ABSTRACT
Embryonic development and adult tissue homeostasis require precise information exchange between cells and their microenvironment to coordinate cell behavior. A specialized class of ultra-long actin-rich filopodia, termed cytonemes, provides one mechanism for this spatiotemporal regulation of extracellular cues. We provide here a mechanism whereby the stem-cell marker Lgr5, and its family member Lgr4, promote the formation of cytonemes. Lgr4- and Lgr5-induced cytonemes exceed lengths of 80 µm, are generated through stabilization of nascent filopodia from an underlying lamellipodial-like network and functionally provide a pipeline for the transit of signaling effectors. As proof-of-principle, we demonstrate that Lgr5-induced cytonemes act as conduits for cell signaling by demonstrating that the actin motor and filopodial cargo carrier protein myosin X (Myo10) and the G-protein-coupled receptor (GPCR) signaling effector β-arrestin-2 (Arrb2) transit into cytonemes. This work delineates a biological function for Lgr4 and Lgr5 and provides the rationale to fully investigate Lgr4 and Lgr5 function and cytonemes in mammalian stem cell and cancer stem cell behavior.
KEY WORDS: Lgr5, GPCR, Cytoneme, Stem cell
INTRODUCTION
The G-protein-coupled receptor (GPCR) Lgr5 genetically identifies a population of intestinal crypt stem cells (Barker et al., 2007). Since this observation, the ability to distinguish stem cells in other tissues on the basis of Lgr5 expression has proved to be a successful strategy in the stomach (Barker et al., 2010), skin (Jaks et al., 2008), liver (Huch et al., 2013b), pancreas (Huch et al., 2013a), kidney (Barker et al., 2012), mammary gland (de Visser et al., 2012; Plaks et al., 2013) and developing hematopoietic system (Liu et al., 2014). Lgr5 is the founding member of a subclass of the leucine-rich G-protein-coupled receptors (Lgrs) that include Lgr4 and Lgr6 (Hsu et al., 2000; Hsu et al., 1998). In addition to Lgr5, Lgr4 and Lgr6 are also used as markers of the stem and progenitor cell lineages within tissues. Lgr4 expression identifies an expanded pool of progenitor cells in the intestinal crypt, which also includes those cells expressing Lgr5 (de Lau et al., 2011). Lgr6 expression identifies a more primordial stem cell with restricted cellular expression compared with Lgr5-expressing hair follicle stem cells (Snippert et al., 2010). The clinical relevance of these discoveries has been underscored by the fact that Lgr5-expressing cells possess greater tumorigenic potential than their differentiated progeny (Barker et al., 2010; Barker et al., 2009), and the demonstration that Lgr5-expressing cells in intestinal adenomas are cancer stem cells (Schepers et al., 2012).
The complement of membrane receptors on stem cells might confer them with intrinsic regulatory capacity by tightly controlling their response to extracellular cues. Lgr4–6 signal by a non-canonical G-protein-independent mechanism by binding to R-spondins (Carmon et al., 2012; de Lau et al., 2011) or Norrin (Deng et al., 2013) and potentiating Wnt–βcatenin signal transduction. However, it is likely that other signaling pathways and biological processes are engaged by Lgr4–6. In fact, GPCR signaling is multifaceted in nature and is tightly regulated by endocytosis of activated receptors (Rajagopal et al., 2010). We have reported on the cellular trafficking properties of Lgr5 using EGFP-tagged forms of the receptor and found that the C-terminal tail regulates its unique constitutive internalization and retrograde trafficking to the trans-Golgi network in the apparent absence of ligand (Snyder et al., 2013b). Therefore, we hypothesized that inhibiting this constitutive internalization would provide new insight into the cellular function of Lgr5. Surprisingly, we discovered that the plasma membrane expression of Lgr5 and Lgr4 provide a mechanism for driving the formation of ultra-long actin-rich membrane protrusions that reach lengths approaching 80 µm. These filopodia are phenotypically and molecularly similar to a specialized class of filopodia previously described by Kornberg in the Drosophila wing imaginal disc as ‘cell threads’ or cytonemes (Ramírez-Weber and Kornberg, 1999).
Filopodia are typically of modest length (often <10 µm) owing to the biophysical forces required to deform the plasma membrane (Mogilner and Rubinstein, 2005). By contrast, ultra-long actin-rich filopodia were first observed in sea urchin embryogenesis by Wolpert in 1961 (Gustafson, 1963) and further investigated by McClay in 1995 (Miller et al., 1995). Since their initial description, these structures have been named cytonemes to distinguish them from shorter filopodia. Cytonemes have many distinctive characteristics including their fragility, width and length; all features shared by Lgr4 and Lgr5-induced protrusions. Cytonemes can compartmentalize signaling pathways (Roy et al., 2011) and direct the transfer of morphogens between cells (Roy et al., 2014). In Drosophila, cytonemes orient towards a morphogen-producing cell or emanate from a morphogen-producing cell towards a target cell. Recent studies in Drosophila indicate that cytonemes provide a platform for transmitting morphogens in the stem cell niche of the Drosophila germarium (Rojas-Ríos et al., 2012). Therefore, cytonemes can be employed to exquisitely regulate the scope and precision of signaling during tissue development and maintenance.
Despite these elegant studies, only a few reports have investigated similar structures in mammalian cells and, until recently, even effectors responsible for their formation in Drosophila were missing entirely (Roy et al., 2014). Therefore, questions regarding the mechanisms that drive cytoneme formation and their utility in signaling remain largely unexplored in mammalian systems (Affolter and Basler, 2011; Kornberg and Roy, 2014). Our study answers these questions. We demonstrate that Lgr5 and Lgr4 provide a receptor-based mechanism for triggering the formation of cytonemes and further illustrate that these cytonemes can be scaffolds for signaling effectors in a mammalian cell system. These findings suggest that stem cells might possess the hardware for regulating signaling at a distance.
RESULTS
Lgr5 and Lgr4 expression in mammalian cells induces the robust formation of membrane protrusions
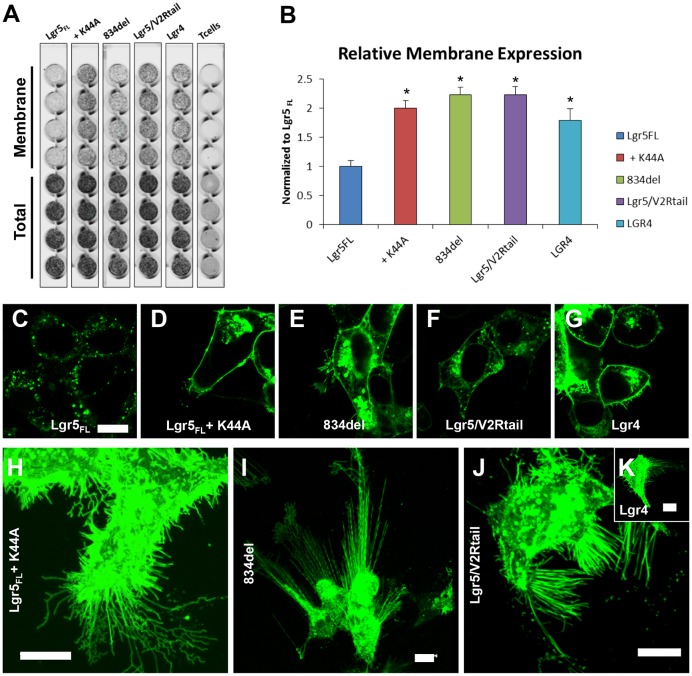
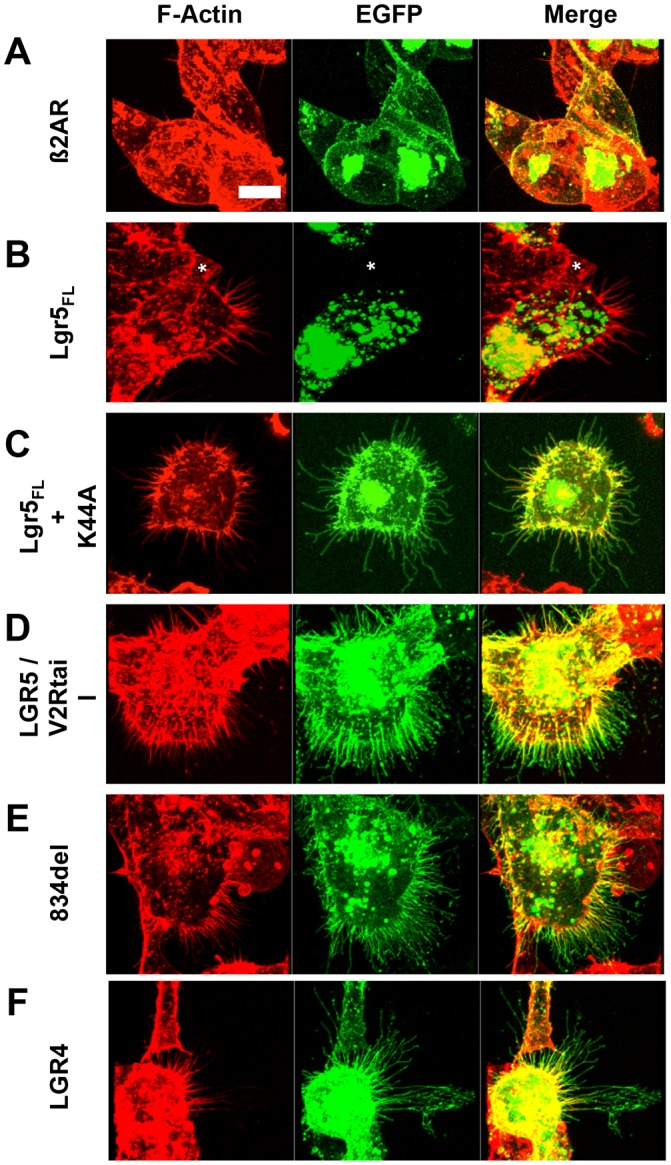
Normally, Lgr5 is constitutively internalized, resulting in intracellular localization of EGFP-tagged receptor (Fig. 1A–C) (Snyder et al., 2013b). However, when the internalization of Lgr5 was blocked either by overexpression of dominant-negative dynamin-1 (K44A), by C-terminal tail truncation at position 834 (834del) or by exchanging the C-terminal tail for that of the human vasopressin V2 receptor (V2R; Lgr5–V2Rtail) (Fig. 1A,B,D–F), we discovered the robust formation of membrane protrusions at the interface between the cell surface and the substrate by confocal microscopy (Fig. 1H–J). Unlike wild-type Lgr5, wild-type Lgr4 was expressed more robustly at the plasma membrane and cells transfected with wild-type Lgr4 displayed extensive protrusions (Fig. 1A,B,G,K). Lgr4 and Lgr5 membrane protrusions are very fragile, and can be severed by over-fixation or even by mild physical perturbations. These structures were often found to be directed towards the basolateral surface and branched upon their contact with the substratum (supplementary material Movie 1). These data demonstrate that membrane protrusions are coincident with the membrane expression for each receptor.
Fig. 1.
Cell-surface expression of Lgr5 or Lgr4 induces the robust formation of membrane protrusions. (A) An on-cell enzyme-linked immunosorbent assay (ELISA) was performed to measure the membrane (live cell stained) and total (fixed, permeabilized and stained) expression of each receptor in HEK cells. The images shown are representative of more than three experiments. (B) Relative membrane expression was determined by calculating the ratio of membrane to total expression values for each receptor and then normalizing to Lgr5FL across multiple experiments. The data show the mean±s.e.m.; *P<0.05 (one-way ANOVA with Bonferroni post-hoc testing). Confocal optical sections of live cells transiently expressing (C) Lgr5FL, (D) Lgr5FL + dynamin-1 K44A (K44A), (E) 834del, (F) Lgr5–V2Rtail (Lgr5/V2Rtail) or (G) Lgr4 to determine their expression at the plasma membrane. HEK cells were transiently transfected with EGFP-tagged (H) Lgr5FL+K44A, (I) 834del, (J) Lgr5–V2Rtail or (K) Lgr4, imaged live on a confocal microscope and rendered in three dimensions. Scale bars: 10 µm.
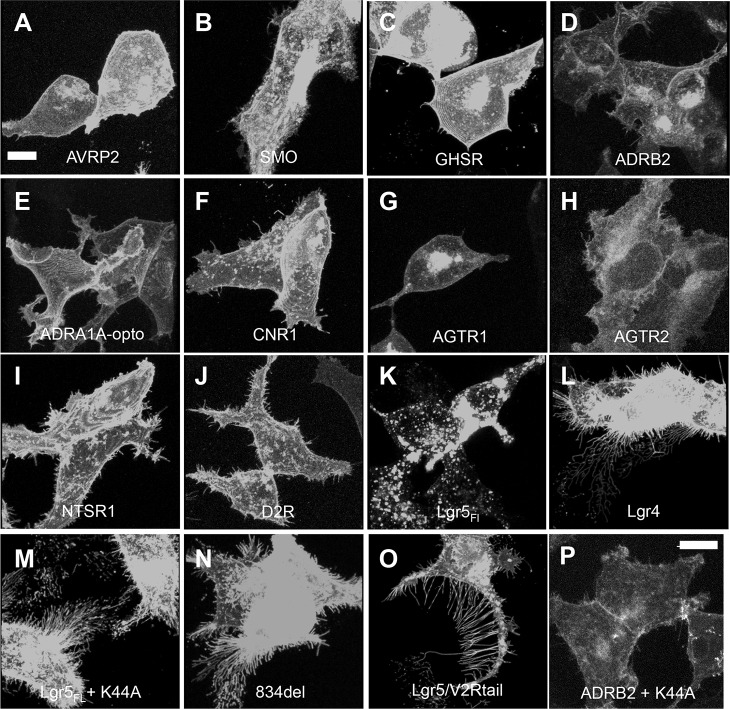
We next tested the hypothesis that this observation is a hallmark of Lgr signaling and not a broader and previously undocumented characteristic of GPCR expression. We transiently transfected cells with 11 EGFP or EYFP-tagged GPCRs, in addition to variants of Lgr5 or Lgr4, and imaged for the presence of membrane protrusions. Cells were blindly scored and then categorized as either an Lgr family member or not based upon their ability to form membrane protrusions. We successfully determined that, as opposed to all other GPCRs tested, all membrane-localized forms of Lgr5 or Lgr4 were competent to form these long thin actin filaments (Fig. 2). Relative to Lgr5, receptors like the β2-AR (ADRB2) do not possess considerable constitutive internalization properties, illustrating that simply preventing GPCR internalization does not cause the formation of membrane protrusions (Snyder et al., 2013b). However, to completely discount the off-target effects of blocking endocytosis, the β2-AR was co-expressed with dynamin K44A. Importantly, no morphological changes were found (Fig. 2P). These data demonstrate that the dramatic induction of membrane protrusions are a specific consequence of overexpressing Lgr4 or Lgr5 at the plasma membrane.
Fig. 2.
Selective generation of membrane protrusions through expression of Lgr4 or Lgr5 and not other GPCRs. (A–P) The indicated EGFP- or EYFP-tagged GPCRs were transiently transfected into cells that were then confocal imaged and scored blindly. The images were scored for the presence or absence of membrane protrusions. Only Lgr5- or Lgr4-transfected cells were scored as having formed extensive protrusions. The presented images are three-dimensionally rendered from a series of optical slices to facilitate coincident viewing of both the apical and basolateral plasma membrane. (A) AVRP2, vasopressin-2 receptor; (B) SMO, smoothened; (C) GHSR, growth hormone secretagogue receptor; (D) ADRB2, β2-adrenergic receptor; (E) ADRA1A-opto, α1-adrenergic receptor–optogenetic chimera (Airan et al., 2009); (F) CNR1, cannabinoid receptor 1; (G) AGTR1, angiotensin II receptor type 1; (H) AGTR2, angiotensin II receptor type 2; (I) NTSR1, neurotensin receptor 1; (J) D2R, dopamine receptor D2; (K) Lgr5FL, (L) Lgr4, (M) Lgr5FL+K44A, (N) Lgr5834del, (O) Lgr5–V2Rtail (Lgr5/V2Rtail), (P) ADRB2:β2-adrenergic receptor+K44A. The latter construct was transfected into cells by using Genecellin. Scale bars: 10 µm.
Morphological and biochemical characterization of Lgr4- and Lgr5-induced membrane protrusions
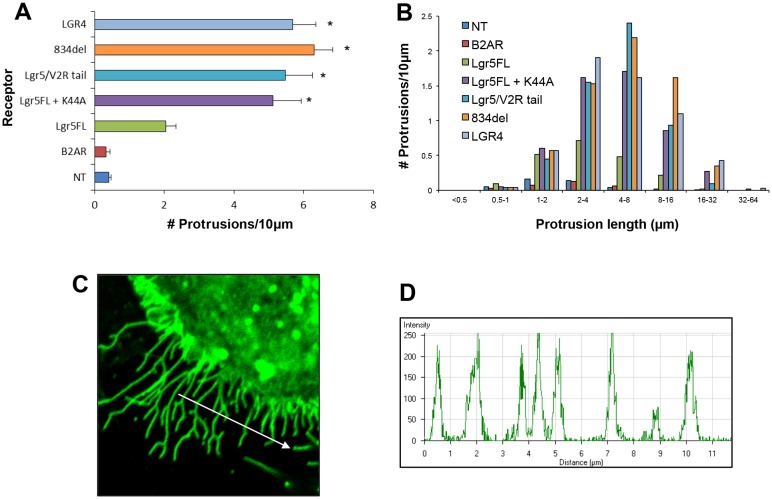
The EGFP tag on the receptor enabled a remarkable visualization of the membrane protrusions. These structures go unnoticed if the receptors are not tagged or can be broken if non-optimal fixation and antibody staining is performed instead of live imaging for EGFP. The membrane protrusions formed gave the appearance of filopodia-like structures that normally comprised polymerized F-actin. Therefore, we tested whether Lgr-induced membrane protrusions are F-actin positive and whether these F-actin structures can be induced by a control GPCR. We performed F-actin staining for each of the Lgr5 or Lgr4-EGFP constructs and compared this to that of non-transfected HEK cells and those expressing human β2-AR. As would be expected from Fig. 2, expression of β2-AR does not induce significant F-actin membrane protrusions (Fig. 3A) nor are these protrusions induced in non-transfected HEK cells (Fig. 3B, asterisk), according to previously published observations (Lin et al., 2007). In previous experiments that utilized GFP to visualize membrane protrusions, we did not notice any apparent membrane protrusions for wild-type full-length Lgr5 (Lgr5FL). However, owing to its labile cell surface expression, GFP expression at the membrane is below the limits of detection. Therefore, we hypothesized that Lgr5FL could induce similar membrane protrusions if instead they were visualized through F-actin staining. As expected, expression of Lgr5FL also induced membrane protrusions, thereby verifying that this is a bona fide property of this receptor when expressed in its wild-type form (Fig. 3B). We also conducted F-actin staining for Lgr5FL that was stabilized on the surface through K44A overexpression or by alterations to its C-terminus. We confirmed that expression of each of these isoforms resulted in F-actin-positive membrane protrusions (Fig. 3C–E). Likewise, Lgr4-induced protrusions also shared this property (Fig. 3F). To test the ability to form actin-rich membrane protrusions in an unbiased manner and further describe this unique property of Lgr proteins, we performed a blinded and quantitative analysis of their number (Fig. 4A) and length (Fig. 4B). These data demonstrated that non-transfected cells and β2-AR-transfected ones have similarly few and short actin-rich membrane protrusions. This is in contrast to cells transfected with Lgr5FL, its membrane stabilized forms and Lgr4, which all drive an increase in the number and length of membrane protrusions. Further analysis also demonstrated the width of these structures to be ∼0.53 µm (±0.019, ±s.e.m.) – consistent with a filopodium-like structure (Fig. 4C,D) (Sheetz et al., 1992).
Fig. 3.
Lgr4- and Lgr5-induced membrane protrusions are rich in polymerized actin. Actin staining (red, left column) in HEK cells transiently expressing the indicated GFP-tagged constructs (green, middle column) was performed for (A) β2-AR, (B) Lgr5FL (the asterisk denotes a non-transfected cell), (C) Lgr5FL+K44A, (D) Lgr5–V2Rtail (Lgr5/V2Rtail), (E) 834del and (F) Lgr4. Three-dimensional reconstructed images were created from Z-stack scans for each cell. Multiple cells across multiple experiments were imaged. Imaging was performed blinded for subsequent utilization in morphometric analysis of protrusion number and length. Scale bar: 10 µm.
Fig. 4.
Morphometric analysis of Lgr4- and Lgr5-induced cytonemes. The number and length of F-actin-positive membrane protrusions in HEK cells that were non-transfected (NT) or transiently expressing β2-AR, Lgr5FL, Lgr5FL + K44A, Lgr5–V2Rtail (Lgr5/V2Rtail), 834del and Lgr4 were counted and are represented in (A) as the total number of protrusions per 10 µm. Data show the mean±s.e.m.; *P<0.05 (one-way ANOVA with Bonferroni post-hoc testing). The data are presented graphically in B to comparatively show the length and number of membrane protrusions as a function of the transiently expressed receptor. (C) HEK cells were transiently transfected with 834del–GFP and imaged live at 100×. A 12-µm line was drawn perpendicular to several protrusions and fluorescence intensity is plotted in D. (D) Pixel intensity as a function of line distance (from left to right in C). These data were imported into GraphPad Prism and fitted with a Gaussian curve, and the width of the cytoneme was calculated by determining the width of the Gaussian curve. A total of 17 membrane protrusions from two different cells were used to calculate the mean width of Lgr5-induced membrane protrusions [0.53 µm; ±0.019 (s.e.m.)].
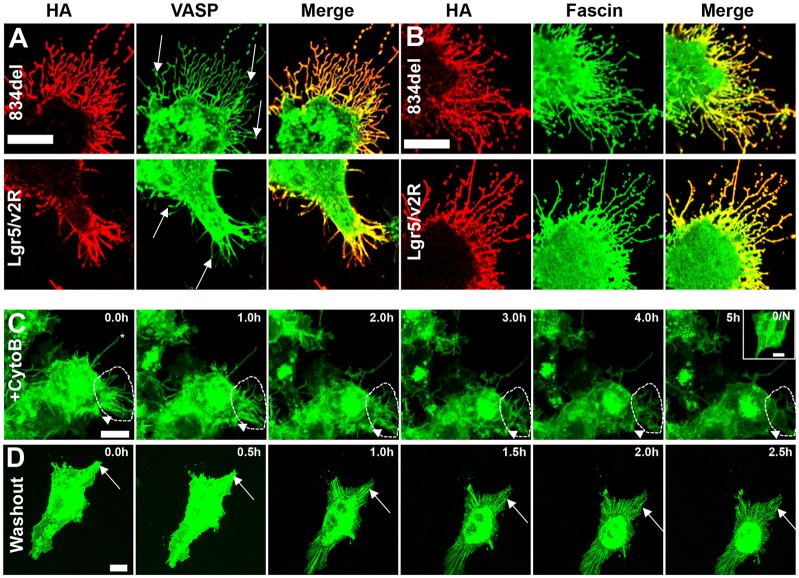
To further characterize Lgr-induced membrane protrusions, we probed for their ability to incorporate other stereotypical filopodia components. Indeed, both 834del and Lgr5–V2Rtail receptor-induced membrane protrusions can incorporate actin effectors, including the anti-capping protein VASP and the actin-bundling protein fascin (Fig. 5A,B) (Svitkina et al., 2003). In contrast to fascin, which is present homogeneously throughout the protrusion, VASP can be found concentrated at the tip, as published previously (Mejillano et al., 2004). These results demonstrate that Lgr5-induced membrane protrusions share similar molecular features to filopodia yet remain unique, owing to their great lengths.
Fig. 5.
Biochemical and functional characterization of Lgr5-induced cytonemes. (A) VASP–GFP (green) or (B) fascin–GFP (green) were cotransfected into cells with 834del- (upper panel) or Lgr5–V2Rtail (Lgr5/V2Rtail, lower panel) HA-tagged receptors (antibody stained in red), and the cells were confocal imaged. The data are presented as single-channel images and merged images. VASP is present throughout the entire cytoneme but can concentrate at the tip (arrows). (C) Overnight treatment with 10 µM cytochalasin B ablates 834del-induced cytonemes (inset). 834del-expressing cells were treated with 10 µM cytochalasin B and imaged every 30 minutes for 5 hours (see supplementary material Movie 2 for full timecourse). The asterisk denotes a cytoneme that remains after treatment; the dotted region demonstrates consolidation and ablation of a cluster of cytonemes; the arrowhead depicts rapid loss of shorter cytonemes. (D) 834del-expressing cells were treated with 10 µM cytochalasin B overnight, washed five times and then imaged every 30 minutes for 2.5 hours (see supplementary material Movie 3 for full timecourse). The arrow denotes rapid appearance of cytonemes from an existing lamellipodial-like framework. Scale bar: 10 µm.
Formation of Lgr5-induced membrane protrusions and their characterization as cytonemes
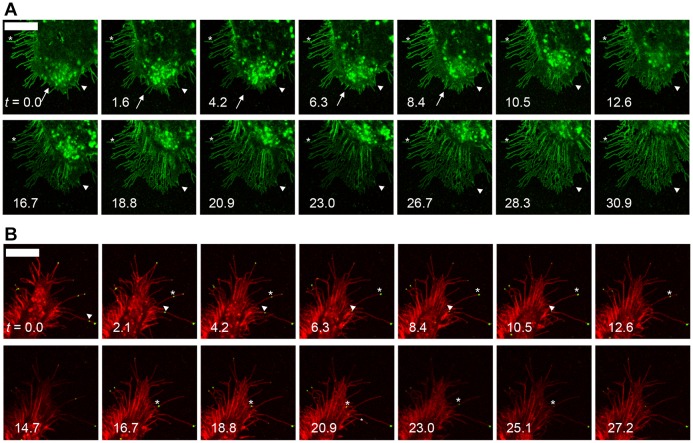
Similar to filopodia, Lgr5-induced protrusions are sensitive to cytochalasin B (Burnette et al., 2007). Overnight treatment with 10 µM cytochalasin B results in total ablation of Lgr5-induced protrusions (Fig. 5C, inset). The majority of Lgr5-induced protrusions are rapidly remodeled, consolidated and ablated in <5 hours of cytochalasin B treatment (Fig. 5C; supplementary material Movie 2). Finally, upon cytochalasin B washout, cells rapidly restore their membrane protrusions within 1 hour by a mechanism that resembles filopodium formation from an underlying Arp2/3 lamellipodial-like interfilopodial veil as described previously (Fig. 5D; supplementary material Movie 3; Korobova and Svitkina, 2008). Next, the formation of Lgr5-induced membrane protrusions in untreated cells was studied in a more focused timecourse. Similar to results observed following cytochalasin washout, the formation of Lgr5-positive membrane protrusions in untreated cells also appeared to occur through a rapid remodeling of a pre-existing lamellipodial-like interfilopodial veil (Fig. 6A; supplementary material Movie 4). De novo branching of Lgr5-induced membrane protrusions was never observed. Rather, fully branched Lgr5-positive membrane protrusions emerged from the underlying lamellipodial-like network. Fully mature Lgr5-induced cytonemes appeared to be relatively static structures (Fig. 6A, asterisk), whereas nascent protrusions were observed to dynamically elongate and retract, closely resembling the dynamic nature of filopodia (Svitkina et al., 2003). In some instances, this elongation was followed by retraction of the lamellipodial-like interfilopodial veil and stabilization of an Lgr5-induced filopodium (Fig. 6A, arrowhead). In other cases, the elongation was immediately followed by retraction and absorption by the plasma membrane (Fig. 6A, arrow). These data indicate that simultaneous elongation of filopodia and dissolution of the lamellipodial-like interfilopodial veil enables the formation of highly elongated and branched Lgr5-induced membrane protrusions with great rapidity.
Fig. 6.
Formation of Lgr5-induced cytonemes and transit of motor proteins. (A) Confocal images (100×) of HEK cells transiently transfected with 834del–GFP. The cells were imaged for 30.9 minutes. Nascent protrusions rapidly elongate and retract by 10.5 minutes (arrow) or stabilize and form cytonemes upon retraction of the cell membrane (arrowhead). The asterisk shows a static Lgr5-induced cytoneme. See supplementary material Movie 4. (B) Strawberry-tagged 834del (red) and GFP-tagged myosin X (green) were imaged for 30.9 minutes. Myosin X travels from the distal tip of the membrane protrusion and is rapidly transported in a retrograde fashion to the cell body by 10.5 minutes (arrowhead) or travels from the membrane protrusion anterograde to the distal tip by 6.3 min, undergoes retrograde transport to the cell body by 16.7 min, and finally anterograde transport from the cell body towards the distal tip at 23.0 min (asterisk). See supplementary material Movie 5. Scale bars: 10 µm.
Previous literature has demonstrated that ultra-long and actin-rich membrane protrusions are a specialized class of actin-rich filopodia termed cytonemes. Cytonemes are biochemically and morphologically similar to Lgr4- and Lgr5-induced membrane protrusions and are also sensitive to cytochalasin treatment (Hsiung et al., 2005; Ramírez-Weber and Kornberg, 1999). The live imaging results suggest that that Lgr4- and Lgr5-induced membrane protrusions most closely resemble cytonemes. However, markers that distinguish cytonemes from other long actin-rich protrusions, such as retraction fibers, do not exist and, as such, alternative classifications cannot be completely discounted.
Lgr5-induced cytonemes as cell signaling platforms
Because cytonemes are well-known cell signaling centers, we tested whether Lgr5-induced cytonemes could serve as a support platform for cell signal transduction. Their great lengths suggested that signaling effectors might require a transport mechanism to reach the extreme distal tips of each membrane protrusion. We thus tested the candidate carrier molecule myosin X (MyoX, also known as Myo10). Myosin X is an actin motor and unconventional myosin, previously shown to travel along actin-rich filopodia and deliver cargo to filopodia tips (Berg and Cheney, 2002; Zhang et al., 2004). Indeed, MyoX can travel along Lgr5-induced cytonemes (Fig. 6B; supplementary material Movie 5). We next sought to establish conditions to test whether Lgr-induced cytonemes could scaffold cell signaling molecules. As a model cell signaling system we focused on GPCR signaling, specifically, the ability of β2-AR to recruit the adaptor protein β-arrestin-2 (βarr2, also known as Arrb2) upon receiving a ligand-mediated stimulus. Translocation of βarr2 is a hallmark of prototypical GPCR signaling and receptor desensitization, and the protein acts as a scaffold for an array of downstream signaling cascades (Barak et al., 1997; Rajagopal et al., 2010). First, we tested whether β2-AR could access cytonemes. We found that, despite the inability of β2-AR to form cytonemes, it localized to them when co-expressed with Lgr5 (Fig. 7A). Next, we tested whether βarr2 could travel along Lgr-induced cytonemes. Surprisingly, βarr2–EGFP was not detected in the Lgr5-induced cytonemes (Fig. 7B). However, following agonist-mediated stimulation of co-expressed β2-AR, we found rapid recruitment of βarr2 throughout the Lgr-induced cytoneme, in addition to its typical plasma membrane distribution described previously (Fig. 7C,D) (Barak et al., 1997). The rapid entry of βarr2 into the Lgr-induced cytoneme suggested the existence of a gating system for enabling βarr2 translocation into Lgr-induced protrusions. Indeed, we were able to demonstrate an interaction between MyoX and βarr2 using in vitro GST pulldowns (Fig. 8A,B). This interaction occurred in vitro both before and after the stimulus, as found through co-immunoprecipitation of MyoX with βarr2 (Fig. 8C). These data demonstrate that Lgr-induced cytonemes harbor the components necessary to serve as a platform for delivery of signaling effectors, and they provide one plausible mechanism whereby signaling effectors might transit into cytonemes.
Fig. 7.
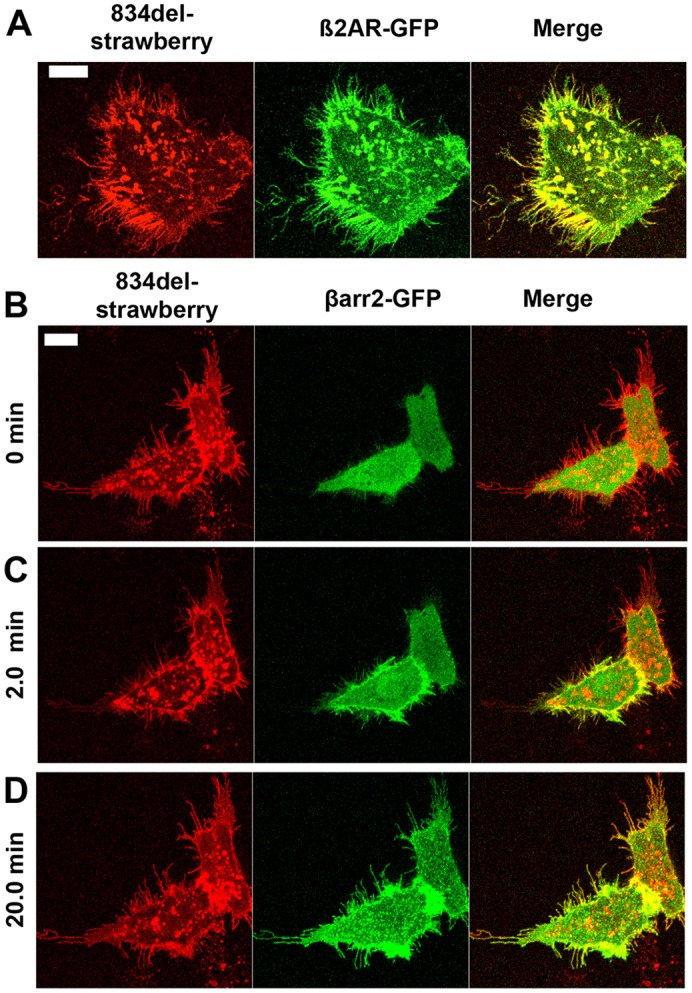
Lgr5-induced cytonemes provide a platform for engaging βarr2. (A) 834del–strawberry (red, left panel) and β2AR–GFP (green, middle panel) were co-transfected and imaged (yellow, colocalization, right panel). (B–D) 834del–strawberry (red, left panel), βarr2–GFP (green, middle panel) and HA–β2AR (not shown) were co-transfected and imaged at steady-state (B) or following stimulus of the β2-AR with 10 µM isoproterenol for (C) 2 minutes or (D) 20 minutes. Yellow depicts colocalization of the βarr2–GFP with the 834del–strawberry receptor (merge, right panel). Scale bars: 10 µm.
Fig. 8.
Myosin X interacts with βarr2. (A) GST–βarr2 pulldown of HEK cells expressing MYO10–GFP demonstrates that myosin X interacts with βarr2. The upper panel shows Coomassie staining; the lower panel shows immunoblotting (IB) for GFP. (B) GST–βarr2 pulldown from mouse brain lysates identifies endogenous myosin X as a βarr2-interacting protein. The upper panel shows Coomassie staining; the lower panel shows immunoblotting. As a positive and negative control for the βarr2 interaction, β-adaptin and actin were probed for, respectively. (C) Cells were transiently co-transfected with 834del–Lgr5 along with the constructs indicated and were stimulated with isoproterenol. The FLAG epitope was immunoprecipitated (IP) to pull down β-arr2–FLAG, and the blots were probed with GFP-, FLAG- or GAPDH-specific antibodies.
DISCUSSION
Lgr4 and Lgr5 are robust markers of the stem cell hierarchy but their function in cells has remained mysterious. We provide cell biological evidence for their roles as robust drivers of actin-rich membrane protrusions termed cytonemes. Cytoneme number and length are dependent upon the expression of Lgr4 and Lgr5 at the cell surface. Lgr4- and Lgr5-induced cytonemes are actin-dependent and contain typical actin-modifier proteins such as VASP and fascin. Their formation occurs through concerted stabilization of nascent filopodia and retraction of the cell membrane. Lgr5-induced cytonemes play a role in cell signaling by providing a conduit for the unconventional myosin motor protein MyoX and its delivery of activated signaling effectors to the tip of an activated cytoneme.
In 1924, Spemann and Mangold first alluded to the presence of what are now referred to as morphogen gradients (De Robertis, 2006). In the years since, this paradigm has provided a foundation for modern day developmental and stem cell biology by aiding in the understanding of the prototypical morphogens Wnt, Shh and BMP, and their roles in cell fate. However, the mechanism whereby gradients are established for these morphogens remains elusive. Many morphogens, Wnt and Shh included, undergo significant post-translational lipid and cholesterol modifications, deeming these secreted proteins highly insoluble and membrane associated. Many postulates have been formed in order to explain how gradients could be formed with insoluble proteins, including delivery by ultra-long filopodia termed cytonemes, as demonstrated in Drosophila (Port and Basler, 2010). Therefore, in a similar manner, Lgr4- or Lgr5-driven cytonemes could provide a mechanism for the delivery of morphogens to a mammalian stem cell niche.
Recent evidence suggests that intestinal Wnt is not provided by the epithelium but most likely by cells from the underlying mesenchyme (Kabiri et al., 2014). Therefore, epithelium-to-mesenchyme projections could provide an avenue for the transit of insoluble Wnts through a dense layer of extracellular matrix and basement membrane. Drosophila Lgr2 is the ortholog to Lgr4–6 and, in contrast to Lgr4 and Lgr5, has a well-defined G-protein-dependent signaling pathway. Lgr2 binds to its cognate heterodimeric hormone ligand Bursicon (Burs or Pburs) and strongly couples to G proteins to stimulate cAMP production. In a recent study, long-distance transmission of Bursicon to Lgr2 has been postulated to indirectly regulate the intestinal stem cell niche (Scopelliti et al., 2014). These data indirectly suggest that Lgr proteins in general might actually utilize cytonemes to regulate the reception of their cognate ligands. Our study provides evidence for Lgr4- and Lgr5-driven cytoneme formation and provides one mechanism whereby directional and compartmentalized signaling in mammalian stem cells could be regulated.
There are two tantalizing reports that might begin to confirm our in vitro findings in mouse model systems. First, Lgr5-positive cells, when observed at the ultrastructural level, possess “slender extensions of apical cytoplasm” in vivo (Barker et al., 2007). Second, mice deficient for Lgr4 have an eyelid closure defect that is coincident with reduced filopodia number and loss of growth factor signaling in the eyelid (Jin et al., 2008). Additionally, conditional deletion of Lgr4 and Lgr5 in adult intestinal stem cells results in marked loss of intestinal crypts. In adults, deletion of Lgr4 or Lgr5 alone has only modest effects, suggesting the existence of a compensatory mechanism provided by each receptor (de Lau et al., 2011) that also exists during development (Kinzel et al., 2014). Taken together, these data point to an important regulatory role for Lgr4 and Lgr5 in vivo that might be the result of cytoneme-based cell signaling.
Lgr4–6 are evolutionarily conserved along with thyroid stimulating hormone receptor (TSHR), luteinizing hormone receptor (LHR) and follicle-stimulating hormone receptor (FSHR), and are likewise marked by seven-transmembrane domains and a uniquely large N-terminal ectodomain composed of leucine-rich repeats. In contrast to TSHR, LHR and FSHR, the signaling cascade that Lgr4–6 modulates is only recently being elucidated and, surprisingly, does not yet include ligands that are capable of coupling Lgr4–6 to G protein or β-arrestins (Carmon et al., 2011; de Lau et al., 1998; Deng et al., 2013). While G-protein-coupling or β-arrestin translocation to Lgr4–6 has not been demonstrated, circumstantial evidence is mounting to suggest that these signaling effectors can be engaged and that the ligands and effectors capable of eliciting this behavior remain unknown. Lgr5 possesses β-arrestin recruitment motifs that are fully active under the appropriate conditions (Snyder et al., 2013a). Recent evidence demonstrates that the cerebellar Purkinje cells of hypomorphic Lgr4 mice have reduced levels of phospho-CREB, which can be restored through treatment with forskolin (Guan et al., 2014). This is further evidence that these receptors might couple to G proteins. These data together illustrate that this family of receptors remains highly enigmatic, but point to the possible role for other ligands that might signal through Lgr4 and Lgr5 and, ultimately, regulate cytoneme formation. Interestingly, recent work has shown that the R-spondin–Lgr4 signaling axis might also converge on actin (Carmon et al., 2014) and suggests that Lgr4 and Lgr5 are uniquely primed for regulating the cytoskeleton. From our experience with GPCRs and the data shown in Fig. 2, this behavior does appear to be highly specific for Lgr4 and Lgr5. However, in a recent report, expression and activation of the GPCR A3-adenosine receptor (A3AR) was also found to initiate the formation of cytonemes in human neutrophils for use in scavenging bacteria (Corriden et al., 2013). In contrast to Lgr4- and Lgr5-induced cytonemes, A3AR-induced cytonemes appear to be distinct based upon the modest number formed per cell and their inability to branch. At this time, further studies will be necessary to distinguish the similarities and differences between these structures. We are unable to determine whether cytonemes can be formed by Lgr6 as we are unable to express this receptor at the plasma membrane. Lgr4–6 are at the crossroads of stem cell biology and cancer. Therefore, elucidating the cellular and biological function of this receptor family is essential to unlock their potential as new drug targets.
Invadopodia are a class of filopodia that are similar to cytonemes. These structures are found in invasive cancer cells and are thought to promote cellular invasion through digestion of extracellular matrix and underlying mesenchyme (Chen, 1989; Linder, 2007). Among the many similarities to Lgr5 cytonemes, invadopodia are also characterized by expression of VASP, fascin and MyoX (Schoumacher et al., 2010). Interestingly, MyoX is able to induce filopodia and delivers integrins to the cell surface to increase cell adhesion (Berg and Cheney, 2002; Zhang et al., 2004). Two recent reports suggest that MyoX is an important marker of cancer invadopodia and cancer cell invasiveness. MyoX-dependent transport of integrins to the filopodium tip is essential for tumor cell invasion (Arjonen et al., 2014) and metastasis (Cao et al., 2014). These reports also demonstrate that MyoX expression is an important marker of aggressive and highly malignant breast cancers. We already know that Lgr5 cells represent the cell of origin in gastrointestinal cancers (Barker et al., 2009; Schepers et al., 2012). Therefore, our data suggests a potentially insidious role for cytonemes in carcinogenesis, whereby they are hijacked and exploited for cancer cell survival, invasion and metastasis. Our study provides the basis for further characterizing Lgr4 and Lgr5 in vivo. The association of Lgr5 and Lgr4 with stem cells, their remarkable ability to induce cytonemes in vitro, and the proposed role for cytonemes in morphogen-mediated stem cell signaling, provide a previously unrealized opportunity for future studies seeking to define roles for these receptors in normal tissue homeostasis and cancer.
MATERIALS AND METHODS
Plasmids, cell lines and transfection
Clones encoding GPCRs were either previously described [Lgr5 and Lgr4 (pcDNA3.1 backbone and CMV promoter) and ADRA1A-opto] or available in our laboratory (Airan et al., 2009; Snyder et al., 2013b). Clones containing bovine GFP–MYO10 (Berg and Cheney, 2002), GFP–fascin-1 (Adams and Schwartz, 2000) and GFP–VASP (Svitkina et al., 2003) were gifts of Dr Richard Cheney, and a clone for rat dynamin-1 K44A was available in our laboratory. HEK239 T/17 (HEK) cells were cultured in 1× DMEM (10-013-CV; Mediatech/Cellgro; Manassas VA) with 10% FBS (F2442; Sigma; St Louis, MO) and 1× antibiotic-antimycotic (15240-062; Life Technologies; Carlsbad, CA) according to the American Type Culture Collection (ATCC; CRL-11268). Cells were transfected by using calcium phosphate (Kingston et al., 2001) or with Genecellin (GC1000; BullDog Bio, Portsmouth, NH), where indicated.
Infrared on-cell internalization assay
Internalization assays were performed as described previously (Snyder et al., 2013b) with a few changes. Briefly, Nunc tissue-culture-treated, clear 96-well plates (167008; Nunc; Rochester NY) were incubated in 50 µl of poly-D-lysine (P0899; Sigma) diluted in H2O to 100 µg/ml for 6 hours at room temperature or overnight at 4°C. Plates were washed with H2O and air dried in a laminar flow hood. HEK cells were plated at 45,000 cells/well and transfected with the appropriate constructs. For transfection, calcium phosphate transfection was scaled down to directly transfect cells in a 96-well format. Briefly, for eight wells of reagent, 2 µg of DNA diluted in 70 µl H2O+10 µl 2.5 M CaCl2 was vortexed, added to 80 µl 2× HBS and aerated, and 20 µl was added per well for 4–6 hours. The medium was then changed to complete cell culture medium. To assess the fraction of the receptor on the surface, cells were stained live with the primary mouse anti-HA antibody (1∶500, 50 µl/well) for 45 minutes in clear MEM with supplements, washed once with 150 µl of clear MEM, fixed (4% PFA) and then stained with goat anti-mouse-IgG antibody conjugated to Alexa Fluor 680 (Invitrogen, Carlsbad, CA) for 1 hour. Total receptor expression was assessed by first fixing and permeabilizing cells (0.1% Triton X-100 in PBS for 10 minutes) and then staining with mouse monoclonal anti-HA for 1 hour in 5% BSA/PBS at room temperature, followed by detection with a goat anti-mouse-IgG antibody conjugated to Alexa Fluor 680 for 1 hour. The plate was washed in PBS for 5 minutes a total of three times and imaged on a LI-COR Odyssey (Li-COR Biosciences; Lincoln, NE) using the 700-nm channel, intensity setting of 5.0 and focal offset of 3.0. Untransfected stained cells were used to subtract background signal from each condition. The fraction of membrane expression for each receptor was calculated by taking the ratio of receptor surface expression to total expression. Data were normalized to the fraction of wild-type membrane expression. The results of nine independent experiments were averaged.
Live-cell confocal imaging and ultra-long filopodia morphometry
A total of 400,000 HEK cells were plated on 35-mm glass-bottomed dishes (P35G-0-10C; MatTek Corporation; Ashland, MA) that were coated (glass center only) with 130 µl of 75 µg/ml fibronectin (33016-015; Life Technologies) for 1 hour in a 37°C incubator. Cells were calcium-phosphate-transfected with 2 µg of the epitope-tagged receptors, with or without 2 µg of K44A, and filopodium markers and normalized to at least 5 µg of DNA with empty vector pcDNA3.1 at 1 day after plating. The next day, cells were imaged live in clear MEM with supplements (51200; Life Technologies), 10 mM HEPES (15630; Life Technologies), 1× GlutaMAX-1 (35050; Life Technologies) on a heated stage set to 37°C on a Zeiss LSM 510 confocal microscope (Carl Zeiss MicroImaging), using a 100× objective (Zeiss Plan-Apochromat, 1.4 NA, Zeiss P/N 44 07 80). Based upon our experience in this study and evidence in the literature (Ramírez-Weber and Kornberg, 1999), long-filopodia are very fragile and, therefore, live imaging appears to better preserve their length and number on each cell. However, to assess their structural components and determine whether these structures were present in non-transfected and therefore non-GFP-expressing cells, we fixed the cells in 4% PFA and performed F-actin staining with Alexa-Fluor-594-conjugated phalloidin, according to the supplier's protocol (A12381, Life Technologies). Briefly, cells were permeabilized with 0.2% Triton X-100 for 20 minutes and then stained with Alexa-Fluor-594-conjugated phalloidin (1∶50) in 0.5% BSA/PBS for 45 minutes at room temperature, followed by two washes with PBS. This procedure slightly decreases the size and number of cytonemes, as many cells can be found that have had their long filopodia severed, especially towards the distal tips. When described in the text, HA-tagged receptors were stained as above for on-cell internalization studies but detected with a goat anti-mouse-IgG secondary antibody conjugated to Alexa Fluor 568 (A11004; Life Technologies). Optical sections of each cell type were acquired on the confocal microscope and rendered in three dimensions using the Zeiss LSM Image Browser v4.2 (Carl Zeiss MicroImaging; Jena, Germany). The number of actin-rich filopodia and their length were then calculated for each cell by manually tracing and counting each actin-positive protrusion in the same software package. We analyzed many cells per genotype using this procedure to provide a large sample size for statistical measurements as follows: N = 16 non-transfected; N = 15 B2AR; N = 21 Lgr5FL; N = 14 K44A; N = 14 Lgr5–V2Rtail; N = 17 834del; N = 13 Lgr4.
Immunoprecipitation and western blotting
100-mm dishes were plated with 3×106 cells, and the cells were calcium phosphate transfected with 2 µg of each construct as indicated in the text. Cells were stimulated with 10 µM isoproterenol for the indicated times and then washed with PBS and scraped in lysis buffer [50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, cOMPLETE EDTA-free protease inhibitors (Roche, Penzberg, Germany) and Halt Phosphatase inhibitor (78420; ThermoFisher Scientific, Waltham, MA)]. Lysates were rotated at 4°C for 30 minutes, cleared by centrifugation at ∼13,000 g for 10 minutes, and quantified using a Pierce bicinchoninic acid assay (BCA) (23225, ThermoFisher Scientific). 2.7 mg of protein per sample was rotated overnight at 4°C with 20 µl of anti-FLAG (M2) beads (A2220; Sigma) that were prepared according to the manufacturer's suggestion. The next day, beads were washed (50 mM Tris, 150 mM NaCl, protease inhibitors) and rotated at 4°C for 5 minutes and then centrifuged at 13,000 g for 1 minute. A total of five washes were performed. Samples were eluted in 100 µl of Laemmli sample buffer and 5% β-mercaptoethanol for 10 minutes at 55°C, loaded onto 10% Tris-glycine gels (EC6075BOX; Life Technologies) electrophoresed and transferred to 0.2 µm nitrocellulose (LC2000; Life Technologies). Blots were Ponceau stained, cut, blocked in 5% milk/TBST for 30 minutes, washed and incubated with primary antibodies diluted in 5% BSA/TBST overnight at 4°C. The primary and secondary antibodies utilized were: rabbit anti-GFP (ab6556; Abcam; Cambridge, MA) with goat anti-rabbit-IgG conjugated to Alexa Fluor 800 (611-132-122; Rockland; Limerick, PA); mouse anti-FLAG (F1804-200ug; Sigma) with goat anti-mouse-IgG (light-chain specific) (115-005-174; Jackson ImmunoResearch; West Grove, PA) and donkey anti-goat-IgG conjugated to Alexa Fluor 680 (A21084; Life Technologies); and mouse anti-GAPDH (MAB374; Millipore; Billerica, MA) with goat anti-mouse-IgG conjugated to Alexa Fluor 680 (A21058; Life Technologies). Blots were incubated with the above secondary antibodies, washed and developed on a LI-COR Odyssey (LI-COR Biosciences).
βarr2–GST pulldowns
GST pulldowns were performed according to a previously published protocol (Marion et al., 2007). Briefly, three whole adult mouse brains or seven 150-mm plates of HEK cells transiently expressing MYO10–GFP were extracted in lysis buffer (10 mM Tris-HCl pH 7.4, 2 mM EDTA, 1% Triton X-100 and protease and phosphatase inhibitors). Extracts were incubated overnight with GST or GST–βarr2 immobilized on glutathione–Sepharose high-performance beads (17-5279-01; GE Biosciences; Pittsburgh, PA). Samples were washed four times, eluted with reduced glutathione, concentrated using a Ultracel-10K centrifugal filters (Millipore) and reduced with Laemmli sample buffer. Western blots were probed with rabbit anti-MYO10 (HPA024223; Sigma), mouse anti-β-adaptin (610281; BD Biosciences; San Jose, CA) and mouse anti-actin (MAB1501R; Millipore).
Statistics
Data were collected in Microsoft Excel and then transferred to GraphPad Prism (GraphPad Software; San Diego, CA). Statistically significant differences between multiple groups were calculated using a one-way ANOVA and post-hoc Bonferroni.
Supplementary Material
Acknowledgments
The authors are thankful for Dr. Scott Soderling's helpful advice in the preparation and editing of this manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
J.C.S., L.S.B. and M.G.C. wrote the manuscript. J.C.S., L.K.R. and S.M. performed experiments. J.C.S., H.K.L., L.S.B. and M.G.C. designed experiments and interpreted experimental results.
Funding
This work was supported by the Susan G. Komen foundation [grant number KG080627 to J.C.S. and H.K.L.]; and the National Cancer Institute (NCI) Clinical Oncology Research Career Development Program [grant number NCI 5K12-CA100639-10 to J.C.S.]; as well as grants from Duke Cancer Center Stewart Trust and Duke Cancer Center Cancer and the Environment to M.G.C.; the NCI [grant number IMAT R21CA173245 to H.K.L., L.S.B. and M.G.C.]; and the National Institute on Drug Abuse [grant number P30 5P30DA029925-05 to L.S.B. and M.G.C.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.166322/-/DC1
References
- Adams J. C., Schwartz M. A. (2000). Stimulation of fascin spikes by thrombospondin-1 is mediated by the GTPases Rac and Cdc42. J. Cell Biol. 150, 807–822 10.1083/jcb.150.4.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affolter M., Basler K. (2011). Cell biology. Cytonemes show their colors. Science 332, 312–313 10.1126/science.1205971 [DOI] [PubMed] [Google Scholar]
- Airan R. D., Thompson K. R., Fenno L. E., Bernstein H., Deisseroth K. (2009). Temporally precise in vivo control of intracellular signalling. Nature 458, 1025–1029 10.1038/nature07926 [DOI] [PubMed] [Google Scholar]
- Arjonen A., Kaukonen R., Mattila E., Rouhi P., Högnäs G., Sihto H., Miller B. W., Morton J. P., Bucher E., Taimen P. et al. (2014). Mutant p53-associated myosin-X upregulation promotes breast cancer invasion and metastasis. J. Clin. Invest. 124, 1069–1082 10.1172/JCI67280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak L. S., Ferguson S. S., Zhang J., Caron M. G. (1997). A beta-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J. Biol. Chem. 272, 27497–27500 10.1074/jbc.272.44.27497 [DOI] [PubMed] [Google Scholar]
- Barker N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J. et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- Barker N., Ridgway R. A., van Es J. H., van de Wetering M., Begthel H., van den Born M., Danenberg E., Clarke A. R., Sansom O. J., Clevers H. (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 10.1038/nature07602 [DOI] [PubMed] [Google Scholar]
- Barker N., Huch M., Kujala P., van de Wetering M., Snippert H. J., van Es J. H., Sato T., Stange D. E., Begthel H., van den Born M. et al. (2010). Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 10.1016/j.stem.2009.11.013 [DOI] [PubMed] [Google Scholar]
- Barker N., Rookmaaker M. B., Kujala P., Ng A., Leushacke M., Snippert H., van de Wetering M., Tan S., Van Es J. H., Huch M. et al. (2012). Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Reports 2, 540–552 10.1016/j.celrep.2012.08.018 [DOI] [PubMed] [Google Scholar]
- Berg J. S., Cheney R. E. (2002). Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat. Cell Biol. 4, 246–250 10.1038/ncb762 [DOI] [PubMed] [Google Scholar]
- Burnette D. T., Schaefer A. W., Ji L., Danuser G., Forscher P. (2007). Filopodial actin bundles are not necessary for microtubule advance into the peripheral domain of Aplysia neuronal growth cones. Nat. Cell Biol. 9, 1360–1369 10.1038/ncb1655 [DOI] [PubMed] [Google Scholar]
- Cao R., Chen J., Zhang X., Zhai Y., Qing X., Xing W., Zhang L., Malik Y. S., Yu H., Zhu X. (2014). Elevated expression of myosin X in tumours contributes to breast cancer aggressiveness and metastasis. Br. J. Cancer 111, 539–550 10.1038/bjc.2014.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon K. S., Gong X., Lin Q., Thomas A., Liu Q. (2011). R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA 108, 11452–11457 10.1073/pnas.1106083108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon K. S., Lin Q., Gong X., Thomas A., Liu Q. (2012). LGR5 interacts and cointernalizes with Wnt receptors to modulate Wnt/β-catenin signaling. Mol. Cell. Biol. 32, 2054–2064 10.1128/MCB.00272-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon K. S., Gong X., Yi J., Thomas A., Liu Q. (2014). RSPO-LGR4 functions via IQGAP1 to potentiate Wnt signaling. Proc. Natl. Acad. Sci. USA 111, E1221–E1229 10.1073/pnas.1323106111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. T. (1989). Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J. Exp. Zool. 251, 167–185 10.1002/jez.1402510206 [DOI] [PubMed] [Google Scholar]
- Corriden R., Self T., Akong-Moore K., Nizet V., Kellam B., Briddon S. J., Hill S. J. (2013). Adenosine-A3 receptors in neutrophil microdomains promote the formation of bacteria-tethering cytonemes. EMBO Rep. 14, 726–732 10.1038/embor.2013.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau W. B., Hurenkamp J., Berendes P., Touw I. P., Clevers H. C., van Dijk M. A. (1998). The gene encoding the granulocyte colony-stimulating factor receptor is a target for deregulation in pre-B ALL by the t(1;19)-specific oncoprotein E2A-Pbx1. Oncogene 17, 503–510 10.1038/sj.onc.1201967 [DOI] [PubMed] [Google Scholar]
- de Lau W., Barker N., Low T. Y., Koo B. K., Li V. S., Teunissen H., Kujala P., Haegebarth A., Peters P. J., van de Wetering M. et al. (2011). Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 10.1038/nature10337 [DOI] [PubMed] [Google Scholar]
- De Robertis E. M. (2006). Spemann's organizer and self-regulation in amphibian embryos. Nat. Rev. Mol. Cell Biol. 7, 296–302 10.1038/nrm1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser K. E., Ciampricotti M., Michalak E. M., Tan D. W., Speksnijder E. N., Hau C. S., Clevers H., Barker N., Jonkers J. (2012). Developmental stage-specific contribution of LGR5(+) cells to basal and luminal epithelial lineages in the postnatal mammary gland. J. Pathol. 228, 300–309. [DOI] [PubMed] [Google Scholar]
- Deng C., Reddy P., Cheng Y., Luo C. W., Hsiao C. L., Hsueh A. J. (2013). Multi-functional norrin is a ligand for the LGR4 receptor. J. Cell Sci. 126, 2060–2068 10.1242/jcs.123471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X., Duan Y., Zeng Q., Pan H., Qian Y., Li D., Cao X., Liu M. (2014). Lgr4 protein deficiency induces ataxia-like phenotype in mice and impairs long term depression at cerebellar parallel fiber-Purkinje cell synapses. J. Biol. Chem. 289, 26492–26504 10.1074/jbc.M114.564138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson T. (1963). Cellular mechanisms in the morphogenesis of the sea urchin embryo. Cell contacts within the ectoderm and between mesenchyme and ectoderm cells. Exp. Cell Res. 32, 570–589 10.1016/0014-4827(63)90195-2 [DOI] [PubMed] [Google Scholar]
- Hsiung F., Ramirez-Weber F-A., Iwaki D. D., Kornberg T. B. (2005). Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature 437, 560–563 10.1038/nature03951 [DOI] [PubMed] [Google Scholar]
- Hsu S. Y., Liang S-G., Hsueh A. J. W. (1998). Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol. Endocrinol. 12, 1830–1845 10.1210/mend.12.12.0211 [DOI] [PubMed] [Google Scholar]
- Hsu S. Y., Kudo M., Chen T., Nakabayashi K., Bhalla A., van der Spek P. J., van Duin M., Hsueh A. J. (2000). The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol. Endocrinol. 14, 1257–1271 10.1210/mend.14.8.0510 [DOI] [PubMed] [Google Scholar]
- Huch M., Bonfanti P., Boj S. F., Sato T., Loomans C. J., van de Wetering M., Sojoodi M., Li V. S., Schuijers J., Gracanin A. et al. (2013a). Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 32, 2708–2721 10.1038/emboj.2013.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M., Dorrell C., Boj S. F., van Es J. H., Li V. S., van de Wetering M., Sato T., Hamer K., Sasaki N., Finegold M. J. et al. (2013b). In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 10.1038/nature11826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V., Barker N., Kasper M., van Es J. H., Snippert H. J., Clevers H., Toftgård R. (2008). Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 40, 1291–1299 10.1038/ng.239 [DOI] [PubMed] [Google Scholar]
- Jin C., Yin F., Lin M., Li H., Wang Z., Weng J., Liu M., Da Dong X., Qu J., Tu L. (2008). GPR48 regulates epithelial cell proliferation and migration by activating EGFR during eyelid development. Invest. Ophthalmol. Vis. Sci. 49, 4245–4253 10.1167/iovs.08-1860 [DOI] [PubMed] [Google Scholar]
- Kabiri Z., Greicius G., Madan B., Biechele S., Zhong Z., Zaribafzadeh H., Edison, Aliyev J., Wu Y., Bunte R. (2014). Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 141, 2206–2215 10.1242/dev.104976 [DOI] [PubMed] [Google Scholar]
- Kingston R. E., Chen C. A., Okayama H. (2001). Calcium phosphate transfection. Current Protocols in Immunology 9.1.1–9.1.11–Hoboken, NJ: John Wiley & Sons, Inc. [DOI] [PubMed] [Google Scholar]
- Kinzel B., Pikiolek M., Orsini V., Sprunger J., Isken A., Zietzling S., Desplanches M., Dubost V., Breustedt D., Valdez R. et al. (2014). Functional roles of Lgr4 and Lgr5 in embryonic gut, kidney and skin development in mice. Dev. Biol. 390, 181–190 10.1016/j.ydbio.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Kornberg T. B., Roy S. (2014). Cytonemes as specialized signaling filopodia. Development 141, 729–736 10.1242/dev.086223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F., Svitkina T. (2008). Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol. Biol. Cell 19, 1561–1574 10.1091/mbc.E07-09-0964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-L., Lei Y-T., Hong C-J., Hsueh Y-P. (2007). Syndecan-2 induces filopodia and dendritic spine formation via the neurofibromin-PKA-Ena/VASP pathway. J. Cell Biol. 177, 829–841 10.1083/jcb.200608121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S. (2007). The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 17, 107–117 10.1016/j.tcb.2007.01.002 [DOI] [PubMed] [Google Scholar]
- Liu D., He X. C., Qian P., Barker N., Trainor P. A., Clevers H., Liu H., Li L. (2014). Leucine-rich repeat-containing G-protein-coupled Receptor 5 marks short-term hematopoietic stem and progenitor cells during mouse embryonic development. J. Biol. Chem. 289, 23809–23816 10.1074/jbc.M114.568170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion S., Fralish G. B., Laporte S., Caron M. G., Barak L. S. (2007). N-terminal tyrosine modulation of the endocytic adaptor function of the beta-arrestins. J. Biol. Chem. 282, 18937–18944 10.1074/jbc.M700090200 [DOI] [PubMed] [Google Scholar]
- Mejillano M. R., Kojima S., Applewhite D. A., Gertler F. B., Svitkina T. M., Borisy G. G. (2004). Lamellipodial versus filopodial mode of the actin nanomachinery: pivotal role of the filament barbed end. Cell 118, 363–373 10.1016/j.cell.2004.07.019 [DOI] [PubMed] [Google Scholar]
- Miller J., Fraser S. E., McClay D. (1995). Dynamics of thin filopodia during sea urchin gastrulation. Development 121, 2501–2511. [DOI] [PubMed] [Google Scholar]
- Mogilner A., Rubinstein B. (2005). The physics of filopodial protrusion. Biophys. J. 89, 782–795 10.1529/biophysj.104.056515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaks V., Brenot A., Lawson D. A., Linnemann J. R., Van Kappel E. C., Wong K. C., de Sauvage F., Klein O. D., Werb Z. (2013). Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell Reports 3, 70–78 10.1016/j.celrep.2012.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F., Basler K. (2010). Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic 11, 1265–1271 10.1111/j.1600-0854.2010.01076.x [DOI] [PubMed] [Google Scholar]
- Rajagopal S., Rajagopal K., Lefkowitz R. J. (2010). Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 9, 373–386 10.1038/nrd3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Weber F-A., Kornberg T. B. (1999). Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 97, 599–607 10.1016/S0092-8674(00)80771-0 [DOI] [PubMed] [Google Scholar]
- Rojas-Ríos P., Guerrero I., González-Reyes A. (2012). Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol. 10, e1001298 10.1371/journal.pbio.1001298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Hsiung F., Kornberg T. B. (2011). Specificity of Drosophila cytonemes for distinct signaling pathways. Science 332, 354–358 10.1126/science.1198949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Huang H., Liu S., Kornberg T. B. (2014). Cytoneme-mediated contact-dependent transport of the Drosophila decapentaplegic signaling protein. Science 343, 1244624 10.1126/science.1244624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers A. G., Snippert H. J., Stange D. E., van den Born M., van Es J. H., van de Wetering M., Clevers H. (2012). Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337, 730–735 10.1126/science.1224676 [DOI] [PubMed] [Google Scholar]
- Schoumacher M., Goldman R. D., Louvard D., Vignjevic D. M. (2010). Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J. Cell Biol. 189, 541–556 10.1083/jcb.200909113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopelliti A., Cordero J. B., Diao F., Strathdee K., White B. H., Sansom O. J., Vidal M. (2014). Local control of intestinal stem cell homeostasis by enteroendocrine cells in the adult Drosophila midgut. Curr. Biol. 24, 1199–1211 10.1016/j.cub.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Wayne D. B., Pearlman A. L. (1992). Extension of filopodia by motor-dependent actin assembly. Cell Motil. Cytoskeleton 22, 160–169 10.1002/cm.970220303 [DOI] [PubMed] [Google Scholar]
- Snippert H. J., Haegebarth A., Kasper M., Jaks V., van Es J. H., Barker N., van de Wetering M., van den Born M., Begthel H., Vries R. G. et al. (2010). Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327, 1385–1389 10.1126/science.1184733 [DOI] [PubMed] [Google Scholar]
- Snyder J. C., Rochelle L. K., Barak L. S., Caron M. G. (2013a). The stem cell-expressed receptor Lgr5 possesses canonical and functionally active molecular determinants critical to β-arrestin-2 recruitment. PLoS ONE 8, e84476 10.1371/journal.pone.0084476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J. C., Rochelle L. K., Lyerly H. K., Caron M. G., Barak L. S. (2013b). Constitutive internalization of the Leucine-rich G protein-coupled Receptor-5 (LGR5) to the trans-Golgi network. J. Biol. Chem. 288, 10286–10297 10.1074/jbc.M112.447540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina T. M., Bulanova E. A., Chaga O. Y., Vignjevic D. M., Kojima S., Vasiliev J. M., Borisy G. G. (2003). Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol. 160, 409–421 10.1083/jcb.200210174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Berg J. S., Li Z., Wang Y., Lång P., Sousa A. D., Bhaskar A., Cheney R. E., Strömblad S. (2004). Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat. Cell Biol. 6, 523–531 10.1038/ncb1136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.