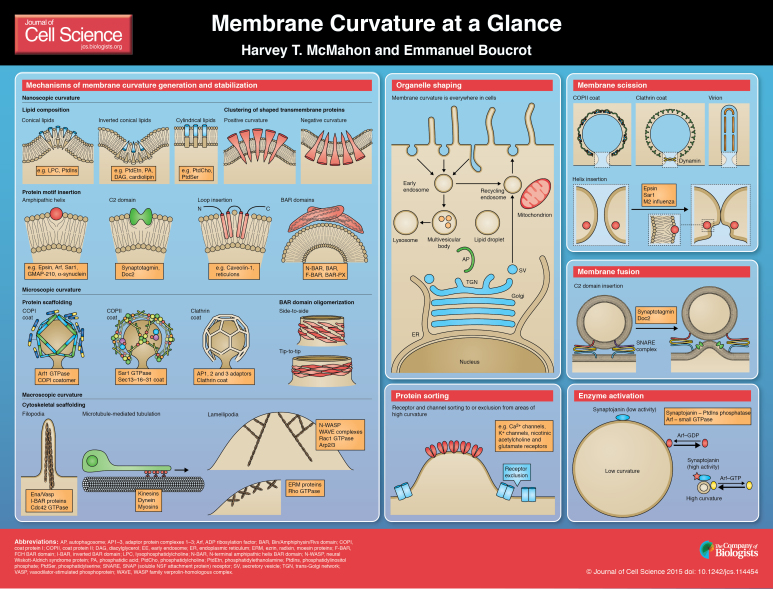
ABSTRACT
Membrane curvature is an important parameter in defining the morphology of cells, organelles and local membrane subdomains. Transport intermediates have simpler shapes, being either spheres or tubules. The generation and maintenance of curvature is of central importance for maintaining trafficking and cellular functions. It is possible that local shapes in complex membranes could help to define local subregions. In this Cell Science at a Glance article and accompanying poster, we summarize how generating, sensing and maintaining high local membrane curvature is an active process that is mediated and controlled by specialized proteins using general mechanisms: (i) changes in lipid composition and asymmetry, (ii) partitioning of shaped transmembrane domains of integral membrane proteins or protein or domain crowding, (iii) reversible insertion of hydrophobic protein motifs, (iv) nanoscopic scaffolding by oligomerized hydrophilic protein domains and, finally, (v) macroscopic scaffolding by the cytoskeleton with forces generated by polymerization and by molecular motors. We also summarize some of the discoveries about the functions of membrane curvature, where in addition to providing cell or organelle shape, local curvature can affect processes like membrane scission and fusion as well as protein concentration and enzyme activation on membranes.
KEY WORDS: Amphipatic helix, BAR domain, Bilayer asymmetry, Lipids, Membrane curvature
Introduction
The formation of many intracellular membrane compartments allows the cell to compartmentalize proteins, thereby supporting the complex and timely coordination of the thousands of biochemical reactions required for eukaryotic life. Because membranes are constantly turned over by trafficking intermediates (vesicles or tubules), the ability to generate high-curvature compartments is necessary for eukaryotic life. Lipid bilayers forming eukaryotic cell membranes have elastic properties that make them resistant to spontaneous bending and, thus, active mechanisms are required to shape them; these have been conserved throughout evolution. Many proteins contain domains or motifs that are specialized in sensing, generating or stabilizing membrane curvature. In addition, there are proteins that act directly by changing lipids, whereas others provide scaffolding and forces that impose tension on membranes.
Mechanisms of membrane curvature generation and stabilization
Change in lipid composition and asymmetry
Lipids have intrinsic shapes depending on the size of their headgroups and their acyl chain compositions (length and saturation). These features determine their side-by-side packing, thereby imposing a shape on the monolayer. If many lipids with similar shape cluster together, then a monolayer will adopt the spontaneous curvature of the local lipids. Owing to bilayer coupling, the whole membrane might also change shape (see poster) (McMahon and Gallop, 2005; Cooke and Deserno, 2006). Thus a bilayer will adopt a spontaneous curvature equal to the difference between the spontaneous curvatures of its inner and outer monolayers, which are themselves dictated by the weighted average of the spontaneous curvature of their constituent lipids (Kozlov et al., 1992; Leikin et al., 1996).
Phosphatidylcholine (PtdCho) and phosphatidylserine (PtdSer) are cylindrical lipids that form a flat monolayer. Lipids such as phosphatidylethanolamine (PtdEtn), phosphatic acid, diacylglycerol (DAG) or cardiolipin, which have a smaller polar headgroup than that of PtdCho, have a roughly conical shape and thus impose a negative curvature, where a monolayer with such lipids bends in such a fashion that the headgroups come closer together. Conversely, a large headgroup to acyl chain ratio, such as in lysophosphatidylcholine (LPC) or the large headgroups in phosphatidylinositol phosphates (PtdIns) confers an inverted conical shape to the lipids, thereby favoring the bending of the membrane into a positive curvature, bending the monolayer away from the headgroups (reviewed in Chernomordik and Kozlov, 2003; Di Paolo and De Camilli, 2006; Zimmerberg and Kozlov, 2006).
Acyl chain saturation also influences lipid geometry; any double bond (such as in oleic acid) induces a kink in the chain and thus forces it to occupy more space than its saturated counterpart. Thus, the ratio between the size of the polar headgroup and acyl chain saturation defines the overall geometry of the lipids (Vanni et al., 2014; Pinot et al., 2014). The artificial combination of a bulky headgroup and monounsaturation of both acyl chains, such as in the artificial lipids 1,2-dioleoyl-sn-glycero-3-phosphoserine (DOPS) or Ni2+-NTA-DOGS, favors spontaneous curvature (Bigay and Antonny, 2012). Conversely, sterols increase the packing of cylindrical sphingolipids (such as sphingomyelin) and of PtdCho, and thus disfavor spontaneous curvature (Ikonen, 2008).
Active maintenance of lipid asymmetry by type IV P-type ATPases (lipid flippases) that translocate phospholipids (e.g. PtdSer or PtdEtn) directionally from the extracellular leaflet to the cytosolic face (Daleke, 2007; Devaux et al., 2008) supports changes in membrane curvature and vesicular budding by generating a difference in the partitioning of lipids between the two leaflets (Poulsen et al., 2008; Ruaud et al., 2009; Xu et al., 2009). Changes in the lipid composition of one monolayer by lysophospholipid acyltransferases, phospholipase A or sphingomyelinases also induce local membrane curvature (reviewed in Graham and Kozlov, 2010). Finally, the clustering of certain lipids by bacterial toxins, such as cholera toxin B or Shiga toxin B, favors the concentration of lipids with the same shape (conical, inverted conical or cylindrical; see poster) on one leaflet and coincides with the generation of negative membrane curvature (Ewers et al., 2010; Römer et al., 2007).
Partitioning of shaped transmembrane domains and protein crowding
Transmembrane proteins (ion channels, transporters and receptors) that have an intrinsic conical or inverted conical shape can bend their associated membranes (Aimon et al., 2014; Fertuck and Salpeter, 1974; Fribourg et al., 2014; MacKinnon, 2003; Unwin, 2005). The asymmetry of extramembrane domains of transmembrane proteins might also result in bending. Thus, receptors might cause membrane bending by protein crowding where the membrane bends away from the side with the largest domains (Copic et al., 2012). In addition, many integral membrane proteins oligomerize – either directly among themselves or through connecting proteins (Boudin et al., 2000; Eckler et al., 2005), which results in local scaffolding of membranes. The clustering of transmembrane receptors, such as transferrin or low-density lipoprotein (LDL) receptors, into forming endocytic pits supports the generation of membrane curvature by stabilizing forming clathrin-coated pits (Ehrlich et al., 2004). These mechanisms might play a role in sorting receptors into (or excluding them from) membrane trafficking carriers. A high local concentration of proteins that bind to the membrane surface has been suggested to induce membrane curvature by a crowding mechanism (Stachowiak et al., 2012), although the contribution of this mechanism is still unclear (recently discussed in Kozlov et al., 2014).
Insertion of hydrophobic protein motifs
Insertion of a small hydrophobic protein motif in between the lipid headgroups is a very efficient way of inducing local membrane curvature (see poster). It acts as a ‘wedge’; given that the shallow insertion is in the outer part of the membrane, this means that the apex of the wedge is in the center of the bilayer and, thus, the radius of curvature is small (the thickness of the monolayer) (Ford et al., 2002). If there is a single insertion, the bending of the membrane is dissipated rapidly in space, but if several insertions are held in close proximity (within the same protein or within complex protein assemblies), the resulting membrane curvature is much more pronounced (Campelo et al., 2008; McMahon et al., 2010). Amphipathic helices are present in many proteins that are involved in membrane remodeling throughout the entire cell, such as epsin, endophilin, amphiphysin, Bin2, nadrin (also known as ARHGAP17), Arf proteins, Sar1 and atlastin GTPases, GRK5, ArfGAP1, ArfGEF GBF1, GMAP-210 (also known as TRIP11), NUP133, barkor, α-synuclein, annexin B12, Atg3 and the endosomal sorting complexes required for transport (ESCRT)-III protein CHMP4B (reviewed in Drin and Antonny, 2010). Amphipathic helices vary in lengths and in their affinities for charged lipids (dictated by the nature of the amino acids on the edges of the hydrophobic face of the helix), and thus have varying abilities to sense or induce membrane curvature. Several bacterial proteins, such as DivIVA or SpoVM, use amphipathic helices for their subcellular targeting, suggesting that curvature sensing is a very ancient mechanism (Lenarcic et al., 2009; Ramamurthi et al., 2009).
The insertion of hydrophobic loops that are present at the tip of the C2 domains of synaptotagmin-1 and Doc2b (Groffen et al., 2010; Hui et al., 2009; Martens et al., 2007) or at the membrane-binding interface of Pacsin and EHD2 also induce membrane curvature by acting as wedges (Daumke et al., 2007; Plomann et al., 2010); although, in the latter case, protein oligomerization and scaffolding also contribute to the generation of curvature. Consistent with their extensive insertion into the lipid bilayer, C2 domains induce a high degree of curvature (McMahon et al., 2010). As C2 domains bind to membrane in a Ca2+-dependent manner, their membrane bending activity is tightly regulated (Hui et al., 2009; Martens et al., 2007).
Nanoscopic scaffolding by oligomerized hydrophilic protein domains
Scaffolding by peripheral proteins supports membrane curvature at a nanoscopic level (curvature on the scale of protein domains), whereby the shape of the membrane-binding interface is imposed on the membrane. Nanoscopic proteins might also assemble into larger structures that have a shape that is formed by the oligomer and thus affect membrane curvature at the microscopic level.
Protein oligomerization
Coat proteins such as clathrin, COPI and COPII are key factors that stabilize membrane curvature during vesicle budding (Jensen and Schekman, 2011; Kirchhausen, 2000; McMahon and Boucrot, 2011; Zanetti et al., 2012). They do not bind to membrane directly and rely on adaptor proteins to link them to membranes (see poster). The capacity of the coat proteins to bend to membranes depends on the rigidity of the assembled polyhedral coat and the transmission of this shape to the membrane (Copic et al., 2012). Members of the dynamin GTPase superfamily induce membrane curvature by self-polymerizing into spirals (reviewed in Faelber et al., 2012; Ferguson and De Camilli, 2012; Morlot and Roux, 2013; Praefcke and McMahon, 2004). However, dynamin requires a pre-existing curvature to assemble efficiently, at least in the presence of membrane tension. Proteins such as caveolin, flotillins or the reticulons oligomerize (directly or through bridging proteins) as well as insert into membranes, thereby facilitating the formation or stabilization of curvature at caveolae and the endoplasmic reticulum (ER), respectively (Hu et al., 2011; Parton and del Pozo, 2013; Shibata et al., 2009).
BAR domains
BAR (Bin/Amphiphysin/Rvs) domains are crescent-shaped dimeric modules that bind to membranes. The prototypical BAR domain protein is amphiphysin, which binds to membrane through its concave face (Peter et al., 2004). The initial binding is mediated by electrostatic interaction between positively charged amino acids (lysine or arginine) of the BAR module and negatively charged lipids [PtdSer and/or phosphatidylinositol phosphates, such as PtdIns(4,5)P2] (Mim and Unger, 2012; Rao and Haucke, 2011). Some BAR domains can further oligomerise by forming ‘tip-to-tip’ or ‘side-to-side’ interactions, thereby increasing their local concentration, which makes it more likely that they can impose their intrinsic curvature locally on the membrane (Mim and Unger, 2012; Rao and Haucke, 2011). BAR domains favor the formation of membrane tubules and disfavor the more extreme curvature needed for membrane scission (Peter et al., 2004; Boucrot et al., 2012).
Macroscopic scaffolding by the cytoskeleton
Scaffolding by the actin, intermediate filament and microtubule cytoskeleton supports cell membrane curvature at the macroscopic scale (in the range of multiple microns in radius), which is present in large intracellular organelles, such as the Golgi or the ER. The cytoskeleton also mediates the macroscopic curvature of the plasma membrane in filopodia and lamellipodia, or of the specific arrangement of the plasma membrane during cell division (i.e. cortical actin supporting mitotic cell rounding), phagocytosis (membrane ruffling) or in specialized cellular shapes (neurons or visual cones) (Rohn and Baum, 2010; Sheetz, 2001). The cytoskeleton maintains cell membrane tension by connecting to the bilayer at regular intervals, and it imposes macroscopic shapes by providing an underlying scaffold (Doherty and McMahon, 2008). Active membrane pulling or pushing by kinesins, dynein and myosin also induces considerable membrane reorganization and supports some of the organelle morphologies (e.g. ER, Golgi and endosomes) (reviewed in Leduc et al., 2010).
Synergistic action of multiple mechanisms
A combination of several of the aforementioned mechanisms usually takes place to efficiently induce membrane sculpting. Proteins with insertion motifs, scaffolding abilities or both bind to numerous other proteins that are involved in the same cellular process and, thus, form a network of interactions that combine sensing, induction and stabilization of curvature (McMahon and Gallop, 2005). For example, during the formation of a clathrin-coated pit, several of the membrane-bending mechanisms presented above take place. The asymmetrical partitioning of inverted conical lipids [PtdSer and PtdIns(4,5)P2] between the two membrane leaflets and their clustering by several proteins – some of which have, in addition, membrane inserting (epsin) or scaffolding modules (amphiphysin) – all occur within an oligomeric protein scaffold that is being held together by the clathrin coat (McMahon and Boucrot, 2011).
Functions of membrane curvature
The control of biological membrane shapes is central to eukaryotic life. Thus, the formation of high-curvature transport intermediates is necessary for compartmentalization, and the characteristic shapes of organelles are likely to be related to their function; indeed, the ability to control fission and fusion, local tethering or enzyme reactions might also be shape dependent (see poster; reviewed in McMahon and Gallop, 2005; Shibata et al., 2009).
Organelle shaping
Most intracellular organelles have characteristic membrane shapes that make them easily recognizable by their morphology in electron micrographs. These shapes are mediated by the extent and localization of membrane curvature, which give rise to the typical shapes of the ER, the Golgi, mitochondria, lipid droplets, autophagosomes or endosomal and secretory carriers (Shibata et al., 2009). However, these characteristic shapes are dynamic, and they change according to the needs of the cell (e.g. mitochondrial fission or fusion) or the maturation of the organelle (e.g. formation or consumption of endocytic or synaptic vesicles) (Friedman and Nunnari, 2014; Haucke et al., 2011; Rafelski and Marshall, 2008).
Membrane scission
Scission between two membranes requires the localized and timely connection in trans of two bilayer membranes to separate an organelle from its membrane of origin, while avoiding leakiness. Membrane scission is a key step in the budding of coated vesicles from the ER (COPII coat), Golgi (COPI coat), trans-Golgi network (AP-1 and AP-3 clathrin coats), endosomes (ESCRT complexes) and plasma membranes (AP-2 clathrin coat), as well as in virus budding (Ferguson and De Camilli, 2012; Hurley and Hanson, 2010; Rossman and Lamb, 2013). Members of the dynamin superfamily are the main drivers of membrane scission. They oligomerize at the neck of nascent vesicles and, upon hydrolysis of GTP, they trigger fusion between the two lipid bilayers, thereby causing the scission of the neck and detachment of the vesicle (Ferguson and De Camilli, 2012; Praefcke and McMahon, 2004). High local concentrations of hydrophobic insertions at the neck of constricted vesicle carriers supports the fission of nascent membrane-trafficking vesicles by creating sufficient stress on the bilayer to favor scission, as it is the case for Sar1 at COPII vesicles (Lee et al., 2005) and epsin, which can support dynamin at clathrin-coated pits (Boucrot et al., 2012). This mechanism is also likely to have a role in virus and exosome budding, as well as in the formation of multivesicular bodies (MVBs) in endosomes, because key proteins involved in these processes have amphipathic helices (Buchkovich et al., 2013; Rossman et al., 2010).
Membrane targeting and tethering
Membrane curvature sensing has an important role in vesicle targeting, which is exerted through tethering proteins such as the Golgin GMAP-210 that acts at the cis Golgi (Drin et al., 2007; Drin et al., 2008). This protein uses amphipathic helices at the tip of long molecular strings to sense high curvature on incoming vesicles before the engagement of soluble NSF attachment protein receptors (SNAREs), thereby increasing the fidelity of vesicle targeting. Other examples are the highly curved membrane sensing by the autophagosome-specific adaptor barkor (also known as Atg14L) that mediates the subsequent recruitment of class III phosphatidylinositol 3-kinase during autophagosome biogenesis (Fan et al., 2011), and the synergy between cargo recruitment by the autophagy receptor Atg19 and membrane bending of Atg8-coated membranes during autophagophore wrapping around the cargo (Sawa-Makarska et al., 2014).
Membrane fusion
The fusion between a vesicle and its target membrane is mediated by the assembly of cognate SNARE complexes that bring the two apposing membranes into close proximity upon assembly (see poster) (Jahn and Scheller, 2006; Martens and McMahon, 2008). However, SNARE complexes might be aided by both the lipid composition of the membrane and the coordinated action of membrane-bending modules, such as the C2 domains of synaptotagmin and Doc2 proteins, or the amphipathic helix of complexin, which facilitate and accelerate membrane fusion and neurotransmitter release at neuronal synapses (Groffen et al., 2010; Hui et al., 2009; Martens et al., 2007; Snead et al., 2014).
Protein sorting
The intrinsic shapes of transmembrane receptors might mediate their partitioning into regions of the membrane that accommodate the relevant shapes. This might be the case for nicotinic acetylcholine and glutamate receptors as well as Ca2+ or K+ channels that appear to concentrate at post-synaptic regions and other areas of high membrane curvature (see poster) (Aimon et al., 2014; Fertuck and Salpeter, 1974; MacKinnon, 2003; Unwin, 2005). In making vesicle trafficking intermediates, the initial bud formation might help to concentrate proteins that favor the bud curvature or, alternatively, help to exclude proteins that either do not have an adaptor to localize them to the bud or have an intrinsic curvature that forces them to leave the bud. The concentration of high-curvature-sensing and -inducing proteins, such as Sar1, the ESCRT complex or sorting nexin proteins, is also important for the organization of ER exit sites or endosomal sorting and recycling (Cullen and Korswagen, 2012; Okamoto et al., 2012).
Enzyme and protein activation
Several enzymes display curvature-sensitive activities. For instance, the phosphoinositide phosphatase synaptojanin has a higher activity on highly curved membrane (Chang-Ileto et al., 2011). The lipid-extraction capacity of the sterol transporter Osh4p (also known as Kes1p) is also greater at a high membrane curvature (de Saint-Jean et al., 2011; Drin et al., 2007), as is lipidation of the autophagy protein LC3 (encoded by MAP1LC3A) or GABARAP by Atg3 (Nath et al., 2014). The small GTPases Arf, Arl and Sar1, as well as some of their guanosine exchange factors (GEFs) and GTPase-activating proteins (GAPs) are also activated by membrane curvature (Bigay et al., 2005; Krauss et al., 2008; Lee et al., 2005; Lundmark et al., 2008). It is likely that future work will uncover many other instances of protein activation by membrane curvature.
Conclusions and perspectives
In addition to the lipid properties that are inherent to the membrane bilayer, several distinct mechanisms exist that allow proteins to sense, stabilize or generate a high local membrane curvature. Several of these mechanisms can be utilized in concert during complex membrane remodeling processes, such as organelle shaping and vesicle budding, scission and fusion. Consistent with its universality within eukaryotic cells, it is very likely that additional mechanisms underlying membrane shaping will be uncovered in the near future and that further cellular functions will be recognized to depend on membrane curvature.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by a Medical Research Council UK grant [grant number U105178805] to H.T.M.; and a Biotechnology and Biological Sciences Research Council David Phillips Research Fellowship [grant number BB/I018921]; and a prize from the Lister Institute for Preventive Medicine to E.B. Deposited in PMC for release after 6 months.
Cell science at a glance
A high-resolution version of the poster is available for downloading in the online version of this article at jcs.biologists.org. Individual poster panels are available as JPEG files at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.114454/-/DC1.
References
- Aimon S., Callan-Jones A., Berthaud A., Pinot M., Toombes G. E., Bassereau P. (2014). Membrane shape modulates transmembrane protein distribution. Dev. Cell 28, 212–218 10.1016/j.devcel.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigay J., Antonny B. (2012). Curvature, lipid packing, and electrostatics of membrane organelles: defining cellular territories in determining specificity. Dev. Cell 23, 886–895 10.1016/j.devcel.2012.10.009 [DOI] [PubMed] [Google Scholar]
- Bigay J., Casella J. F., Drin G., Mesmin B., Antonny B. (2005). ArfGAP1 responds to membrane curvature through the folding of a lipid packing sensor motif. EMBO J. 24, 2244–2253 10.1038/sj.emboj.7600714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucrot E., Pick A., Çamdere G., Liska N., Evergren E., McMahon H. T., Kozlov M. M. (2012). Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains. Cell 149, 124–136 10.1016/j.cell.2012.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudin H., Doan A., Xia J., Shigemoto R., Huganir R. L., Worley P., Craig A. M. (2000). Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain binding site. Neuron 28, 485–497 10.1016/S0896-6273(00)00127-6 [DOI] [PubMed] [Google Scholar]
- Buchkovich N. J., Henne W. M., Tang S., Emr S. D. (2013). Essential N-terminal insertion motif anchors the ESCRT-III filament during MVB vesicle formation. Dev. Cell 27, 201–214 10.1016/j.devcel.2013.09.009 [DOI] [PubMed] [Google Scholar]
- Campelo F., McMahon H. T., Kozlov M. M. (2008). The hydrophobic insertion mechanism of membrane curvature generation by proteins. Biophys. J. 95, 2325–2339 10.1529/biophysj.108.133173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Ileto B., Frere S. G., Chan R. B., Voronov S. V., Roux A., Di Paolo G. (2011). Synaptojanin 1-mediated PI(4,5)P2 hydrolysis is modulated by membrane curvature and facilitates membrane fission. Dev. Cell 20, 206–218 10.1016/j.devcel.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. V., Kozlov M. M. (2003). Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 72, 175–207 10.1146/annurev.biochem.72.121801.161504 [DOI] [PubMed] [Google Scholar]
- Cooke I. R., Deserno M. (2006). Coupling between lipid shape and membrane curvature. Biophys. J. 91, 487–495 10.1529/biophysj.105.078683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copic A., Latham C. F., Horlbeck M. A., D'Arcangelo J. G., Miller E. A. (2012). ER cargo properties specify a requirement for COPII coat rigidity mediated by Sec13p. Science 335, 1359–1362 10.1126/science.1215909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P. J., Korswagen H. C. (2012). Sorting nexins provide diversity for retromer-dependent trafficking events. Nat. Cell Biol. 14, 29–37 10.1038/ncb2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daleke D. L. (2007). Phospholipid flippases. J. Biol. Chem. 282, 821–825 10.1074/jbc.R600035200 [DOI] [PubMed] [Google Scholar]
- Daumke O., Lundmark R., Vallis Y., Martens S., Butler P. J., McMahon H. T. (2007). Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature 449, 923–927 10.1038/nature06173 [DOI] [PubMed] [Google Scholar]
- de Saint-Jean M., Delfosse V., Douguet D., Chicanne G., Payrastre B., Bourguet W., Antonny B., Drin G. (2011). Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J. Cell Biol. 195, 965–978 10.1083/jcb.201104062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P. F., Herrmann A., Ohlwein N., Kozlov M. M. (2008). How lipid flippases can modulate membrane structure. Biochim. Biophys. Acta 1778, 1591–1600 10.1016/j.bbamem.2008.03.007 [DOI] [PubMed] [Google Scholar]
- Di Paolo G., De Camilli P. (2006). Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657 10.1038/nature05185 [DOI] [PubMed] [Google Scholar]
- Doherty G. J., McMahon H. T. (2008). Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annu. Rev. Biophys. 37, 65–95 10.1146/annurev.biophys.37.032807.125912 [DOI] [PubMed] [Google Scholar]
- Drin G., Antonny B. (2010). Amphipathic helices and membrane curvature. FEBS Lett. 584, 1840–1847 10.1016/j.febslet.2009.10.022 [DOI] [PubMed] [Google Scholar]
- Drin G., Casella J. F., Gautier R., Boehmer T., Schwartz T. U., Antonny B. (2007). A general amphipathic α-helical motif for sensing membrane curvature. Nat. Struct. Mol. Biol. 14, 138–146 10.1038/nsmb1194 [DOI] [PubMed] [Google Scholar]
- Drin G., Morello V., Casella J. F., Gounon P., Antonny B. (2008). Asymmetric tethering of flat and curved lipid membranes by a golgin. Science 320, 670–673 10.1126/science.1155821 [DOI] [PubMed] [Google Scholar]
- Eckler S. A., Kuehn R., Gautam M. (2005). Deletion of N-terminal rapsyn domains disrupts clustering and has dominant negative effects on clustering of full-length rapsyn. Neuroscience 131, 661–670 10.1016/j.neuroscience.2004.11.035 [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Boll W., Van Oijen A., Hariharan R., Chandran K., Nibert M. L., Kirchhausen T. (2004). Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118, 591–605 10.1016/j.cell.2004.08.017 [DOI] [PubMed] [Google Scholar]
- Ewers H., Romer W., Smith A. E., Bacia K., Dmitrieff S., Chai W., Mancini R., Kartenbeck J., Chambon V., Berland L. et al. (2010). GM1 structure determines SV40-induced membrane invagination and infection. Nat. Cell Biol. 12, 11–18 10.1038/ncb1999 [DOI] [PubMed] [Google Scholar]
- Faelber K., Held M., Gao S., Posor Y., Haucke V., Noé F., Daumke O. (2012). Structural insights into dynamin-mediated membrane fission. Structure 20, 1621–1628 10.1016/j.str.2012.08.028 [DOI] [PubMed] [Google Scholar]
- Fan W., Nassiri A., Zhong Q. (2011). Autophagosome targeting and membrane curvature sensing by Barkor/Atg14(L). Proc. Natl. Acad. Sci. USA 108, 7769–7774 10.1073/pnas.1016472108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. M., De Camilli P. (2012). Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 13, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. (1974). Localization of acetylcholine receptor by 125I-labeled alpha-bungarotoxin binding at mouse motor endplates. Proc. Natl. Acad. Sci. USA 71, 1376–1378 10.1073/pnas.71.4.1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M. G., Mills I. G., Peter B. J., Vallis Y., Praefcke G. J., Evans P. R., McMahon H. T. (2002). Curvature of clathrin-coated pits driven by epsin. Nature 419, 361–366 10.1038/nature01020 [DOI] [PubMed] [Google Scholar]
- Fribourg P. F., Chami M., Sorzano C. O. S., Gubellini F., Marabini R., Marco S., Jault J. M., Lévy D. (2014). 3D cryo-electron reconstruction of BmrA, a bacterial multidrug ABC transporter in an inward-facing conformation and in a lipidic environment. J. Mol. Biol. 426, 2059–2069 10.1016/j.jmb.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Friedman J. R., Nunnari J. (2014). Mitochondrial form and function. Nature 505, 335–343 10.1038/nature12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. R., Kozlov M. M. (2010). Interplay of proteins and lipids in generating membrane curvature. Curr. Opin. Cell Biol. 22, 430–436 10.1016/j.ceb.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen A. J., Martens S., Díez Arazola R., Cornelisse L. N., Lozovaya N., de Jong A. P., Goriounova N. A., Habets R. L., Takai Y., Borst J. G. et al. (2010). Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science 327, 1614–1618 10.1126/science.1183765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V., Neher E., Sigrist S. J. (2011). Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat. Rev. Neurosci. 12, 127–138 10.1038/nrn2948 [DOI] [PubMed] [Google Scholar]
- Hu J., Prinz W. A., Rapoport T. A. (2011). Weaving the web of ER tubules. Cell 147, 1226–1231 10.1016/j.cell.2011.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E., Johnson C. P., Yao J., Dunning F. M., Chapman E. R. (2009). Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell 138, 709–721 10.1016/j.cell.2009.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. H., Hanson P. I. (2010). Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat. Rev. Mol. Cell Biol. 11, 556–566 10.1038/nrm2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E. (2008). Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 9, 125–138 10.1038/nrm2336 [DOI] [PubMed] [Google Scholar]
- Jahn R., Scheller R. H. (2006). SNAREs – engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7, 631–643 10.1038/nrm2002 [DOI] [PubMed] [Google Scholar]
- Jensen D., Schekman R. (2011). COPII-mediated vesicle formation at a glance. J. Cell Sci. 124, 1–4 10.1242/jcs.069773 [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. (2000). Three ways to make a vesicle. Nat. Rev. Mol. Cell Biol. 1, 187–198 10.1038/35043117 [DOI] [PubMed] [Google Scholar]
- Kozlov M. M., Kuzmin P. I., Popov S. V. (1992). Formation of cell protrusions by an electric field: a thermodynamic analysis. Eur. Biophys. J. 21, 35–45 10.1007/BF00195442 [DOI] [PubMed] [Google Scholar]
- Kozlov M. M., Campelo F., Liska N., Chernomordik L. V., Marrink S. J., McMahon H. T. (2014). Mechanisms shaping cell membranes. Curr. Opin. Cell Biol. 29, 53–60 10.1016/j.ceb.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M., Jia J. Y., Roux A., Beck R., Wieland F. T., De Camilli P., Haucke V. (2008). Arf1-GTP-induced tubule formation suggests a function of Arf family proteins in curvature acquisition at sites of vesicle budding. J. Biol. Chem. 283, 27717–27723 10.1074/jbc.M804528200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc C., Campàs O., Joanny J. F., Prost J., Bassereau P. (2010). Mechanism of membrane nanotube formation by molecular motors. Biochim. Biophys. Acta 1798, 1418–1426 10.1016/j.bbamem.2009.11.012 [DOI] [PubMed] [Google Scholar]
- Lee M. C., Orci L., Hamamoto S., Futai E., Ravazzola M., Schekman R. (2005). Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell 122, 605–617 10.1016/j.cell.2005.07.025 [DOI] [PubMed] [Google Scholar]
- Leikin S., Kozlov M. M., Fuller N. L., Rand R. P. (1996). Measured effects of diacylglycerol on structural and elastic properties of phospholipid membranes. Biophys. J. 71, 2623–2632 10.1016/S0006-3495(96)79454-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarcic R., Halbedel S., Visser L., Shaw M., Wu L. J., Errington J., Marenduzzo D., Hamoen L. W. (2009). Localisation of DivIVA by targeting to negatively curved membranes. EMBO J. 28, 2272–2282 10.1038/emboj.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark R., Doherty G. J., Vallis Y., Peter B. J., McMahon H. T. (2008). Arf family GTP loading is activated by, and generates, positive membrane curvature. Biochem. J. 414, 189–194 10.1042/BJ20081237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R. (2003). Potassium channels. FEBS Lett. 555, 62–65 10.1016/S0014-5793(03)01104-9 [DOI] [PubMed] [Google Scholar]
- Martens S., McMahon H. T. (2008). Mechanisms of membrane fusion: disparate players and common principles. Nat. Rev. Mol. Cell Biol. 9, 543–556 10.1038/nrm2417 [DOI] [PubMed] [Google Scholar]
- Martens S., Kozlov M. M., McMahon H. T. (2007). How synaptotagmin promotes membrane fusion. Science 316, 1205–1208 10.1126/science.1142614 [DOI] [PubMed] [Google Scholar]
- McMahon H. T., Boucrot E. (2011). Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517–533 10.1038/nrm3151 [DOI] [PubMed] [Google Scholar]
- McMahon H. T., Gallop J. L. (2005). Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438, 590–596 10.1038/nature04396 [DOI] [PubMed] [Google Scholar]
- McMahon H. T., Kozlov M. M., Martens S. (2010). Membrane curvature in synaptic vesicle fusion and beyond. Cell 140, 601–605 10.1016/j.cell.2010.02.017 [DOI] [PubMed] [Google Scholar]
- Mim C., Unger V. M. (2012). Membrane curvature and its generation by BAR proteins. Trends Biochem. Sci. 37, 526–533 10.1016/j.tibs.2012.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlot S., Roux A. (2013). Mechanics of dynamin-mediated membrane fission. Annu. Rev. Biophys. 42, 629–649 10.1146/annurev-biophys-050511-102247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath S., Dancourt J., Shteyn V., Puente G., Fong W. M., Nag S., Bewersdorf J., Yamamoto A., Antonny B., Melia T. J. (2014). Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nat. Cell Biol. 16, 415–424 10.1038/ncb2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M., Kurokawa K., Matsuura-Tokita K., Saito C., Hirata R., Nakano A. (2012). High-curvature domains of the ER are important for the organization of ER exit sites in Saccharomyces cerevisiae. J. Cell Sci. 125, 3412–3420 10.1242/jcs.100065 [DOI] [PubMed] [Google Scholar]
- Parton R. G., del Pozo M. A. (2013). Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 14, 98–112 10.1038/nrm3512 [DOI] [PubMed] [Google Scholar]
- Peter B. J., Kent H. M., Mills I. G., Vallis Y., Butler P. J., Evans P. R., McMahon H. T. (2004). BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, 495–499 10.1126/science.1092586 [DOI] [PubMed] [Google Scholar]
- Pinot M., Vanni S., Pagnotta S., Lacas-Gervais S., Payet L. A., Ferreira T., Gautier R., Goud B., Antonny B., Barelli H. (2014). Lipid cell biology. Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science 345, 693–697 10.1126/science.1255288 [DOI] [PubMed] [Google Scholar]
- Plomann M., Wittmann J. G., Rudolph M. G. (2010). A hinge in the distal end of the PACSIN 2 F-BAR domain may contribute to membrane-curvature sensing. J. Mol. Biol. 400, 129–136 10.1016/j.jmb.2010.05.008 [DOI] [PubMed] [Google Scholar]
- Poulsen L. R., López-Marqués R. L., Palmgren M. G. (2008). Flippases: still more questions than answers. Cell. Mol. Life Sci. 65, 3119–3125 10.1007/s00018-008-8341-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praefcke G. J., McMahon H. T. (2004). The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 5, 133–147 10.1038/nrm1313 [DOI] [PubMed] [Google Scholar]
- Rafelski S. M., Marshall W. F. (2008). Building the cell: design principles of cellular architecture. Nat. Rev. Mol. Cell Biol. 9, 593–602 10.1038/nrm2460 [DOI] [PubMed] [Google Scholar]
- Ramamurthi K. S., Lecuyer S., Stone H. A., Losick R. (2009). Geometric cue for protein localization in a bacterium. Science 323, 1354–1357 10.1126/science.1169218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y., Haucke V. (2011). Membrane shaping by the Bin/amphiphysin/Rvs (BAR) domain protein superfamily. Cell. Mol. Life Sci. 68, 3983–3993 10.1007/s00018-011-0768-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn J. L., Baum B. (2010). Actin and cellular architecture at a glance. J. Cell Sci. 123, 155–158 10.1242/jcs.049759 [DOI] [PubMed] [Google Scholar]
- Römer W., Berland L., Chambon V., Gaus K., Windschiegl B., Tenza D., Aly M. R., Fraisier V., Florent J. C., Perrais D. et al. (2007). Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 450, 670–675 10.1038/nature05996 [DOI] [PubMed] [Google Scholar]
- Rossman J. S., Lamb R. A. (2013). Viral membrane scission. Annu. Rev. Cell Dev. Biol. 29, 551–569 10.1146/annurev-cellbio-101011-155838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman J. S., Jing X., Leser G. P., Lamb R. A. (2010). Influenza virus M2 protein mediates ESCRT-independent membrane scission. Cell 142, 902–913 10.1016/j.cell.2010.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruaud A. F., Nilsson L., Richard F., Larsen M. K., Bessereau J. L., Tuck S. (2009). The C. elegans P4-ATPase TAT-1 regulates lysosome biogenesis and endocytosis. Traffic 10, 88–100 10.1111/j.1600-0854.2008.00844.x [DOI] [PubMed] [Google Scholar]
- Sawa-Makarska J., Abert C., Romanov J., Zens B., Ibiricu I., Martens S. (2014). Cargo binding to Atg19 unmasks additional Atg8 binding sites to mediate membrane-cargo apposition during selective autophagy. Nat. Cell Biol. 16, 425–433 10.1038/ncb2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P. (2001). Cell control by membrane-cytoskeleton adhesion. Nat. Rev. Mol. Cell Biol. 2, 392–396 10.1038/35073095 [DOI] [PubMed] [Google Scholar]
- Shibata Y., Hu J., Kozlov M. M., Rapoport T. A. (2009). Mechanisms shaping the membranes of cellular organelles. Annu. Rev. Cell Dev. Biol. 25, 329–354 10.1146/annurev.cellbio.042308.113324 [DOI] [PubMed] [Google Scholar]
- Snead D., Wragg R. T., Dittman J. S., Eliezer D. (2014). Membrane curvature sensing by the C-terminal domain of complexin. Nat. Commun. 5, 4955 10.1038/ncomms5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak J. C., Schmid E. M., Ryan C. J., Ann H. S., Sasaki D. Y., Sherman M. B., Geissler P. L., Fletcher D. A., Hayden C. C. (2012). Membrane bending by protein-protein crowding. Nat. Cell Biol. 14, 944–949 10.1038/ncb2561 [DOI] [PubMed] [Google Scholar]
- Unwin N. (2005). Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 346, 967–989 10.1016/j.jmb.2004.12.031 [DOI] [PubMed] [Google Scholar]
- Vanni S., Hirose H., Barelli H., Antonny B., Gautier R. (2014). A sub-nanometre view of how membrane curvature and composition modulate lipid packing and protein recruitment. Nat. Commun. 5, 4916 10.1038/ncomms5916 [DOI] [PubMed] [Google Scholar]
- Xu P., Okkeri J., Hanisch S., Hu R. Y., Xu Q., Pomorski T. G., Ding X. Y. (2009). Identification of a novel mouse P4-ATPase family member highly expressed during spermatogenesis. J. Cell Sci. 122, 2866–2876 10.1242/jcs.047423 [DOI] [PubMed] [Google Scholar]
- Zanetti G., Pahuja K. B., Studer S., Shim S., Schekman R. (2012). COPII and the regulation of protein sorting in mammals. Nat. Cell Biol. 14, 20–28 10.1038/ncb2390 [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Kozlov M. M. (2006). How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 7, 9–19 10.1038/nrm1784 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.