Abstract
Background/Aim
Transarterial chemoembolization (TACE) is the recommended treatment for patients with Barcelona stage B hepatocellular carcinoma; however, community practice varies from these American Association for the Study of Liver Diseases guidelines. In this study, we sought to assess factors determining outcome after TACE and examine adherence to guidelines.
Methods
From January 2006 to December 2012, 308 patients with newly diagnosed HCC were treated at the Veterans Affairs (VA) Ann Arbor Healthcare System. Of these, 109 patients underwent TACE. The primary outcome measured mortality. Kaplan–Meier analysis was used to determine the cumulative probability of death. Cox regression was used to assess the predictors of mortality.
Results
The median age of the 109 patients was 60 years (48–90), 97 % were males and 82 % had chronic HCV infection. The median size of the largest lesion was 4 cm, 51 % were multifocal, and portal vein thrombosis was present in 3.6 %. Sixty-two patients died after median 333 days from the index TACE treatment. Median overall survival from index TACE was 11.2 months. Unadjusted 1-, 2-, and 3-year survival was 64, 35, and 24 %, respectively. CTP score (B vs. A: HR 2.51, p = 0.002; C vs. A: HR 7.96, p < 0.0001) and presence of complete response to TACE (HR 0.51, p = 0.004) were independent predictors of mortality. Barcelona stage (p = 0.88) and performance status as measured by ECOG (p = 0.98) were not associated with mortality after TACE.
Conclusions
In this community based, single VA center study, we found a significant number of patients beyond Barcelona stage B were treated with TACE. Advanced TNM stage, poor liver synthetic function and achieving CR with TACE were better predictors of mortality than guideline-directed decisions based on Barcelona stage. These factors may be useful to guide future patient selection for TACE.
Keywords: Hepatocellular carcinoma, Transarterial chemoembolization, Barcelona Clinic Liver Cancer, Mortality, Predictors
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and represents 4 % of all new cancer diagnoses [1]. HCC has been identified as the fastest rising cause of cancer-related death in the USA, with a dismal 5-year survival of <12 % [2]. Low survival rates are due to lack of observable symptoms in the early stages, aggressiveness of the cancer with concomitant hepatic failure, and limited curative options in the later stages.
HCC surveillance can be effective in early detection of malignant lesions and improving mortality. However, it is significantly underutilized with <20 % of eligible patients receiving appropriate screening [3, 4]. A survey studied showed that one quarter of physicians did not know the appropriate management of a positive result, thereby limiting the overall effectiveness of HCC surveillance [5]. The natural history of HCC is such that nearly two-thirds of the diagnoses are thus made at an advanced stage [6]. Only a minority are diagnosed at a stage where potentially effective curative therapies such as liver transplantation (LT) and resection are available, as outlined by Barcelona Clinic Liver Cancer (BCLC) guidelines [7].
In the absence of viable curative options, transarterial chemoembolization (TACE) has become the first-line therapy for many patients who exceed transplantation criteria and for whom radio frequency ablation (RFA) are precluded due to tumor size and location [8]. Although the American Association for the Study of Liver Diseases (AASLD) guideline recommends TACE for only intermediate stage HCC (Barcelona stage B) [7], it is not clear whether these guidelines are strictly adhered to in the community or whether other factors may be useful in determining outcomes after TACE.
The primary aim of this study was to determine the factors associated with mortality after TACE procedure among patients with HCC receiving TACE at a single center. The secondary aim was to determine the incidence of response and recurrence as well as factors associated with HCC response and recurrence after the TACE procedure.
Methods
Patients/Materials
From the period of 2006 to 2012, 308 patients with newly diagnosed HCC were treated at the VA Ann Arbor Healthcare System. The patients were identified through the Liver Tumor Board as well as from clinical administrative databases using the ICD-9 codes of 155.0 and 155.2. Retrospective chart review was performed to verify all diagnosis. Of the 308 patients, 109 patients received TACE procedure, whose data were analyzed.
Diagnosis of HCC was made based on radiological criteria or histology per AASLD guidelines [7]. Ninety-eight percent of the patients were seen by the Gastroenterology Consultation service, and 91 % were formally reviewed by the Liver Tumor Board. A treatment algorithm was used where all patients were assessed for resection or LT if they were within Milan criteria and/or met the United Network for Organ Sharing (UNOS) criteria. Patients who were not candidates for resection or LT were then considered for RFA or TACE. Of note, six patients did receive subsequent LT after initial intervention with RFA (n = 2), TACE (n = 3), or RFA followed by TACE (n = 1).
Clinical data were abstracted from medical chart review including liver tumor board notes and cancer-staging forms. Tumor assessment and staging as well as clinical diagnosis of diabetes, hypertension, and laboratory studies were based on the data, which were closest to the diagnostic imaging study (either MRI or CT) and never >6 months from the date of imaging. Hepatitis C diagnosis was confirmed by serum HCV RNA. Tobacco use included present and past use. Survival was based on date of first intervention to death or censor. Follow-up was censored at date of last visit. No patients were lost to follow-up.
TACE Procedure
We used standard fashion TACE using up to 10 mg mitomycin, 50 mg doxorubicin, 100 mg cisplatin and 20 mL lipodiol until March 2011. We then switched to doxorubicin drug-eluting beads (DEB) with doses up to 150 mg. A follow-up CT scan or MRI with contrast was obtained 6 weeks after the TACE procedure to assess response. Response to initial procedure was determined by mRECIST criteria [9]. Subsequent surveillance imaging was obtained every 3 months for 1 year and 6 months for the following subsequent 2 years. Patients were then continuously followed until either death or last follow-up, and presence of recurrence or progression-free survival was recorded. If disease recurred or progressed, treatment was then decided based on primary hepatologist opinion and recommendation of tumor board, as above. This included further TACE, RFAs, or best supportive care including sorafenib and/or palliative care.
Statistical Analysis
The continuous variables were expressed as median and range, and categorical variables were expressed as percentage. The primary outcome was mortality, and the secondary outcome was recurrence of HCC. Patients were followed from the time of initial diagnosis until death or June 30, 2013.
Kaplan–Meier method was used to estimate the cumulative probability of death. Time to death was counted from the first TACE procedure to death or last follow-up visit. Backwards stepwise Cox regression models were used to assess the predictors of mortality. The variables with p < 0.05 in the univariate analysis were further investigated in the multivariate Cox regression analysis. The model was adjusted for age, size of largest lesion, stage, CTP score, MELD, Log AFP, etiology, and response to TACE and ECOG score.
Logistic regression was used to examine the predictors of complete response. A p value <0.05 was considered statistically significant. All analyses were performed in SPSS version 20.
Results
Description and Outcomes of Cohort
One hundred and nine patients underwent TACE procedures as a primary intervention for HCC during the study period. The demographic, clinical, and tumor features are shown in Table 1. The median age was 60 years old (48–90); 97 % males and 82 % had hepatitis C as their primary liver disease. The median MELD score was 9, with 61, 34, and 5 % of patients in Child’s class A, B, and C, respectively, and 2, 20, 22, 50, and 7 % in BCLC stage 0, A, B, C, and D, respectively.
Table 1.
Baseline characteristics of cohort
| Variables | Median (range)/number (%) N = 109 |
|---|---|
| Age at diagnosis (years) | 60 (48–90) |
| Male | 106 (97 %) |
| Female | 3 (3 %) |
| White | 46 (42 %) |
| AA | 30 (27.5 %) |
| Other | 1 (0.1 %) |
| Unknown | 32 (29.4 %) |
| HCV | 89 (82 %) |
| Size of largest lesion | 4 (1–24) |
| Multifocal | 56 (51 %) |
| PVT | 8 (7.3 %) |
| ECOG score | 1 (0–3) |
| MELD score | 9 (6–18) |
| CTP | 6 (5–11) |
| A/B/C | 67 (61 %)/37 (34 %)/5 (5 %) |
| Barcelona stage 0–A–B–C–D | 2 (1.8 %)–21 (19.3 %)–24 (22 %)–54 (49.5 %)–8 (7.3 %) |
| Stage I/II/III/IV | 50 (45.8 %)/41 (37.6 %)/17 (15.6 %)/1 (1 %) |
| BMI | 26.6 (20–42) |
| Log AFP | 1.48 (0.02–4.65) |
| Albumin | 3.2 (1.9–4.6) |
| Ascites: none/mild/mod-severe | 87 (80 %)/21 (19 %)/1 (1 %) |
| HE: none/Gr 1–2/Gr 3–4 | 98 (90 %)/10 (9 %)/1 (1 %) |
| DM | 27 (25 %) |
| HTN | 73 (67 %) |
| Smoking | 88 (81 %) |
The median size of the largest lesion was 4 cm, and 51 % of the patients had multifocal tumor. Of the patients with portal vein thrombosis, only 4 (3.6 %) patients had true tumor thrombus. Two invaded the main/right portal veins and left portal vein, respectively. The other two instances involved thrombus in the main/left portal veins without invasion. The median time from diagnosis to index procedure in the treatment session was 81 days. The average number TACE per patient was 1, with a range of 1–5. The results of the TACE procedures are given in Table 2.
Table 2.
Results of TACE procedures
| Variables | Median (range)/number (%) N = 109 |
|---|---|
| Number of TACE procedures/patient | 1 (1–5) |
| Complete response | 56 (51.4 %) |
| Recurrence | 27 |
| No recurrence | 29 |
| Incomplete response (> 30 % necrosis) | 10 (9.2 %) |
| Partial response (< 30 % necrosis) | 9 (8.3 %) |
| Progression (increase in size) | 23 (21 %) |
| Unknown | 11 (10.1 %) |
| Deaths | 62 (57 %) |
| Time from diagnosis to death (days) | 413 (41–2,491) |
| Time from diagnosis to TACE | 81 (8–648) |
| Time from TACE to death | 333 (28–2,351) |
Unadjusted Mortality After TACE Procedure
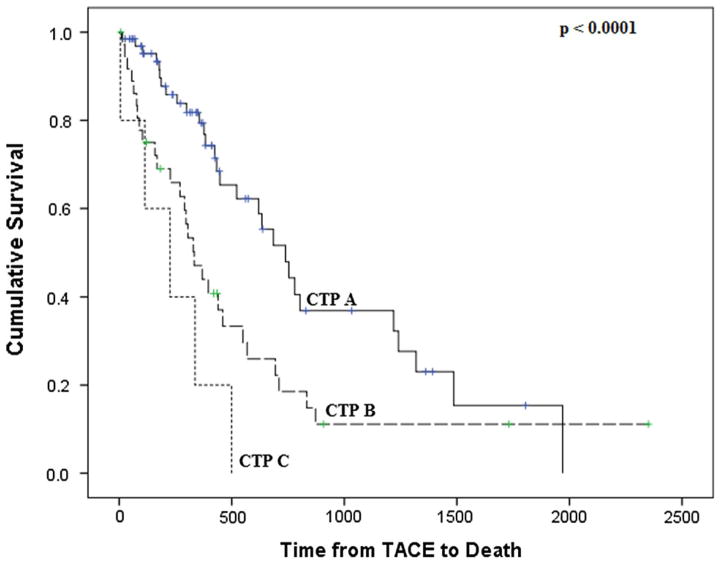
Sixty-two of the 109 patients died after a median of 333 days from the index TACE treatment. Median overall survival from index TACE was 11.2 months. Median overall survival in patients who achieved CR was 14.5 months compared with 7.7 months in those who did not. The unadjusted 1-, 2-, and 3-year patient survival was 64, 35, and 24 %, respectively. Figure 1 shows the cumulative probability of survival stratified by CTP score.
Fig. 1.
Unadjusted patient survival by CTP score
Independent Predictors of Mortality After TACE Procedure
Table 3 shows the independent predictors of mortality. Patients with Child’s Class B and C cirrhosis had 2.1-fold and 3.7-fold higher hazard of death compared with Child’s Class A, respectively. The presence of complete response was associated with a 49 % lower risk of death (HR 0.51, p = 0.004). Interestingly, Barcelona stage and performance status as measured by ECOG were not associated with mortality after TACE.
Table 3.
Independent predictors of survival
| Covariates | HR (95 % CI) | p value |
|---|---|---|
| Log AFP | 1.34 (0.96–1.86) | 0.085 |
| CTP | 2.13 (1.25–3.6) | 0.006 |
| B (vs. A) | 3.72 (2.34–10.4) | 0.012 |
| C (vs. A) | ||
| Complete response to TACE (vs. not) | 0.51 (0.28–0.92) | 0.004 |
Response to TACE and HCC Recurrence
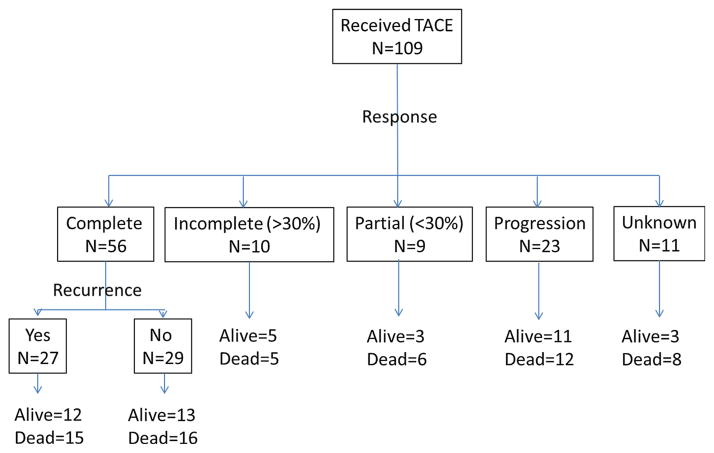
Figure 2 shows the spectrum of response and outcomes based upon response to TACE. Approximately half of the patients (51 %) achieved complete response after a median of one TACE procedure (range 1–5). Of those with a complete response, 48 % had HCC recurrence during the follow-up period (Fig. 2).
Fig. 2.
Outcomes based upon response to TACE
Independent predictors of complete response (Table 4) included number of TACE procedures (OR 0.43; p = 0.023), TNM stage (OR 0.43; p = 0.006), and lesion size per cm (OR 0.78; p = 0.029).
Table 4.
Independent predictors of complete response
| Covariates | OR (95 % CI) | p value |
|---|---|---|
| Number of TACE procedures | 0.43 (0.21–0.89) | 0.023 |
| TNM stage | 0.43 (0.23–0.78) | 0.006 |
| Largest lesion (per cm) | 0.78 (0.60–0.97) | 0.029 |
Discussion
In this large VA-based study of American patients undergoing TACE as primary intervention, the 1-year overall survival was 64 % which is similar to prior studies showing a median survival of approximately 20 months with TACE [10, 11]. The cumulative incidence of survival was highest among those with well-preserved liver synthetic function. Our results validate previous findings that residual liver function is important to the success of TACE and survival of patients. Uniquely, our analysis did not demonstrate that a single component of CTP scoring (bilirubin, INR, albumin, ascites, or encephalopathy) was predictive of survival as seen in previous studies: albumin [12], bilirubin [13], and INR [14].
The role of performance status as measured by ECOG score is a unique component of BCLC staging. While previous studies have shown that lower ECOG score has a significant positive impact on survival [12, 13], we did not find an association between post-TACE survival and ECOG status. Although the AASLD guideline recommends TACE procedure for intermediate stage (BCLC-B) HCC [7], 57 % of our patients who received TACE were advanced stage, BCLC stage C and D. In our patients, advanced BCLC stage did not predict the mortality after TACE, suggesting that with careful patient selection, TACE may be a reasonable option for palliation.
Our study also confirms the findings of Cabibbo et al. [13] that achieving a complete response-affected survival. However, we found that an increasing number of TACE procedures were associated with lower complete response. We speculate that an increase in the number of TACE procedures might be a surrogate for aggressive or harder to treat tumor biology such as undifferentiated or anaplastic HCC.
Curative as well as palliative modalities for HCC tend to vary clinically, especially for intermediate stage. TACE has been used in Asia for many years for intermediate stages and two major RCTs and a systematic review in the early 2000s validated the procedure [15–17]. Since then, the majority of studies examining the predictors of outcomes after TACE are from Asia. The epidemiology of HCC in Asia is different from the USA because of high incidence of hepatitis B and occurrence of HCC in patients with well-preserved hepatic function. Results from those studies cannot be generalized to patients in the USA. Our results may help guide the patient selection for TACE procedure in this country. Additional unique features strengthening our study are the uniformity of patient selection, standard decision-making criteria using a multidisciplinary tumor board and extremely low loss to follow-up.
Finally, the limitations to our study include that it is a single center study with a predominantly male population and retrospective study design that can result in some bias from unmeasured components. Despite these limitations, this is one of the largest studies that evaluated the risk factors of mortality as well as factors associated with response after TACE procedure in the USA.
In this community based, single VA center study, many patients were beyond BCLC-B stage, with poor ECOG performance status, but these factors were not associated with decrease survival after TACE. In conclusion, patients should be carefully selected based upon their CTP score. Complete response to TACE is associated with lower mortality and dependent upon lower TNM stage and small size of the lesion. Patients who do not respond to TACE after index procedure are less likely to achieve complete response. These results may guide caregivers regarding patient selection for TACE and counseling regarding the outcomes of TACE procedures.
Acknowledgments
Pratima Sharma is supported by National Institutes of Health (NIH) Grant KO8 DK-088946.
Abbreviations
- HCC
Hepatocellular carcinoma
- TACE
Transarterial chemoembolization
- BCLC
Barcelona clinic liver cancer
- CTP
Child–Turcotte–Pugh
- AASLD
American association for the study of liver diseases
- mRECIST
Modified response evaluation criteria in solid tumors
Footnotes
Conflict of interest None.
Contributor Information
Pranab M. Barman, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI, USA
Pratima Sharma, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI, USA.
Venkat Krishnamurthy, Department of Radiology, University of Michigan, Ann Arbor, MI, USA. VA Ann Arbor Healthcare System, Ann Arbor, MI, USA.
Jonathon Willatt, Department of Radiology, University of Michigan, Ann Arbor, MI, USA. VA Ann Arbor Healthcare System, Ann Arbor, MI, USA.
Heather McCurdy, VA Ann Arbor Healthcare System, Ann Arbor, MI, USA.
Richard H. Moseley, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI, USA. VA Ann Arbor Healthcare System, Ann Arbor, MI, USA
Grace L. Su, Email: gsu@umich.edu, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI, USA. VA Ann Arbor Healthcare System, Ann Arbor, MI, USA
References
- 1.Serag HBE. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37:S88–S94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 2.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47:S2–S6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singal AG, Conjeevaram HS, Volk ML, et al. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol Biomark Prev. 2012;21:793–799. doi: 10.1158/1055-9965.EPI-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Saini SD, Kuhn LB, et al. Knowledge of hepatocellular carcinoma screening guidelines and clinical practices among gastroenterologists. Dig Dis Sci. 2011;56:569–577. doi: 10.1007/s10620-010-1453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padhya KT, Marrero JA, Singal AG. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol. 2013;29:285–292. doi: 10.1097/MOG.0b013e32835ff1cf. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M, American D. Association for the Study of Liver Diseases, Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrero JA. Multidisciplinary management of hepatocellular carcinoma: where are we today? Semin Liver Dis. 2013;33:S3–S10. doi: 10.1055/s-0033-1333631. [DOI] [PubMed] [Google Scholar]
- 9.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 10.Sherman M, Burak K, Maroun J, et al. Multidisciplinary Canadian consensus recommendations for the management and treatment of hepatocellular carcinoma. Curr Oncol. 2011;18:228–240. doi: 10.3747/co.v18i5.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10:34–42. doi: 10.1038/nrgastro.2012.199. [DOI] [PubMed] [Google Scholar]
- 12.Tsai YJ, Hsu CY, Huang YH, et al. Early identification of poor responders to transarterial chemoembolization for hepatocellular carcinoma. Hepatol Int. 2011;5:975–984. doi: 10.1007/s12072-011-9276-9. [DOI] [PubMed] [Google Scholar]
- 13.Cabibbo G, Genco C, Di Marco V, et al. Predicting survival in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Aliment Pharmacol Ther. 2011;34:196–204. doi: 10.1111/j.1365-2036.2011.04694.x. [DOI] [PubMed] [Google Scholar]
- 14.Shen H, Agarwal D, Qi R, et al. Predictors of outcome in patients with unresectable hepatocellular carcinoma receiving transcatheter arterial chemoembolization. Aliment Pharmacol Ther. 2007;26:393–400. doi: 10.1111/j.1365-2036.2007.03395.x. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 17.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]