Abstract
Neuropeptide Y (NPY) is involved in the regulation of emotional behavior, and there is indirect evidence for a role of NPY in the cerebral responses to peripheral immune challenge. Since the NPY receptors involved in these reactions are not known, we investigated the effect of E. coli lipopolysaccharide (LPS) on emotional, locomotor and social behavior, body temperature and circulating corticosterone in female Y2 (Y2−/−) and Y4 (Y4−/−) receptor knockout mice. LPS (0.1 mg/kg injected IP 2.5 h before testing) increased rectal temperature in control and Y4−/− mice to a larger degree than in Y2−/− animals. Both Y2−/− and Y4−/− mice exhibited reduced anxiety-related and depression-like behavior in the open field, elevated plus maze and tail suspension test, respectively. While depression-like behavior was not changed by LPS, anxiety-related behavior was enhanced by LPS in Y2−/−, but not control and Y4−/− animals. Y2−/− mice were also particularly susceptible to the effect of LPS to attenuate locomotor behavior and social interaction with another mouse. The corticosterone response to LPS was blunted in Y2−/− mice which presented elevated levels of circulating corticosterone following vehicle treatment. These data show that Y2−/− mice are particularly sensitive to the effects of LPS-evoked immune stress to attenuate locomotion and social interaction and to increase anxiety-like behavior, while the LPS-induced rise of temperature and circulating corticosterone is suppressed by Y2 receptor knockout. Our observations attest to an important role of endogenous NPY acting via Y2 receptors in the cerebral response to peripheral immune challenge.
Keywords: Neuropeptide-Y Y2 receptors, neuropeptide-Y Y4 receptors, bacterial lipopolysaccharide (endotoxin), fever, anxiety-related behavior, locomotor behavior, depression-like behavior, social interaction, corticosterone
INTRODUCTION
Neuropeptide Y (NPY) is a messenger widely distributed in the peripheral and central nervous system. Its many functional implications include the control of sympathetic nervous system activity and immune function and the central regulation of energy balance, cognition, mood, anxiety and stress sensitivity (Kask et al., 2002; Heilig, 2004; Lin et al., 2004; Karl and Herzog, 2007; Bedoui et al., 2007). The physiological actions of NPY are mediated by several classes of NPY receptors, five of which (Y1, Y2, Y4, Y5 and Y6) have been elucidated at the gene and protein level (Michel et al., 1998; Redrobe et al., 2004). Coupled to Gi/o signaling pathways, these Y receptors mediate the biologic actions of NPY.
Gene knockout studies have revealed that endogenous NPY acting via Y2 and Y4 receptors is involved in the regulation of anxiety, stress coping and energy homeostasis. Thus, anxiety- and depression-like behavior is significantly reduced in Y2 receptor knockout (Y2−/−) mice (Redrobe et al., 2003; Tschenett et al., 2003), and a similar anxiolytic and antidepressant phenotype has been observed in Y4 receptor knockout (Y4−/−) mice (Painsipp et al., 2008).
There is indirect evidence that NPY-expressing neurons in the arcuate and paraventricular nuclei of the hypothalamus participate in the behavioral responses to immune stress and infection (McCarthy et al., 1995; Sonti et al., 1996; McMahon et al., 1999; Konsman and Dantzer, 2001; Romanovsky et al., 2005). These reactions are embodied in the term “sickness response” which is mediated by proinflammatory cytokines such as interleukin-1β and tumor necrosis-factor-α, which can excite vagal afferent neurons but also directly gain access to the brain (Goehler et al., 2000; Konsman et al., 2002; Romanovsky et al., 2005). As a result, fever, anorexia, a decrease in locomotor activity and social interaction and other pathophysiological changes (e.g., release of adrenal corticosteroids, altered brain monoamine activity and sleep disturbances) are brought about as typical features of the sickness response. This reaction can be reproduced by intraperitoneal (IP) injection of bacterial lipopolysaccharide (LPS; endotoxin) which causes the generation of proinflammatory cytokines. The behavioral responses to peripheral immune challenge involve changes in the central monoamine systems and in the hypothalamic-pituitary-adrenal (HPA) axis (Lacosta et al., 1999; Turnbull and Rivier, 1999; Dunn et al., 2005).
Although NPY has been implicated in the sickness response (Konsman and Dantzer, 2001; Romanovsky et al., 2005), the NPY receptors mediating the link between peripheral immune system and brain have not yet been characterized. Therefore, the overall aim of this study was to investigate whether some behavioral effects of LPS are altered by deletion of the Y2 or Y4 receptor gene. Our study addressed 5 specific issues in control, Y2−/− and Y4−/− mice: the influence of LPS on (i) fever, (ii) locomotor and anxiety-related behavior, (iii) social interaction, (iv) depression-like behavior, and (v) circulating corticosterone levels post-stress.
METHODS
Experimental animals
This study was carried out with age-matched adult female mice which were housed under controlled temperature (21 °C), relative air humidity (50 ± 15 %) and light conditions (lights on at 7:00 h, lights off at 19:00 h, maximal intensity 150 lux). The experimental procedures and number of animals used in this study were approved by an ethical committee at the Federal Ministry of Science and Research of the Republic of Austria and conducted according to the Directive of the European Communities Council of 24 November 1986 (86/609/EEC). The experiments were designed in such a way that the number of animals used and their suffering was minimized. For this reason, only female mice were studied because in a preceding study only female control, Y2−/− and Y4−/−mice had been phenotyped with regard to their emotional-affective behavior (Painsipp et al., 2008). The limited number of animals approved for the study made it necessary to inject LPS repeatedly to the animals in order to examine its influence on the behavioral parameters under study.
The germline Y2−/− and Y4−/− mice and non-induced conditional Y2 and Y4 receptor knockout (FY2 and FY4) mice were bred in the Department of Pharmacology of the Medical University of Innsbruck (Innsbruck, Austria), while all experiments were carried out at the Medical University of Graz. The genetic design of these animals has been described previously (Sainsbury et al., 2002a; Sainsbury et al., 2002c). Germline Y2−/− and Y4−/− mice were generated from the same founders on the same mixed C57BL/6: 129/SvJ (50 %: 50 %) background as the conditional FY2 and FY4 knockout mice. Germline Y2−/− and Y4−/− mice were obtained by crossing chimeric mice carrying a Y2 floxed gene (Y2lox/lox) or a Y4 floxed gene (Y4lox/lox), respectively, with oocyte-specific Cre recombinase-expressing C57BL/6 mice (Sainsbury et al., 2002a; Sainsbury et al., 2002c). Non-induced conditional FY2 and FY4 knockout mice were used as controls in all experiments and termed control mice throughout the paper. As demonstrated before, these non-induced conditional Y2lox/lox and Y4lox/lox mice do not differ from wild-type mice, as the level of expression of Y2 and Y4 receptors is not influenced by the introduction of the loxP sites (Sainsbury et al., 2002a; Sainsbury et al., 2002c). The deletion or presence of Y2 and Y4 receptors in the germline and non-induced conditional knockout mice was verified by receptor autoradiography using [125I]PYY3-36 and [125I]PP, respectively, in situ hybridization (data not shown) as well as by polymerase chain reaction using oligonucleotide primers recognizing DNA sequences adjacent to the loxP sites flanking the deleted or residing Y2 and Y4 receptor gene (Sainsbury et al., 2002a; Sainsbury et al., 2002c).
For the social interaction test, adult female mice of the outbred strain Him:OF1 (Division of Laboratory Animal Science and Genetics, Department of Biomedical Research, Medical University of Vienna, Himberg, Austria) were used as the partners which the test mice could interact with.
Experimental protocols
Three different cohorts of animals of each genotype were used to address the questions under study. The first cohort of animals was used to examine the ability of LPS (0.1 mg/kg administered IP 2.5 h before the behavioral tests), relative to vehicle, to modify (i) locomotor and anxiety-related behavior in the open field (OF) and social interaction with another mouse of the same age and gender but different genotype, elevated plus-maze (EPM) and stress-induced hyperthermia (SIH) tests, (ii) rectal temperature, (iii) depression-like behavior in the tail suspension test (TST), and (iv) circulating corticosterone levels following exposure to the TST. In order to avoid tolerance to endotoxin (Beishuizen and Thijs, 2003), LPS was administered IP to the mice at intervals of at least 2 weeks, each administration being followed by a different test. The series of tests was started with the EPM test, continued with the SIH test, followed by the OF test combined with the social interaction test, and completed with the TST test combined with determination of circulating corticosterone 45 min post-TST.
This series of tests under the influence of LPS was replicated with a second cohort of animals. Since the results of the two test series were very similar, the data were pooled and are presented as one data set. A third cohort of animals was used to examine the effect of a high dose of LPS (0.83 mg/kg administered IP 2.5 h before the test) on OF behavior in naïve control and Y2−/− mice, i.e., in animals that had not been exposed to LPS before.
Throughout the experiments the animals were housed in groups of 3 – 4 animals per cage. After completion of each test, the animals were immediately returned to their cage mates in the home cage. Care was taken not to change the cage mates during the experiments.
Administration of lipopolysaccharide
LPS extracted from E. coli 0127:B8 (Sigma, Vienna, Austria) was dissolved in pyrogen-free sterile saline (0.9 % NaCl) at a concentration of 1 mg/ml. This stock solution was diluted with pyrogen-free sterile saline to yield injection solutions of 0.01 and 0.083 mg/ml LPS, which were injected IP at a volume of 0.01 ml/g, equivalent to doses of 0.1 and 0.83 mg/kg LPS, respectively. Pyrogen-free sterile saline injected at the same volume was used as vehicle control. LPS was administered 2.5 h before the tests in question were carried out. This interval was chosen because the effect of LPS to depress social interaction has previously been found to become maximal 2 – 3 h post-LPS (Fishkin and Winslow, 1997; Konsman et al., 2000).
Behavioral tests
General precautions
Prior to all behavioral tests, the mice were allowed to adapt to the test room (22 ± 1 °C, 50 ± 15 % relative air humidity, lights on at 7:00 h, lights off at 19:00 h, maximal light intensity 100 lux) for at least two days. The EPM, OF and social interaction test and TST were performed 2.5 h after the IP injection of LPS during the period of 10:30 to 13:30 h. The SIH test was carried out between 13:00 h and 13:30 h.
Elevated plus-maze test
The animals were placed in the center of a maze with 4 arms arranged in the shape of a plus (Belzung and Griebel, 2001). The maze consisted of a central quadrangle (5 × 5 cm), two opposing open arms (30 cm long, 5 cm wide) and two opposing closed arms of the same size but equipped with 15 cm high walls at their sides and the far end. The device was made of opaque gray plastic and elevated 70 cm above the floor. The light intensity at the central quadrangle was 70 lux, on the open arms 80 lux and in the closed arms 40 lux.
At the beginning of each trial, the animals were placed on the central quadrangle facing an open arm. The movements of the animals during a 5 min test period were tracked by a video camera above the center of the maze and recorded with the software VideoMot2 (TSE Systems, Bad Homburg, Germany). This software was used to evaluate the animal tracks and to determine the number of their entries into the open arms, the time spent on the open arms, the total distance traveled on the EPM and the total number of entries into any arm. Entry into an arm was defined as the instance when the mouse placed its four paws on that arm.
Open field and social interaction test
The OF consisted of a box (50 × 50 × 30 cm) that was made of opaque gray plastic and illuminated by 80 lux at floor level. The ground area of the box was divided into a 36 × 36 cm central area and the surrounding border zone. Mice were individually placed in a corner of the OF, and their behavior during a 5 min test period was tracked by a video camera positioned above the center of the OF and recorded with the software VideoMot2 (TSE Systems, Bad Homburg, Germany). This software was used to evaluate the time spent in the central area, the number of entries into the central area and the total distance traveled in the OF.
In the experiments, the mice were placed in the OF arena for two consecutive 5 min periods. During the first period, the locomotor behavior of the test mice was recorded in the absence of another mouse. During the second period, the behavior of the test mice was evaluated in the presence of a female partner mouse which the test mice could interact with. The time spent in the central area, the number of entries into the central area, the total distance traveled and the number of social contacts which the test mouse initiated with the partner mouse were counted. Social contacts were defined as direct body-to-body contacts.
Stress-induced hyperthermia test
Measurement of the basal temperature in mice with a rectal probe represents a stressor that causes an increase in the temperature by about 1-1.5 °C within 15 min (Olivier et al., 2003). Measurement of the basal temperature (T1) was followed by a second measurement of the temperature (T2) 13 min later. This time interval had been found in pilot experiments to best portray the maximal increase in temperature which returned to baseline levels within the following hour. Rectal temperature was determined with a digital thermometer (BAT-12, Physitemp Instruments, Clifton, New Jersey, USA) equipped with a rectal probe for mice. The stress-induced rise of temperature was expressed as the difference ΔT = T2-T1. Since SIH depends both on the time of the day and the light conditions (Peloso et al., 2002), the SIH test was carried out at 13:00 – 13:30 h when the stress-induced rise of temperature is maximal.
Tail suspension test
Following exposure to the inescapable stress of being suspended by their tail, mice first struggle to escape but sooner or later attain a posture of immobility (Cryan et al., 2005). Mice were suspended by their tail with a 1.9 cm wide strapping tape (OmnitapeR, Paul Hartmann AG, Heidenheim, Germany) to the lever of a force displacement transducer (K30 type 351, Hugo Sachs Elektronik, Freiburg, Germany) which was connected to a bridge amplifier (type 301, Hugo Sachs Elektronik, Freiburg, Germany). The force displacement signals caused by the struggling animal were fed, via an A/D converter (PCI-AD16LC; Kolter Electronic, Erftstadt, Germany), into a personal computer and evaluated with a custom-made software. The sampling frequency was 20 Hz. Each trial took 6 min and was carried out at a light intensity of 20 lux. The total duration of immobility was calculated as the time during which the force of the animal’s movements was below a preset threshold. This threshold was determined to be ± 7 % of the animal’s body weight, and immobility was assumed when at least 4 digits recorded in continuity (equivalent to a time of 0.2 s) were within this threshold range. The validity of the threshold parameters was proved by a highly significant (P < 0.001) Pearson correlation coefficient (r = 0.641) between the software output data and the duration of immobility recorded with a stop watch in 22 animals.
Circulating corticosterone
The plasma levels of corticosterone were determined between 12:00 h and 14:00 h, 45 min after the TST had begun. The animals were deeply anaesthetized with pentobarbital (150 mg/kg IP) before they were decapitated. Trunk blood was collected into vials coated with ethylenediamine tetraacetate (EDTA; Greiner, Kremsmünster, Austria) kept on ice. Following centrifugation for 20 min at 4 °C and 1200 × g, blood plasma was collected and stored at −20 °C until assay. The plasma levels of corticosterone were determined with an enzyme immunoassay kit (Assay Designs, Ann Arbor, Michigan, USA). According to the manufacturer’s specifications, the sensitivity of the assay is 27 pg/ml, and the intra- and inter-assay coefficient of variation amount to 7.7 and 9.7 %, respectively.
Statistics
Statistical evaluation of the results was performed on SPSS 14.0 (SPSS Inc., Chicago, Illinois, USA). Since differences in the emotional behavior between Y2−/− and Y4−/− mice have previously been reported (Redrobe et al., 2003; Tschenett et al., 2003; Painsipp et al., 2008), all data were analyzed by planned comparisons (Kirk, 1995) and two-way or three-way analysis of variance (ANOVA). If planned comparisons and ANOVA yielded the same results, only those obtained by ANOVA are reported. Planned comparisons were made with the t-test or one-way ANOVA. Whenever two-way or three-way ANOVA was performed, the homogeneity of variances was assessed with the Levene test. If a significant interaction between the test factors (genotype, treatment, and time in the SIH test) was found, post-hoc analysis of group differences was made with the Tukey HSD (honestly significant difference) test or, in case of inhomogeneity of variances, with the Games-Howell test. In view of the exploratory nature of the study, probability values ≤ 0.1 (Winer et al., 1991; Kirk, 1995; Hays, 2007) were regarded as statistically significant. All data are presented as means ± SEM, n referring to the number of mice in each group.
RESULTS
General observations
As reported previously (Sainsbury et al., 2002a; Sainsbury et al., 2002b; Sainsbury et al., 2002c; Redrobe et al., 2003; Tschenett et al., 2003), Y2−/− and Y4−/− mice did not have any gross abnormalities, did not exhibit any obvious signs of sensory deficits and appeared healthy. There was, however, a significant difference in the body weight between the three genotypes under study (F(2,59) = 21.22, P < 0.001). Thus, both Y2−/− (24.2 ± 0.48 g, n = 17) and Y4−/− (23.5 ± 0.44 g, n = 22) animals had a lower body weight than control mice (27.6 ± 0.55 g, n = 22). In none of the experimental groups did the body weight change significantly (P > 0.1) during the course of the experiments.
Effect of LPS on behavior in the EPM test
As shown in Figure 1, Y2−/− and Y4−/− mice differed from control mice in their behavior on the EPM, and LPS had a particular effect in Y2−/− mice.
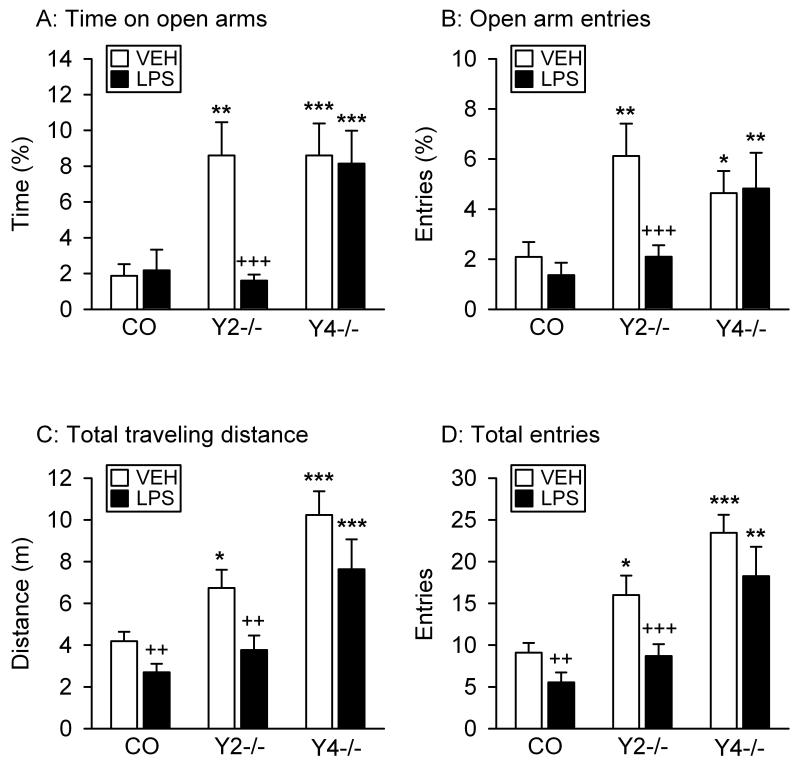
Figure 1.
Effect of LPS on the behavior of control (CO), Y2−/− and Y4−/− mice in the EPM test. LPS (0.1 mg/kg) or vehicle (VEH, sterile saline) was injected IP 2.5 h before the behavioral test. The graphs show (A) the time spent on the open arms, (B) the number of entries into the open arms, (C) the total distance traveled, and (D) the total number of entries into any arm. The parameters in panels A and B are expressed as a percentage of the total time spent on any arm and of the total number of entries into any arm, respectively. The values represent means ± SEM, n = 8 – 11. * P ≤ 0.1, ** P < 0.05, *** P < 0.01 versus control mice with the same treatment, ++ P < 0.05, +++ P < 0.01 versus vehicle-treated mice of the same genotype.
Two-way ANOVA revealed that the time spent on the open arms differed with genotype (F(2,56) = 11.10, P < 0.001) and treatment (F(1,56) = 4.35, P = 0.04), with a significant interaction between these factors (F(2,56) = 3.84, P = 0.03). Post-hoc analysis confirmed that vehicle-treated Y2−/− and Y4−/− mice spent more time on the open arms than vehicle-treated control mice (Figure 1A). LPS had no effect in control and Y4−/− mice, but reduced the time which Y2−/− mice spent on the open arms to that recorded in control mice (Figure 1A).
Similarly, the number of entries into the open arms varied according to genotype (F(2,56) = 6.23, P = 0.004) and treatment (F(1,56) = 4.06, P = 0.05), with a significant interaction between these factors (F(2,56) = 2.86, P = 0.08). Post-hoc analysis showed that vehicle-treated Y2−/− and Y4−/− mice entered the open arms more often than vehicle-treated control mice (Figure 1B). LPS did not modify this parameter in control and Y4−/− mice, but reduced the number of open arm entries of Y2−/− mice to that of control mice (Figure 1B).
The changes in open arm behavior were paralleled by changes in the total distance traveled on the open and closed arms and the total number of entries into any arm during the test session (Figure 1C,D). Planned comparisons indicated that vehicle-treated Y2−/− mice and in particular vehicle-treated Y4−/− animals traveled longer distances than vehicle-treated control mice (Figure 1C). LPS shortened the total traveling distance in control and Y2−/− mice, but not in Y4−/− animals (Figure 1C). Two-way ANOVA confirmed that the total traveling distance differed with genotype (F(2,56) = 19.58, P < 0.001) and treatment (F(1,56) = 9.67, P = 0.003), without a significant interaction between these factors.
Similar observations were made with regard to the total number of entries into any arm (Figure 1D). As revealed by planned comparisons, vehicle-treated Y2−/− and Y4−/− mice entered any arm more often than vehicle-treated control mice, and LPS reduced the number of total arm entries in control and Y2−/− animals, but not in Y4−/− mice (Figure 1D). Two-way ANOVA unveiled differences related to genotype (F(2,56) = 21.64, P < 0.001) and treatment (F(1,56) = 9.19, P = 0.004), without a significant interaction between these factors.
Effect of LPS on behavior in the SIH test
In this test rectal temperature was recorded twice, before (T1) and after an interval of 13 min (T2). Planned comparisons showed that baseline rectal temperature (T1) of vehicle-treated control, Y2−/− and Y4−/− mice did not differ from each other (Figure 2A). LPS enhanced T1 in control and Y4−/− mice to a larger extent than in Y2−/− mice, and T1 in LPS-treated Y2−/− mice was significantly lower than in LPS-treated control mice (Figure 2A). T2 of LPS-treated Y2−/− mice differed from T2 of LPS-treated control animals to a larger extent than T2 of LPS-treated Y4−/− mice (Figure 2B).
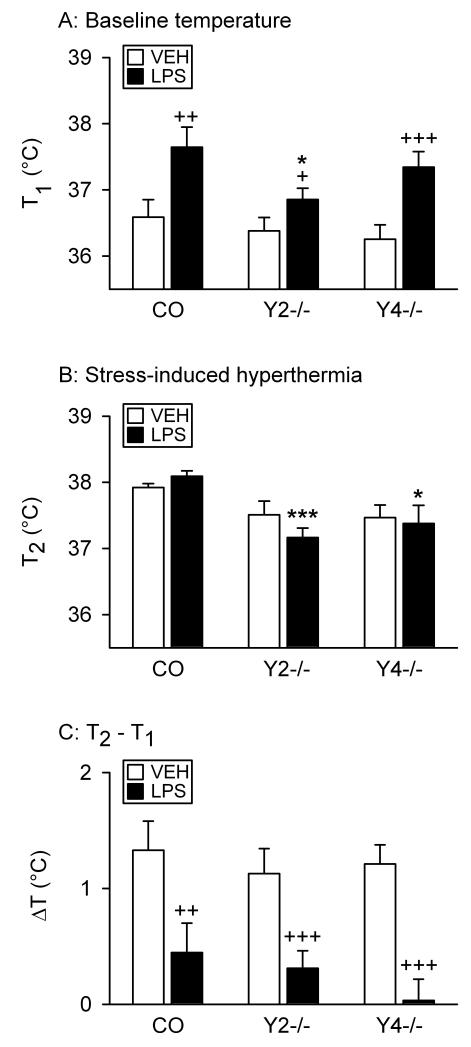
Figure 2.
Effect of LPS on the behavior of control (CO), Y2−/− and Y4−/− mice in the SIH test. LPS (0.1 mg/kg) or vehicle (VEH, sterile saline) was injected IP 2.5 h before the behavioral test. In the test, the rectal temperature was measured twice at an interval of 13 min. The graphs show (A) the baseline temperature (T1) recorded at the first measurement, (B) the temperature (T2) recorded at the second measurement, and (C) the SIH defined as ΔT = T2 - T1. The values represent means ± SEM, n = 9 – 11. * P ≤ 0.1, *** P < 0.01 versus control mice with the same treatment, + P ≤ 0.1, ++ P < 0.05, +++ P < 0.01 versus vehicle-treated mice of the same genotype.
These results were consistent with the outcome of three-way ANOVA which revealed differences in rectal temperature related to time (F(1,53) = 71.39, P < 0.001), genotype (F(2,53) = 6.01, P = 0.004) and treatment (F(1,53) = 7.12, P = 0.01), with a strong interaction between time and treatment (F(1,53) = 29.76, P < 0.001) but without a significant interaction between treatment and genotype, time and genotype as well as time, genotype and treatment. Using two-way ANOVA we found that T1 did not differ with genotype but was significantly modified by treatment (F(1,53) = 19.50, P < 0.001), without any significant interaction between these factors. T2 differed with genotype (F(2,53) = 9.28, P < 0.001) but not treatment, without a significant interaction between these factors.
The stress-induced rise of temperature was expressed as the difference ΔT = T2 - T1. Planned comparisons revealed that, independently of genotype, ΔT in vehicle-treated animals was significantly higher than in LPS-treated mice in which SIH was largely absent (Figure 2C). Similarly, two-way ANOVA indicated that ΔT varied with treatment (F(1,53) = 29.76, P < 0.001) but not genotype, without a significant interaction between these factors.
Effect of LPS on behavior in the OF test
Y2−/− and Y4−/− mice differed from control mice in their behavior on the OF, with LPS having an effect on 2 of the 3 OF parameters recorded in Y2−/− mice (Figure 3).
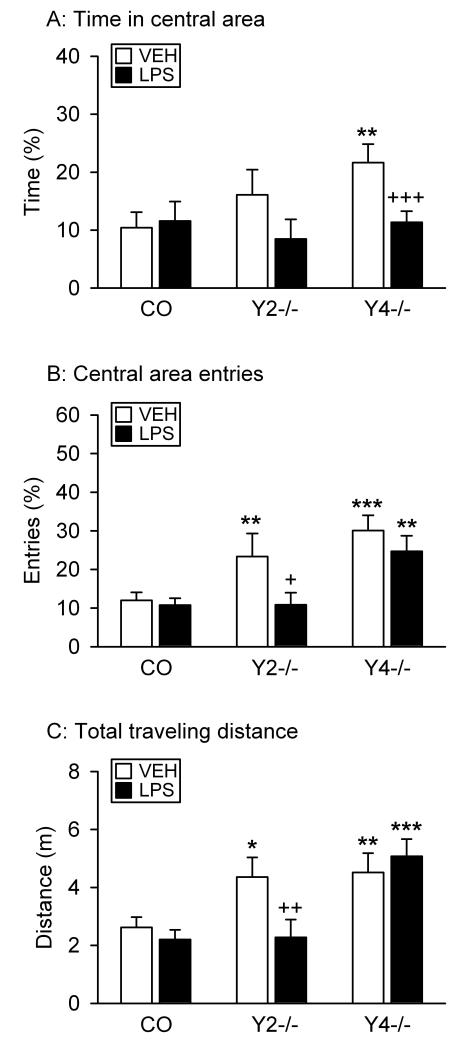
Figure 3.
Effect of LPS on the behavior of control (CO), Y2−/− and Y4−/− mice in the OF test. LPS (0.1 mg/kg) or vehicle (VEH, sterile saline) was injected IP 2.5 h before the behavioral test. The graphs show (A) the time spent in the central area, (B) the number of entries into the central area, and (C) the total distance traveled. The parameters in panels A and B are expressed as a percentage of the total time spent in the OF and of the total number of entries into any zone, respectively. The values represent means ± SEM, n = 7 – 13. * P ≤ 0.1, ** P < 0.05, *** P < 0.01 versus control mice with the same treatment, + P ≤ 0.1, ++ P < 0.05, +++ P < 0.01 versus vehicle-treated mice of the same genotype.
Planned comparisons confirmed that vehicle-treated Y4−/− but not Y2−/− mice spent more time in the central area of the OF than vehicle-treated control mice (Figure 3A). LPS had no significant effect in control and Y2−/− mice, but reduced the time which Y4−/− mice spent in the central area (Figure 3A). Two-way ANOVA of the time spent in the central area revealed differences related to treatment (F(1,62) = 4.55, P = 0.04) but not genotype, without a significant interaction between these factors.
Vehicle-treated Y2−/− and Y4−/− mice entered the central area of the OF more often than vehicle-treated control mice (Figure 3B). Planned comparisons unveiled that LPS did not modify this parameter in control and Y4−/− mice, but reduced the number of entries of Y2−/− mice to that counted in control mice (Figure 3B). A similar result emerged from two-way ANOVA, given that the number of entries into the central area differed with genotype (F(2,62) = 11.52, P < 0.001) and treatment (F(1,62) = 4.41, P = 0.04), without a significant interaction between these factors.
The changes in central area entries were paralleled by changes in the total distance traveled in the OF (Figure 3C). Two-way ANOVA indicated that this parameter varied with genotype (F(2,62) = 11.04, P < 0.001) but not treatment, and that there was a significant interaction between these factors (F(2,62) = 2.51, P = 0.09). Post-hoc analysis showed that vehicle-treated Y2−/− and Y4−/− mice traveled longer distances than vehicle-treated control mice (Figure 3C). LPS shortened the total traveling distance in Y2−/− mice, but not in control and Y4−/− animals (Figure 3C).
Effect of LPS on behavior in the combined OF and social interaction test
After observation of the exploratory/locomotor behavior of the test mice on the OF in the absence of another mouse (Figure 3), a partner animal was introduced and the exploratory behavior of the test mice recorded during a second 5 min period (Figure 4). In addition, the social interaction of the test mice with the partner mouse was recorded (Figure 5).
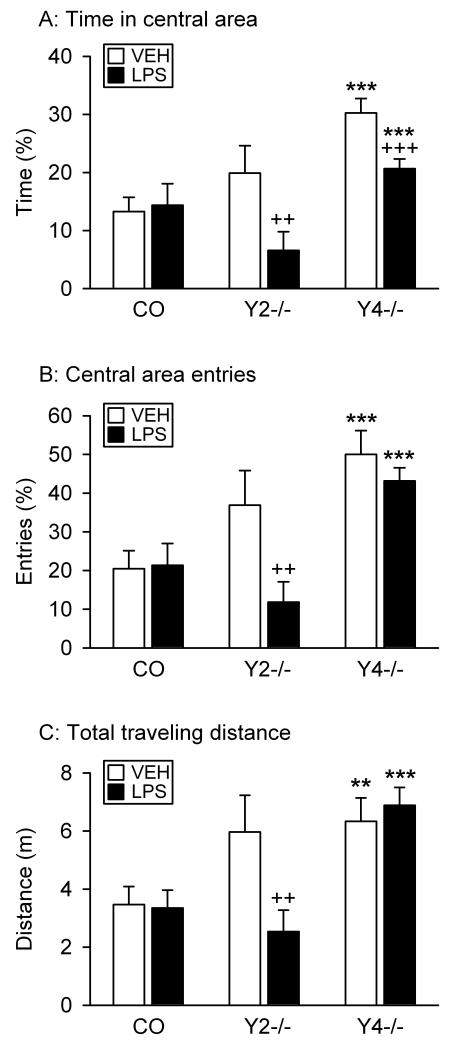
Figure 4.
Effect of LPS on the behavior of control (CO), Y2−/− and Y4−/− mice in the OF test in the presence of a partner mouse. LPS (0.1 mg/kg) or vehicle (VEH, sterile saline) was injected IP 2.5 h before the behavioral test. The graphs show (A) the time spent in the central area, (B) the number of entries into the central area, and (C) the total distance traveled. The parameters in panels A and B are expressed as a percentage of the total time spent in the OF and of the total number of entries into any zone, respectively. The values represent means ± SEM, n = 7 – 13. ** P < 0.05, *** P < 0.01 versus control mice with the same treatment, ++ P < 0.05, +++ P < 0.01 versus vehicle-treated mice of the same genotype.
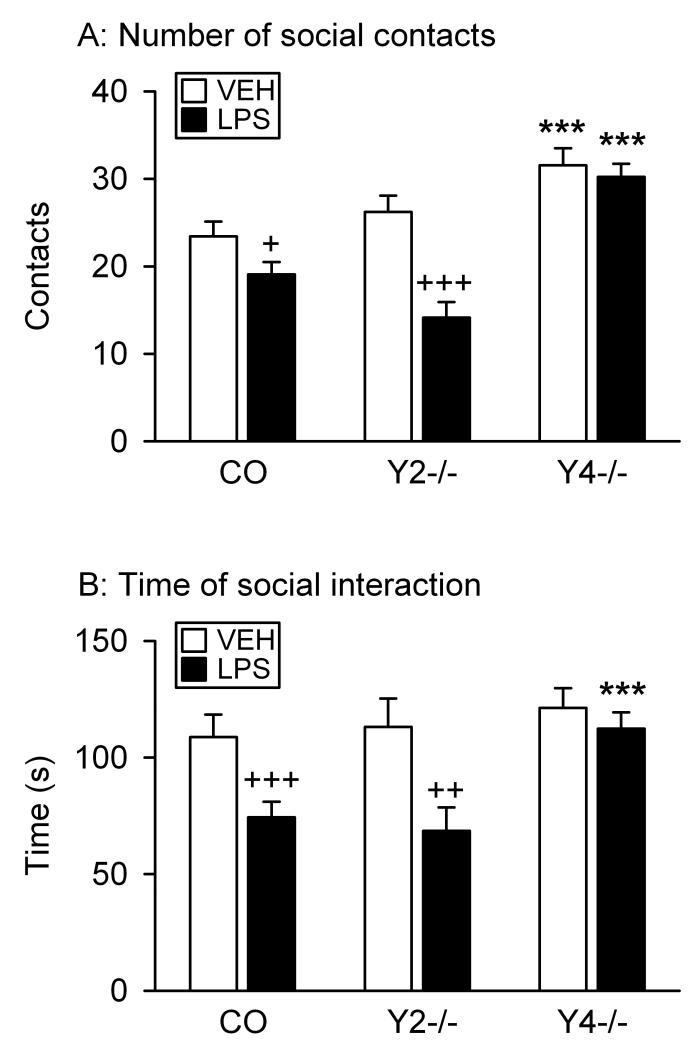
Figure 5.
Effect of LPS on the behavior of control (CO), Y2−/− and Y4−/− mice in the social interaction test. LPS (0.1 mg/kg) or vehicle (VEH, sterile saline) was injected IP 2.5 h before the behavioral test. The social contacts which the test mice initiated with the partner animal were counted during a 5 min test period. The graphs show (A) the number and (B) the total duration of social contacts. The values represent means ± SEM, n = 7 – 13. *** P < 0.01 versus control mice with the same treatment, + P ≤ 0.1, ++ P < 0.05, +++ P < 0.01 versus vehicle-treated mice of the same genotype.
The locomotor behavior of the test mice in the presence of a partner showed the same genotype- and treatment-related pattern as that observed in the absence of a partner (compare Figures 3 and 4). Two-way ANOVA revealed that the time spent in the central area of the OF differed with genotype (F(2,62) = 10.72, P < 0.001) and treatment (F(1,62) = 8.10, P = 0.006), with a significant interaction between these factors (F(2,62) = 2.95, P = 0.06). Post-hoc analysis confirmed that vehicle-treated Y4−/− but not Y2−/− mice spent more time in the central area than vehicle-treated control mice (Figure 4A). LPS had no effect in control animals, but reduced the time which Y2−/− and Y4−/− mice spent in the central area, its effect being particularly pronounced in Y2−/− mice (Figure 4A).
The number of entries into the central area varied likewise with genotype (F(2,62) = 13.04, P < 0.001) and treatment (F(1,62) = 4.63, P = 0.04), but there was no significant interaction between these factors. Post-hoc analysis showed that vehicle-treated Y4−/− but not Y2−/− mice entered the central area more often than vehicle-treated control mice (Figure 4B). LPS did not modify this behavior in control and Y4−/− mice, but suppressed it in Y2−/− mice (Figure 4B).
The changes in central area exploration were associated with changes in the total distance traveled in the OF during the test session (Figure 4C). Two-way ANOVA of this parameter uncovered differences related to genotype (F(2,62) = 10.44, P < 0.001) but not treatment, with a significant interaction between these factors (F(2,62) = 3.11, P = 0.05). Post-hoc analysis showed that vehicle-treated Y4−/− mice traveled longer distances than vehicle-treated control mice (Figure 4C). LPS shortened the total traveling distance in Y2−/− mice, but not in control and Y4−/− animals (Figure 4C).
The number of social contacts initiated by the test mice differed with genotype (F(2,61) = 24.46, P < 0.001) and treatment (F(1,61) = 16.77, P < 0.001), and there was a significant interaction between these factors (F(2,61) = 4.38, P = 0.02). Post-hoc analysis revealed that vehicle-treated Y4−/− but not Y2−/− mice initiated more social contacts than vehicle-treated control animals (Figure 5A). LPS had no effect on the number of social contacts in Y4−/− mice, but reduced it in control mice and particularly in Y2−/− animals (Figure 5A).
A similar result emerged with regard to the duration of social contacts (Figure 5B) which differed with genotype (F(2,61) = 5.93, P = 0.004) and treatment (F(1,61) = 15.53, P < 0.001), while there was no significant interaction between these factors. Post-hoc analysis revealed that there was no significant difference between vehicle-treated control, Y2−/− and Y4−/− mice with regard to the time of social interaction (Figure 5B). LPS reduced this parameter in control and Y2−/− but not Y4−/− mice (Figure 5B).
Effect of LPS on behavior in the TST
The time which vehicle-treated Y2−/− and Y4−/− mice spent immobile in the TST was significantly less than that spent immobile by vehicle-treated control mice (Figure 6A). Planned comparisons also showed that LPS failed to modify TST behavior in any of the genotypes studied (Figure 6A). Two-way ANOVA confirmed that the time of immobility varied with genotype (F(2,58) = 47.64, P < 0.001) but not treatment and that there was no significant interaction between these factors.
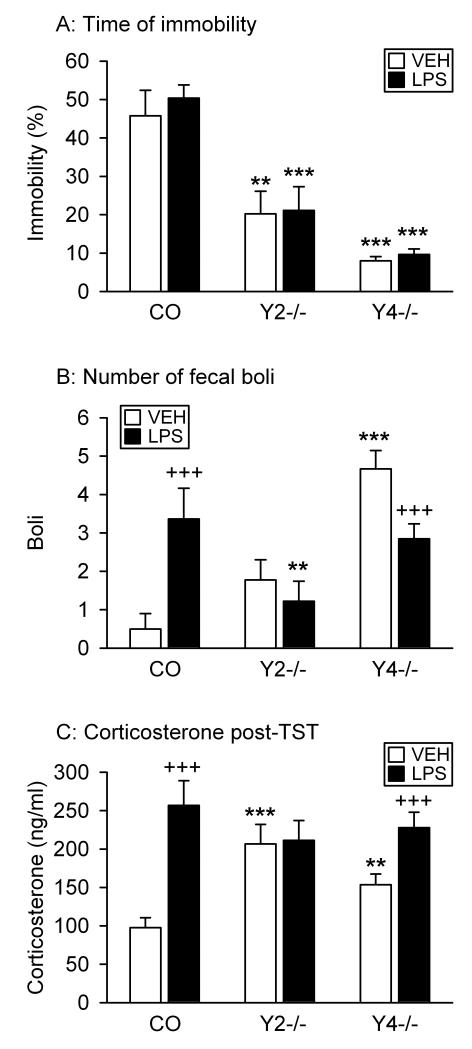
Figure 6.
Effect of LPS on the behavior of control (CO), Y2−/− and Y4−/− mice in the TST. LPS (0.1 mg/kg) or vehicle (VEH, sterile saline) was injected IP 2.5 h before the behavioral test. The graphs show (A) the time of immobility in the TST, (B) the number of fecal boli shed during the TST, and (C) circulating corticosterone 45 min post-TST. The time of immobility in the TST is expressed as a percentage of the total test duration. The values represent means ± SEM, n = 9 – 13. ** P < 0.05, *** P < 0.01 versus control mice with the same treatment, +++ P < 0.01 versus vehicle-treated mice of the same genotype.
In addition to immobility, the number of fecal boli shed by the mice during the TST was counted (Figure 6B). As indicated by two-way ANOVA, defecation differed with genotype (F(2,58) = 10.42, P < 0.001) but not treatment, and there was a significant interaction between these factors (F(2,58) = 10.55, P < 0.001). Post-hoc analysis revealed that vehicle-treated Y4−/− but not Y2−/− mice expelled more fecal boli than vehicle-treated control animals (Figure 6B). LPS enhanced defecation in control animals, failed to modify it in Y2−/− mice and reduced it in Y4−/− mice (Figure 6B).
Effect of LPS on circulating corticosterone levels
The circulating levels of corticosterone, determined 45 min after exposure to the TST, differed with treatment (F(1,50) = 19.67, P < 0.001) but not genotype. In addition, there was a significant interaction between these factors (F(2,50) = 5.99, P = 0.005). Post-hoc analysis indicated that the corticosterone levels in vehicle-treated Y2−/− and Y4−/− mice were significantly higher than in vehicle-treated control animals (Figure 6C). LPS caused the corticosterone concentration to rise significantly in control and Y4−/− but not Y2−/− mice (Figure 6C). As a result, the corticosterone levels in LPS-treated animals did not differ between the three genotypes (Figure 6C).
Effect of a high dose of LPS on OF behavior in naïve animals
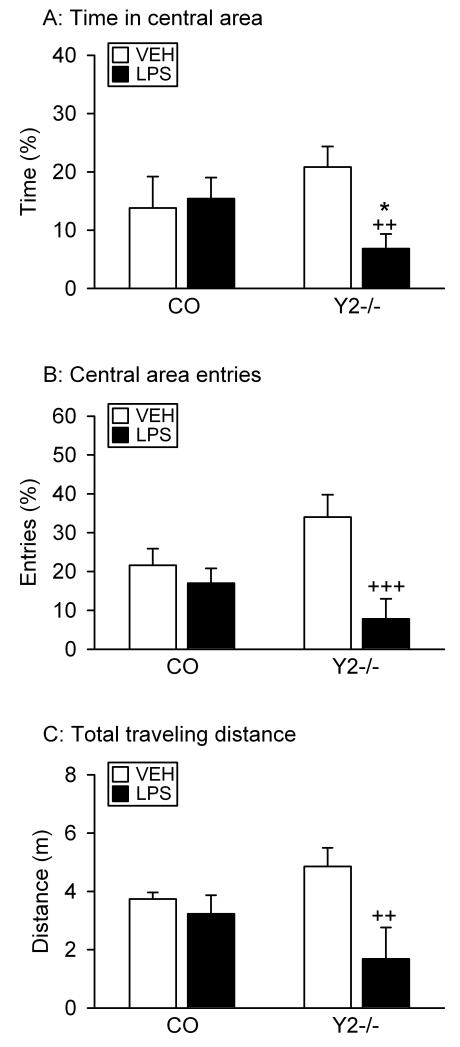
The effect of a high dose of LPS (0.83 mg/kg) on the OF behavior was examined in naïve control and Y2−/− mice, i.e., in animals that had not been exposed to LPS before. Numerically, vehicle-treated Y2−/− mice entered more often (P = 0.31) and spent more time (P = 0.12) on the central area of the OF and traveled a longer distance (P = 0.14) than control mice (Figure 7A,B,C). These changes were similar to those presented and analyzed above (Figures 3 and 4) but did not reach statistical significance due to the small number of animals available for these experiments. Two-way ANOVA of the time spent in the central area failed to reveal differences due to treatment (F(1,16) = 2.53, P = 0.13) and genotype (F(1,16) = 0.04, P = 0.84) but showed a significant interaction between these factors (F(1,16) = 4.03, P = 0.06). In contrast, the number of entries into the central area differed with treatment (F(1,16) = 10.23, P < 0.01) but not genotype (F(1,16) = 0.11, P = 0.74), with a significant interaction between these factors (F(1,16) = 5.03, P = 0.04). Two-way ANOVA of the total traveling distance indicated that this parameter varied with treatment (F(1,16) = 6.69, P = 0.02) but not genotype (F(1,16) = 0.09, P = 0.77) and that there was a significant interaction between these factors (F(1,16) = 3.51, P = 0.08). Post-hoc analysis showed that LPS had no significant effect on the OF behavior in control mice but reduced all parameters (time in central area, central area entries, total traveling distance) in Y2−/− mice (Figure 7A,B,C).
Figure 7.
Effect of a high dose of LPS on the behavior of naïve control (CO) and Y2−/− mice in the OF test. LPS (0.83 mg/kg) or vehicle (VEH, sterile saline) was injected IP 2.5 h before the behavioral test. The graphs show (A) the time spent in the central area, (B) the number of entries into the central area, and (C) the total distance traveled. The parameters in panels A and B are expressed as a percentage of the total time spent in the OF and of the total number of entries into any zone, respectively. The values represent means ± SEM, n = 5. * P ≤ 0.1 versus control mice with the same treatment, ++ P < 0.05, +++ P < 0.01 versus vehicle-treated mice of the same genotype.
DISCUSSION
The present data indicate that knockout of Y2 receptors alters the ability of immune stress to modify anxiety-like, locomotor and social behavior, rectal temperature and circulating corticosterone levels. Anxiety-related and locomotor behavior was explored with the EPM and OF tests in which a reduction of open arm entries and open arm time as well as of central area entries and central area time, respectively, is considered to reflect enhanced anxiety (Belzung and Griebel, 2001). Depression-like behavior was assessed in the TST in which prolonged immobility is thought to mirror behavioral despair (Cryan et al., 2005). For consistency and comparability, the protocols and methods used here were identical with those used in a previous study in which anxiety-related and depression-like behavior was found to be reduced and locomotion enhanced in female Y2−/− and Y4−/− mice (Painsipp et al., 2008).
The experimental approach in this study involved IP injection of LPS, which was validated by the inability of IP injected sterile saline to influence the low-anxiety and low-depression phenotype of Y2−/− and Y4−/− mice. Since the sickness response to endotoxin involves behavioral alterations that are in part opposite to those caused by Y2 and Y4 receptor knockout, we hypothesized that endogenous NPY acting via these receptors could be involved. In addition, NPY-expressing neurons in the hypothalamus have been suggested to participate in the anorexia caused by systemic LPS and proinflammatory cytokine administration (McCarthy et al., 1995; Sonti et al., 1996; McMahon et al., 1999; Romanovsky et al., 2005).
The systemic effects of LPS are initiated by activation of Toll-like receptor-4, which triggers the release of interleukin-1β, interleukin-6 and tumor necrosis factor-α from phagocytic cells. These proinflammatory cytokines alter behavior both via a humoral route and excitation of vagal afferent neurons (Goehler et al., 2000; Konsman et al., 2002). The type, magnitude and mechanism of the behavioral reaction depend on the dose of LPS (Konsman et al., 2000; Konsman et al., 2002; Mormede et al., 2004; Romanovsky et al., 2005). In most of our experiments LPS was tested at a dose of 0.1 mg/kg which is too low to elicit systemic inflammation (Teeling et al., 2007) and a full-spectrum sickness response (Mormede et al., 2004). However, this dose was able to increase rectal temperature and circulating corticosterone in the control mice. Since the systemic effects of IP administered LPS peak 2 – 3 h post-injection (Fishkin and Winslow, 1997; Konsman et al., 2000), the parameters under study were recorded 2.5 h post-LPS.
LPS (0.1 mg/kg) failed to alter anxiety-related behavior on the EPM and OF in control mice, although it reduced locomotor activity on the EPM to a significant extent. Our observations made in mice with a mixed C57BL/6–129/SvJ background differ from observations made in CD-1 mice in which LPS doses ranging from 0.02 to 0.2 mg/kg increased anxiety-like behavior on the EPM and OF, although this outcome may reflect reduced locomotion rather than enhanced anxiety (Lacosta et al., 1999; Swiergiel and Dunn, 2007). The inability of LPS to increase the anxiety-like behavior of control mice on the EPM is likely due to their property to spend little time on and rarely enter the open arms of the EPM even after vehicle treatment. Differences in mouse strain and endotoxin dosing may explain why LPS failed to change the behavior of C57BL/6–129SvJ mice in the TST, whereas in CD-1 mice LPS enhances immobility in the TST and forced swim test (Jain et al., 2001; Dunn and Swiergiel, 2005; Frenois et al., 2007).
The limited number of animals approved for this study entailed that LPS (0.1 mg) was repeatedly injected to the animals in order to examine its influence in multiple behavioral tests. Since daily injections of LPS can induce endotoxin tolerance (Beishuizen and Thijs, 2003), LPS was administered IP to the mice at intervals of at least 2 weeks. We conclude that this protocol precluded the development of endotoxin tolerance as deduced from the result of a control experiment in which the high dose of 0.83 mg/kg LPS (Mormede et al., 2004; Frenois et al., 2007) was tested on naïve control and Y2−/− mice. The effect of this LPS dose on OF behavior (Figure 7) was very similar to that of 0.1 mg/kg LPS administered the third time (Figure 3).
In exploring the involvement of Y2 and Y4 receptors in the behavioral response to immune stress we observed that Y2−/− mice differed from control and Y4−/− mice in terms of their behavioral reactions to LPS challenge. Specifically, the LPS responses under study were either positively or negatively modified by Y2 receptor knockout. While the LPS-induced fever, rise of circulating corticosterone and stimulation of defecation were attenuated in Y2−/− animals, the ability of LPS to enhance anxiety-like behavior and to reduce locomotor activity and social interaction was most pronounced in Y2−/− mice. These observations suggest that Y2 receptors play diverse roles in the various aspects of the sickness response to endotoxin challenge.
Most striking was the finding that the reduction of anxiety-related behavior due to Y2 receptor knockout was completely reversed by LPS, as revealed in both the EPM and OF tests. Since this behavioral change in Y2−/− mice was associated with a decrease in locomotion, we cannot rule out that the apparently anxiogenic effect of LPS reflected suppression of locomotor activity. In addressing this uncertainty we employed the SIH test which, relative to the EPM and OF tests, assesses anxiety in a locomotion-independent manner. The magnitude of ΔT is thought to be proportional to anxiety (Olivier et al., 2003) as long as T1 remains unaltered by the experimental manipulation under study (Painsipp et al., 2008). However, the SIH test was invalidated by the property of LPS to increase T1, which will cut short any stress-induced increase in rectal temperature by a ceiling effect. The attenuation of social interaction which LPS caused in Y2−/− mice may likewise be the result of reduced locomotion.
The reduction of depression-like behavior which Y2−/− mice displayed in the TST remained unchanged by LPS. Two other TST-associated responses were, however, altered by LPS: defecation and circulating corticosterone. Defecation is stimulated by stress (Taché et al., 2004) and thought to reflect an aspect of emotionality (DeFries et al., 1978), but in the case of IP administered LPS may arise from altered intestinal function (Fruhwald et al., 2004). Our observation that LPS-induced defecation, seen in control mice, was absent in Y2−/− animals, points to a role of Y2 receptors in this intestinal reaction to endotoxin challenge. Another response to LPS-evoked immune stress is activation of the HPA axis as reflected by a rise of circulating corticosterone (Turnbull and Rivier, 1999; Beishuizen and Thijs, 2003). After injection of vehicle and exposure to the TST, Y2−/− mice exhibited higher levels of circulating corticosterone than control mice, whereas the effect of LPS to boost the TST-induced rise of corticosterone, seen in control and Y4−/− mice, was absent in Y2−/− mice. These data suggest that the HPA axis of Y2−/− mice responds to LPS in an exaggerated manner so that the TST procedure fails to further stimulate HPA axis activity. A detailed analysis of HPA axis dynamics is required to understand the relationship between our observations and the reported reduction of baseline corticosterone and corticotropin-releasing factor (CRF) mRNA in the hypothalamic paraventricular nucleus of Y2−/− mice (Sainsbury et al., 2002b).
The particular sensitivity of Y2−/− mice to immune stress could arise from an interaction of LPS with the NPY system at the peripheral and/or central level. Activation of Y2 receptors on monocytes counteracts the adverse effects of endotoxinemia by inhibiting tissue infiltration with immunocytes (Nave et al., 2004) and the ensuing fever and hypotension (Felies et al., 2004). At the central level, only high doses of systemic LPS (0.5 mg/kg) have been reported to decrease the expression of NPY in the rat hypothalamus (Kim et al., 2007), whereas 0.1 mg/kg LPS is ineffective (Sergeyev et al., 2001; Borges et al., 2007). The diverse implications of Y2 receptors in the behavioral responses to LPS could be related to the pre- and postsynaptic location of Y2 receptors in the brain. Genetic deletion of postsynaptic Y2 receptors is likely to blunt responses in which this type of NPY receptor subserves feed-forward excitatory signaling, whereas knockout of presynaptic Y2 receptors with a negative feedback role will disinhibit processes that normally are under negative control. Systemic LPS activates neurons in the extended amygdala, hippocampus and hypothalamus (Frenois et al., 2007), which are involved in the control of anxiety- and depression-related behavior and contain many Y2 receptors with a predominantly presynaptic location (Gustafson et al., 1997; Parker and Herzog, 1999; Fetissov et al., 2004; Stanic et al., 2006).
Unlike Y2−/− animals whose behavioral phenotype was markedly changed by LPS, Y4−/− mice were relatively resistant to behavioral modifications by immune stress. This finding indicates that Y4 receptors play a minor role in the responses to immune challenge, which is in keeping with their restricted distribution in the rodent brain (Whitcomb et al., 1997; Parker and Herzog, 1999; Campbell et al., 2003; Fetissov et al., 2004).
In summary, the current data show that Y2 receptors contribute to the effect of immune stress to modify anxiety-like, locomotor and social behavior, rectal temperature and circulating corticosterone levels. Although developmental compensations in germline gene knockout mice may mask the full implication of Y2 receptors, our findings indicate that, if such adaptations occurred, they were insufficient to balance the deficit in Y2 receptors. In attesting to an important role of NPY acting via Y2 receptors in the cerebral response to peripheral immune challenge, our data generate a hypothesis that warrants further investigation in neurochemical, endocrinological and pharmacological terms.
ACKNOWLEDGEMENTS
This study was supported by the Zukunftsfonds Steiermark (grant 262) and the Austrian Scientific Research Funds (FWF grant L25-B05). The authors thank Professor Günther Sperk (Medical University of Innsbruck, Austria) for breeding the knockout mice and critically reading the manuscript, Professor Helmuth P. Huber (University of Graz, Austria) for advice on statistics, and Dr. Anaid Shahbazian and Dr. Thomas Wultsch for technical help with the experiments. The support by the director and staff of the Center for Medical Research (ZMF I) of the Medical University of Graz is greatly appreciated.
REFERENCES
- Bedoui S, von Hörsten S, Gebhardt T. A role for neuropeptide Y (NPY) in phagocytosis: implications for innate and adaptive immunity. Peptides. 2007;28:373–376. doi: 10.1016/j.peptides.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Beishuizen A, Thijs LG. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J. Endotoxin Res. 2003;9:1–22. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav. Brain Res. 2002;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Borges BC, Antunes-Rodrigues J, Castro M, Bittencourt JC, Elias CF, Elias LL. Expression of hypothalamic neuropeptides and the desensitization of pituitary-adrenal axis and hypophagia in the endotoxin tolerance. Horm. Behav. 2007;52:508–519. doi: 10.1016/j.yhbeh.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Campbell RE, Smith MS, Allen SE, Grayson BE, Ffrench-Mullen JM, Grove KL. Orexin neurons express a functional pancreatic polypeptide Y4 receptor. J. Neurosci. 2003;23:1487–1497. doi: 10.1523/JNEUROSCI.23-04-01487.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- DeFries JC, Gervais MC, Thomas EA. Response to 30 generations of selection for open-field activity in laboratory mice. Behav. Genet. 1978;8:3–13. doi: 10.1007/BF01067700. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol. Biochem. Behav. 2005;81:688–693. doi: 10.1016/j.pbb.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci. Biobehav. Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Felies M, von Hörsten S, Pabst R, Nave H. Neuropeptide Y stabilizes body temperature and prevents hypotension in endotoxaemic rats. J. Physiol. 2004;561:245–252. doi: 10.1113/jphysiol.2004.073635. (London) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetissov SO, Kopp J, Hökfelt T. Distribution of NPY receptors in the hypothalamus. Neuropeptides. 2004;38:175–188. doi: 10.1016/j.npep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Fishkin RJ, Winslow JT. Endotoxin-induced reduction of social investigation by mice: interaction with amphetamine and anti-inflammatory drugs. Psychopharmacology. 1997;132:335–341. doi: 10.1007/s002130050353. [DOI] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhwald S, Herk E, Schöll G, Shahbazian A, Hammer HF, Metzler H, Holzer P. Endotoxin pretreatment modifies peristalsis and attenuates the antipropulsive action of adrenoceptor agonists in the guinea-pig small intestine. Neurogastroenterol. Motil. 2004;16:213–222. doi: 10.1111/j.1365-2982.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton. Neurosci. Basic Clin. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Smith KE, Durkin MM, Walker MW, Gerald C, Weinshank R, Branchek TA. Distribution of the neuropeptide Y Y2 receptor mRNA in rat central nervous system. Mol. Brain Res. 1997;46:223–235. doi: 10.1016/s0169-328x(97)00017-x. [DOI] [PubMed] [Google Scholar]
- Hays WL. Statistics. 6th Edition Wadsworth; Belmont: 2007. [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Jain NK, Kulkarni SK, Singh A. Lipopolysaccharide-mediated immobility in mice: reversal by cyclooxygenase enzyme inhibitors. Methods Find. Exp. Clin. Pharmacol. 2001;23:441–444. doi: 10.1358/mf.2001.23.8.662131. [DOI] [PubMed] [Google Scholar]
- Karl T, Herzog H. Behavioral profiling of NPY in aggression and neuropsychiatric diseases. Peptides. 2007;28:326–333. doi: 10.1016/j.peptides.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Kask A, Harro J, von Hörsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci. Biobehav. Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Kim YW, Kim KH, Ahn DK, Kim HS, Kim JY, Lee DC, Park SY. Time-course changes of hormones and cytokines by lipopolysaccharide and its relation with anorexia. J. Physiol. Sci. 2007;57:159–165. doi: 10.2170/physiolsci.RP003407. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design. Procedures for the Behavioral Sciences. Third edition Brooks/Cole; Pacific Grove, California: 1995. [Google Scholar]
- Konsman JP, Dantzer R. How the immune and nervous systems interact during disease-associated anorexia. Nutrition. 2001;17:664–668. doi: 10.1016/s0899-9007(01)00602-5. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Luheshi GN, Bluthe RM, Dantzer R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur. J. Neurosci. 2000;12:4434–4446. doi: 10.1046/j.0953-816x.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Behavioral and neurochemical consequences of lipopolysaccharide in mice: anxiogenic-like effects. Brain Res. 1999;818:291–303. doi: 10.1016/s0006-8993(98)01288-8. [DOI] [PubMed] [Google Scholar]
- Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38:189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- McCarthy HD, Dryden S, Williams G. Interleukin-1β-induced anorexia and pyrexia in rat: relationship to hypothalamic neuropeptide Y. Am. J. Physiol. 1995;269:E852–E857. doi: 10.1152/ajpendo.1995.269.5.E852. [DOI] [PubMed] [Google Scholar]
- McMahon CD, Buxton DF, Elsasser TH, Gunter DR, Sanders LG, Steele BP, Sartin JL. Neuropeptide Y restores appetite and alters concentrations of GH after central administration to endotoxic sheep. J. Endocrinol. 1999;161:333–339. doi: 10.1677/joe.0.1610333. [DOI] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- Mormede C, Palin K, Kelley KW, Castanon N, Dantzer R. Conditioned taste aversion with lipopolysaccharide and peptidoglycan does not activate cytokine gene expression in the spleen and hypothalamus of mice. Brain Behav. Immun. 2004;18:186–200. doi: 10.1016/S0889-1591(03)00133-8. [DOI] [PubMed] [Google Scholar]
- Nave H, Bedoui S, Moenter F, Steffens J, Felies M, Gebhardt T, Straub RH, Pabst R, Dimitrijevic M, Stanojevic S, von Hörsten S. Reduced tissue immigration of monocytes by neuropeptide Y during endotoxemia is associated with Y2 receptor activation. J. Neuroimmunol. 2004;155:1–12. doi: 10.1016/j.jneuroim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Olivier B, Zethof T, Pattij T, van Boogaert M, van Oorschot R, Leahy C, Oosting R, Bouwknecht A, Veening J, van der Gugten J, Groenink L. Stress-induced hyperthermia and anxiety: pharmacological validation. Eur. J. Pharmacol. 2003;463:117–132. doi: 10.1016/s0014-2999(03)01326-8. [DOI] [PubMed] [Google Scholar]
- Painsipp E, Wultsch T, Edelsbrunner ME, Tasan RO, Singewald N, Herzog H, Holzer P. Reduced anxiety-like and depression-related behavior in neuropeptide Y4 receptor knockout mice. Genes Brain Behav. 2008 doi: 10.1111/j.1601-183X.2008.00389.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur. J. Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- Peloso E, Wachulec M, Satinoff E. Stress-induced hyperthermia depends on both time of day and light condition. J. Biol. Rhythms. 2002;17:164–170. doi: 10.1177/074873002129002456. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Carvajal C, Kask A, Dumont Y, Quirion R. Neuropeptide Y and its receptor subtypes in the central nervous system: emphasis on their role in animal models of psychiatric disorders. In: Michel MC, editor. Neuropeptide Y and Related Peptides. Springer; Berlin: 2004. pp. 101–136. (Handbook of Experimental Pharmacology, Volume 162). [Google Scholar]
- Redrobe JP, Dumont Y, Herzog H, Quirion R. Neuropeptide Y (NPY) Y2 receptors mediate behaviour in two animal models of anxiety: evidence from Y2 receptor knockout mice. Behav. Brain Res. 2003;141:251–255. doi: 10.1016/s0166-4328(02)00374-1. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front. Biosci. 2005;10:2193–2216. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Fetissov S, Fürtinger S, Jenkins A, Cox HM, Sperk G, Hökfelt T, Herzog H. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc. Natl. Acad. Sci. USA. 2002a;99:8938–8943. doi: 10.1073/pnas.132043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Herzog H. Y2 receptor deletion attenuates the type 2 diabetic syndrome of ob/ob mice. Diabetes. 2002b;51:3420–3427. doi: 10.2337/diabetes.51.12.3420. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, Herzog H. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 2002c;16:1077–1088. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyev V, Broberger C, Hökfelt T. Effect of LPS administration on the expression of POMC, NPY, galanin, CART and MCH mRNAs in the rat hypothalamus. Mol. Brain Res. 2001;90:93–100. doi: 10.1016/s0169-328x(01)00088-2. [DOI] [PubMed] [Google Scholar]
- Sonti G, Ilyin SE, Plata-Salaman CR. Neuropeptide Y blocks and reverses interleukin-1β-induced anorexia in rats. Peptides. 1996;17:517–520. doi: 10.1016/0196-9781(96)00016-2. [DOI] [PubMed] [Google Scholar]
- Stanic D, Brumovsky P, Fetissov S, Shuster S, Herzog H, Hökfelt T. Characterization of neuropeptide Y2 receptor protein expression in the mouse brain. I. Distribution in cell bodies and nerve terminals. J. Comp. Neurol. 2006;499:357–390. doi: 10.1002/cne.21046. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ. Effects of interleukin-1β and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol. Biochem. Behav. 2007;86:651–659. doi: 10.1016/j.pbb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taché Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br. J. Pharmacol. 2004;141:1321–1330. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeling JL, Felton LM, Deacon RM, Cunningham C, Rawlins JN, Perry VH. Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain Behav. Immun. 2007;21:836–850. doi: 10.1016/j.bbi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Tschenett A, Singewald N, Carli M, Balducci C, Salchner P, Vezzani A, Herzog H, Sperk G. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur. J. Neurosci. 2003;18:143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol. Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Whitcomb DC, Puccio AM, Vigna SR, Taylor IL, Hoffman GE. Distribution of pancreatic polypeptide receptors in the rat brain. Brain Res. 1997;760:137–149. doi: 10.1016/s0006-8993(97)00295-3. [DOI] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. 3rd Edition McGraw-Hill; New York: 1991. [Google Scholar]