Abstract
Cigarette smoking is a major risk factor in the development of non-small cell lung cancer (NSCLC), which accounts for 80% of all lung cancers. Nicotine, the major addictive component of tobacco smoke, can induce proliferation, invasion and epithelial-mesenchymal transition (EMT) in NSCLC cell lines and promote metastasis of NSCLC in mice. Here we demonstrate that the scaffolding protein β-arrestin-1 is necessary for nicotine-mediated induction of mesenchymal genes vimentin and fibronectin as well as EMT regulators ZEB1 and ZEB2. Nicotine induced changes in cell morphology and ablate tight junctions consistent with EMT; β-arrestin-1, but not β-arrestin-2, was required for these changes. β-arrestin-1 promoted the expression of the mesenchymal genes as well as ZEB1 and ZEB2 through the mediation of the E2F1 transcription factor; this required Src kinase activity. Stimulation of multiple NSCLC cell lines with nicotine led to enhanced recruitment of β-arrestin-1 and E2F1 on vimentin, fibronectin, ZEB1 and ZEB2 promoters. Further, there was significantly more β-arrestin-1 and E2F1 associated with these promoters in human NSCLC tumors and β-arrestin-1 levels correlated with vimentin and fibronectin levels in human NSCLC samples. A549-luciferase cells lacking β-arrestin-1 showed a significantly reduced capacity for tumor growth and metastasis when orthotopically implanted into the lungs of SCID-beige mice. Taken together, these studies reveal a novel role for β-arrestin-1 in the growth and metastasis of NSCLC.
Keywords: Rb, E2F1, Zeb1, Zeb2, fibronectin, vimentin
Introduction
Cigarette smoking is highly correlated with the development of non-small cell lung cancer (NSCLC), which accounts for 80% of all lung cancers (1). Nicotine, the addictive component of tobacco smoke has been found to induce cell proliferation, angiogenesis and epithelial to mesenchymal transition (EMT) through nicotinic acetylcholine receptors (nAChRs) (2, 3). In addition, nicotine confers resistance to apoptosis induced by chemotherapeutic agents (4). Although nicotine is not known to initiate tumors, it can promote the growth and metastasis of solid tumors in vivo suggesting that nicotine might promote the progression of tumors already initiated by tobacco-specific nitrosamines (5, 6).
The arrestin family consists of four members, including visual arrestins (arrestin1&4) that are expressed exclusively in the retina and ubiquitously expressed non-visual arrestins (β-arrestin-1&2). While it is established that β-arrestins promote internalization and desensitization of GPCRs (G protein-coupled receptors), they also regulate signaling by Notch, endothelin A receptor, frizzled, smoothened and T-cell receptors (7–11). Our earlier studies demonstrated that nicotinic acetylcholine receptor (nAChR) signaling induces nuclear translocation of β-arrestin-1 in a Src dependent manner, where it recruited the histone acetyltransferase p300 to E2F1-regulated proliferative promoters, facilitating their transcription (12).
Nicotine can induce changes in gene expression consistent with EMT, a signature of more advanced and less differentiated cancers. The EMT program is a highly conserved developmental event that promotes epithelial cell dissociation and migration to appropriate sites during embryogenesis (13); it also facilitates the metastasis of tumors (14). During EMT, epithelial markers such as E-cadherin and catenins are downregulated with a concomitant upregulation of mesenchymal genes including vimentin and fibronectin (15). TGF-β is known to induce EMT robustly in many cell types (16). Here we demonstrate that fibronectin and vimentin promoters are E2F1 regulated and the expression of these genes is induced by nicotine in a β-arrestin-1 dependent manner. There was a direct correlation between the levels of these genes and β-arrestin-1 in human NSCLC samples. In addition, β-arrestin-1 regulated the expression of EMT promoting transcription factors such as ZEB1 and ZEB2. β-arrestin-1 depleted cells could not form tumors or metastasize in SCID mice in response to nicotine stimulation. Our results suggest that β-arrestin-1 plays a key and specific role in nicotine-induced EMT and metastasis.
Materials and Methods
Cell Lines and Reagents
NSCLC cell lines A549, H1650, H358, H1975, Calu6, H23 and PC9 were obtained from American Type Culture Collection (ATCC) and were used within 6 months; they have been re-authenticated by STR analysis. A549 NSCLC cells were cultured in F12K medium with 10% serum (Cellgro). H358, H1975, Calu6, H23, PC9 and H1650 human NSCLC cells were grown in RPMI with 10% serum. A549 cells stably expressing the firefly luciferase gene (A549-luc) were obtained from Caliper and grown in RPMI with neomycin (200 ng/ml). shRNA cell lines were made by stably transfecting A549 cells with shRNA constructs that specifically target β-arrestin-1 or a control vector obtained from a shRNAmir library from Open Biosystems, Huntsville, AL. A549 cells transfected with empty vector (called shcontrol) were used as the control. All shRNA cells lines were maintained in media containing 1 μg/ml puromycin. For treatment with nicotine or TGF-β, cells were rendered quiescent by serum starvation for 36 hours, and subsequently stimulated with nicotine (Sigma) at 1 μM or TGF-β at 5ng/ml concentration. TGF-β receptor inhibitor, SB 431542, was purchased from Cayman Chemical and was used at 1 μM. For treatment with Src inhibitors Dasatinib and PP2, cells were serum starved for 36 hours and treated with nicotine 1 μM for 24 hours in the presence of 0. 2μM Dasatinib or 0.5 μM PP2.
Detailed experimental procedures for orthotopic lung tumor model, migration assay, 3-D culture, Immunofluorescence, western blots, ChIP assays and transfections are provided in the supplementary methods section.
Constructs and transfections
Fibronectin (pFN 1.2kb) and Vimentin (VimPro-1.5Kb) promoter luciferase constructs were kindly provided by Dr. Jesse Roman (Emory University) (17)and Dr. C. Gilles (Liege University, Belgium) (18). A549 and H1650 cells were transfected using Fugene HD reagent (Roche) following manufacturer’s instruction. Luciferase assays were done 48 hours post transfection, using Dual Luciferase Assay system (Promega) following the manufacturer’s protocol.
siRNA transfections and Real-time PCR
For siRNA transfections 50, 75 or 100 pmol of siRNAs (Santa Cruz) with Oligofectamine were added to cells. For real-time PCR, total RNA was isolated using RNeasy miniprep kit (QIAGEN) following manufacturer’s protocol, followed by first-strand cDNA synthesis using iScript cDNA synthesis kit (Bio-Rad). Data was analyzed by ΔΔCT method, where gene of interested was normalized to 18s rRNA, then compared to the non-targeting siRNA control sample. Error bars represent the standard deviation of three independent experiments. Details of siRNAs and sequences of primers used for RT-PCR are given in supplemental methods.
Wound healing assay
A549 cells were grown in a 6-well plate (Falcon Becton Dickinson) transfected with siRNAs. These cells were starved in serum free media for 24 hours and then washed with 1x Dulbecco’s Phosphate-buffered saline (MediaTech). The cells were scratched with a sterilized 200 μl pipette tip in three separate places in each well and medium containing 1 μM nicotine or starvation media was added to the wells. After 24 hours, the wounds were observed and images were taken in 20X magnification using Zeiss inverted phase contrast microscope.
Invasion Assays
Boyden Chamber assays were used to assess the invasive ability of cells as described previously (2). The upper surface of the 6.5 mm filters (Corning) were coated with collagen (100 μg/filter) and Matrigel (BD Bioscience) (50 μg/filter). Twenty thousand cells were plated in the upper chamber with 0.1% bovine serum albumin (Sigma). Media containing 20% fetal bovine serum was placed in the lower well as chemoattractant. The cells that invaded through the filters were quantified by counting three fields under 20X objective magnifications.
Statistical Methods and Analysis
A log transformation was performed to make mRNA expression values of four genes (β arrestin1, vimentin (VIM) fibronectin (FN1) and 18SrRNA) approximately normal. Pearson rank correlation (r) was used to assess correlation among these three genes. An exact normal scores test was used to assess the association between mRNA expression values of these genes and pathological stage. The reduced monotonic regression model (19) was used to assess the association between mRNA expression values of these genes and smoking pack years. An optimal cut point for disease-free survival, defined as the time for surgical resection to disease recurrence or death, was tested for using the maximal chi-square test. All statistical analyses were performed using SAS (Version 9.2; SAS Institute; Cary, NC).
Results
β-arrestin-1 is necessary for nicotine induced disruption of tight junctions to facilitate EMT and invasion
We previously observed that nAChR stimulation results in changes reminiscent of EMT (2, 20). To investigate the role of β-arrestin-1 in this process, A549 cells that lack β-arrestin-1 were generated by stably expressing β-arrestin-1 specific shRNA (shβ-arrestin1); cells transfected with empty vector was used as control (shcontrol). These cells were rendered quiescent by serum starvation and stimulated with 1μM nicotine, which is equivalent to the levels observed in the blood stream of heavy smokers. Immunofluorescence experiments revealed membranous staining of tight junction protein ZO-1 in quiescent cells; nicotine treatment reduced ZO1 levels in shcontrol cells, but not in shβ-arrestin1 cells (Fig. 1A, top panels & Supplementary Fig. 1E). Selective knockdown of β-arrestin-1 by shRNA and siRNAs is shown in Supplementary Figure 1A–D. Similar results were observed when β-arrestin-1 was depleted by siRNA in H1650 cells (Fig. 1A, bottom panels & Supplementary Fig. 1F–G) suggesting that β-arrestin-1 is necessary for reducing tight junctions upon nAChR activation. In addition to ZO-1, the levels of E-cadherin, an epithelial marker, were significantly decreased upon nicotine stimulation only in shcontrol cells but not in shβ-arrestin1 cells (Supplementary Fig. 2A&B). Shcontrol cells and shβ-arrestin1 cells were grown as 3-D cultures on collagen in the presence or absence of nicotine; shcontrol cells treated with nicotine acquired a more elongated, migratory morphology, but shβ-arrestin1 cells did not show such changes (Fig. 1B). Shβ-arrestin1 cells were also impaired in their ability to migrate (Supplementary Fig. 2C) or invade in response to nicotine stimulation (Fig. 1C). Depletion of β-arrestin-1 impaired nicotine-mediated invasion of a variety of NSCLC cell lines (Fig.1D), indicating a definite role for β-arrestin-1 in the induction of mesenchymal features in response to nAChR signaling.
Figure 1. β-arrestin-1 is necessary for nicotine induced disruption of tight junctions that correlate with EMT and invasion.
(A) Nicotine downregulated the membrane localization of the tight junction protein ZO-1(green) in shcontrol cells but not in shβ-arrestin1 cells. Nuclei were counterstained with DAPI (blue). Scale bar represents 50μm. SS denotes serum starved cells. (B) Shcontrol cells grown in presence of nicotine acquired more elongated, migratory morphology in 3D cultures while shβ-arrestin1 cells did not. Scale bar: 20 μm. (C) Depletion of β-arrestin-1 abrogates nicotine induced invasive capacity of A549 and H1650 cells (D) and H1975, H23 and H358 cells as seen by Boyden chamber assays. (*=P<0.05).
β-arrestin-1 is required for the expression of vimentin and fibronectin in response to nAChR signaling
We examined whether β-arrestin-1 regulates the expression of the mesenchymal genes fibronectin and vimentin upon nAChR stimulation. Western blotting showed that nicotine treatment induced the expression of fibronectin and vimentin in shcontrol cells, but not in shβ-arrestin1 cells (Fig. 2A); this induction occurred at the transcriptional level (Fig. 2B). In contrast, depletion of β-arrestin-1 did not alter TGF-β mediated vimentin or fibronectin expression, suggesting that β-arrestin-1 plays a role in mediating EMT downstream of nAChRs. Transient transfection assays were then conducted using luciferase reporter constructs driven by human vimentin and fibronectin promoters. Shcontrol and shβ-arrestin1 cells were transfected with the reporters, rendered quiescent by serum starvation and stimulated with nicotine or TGF-β. Both promoters were induced by nicotine in shcontrol cells but not in shβ-arrestin1 cells while TGF-β could induce them in cells (Fig. 2D). Similar pattern of induction of fibronectin and vimentin was observed when H358 cells transfected with either control siRNA or β-arrestin-1 siRNA were stimulated with nicotine or TGF-β (Fig. 2E), showing that β-arrestin-1 plays a role in the nicotine-mediated induction of these genes. Interestingly, β-arrestin-2, a closely related arrestin, did not play a role in nicotine induced invasion or expression of mesenchymal markers (Supplementary Figure 3A–F)
Figure 2. β-arrestin-1 is necessary for nicotine induced upregulation of fibronectin and vimentin expression.
(A) Western blots or RT-PCR (B) showing the induction of vimentin and fibronectin in shcontrol A549 cells, but not in β-arrestin-1 null cells. (C) RT-PCR showing the levels of fibronectin and vimentin upon nicotine and TGF-β stimulation, after transfecting a different siRNA targeting β-arrestin-1 (D) Transient transfection assays in shcontrol and shβ-arrestin1 (#1and #2 are two clones) cells using vimentin and fibronectin promoter luciferase constructs demonstrating the role of β-arrestin-1 in nicotine induced promoter induction. (E) Western blot showing the levels of vimentin and fibronectin after siRNA transfection in H358. (F) Depletion of Src and α7nAChR abrogated nicotine induced vimentin and fibronectin expression as seen by western blot and RT- PCR (G). (H) Treatment of cells with a TGF-β inhibitor prevented the TGF-β-mediated, but not nicotine-mediated, vimentin or fibronectin. (I) Levels of E-cadherin, β-catenin and ZO1 in the membrane fractions of serum starved and nicotine treated shcontrol or shβ-arrestin1 cells. Levels of Na+/K+ ATPase, a marker for membrane fraction, were not downregulated upon nicotine stimulation. (*=p<0.05)
Since nicotine induces cell proliferation by α7nAChR (CHRNA7) mediated activation of Src, experiments were conducted to assess whether they contribute to the induction of these mesenchymal promoters. Western blot analysis (Fig. 2F) and RT-PCR (Fig. 2G) showed that depletion of Src and α7nAChR by siRNAs abrogated induction of vimentin and fibronectin by nicotine, but not TGF-β. Since nicotine is known to induce TGF-β in certain cell lines (21, 22), we examined whether nAChR mediated mesenchymal gene expression is indirectly mediated through the activation of TGF-β pathway. A549 cells were serum starved and treated with nicotine or TGF-β in the presence or absence of TGF-β1 receptor inhibitor SB431542. SB431542 abrogated the induction of vimentin and fibronectin expression by TGF-β but not nicotine (Fig. 2H), suggesting that nicotine mediated induction of these genes is independent of TGF-β. To further establish the role of β-arrestin-1 in nicotine induced EMT, we determined the levels of tight junction protein ZO1 and epithelial markers E-cadherin and β-catenin from membrane fractions of shcontrol and shβ-arrestin1 cells. As seen in Fig. 2I, nicotine treatment decreased the levels of E-cadherin, β-catenin and ZO1 in shcontrol cells, but not in shβ-arrestin1 cells.
Vimentin and Fibronectin promoters are E2F1 and Rb responsive
Recent studies have indicated that E2Fs function in a wide range of biological processes in addition to cell cycle progression (23–26). Since β-arrestin-1 interacts with E2F1 (12), attempts were made to assess whether E2F transcription factors played a role in the induction of fibronectin and vimentin during EMT. An analysis of the promoter regions (1 kb) upstream of transcription start site using MatInspector program (Genomatix) revealed the presence of six potential E2F binding sites on the vimentin promoter (Supplementary Figure 4A) and two sites on the fibronectin promoter (Supplementary Table 1).
Chromatin immunoprecipitation (ChIP) assays conducted on A549 cells detected the binding of E2F1 and Rb to vimentin and fibronectin promoters (Supplementary Fig. 4B); further, transient transfection experiments showed that E2F1 could induceusing vimentin-Luc and fibronectin-Luc reporters, while Rb suppressed this induction (Supplementary Fig. 3C). Similar results were obtained in additional NSCLC cell lines (Supplementary Fig. 4D&E). Although E2Fs 2–5 could also induce these promoters, E2F1 had the maximal effect (Supplementary Fig. 3F). Supporting these results, transfection of 75 pmols of an E2F1 siRNA significantly reduced the levels of endogenous vimentin and fibronectin, (Supplementary Fig. 4G&H).
Transient transfections were conducted using deletion mutants of vimentin promoter to fine map the promoter region required for E2F mediated induction (Supplementary Fig. 5A); it was found that the full length promoter (1.5kb; six E2F binding sites) and the deletion mutant vim-387+24 (Vim-411; three sites) could be transcriptionally induced by E2F1, but the shortest mutant vim-245+24 (Vim-269) was not E2F1 responsive (Supplementary Fig. 5B), suggesting that the three proximal sites were sufficient for E2F mediated transcriptional induction. Similar experiments on the deletion mutants of fibronectin promoter revealed that only a proximal binding site was necessary for induction by E2F1 (Supplementary Fig. 5C&D); this was confirmed by mutating this site (Supplementary Fig. 5E). These experiments demonstrate a direct role for E2F1 in regulating these mesenchymal promoters.
nAChR stimulation leads to recruitment of E2F1 and β-arrestin-1 on fibronectin and vimentin promoters
Since nAChR stimulation induced the nuclear translocation of a subset of β-arrestin-1 (12), ChIP assays were conducted to determine whether it could associate with vimentin and fibronectin promoters. A549 cells were serum starved and stimulated with nicotine; quiescent cells had robust amount of Rb on these promoters. Nicotine stimulation led to an increase in the association of E2F1 and β-arrestin-1 with a concomitant dissociation of Rb from these promoters (Fig. 3A and Supplementary Fig. S6). ChIP assays followed by real time PCR also showed the increased association of E2F1, β-arrestin-1, p300, and Ac-H3 with vimentin and fibronectin promoters in nicotine treated shcontrol cells, but not on shβ-arrestin1 cells. Further, Rb was present on vimentin and fibronectin promoters in shβ-arrestin1 cells even after nicotine stimulation (Fig. 3B). An unrelated promoter, c-Fos, was used as the negative control and there was only minimal association of E2F1, Rb, β-arrestin-1, p300, and Ac-H3 on this promoter (Supplementary Fig. S7); further, the fibronectin and vimentin promoters could not be detected in immunoprecipitations performed with a control irrelevant antibody (IgG), confirming the specificity of the assay. These results suggest that β-arrestin-1 mediated signaling events facilitated the dissociation of Rb and enhanced recruitment of E2F1, p300 and acetylation of histones on vimentin and fibronectin promoters.
Figure 3. Nicotine induces recruitment of E2F1 and β-arrestin-1 on fibronectin and vimentin promoters.
(A) ChIP assays showed enhanced binding of E2F1 and β-arrestin-1 to fibronectin and vimentin promoters, but not the control c-Fos promoter, after nicotine stimulation. (B) ChIP assays performed on quiescent and nicotine stimulated shcontrol as well as shβ-arrestin1 cells demonstrating that β-arrestin-1 is required for the recruitment of p300 and AcH3 to vimentin and fibronectin promoters in response to nicotine. There was no detectable level of amplification from an irrelevant antibody control IgG. (C) Depletion of E2F1 by a siRNA prevents nicotine induced expression of vimentin and fibronectin; a second siRNA is used in (D). (E) Real-time PCR showing the expression of fibronectin and vimentin after E2F1 depletion. (F&G) Transient transfection experiments showing that E2F1 could induce fibronectin and vimentin promoters only in shControl cells, but not Shβ-arrestin1 cells. (H) Depletion of E2F1 ablates nicotine mediated induction, but not TGF-β-mediated induction, of fibronectin (H) and vimentin (I) promoters (**=p<0.005, *=p<0.05).
To examine whether nicotine or TGF-β induced expression of these mesenchymal markers is dependent on E2F1, we transfected A549 cells with control siRNA or two different E2F1 siRNAs, and stimulated with nicotine or TGF-β. Western blotting (Fig. 3C&D) and RT-PCR showed that (Fig. 3E) E2F1 was necessary for nicotine-mediated, but not TGF-β mediated, induction of vimentin and fibronectin. To assess whether β-arrestin-1 is required for the transcriptional induction of these promoters by E2F1, shcontrol and shβ-arrestin1 cells were transfected with vimentin or fibronectin promoter constructs along with E2F1. Interestingly, fibronectin and vimentin promoters were induced by E2F1 in shcontrol cells but not in shβ-arrestin1 cells (Figure 3F&G) suggesting that β-arrestin-1 was necessary for the E2F1-mediated transcription of these genes. Experiments on control siRNA or E2F1 siRNA transfected cells also showed that E2F1 was required for the induction of vimentin and fibronectin promoters upon nicotine stimulation, but not TGF-β stimulation (Figure 3H&I). Collectively these results indicate that E2F1 and β-arrestin-1 are required for the nAChR mediated, but not TGFβ-mediated, expression of fibronectin and vimentin.
Nuclear function of β-arrestin-1 is necessary for induction of EMT
To examine whether nuclear translocation of β-arrestin-1 regulates the expression of mesenchymal genes, we performed transient transfection assays using the β-arrestin-1 mutant Q394L, in which glutamine 394 has been mutated to leucine to create a nuclear export signal (27, 28). The mutant Q394L did not enhance E2F1 mediated transcription from fibronectin and vimentin promoters, suggesting that nuclear translocation of β-arrestin-1 is required for the transcription of these genes (Fig. 4A).
Figure 4. Rescue of β-arrestin-1 function by wild type β-arrestin-1 construct.
(A) Transient transfection assays demonstrating that Q394L β-arrestin-1 mutant does not induce vimentin or fibronectin promoters. 3μg of wild type or mutant β-arrestin-1 along with 0.5 μg of promoter construct and 0.5 μg of E2F1 was used in transfections. (B) RT-PCR showing the rescue of nicotine induced expression of vimentin and fibronectin in shβ-arrestin1 cells transfected with wild type rat β-arrestin-1 construct (βarr-RFP); Q394L-βarr-Q394L could not rescue the nicotine response. (*=P 0.05). (C) Transfection of WT-β-arrestin-1, but not Q394L-β-arrestin-1, could facilitate nicotine induced migration of shβ-arrestin1 cells in wound healing assays. (D) Similar results were observed on invasion (*=p<0.05). (E) Dominant-negative Src inhibits the transcription from vimentin and fibronectin promoters in A549 and H1650 cells; Dasatinib and PP2 exerted similar effects, as seen in transient transfections (F). (G&H) ChIP assays followed by qRT-PCR on A549 cells show that nicotine treatment enhanced the recruitment of E2F1, β-arrestin-1 and p300 with a concomitant release of Rb; these changes were abrogated by Src inhibitors. There was no detectable level of amplification from an irrelevant antibody control IgG.
To further explore the requirement of the nuclear function of β-arrestin-1 in inducing EMT, shβ-arrestin1 cells were transfected with pcDNA3, WT-β-arrestin1-RFP or Q394L-β-arrestin-1 constructs. Shcontrol cells transfected with pcDNA3 were used as the control. As shown in Fig. 4B, nicotine-induced expression of fibronectin and vimentin in Shβ-arrestin1 cells was rescued by the WT-β-arrestin-1-RFP construct but not the Q394L-β-arrestin-1 construct. Further, WT-β-arrestin-1, but not Q394L-β-arrestin-1 could rescue the nicotine induced migration and invasion (Fig. 4C&D) of shβ-arrestin1 cells, supporting the contention that β-arrestin-1 facilitates the transcriptional induction of mesenchymal genes upon nicotine stimulation. The level of β-arrestin-1 after rescue was examined by RT-PCR and western blots (Supplementary Fig. S8A–C).
We next examined if nuclear functions of β-arrestin-1 require Src activity. A549 and H1650 cells were transfected with vimentin and fibronectin promoters along with a dominant negative or constitutively active Src expression vector. Luciferase assays revealed that dominant negative Src inhibited transactivation of these promoters (Figure 4E) by E2F1. In addition, Src inhibitors Dasatinib and PP2 could strongly inhibit E2F1 and β-arrestin-1 mediated transcription of fibronectin and vimentin reporters (Figure 4F), suggesting that Src was necessary for the nuclear functions of β-arrestin-1. In addition, we performed ChIP assays on A549 cells treated with nicotine in the presence or absence of Dasatinib and PP2. Nicotine treatment enhanced recruitment of E2F1, β-arrestin-1 and p300 on these promoters with concomitant release of Rb; these effects were abrogated in cells treated with the two Src inhibitors (Figure 4G&H). Though less specific than Dasatinib, treatment with both the agents led to a near complete dissociation of Rb, E2F1 β-arrestin-1 and p300 from the vimentin promoter; a minimal amount of Rb, comparable to that present in serum-starved cells, was retained on the FN promoter. There was no detectable amplification of the promoters in a control IP conducted with normal IgG, and these proteins were not associated with the unrelated c-Fos promoter. These results indicate that Src mediated signaling events facilitate the nicotine-mediated induction of these promoters, by targeting Rb, E2F1 and β-arrestin-1.
E2F1 - β-arrestin-1 interaction is necessary for the expression of mesenchymal genes
Previous studies from our lab had shown that amino acids 1-163 of β-arrestin-1 were required for its binding to E2F1 (Supplementary data in (12)). We conducted IP-Western blot assays to determine if delivery of the 1-163 fragment of β-arrestin-1 disrupts the binding of endogenous E2F1 to β-arrestin-1. A549 cells were transfected with either the control vector (pcDNA3) or two levels of pcDNA3-β-arrestin-1 1-163 construct (4μg or 8 μg). Immunoprecipitation-western blots showed that the binding of endogenous E2F1 and β-arrestin-1 was disrupted by β-arrestin-1 1-163 construct in a dose dependent manner (Figure 5A). Similar results were obtained when we overexpressed E2F1 and WT-β-arrestin-1 along with two different levels of β-arrestin-1 1-163 fragment (Figure 5B).
Figure 5. E2F1-βarrestin-1 interaction is necessary for the expression of EMT regulating genes.
(A) β-arrestin-1 fragment 1-163 can prevent the interaction of endogenous E2F1 with β-arrestin-1, as seen by an IP-western blot experiment. (B) IP-western blots showing the inhibition of E2F1-β-arrestin-1 interaction by β-arrestin-1 fragment 1-163. A549 cells were transfected with pCDNA3-E2F1 and β-arrestin-1 along with β-arrestin-1 fragment 1-163. (C) Cotransfection of β-arrestin-1 fragment 1-163 abrogates induction of vimentin and fibronectin promoters by E2F1, suggesting that binding of E2F1 to β-arrestin-1 is necessary for E2F1 mediated induction of vimentin and fibronectin. (D) Transfection of β-arrestin-1 1-163 fragment inhibits nicotine mediated induction of fibronectin and vimentin as shown by RT-PCR. (E) A549 cells transfected with β-arrestin-1 1-163 fragment did not invade or migrate (F) in presence of nicotine.
We next examined whether disrupting the binding of β-arrestin-1 prevented the E2F-1 mediated induction of vimentin and fibronectin. A549 cells were transfected with the promoter luciferase constructs along with E2F1 and β-arrestin-1 alone or with different levels of β-arrestin-1 1-163; co-transfection of β-arrestin-1 1-163 abrogated promoter induction by E2F1 (Figure 5C), suggesting that binding of β-arrestin-1 is necessary for E2F1 mediated induction of vimentin and fibronectin.
We performed functional assays to explore whether binding of β-arrestin-1 to E2F1 is required for nicotine induced EMT. A549 cells were transfected with either pcDNA3 or β-arrestin-1 1-163 fragment, serum starved for 24 hours and subsequently induced with 1 μM nicotine. As seen in Figure 5D, β-arrestin-1 1-163 fragment could effectively inhibit nicotine mediated expression of vimentin and fibronectin, as well as nicotine mediated invasion (Figure 5E) and migration (Figure 5F) of cells. In conclusion, disruption of E2F1- β-arrestin-1 binding by β-arrestin-1 1-163 fragment could significantly prevent nicotine mediated mesenchymal gene expression, invasion and migration of cells.
β-arrestin-1 mediates the induction of EMT promoting transcription factors
Attempts were made to analyze the global association of β-arrestin-1 with promoters upon nicotine stimulation by ChIP-sequencing. It was found that β-arrestin-1 is recruited on the promoters of many genes that regulate EMT such as ZEB2 as well as other regulatory pathways (Supplementary Table 2; GEO accession number GSE40689). Since ZEB1 and ZEB2 are involved in the repression of epithelial genes during EMT, it was examined whether β-arrestin-1 contributes to the expression of these factors. Real-time PCR experiments showed that ZEB1 and ZEB2 are upregulated by nAChR stimulation and β-arrestin-1 was necessary for this (Supplementary Figure 9A–F).
Since β-arrestin-1 does not possess a DNA binding domain, we hypothesized that β-arrestin-1 associates with these promoters probably through E2F1. It was found that ZEB1 had six and ZEB2 had two E2F binding sites (Supplementary Table 1) in the 1000bp promoter region upstream of TSS (Supplementary Figure 10A). ChIP assays revealed the association of β-arrestin-1 and E2F1 on these promoters, along with dissociation of Rb upon nAChR stimulation (Supplementary Figure 10B). Transient transfection assays demonstrated that these promoters are indeed E2F1 responsive (Supplementary Figure 10C).
Role of β-arrestin-1 in lung cancer metastasis
Given the above results, we examined whether β-arrestin-1 was necessary for nicotine-mediated growth and metastasis of tumors in mice. Shβ-arrestin1 cells showed markedly reduced ability to form tumors compared to shcontrol cells when implanted subcutaneously into athymic nude mice (Fig.6A). The potential role of β-arrestin-1 in nicotine-mediated induction of metastasis was next examined. A549 cells stably expressing luciferase gene (A549-luc) along with β-arrestin-1 specific shRNA (shβarr1-luc) or A549-luc cells stably transfected with an empty vector as control (shcontrol-luc) were orthotopically implanted into the left lung of SCID-Beige mice (12/group). Mice were randomized into two groups and administered nicotine (n=6) or vehicle (n=6) every other day by intraperitoneal injection for seven weeks and tumor growth monitored weekly using the IVIS-Caliper 200 system. Mice implanted with shcontrol-luc cells that received nicotine had significantly larger tumors compared to those received vehicle (Fig. 6B&E); shcontrol-luc tumors displayed metastases to brain, adrenal glands and liver (Fig. 6D) and nicotine treatment further enhanced metastases. Mice implanted with shβarr1-luc had significantly smaller tumors and nicotine treatment did not increase tumor growth or metastasis; ex vivo imaging of organs at the termination of the experiment confirmed these results (Fig. 6C).
Figure 6. Role of β-arrestin-1 in nicotine induced metastasis.
(A) Shβ-arrestin-1 cells showed significantly lower tumor growth in a subcutaneous xenograft model. (B) Tumor growth of orthotopically implanted cells, as seen by weekly bioluminescence imaging for 7 weeks. Mice implanted with shβarr1-luc had significantly smaller tumors and nicotine did not increase tumor growth or metastasis; mice implanted with shcontrol-luc cells displayed metastases to brain, adrenal glands and liver. (C) Luminescence from lung after ex vivo imaging. (***=p<0.0005, **=p<0.005, *=p<0.05) (D) Luminescence from liver, brain, adrenal and liver after imaging in vivo. (E) Representative images of mice from each group in the orthotopic lung experiment. (F) Lung tissues from mice implanted with β-arrestin-1depleted cells show reduced levels of vimentin and fibronectin, as seen by IHC. Scale bar: 200 μm (G) Western blots showing the levels of β-arrestin-1 in shcontrol-luc and shβarr1-luc cells.
Vimentin and fibronectin expression was significantly higher in tumors from nicotine treated mice compared to vehicle treated mice implanted with shcontrol cells; tumors from mice implanted with shβ-arrestin-1 cells displayed minimal staining for vimentin and fibronectin (Fig. 6F). The levels of β-arrestin-1 depletion in the stable β-arrestin-1 knockdown cells are shown in Fig. 6G. Taken together, these results confirm that β-arrestin-1 is indispensable for the growth and metastasis of lung tumors, especially in the context of nicotine exposure.
β-arrestin-1 expression levels correlate with fibronectin and vimentin levels in lung tumor tissues from patients
We next examined whether the expression of β-arrestin-1 in human lung tumor samples correlates with levels of vimentin and fibronectin by conducting quantitative RT-PCR on 116 patient samples. As shown in Fig. 7A, B and Supplementary table 3, β-arrestin-1 expression showed a very strong positive correlation with vimentin (r=0.59, p<0.0001) and fibronectin (r=0.52; p<0.0001), which are known to be overexpressed in more aggressive tumors (29, 30). No significant association was observed between these gene expression patterns and pathological stages or smoking history (pack years) and disease-free survival (data not shown); suggesting that β-arrestin-1 might be contributing to the growth and metastasis of lung cancer in both smokers and non-smokers.
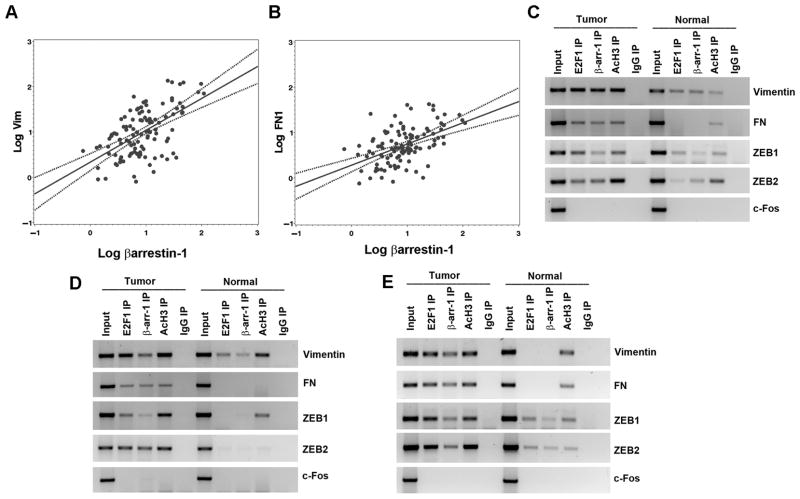
Figure 7. β-arrestin-1 expression correlates with vimentin and fibronectin expression.
(A and B) Scatter plots showing the correlation of β-arrestin-1 expression with the expression of vimentin and fibronectin from lung tumors of 116 patients. (C–E) ChIP assay performed on NSCLC tumors showed enhanced association of E2F1 and β-arrestin-1 on vimentin, fibronectin, ZEB1 and ZEB2 promoters compared to normal tissues.
ChIP assays were conducted to examine whether β-arrestin-1 is recruited to the promoters of vimentin, fibronectin, ZEB1 and ZEB2 in human lung tumors. There was enhanced association of E2F1 and β-arrestin-1 and elevated levels of acetylated histone H3 on vimentin, fibronectin, ZEB1 and ZEB2 promoters in tumors compared to normal lung tissues in three sets of tumor samples (Figure 7C–E). Taken together, these results indicate that β-arrestin-1 might have contributed to the growth and progression of NSCLCs by regulating the expression of E2F-regulated mesenchymal genes and EMT transcription factors.
Discussion
β-arrestins function as scaffold proteins that recruit a broad spectrum of signaling molecules to membrane bound receptors (11, 31). In addition to their established roles in promoting internalization and desensitization of GPCRs, recent studies have implicated involvement of β-arrestins in Notch, frizzled, Wnt/β-catenin, nAChR signaling and regulation of gene expression by facilitating histone acetylation (32, 33). Accumulating evidences indicate a functional role for β-arrestin-1 as a mediator of cellular migration, invasion and metastatic progression of colorectal, ovarian and breast cancer (34–39). In this study, we demonstrate that β-arrestin-1, but not β-arrestin-2, plays a major role in nicotine induced EMT and metastasis, thus contributing to invasive properties of nicotine. The elevated levels of β-arrestin-1, vimentin and FN in tumors from both smokers and non-smokers demonstrate that the molecules contribute to the growth and progression of NSCLCs; at the same time, the induction of these genes could be an impoartant mechanism by which nicotine exerts its tumor promoting functions. Interestingly, β-arrestin-1 was necessary for the induction of mesenchymal promoters upon nAChR stimulation, it did not play a role in TGFβ-mediated induction of these genes. The induction of these genes by β-arrestin-1 and E2F1 in response to nAChR signaling required Src activity; at the same time, TGF-β induces these mesenchymal genes through SMAD proteins suggesting a unique role for β-arrestin-1 in mediating signals downstream of nAChRs (40, 41).
In addition, the present study demonstrates the critical role of E2F1 in regulating genes involved in EMT, including ZEB1 and ZEB2. ZEB1 suppresses the expression of basement membrane components, cell polarity factors and epithelial genes including E-cadherin (42–44), promoting tumor invasion and metastasis (45, 46). ZEB2 collaborates with the TGF-β signaling pathway by interacting with SMAD factors, and induces tumor cell invasion (47). A recent analysis of gene expression database of NSCLC cell lines identified a mesenchymal gene pattern (low E-cadherin, high vimentin) significantly associated with ZEB1 and ZEB2 expression but not with snail, slug, twist1 or twist2 (48). However, correlations between these genes with proliferative signaling cascades have not been elucidated. Our studies show that mitogenic signaling through the nAChRs can also activate components of invasive and metastatic phenotype of cancer.
The Rb-E2F transcriptional regulatory pathway plays a major role in cell cycle regulation, but its role in other aspects of tumor progression, invasion and metastasis is relatively less explored. E2F1 has been shown to induce VEGF receptors and MMPs, indicating the importance of Rb-E2F pathway in promoting tumor angiogenesis and metastasis (23, 24). Recent studies demonstrated that Rb depletion results in disruption of cell-cell adhesion and downregulation of E-cadherin (49). Additionally, other EMT-related transcription factors including slug and ZEB1 are also induced by Rb depletion suggesting that inhibition of EMT is a novel tumor suppressor function of Rb (50). Our finding that Rb-E2F pathway is involved in the regulation of mesenchymal proteins and EMT-related transcription factors provides a molecular mechanism by which Rb and E2F1 facilitate tumor progression. This also raises the possibility that signaling events that inactivate Rb and promote cell proliferation might also promote EMT and metastasis upon the acquisition of additional mutational events or signaling cues, suggesting an interrelated circuitry of signaling and transcriptional events that promote the initiation as well as progression of cancers.
Supplementary Material
Acknowledgments
We thank Michael Damit and Jonathan Nguyen for technical assistance. These studies were supported by the grant CA127725 from the NCI. Assistance of the Core Facilities at Moffitt Cancer Center is gratefully acknowledged. We thank the Lung Cancer SPORE at Moffitt for continued support.
References
- 1.US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress: a Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2.Dasgupta P, Rizwani W, Pillai S, Kinkade R, Kovacs M, Rastogi S, et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124:36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, et al. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116:2208–17. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasgupta P, Kinkade R, Joshi B, Decook C, Haura E, Chellappan S. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci U S A. 2006;103:6332–7. doi: 10.1073/pnas.0509313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis R, Rizwani W, Banerjee S, Kovacs M, Haura E, Coppola D, et al. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer. PLoS One. 2009;4:e7524. doi: 10.1371/journal.pone.0007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paleari L, Catassi A, Ciarlo M, Cavalieri Z, Bruzzo C, Servent D, et al. Role of alpha7-nicotinic acetylcholine receptor in human non-small cell lung cancer proliferation. Cell Prolif. 2008;41:936–59. doi: 10.1111/j.1365-2184.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Fernandez-Arenas E, Calleja E, Martinez-Martin N, Gharbi SI, Navajas R, Garcia-Medel N, et al. beta-arrestin-1 mediates the TCR-triggered re-routing of distal receptors to the immunological synapse by a PKC-mediated mechanism. EMBO J. 2014 doi: 10.1002/embj.201386022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGovern KW, Defea KA. Molecular mechanisms underlying Beta-arrestin-dependent chemotaxis and actin-cytoskeletal reorganization. Handbook of experimental pharmacology. 2014;219:341–59. doi: 10.1007/978-3-642-41199-1_17. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee A, Veraksa A, Bauer A, Rosse C, Camonis J, Artavanis-Tsakonas S. Regulation of Notch signalling by non-visual beta-arrestin. Nat Cell Biol. 2005;7:1191–201. doi: 10.1038/ncb1327. [DOI] [PubMed] [Google Scholar]
- 10.Schulte G, Shenoy SK. beta-Arrestin and dishevelled coordinate biased signaling. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19839–40. doi: 10.1073/pnas.1117444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shenoy SK. Arrestin interaction with e3 ubiquitin ligases and deubiquitinases: functional and therapeutic implications. Handbook of experimental pharmacology. 2014;219:187–203. doi: 10.1007/978-3-642-41199-1_10. [DOI] [PubMed] [Google Scholar]
- 12.Dasgupta P, Rizwani W, Pillai S, Davis R, Banerjee S, Hug K, et al. ARRB1-mediated regulation of E2F target genes in nicotine-induced growth of lung tumors. Journal of the National Cancer Institute. 2011;103:317–33. doi: 10.1093/jnci/djq541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. The Journal of clinical investigation. 2009;119:1417–9. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saitoh M, Miyazawa K. Transcriptional and post-transcriptional regulation in TGF-beta-mediated epithelial-mesenchymal transition. J Biochem. 2012;151:563–71. doi: 10.1093/jb/mvs040. [DOI] [PubMed] [Google Scholar]
- 15.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michaelson JE, Ritzenthaler JD, Roman J. Regulation of serum-induced fibronectin expression by protein kinases, cytoskeletal integrity, and CREB. Am J Physiol Lung Cell Mol Physiol. 2002;282:L291–301. doi: 10.1152/ajplung.00445.2000. [DOI] [PubMed] [Google Scholar]
- 18.Gilles C, Polette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P, et al. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer research. 2003;63:2658–64. [PubMed] [Google Scholar]
- 19.Schell MaSB. The reduced monotonic regression method. The Journal of American Statistical Association. 1997;92:128–35. [Google Scholar]
- 20.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cucina A, Corvino V, Sapienza P, Borrelli V, Lucarelli M, Scarpa S, et al. Nicotine regulates basic fibroblastic growth factor and transforming growth factor beta1 production in endothelial cells. Biochem Biophys Res Commun. 1999;257:306–12. doi: 10.1006/bbrc.1999.0478. [DOI] [PubMed] [Google Scholar]
- 22.Cucina A, Sapienza P, Corvino V, Borrelli V, Mariani V, Randone B, et al. Nicotine-induced smooth muscle cell proliferation is mediated through bFGF and TGF-beta 1. Surgery. 2000;127:316–22. doi: 10.1067/msy.2000.104249. [DOI] [PubMed] [Google Scholar]
- 23.Pillai S, Kovacs M, Chellappan S. Regulation of vascular endothelial growth factor receptors by Rb and E2F1: role of acetylation. Cancer research. 2010;70:4931–40. doi: 10.1158/0008-5472.CAN-10-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson JL, Pillai S, Pernazza D, Sebti SM, Lawrence NJ, Chellappan SP. Regulation of matrix metalloproteinase genes by E2F transcription factors: Rb-Raf-1 interaction as a novel target for metastatic disease. Cancer research. 2012;72:516–26. doi: 10.1158/0008-5472.CAN-11-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelmann D, Putzer BM. The dark side of E2F1: in transit beyond apoptosis. Cancer research. 2012;72:571–5. doi: 10.1158/0008-5472.CAN-11-2575. [DOI] [PubMed] [Google Scholar]
- 26.Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nature reviews Cancer. 2009;9:785–97. doi: 10.1038/nrc2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott MG, Le Rouzic E, Perianin A, Pierotti V, Enslen H, Benichou S, et al. Differential nucleocytoplasmic shuttling of beta-arrestins. Characterization of a leucine-rich nuclear export signal in beta-arrestin2. J Biol Chem. 2002;277:37693–701. doi: 10.1074/jbc.M207552200. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Gao H, Ni Y, Wang B, Wu Y, Ji L, et al. Beta-arrestin 2 functions as a G-protein-coupled receptor-activated regulator of oncoprotein Mdm2. J Biol Chem. 2003;278:6363–70. doi: 10.1074/jbc.M210350200. [DOI] [PubMed] [Google Scholar]
- 29.Dauphin M, Barbe C, Lemaire S, Nawrocki-Raby B, Lagonotte E, Delepine G, et al. Vimentin expression predicts the occurrence of metastases in non small cell lung carcinomas. Lung cancer. 2013;81:117–22. doi: 10.1016/j.lungcan.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Labat-Robert J. Fibronectin in malignancy. Seminars in cancer biology. 2002;12:187–95. doi: 10.1016/S1044-579X(02)00022-6. [DOI] [PubMed] [Google Scholar]
- 31.Kommaddi RP, Shenoy SK. Arrestins and protein ubiquitination. Prog Mol Biol Transl Sci. 2013;118:175–204. doi: 10.1016/B978-0-12-394440-5.00007-3. [DOI] [PubMed] [Google Scholar]
- 32.Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, et al. A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell. 2005;123:833–47. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Ma L, Pei G. Beta-arrestin signaling and regulation of transcription. J Cell Sci. 2007;120:213–8. doi: 10.1242/jcs.03338. [DOI] [PubMed] [Google Scholar]
- 34.Decaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. The Journal of biological chemistry. 2011;286:32188–97. doi: 10.1074/jbc.M111.277038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchanan FG, Gorden DL, Matta P, Shi Q, Matrisian LM, DuBois RN. Role of beta-arrestin 1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci U S A. 2006;103:1492–7. doi: 10.1073/pnas.0510562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li TT, Alemayehu M, Aziziyeh AI, Pape C, Pampillo M, Postovit LM, et al. Beta-arrestin/Ral signaling regulates lysophosphatidic acid-mediated migration and invasion of human breast tumor cells. Mol Cancer Res. 2009;7:1064–77. doi: 10.1158/1541-7786.MCR-08-0578. [DOI] [PubMed] [Google Scholar]
- 37.Min J, Defea K. beta-arrestin-dependent actin reorganization: bringing the right players together at the leading edge. Molecular pharmacology. 2011;80:760–8. doi: 10.1124/mol.111.072470. [DOI] [PubMed] [Google Scholar]
- 38.Rosano L, Cianfrocca R, Tocci P, Spinella F, Di Castro V, Spadaro F, et al. beta-arrestin-1 is a nuclear transcriptional regulator of endothelin-1-induced beta-catenin signaling. Oncogene. 2013;32:5066–77. doi: 10.1038/onc.2012.527. [DOI] [PubMed] [Google Scholar]
- 39.DeFea KA. Arrestins in actin reorganization and cell migration. Prog Mol Biol Transl Sci. 2013;118:205–22. doi: 10.1016/B978-0-12-394440-5.00008-5. [DOI] [PubMed] [Google Scholar]
- 40.Piek E, Moustakas A, Kurisaki A, Heldin CH, ten Dijke P. TGF-(beta) type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci. 1999;112 (Pt 24):4557–68. doi: 10.1242/jcs.112.24.4557. [DOI] [PubMed] [Google Scholar]
- 41.Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Molecular biology of the cell. 2005;16:1987–2002. doi: 10.1091/mbc.E04-08-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66:773–87. doi: 10.1007/s00018-008-8465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop--a motor of cellular plasticity in development and cancer? EMBO reports. 2010;11:670–7. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez-Tillo E, de Barrios O, Siles L, Amendola PG, Darling DS, Cuatrecasas M, et al. ZEB1 Promotes invasiveness of colorectal carcinoma cells through the opposing regulation of uPA and PAI-1. Clin Cancer Res. 2013;19:1071–82. doi: 10.1158/1078-0432.CCR-12-2675. [DOI] [PubMed] [Google Scholar]
- 45.Argast GM, Krueger JS, Thomson S, Sujka-Kwok I, Carey K, Silva S, et al. Inducible expression of TGFbeta, snail and Zeb1 recapitulates EMT in vitro and in vivo in a NSCLC model. Clinical & experimental metastasis. 2011;28:593–614. doi: 10.1007/s10585-011-9394-8. [DOI] [PubMed] [Google Scholar]
- 46.Takeyama Y, Sato M, Horio M, Hase T, Yoshida K, Yokoyama T, et al. Knockdown of ZEB1, a master epithelial-to-mesenchymal transition (EMT) gene, suppresses anchorage-independent cell growth of lung cancer cells. Cancer letters. 2010;296:216–24. doi: 10.1016/j.canlet.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miura N, Yano T, Shoji F, Kawano D, Takenaka T, Ito K, et al. Clinicopathological significance of Sip1-associated epithelial mesenchymal transition in non-small cell lung cancer progression. Anticancer Res. 2009;29:4099–106. [PubMed] [Google Scholar]
- 48.Gemmill RM, Roche J, Potiron VA, Nasarre P, Mitas M, Coldren CD, et al. ZEB1-responsive genes in non-small cell lung cancer. Cancer letters. 2011;300:66–78. doi: 10.1016/j.canlet.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arima Y, Hayashi H, Sasaki M, Hosonaga M, Goto TM, Chiyoda T, et al. Induction of ZEB proteins by inactivation of RB protein is key determinant of mesenchymal phenotype of breast cancer. The Journal of biological chemistry. 2012;287:7896–906. doi: 10.1074/jbc.M111.313759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arima Y, Inoue Y, Shibata T, Hayashi H, Nagano O, Saya H, et al. Rb depletion results in deregulation of E-cadherin and induction of cellular phenotypic changes that are characteristic of the epithelial-to-mesenchymal transition. Cancer Res. 2008;68:5104–12. doi: 10.1158/0008-5472.CAN-07-5680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.