Abstract
Background
Resveratrol has beneficial effects on cardiovascular system. This study aimed at examining antidiabetic and antihypertensive effects of resveratrol in rats with simultaneous type 2 diabetes and renal hypertension.
Methods
Eight groups (8-10 each) of male Spargue-Dawley rats, including a control, a diabetic (induced by streptozotocin and nicotinamide), a renal hypertensive (induced by placing plexiglas clips on the left renal arteries), a sham, a simultaneously hypertensive-diabetic receiving vehicle, and 3 simultaneous hypertensive-diabetic receiving resveratrol at 5, 10 or 20 mg/kg/day were used. Four weeks after the induction of diabetes, renal hypertension was induced and animals were given vehicle or resveratrol for the next four weeks. Afterwards, blood pressure and glucose, serum markers of oxidative stress were measured and animal’s aortic rings were used for isolated studies.
Results
Serum malondialdehyde, systolic blood pressure, heart rate, fasting blood glucose, maximal response and effective concentration 50 of phenylephrine, and inhibitory concentration 50 of acetylcholine of hypertensive-diabetic group receiving vehicle were significantly higher than those of the control group, and treatment with resveratrol caused significant reduction of these variables. Moreover, serum superoxide dismutase, glutathione reductase, and maximal response to acetylcholine of hypertensive-diabetic group receiving vehicle were significantly lower than those of the control group, and treatment with resveratrol caused significant increase of these variables.
Conclusion
The findings indicate that resveratrol has antidiabetic and antihypertensive effects, which may be partly due to antioxidant mechanism. They also show that antihypertensive effect of resveratrol may be additionally mediated by improving the release of nitric oxide and sympathoplegic activities.
Keywords: Resveratrol, Diabetes mellitus, Renal hypertension, Oxidative stress, Nitric oxide, Rat
Introduction
Resveratrol (3,5,4-trihydroxystilbene ), an important polyphenolic component of red grapes, has been hypothesized to mediate its cardioprotective effects.1 Clinical and experimental studies have shown that resveratrol has beneficial cardiovascular effects. It has been shown to reduce blood pressure in spontaneously hypertensive rats,2 DOCA-salt hypertensive rats,3 two-kidney, one-clip hypertensive rats,4 relaxed precontracted vascular segments from spontaneously hypertensive rats,2 DOCA-salt hypertensive rats,3 and female normotensive guinea-pigs.5 The antihypertensive or vasorelaxing effects of resveratrol have been attributed to increased expression of endothelial nitric synthase,6 improved nitric oxide (NO) release and endothelium-dependent vasorelaxation,2 decreased endothelin-1 and angiotensin II production,7 decreased sympathetic activity,8 and decreased oxidative stress.9
Earlier studies have shown the beneficial effects of resveratrol in experimental and human diabetes. Resveratrol was shown to reduce blood glucose in experimental10 and human type 2 diabetes.11 Antidiabetic effects of resveratrol have been attributed to preservation of beta cells,12 increase of insulin release,13 enhancement of insulin sensitivity,14 and decrease of oxidative stress.15
Hypertension and diabetes mellitus are increasingly occurring together in human, and when occur concurrently; they make patients more vulnerable to other cardiovascular disease and death.16 Although the effects of resveratrol have been investigated in experimental hypertension and diabetes separately, its effects have rarely been investigated in a model simultaneous hypertension and diabetes. The objective of this study was to examine the antidiabetic and antihypertensive effects of resveratrol in a rat model of simultaneous type 2 diabetes mellitus and renal hypertension.
Materials and Methods
Animals
Male Sprague-Dawley rats (n=80), weighting 200-250 g, were obtained from Laboratory Animal Breeding Centre, Shiraz University of Medical Sciences (Shiraz, Iran) and kept under standard conditions (12 h light⁄dark cycle, 25-35% humidity, 20-22°C temperature) with standard diet and water ad libitum. All procedures were approved by the University Committee for Care and Use of Animals.
Induction of Type 2 Diabetes and Renal Hypertension
Rats were injected intraperitoneally with 110 mg/kg nicotinamide (NA) (Sigma-Aldrich Chemical Co. (Steinheim, Germany) and 65 mg/kg streptozotocin (STZ) (Teva Parenteral Medicine Inc., Irvine, CA, USA) or distilled water as vehicle (to constitute the control group). Seven days later, animals’ blood glucose levels were determined using a glucometer (Accu-check® active, Germany), and those with a fasting blood glucose higher than 126 mg/dl were considered as having type 2 diabetes.17
Four weeks after the induction of diabetes, control and type 2 diabetic animals were subjected to sham-operation or induction of two-kidney, one clip renal hypertension by placing self-made solid plexiglass clips on the left renal arteries as previously described.18 Briefly, animals were anesthetized with 60 mg/kg ketamine (Pan Pharam, Trittau, Schleswig Holstein, Germany) and 8 mg/kg Xylazine (Alfasan, Woerden, The Netherland), and through a left flank incision the left renal arteries were exposed and dissected away from renal veins and surrounding tissues. Afterwards, plexiglass clips (internal diameter of 0.20-0.22 mm) were placed on the arteries. Penicillin powder (Jaber Ebne Hayyan, Tehran, Iran) was applied to the incision sites and abdominal wall and skin were sutured using absorbable (catgut) and non-absorbable (silk) suture materials, respectively. Sham-operated animals were subjected to a similar procedure, but no clip was placed around renal arteries. The animals were then recovered from anesthesia and kept in cages of two rats each for 4 weeks under standard condition.
Starting from the day after the operations, 8 groups of animals emerged including: a diabetic control group assigned to receive vehicle (DMC-V), a sham group for renal hypertensive rats assigned to receive vehicle (Sham-V), a renal hypertensive group assigned to receive vehicle (HTN-V), a type 2 diabetic group assigned to receive vehicle (DM-V), a simultaneous type 2 diabetes and renal hypertensive group assigned to receive vehicle (DM+HTN-V), and 3 simultaneous type 2 diabetes and renal hypertensive groups assigned to received resveratrol (R) (Serva Co, Oklahoma, USA) at 5 mg/kg/day (DM+HTN-R5), 10 mg/kg/day (DM+HTN-R10), or 20 mg/kg/day (DM+HTN-R20). Vehicle or resveratrol was administered by oral gavage for the next 4 weeks. On day 28, systolic blood pressure (SBP) and heart rate (HR) were measured using non-invasive tail-cuff method (Chart 5.0 software, PowerLab 4/30, AD Instruments Inc., MA, Australia). Three consecutive blood pressure measurements, which had a difference of less than 5 mmHg, were considered as valid. Those measurements were averaged and their means were taken as values of blood pressure at each time.
After the measurement of SBP and HR, animals were anesthetized with 70 mg/kg thiopental sodium (Biochem GmbH, Vienna, Austria). Fasting blood glucose (FBG) was measured using the glucometer, and blood samples for measurement of serum levels of malondialdehyde (MDA), superoxide dismutase (SOD), glutathione reductase (GR) were collected. The samples were allowed to clot for 30 minutes, centrifuged at 3000 rpm for 20 minutes, and their serums were separated and stored at -80ºC until analysis. The animals were then sacrificed and their thoracic aortas were used for isolated tissue studies.
Isolated Aortic Ring Studies
Isolated thoracic aortas were cleaned from the surrounding connective tissues and cut into 4-5 mm rings. The rings were mounted on hooks connected to force transducers in isolated tissue organ baths (K30, Hugo Sachs Electronik, Germany) filled with 20 ml physiological solution containing the following composition (mmol/L): NaCl 118, KCl 4.7, KH2PO4 1.2, CaCl2 2.5, MgSO4 1.2, NaHCO3 25, D-glucose 11.1, bubbled constantly with 95% O2 and 5% CO2 at a pH of 7.4 and a temperature of 37ºC. Tension was recorded with a four-channel polygraph (model 705/1, Hugo Sachs Electronik, Germany). The tissues were allowed to stabilize for 60 minutes. Then, a full concentration-response to phenylephrine (Phe) (Sigma-Aldrich Chemical Co., Steinheim, Germany) was performed. After two washes and 30 minutes equilibration, the rings were contracted with Phe concentrations that made similar contraction responses (50% of maximal response in the DMC-Veh group) in all groups. Concentration-response curves to acetylcholine (ACh) or sodium nitroprusside (SNP) (both from Sigma-Aldrich Chemical Co., Steinheim, Germany) were performed at the plateau of contractile response to Phe. Concentration-responses to Phe were compared using EC50 and maximal response (Emax). Concentration-responses to ACh or SNP were compared using IC50 and Emax.
Biochemical Measurements
Serum level of MDA was determined using MDA ELISA (Bioassay technology laboratory, Shanghai, China) kit. Serum level of SOD and glutathione reductase (GR) was determined using Biorexfars chemical kits (Shiraz, Iran).
Statistical Analysis
Data, presented as mean±SEM, were analyzed using One-way Analysis of Variance (ANOVA). Where a significant difference was obtained with one-way ANOVA, the source of the difference was located using Duncan’s Multiple Range test. The data were analyzed using Sigmastat statistical software version 3.0. P value ≤0.05 was considered statistically significant.
Results
Blood Pressure and Heart Rate
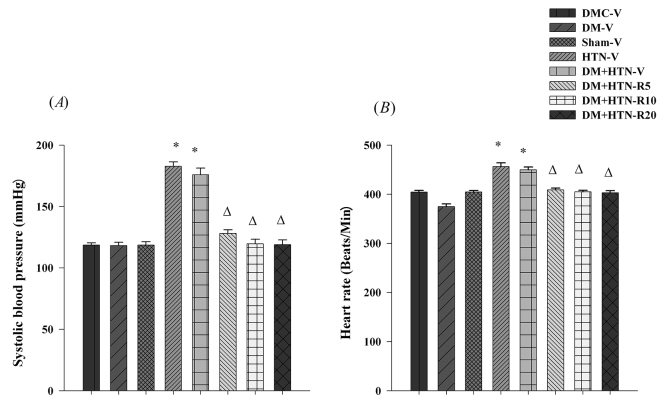
There was no significant difference in the SBP of DMC-V, DM-V and Sham-V groups (figure 1A). Systolic blood pressures of HTN-V, DM+HTN-V were significantly higher than those of DM-C-V, Sham-V and DM-V (figure 1A). Systolic blood pressures of resveratrol-treated groups (DM+HTN-R5, DM+HTN-R10 and DM+HTN-R20) were significantly lower than those of the DM+HTN-V group (figure 1A).
Figure 1.
(A) Systolic blood pressure and (B) heart rate for all experimental groups after 4 weeks of treatment with vehicle or resveratrol. DMC-V: Diabetic control group receiving vehicle; Sham-V: Sham-operated receiving vehicle; DM-V: Diabetic receiving vehicle; HTN-V: Hypertensive receiving vehicle; DM+HTN-V: Diabetic hypertensive receiving vehicle; DM+HTN-R5: Diabetic hypertensive receiving 5 mg/kg/day resveratrol; DM+HTN-R10: Diabetic hypertensive receiving 10 mg/kg/day resveratrol; DM+HTN-R20: Diabetic hypertensive receiving 20 mg/kg/day resveratrol; Data are mean±SEM and n=8-10 in each group; *Significant difference (P<0.05) from DMC-V or Sham-V; ∆Significant difference (P<0.05) from DM+HTN-V
The HR of the DM-V group was significantly lower than those of the DMC-V group (figure 1B). The HR of the HTN-V and DM+HTN-V groups were significantly higher than those of the DMC-V, Sham-V and DM-V groups (figure 1B). The HR of DM+HTN-R5, DM+HTN-R10 and DM+HTN-R20 groups were significantly lower than those of the DM+HTN-V group (figure 1B).
Serum Biochemistry
Fasting blood glucose of the DM-V and DM+HTN-V groups were significantly higher than those of the DMC-V, Sham-V and HTN-V groups. Fasting blood glucose of DM+HTN-R5, DM+HTN-R10 and DM+HTN-R20 groups were significantly lower than that of the DM+HTN-V group (table 1).
Table 1.
The values of biochemical markers of experimental groups after 4 weeks of treatment with vehicle or resveratrol
|
FBS
(mg/dl) |
MDA
(nmol/ml) |
SOD
(Unit/ml) |
GR
(Unit/L) |
|
|---|---|---|---|---|
| DMC-V | 104.9±2.4 | 0.63±0.07 | 264.2±39.2 | 70.6±4.1 |
| DM-V | 209.0±4.4* | 1.55±0.11* | 92.9±11.3* | 26.4±2.8* |
| Sham-V | 106.1±2.6 | 0.64±0.07 | 243.5±43.9 | 68.8±3.9 |
| HTN-V | 120.4±1.5 | 1.32±0.13* | 147.4±19.7* | 39.4±3.1* |
| DM+HTN-V | 199.4±5.8* | 1.81±0.45* | 116.1±13.8* | 30.1±2.4* |
| DM+HTN-R5 | 123.6±2.7Δ | 0.63±0.08Δ | 265.6±51.1Δ | 62.8±5.1Δ |
| DM+HTN-R10 | 106.1±4.2Δ | 0.64±0.09Δ | 302.3±62.1Δ | 64.7±5.1Δ |
| DM+HTN-R20 | 102.6±5.4Δ | 0.56±0.07Δ | 276.1±61.5Δ | 65.4±3.7Δ |
DMC-V: Diabetic control group receiving vehicle; Sham-V: Sham-operated receiving vehicle; DM-V: Diabetic receiving vehicle; HTN-V: Hypertensive receiving vehicle; DM+HTN-V: Diabetic hypertensive receiving vehicle; DM+HTN-R5: Diabetic hypertensive receiving 5 mg/kg/day resveratrol; DM+HTN-R10: Diabetic hypertensive receiving 10 mg/kg/day resveratrol; DM+HTN-R20: Diabetic hypertensive receiving 20 mg/kg/day resveratrol; FBS: Fasting blood glucose; MDA: Malondialdehyde; SOD: Superoxide dismutase; GR: Glutathione reductase; Data are mean±SEM and n=8-10 in each group; *Significant difference (P<0.05) with DMC-Veh or Sham-V; ∆Significant difference (P<0.05) with DM+HTN-V
Serum levels of MDA of the HTN-V, DM-V and DM+HTN-V groups were significantly higher than those of the DMC-V and Sham-V groups. Serum levels of MDA of DM+HTN-R5, DM+HTN-R10 and DM+HTN-R20 groups were significantly lower than the DM+HTN-V group (table 1).
Serum levels of SOD of the HTN-V, DM-V and DM+HTN-V groups were significantly lower than those of the DMC-V and Sham-V groups. Serum levels of SOD of DM+HTN-R5, DM+HTN-R10 and DM+HTN-R20 groups were significantly higher than the DM+HTN-V group (table 1).
Serum levels of GR of the HTN-V, DM-V and DM+HTN-V groups were significantly lower than those of the DMC-V and Sham-V groups. Serum levels of GR of DM+HTN-R5, DM+HTN-R10 and DM+HTN-R20 groups were significantly higher than the DM+HTN-V group (table 1).
Isolated Aortic Ring Studies
The Emaxs of Phe concentration-responses in DM-V, HTN-V and DM+HTN-V groups were significantly higher than those of DMC-V and Sham-V groups. The Emaxs of concentration-responses to Phe of DM+HTN-R5, DM+HTN-R10 and DM+HTN-R20 groups were significantly lower than that of the DM+HTN-V group (figure 2A, table 2). The EC50 of contraction responses to Phe of HTN-V, DM-V and DM+HTN-V groups were significantly lower than those of the DMC-V or Sham-V groups. The EC50s of contraction response to Phe from DM+HTN-R5, DM+HTN-R10 and DM+HTN-R20 groups were significantly higher than that of the DM+HTN-V group (table 2).
Figure 2.
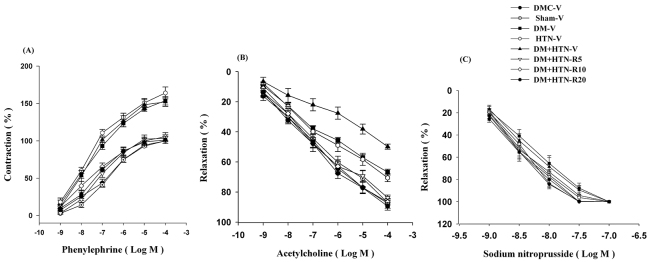
Dose responses from isolated aortic ring studies for all experimental groups after 4 weeks of treatment with vehicle or resveratrol. DMC-V: Diabetic control group receiving vehicle; Sham-V: Sham-operated receiving vehicle; DM-V: Diabetic receiving vehicle; HTN-V: Hypertensive receiving vehicle; DM+HTN-V: Diabetic hypertensive receiving vehicle; DM+HTN-R5: Diabetic hypertensive receiving 5 mg/kg/day resveratrol; DM+HTN-R10: Diabetic hypertensive receiving 10 mg/kg/day resveratrol; DM+HTN-R20: Diabetic hypertensive receiving 20 mg/kg/day resveratrol; Data are mean±SEM and n=8-10 in each group; *Significant difference (P<0.05) from DMC-V or Sham-V; ∆Significant difference (P<0.05) from DM+HTN-V
Table 2.
The values of pharmacological variables obtained from isolated aortic ring studies after 4 weeks of treatment with vehicle or resveratrol
| Phenylephrine | Acetylcholine | |||
|---|---|---|---|---|
| Emax | EC50 | Emax | IC50 | |
| DM2-C-Veh | 100.0±0.0 | -6.76±0.08 | 87.5±3.2 | -7.23±0.13 |
| DM2-Veh | 153.7±26.8* | -7.04±0.09* | 66.7±1.9* | -6.63±0.26* |
| Sham-Veh | 100.0±0.0 | -6.77±0.10 | 87.0±1.5 | -7.13±0.13 |
| HTN-Veh | 173.8±18.2* | -7.37±0.08* | 70.5±2.6* | -6.69±0.28* |
| DM2+HTN-Veh | 165.1±16.0* | -7.29±0.10* | 49.8±1.7* | -6.15±0.37* |
| DM2+HTN-Res5 | 103.6±9.3Δ | -6.10±0.08Δ | 83.8±1.7Δ | -7.07±0.16Δ |
| DM2+HTN-Res10 | 105.5±13.4Δ | -6.91±0.12Δ | 86.7±2.0Δ | -7.16±0.21Δ |
| DM2+HTN-Res20 | 101.4±8.8Δ | -6.70±0.15Δ | 87.5±3.2Δ | -7.3±0.12Δ |
DMC-V: Diabetic control group receiving vehicle; Sham-V: Sham-operated receiving vehicle; DM-V: Diabetic receiving vehicle; HTN-V: Hypertensive receiving vehicle; DM+HTN-V: Diabetic hypertensive receiving vehicle; DM+HTN-R5: Diabetic hypertensive receiving 5 mg/kg/day resveratrol; DM+HTN-R10: Diabetic hypertensive receiving 10 mg/kg/day resveratrol; DM+HTN-R20: Diabetic hypertensive receiving 20 mg/kg/day resveratrol; Emax: Maximal response; EC50: Effective concentration 50; IC50: Inhibitory concentration 50; EC50 and IC50 are presented as antilog of the concentrations; Data are mean±SEM and n=8-10 each; *Significant difference (P<0.05) with DMC-V or Sham-V; ∆Significant difference (P<0.05) with DM+HTN-V
The Emaxsof ACh concentration-responses of DM-V, HTN-V and DM+HTN-V groups were significantly lower than those of DMC-V or Sham-V groups. The Emaxs of ACh concentration-responses of DM+HTN-R5, DM+HTN-R10 and DM+HTN-R20 groups were significantly higher than those of the DM+HTN-V group (figure 2B, table 2). The IC50s of relaxation response to ACh of HTN-V, DM-V and DM+HTN-V groups were significantly higher than those of the DMC-V and Sham-V groups. The IC50s of concentration-response to ACh of DM+HTN-R5, DM+HTN-R10 and DM+HTN-R20 groups were significantly lower than that of the DM+HTN-V group (figure 2B, table 2). There was no significant difference in IC50 or Emaxsof SNP concentration-responses in all groups (figure 2C).
Discussion
The main objective of the present study was to examine the resveratrol’s antihypertensive and antidiabetic effects and their possible mechanisms in rats with simultaneous type 2 diabetes mellitus and renal hypertension. The study showed that resveratrol had antihypertensive, antidiabetic and antioxidative stress activities, and prevented the impairment of endothelial release of NO.
The present study showed that administration of NA and STZ resulted in type 2 diabetes, which its blood glucose was in the range reported by us19 and others.20 This study also shows that, similar to our previous studies19 and those of others,8 the present model was associated with increased oxidative stress.15 Our findings show that placement of plexiglass clips on the left renal arteries led to renal hypertension, in which the increase in blood pressure was comparable to those obtained in our earlier studies21,22 and those of others.23 The findings also shows that administration of NA and STZ and placement of plexiglass clips resulted in a model of simultaneous type 2 diabetes and renal hypertension, which similar to our previous studies22 was associated with the increased blood glucose, hypertension, impaired cardiac function, impaired endothelial function, and increased oxidative stress. Possible mechanisms of increased blood pressure, blood glucose, and cardiac dysfunction in the present models of type 2 diabetes, renal hypertension, and simultaneous type 2 diabetes and renal hypertension were discussed in our earlier publications.17,22
The present study represents the first to report that resveratrol, at all examined doses, was antihypertensive in a rat model of simultaneous type 2 diabetes and renal hypertension. This finding is in agreement with previous studies demonstrating that resveratrol had antihypertensive effects in DOCA-salt hypertensive rats,3 spontaneously hypertensive rats,2 experimental model of malignant hypertension24 and two-kidney, one-clip hypertensive rats.4
The mechanism of antihypertensive activity of resveratrol is not exactly known, but a previous report has attributed that to increased release of endothelial NO rats.25 In order to investigate the mechanism of resveratrol’s antihypertensive effects, we examined whether it might be related to the status endothelial-derived NO release. Similar to experimental diabetes26 or experimental hypertension,3 our model of simultaneous type 2 diabetes and renal hypertension was associated with impaired release of endothelial NO. The Impaired relaxation response to ACh is an indication of reduced release of nitric oxide.17,27 Our findings show that Emax and EC50 of relaxation response to ACh in aortic rings from rats with simultaneous type 2 diabetes and renal hypertension were significantly lower and higher than those of the control group, respectively. These findings indicate that the model was associated with impaired release of nitric oxide. However, resveratrol restored the reduced Emax and increased IC50 of ACh relaxation response, which indicate that it prevented the impairment of endothelial release of NO. Enhancement of endothelial-derived NO activity underlies the antihypertensive activity of a number of other drugs such as enalapril and quinapril,28 nebivolol and carvedilol,29 losartan30 or extracts of herbs such as garlic31 and crataegus.32 The resveratrol’s preservation of endothelium-derived NO release is in agreement with previous studies demonstrating that it increased the release of endothelial NO in spontaneously hypertensive,2 obese Zucker,33 two-kidney, one-clip hypertensive,4 fructose-fed,9 and healthy34 rats.
We also examined whether resveratrol’s sympatholytic activity might be involved in its antihypertensive effects. Our findings show that resveratrol significantly prevented the increase of heart rate in rats with simultaneous type 2 diabetes and renal hypertension. It also prevented the increase in contraction response to Phe in aortic rings from such rats. Both such effects may have contributed to antihypertensive activity of resveratrol. The prevention of heart rate increase and attenuation of contractile response to Phe may be indicative of resveratrol’s beta and alpha antagonism, respectively. Alternatively, resveratrol may have acted somewhere upstream to the alpha and beta receptors, and have caused the attenuation of sympathetic nervous system activity, which has been reported to be elevated in renal hypertension.4
The increased oxidative stress has been reported to be involved in the development of hypertension by causing up-regulation of angiotensin II signaling,35 decreasing NO bioavailability,34 dysfunction of endothelial NO synthase,9 and reduction of the levels of reactive oxygen species scavenger.34 We therefore, measured the concentration of serum markers of oxidative stress. The findings of the present study indicate that the model of simultaneous type 2 diabetes and renal hypertension was associated with the increased oxidative stress, and treatment with resveratrol prevented the increase of oxidative stress, characterized by decreased serum levels of MDA, and increased serum levels of SOD. This finding is similar to those of previous studies showing that resveratrol decreased oxidative stress in stroke prone spontaneously hypertensive,36 two-kidney, one clip hypertensive,4 partially nephrectomized37 and fructose-fed9 rats. Given the role of increased oxidative stress in the development of hypertension, it might be possible to speculate that, by virtue of antioxidative stress activities, resveratrol might have offset one or more of above-mentioned mechanisms, and thereby prevented the increase of blood pressure. However, a recent publication suggests that resveratrol, by virtue of its antioxidative effects, increased phosphorylation of adenosine-monophosphate-activated protein kinase, and thereby reduced blood pressure.9
The present study shows that resveratrol did decrease the blood glucose in rats with simultaneous type 2 diabetes and renal hypertension. This finding is similar to those of previous studies showing that resveratrol decreased blood glucose in streptozotocin-induced,13 and streptozotocin and nicotinamide-induced15 diabetes. Several mechanisms such as preservation of beta cells,12 increased release of insulin,13 enhancement of insulin sensitivity,14 increasing insulin receptor substrate-1 and glucose transporter 1 and 4,38 and decrease of oxidative stress15 have been proposed to mediate resveratrol’s antidiabetic effect.
In order to investigate whether antidiabetic effect of resveratrol may be attributed to antioxidative stress properties, serum concentrations of MDA and SOD were measured. Treatment of rats with simultaneous type 2 diabetes and renal hypertension was associated with reduced oxidative stress activities. Such findings are in agreement with previous studies showing that resveratrol decreased oxidative stress in type 2 diabetic patients,11 streptozotocin-induced diabetes,13 streptozotocin and nicotinamide-induced diabetes,15 and fructose-fed rat.9 Therefore, it is appropriate to suggest that by reducing the oxidative stress, resveratrol may have reduced blood glucose in the present study.
Diabetes mellitus and hypertension often occur simultaneously in human. Data from Farmingham study indicate that at the time of diabetes diagnosis, 58% of patients had hypertension.39 Simultaneous occurrence of these diseases is associated with a higher cardiovascular risk and mortality in human.40 Given the grave consequence of simultaneous occurrence of the two diseases, the use of a single remedy that is effective for both diseases are of vital importance. Our findings show that resveratrol did have both antihypertensive and antidiabetic activities. Therefore, our findings may open a further horizon for resveratrol’s research, and its future use as a supplement or a drug in situations that the two diseases occur concurrently.
Conclusion
The findings of the present study indicate that resveratrol has antihypertensive and antidiabetic effects in rats with simultaneous renal hypertension and type 2 diabetes. Resveratrol effects might be partly attributed to relate to restoration of endothelial NO release, a sympatholytic or α and β receptor blocking activity, and antioxidative stress activity.
Acknowledgment
This work was supported by the Vice President for Research Affairs, Shiraz University of Medical Sciences. This paper is an extract from Masoud Mozafari’s PhD thesis.
Conflict of Interest: None declared.
References
- 1.Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 2000;47:549–55. doi: 10.1016/s0008-6363(00)00102-4. doi: 10.1016/S0008-6363(00)00102-4. PubMed PMID: 10963727. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt SR, Lokhandwala MF, Banday AA. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur J Pharmacol. 2011;667:258–64. doi: 10.1016/j.ejphar.2011.05.026. doi: 10.1016/j.ejphar.2011.05.026. PubMed PMID: 21640096. [DOI] [PubMed] [Google Scholar]
- 3.Chan V, Fenning A, Iyer A, Hoey A, Brown L. Resveratrol improves cardiovascular function in DOCA-salt hypertensive rats. Curr Pharm Biotechnol. 2011;12:429–36. doi: 10.2174/138920111794480552. PubMed PMID: 20874677. [DOI] [PubMed] [Google Scholar]
- 4.de Oliveira JC, Antonietto CRK, Scalabrini AC, Marinho TS, Pernomian L, Corrêa JWN, et al. Antioxidant protective effects of the resveratrol on the cardiac and vascular tissues from renal hypertensive rats. Open Journal of Medicinal Chemistry. 2012;2:61–71. [Google Scholar]
- 5.Naderali EK, Doyle PJ, Williams G. Resveratrol induces vasorelaxation of mesenteric and uterine arteries from female guinea-pigs. Clin Sci (Lond) 2000;98:537–43. doi: 10.1042/CS19990303. PubMed PMID: 10781384. [PubMed] [Google Scholar]
- 6.Nicholson SK, Tucker GA, Brameld JM. Physiological concentrations of dietary polyphenols regulate vascular endothelial cell expression of genes important in cardiovascular health. Br J Nutr. 2010;103:1398–403. doi: 10.1017/S0007114509993485. doi: 10.1017/S0007114509993485. PubMed PMID: 20021702. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Song Y, Zhang X, Liu Z, Zhang W, Mao W, et al. Effects of trans-resveratrol on hypertension-induced cardiac hypertrophy using the partially nephrectomized rat model. Clin Exp Pharmacol Physiol. 2005;32:1049–54. doi: 10.1111/j.1440-1681.2005.04303.x. doi: 10.1111/j.1440-1681.2005.04303.x. PubMed PMID: 16445570. [DOI] [PubMed] [Google Scholar]
- 8.Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1–7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol. 2008;28:25–33. doi: 10.1159/000108758. PubMed PMID: 17890855. [DOI] [PubMed] [Google Scholar]
- 9.Cheng PW, Ho WY, Su YT, Lu PJ, Chen BZ, Cheng WH, et al. Resveratrol decreases fructose‐induced oxidative stress, mediated by NADPH oxidase via an AMPK‐dependent mechanism. Br J Pharmacol. 2014;171:2739–50. doi: 10.1111/bph.12648. PubMed PMID: 24547812; PubMed Central PMCID: PMC4243851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akar F, Pektas MB, Tufan C, Soylemez S, Sepici A, Ulus AT, et al. Resveratrol shows vasoprotective effect reducing oxidative stress without affecting metabolic disturbances in insulin-dependent diabetes of rabbits. Cardiovasc Drugs Ther. 2011;25:119–31. doi: 10.1007/s10557-010-6255-7. doi: 10.1007/s10557-010-6255-7. PubMed PMID: 20676927. [DOI] [PubMed] [Google Scholar]
- 11.Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383–9. doi: 10.1017/S0007114511000316. doi: 10.1017/S0007114511000316. PubMed PMID: 21385509. [DOI] [PubMed] [Google Scholar]
- 12.Szkudelski T, Szkudelska K. Anti-diabetic effects of resveratrol. Ann N Y Acad Sci. 2011;1215:34–9. doi: 10.1111/j.1749-6632.2010.05844.x. doi: 10.1111/j.1749-6632.2010.05844.x. PubMed PMID: 21261639. [DOI] [PubMed] [Google Scholar]
- 13.Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:E1339–46. doi: 10.1152/ajpendo.00487.2005. PubMed PMID: 16434553. [DOI] [PubMed] [Google Scholar]
- 14.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. doi: 10.1016/j.cell.2006.11.013. PubMed PMID: 17112576. [DOI] [PubMed] [Google Scholar]
- 15.Palsamy P, Subramanian S. Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic beta-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J Cell Physiol. 2010;224:423–32. doi: 10.1002/jcp.22138. doi: 10.1002/jcp.22138. PubMed PMID: 20333650. [DOI] [PubMed] [Google Scholar]
- 16.Du X, Ninomiya T, de Galan B, Abadir E, Chalmers J, Pillai A, et al. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: results of the ADVANCE study. Eur Heart J. 2009;30:1128–35. doi: 10.1093/eurheartj/ehp055. doi: 10.1093/eurheartj/ehp055. PubMed PMID: 19282274. [DOI] [PubMed] [Google Scholar]
- 17.Nekooeian AA, Eftekhari MH, Adibi S, Rajaeifard A. Effects of pomegranate seed oil on insulin release in rats with Type 2 diabetes. Iran J Med Sci. 2014;39:130–5. PubMed PMID: 24453394; PubMed Central PMCID: PMC3957012. [PMC free article] [PubMed] [Google Scholar]
- 18.Nekooeian AA, Mashhoodi T. Solid plexiglass clips to induce reproducible renal hypertension in the rat. Indian Journal of Pharmacology. 2007;39:25–6. doi: 10.4103/0253-7613.30758. [Google Scholar]
- 19.Khalili A, Nekooeian AA, Khosravi MB, Fakher S. Simultaneous renal hypertension and type 2 diabetes exacerbate vascular endothelial dysfunction in rats. Int J Exp Pathol. 2012;93:210–7. doi: 10.1111/j.1365-2613.2012.00811.x. doi: 10.1111/j.1365-2613.2012.00811.x. PubMed PMID: 22458508; PubMed Central PMCID: PMC3385919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pari L, Srinivasan S, Saddiq M. Preventive effect of diosmin, a bioflavonoid, on glycoprotein changes in streptozotocin-nicotinamide-induced type 2 diabetic rats. IJPSR. 2010;1:89–95. [Google Scholar]
- 21.Nekooeian AA, Dehghani GA, Mostafavi H, Khalili A. The Effect of Hydroalcoholic Extract of Olive Leaves on Blood Pressure in Rat Model of Two-Kidney, One-Clip Goldblatt Hypertension. Iranian Cardiovascular Research Journal. 2011;5:1–6. [Google Scholar]
- 22.Khalili A, Nekooeian AA, Khosravi MB, Fakher S. Simultaneous renal hypertension and type 2 diabetes exacerbate vascular endothelial dysfunction in rats. Int J Exp Pathol. 2012;93:210–7. doi: 10.1111/j.1365-2613.2012.00811.x. doi: 10.1111/j.1365-2613.2012.00811.x. PubMed PMID: 22458508; PubMed Central PMCID: PMC3385919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soylemez S, Sepici A, Akar F. Resveratrol supplementation gender independently improves endothelial reactivity and suppresses superoxide production in healthy rats. Cardiovasc Drugs Ther. 2009;23:449–58. doi: 10.1007/s10557-009-6198-z. doi: 10.1007/s10557-009-6198-z. PubMed PMID: 19809869. [DOI] [PubMed] [Google Scholar]
- 24.Grujic Milanovic J, Mihailovic-Stanojevic N, Miloradovic Z, Jacevic V, Milosavljevic I, Milanovic S, et al. Resveratrol Reduces Blood Pressure, Changes of Antioxidant Enzyme Activity and Histological Parameters in Experimental Model of Malignant Hypertension. Journal of Hypertension. 2010;28:29–171. doi: 10.1097/01.hjh.0000379709.75759.98. [Google Scholar]
- 25.López-Sepúlveda R, Jiménez R, Romero M, Zarzuelo MJ, Sánchez M, Gómez-Guzmán M, et al. Wine polyphenols improve endothelial function in large vessels of female spontaneously hypertensive rats. Hypertension. 2008;51:1088–95. doi: 10.1161/HYPERTENSIONAHA.107.107672. PubMed PMID: 18259008. [DOI] [PubMed] [Google Scholar]
- 26.Roghani M, Baluchnejadmojarad T. Mechanisms underlying vascular effect of chronic resveratrol in streptozotocin‐diabetic rats. Phytother Res. 2010;24:S148–S54. doi: 10.1002/ptr.3032. doi: 10.1002/ptr.3032. PubMed PMID: 20013818. [DOI] [PubMed] [Google Scholar]
- 27.Wong CM, Yao X, Au CL, Tsang SY, Fung KP, Laher I, et al. Raloxifene prevents endothelial dysfunction in aging ovariectomized female rats. Vascul Pharmacol. 2006;44:290–8. doi: 10.1016/j.vph.2005.12.005. doi: 10.1016/j.vph.2005.12.005. PubMed PMID: 16542882. [DOI] [PubMed] [Google Scholar]
- 28.De Gennaro Colonna V, Rossoni G, Rigamonti AE, Bonomo S, Manfredi B, Berti F, et al. Enalapril and quinapril improve endothelial vasodilator function and aortic eNOS gene expression in L-NAME-treated rats. Eur J Pharmacol. 2002;450:61–6. doi: 10.1016/s0014-2999(02)02046-0. doi: 10.1016/S0014-2999(02)02046-0. PubMed PMID: 12176110. [DOI] [PubMed] [Google Scholar]
- 29.Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, Jankowski M, Martyniec L, Angielski S, et al. Third-generation β-blockers stimulate nitric oxide release from endothelial cells through ATP efflux a novel mechanism for antihypertensive action. Circulation. 2003;107:2747–52. doi: 10.1161/01.CIR.0000066912.58385.DE. PubMed PMID: 12742996. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto T, Ishida K, Nakayama N, Taguchi K, Kobayashi T, Kamata K. Mechanisms underlying the losartan treatment-induced improvement in the endothelial dysfunction seen in mesenteric arteries from type 2 diabetic rats. Pharmacol Res. 2010;62:271–81. doi: 10.1016/j.phrs.2010.03.003. doi: 10 10.1016/j.phrs.2010.03.003. PubMed PMID: 20304070. [DOI] [PubMed] [Google Scholar]
- 31.Ashraf MZ, Hussain ME, Fahim M. Endothelium mediated vasorelaxant response of garlic in isolated rat aorta: role of nitric oxide. J Ethnopharmacol. 2004;90:5–9. doi: 10.1016/j.jep.2003.06.001. doi: 10.1016/j.jep.2003.06.001. PubMed PMID: 14698500. [DOI] [PubMed] [Google Scholar]
- 32.Brixius K, Willms S, Napp A, Tossios P, Ladage D, Bloch W, et al. Crataegus special extract WS® 1442 induces an endothelium-dependent, NO-mediated vasorelaxation via eNOS-phosphorylation at serine 1177. Cardiovasc Drugs Ther. 2006;20:177–84. doi: 10.1007/s10557-006-8723-7. PubMed PMID: 16779533. [DOI] [PubMed] [Google Scholar]
- 33.Rivera L, Morón R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053–63. doi: 10.1016/j.bcp.2008.11.027. PubMed PMID: 19100718. [DOI] [PubMed] [Google Scholar]
- 34.Gordish KL, Beierwaltes WH. Resveratrol induces acute endothelium-dependent renal vasodilation mediated through nitric oxide and reactive oxygen species scavenging. Am J Physiol Renal Physiol. 2014;306:F542–50. doi: 10.1152/ajprenal.00437.2013. doi: 10.1152/ajprenal.00437.2013. PubMed PMID: 24431202; PubMed Central PMCID: PMC3949032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Touyz RM, Tabet F, Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol. 2003;30:860–6. doi: 10.1046/j.1440-1681.2003.03930.x. PubMed PMID: 14678251. [DOI] [PubMed] [Google Scholar]
- 36.Mizutani K, Ikeda K, Yamori Y. Resveratrol inhibits AGEs-induced proliferation and collagen synthesis activity in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Biochemical and biophysical research communications. Biochem Biophys Res Commun. 2000;274:61–7. doi: 10.1006/bbrc.2000.3097. PubMed PMID: 10903896. [DOI] [PubMed] [Google Scholar]
- 37.Liu ZP, Song Y, Liu ZQ, Zhang XP. Preventive effect of trans-resveratrol on hypertension-induced cardiac hypertrophy in partially nephrectomized rats. Wei Sheng Yan Jiu. 2005;34:756–8. PubMed PMID: 16535856. [PubMed] [Google Scholar]
- 38.Robich MP, Osipov RM, Chu LM, Han Y, Feng J, Nezafat R, et al. Resveratrol modifies risk factors for coronary artery disease inswine with metabolic syndrome and myocardial ischemia . European journal of pharmacology. 2011;664:45–53. doi: 10.1016/j.ejphar.2011.04.059. doi: 10.1016/j.ejphar.2011.04.059. PubMed PMID: 21575630; PubMed Central PMCID: PMC3107708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen G, McAlister FA, Walker RL, Hemmelgarn BR, Campbell NR. Cardiovascular Outcomes in Framingham Participants With Diabetes The Importance of Blood Pressure. Hypertension. 2011;57:891–7. doi: 10.1161/HYPERTENSIONAHA.110.162446. doi: 10.1161/HYPERTENSIONAHA.110.162446. PubMed PMID: 21403089; PubMed Central PMCID: PMC3785072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Endemann DH, Pu Q, De Ciuceis C, Savoia C, Virdis A, Neves MF, et al. Persistent remodeling of resistance arteries in type 2 diabetic patients on antihypertensive treatment. Hypertension. 2004;43:399–404. doi: 10.1161/01.HYP.0000112029.03691.e7. doi: 10.1161/01.HYP.0000112029.03691.e7. PubMed PMID: 14707158. [DOI] [PubMed] [Google Scholar]