a 6 cage LabMaster system (TSE Systems, Bad Homburg, Germany). Each cage was fitted with a photobeam-based activity monitoring system that recorded every ambulatory movement (Theander-Carrillo et al. 2006). Locomotion was evaluated for the time window (10:00 – 14:00 h) during which the TST, EPM and OF tests were carried out. Locomotor activity during this time window was recorded twice. The first recording was made immediately after the animals had been placed for the first time in the homecage, while the second recording was taken two days later when the mice had become familiar with the homecage.
Open field test
The open field consisted of a box (50 × 50 × 30 cm) that was made of opaque gray plastic and illuminated by 80 lux at floor level. The ground area of the box was divided into a 36 × 36 cm central area and the surrounding border zone. Mice were individually placed in a corner of the open field, and their behavior during a 5 min test period was tracked by a video camera positioned above the center of the open field and recorded with the software VideoMot2 (TSE Systems, Bad Homburg, Germany). This software was used to evaluate the time spent in the central area, the number of entries into the central area and the total distance traveled in the central area as well as in the whole open field. The OF test was carried out between 10:00 h and 14:00 h.
Elevated plus-maze test
The animals were placed in the center of a maze with 4 arms arranged in the shape of a plus (Pellow & File 1986; Belzung & Griebel 2001). The maze consisted of a central quadrangle (5 × 5 cm), two opposing open arms (30 cm long, 5 cm wide) and two opposing closed arms of the same size but equipped with 15 cm high walls at their sides and the far end. The device was made of opaque gray plastic and elevated 70 cm above the floor. The light intensity at the central quadrangle was 70 lux, on the open arms 80 lux and in the closed arms 40 lux.
At the beginning of each trial, the animals were placed on the central quadrangle facing an open arm. The movements of the animals during a 5 min test period were tracked by a video camera above the center of the maze and recorded with the software VideoMot2 (TSE Systems, Bad Homburg, Germany). This software was used to evaluate the animal tracks and to determine the number of their entries into the open and closed arms, the time spent on the open and closed arms and the total distance traveled in the open and closed arms during the test session. Entry into an arm was defined as the instance when the mouse placed its four paws on that arm. The EPM test was carried out between 10:00 h and 14:00 h.
Stress-induced hyperthermia test
Measurement of the basal temperature in mice with a rectal probe represents a stressor that causes an increase in the temperature by about 1-1.5 °C within 15 min (Zethof et al. 1994; Olivier et al. 2003). Measurement of the basal temperature (T1) was followed by a second measurement of the temperature (T2) 13 min later. This time interval had been found in pilot experiments to best portray the maximal increase in temperature which returned to baseline levels within the following hour. Being determined with a digital thermometer (BAT-12, Physitemp Instruments, Clifton, New Jersey, USA) equipped with a rectal probe for mice, the stress-induced hyperthermia was calculated as the difference ΔT = T2-T1. Since stress-induced hyperthermia depends both on the time of the day and the light conditions (Peloso et al. 2002), the SIH test was carried out at 4 time slots within a 24 h cycle starting at 7:00 h in the morning. The same mice were consecutively tested at all 4 time slots. The tests at 07:00 – 07:30 h and at 13:00 – 13:30 h were performed at a light intensity of 100 lux. The following two tests were conducted at 19:00 – 19:30 h and 01:00 – 01:30 h at red light conditions, i.e., in complete darkness for the rodents.
Tail suspension test
Following exposure to the inescapable stress of being suspended by their tail, mice first struggle to escape but sooner or later attain a posture of immobility (Steru et al. 1985; Liu & Gershenfeld 2001; Cryan et al. 2005). Mice were suspended by their tail with a 1.9 cm wide strapping tape (OmnitapeR, Paul Hartmann AG, Heidenheim, Germany) to the lever of a force displacement transducer (K30 type 351, Hugo Sachs Elektronik, Freiburg, Germany) which was connected to a bridge amplifier (type 301, Hugo Sachs Elektronik, Freiburg, Germany). The force displacement signals caused by the struggling animal were fed, via an A/D converter (PCI-AD16LC; Kolter Electronic, Erftstadt, Germany), into a personal computer and evaluated with a custom-made software. The sampling frequency was 20 Hz. Each trial took 6 min and was carried out at a light intensity of 20 lux. The total duration of immobility was calculated as the time during which the force of the animals’s movements was below a preset threshold. This threshold was determined to be ± 7 % of the animal’s body weight, and immobility was assumed when at least 4 digits recorded in continuity (equivalent to a time of 0.2 s) were within this threshold range. The validity of the threshold parameters was proved by a highly significant (P < 0.001) Pearson correlation coefficient (r = 0.641) between the software output data and the duration of immobility recorded with a stop watch in 22 animals. The TST was carried out between 10:00 h and 14:00 h.
Object recognition test
The ORT was performed in the open field box (50 × 50 × 30 cm) which was made of opaque grey plastic and illuminated by 80 lux at floor level. The objects to be discriminated were a rough metal tube (outer diameter 3.1 cm, inner diameter 1.9 cm, length 3.8 cm) and a rough teflon column (3.2 × 2.9 × 5.8 cm, length × width × height) with a hole (diameter 1.3 cm) at half-height. After each trial the objects and the open field were cleaned with ethanol (96 %) to eliminate olfactory cues (Dodart et al. 1997). Mice were habituated to the open field for 20 min each day during 4 consecutive days, this period being followed by a pause of 4 days before the ORT. On the test day, two identical objects were placed on the center line of the open field, 9 cm from each box end (Redrobe et al. 2004b). The animals were allowed to explore the two objects for 5 min, during which the exploratory activity directed at each object was tracked by a video camera above the center of the open field and recorded with the software VideoMot2 (TSE Systems, Bad Homburg, Germany). After a delay of 6 h, the animals were reexposed to one familiar object together with a novel object not used in the acquisition phase, and the exploratory behavior directed at each object recorded during another 5 min test period (Redrobe et al. 2004b). The position of each object was alternated between the trials, and the object chosen to be familiar and novel was changed from mouse to mouse. The performance of each mouse was expressed by the memory index (MI) which was calculated according to the formula MI = (tn − to) / (tn + to), where to represents the time exploring the familiar object and tn represents the time exploring the novel object (Redrobe et al. 2004b).
Circulating corticosterone
The plasma levels of corticosterone were determined between 12:00 h and 14:00 h, both at baseline and following exposure to stress. Baseline levels of circulating corticosterone were measured in animals that stayed in their homecage undisturbed until the time of trunk blood collection. For exposure to moderate restraint stress at room temperature, the mice were placed in a tube of 3 cm diameter and 11 cm length, either end of which had an opening of 0.5 cm diameter to permit exchange of air. After a period of 30 min restraint the animals were returned to their home cage for a period of 30 min. At the end of this period the animals were deeply anaesthetized with pentobarbital (100 mg/kg intraperitoneally) and decapitated within 3 min of the pentobarbital injection. The same procedure was used to collect blood for determination of baseline corticosterone levels. Trunk blood was collected into vials coated with ethylenediamine tetraacetate (EDTA; Greiner, Kremsmünster, Austria) kept on ice. Following centrifugation for 20 min at 4 °C and 1200 × g, blood plasma was collected and stored at −20 °C until assay. The plasma levels of corticosterone were determined with an enzyme immunoassay kit (Assay Designs, Ann Arbor, Michigan, USA). According to the manufacturer’s specifications, the sensitivity of the assay was 27 pg/ml, and the intra- and inter-assay coefficient of variation amounted to 7.7 and 9.7 %, respectively.
Statistics
Statistical evaluation of the results was performed on SPSS 14.0 (SPSS Inc., Chicago, IL, USA). Oneway, two-way or three-way analysis of variance (ANOVA), for single or repeated measurements, was used to dissect statistical differences for the factors genotype and, if applicable, time and/or treatment. In case of sphericity violations the Greenhouse-Geisser correction was applied. The homogeneity of variance was analyzed with the Levene test. Post-hoc analysis of group differences was performed with the Tukey HSD (honestly significant difference) test, when the variances were homogeneous, and with the Games-Howell test, when the variances were unequal. Probability values of P < 0.05 were regarded as statistically significant. All data are presented as means ± SEM, n referring to the number of mice in each group.
RESULTS
Homecage activity
Homecage activity was recorded during the photophase from 10:00 to 14:00 h. Locomotor activity recorded immediately after the animals had been placed in the homecage on day 1 (Figure 1A,C) was considerably higher than that on day 3 (Figure 1B,D) when the mice had become familiar with their environment. This difference was seen for control, Y4−/− and Y2−/− mice irrespectively of whether a 1 h period (Figure 1A,B) or a 4 hour period (Figure 1C,D) was analyzed. ANOVA revealed genotype-related differences in locomotion on day 1 (10:00 – 11:00 h: F(2,29) = 8.82, P = 0.001; 10:00 – 14:00 h: F(2,29) = 10.55, P < 0.001) and day 3 (10:00 – 11:00 h: F(2,29) = 2.16, P = 0.13; 10:00 – 14:00 h: F(2,29) = 19.74, P < 0.001). Post-hoc analysis indicated that, in the non-familiar environment, Y4−/− mice but not Y2−/− mice displayed higher locomotor activity than control mice (Figure 1A,C), whereas in the familiar environment Y4−/− mice did not differ from controls and Y2−/− mice moved less than control animals (Figure 1B,D).
Figure 1.
Locomotor activity in the homecage recorded in control, Y4−/− and Y2−/− mice between 10:00 h and 14:00 h. Two recordings were taken from each mouse: immediately after the animals had been placed in the homecage on day 1 (A,C) and two days later (day 3) when the mice had become familiar with the homecage (B,D). The data show the counts of photobeam crossings accumulated for periods of 1 h (A,B) and 4 h (C,D). The values represent means ± SEM, n as indicated in brackets. **P < 0.01 versus control mice.
Open field test
The OF test was first used to examine the locomotor/exploratory and anxiety-related behavior of control, Y4−/− and Y2−/− mice (Figure 2). The time spent in the central area and the number of entries into the central area were considered as indices of anxiety and expressed as a percentage of the total test duration and of the total number of entries into any zone during the whole test session, respectively. ANOVA revealed a genotype-related difference in the time spent in the central area (F(2,23) = 4.585, P = 0.02), and post-hoc analysis showed that Y4−/− as well as Y2−/− mice spent significantly more time in the central area than the control mice (Figure 2A). A similar observation was made with regard to the number of central area entries which exhibited a genotype-related difference (F(2,23) = 10.043, P < 0.001), given that Y4−/−, but not Y2−/− mice entered the central area significantly more often than the control animals (Figure 2B).
Figure 2.
Behavior of Y4−/−, Y2−/− and control mice in the OF test. The graphs show the time spent in the central area (A), the number of entries into the central area (B), and the total distance traveled (C) during the 5 min test session. The time spent in the central area is expressed as a percentage of the total test duration, and the number of entries into the central area is given as a percentage of the total number of entries into any zone during the whole test session. The values represent means ± SEM, n as indicated in brackets. *P < 0.05, **P < 0.01 versus control mice.
Further analysis revealed that knockout of the Y4 receptor gene caused an increase in the locomotor/exploratory activity in the open field (Figure 2C). Thus, the total traveling distance in the open field (F(2,23) = 16.598, P < 0.001) during the test session differed between the genotypes, and post-hoc analysis revealed that Y4−/−, but not Y2−/− mice exhibited greater locomotor activity than the control mice (Figure 2C).
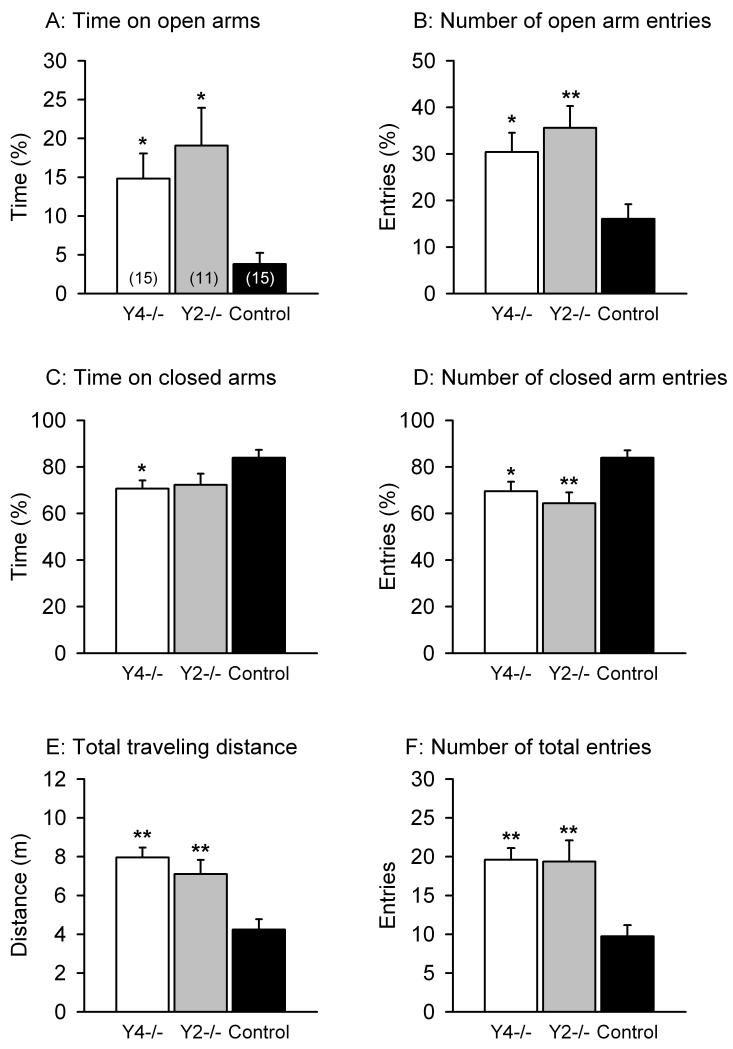
Elevated plus-maze test
The anxiety-related behavior of control, Y4−/− and Y2−/− mice was further assessed with the EPM test (Figure 3A,B,C,D,E,F), in which the time spent on the open arms and the number of entries into the open arms were taken as established indices of anxiety. These parameters were expressed as a percentage of the total time spent on any arm and of the total number of entries into any arm during the 5 min test session. ANOVA demonstrated a genotype-related difference in the time spent on the open arms (F(2,38) = 6.07, P < 0.01). Post-hoc analysis revealed that both Y4−/− and Y2−/− mice spent significantly more time on the open arms than the control mice (Figure 3A). This result was reproduced by the number of open arm entries which exhibited a genotype-related difference (F(2,38) = 6.58, P < 0.01), given that both Y4−/− and Y2−/− mice entered the open arms significantly more often than the control animals (Figure 3B). In contrast, the number of entries into the closed arms was significantly smaller (F(2,38) = 6.58, P < 0.01) in Y4−/− and Y2−/− mice than in the control mice (Figure 3D). Likewise, the time spent on the closed arms was significantly (F(2,38) = 3.88, P < 0.05) shortened in Y4−/− mice, but insignificantly (P = 0.11) reduced in Y2−/− mice (Figure 3C).
Figure 3.
Behavior of Y4−/−, Y2−/− and control mice in the EPM test. The graphs show the time spent on the open arms (A), the number of entries into the open arms (B), the time spent on the closed arms (C), the number of entries into the closed arms (D), the total distance traveled in the open and closed arms (E) and the total number of entries into any arm (F) during the 5 min test session. The time spent on the open or closed arms is expressed as a percentage of the total time spent on any arm, and the number of entries into the open or closed arms is given as a percentage of the total number of entries into any arm during the 5 min test session. The values represent means ± SEM, n as indicated in brackets. *P < 0.05, **P < 0.01 versus control mice.
In order to assess locomotor activity on the EPM, the total distance traveled in the open and closed arms and the total number of entries into any arm during the 5 min test session was analyzed. Both the total traveling distance (F(2,38) = 12.27, P < 0.001) and the number of total arm entries (F(2,38) = 9.84, P < 0.001) differed between the genotypes, and post-hoc analysis revealed that both Y4−/− and Y2−/− mice exhibited greater locomotor activity than the control mice (Figure 3E,F).
Stress-induced hyperthermia test
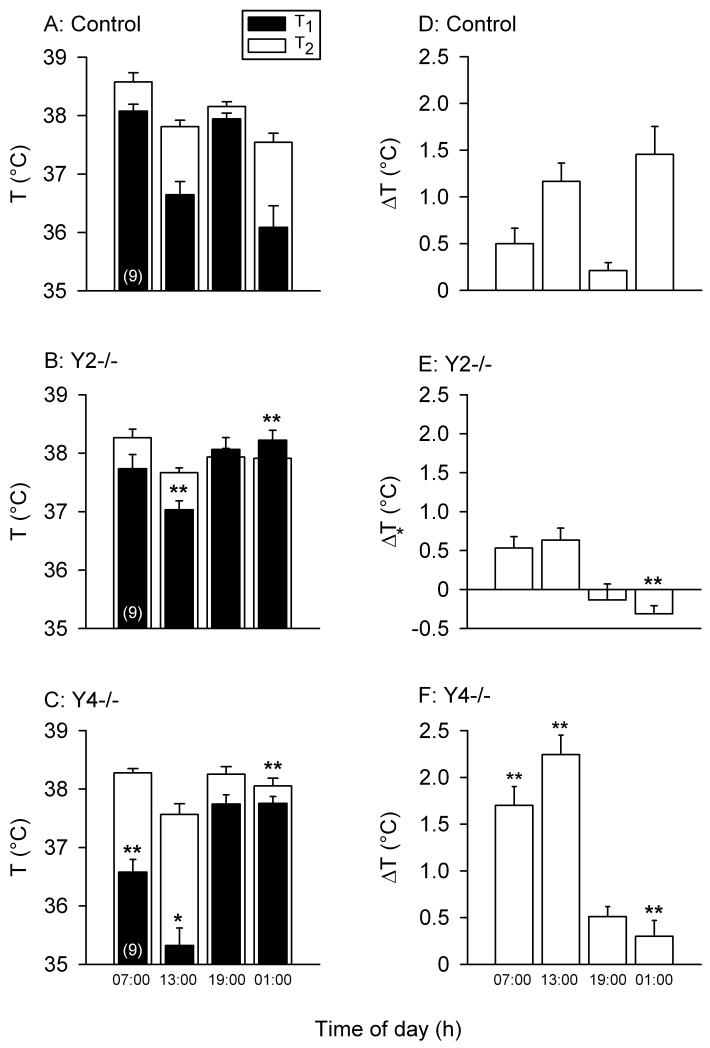
The SIH test was carried out at 4 time slots within a 24 h cycle, i.e., at 07:00 – 07:30 h and 13:00 – 13:30 h at 100 lux as well as at 19:00 – 19:30 h and 01:00 – 01:30 h under red light conditions. The baseline rectal temperature (T1) of control mice showed characteristic diurnal fluctuations (Figure 4A), with minima at 13:00 – 13:30 h and 01:00 – 01:30 h, and maxima at 07:00 – 07:30 h and 19:00 – 19:30 h, i.e., following the change in the light conditions (lights off at 18.00 h and lights on at 06:00 h). ANOVA revealed that T1 differed with regard to both the time of the day (F(3.73,89.60) = 39.81, P < 0.001) and the genotype (F(2,24) = 6.95, P < 0.01). In addition, there was a significant interaction between the factors genotype and time of the day (F(7.47,89.60) = 12.32, P < 0.001). The circadian oscillations in T1 were flattened in Y2−/− mice in which only one minimum at 13.00 h was discernible; in addition, the T1 of Y2−/− animals at 01:00 – 01:30 h was significantly (P < 0.001) higher than that of control animals (Figure 4B). Y4−/− mice had a lower T1 at 07:00 – 07:30 h and 13:00 – 13:30 h and a higher T1 at 01:00 – 01:30 h (P < 0.001) than control mice (Figure 4C).
Figure 4.
Behavior of control, Y2−/− and Y4−/− mice in the SIH test in which the rectal temperature was measured twice at an interval of 13 min. In the graphs on the left-hand side (A,B,C), the black columns depict the rectal temperature recorded at the first measurement (T1), and the white columns show the rectal temperature recorded at the second measurement (T2). The graphs on the right-hand side (D,E,F) show the stress-induced hyperthermia (ΔT = T2 - T1). The values represent means ± SEM, n as indicated in brackets. *P < 0.05 and **P < 0.01 refer to significant differences in T1 and ΔT versus control mice.
Stress induced hyperthermia was determined by a second measurement of rectal temperature (T2) 13 min after recording of T1 and expressed as the difference ΔT = T2 - T1. At this second measurement, the rectal temperature (T2) did not exhibit any genotype-related difference, but there was a significant interaction between the factors genotype and time of the day (F(7.47,89.60) = 12.32, P < 0.001). Thus, T2 varied with the time of the day (F(3.73,89.60) = 39.81, P < 0.001) and exhibited a minimum at 13:00 – 13:30 h (Figure 4A,B,C). ANOVA of ΔT (stress-induced hyperthermia) revealed that this parameter depended both on the time of the day (F(3,96) = 23,76, P < 0.01) and on the genotype (F(2,96) = 32.64, P < 0.01) and that there was a significant interaction between the factors genotype and time of the day (F(6,96) = 10.44, P < 0.01). In control animals, ΔT showed characteristic diurnal fluctuations (Figure 4D), with minima at 07:00 – 07:30 h and 19:00 – 19:30 h and maxima at 13:00 – 13:30 h and 01:00 – 01:30 h. ΔT in Y2−/− mice did not differ from that of control mice, except at the time slot of 01:00 – 01:30 h when there was no longer any stress-induced hyperthermia and the negative ΔT was significantly smaller (P < 0.01) than in control mice (Figure 4E). Y4−/− mice exhibited a higher ΔT (P < 0.01) at 07:00 – 07:30 h and 13:00 – 13:30 h and a lower ΔT (P < 0.01) at 01:00 – 01:30 h than control mice (Figure 4F).
Tail suspension test
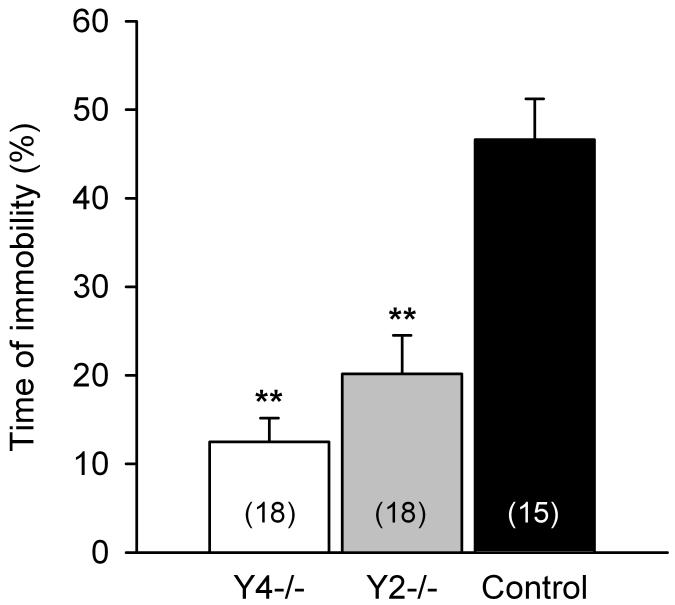
The time of immobility during a 6 min test period was assessed as a measure of depression-like behavior and expressed as a percentage of the test duration. ANOVA revealed that the three genotypes of mice investigated here differed significantly in this parameter (F(2,48) = 19.87, P < 0.001). Specifically, the time that Y4−/− and Y2−/− mice spent immobile was significantly less than that spent immobile by control mice (Figure 5).
Figure 5.
Behavior of control, Y4−/− and Y2−/− mice in the TST. The graph shows the time of immobility during a 6 min test period and is expressed as a percentage of the test duration. The values represent means ± SEM, n as indicated in brackets. **P < 0.01 versus control mice.
Circulating corticosterone levels
The baseline plasma levels of corticosterone determined in control, Y4−/− and Y2−/− mice that stayed in their homecage undisturbed until the time of trunk blood collection did not differ significantly between the three genotypes. Exposure to a moderate restraint stress at room temperature for 30 min caused a significant rise of circulating corticosterone in all mouse genotypes under study (ANOVA for factor treatment: F(1,27) = 89.87, P < 0.001). This stress-induced increase in the plasma levels of corticosterone was similar in control, Y4−/− and Y2−/− mice (Figure 6).
Figure 6.
Corticosterone levels in blood plasma of control, Y4−/− and Y2−/− mice determined at baseline or 30 min after the end of a 30-min exposure to restraint stress. The values represent means ± SEM, n as indicated in brackets. * P < 0.05, ** P < 0.01 versus respective levels at baseline. There were no genotype-related statistically significant differences.
Object recognition test
Figure 7 presents the data of memory performance 6 h after initial object exposure. ANOVA demonstrated that the performance in the ORT, expressed by the MI, differed significantly with the genotype (F(2,21) = 6.299, P = 0.007). Post-hoc analysis showed that the performance of Y2−/− mice in the object recognition task was significantly impaired relative to the performance of control animals, whereas the performance of Y4−/− mice was at least as good as that of control mice (Figure 7). The relative time that the mice spent exploring one or the other object in the initial acquisition phase did not differ significantly in any of the genotypes investigated (data not shown).
Figure 7.
Behavior of control, Y4−/− and Y2−/− mice in the ORT. The graph shows the memory index (MI) which was calculated according to the formula MI = (tn − to) / (tn + to), where to represents the time exploring the familiar object and tn represents the time exploring the novel object. The values represent means ± SEM, n as indicated in brackets. *P < 0.05 versus control mice.
DISCUSSION
The current data show that, relative to control animals, Y4−/− mice exhibit reduced anxiety-like and depression-related behavior on the OF and EPM and in the TST, respectively. These effects of Y4 receptor deletion resemble those of Y2 receptor knockout (Redrobe et al. 2003; Tschenett et al. 2003) and Y2 receptor blockade (Bacchi et al. 2006). In contrast, Y4−/− and Y2−/− mice differ in their cognitive behavior, given that Y4−/− mice perform as well as control animals whereas Y2−/− mice have a deficit in object memory as shown before (Redrobe et al. 2004b).
Relative to control animals, Y4−/− and Y2−/− mice exhibited diminished anxiety-related behavior as assessed in the EPM and OF tests. Knockout of either the Y4 or Y2 receptor gene increased the time spent in the central area of the OF and on the open arms of the EPM. Overall locomotor activity as assessed by the total traveling distance on the EPM was also enhanced in Y4−/− and Y2−/− mice, whereas in the OF test only Y4−/− mice traveled a significantly longer distance than control mice. Although the magnitude of anxiety-related behavior in the EPM and OF tests can be influenced by locomotion (File 2001), we conclude that the anxiolytic effect of Y4 and Y2 receptor deletion is not directly related to increased locomotor activity for a number of reasons. First, Y4 and Y2 receptor gene knockout was associated with a selective increase in open arm entry and open arm time on the EPM, while the respective parameters for the closed arms were diminished. Enhanced locomotor activity in male Y2−/− mice was noted in the OF but not on the EPM (Redrobe et al. 2003; Tschenett et al. 2003). Second, the test-dependent increase in locomotor activity in Y2−/− mice is conceivably related to the decrease in visual attention and increase in impulsivity caused by Y2 receptor knockout (Greco & Carli 2006). Third, the increased locomotion of Y4−/− mice on the OF and EPM seems to be related to the novelty of the test environment, because a similar increase in locomotion was seen when the animals were put into a novel homecage, whereas in a familiar homecage locomotion was unchanged in Y4−/− mice and even decreased in Y2-/ mice.
Most experimental studies of emotional behavior are performed with male rather than female rodents (Palanza 2001). If seen as a model for human disease, this experimental approach is at variance with epidemiological evidence that anxiety and mood disorders have a higher prevalence in women than in men (Palanza 2001; Simonds and Whiffen, 2003; Gorman, 2006). For this reason, we decided to study female mice and to explore the role of Y4 and Y2 receptors in the emotional behavior of this gender. Although the estrus cycle was not determined, we consider it unlikely that our data were biased by this potentially confounding factor. Thus, the experiments were performed in the strict absence of male mice, and the coefficient of variation for the EPM data in female control and Y2−/− mice was not greater than that in male mice of identical genetic background (Tschenett et al. 2003). Furthermore, the behavior of mice on the EPM does not vary significantly with the different phases of the estrus cycle which is synchronized not only among cage mates but also across cages (Painsipp et al. 2007). Fourth, male Y4−/− mice have the same anxiolytic-like and antidepressant-like phenotype as female Y4−/− mice (G. Sperk, personal communication).
NPY acting via Y1 receptors has been involved in the circadian control of homeostatic functions such as motor activity, exploration and anxiety-related behavior (Yannielli & Harrington 2001; Karl et al. 2006). We have found here that knockout of either the Y4 or Y2 receptor has an impact on the diurnal fluctuation of baseline rectal temperature (T1). The high value of T1 in Y4−/− and Y2−/− mice throughout the scotophase is conceivably related to the enhanced intake of water during that period (Wultsch et al. 2006). In keeping with previous data (Sainsbury et al. 2002a, 2002c), our results indicate that the circadian regulation of body temperature and energy homeostasis is altered in Y4−/− and Y2−/− mice, and it awaits to be elucidated which mechanisms (e.g., motor activity, water and food intake) account for the changes in the diurnal T1 profile.
Relative to the EPM test, the SIH test has the advantage of assessing anxiety in a locomotion-independent manner. In the current study, however, this test was complicated by the circadian and genotype-related alterations in T1 and the interaction between these factors. Stress-induced hyperthermia (ΔT) is thought to be a homeostatic reaction that involves the central as well as sympathetic nervous system (Oka et al. 2001; Liu et al. 2003; DiMicco et al. 2006) and depends on light conditions and day time (Peloso et al. 2002). The present study showed that ΔT in control mice was maximal at noon and midnight when T1 was lowest. In Y4−/− and Y2−/− mice stress-induced hyperthermia was practically absent during the dark phase when T1 was highest. It is very likely, therefore, that SIH in Y4−/− and Y2−/− mice during the scotophase has been cut short by a ceiling effect. As a consequence, the SIH test cannot be used to assess anxiety if T1 is changed by the experimental manipulation under study (Painsipp et al. 2007).
In the TST, the immobility time of Y4−/− mice was shortened, which is thought to reflect a reduction of depression-like behavior (Cryan et al. 2005). A similar observation in female Y2−/− mice is consistent with a previous report that male Y2−/− mice spend less time immobile in the forced swim test than control animals (Tschenett et al. 2003).
The deficit of male Y2−/− mice in novel object recognition and object memory (Redrobe et al. 2004b) has been confirmed here with female Y2−/− mice. Since Y4−/− mice failed to display a similar cognitive impairment, Y4 receptors do not seem to play a significant role in nonspatial working memory which the ORT is thought to evaluate (Ennaceur & Delacour 1988; Dodart et al. 1997; Redrobe et al. 2004b). The cognitive deficits associated with Y2 receptor knockout are consistent with the region-specific effects of intracerebral NPY injections and the amnesia resulting from NPY overexpression in the hippocampus (Flood et al. 1989; Thorsell et al. 2000; Redrobe et al. 2004a). A more complete analysis of cognition in Y4−/− mice was beyond the scope of this study.
NPY as well as Y2 and Y4 receptors are present in the hypothalamus including the paraventricular nucleus (Dumont et al. 1998; Parker & Herzog 1999; Fetissov et al. 2004), in which knockout of the Y2 receptor causes a decrease in corticotropin-releasing factor (CRF) mRNA expression (Sainsbury et al. 2002b). There is evidence that NPY also interacts with CRF in the amygdala (Heilig 2004; Sajdyk et al. 2004, 2006) and that emotional-affective behavior is regulated by both extrahypothalamic and hypothalamic CRF, the latter controlling HPA axis activity (Holsboer 2000; de Kloet 2003; Cryan & Mombereau 2004; Shekhar et al. 2005). Since neither baseline nor stress-induced release of corticosterone, the neuroendocrine endpoint of HPA axis activity, was altered by Y4 or Y2 receptor knockout, the behavioral alterations in Y4−/− and Y2−/− mice appear to be unrelated to alterations in HPA axis activity. This claim needs to be substantiated, however, by a more detailed analysis of the release profile of corticosterone (Müller et al. 2003; Oshima et al. 2003).
The similarity in the emotional traits of Y2−/− and Y4−/− mice raises the question as to the location of the Y4 and Y2 receptors involved and the nature of their endogenous ligand. While Y2 receptors have high affinity for NPY and peptide YY, Y4 receptors are particularly sensitive to pancreatic polypeptide (PP) (Lundell et al. 1996; Michel et al. 1998; Redrobe et al. 2004a). NPY has anxiolytic and antidepressant actions which are primarily mediated by Y1 receptors (Kask et al. 2002; Redrobe et al. 2002; Heilig 2004; Sajdyk et al. 2004, 2006; Primeaux et al. 2005). The anxiolytic-like and antidepressant-like effect of Y2 receptor knockout is most probably due to deletion of presynaptic Y2 receptors, which will disinhibit the release of NPY and other transmitters and thus lead to an increased drive at Y1 receptors (Redrobe et al. 2003; Tschenett et al. 2003; Heilig 2004; Sajdyk et al. 2004). Microinjection experiments (Sajdyk et al. 2002) and region-specific deletion of Y2 receptors (Tasan et al. 2007) indicate that the action of Y2 receptors to modify anxiety- and depression-like behavior takes place in the amygdala.
Compared with Y2 receptors, Y4 receptors are less abundant in the brain and their functional implications are little understood because of a lack of selective Y4 receptor antagonists. Although PP, the preferential agonist at Y4 receptors, is largely absent from the brain, Y4 receptors have been localized to the medial and basolateral amygdala, ventral tegmental area, hippocampus, hypothalamus, locus coeruleus and medulla of the rodent brain (Dumont et al. 1998; Parker & Herzog 1999; Campbell et al. 2003; Fetissov et al. 2004). In the hypothalamus, Y4 receptors are involved in presynaptic inhibition of transmitter release (Acuna-Goycolea et al. 2005), a mechanism that could explain why Y4 receptor knockout results in similar alterations of emotional behavior as Y2 receptor deletion. The anxiolytic-like phenotype of Y4−/− mice is consistent with the anxiogenic phenotype of PP-overexpressing mice (Ueno et al. 2007). Since intracerebroventricular PP fails to alter anxiety-related behavior (Asakawa et al. 1999) while chronic peripheral administration of PP reduces anxiety (Asakawa et al. 2003), it is conceivable that PP modifies anxiety- and depression-like behavior via an action in the periphery or in the area postrema outside the blood-brain barrier (Larsen & Kristensen 1997; Dumont et al. 2007). In this context it is worth mentioning that both Y2−/− and Y4−/− mice exhibit increased levels of circulating PP (Sainsbury et al. 2002a, 2002c).
In conclusion, our data show that deletion of Y4 receptors, like that of Y2 receptors, reduces anxiety-like and depression-related behavior. Although developmental compensations in germline gene knockout mice may be a confounding factor, our data indicate that, if such adaptations occurred, they were insufficient to balance the deficit in Y4 and Y2 receptors, respectively. This instance attests to a novel and important role of Y4 receptors in the control of emotional behavior and diurnal homeostasis and warrants further examination of Y4 receptor function at the cellular level and exploration of Y4 receptors as a novel drug target.
ACKNOWLEDGEMENTS
This study was supported by the Zukunftsfonds Steiermark (grant 262), the Austrian Scientific Research Funds (FWF grants L25-B05 and S-10204), and the Deutsche Forschungsgemeinschaft (grant KFO 125/1-1). The authors thank Professor Günther Sperk (Medical University of Innsbruck, Austria) for critically reading the manuscript, Dr. Michael Trappitsch (Graz, Austria) for help in the development of the TST software and Dr. Christoph K. Thoeringer (Max Planck Institute of Psychiatry, Munich, Germany) for advice on the behavioral experiments. The support by the director and staff of the Center for Medical Research (ZMF I) of the Medical University of Graz is greatly appreciated.
REFERENCES
- Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol AN. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J Neurosci. 2005;25:7406–7419. doi: 10.1523/JNEUROSCI.1008-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Ueno N, Fujimiya M, Fujino MA, Kasuga M. Mouse pancreatic polypeptide modulates food intake, while not influencing anxiety in mice. Peptides. 1999;20:1445–1448. doi: 10.1016/s0196-9781(99)00155-2. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Yuzuriha H, Ueno N, Katsuura G, Fujimiya M, Fujino MA, Niijima A, Meguid MM, Kasuga M. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology. 2003;124:1325–1336. doi: 10.1016/s0016-5085(03)00216-6. [DOI] [PubMed] [Google Scholar]
- Bacchi F, Mathe AA, Jimenez P, Stasi L, Arban R, Gerrard P, Caberlotto L. Anxiolytic-like effect of the selective Neuropeptide Y Y2 receptor antagonist BIIE0246 in the elevated plus-maze. Peptides. 2006;27:3202–3207. doi: 10.1016/j.peptides.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Campbell RE, Smith MS, Allen SE, Grayson BE, Ffrench-Mullen JM, Grove KL. Orexin neurons express a functional pancreatic polypeptide Y4 receptor. J Neurosci. 2003;23:1487–1497. doi: 10.1523/JNEUROSCI.23-04-01487.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiat. 2004;9:326–57. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Hormones, brain and stress. Endocr Regul. 2003;37:51–68. [PubMed] [Google Scholar]
- DiMicco JA, Sarkar S, Zaretskaia MV, Zaretsky DV. Stress-induced cardiac stimulation and fever: common hypothalamic origins and brainstem mechanisms. Auton Neurosci. 2006;126-127:106–119. doi: 10.1016/j.autneu.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Mathis C, Ungerer A. Scopolamine-induced deficits in a two-trial object recognition task in mice. NeuroReport. 1997;8:1173–1178. doi: 10.1097/00001756-199703240-00023. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Jacques D, Bouchard P, Quirion R. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J Comp Neurol. 1998;402:372–384. [PubMed] [Google Scholar]
- Dumont Y, Moyse E, Fournier A, Quirion R. Distribution of peripherally injected peptide YY ([125I] PYY (3-36)) and pancreatic polypeptide ([125I] hPP) in the CNS: enrichment in the area postrema. J Mol Neurosci. 2007;33:294–304. doi: 10.1007/s12031-007-9007-9. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Kopp J, Hökfelt T. Distribution of NPY receptors in the hypothalamus. Neuropeptides. 2004;38:175–188. doi: 10.1016/j.npep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- Flood JF, Baker ML, Hernandez EN, Morley JE. Modulation of memory processing by neuropeptide Y varies with brain injection site. Brain Res. 1989;503:73–82. doi: 10.1016/0006-8993(89)91706-x. [DOI] [PubMed] [Google Scholar]
- Gorman JM. Gender differences in depression and response to psychotropic medication. Gend Med. 2006;3:93–109. doi: 10.1016/s1550-8579(06)80199-3. [DOI] [PubMed] [Google Scholar]
- Greco B, Carli M. Reduced attention and increased impulsivity in mice lacking NPY Y2 receptors: relation to anxiolytic-like phenotype. Behav Brain Res. 2006;169:325–334. doi: 10.1016/j.bbr.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Harro J. CCK and NPY as anti-anxiety treatment targets: promises, pitfalls, and strategies. Amino Acids. 2006;31:215–230. doi: 10.1007/s00726-006-0334-x. [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Karl T, Burne TH, Herzog H. Effect of Y1 receptor deficiency on motor activity, exploration, and anxiety. Behav Brain Res. 2006;167:87–93. doi: 10.1016/j.bbr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Karl T, Herzog H. Behavioral profiling of NPY in aggression and neuropsychiatric diseases. Peptides. 2007;28:326–333. doi: 10.1016/j.peptides.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Kristensen P. The neuropeptide Y (Y4) receptor is highly expressed in neurones of the rat dorsal vagal complex. Mol Brain Res. 1997;48:1–6. doi: 10.1016/s0169-328x(97)00069-7. [DOI] [PubMed] [Google Scholar]
- Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38:189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biol Psychiat. 2001;49:575–581. doi: 10.1016/s0006-3223(00)01028-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Peprah D, Gershenfeld HK. Tail-suspension induced hyperthermia: a new measure of stress reactivity. J Psychiat Res. 2003;37:249–259. doi: 10.1016/s0022-3956(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Lundell I, Statnick MA, Johnson D, Schober DA, Starbäck P, Gehlert DR, Larhammar D. The cloned rat pancreatic polypeptide receptor exhibits profound differences to the orthologous receptor. Proc Natl Acad Sci USA. 1996;93:5111–5115. doi: 10.1073/pnas.93.10.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- Müller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kuhn R, Reul JM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nature Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Oka T, Oka K, Hori T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosom Med. 2001;63:476–486. doi: 10.1097/00006842-200105000-00018. [DOI] [PubMed] [Google Scholar]
- Olivier B, Zethof T, Pattij T, van Boogaert M, van Oorschot R, Leahy C, Oosting R, Bouwknecht A, Veening J, van der Gugten J, Groenink L. Stress-induced hyperthermia and anxiety: pharmacological validation. Eur J Pharmacol. 2003;463:117–132. doi: 10.1016/s0014-2999(03)01326-8. [DOI] [PubMed] [Google Scholar]
- Oshima A, Flachskamm C, Reul JM, Holsboer F, Linthorst AC. Altered serotonergic neurotransmission but normal hypothalamic-pituitary-adrenocortical axis activity in mice chronically treated with the corticotropin-releasing hormone receptor type 1 antagonist NBI 30775. Neuropsychopharmacology. 2003;28:2148–2159. doi: 10.1038/sj.npp.1300267. [DOI] [PubMed] [Google Scholar]
- Painsipp E, Wultsch T, Shahbazian A, Edelsbrunner M, Kreissl MC, Schirbel A, Bock E, Pabst MA, Thoeringer CK, Huber HP, Holzer P. Experimental gastritis in mice enhances anxiety in a gender-related manner. Neuroscience. 2007;150:522–536. doi: 10.1016/j.neuroscience.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Peloso E, Wachulec M, Satinoff E. Stress-induced hyperthermia depends on both time of day and light condition. J Biol Rhythms. 2002;17:164–170. doi: 10.1177/074873002129002456. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, Cusick MC, York DA, Wilson MA. Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology. 2005;30:1589–1597. doi: 10.1038/sj.npp.1300705. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Fournier A, Quirion R. The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY-induced antidepressant-like activity in the mouse forced swimming test. Neuropsychopharmacology. 2002;26:615–624. doi: 10.1016/S0893-133X(01)00403-1. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Herzog H, Quirion R. Neuropeptide Y (NPY) Y2 receptors mediate behaviour in two animal models of anxiety: evidence from Y2 receptor knockout mice. Behav Brain Res. 2003;141:251–255. doi: 10.1016/s0166-4328(02)00374-1. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Carvajal C, Kask A, Dumont Y, Quirion R. Neuropeptide Y and its receptor subtypes in the central nervous system: emphasis on their role in animal models of psychiatric disorders. In: Michel MC, editor. Neuropeptide Y and Related Peptides. Springer; Berlin: 2004a. pp. 101–136. (Handbook of Experimental Pharmacology Volume 162). [Google Scholar]
- Redrobe JP, Dumont Y, Herzog H, Quirion R. Characterization of neuropeptide Y, Y2 receptor knockout mice in two animal models of learning and memory processing. J Mol Neurosci. 2004b;22:159–166. doi: 10.1385/JMN:22:3:159. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Fetissov S, Fürtinger S, Jenkins A, Cox HM, Sperk G, Hökfelt T, Herzog H. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc Natl Acad Sci USA. 2002a;99:8938–8943. doi: 10.1073/pnas.132043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Herzog H. Y2 receptor deletion attenuates the type 2 diabetic syndrome of ob/ob mice. Diabetes. 2002b;51:3420–3427. doi: 10.2337/diabetes.51.12.3420. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, Herzog H. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Development. 2002c;16:1077–1088. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Fitz SD, Shekhar A. The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in the rat. Stress. 2006;9:21–28. doi: 10.1080/10253890600557315. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Smiley DL, Gehlert DR. Neuropeptide Y-Y2 receptors mediate anxiety in the amygdala. Pharmacol Biochem Behav. 2002;71:419–423. doi: 10.1016/s0091-3057(01)00679-7. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A, Gehlert DR. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38:225–234. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8:209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- Simonds VM, Whiffen VE. Are gender differences in depression explained by gender differences in co-morbid anxiety? J Affect Disord. 2003;77:197–202. doi: 10.1016/s0165-0327(02)00113-1. [DOI] [PubMed] [Google Scholar]
- Stanic D, Brumovsky P, Fetissov S, Shuster S, Herzog H, Hökfelt T. Characterization of neuropeptide Y2 receptor protein expression in the mouse brain. I. Distribution in cell bodies and nerve terminals. J Comp Neurol. 2006;499:357–390. doi: 10.1002/cne.21046. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berlin) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Tasan RO, Weger S, Heilbronn R, Nguyen NK, Singewald N, Herzog H, Sperk G. Experiments to localize the site for the anxiogenic action of NPY mediated by Y2 receptors in the mouse brain. BMC Pharmacol. 2007;7(Suppl 2):A14. [Google Scholar]
- Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda TR, Muzzin P, Schürmann A, Szanto I, Tschöp MH, Rohner-Jeanrenaud F. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest. 2006;116:1983–1993. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, Mathe AA, Heilig M. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci USA. 2000;97:12852–12857. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschenett A, Singewald N, Carli M, Balducci C, Salchner P, Vezzani A, Herzog H, Sperk G. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur J Neurosci. 2003;18:143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- Ueno N, Asakawa A, Satoh Y, Inui A. Increased circulating cholecystokinin contributes to anorexia and anxiety behavior in mice overexpressing pancreatic polypeptide. Regul Pept. 2007;141:8–11. doi: 10.1016/j.regpep.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Wultsch T, Painsipp E, Donner S, Sperk G, Herzog H, Peskar BA, Holzer P. Selective increase of dark phase water intake in neuropeptide-Y Y2 and Y4 receptor knockout mice. Behav Brain Res. 2006;168:255–260. doi: 10.1016/j.bbr.2005.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannielli PC, Harrington ME. Neuropeptide Y in the mammalian circadian system: effects on light-induced circadian responses. Peptides. 2001;22:547–556. doi: 10.1016/s0196-9781(01)00356-4. [DOI] [PubMed] [Google Scholar]
- Zethof TJ, Van der Heyden JA, Tolboom JT, Olivier B. Stress-induced hyperthermia in mice: a methodological study. Physiol Behav. 1994;55:109–115. doi: 10.1016/0031-9384(94)90017-5. [DOI] [PubMed] [Google Scholar]