SUMMARY
Gastric arrhythmia continues to be of uncertain diagnostic and therapeutic significance. However, recent progress has been substantial, with technical advances, theoretical insights and experimental discoveries offering new translational opportunities. The discoveries that interstitial cells of Cajal (ICC) generate slow waves and that ICC defects are associated with dysmotility have reinvigorated gastric arrhythmia research. Increasing evidence now suggests that ICC depletion and damage, network disruption and channelopathies may lead to aberrant slow wave initiation and conduction. Histological and high-resolution (HR) electrical mapping studies have now redefined the human ‘gastric conduction system’, providing an improved baseline for arrhythmia research. The application of HR mapping to arrhythmia has also generated important new insights into the spatiotemporal dynamics of arrhythmia onset and maintenance, resulting in the emergence of new provisional classification schemes. Meanwhile, the strong associations between gastric functional disorders and electrogastrography (EGG) abnormalities (e.g. in gastroparesis, unexplained nausea and vomiting and functional dyspepsia) continue to motivate deeper inquiries into the nature and causes of gastrointestinal arrhythmias. In future, technical progress in EGG methods, new HR mapping devices and software, wireless slow wave acquisition systems and improved gastric pacing devices may achieve validated applications in clinical practice. Neurohormonal factors in arrhythmogenesis also continue to be elucidated and a deepening understanding of these mechanisms may open opportunities for drug design for treating arrhythmias. However, for all translational goals, it remains to be seen whether arrhythmia can be corrected in a way that meaningfully improves organ function and symptoms in patients.
Keywords: electrogastrography, gastric electrical activity, high-resolution mapping, interstitial cells of Cajal, tachygastria
INTRODUCTION
Almost a century has passed since the esteemed physiologist Alvarez made his ‘little prophesy’ that ‘gastroenterologists would come to rely upon electrical measures for the routine diagnosis of stomach disorders, just as the heart specialist [does]’.1 Despite intensive investigations through the intervening years, Alvarez's prophesy remains unfulfilled. There continues to be little uptake of gastric electrical diagnostics beyond a few specialist research centres due to ongoing uncertainty regarding the reliability and value of current clinical tests.2,3
However, recent progress has been substantial, with technical advances, theoretical insights and experimental discoveries offering new translational opportunities in gastric electrophysiology. This update evaluates several of these advances and addresses future horizons in the basic and clinical science of gastric arrhythmia.
ROLE OF THE INTERSTITIAL CELLS OF CAJAL
It is now widely accepted that slow waves are generated and propagated by interstitial cells of Cajal (ICC) and this discovery has been accompanied by intense interest in what roles ICC defects may play in disease.4,5 These advances have critically informed new directions in arrhythmia research and reinvigorate clinical interest in evaluating gastric electrical activity.
In the stomach, the strongest evidence for a pathophysiological role for ICC is in gastroparesis.6,7 Although gastroparesis is multifactorial and also associated with autonomic, smooth muscle, enteric nervous system and immune cellular changes and fibrosis,6,8 the ICC loss appears to be of particular significance. Abnormalities in ICC are the most common histological finding in both idiopathic and diabetic gastroparesis,6 and the severity of ICC loss has been correlated with the severity of gastric retention, as well as electrogastrography (EGG) abnormalities.9,10 Animal model research supports a pathophysiological role for ICC in gastroparesis; for example, induction of heme oxygenase-1, a cytoprotective molecule against oxidative stress, has been shown to both restore ICC and normalize gastric emptying in non-obese diabetic mice.11
Another emerging ICC research area with implications for arrhythmia is ion channel defects (channelopathies).12 Early work in this area has linked mutations in the mechanosensitive Na(v)1.5 sodium channel, encoded by the sodium channel, voltage-gated, type V, alpha subunit (SCN5A) gene and found in human ICC and smooth muscle cells, with irritable bowel syndrome.13 Channel defects of this type can substantially modify whole cell slow wave activation,14 potentially inducing arrhythmias in a manner analogous to defined types of cardiac arrhythmia.15 Further research is needed to determine whether such mechanisms are also important in disorders such as functional dyspepsia.16 Another recent focus of substantial current interest in ICC are calcium-activated chloride channels, which likely play a key role in gastric excitability.17,18
The ICC also serve several other functions in addition to slow wave pacemaking, including mediating neurotransmission, mechanotransduction and establishing smooth muscle resting membrane potential gradients.4,5 Importantly, ICC pathologies will therefore impact on gastric motility by other pathways beyond just slow wave arrhythmia.19
THE GASTRIC CONDUCTION SYSTEM
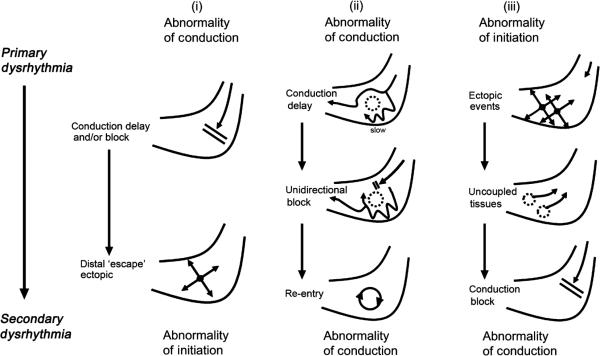
In the normal stomach, ICC generate slow waves synchronously at a common frequency, achieved by their ‘entrainment’ to the highest frequency expressed in their syncytium within the gastric wall.20 Entrainment is maintained by an underlying frequency gradient intrinsic to ICC, which declines in the aboral direction, as may be revealed by partitioning the stomach into isolated segments.21,22 It is helpful to consider the pathogenesis of arrhythmia mechanisms in terms of disruption to normal entrainment (‘disorders of conduction’) or to the intrinsic ICC frequencies (‘disorders of initiation’; Fig. 1) and each of these abnormalities may, in turn, promote secondary forms of arrhythmia (Fig. 2).
Figure 1.
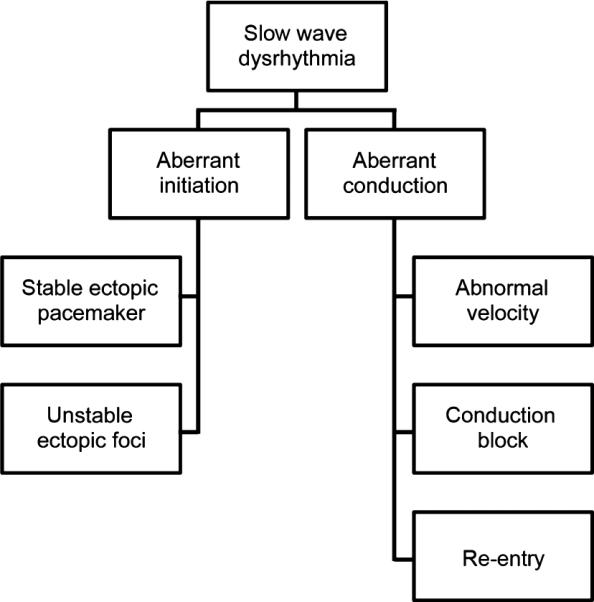
An example of the classification schemes for gastric arrhythmia that are emerging, which are now being based on mechanisms and spatial patterns of slow wave activation. Aberrant initiation relates to abnormalities of intrinsic interstitial cells of Cajal frequencies and example activities include stable ectopic pacemakers and unstable regions of ectopic foci. Aberrant conduction involves disruption of the normal slow wave entrainment and examples include abnormal velocities, conduction blocks and re-entrant activities.
Figure 2.
Examples of secondary gastric arrhythmia mechanisms, as observed during multi-electrode (high-resolution) mapping, whereby one class of arrhythmia may induce secondary arrhythmic events. (i) Conduction delay or block may give rise to distal ‘escape’ events, due to the inherent automaticity of distal interstitial cells of Cajal;7,86 (ii) conduction inhomogeneity, in the presence of unidirectional block, may give rise to re-entrant slow wave activity;36,44,86 and (iii) ectopic events in the distal stomach may cause uncoupled areas of tissue, resulting in block of the normal antegrade wavefronts.7,44,86
In the human stomach, an anastomosing network of ICC is distributed throughout the myenteric plexus (ICC-MP) and circular and longitudinal muscle layers (intramuscular ICC; ICC-IM).23,24 The ICC-IM run parallel to the smooth muscle fibres within the muscle layers, forming a loosely connecting network, with a further anatomical subclass of ICC encasing and connecting bundles (ICC-SEP).24 In animal models (but yet to be shown in humans), a decrease in ICC-MP within the pylorus functions as an electrical barrier, helping to isolate gastric and duodenal electrical events.25
Extracellular recordings remain the primary tool for evaluating in vivo gastric slow wave activation patterns over large tissue areas.26 Classically, gastric slow wave propagation was studied using sparse arrangements of extracellular electrodes, typically up to eight, placed at regular intervals along the gastrointestinal (GI) tract. However, such results were incomplete due to the limitations of sparse sampling and could be misleading due to spatial aliasing. 27 Therefore, these limitations motivated the introduction of GI high-resolution (HR) electrical mapping by Lammers et al., whereby dense arrays of electrodes are applied to accurately track activation in spatiotemporal detail.28,29 The analysis of gastric conduction patterns using HR mapping has now yielded a much more accurate description of the normal origin and propagation of gastric slow waves in large animal models and in humans,27,30,31 serving as a reliable baseline for arrhythmia investigations.
GASTRIC ANISOTROPY
Velocity anisotropy has proved to be an important finding from HR mapping studies for arrhythmia interpretation, whereby extracellular slow waves in the region of the normal gastric pacemaker show a much higher velocity and amplitude activity than events in the surrounding corpus (~2.5-fold greater in humans).27,30 The reason for the high velocity and amplitude pacemaker pattern was only recently resolved in a combined theoretical–experimental study,32 aided by novel methods for analysing the spatial properties of slow wave activation.33–35 The high local velocity is the consequence of the fact that the impulse propagates from the pacemaker area initially and predominantly in the circumferential direction.32 In human stomach, propagation in the circumferential direction is approximately 2.5-fold faster than in the longitudinal direction (termed ‘anisotropic’ propagation). The accompanying increase in extracellular amplitudes is due to changes in current distribution associated with the higher impulse velocity.32 However, by the time slow waves reach the mid to lower corpus they have formed into complete ring wavefronts, such that the rapid circumferential conduction ceases and wavefronts propagate with slower antegrade activation towards the pylorus.27,32
Velocity anisotropy is critical to the interpretation of arrhythmic activity, because disorders of both initiation and conduction disrupt normal gastric ring wavefronts.7,32 This allows ‘ectopic circumferential propagation’ to emerge, such that arrhythmias are characterized by high-velocity wavefronts and high-amplitude signals local to their source, which directly determines the patterns of arrhythmic activation. This anisotropy also plays a key role in the maintenance and stability of arrhythmic patterns, such as slow wave re-entry36 (for a recent review, see Cheng, Du and O'Grady37).
It is not yet known which ICC network structures underlie the anisotropic properties outlined above. There are likely to be two complimentary gastric conduction pathways active, supporting circumferential conduction at high velocity and longitudinal conduction at low velocity.38 For humans, it was recently proposed that within a bidirectionally coupled ICC system, the leading network may switch between ICC-MP (dominant during longitudinal conduction) and ICC-IM (dominant during circumferential conduction), depending on the presence or absence of complete gastric ring wavefronts.32 This theory awaits validation, but could offer mechanistic insights into arrhythmogenesis. For example, slow wave frequencies are known to increase in ICC-IM at sites of sustained cholinergic stimulation,39 which could then act as sites of arrhythmic initiation within a bidirectionally coupled system.
ELECTROGASTROGRAPHY AND ARRHYTHMIA
Much of what is known about gastric arrhythmia, particularly in human disease, continues to be derived from electrogastrography (EGG). Electrogastrography is a non-invasive test and is relatively simple to perform,40 but the signals are currently difficult to relate to the underlying slow waves. In addition, EGG signals are of low amplitude and susceptible to motion artefacts induced by even fine patient movements, such that subjects must lie completely still during the recording period. Signal acquisition and processing for effective EGG generally requires the use of bipolar electrodes, tight band-pass filtering (e.g. 0.016–0.3 Hz), careful attention to noise discrimination and, ideally, concurrent respiratory motion sensing.41
Electrogastrography recordings are typically taken before and after a test meal and variables analysed include the dominant slow wave frequency and its consistency, dominant power (reflecting signal amplitude and regularity over time), the ‘fasting–fed power ratio’ (the dominant power increases after a test meal in normal subjects), as well as measures of signal instability.3,40 Analysis of the EGG waveform morphology has no defined role, but EGG may also be applied to detect ‘gastric uncoupling’ (where discrete areas of the stomach are entrained to different sources and frequencies),42 particularly using modern multichannel approaches (e.g. four bipolar electrodes).43
The normal frequency range defined by EGG is approximately 2.4–3.7 cycles/min, with frequency abnormalities outside this range classed as bradygastria, tachygastria or non-specific.3,40 However, a limitation of this approach is that it is not yet certain how and from where the gastric slow wave potentials actually summate to form the EGG signal, especially because it has now been shown that there may be three to five slow waves propagating at any time in the stomach.27,30 This issue is even more problematic during arrhythmias, when different frequencies may be simultaneously active in multiple uncoupled gastric regions.7,44
Several decades of EGG investigations have produced an extensive literature associating electrical abnormalities with gastric disorders (Table 1). Multiple studies have also demonstrated that hyperglycaemia is associated with arrhythmia, possibly contributing to arrhythmia in diabetic gastroparesis,45,46 leading to recommendations that blood glucose be tested and normalized prior to testing.3 In addition, EGG abnormalities have been associated with chronic renal failure,47 anorexia nervosa (after a prolonged period)48 and chronic intestinal pseudo-obstruction.49
Table 1.
Published associations between electrogastrography measures and gastric disorders. EGG (electrogastrography), HR mapping (high-resolution mapping), GORD (gastro-oesophageal reflux disease).
| Disorder | Reference | EGG Measure | Prevalence of Abnormalities | Comments |
|---|---|---|---|---|
| Gastroparesis | 50 | Any abnormality | 6/6 patients | Arrhythmias variably reported to be frequent or rare in gastroparesis by serosal or mucosal recording,51–53 whereas HR mapping showed a high rate7 |
| 54 | Any abnormality | 72% | ||
| 55 | Arrhythmia; pooled analysis | Increased | ||
| 56 | Any abnormality | 75% vs 0% controls | ||
| Chronic unexplained nausea and vomiting | 57 | Arrhythmia by cutaneous EGG | Undefined | High arrhythmia rate also described in direct organ recording studies58,59 |
| 57 | Arrhythmia by serosal recording | 95% | ||
| Functional dyspepsia | 60 | Arrhythmias | 36% vs 7% controls | Arrhythmias increased postprandially61 and were higher in patients also having delayed gastric emptying62 |
| 63 | Arrhythmias | 33% vs 0% controls | Increased detection using multichannel EGG64 | |
| 63 | Any abnormality | 66% vs 0% controls | ||
| 65 | Multichannel; any abnormality | 83%; (tachygastria 36%; bradygastria 15%) | ||
| 61 | Post-prandial arrhythmias | 43% vs 0% controls | ||
| Gastro-oesophageal Reflux Disease | 60 | Arrhythmias | 10% vs 7% controls | Arrhythmia only significantly present in GORD patient subsets with regurgitation,60 delayed gastric emptying66 and dyspepsia symptoms67 |
| 68 | Arrhythmias | 50% of GORD patients with food regurgitation | ||
| 66 | Arrhythmias | 22–24% vs 10% controls | ||
| 67 | Arrhythmias | 75% GORD with dyspepsia symptoms vs 11% GORD alone | ||
| Cyclic Vomiting Syndrome | 69 | Tachygastria | 5/8 symptomatic children preprandially; 8/8 post-prandially | High arrhythmia rate observed when patients symptomatic |
| Ischaemic gastropathy | 70 | Arrhythmias | 2/2 patients (case report) | Resolved after revascularization |
In terms of symptoms, the strongest association with EGG-diagnosed frequency abnormality is with nausea and vomiting.71 Arrhythmia is common when vomiting is likely, as seen in chronic unexplained nausea and vomiting,58,72 after chemotherapy,73 in motion sickness74 and in nausea of pregnancy.75 Indeed, physiological evidence suggests that myoelectrical derangements may be part of the normal physiological sequence of vomiting.76–78 However, some controversy remains as to whether arrhythmia is truly a cause of nausea and vomiting or merely an associated epiphenomenon. More research on this point is needed, but it is notable that gastric electrical pacing therapy may have potential to both revert arrhythmia and reduce nausea, suggesting arrhythmia does have a role in symptom genesis.79 A pathogenic role for arrhythmia in the motion sickness induced by circular vection80 has also been suggested by the fact that the rhythm disturbances arise 1–2 min before symptoms and their severity correlates with nausea intensity.71 In addition, in patients with gastroparesis, the resolution of nausea and vomiting has been associated with improvements in EGG abnormalities.50
Another documented EGG abnormality is the loss of normal power increase after a test meal observed in gastroparetic patients.54 The EGG signal is considered summative of slow wave activity and smooth muscle potentials and/or contractions.81 Therefore, the normal postprandial power increase may reflect increased electromechanical activity and/or perhaps gastric distension, 82,83 whereas its loss in gastroparesis may reflect reduced electromechanical activity,83 potentially related to hypomotility or reduced current density output caused by ICC loss.84 Consistent with this latter hypothesis, a recent extracellular study has shown reduced serosal slow wave amplitudes in patients with gastroparesis.7
Despite the many studies showing the potential value of EGG, clinical adoption remains weak. In addition to the limitations outlined above, EGG is perceived as having incomplete sensitivity and specificity and inconsistent associations with symptoms, and there are uncertainties regarding its functional significance and role in management.2,3 The positive predictive value of EGG for normal gastric emptying ranges from 65% to 100% in published studies (average 85%) and from 50% to 81% for predicting abnormal gastric emptying (average 65%).3 However, it should be noted that EGG and gastric emptying tests likely reflect and characterize different patient subgroups. More significantly, additional work is needed to define validated roles for EGG in therapeutic algorithms.3 There is also considerable scope to further refine the principles and practice of EGG, through biophysical modelling, technical advances and basic experimental studies.85
NEW INSIGHTS INTO ARRHYTHMIA FROM HR MAPPING
The advent of HR mapping has been a major advance in gastric arrhythmia research. The first HR analyses of slow wave arrhythmias were performed in dogs in 2008, reporting novel abnormalities of both initiation (e.g. focal or ectopic activities) and conduction (e.g. re-entrant propagations) as mechanisms of antral tachygastrias.44 These concepts were extended in a subsequent porcine study, demonstrating that complex slow wave abnormalities also underlie tachygastria in the corpus, including re-entry, self-perpetuating conduction block and ectopic initiation and showing ‘escape rhythms’ in bradygastria.86 These two studies are representative of a new era for gastric arrhythmia, in which modern methods of cardiac rhythm analysis are brought to bear to achieve accurate analyses of arrhythmic onset and maintenance. 87
Clinical translation of HR mapping was recently achieved in a study of patients with idiopathic and diabetic gastroparesis, providing the first spatially detailed description of human arrhythmic patterns.7 Whereas previous studies had focused almost exclusively on human abnormalities of slow wave initiation (e.g. ectopic activity), that study demonstrated that conduction abnormalities (e.g. slow conduction and conduction blocks) were also prevalent in gastroparesis.7 These conduction abnormalities could be a consequence of ICC loss, which results in network remodelling and haphazard or delayed conduction.84,88 The complex arrhythmic propagation patterns described in that recent study7 also have pathophysiological implications, because disorganized patterns, such as competing pacemakers, colliding wavefronts, retrograde propagation and uncoupling, must be highly disruptive to gastric mixing and motility, in a manner akin to cardiac fibrillation disrupting cardiac contractility and output. This notion is supported by canine studies that show slow wave arrhythmia impairs gastric contractions and induces hypomotility. 89,90
With a range of abnormalities now being discovered and examined by HR mapping, early attempts are being made to classify these arrhythmias. In their canine study, Lammers et al.44 proposed a first classification mostly based on rate and rhythm, very similar to what is used in cardiac arrhythmias.91 In contrast, in the human gastroparesis mapping study,7 the changes in rate were less dramatic and arrhythmias were classified in patterns of propagation, as elucidated by HR mapping (e.g. Fig. 1). The limited alterations in slow wave rate during human arrhythmia is important, because it means that many such abnormalities could be missed by tests relying predominantly on frequency analysis, perhaps partly explaining the limited sensitivity of EGG.
Given that the HR mapping analysis of arrhythmias has only recently begun, there may well be additional types of arrhythmias to be discovered and analysed and it therefore seems premature at present to suggest a complete or comprehensive classification system. Furthermore, future classifications could also be based directly on the mechanisms of these arrhythmias, as is starting to occur in the nascent field of small intestine arrhythmias.36,92
Despite its advantages, a major limitation of HR mapping is that all studies to date have been performed in fasted anaesthetized subjects subjected to laparotomy. Although routine anaesthesia is substantially unlikely to alter slow wave activity,29 this research context is limiting because mechanical and nutrient factors are likely to be significant in slow wave arrhythmogenesis. For example, balloon distension of the antrum can provoke arrhythmias,93 as can duodenal perfusion with lipid- and protein-rich solutions,71 suggesting that it would also be productive to evaluate arrhythmias in the post-prandial period, as occurs with EGG. Major technical advances would be required to achieve multi-electrode mapping in fed, awake subjects (discussed below).
NEUROHORMONAL INFLUENCES AND THERAPIES
Multiple neurohormonal factors have shown potential to induce gastric arrhythmias, some of which are discussed here. Hormonal factors that may affect slow wave rhythms include insulin, cholecystokinin, pentagastrin and glucagon.71 Administration of progesterone and oestrogen to non-pregnant women, in levels equivalent to that occurring in the pregnant state, can also induce gastric arrhythmia and nausea,94 and adrenaline and noradrenaline have also been shown to be arrhythmogenic in susceptible individuals and animals.71,90 In general, however, the clinical significance of these associations remains quite uncertain. For example, although female sex steroids can induce arrhythmias and arrhythmias are associated with nausea and vomiting in pregnancy,75 proof of causality within this chain of events is lacking.
Neural influences may also promote or suppress arrhythmia. As discussed above, cholinergic stimulation may modulate intrinsic ICC frequencies, potentially inducing arrhythmias.39 In addition, anticholinergic agents, such as atropine and scopolamine, can inhibit tachygastria and reduce nausea during circular vection, implying a cholinergic influence, which is likely to be centrally mediated.74,95 Phentolamine has also been shown to blunt nausea and tachygastria induced by circular vection and ephedrine infusion, implying that α-adrenoceptor pathways are also involved.71,74 In contrast, arrhythmias induced by gastric antral distension cannot be inhibited by anticholinergic administration, 93 and other influences could also be active in this context, such as mechanosensitive ion channels within the ICC network.96
The exact pathways by which neural influences may mediate arrhythmias remain incompletely understood. In general, ICC show extensive innervation, having synapse-like contacts with enteric nervous system (ENS) varicosities, such that ENS motor neurons may impact directly on ICC excitability and frequency and ICC also show anatomical associations with vagal fibres.5,97 Through these connections, ICC likely play a mediating role in nitrergic and cholinergic modulation of gut smooth muscle function, whereas purinergic and peptidergic innervation appear to act via direct neurotransmitter diffusion, such that parallel influences are ultimately integrated in smooth muscle function.5
Paracrine factors are also known to induce gastric arrhythmias, such as the endogenous prostaglandin E2,98 which may act through prostanoid EP3 receptors with chronotropic responses mediated by inositol 1,4,5-trisphosphate (IP3) generation.99 Indomethacin has been shown to reduce arrhythmia in hyperglycaemia, among other conditions,100 but the clinical applicability and safety of prostaglandin synthesis inhibitors in arrhythmia management currently remain uncertain.
Finally, dopamine and serotonin pathways have also been associated with gastric arrhythmia pathogenesis. Domperidone, cisapride and ondansetron are all known to stabilize gastric rhythm; however, it is unclear whether these are simply associations or pharmacological mechanisms of action.71 Interestingly, ginger has similar effects, mediated by unknown means.101
ADVANCES IN GASTRIC ARRHYTHMIA MONITORING DEVICES
Future advances in gastric arrhythmia research will continue to be underpinned by technical advances and some of these opportunities are addressed here. To date, human HR mapping studies have been facilitated by a flexible printed circuit board (PCB) electrode platform.102 In addition to being flexible, these arrays are cheap to mass produce, are readily sterilized and potentially disposable. However, their relatively low signal-to-noise ratio (SNR) can make signal interpretation problematic in the electrically noisy clinical environment, and further refinements to human serosal mapping arrays will be important.
Data management remains a major technical challenge in HR mapping research, with vast volumes of electrograms being recorded in each study. This problem has been partly overcome through a recent series of signal processing advances, which have generated an efficient data analysis package (i.e. the Gastrointestinal Electrical Mapping Suite or ‘GEMS’) that is largely automated when data quality is sufficient.103 The back end of this analysis package is supported by a number of signal processing algorithms specifically developed for slow wave identification; these include validated algorithms for filtering,104 identifying slow wave activation times,105 grouping and mapping propagation cycles,106 and mapping amplitude and velocity fields.33,34 Efforts are also underway to apply similar methods in real time, allowing live mapping during experiments or clinical studies.107
To progress the goal of HR mapping arrhythmias in conscious, fed patients, Farajidavar et al.108 recently presented the first telemetric platform for wireless slow wave data acquisition and transmission. In future, wireless transmission could be coupled with secure mucosal recording methods,109 allowing detailed clinical studies to be performed over several days and in the awake state.
Gastric pacing (i.e. low-frequency stimulation to entrain slow waves) has shown potential to revert arrhythmias, modulate symptoms and accelerate gastric emptying,79 and continues to be explored as a treatment modality. In a recent porcine study, HR mapping was applied to evaluate the effects of gastric pacing on slow wave entrainment in spatiotemporal detail,110 and it would be valuable to reproduce this work in humans. An endoscopically implantable gastric stimulation device was recently proposed that could reduce invasiveness.111
Another critical advance will be development of effective devices for endoscopic HR mapping, which holds the potential for a routinely deployable diagnostic device. This task is complicated by the high impedance of the gastric mucosa, which attenuates signals that are already low in amplitude. However, past efforts at mucosal recordings in humans show that the approach is feasible109,112 and we anticipate that endoscopic prototypes will be proposed within the coming years. Laparoscopic approaches are also being developed, which could be usefully applied to investigate the consequences of operative procedures on slow wave activity.113
CONCLUDING REMARKS
Despite substantial recent progress, gastric arrhythmia remains a clinical enigma, with uncertain pathogenesis, pathophysiology and therapeutic implications. Many lines of enquiry must be further developed before we will know the full potential of Alvarez's ‘little prophesy’ that ‘gastroenterologists would come to rely upon electrical measures for the routine diagnosis of stomach disorders’. However, like Alvarez, we continue to see significant unmet potential in this field. The ultimate test will be to show that arrhythmias can be reversed in a way that meaningfully improves organ function and symptoms for the benefit of patients. In light of the many advances discussed here, such a goal may finally be within reach.
ACKNOWLEDGEMENTS
The authors are supported by the New Zealand Health Research Council, the National Institutes of Health (R01 DK64775), the Riddet Institute, the New Zealand Rutherford Foundation Trust and the Marsden Fund.
REFERENCES
- 1.Alvarez WC. The electrogastrogram and what it shows. JAMA. 1922;78:1116–19. [Google Scholar]
- 2.Bortolotti M. Electrogastrography: A seductive promise, only partially kept. Am. J. Gastroenterol. 1998;93:1791–4. doi: 10.1111/j.1572-0241.1998.01791.x. [DOI] [PubMed] [Google Scholar]
- 3.Parkman HP, Hasler WL, Barnett JL, Eaker EY. Electrogastrography: A document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol. Motil. 2003;15:89–102. doi: 10.1046/j.1365-2982.2003.00396.x. [DOI] [PubMed] [Google Scholar]
- 4.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol. Motil. 2008;20:54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 5.Huizinga JD, Zarate N, Farrugia G. Physiology, injury, and recovery of interstitial cells of Cajal: Basic and clinical science. Gastroenterology. 2009;137:1548–56. doi: 10.1053/j.gastro.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–85.e8. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Grady G, Angeli T, Du P, et al. Abnormal initiation and conduction of slow wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology. 2012;143:589–98. doi: 10.1053/j.gastro.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faussone-Pellegrini MS, Grover M, Pasricha PJ, et al. Ultrastructural differences between diabetic and idiopathic gastroparesis. J. Cell Mol. Med. 2012;16:1573–81. doi: 10.1111/j.1582-4934.2011.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grover M, Bernard CE, Pasricha PJ, et al. Clinical–histological associations in gastroparesis: Results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol. Motil. 2012;24:531–9. doi: 10.1111/j.1365-2982.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Z, Sarosiek I, Forster J, Damjanov I, Hou Q, McCallum RW. Association of the status of interstitial cells of Cajal and electrogastrogram parameters, gastric emptying and symptoms in patients with gastroparesis. Neurogastroenterol. Motil. 2010;22:56–61. doi: 10.1111/j.1365-2982.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 11.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–64. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyder A, Farrugia G. Targeting ion channels for the treatment of gastrointestinal motility disorders. Therap. Adv. Gastroenterol. 2012;5:5–21. doi: 10.1177/1756283X11415892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito YA, Strege PR, Tester DJ, et al. Sodium channel mutation in irritable bowel syndrome: Evidence for an ion channelopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G211–18. doi: 10.1152/ajpgi.90571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poh YC, Beyder A, Strege PR, Farrugia G, Buist ML. Quantification of gastrointestinal sodium channelopathy. J. Theor. Biol. 2012;293:41–8. doi: 10.1016/j.jtbi.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter S, Brugada P. Role of mapping in channelopathies. Brugada syndrome, long-QT syndrome, and idiopathic VF. In: Shenasa M, Hindricks G, Borggrefe M, Breithardt G, editors. Cardiac Mapping. 3rd edn. Wiley-Blackwell; Oxford: 2009. pp. 434–53. [Google Scholar]
- 16.Jung KT, Park H, Kim JH, et al. The relationship between gastric myoelectric activity and SCN5A mutation suggesting sodium channelopathy in patients with Brugada syndrome and functional dyspepsia: A pilot study. J. Neurogastroenterol. Motil. 2012;18:58–63. doi: 10.5056/jnm.2012.18.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang F, Rock JR, Harfe BD, et al. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc. Natl Acad. Sci. USA. 2009;106:21413–8. doi: 10.1073/pnas.0911935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu MH, Kim TW, Ro S, et al. A Ca(2+)-activated Cl(-) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J. Physiol. 2009;587:4905–18. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein S, Seidler B, Kettenberger A, et al. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat. Commun. 2013;4:1630. doi: 10.1038/ncomms2626. [DOI] [PubMed] [Google Scholar]
- 20.van Helden DF, Laver DR, Holdsworth J, Imtiaz MS. The generation and propagation of gastric slow waves. Clin. Exp. Pharmacol. Physiol. 2010;37:516–24. doi: 10.1111/j.1440-1681.2009.05331.x. [DOI] [PubMed] [Google Scholar]
- 21.Kelly KA, Code CF. Canine gastric pacemaker. Am. J. Physiol. 1971;220:112–18. doi: 10.1152/ajplegacy.1971.220.1.112. [DOI] [PubMed] [Google Scholar]
- 22.Hinder RA, Kelly KA. Human gastric pacesetter potential. Site of origin, spread, and response to gastric transection and proximal gastric vagotomy. Am. J. Surg. 1977;133:29–33. doi: 10.1016/0002-9610(77)90187-8. [DOI] [PubMed] [Google Scholar]
- 23.Torihashi S, Horisawa M, Watanabe Y. c-Kit immunoreactive interstitial cells in the human gastrointestinal tract. J. Auton. Nerv. Syst. 1999;75:38–50. doi: 10.1016/s0165-1838(98)00174-x. [DOI] [PubMed] [Google Scholar]
- 24.Rhee PL, Lee JY, Son HJ, et al. Analysis of pacemaker activity in the human stomach. J. Physiol. 2011;589:6105–18. doi: 10.1113/jphysiol.2011.217497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang XY, Lammers WJ, Bercik P, Huizinga JD. Lack of pyloric interstitial cells of Cajal explains distinct peristaltic motor patterns in stomach and small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G539–49. doi: 10.1152/ajpgi.00046.2005. [DOI] [PubMed] [Google Scholar]
- 26.Angeli TR, Du P, Paskaranandavadivel N, et al. The bioelectrical basis and validity of gastrointestinal extracellular slow wave recordings. J. Physiol. 2013;591:4567–79. doi: 10.1113/jphysiol.2013.254292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Grady G, Du P, Cheng LK, et al. Origin and propagation of human gastric slow-wave activity defined by high-resolution mapping. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G585–92. doi: 10.1152/ajpgi.00125.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lammers WJ, al-Kais A, Singh S, Arafat K, el-Sharkawy TY. Multielectrode mapping of slow-wave activity in the isolated rabbit duodenum. J. Appl. Physiol. 1993;74:1454–61. doi: 10.1152/jappl.1993.74.3.1454. [DOI] [PubMed] [Google Scholar]
- 29.O'Grady G, Angeli T, Lammers WJ. The principles and practice of gastrointestinal high-resolution mapping. In: Cheng LK, Farrugia G, Pullan AJ, editors. New Advances in Gastrointestinal Motility Research. Springer; New York: 2013. pp. 51–69. [Google Scholar]
- 30.Lammers WJ, Ver Donck L, Stephen B, Smets D, Schuurkes JA. Origin and propagation of the slow wave in the canine stomach: The outlines of a gastric conduction system. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G1200–10. doi: 10.1152/ajpgi.90581.2008. [DOI] [PubMed] [Google Scholar]
- 31.Egbuji JU, O'Grady G, Du P, et al. Origin, propagation and regional characteristics of porcine gastric slow wave activity determined by high-resolution mapping. Neurogastroenterol. Motil. 2010;22:e292–300. doi: 10.1111/j.1365-2982.2010.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Grady G, Du P, Paskaranandavadivel N, et al. Rapid high amplitude circumferential slow wave conduction during normal gastric pacemaking and dysrhythmia. Neurogastroenterol. Motil. 2012;24:e299–312. doi: 10.1111/j.1365-2982.2012.01932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paskaranandavadivel N, Cheng LK, Du P, O'Grady G, Pullan AJ. Improved signal processing techniques for the analysis of high resolution serosal slow wave activity in the stomach. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011;2011:1737–40. doi: 10.1109/IEMBS.2011.6090497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paskaranandavadivel N, O'Grady G, Du P, Pullan AJ, Cheng LK. An improved method for the estimation and visualization of velocity fields from gastric high-resolution electrical mapping. IEEE Trans. Biomed. Eng. 2012;59:882–9. doi: 10.1109/TBME.2011.2181845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du P, O'Grady G, Paskaranandavadivel N, et al. Quantification of velocity anisotropy during gastric electrical arrhythmia. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011;2011:4402–5. doi: 10.1109/IEMBS.2011.6091092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angeli TR, O'Grady G, Du P, et al. Circumferential and functional re-entry of in vivo slow-wave activity in the porcine small intestine. Neurogastroenterol. Motil. 2013;25:e304–14. doi: 10.1111/nmo.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng LK, Du P, O'Grady G. Mapping and modeling gastrointestinal bioelectricity: From engineering bench to bedside. Physiology. 2013;28:310–17. doi: 10.1152/physiol.00022.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirst GD, Garcia-Londono AP, Edwards FR. Propagation of slow waves in the guinea-pig gastric antrum. J. Physiol. 2006;571:165–77. doi: 10.1113/jphysiol.2005.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forrest AS, Ordog T, Sanders KM. Neural regulation of slow wave frequency in the murine gastric antrum. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G486–95. doi: 10.1152/ajpgi.00349.2005. [DOI] [PubMed] [Google Scholar]
- 40.Yin J, Chen JD. Electrogastrography: Methodology, validation and applications. J. Neurogastroenterol. Motil. 2013;19:5–17. doi: 10.5056/jnm.2013.19.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verhagen MA, Van Schelven LJ, Samsom M, Smout AJ. Pitfalls in the analysis of electrogastrographic recordings. Gastroenterology. 1999;117:453–60. doi: 10.1053/gast.1999.0029900453. [DOI] [PubMed] [Google Scholar]
- 42.Mintchev MP, Otto SJ, Bowes KL. Electrogastrography can recognise gastric electrical uncoupling in dogs. Gastroenterology. 1997;112:2006–11. doi: 10.1053/gast.1997.v112.pm9178693. [DOI] [PubMed] [Google Scholar]
- 43.Simonian HP, Panganamamula K, Chen JZ, Fisher RS, Parkman HP. Multichannel electrogastrography (EGG) in symptomatic patients: A single center study. Am. J. Gastroenterol. 2004;99:478–85. doi: 10.1111/j.1572-0241.2004.04103.x. [DOI] [PubMed] [Google Scholar]
- 44.Lammers WJEP, Ver Donck L, Stephen B, Smets D, Schuurkes JAJ. Focal activities and re-entrant propagations as mechanisms of gastric tachyarrhythmias. Gastroenterology. 2008;135:1601–11. doi: 10.1053/j.gastro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 45.Abell TL, Malagelada JR. Glucagon-evoked gastric dysrhythmias in humans shown by an improved electrogastrographic technique. Gastroenterology. 1985;88:1932–40. doi: 10.1016/0016-5085(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 46.Jebbink RJ, Samsom M, Bruijs PP, et al. Hyperglycemia induces abnormalities of gastric myoelectrical activity in patients with type I diabetes mellitus. Gastroenterology. 1994;107:1390–7. doi: 10.1016/0016-5085(94)90541-x. [DOI] [PubMed] [Google Scholar]
- 47.Lin X, Mellow MH, Southmayd L, Pan J, Chen JD. Impaired gastric myoelectrical activity in patients with chronic renal failure. Dig. Dis. Sci. 1997;42:898–906. doi: 10.1023/a:1018856112765. [DOI] [PubMed] [Google Scholar]
- 48.Abell TL, Malagelada JR, Lucas AR, et al. Gastric electromechanical and neurohormonal function in anorexia nervosa. Gastroenterology. 1987;93:958–65. doi: 10.1016/0016-5085(87)90557-9. [DOI] [PubMed] [Google Scholar]
- 49.Debinski HS, Ahmed S, Milla PJ, Kamm MA. Electrogastrography in chronic intestinal pseudoobstruction. Dig. Dis. Sci. 1996;41:1292–7. doi: 10.1007/BF02088549. [DOI] [PubMed] [Google Scholar]
- 50.Koch KL, Stern RM, Stewart WR, Vasey MW. Gastric emptying and gastric myoelectrical activity in patients with diabetic gastroparesis: Effect of long-term domperidone treatment. Am. J. Gastroenterol. 1989;84:1069–75. [PubMed] [Google Scholar]
- 51.Lin ZY, McCallum RW, Schirmer BD, Chen JD. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am. J. Physiol. 1998;274:G186–91. doi: 10.1152/ajpgi.1998.274.1.G186. [DOI] [PubMed] [Google Scholar]
- 52.Chen JD, Schirmer BD, McCallum RW. Serosal and cutaneous recordings of gastric myoelectrical activity in patients with gastroparesis. Am. J. Physiol. 1994;266:G90–8. doi: 10.1152/ajpgi.1994.266.1.G90. [DOI] [PubMed] [Google Scholar]
- 53.Bortolotti M, Sarti P, Barara L, Brunelli F. Gastric myoelectrical activity in patients with chronic idiopathic gastroparesis. J. Gastroint Motil. 1990;2:104–8. [Google Scholar]
- 54.Chen JD, Lin Z, Pan J, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig. Dis. Sci. 1996;41:1538–45. doi: 10.1007/BF02087897. [DOI] [PubMed] [Google Scholar]
- 55.Brzana RJ, Koch KL, Bingaman S. Gastric myoelectrical activity in patients with gastric outlet obstruction and idiopathic gastroparesis. Am. J. Gastroenterol. 1998;93:1803–9. doi: 10.1111/j.1572-0241.1998.00524.x. [DOI] [PubMed] [Google Scholar]
- 56.Chen J, McCallum RW. Gastric slow wave abnormalities in patients with gastroparesis. Am. J. Gastroenterol. 1992;87:477–82. [PubMed] [Google Scholar]
- 57.Abell TL, Familoni B, Voeller G, et al. Electrophysiologic, morphologic, and serologic features of chronic unexplained nausea and vomiting: Lessons learned from 121 consecutive patients. Surgery. 2009;145:476–85. doi: 10.1016/j.surg.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 58.You CH, Lee KY, Chey WY, Menguy R. Electrogastrographic study of patients with unexplained nausea, bloating, and vomiting. Gastroenterology. 1980;79:311–14. [PubMed] [Google Scholar]
- 59.You CH, Chey WY, Lee KY, Menguy R, Bortoff A. Gastric and small intestinal myoelectric dysrhythmia associated with chronic intractable nausea and vomiting. Ann. Intern. Med. 1981;95:449–51. doi: 10.7326/0003-4819-95-4-449. [DOI] [PubMed] [Google Scholar]
- 60.Leahy A, Besherdas K, Clayman C, Mason I, Epstein O. Abnormalities of the electrogastrogram in functional gastrointestinal disorders. Am. J. Gastroenterol. 1999;94:1023–8. doi: 10.1111/j.1572-0241.1999.01007.x. [DOI] [PubMed] [Google Scholar]
- 61.Lin X, Levanon D, Chen JD. Impaired postprandial gastric slow waves in patients with functional dyspepsia. Dig. Dis. Sci. 1998;43:1678–84. doi: 10.1023/a:1018806913907. [DOI] [PubMed] [Google Scholar]
- 62.Pfaffenbach B, Adamek RJ, Bartholomaus C, Wegener M. Gastric dysrhythmias and delayed gastric emptying in patients with functional dyspepsia. Dig. Dis. Sci. 1997;42:2094–9. doi: 10.1023/a:1018826719628. [DOI] [PubMed] [Google Scholar]
- 63.Lin Z, Eaker EY, Sarosiek I, McCallum RW. Gastric myoelectrical activity and gastric emptying in patients with functional dyspepsia. Am. J. Gastroenterol. 1999;94:2384–9. doi: 10.1111/j.1572-0241.1999.01362.x. [DOI] [PubMed] [Google Scholar]
- 64.Lin X, Chen JZ. Abnormal gastric slow waves in patients with functional dyspepsia assessed by multichannel electrogastrography. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G1370–5. doi: 10.1152/ajpgi.2001.280.6.G1370. [DOI] [PubMed] [Google Scholar]
- 65.Sha W, Pasricha PJ, Chen JD. Rhythmic and spatial abnormalities of gastric slow waves in patients with functional dyspepsia. J. Clin. Gastroenterol. 2009;43:123–9. doi: 10.1097/MCG.0b013e318157187a. [DOI] [PubMed] [Google Scholar]
- 66.Cucchiara S, Salvia G, Borrelli O, et al. Gastric electrical dysrhythmias and delayed gastric emptying in gastroesophageal reflux disease. Am. J. Gastroenterol. 1997;92:1103–8. [PubMed] [Google Scholar]
- 67.Chen CL, Lin HH, Huang LC, Huang SC, Liu TT. Electrogastrography differentiates reflux disease with or without dyspeptic symptoms. Dig. Dis. Sci. 2004;49:715–19. doi: 10.1023/b:ddas.0000030079.20501.62. [DOI] [PubMed] [Google Scholar]
- 68.Leahy A, Besherdas K, Clayman C, Mason I, Epstein O. Gastric dysrhythmias occur in gastro-oesophageal reflux disease complicated by food regurgitation but not in uncomplicated reflux. Gut. 2001;48:212–15. doi: 10.1136/gut.48.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chong SK. Electrogastrography in cyclic vomiting syndrome. Dig Dis Sci. 1999;44:64S–73S. [PubMed] [Google Scholar]
- 70.Liberski SM, Koch KL, Atnip RG, Stern RM. Ischemic gastroparesis: Resolution after revascularization. Gastroenterology. 1990;99:252–7. doi: 10.1016/0016-5085(90)91255-5. [DOI] [PubMed] [Google Scholar]
- 71.Owyang C, Hasler WL. Physiology and pathophysiology of the interstitial cells of Cajal: From bench to bedside. VI. Pathogenesis and therapeutic approaches to human gastric dysrhythmias. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G8–15. doi: 10.1152/ajpgi.00095.2002. [DOI] [PubMed] [Google Scholar]
- 72.Geldof H, van der Schee EJ, van Blankenstein M, Grashuis JL. Electrogastrographic study of gastric myoelectrical activity in patients with unexplained nausea and vomiting. Gut. 1986;27:799–808. doi: 10.1136/gut.27.7.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song J, Zhong DX, Qian W, Hou XH, Chen JD. Short pulse gastric electrical stimulation for cisplatin-induced emesis in dogs. Neurogastroenterol. Motil. 2011;23:468–74. e178. doi: 10.1111/j.1365-2982.2011.01684.x. [DOI] [PubMed] [Google Scholar]
- 74.Hasler WL, Kim MS, Chey WD, Stevenson V, Stein B, Owyang C. Central cholinergic and alpha-adrenergic mediation of gastric slow wave dysrhythmias evoked during motion sickness. Am. J. Physiol. 1995;268:G539–47. doi: 10.1152/ajpgi.1995.268.4.G539. [DOI] [PubMed] [Google Scholar]
- 75.Koch KL, Stern RM, Vasey M, Botti JJ, Creasy GW, Dwyer A. Gastric dysrhythmias and nausea of pregnancy. Dig. Dis. Sci. 1990;35:961–8. doi: 10.1007/BF01537244. [DOI] [PubMed] [Google Scholar]
- 76.Lee KY, Park HJ, Chey WY. Studies on mechanism of retching and vomiting in dogs. Effect of peripheral dopamine blocker on myoelectric changes in antrum and upper small intestine. Dig. Dis. Sci. 1985;30:22–8. doi: 10.1007/BF01318366. [DOI] [PubMed] [Google Scholar]
- 77.Lang IM, Sarna SK. Motor and myoelectric activity associated with vomiting, regurgitation, and nausea. [1 August 2014];Compr. Physiol. 1989 :1179–98. Available online at: http://www.comprehensivephysiology.com/WileyCDA/CompPhysArticle/refId-cp060132.html.
- 78.Ueno T, Chen JD. Vomiting and gastric electrical dysrhythmia in dogs. Scand. J. Gastroenterol. 2004;39:344–52. doi: 10.1080/00365520310008601. [DOI] [PubMed] [Google Scholar]
- 79.McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456–61. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 80.Stern RM, Koch KL, Stewart WR, Lindblad IM. Spectral analysis of tachygastria recorded during motion sickness. Gastroenterology. 1987;92:92–7. doi: 10.1016/0016-5085(87)90843-2. [DOI] [PubMed] [Google Scholar]
- 81.Smout AJPM, Van der schee EJ, Grashuis JL. What is measured in electrogastrography? Dig. Dis. Sci. 1980;25:179–87. doi: 10.1007/BF01308136. [DOI] [PubMed] [Google Scholar]
- 82.Mintchev MP, Kingma YJ, Bowes KL. Accuracy of cutaneous recordings of gastric electrical activity. Gastroenterology. 1993;104:1273–80. doi: 10.1016/0016-5085(93)90334-9. [DOI] [PubMed] [Google Scholar]
- 83.Lin Z, Chen JD, Schirmer BD, McCallum RW. Postprandial response of gastric slow waves: Correlation of serosal recordings with the electrogastrogram. Dig. Dis. Sci. 2000;45:645–51. doi: 10.1023/a:1005434020310. [DOI] [PubMed] [Google Scholar]
- 84.Du P, O'Grady G, Gibbons SJ, et al. Tissue-specific mathematical models of slow wave entrainment in wild-type and 5-HT2B knockout mice with altered interstitial cell of Cajal networks. Biophys. J. 2010;98:1772–81. doi: 10.1016/j.bpj.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Du P, O'Grady G, Cheng LK, Pullan AJ. A multi-scale model of the electrophysiological basis of the human electrogastrogram. Biophys. J. 2010;99:2784–92. doi: 10.1016/j.bpj.2010.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O'Grady G, Egbuji JU, Du P, et al. High-resolution spatial analysis of slow wave initiation and conduction in porcine gastric dysrhythmia. Neurogastroenterol. Motil. 2011;23:e345–55. doi: 10.1111/j.1365-2982.2011.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lammers WJ. Arrhythmias in the gut. Neurogastroenterol. Motil. 2013;25:353–7. doi: 10.1111/nmo.12116. [DOI] [PubMed] [Google Scholar]
- 88.Gao J, Du P, O'Grady G, et al. Numerical metrics for automated quantification of interstitial cell of Cajal network structural properties. J. R. Soc. Interface. 2013;10:20130421. doi: 10.1098/rsif.2013.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xing J, Qian L, Chen J. Experimental gastric dysrhythmias and its correlation with in vivo gastric muscle contractions. World J. Gastroenterol. 2006;12:3994–8. doi: 10.3748/wjg.v12.i25.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.You CH, Chey WY. Study of electromechanical activity of the stomach in humans and in dogs with particular attention to tachygastria. Gastroenterology. 1984;86:1460–8. [PubMed] [Google Scholar]
- 91.Waldo AL, Wit AL. Mechanisms of cardiac arrhythmias. Lancet. 1993;341:1189–93. doi: 10.1016/0140-6736(93)91012-b. [DOI] [PubMed] [Google Scholar]
- 92.Lammers WJ, Stephen B, Karam SM. Functional reentry and circus movement arrhythmias in the small intestine of normal and diabetic rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G684–9. doi: 10.1152/ajpgi.00332.2011. [DOI] [PubMed] [Google Scholar]
- 93.Ladabaum U, Koshy SS, Woods ML, Hooper FG, Owyang C, Hasler WL. Differential symptomatic and electrogastrographic effects of distal and proximal human gastric distension. Am. J. Physiol. 1998;275:G418–24. doi: 10.1152/ajpgi.1998.275.3.G418. [DOI] [PubMed] [Google Scholar]
- 94.Walsh JW, Hasler WL, Nugent CE, Owyang C. Progesterone and estrogen are potential mediators of gastric slow-wave dysrhythmias in nausea of pregnancy. Am. J. Physiol. 1996;270:G506–14. doi: 10.1152/ajpgi.1996.270.3.G506. [DOI] [PubMed] [Google Scholar]
- 95.Uijtdehaage SH, Stern RM, Koch KL. Effects of scopolamine on autonomic profiles underlying motion sickness susceptibility. Aviat. Space Environ. Med. 1993;64:1–8. [PubMed] [Google Scholar]
- 96.Beyder A, Rae JL, Bernard C, Strege PR, Sachs F, Farrugia G. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J. Physiol. 2010;588:4969–85. doi: 10.1113/jphysiol.2010.199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Powley TL, Wang XY, Fox EA, Phillips RJ, Liu LW, Huizinga JD. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol. Motil. 2008;20:69–79. doi: 10.1111/j.1365-2982.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 98.Kim CH, Zinsmeister AR, Malagelada JR. Mechanisms of canine gastric dysrhythmia. Gastroenterology. 1987;92:993–9. doi: 10.1016/0016-5085(87)90975-9. [DOI] [PubMed] [Google Scholar]
- 99.Forrest AS, Hennig GW, Jokela-Willis S, Park CD, Sanders KM. Prostaglandin regulation of gastric slow waves and peristalsis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G1180–90. doi: 10.1152/ajpgi.90724.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hasler WL, Soudah HC, Dulai G, Owyang C. Mediation of hyperglycemia-evoked gastric slow-wave dysrhythmias by endogenous prostaglandins. Gastroenterology. 1995;108:727–36. doi: 10.1016/0016-5085(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 101.Lien HC, Sun WM, Chen YH, Kim H, Hasler W, Owyang C. Effects of ginger on motion sickness and gastric slow-wave dysrhythmias induced by circular vection. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G481–9. doi: 10.1152/ajpgi.00164.2002. [DOI] [PubMed] [Google Scholar]
- 102.Du P, O'Grady G, Egbuji JU, et al. High-resolution mapping of in vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: Methodology and validation. Ann. Biomed. Eng. 2009;37:839–46. doi: 10.1007/s10439-009-9654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yassi R, O'Grady G, Paskaranandavadivel N, et al. The Gastrointestinal Electrical Mapping Suite (GEMS): Software for analysing and visualising gastrointestinal multi-electrode recordings. BMC Gastroenterol. 2012;12:60. doi: 10.1186/1471-230X-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paskaranandavadivel N, O'Grady G, Du P, Cheng LK. Comparison of filtering methods for extracellular gastric slow wave recordings. Neurogastroenterol. Motil. 2013;25:79–83. doi: 10.1111/nmo.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Erickson JC, O'Grady G, Du P, et al. Falling-edge, variable threshold (FEVT) method for the automated detection of gastric slow wave events in serosal high-resolution electrical recordings. Ann. Biomed. Eng. 2010;38:1511–29. doi: 10.1007/s10439-009-9870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Erickson JC, O'Grady G, Du P, Egbuji JU, Pullan AJ, Cheng LK. Automated cycle partitioning and visualization of high-resolution activation time maps of gastric slow wave recordings: The Region Growing Using Polynomial Surface-estimate stabilization (REGroupS) algorithm. Ann. Biomed. Eng. 2011;39:469–83. doi: 10.1007/s10439-010-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bull S, O'Grady G, Cheng LK, Pullan AJ. A framework for the online analysis of multi-electrode gastric slow wave recordings. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011:1741–4. doi: 10.1109/IEMBS.2011.6090498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Farajidavar A, O'Grady G, Rao SM, Cheng LK, Abell T, Chiao JC. A miniature bidirectional telemetry system for in vivo gastric slow wave recordings. Physiol. Meas. 2012;33:N29–37. doi: 10.1088/0967-3334/33/6/N29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coleski R, Hasler WL. Coupling and propagation of normal and dysrhythmic gastric slow waves during acute hyperglycaemia in healthy humans. Neurogastroenterol. Motil. 2009;21:492–9. doi: 10.1111/j.1365-2982.2008.01235.x. [DOI] [PubMed] [Google Scholar]
- 110.O'Grady G, Du P, Lammers WJ, et al. High-resolution entrainment mapping for gastric pacing: A new analytic tool. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G314–21. doi: 10.1152/ajpgi.00389.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deb S, Tang SJ, Abell TL, et al. An endoscopic wireless gastrostimulator (with video). Gastrointest. Endosc. 2012;75:411–15. doi: 10.1016/j.gie.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Monges H, Salducci J. A method of recording the gastric electrical activity in man. Am. J. Dig. Dis. 1970;15:271. doi: 10.1007/BF02233459. [DOI] [PubMed] [Google Scholar]
- 113.O'Grady G, Du P, Egbuji JU, et al. A novel laparoscopic device for measuring gastrointestinal slow-wave activity. Surg. Endosc. 2009;23:2842–8. doi: 10.1007/s00464-009-0515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]