Abstract
Short-term starvation or fasting can augment cancer treatment efficacy and can be effective in delaying cancer progression in the absence of chemotherapy, but the underlying molecular mechanisms of action remain elusive. Here we describe the role of REV1, a specialized DNA polymerase involved in DNA repair, as an important signaling node linking nutrient sensing and metabolic control to cell fate. We show that REV1 is a novel binding partner of the tumor suppressor p53 and regulates its activity. Under starvation, REV1 is modified by SUMO2/3, resulting in the relief of REV1’s inhibition of p53 and enhancing p53’s effects on pro-apoptotic genes expression and apoptosis in breast cancer and melanoma cells. Thus, fasting, through its effect on REV1, is a promising non-toxic strategy to increase p53-dependent cell death and to enhance the efficacy of cancer therapies.
Keywords: starvation, STS, REV1, p53, sumoylation
INTRODUCTION
Nutrient-sensing signaling pathways play a pivotal role in regulating cellular protection and lifespan in a wide range of organisms (1). A chronic 20~30% reduction in calorie intake, or calorie restriction, has been a well-studied strategy for increasing survival and preventing carcinogenesis in mammals. However, its application in the treatment of existing tumors has been hindered by a lack of consistent and potent effects in animal studies and the incompatibility of the resulting chronic reduction in weight with cancer treatment in humans. We have recently shown that short-term starvation (STS) or prolonged fasting (PF) is an effective intervention enhancing the treatment of a wide variety of tumors in mice but possibly also protecting human against chemotherapy toxicity (1–4).
REV1 was originally characterized as a member of specialized Y-family DNA polymerases, which exhibit low replication fidelity that can overcome lesion-induced DNA replication arrest, a process known as translesion synthesis (TLS) (5). REV1 has been shown to contribute to genomic instability during yeast aging and under genotoxic stress (6), and to be partly responsible for carcinogen-induced mutagenesis, tumor formation and chemoresistance in mammals (7,8). Our previous findings point to the pivotal role of REV1 in promoting point mutations while preventing gross chromosomal rearrangements and cell death under genotoxic stress (6). Interestingly, recent studies have indicated that REV1’s polymerase activity may not be required for TLS, and that REV1 may play important roles in genome maintenance through its non-catalytic functions (9). REV1 protein can interact with multiple specialized DNA polymerases, such as Polκ, Polι, Polη, and Polζ, as well as with the critical regulators of DNA replication and repair processes, such as proliferating cell nuclear antigen (PCNA) (10,11).
The tumor suppressor p53 plays a key role in multiple signaling pathways in response to genotoxic and cellular stresses (12). In response to a broad array of stimuli, p53 is activated and regulates the expression of hundreds of target genes involved in cellular processes including cell growth arrest, DNA repair or apoptotic cell death. The p53-dependent responses are regulated by p53 abundance, cellular localization and activity, all of which are affected by p53’s post-translational modifications and its interactions with various binding partners, such as BRCA1, ATM, NFκB, MDM2, PML, and p300/CBP (12). In fact, the development of small molecule activators of the p53 network holds the promise to advance current cancer therapy.
Post-translational modification by the small ubiquitin-like modifier (SUMO) has emerged as an essential regulatory mechanism for diverse cellular processes (13,14). SUMOylation regulates the subcellular localization, activity, interaction, and stability of target proteins. Mammalian cells express three major SUMO isoforms: SUMO1 and the highly-related SUMO2 and SUMO3 (SUMO2/3). These small proteins are covalently conjugated to lysine residues of target proteins via an enzymatic cascade involving E1 (activating), E2 (conjugating), and E3 (ligating) enzymes and regulate the activity, interaction, and stability of target proteins. Interestingly, SUMOylation plays important roles in DNA repair and genomic maintenance, and the accumulated data supports that this modification is directly involved in modulating tumor cell sensitivity to the chemotherapeutic agents (15).
In this study, we show that REV1 interacts with p53 and regulates its stability and transactivation. Under starvation conditions, REV1 is rapidly modified by SUMO2/3, resulting in increased REV1 stability with a consequent relief of its inhibition of p53 activation. Starvation simultaneously causes p53 phosphorylation/acetylation and its activation, leading to the death of cancer cells. Thus, our data support an important new role for REV1 as a regulator of p53-dependent death of cancer cells in response to starvation conditions.
MATERIALS AND METHODS
Cell culture and transfection
MCF7 human breast carcinoma cells, B16 mouse melanoma cells, human embryonic kidney (HEK) 293 cells, and p53-null mouse embryonic fibroblasts (MEFs) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) and 100 units/mL penicillin plus 100 μg/mL streptomycin (Invitrogen). p53-null MEFs were obtained from Dr. Shengkan Jin at University of Medicine and Dentistry of New Jersey. Non-small cell lung cancer H1299 cells were obtained from Dr. Deborah Johnson at University of Southern California and cultured in RPMI-1640 medium with 10% FBS. Cells were all maintained in a humidified incubator at 37°C under 5% CO2. Transient transfection was carried out using the X-tremeGENE HP Transfection Reagent (Roche Applied Science) according to the manufacturer’s instructions. For RNAi experiments, SMART Pool siRNAs (Dharmacon) against mouse or human REV1 (M-041898-01 and M-008234-01) or non-targeting siRNA (D-001210-01) were transfected into the cells using Lipofectamine RNAiMAX reagent (Invitrogen), according to the manufacturer’s protocols.
Plasmid expressing Myc-REV1 (11) was gifted from Dr. Errol C. Friedberg (University of Texas Southwestern Medical Center, USA). Expression vectors for Flag-p53 (16) (10838), HA-SUMO2 (17) (17360), HA-SUMO3 (17) (17361), HA-Ubc9 (18) (14438), Flag-PIASy (19) (15208), Flag-SENP1 (20) (17357), Flag-SENP6 (14) (18065), pG13-luc (21) (16442) and p21/WAF1-luc (7) (16451) have been described previously and were obtained from Addgene. The full length cDNA of REV7 was cloned into pcDNA3-HA (Invitrogen) using standard techniques. A series of deletion constructs of Myc-REV1 were generated by ExoIII-S1 nuclease digestion (Promega). Mutations of lysine residues to arginine were generated by PCR-based site-directed mutagenesis using the Quik Change II XL Site-Directed Mutagenesis Kit (Stratagene). All mutants were verified by DNA sequencing.
LDH cytotoxicity assay
At 24 h following siRNA introduction, MCF7 cells were incubated in normal or starvation media for additional 24 h. Chemotherapeutic agent was treated the following day and cell cytotoxicity was measured 24 h later by LDH release assay (Promega).
Luciferase assays
Cells were transfected with the reporter plasmids with the indicated expression constructs using X-tremeGENE HP Transfection Reagent following the protocol provided by Roche Applied Science. At the indicated time, cells were harvested and assayed for luciferase activity using the dual-luciferase reporter assay system according to the manufacturer’s instructions (Promega).
Immunoprecipitation and immunoblot analysis
Immunoprecipitation and immunoblot analyses were performed as previously described (22), with minor modifications. For co-immunoprecipitation assay, mouse tissues or cells were lysed on ice in a modified RIPA buffer containing 50 mM HEPES at pH 7.4, 150 mM NaCl, 1% NP 40, 1 mM EDTA, 1X phosphatase and phosphatase inhibitor cocktail (Pierce), and the whole lysates were clarified by centrifugation. The supernatants were incubated overnight at 4°C with 2 μg of indicated antibody; and the protein A/G plus agarose beads (Pierce) were then added and incubated for an additional hour at 4°C. Co-immunoprecipitation of transfected Flag-p53 and Myc-REV1 was carried out using anti-Flag M2 affinity gel (Sigma) and anti-c-Myc-agarose beads (Pierce) according to manufacturers’ instructions in order to minimize interference by IgG heavy chain with p53. Immunoprecipitates were recovered with SDS sample buffer and subjected to Western blot analysis.
For analysis of SUMO-modified REV1 protein, immunoprecipitation was performed under denaturing conditions in the presence of NEM, a deSUMOylation inhibitor, as described previously (23), with minor modification. The cells were washed with ice-cold PBS, lysed by adding SUMO lysis buffer (62.5 mM Tris at pH 6.8, 2% SDS), and boiled for 10 min. The samples were centrifuged for 20 min at full speed and the supernatant was diluted 1/20 with NEM-RIPA buffer supplemented with 20 mM N-ethylmaleimide (NEM; Calbiochem). The same amount of total protein was used for immunoprecipitation, followed by Western blot analysis.
Antibodies against Myc (9E10; Santa Cruz Biotechnology), Flag (M2; Sigma-Aldrich), HA (F-7; Santa Cruz Biotechnology), REV1 (H-300; Santa Cruz Biotechnology), p53 (FL-393; Santa Cruz Biotechnology), phospho-p53 (Ser18, Cell Signaling Technology), acetyl-p53 (Lys379, Cell Signaling Technology), SUMO2/3 (Zymed Laboratories Inc), Ubiquitin (P4D1; Santa Cruz Biotechnology), PCNA (NCL-PCNA; Novocastra Laboratories) and Tubulin (Cell Signaling Technology) were obtained from commercial sources.
RNA isolation and quantitative RT-PCR
Total RNA was isolated using TRI Reagent (Invitrogen), reverse-transcribed with M-MLV Reverse Transcriptase (Promega), and amplified with SYBR Green PCR maser mix (Invitrogen) according to the manufacturer’s recommendation. The following PCR primers were used: human REV1 forward 5′-TTG TGA TGA AGC GCT GGT AG-3′, REV1 reverse 5′-TTG GTC ACT AGC TGG CCT CT-3′, human BAX forward 5′-TTT TGC TTC AGG GTT TCA TC -3′, BAX reverse 5′-CAG TTG AAG TTG CCG TCA GA -3′, human NOXA forward 5′-AGA GCT GGA AGT CGA GTG T -3′, NOXA reverse 5′-GCA CCT TCA CAT TCC TCT C -3′, human PUMA forward 5′-TCA ACG CAC AGT ACG AGC G -3′, PUMA reverse 5′-TGG GTA AGG GCA GGA GTC C -3′, human KILLER/DR5 forward 5′-TGC AGC CGT AGT CTT GAT TG -3′, KILLER/DR5 reverse 5′-TCC TGG ACT TCC ATT TCC TG -3′, human TIGAR forward 5′-TCC AAG CAA CTG TCT GGA AA -3′, TIGAR reverse 5′-ATC TGC TCA GAG TGG CTG GT -3′, human SESTRIN2 forward 5′-TCA AGG ACT ACC TGC GGT TC -3′, SESTRIN2 reverse 5′-GTT GTC TAC TCG CCC AGA GG -3′, mouse Puma forward 5′-GCC CAG CAG CAC TTA GAG TC -3′, Puma reverse 5′-TGT CGA TGC TGC TCT TCT TG -3′, mouse Noxa forward 5′-GGC AGA GCT ACC ACC TGA GT -3′, Noxa reverse 5′-TTG AGC ACA CTC GTC CTT CA -3′, mouse Bax forward 5′-TGG AGA TGA ACT GGA CAG CA -3′, Bax reverse 5′-GAT CAG CTC GGG CAC TTT AG -3′, and β-ACTIN forward 5′-GGA CTT CGA GCA AGA GAT GG -3′, β-ACTIN reverse 5′-AGC ACT GTG TTG GCG TAC AG -3′. The expression levels were normalized with β-ACTIN mRNA in each sample.
Starvation treatment
Short-term starvation (STS) in a cell culture model was performed by glucose and serum restriction. The culture media were supplemented with 0.5 g/L or 2.0 g/L glucose to match blood glucose levels in starved and normally fed mice, respectively (3). FBS was supplemented at 1% for starvation conditions as compared to the normal 10%. For STS in vivo, mice were fasted for 24–48 h by complete deprivation of food, but with free access to water.
Mouse allografts
Twelve-week-old female C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). 2×105 B16 cells suspended in PBS were injected subcutaneously in the right flank of mice. Implanted melanoma cells were allowed to form palpable tumors, and mice bearing these tumors were subjected to fasting for 48 h with chemotreatment (3). Two cycles of fasting and chemotreatment were performed, and body weights and tumor size were measured periodically. All of the experiments were approved by University of Southern California’s Institutional Animal Care and Use Committee before the experiments were started.
Detection of cellular reactive oxygen species (ROS) level
Cells were treated as indicated, stained with fluorescent indicator 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) for 30 min, and then analyzed by fluorescence microscopy according to the manufacturer’s instructions (Invitrogen).
Clonogenic colony assay
MCF7 cells were transfected with either siCTL or siREV1 RNAi. The next day, cells were incubated in normal or starvation medium. After 48 h, cells were split and seeded at equal densities in triplicate into 6-well plates (24). After 10–14 days, colonies were fixed and stained using a crystal violet solution, and visible colonies were counted.
RESULTS
Short-term starvation induces REV1 modification
We have recently shown that short-term starvation or fasting can promote sensitization of a variety of cancer cells to chemotherapeutic agents but can also delay cancer progression independently of chemotherapy (3,25). In agreement with our previous studies, STS had a potent effect on sensitizing human MCF7 breast carcinoma cells to the chemotherapeutic drug doxorubicin (DXR) (Fig. 1A).
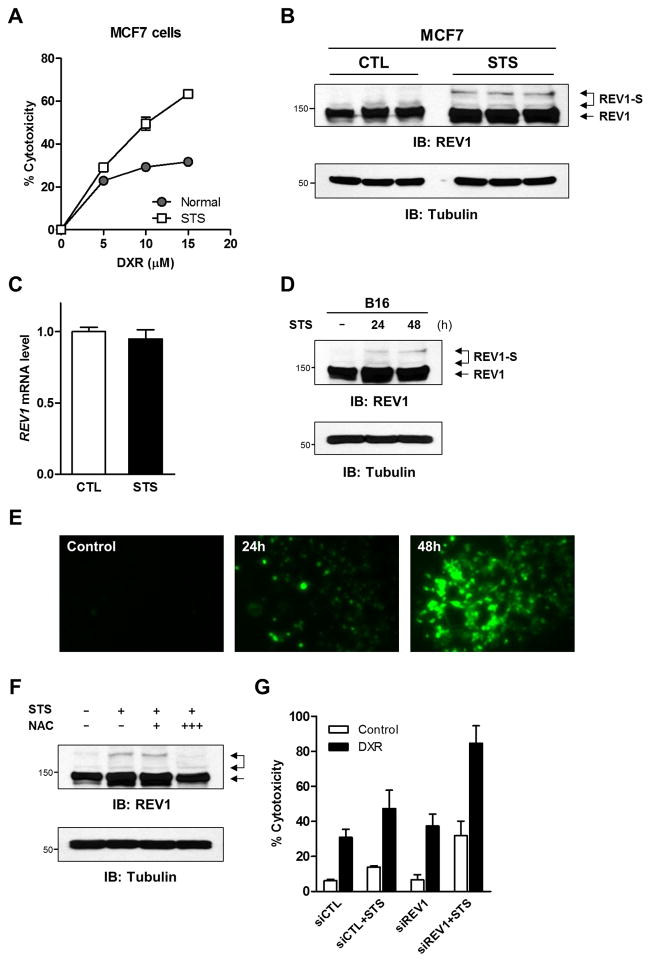
Figure 1. Short-term starvation (STS) leads to REV1 modification via ROS.
(A) Doxorubicin dose-response curves in MCF7 cells upon normal or starvation conditions. Starvation was applied to cells 24 h before and 24 h during DXR treatment. Cytotoxicity values were obtained by evaluation of LDH release to assess cell viability. (B, D) MCF7 and B16 cells were fed complete or starvation medium for the indicated times, lysed and immunoblotted with anti-REV1 antibody. A tubulin blot was presented as a loading control (bottom panel). (C) Relative mRNA expression level of REV1 in MCF7 cells was measured after 48 h of starvation condition. (E) ROS levels under STS were examined by carboxy-H2DCFDA. Cells were incubated with starvation medium for the indicate times, and the fluorescence intensity of carboxy-H2DCFDA was analyzed by fluorescence microscopy. (F) Cells were starved for 24 h, followed by treatment with 10 mM NAC for additional 24 h. The cell extracts were separated by SDS-PAGE and blotted with anti-REV1 antibody. (G) REV1’s effect on STS-induced sensitization of MCF7 cells to DXR. MCF7 cells were transfected with non-targeting control or REV1 siRNAs. 24 h following siRNA transfection, cells were incubated in normal or starvation conditions for additional 24 h. After DXR treatment for 24 h, cells were prepared and analyzed by LDH assay. Data are shown as average ± SD.
We have previously shown that REV1, an error-prone DNA repair enzyme, is a major contributor to age-dependent genomic instability and to cell survival in response to genotoxic stress in S. cerevisiae (6). Since recent studies have also shown that mammalian REV1 is implicated in cancer drug-induced mutagenesis and drug resistance (8,26), we have sought to determine the role of REV1 in cancer cells in response to starvation. Interestingly, STS of human MCF7 breast cancer cells and of mouse B16 melanoma cells resulted in the generation of slower-migrating forms (the upper bands) of endogenous REV1 proteins in SDS-PAGE (Figs 1B and 1D). However, no significant change in REV1 mRNA levels was observed during the same time period (Fig. 1C).
Nutrient starvation can induce the accumulation of intracellular reactive oxygen species (ROS) which contributes to cell death selectively in cancer cells (3,4). In agreement with previous results, we observed that the ROS levels were elevated at 24 h and increased further at 48 h of STS (Fig. 1E). Since ROS operate in cellular signaling events (27), we next determined their effect on REV1 response to starvation. Treatment of starved cells with the ROS scavenger N-acetyl cysteine (NAC) significantly attenuated REV1 modification (Fig. 1F), indicating a ROS-induced REV1 modification upon STS.
We next investigated REV1’s effect on cancer chemotherapy. We used short-interfering RNA (siRNA) to knock down REV1 expression (Supplementary Fig. S1). We tested the cytotoxicity of MCF7 cells after exposure to different combinations of DXR and STS treatments. Before DXR treatment, cells were transfected with siRNA for 48 h to achieve reduced REV1 expression. Whereas siREV1 did not affect DXR toxicity, the combination of REV1-knockdown and STS doubled the killing of MCF7 cells by DXR treatment (Fig. 1G), providing evidence that REV1 suppression combined with STS can enhance chemosensitization of cancer cells.
REV1 is post-translationally modified by SUMO2/3 in response to ROS
We investigated further the molecular mechanisms underlying REV1 modification by STS-induced ROS. Consistent with our previous data, slower-migrating forms of endogenous REV1 protein in SDS-PAGE were also observed in response to hydrogen peroxide treatment (Fig. 2A). Post-translational modification with SUMO, especially SUMO2/3, has recently been established as a key step in cellular stress response (13). SUMO conjugation is a rapid and reversible process which regulates numerous protein functions. To determine whether REV1 protein is modified by SUMO, cell extracts treated with H2O2 were immunoprecipitated with anti-SUMO2/3 antibody under denaturing conditions. Immunoprecipitated SUMO2/3 also pulled down REV1, and the anti-SUMO2/3 signal overlapped with the slower-migrating bands of REV1 (Fig. 2B, upper panel). Although REV1 can also be modified by ubiquitin (28), the ubiquitination level of REV1 was not affected by H2O2 (Fig. 2B, lower panel). These data indicate that endogenous REV1 is modified by endogenous SUMO2/3 in response to both exogenous and endogenous ROS.
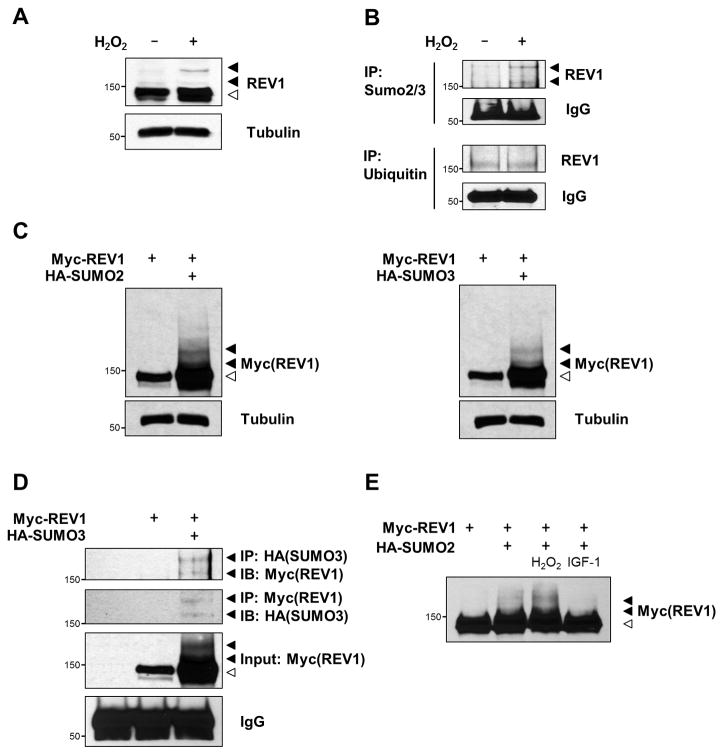
Figure 2. SUMO2/3 modification of REV1 in response to ROS.
(A) HEK293 cells were treated with 200 μM H2O2 for 15 min. Equal amounts of total cellular proteins were immunoblotted with anti-REV1 antibody. A tubulin blot was presented as a loading control (bottom panel). (B) Cell extracts from HEK293 cells treated with or without 200 μM H2O2 for 15 min were immunoprecipitated with anti-SUMO2/3 or anti-Ubiquitin antibody under denaturing conditions and immunoblotted with anti-REV1 antibody. IgG heavy chain is shown as loading control. (C) Either HA-SUMO2 or HA-SUMO3 was transiently co-expressed with Myc-REV1 in HEK293 cells, and cell extracts were immunoblotted with anti-Myc antibody. (D) HEK293 cells were co-transfected with Myc-REV1 and HA-SUMO3, and extracted under denaturing conditions. SUMOylation of REV1 was determined by reciprocal immunoprecipitation and immunoblot analyses. Whole cell lysates were immunoblotted for Myc-REV1 as input control, and IgG heavy chain as loading control. (E) Cells were co-transfected with Myc-REV1 and HA-SUMO2, treated with H2O2 (200 μM) or IGF-1 (200 ng/ml) for 15 min, and immunoblotted with anti-Myc antibody. Arrowheads indicate the SUMOylated form (filled) and the unmodified form (open).
SUMOylation of REV1 was further confirmed by co-transfection assay with Myc-tagged REV1 and HA-tagged SUMO2 or SUMO3 expression constructs. The slower-migrating forms of REV1 were detected in immunoblots of whole cell lysates from co-transfected cells, but not from cells expressing Myc-REV1 alone (Fig. 2C). Moreover, reciprocal immunoprecipitation and immunoblot analyses confirmed that the slower-migrating forms corresponded to SUMO-conjugated forms of REV1 (Fig. 2D). H2O2 treatment also led to an increase in REV1 SUMOylation, but introduction of insulin-like growth factor 1 (IGF-1), whose decreased levels are central in the effects of STS in cancer treatment (3), reversed the ROS-induced REV1 SUMOylation (Fig. 2E). These data provide direct evidence that endogenous, as well as ectopically expressed, REV1 is SUMOylated in vivo via ROS.
PIASy E3 SUMO ligase modulates REV1 SUMOylation
SUMOylation is a reversible and dynamic process (14). SUMO can be removed from targets by a family of SUMO-specific peptidases (SENPs), and at least six members (SENP1-3 and SENP5-7) have been identified in mammalian cells. To examine whether SUMO proteases act on SUMO-modified REV1, HEK293 cells were co-transfected with REV1, SUMO2, and either SENP1 or SENP6. Over-expression of either SENP1 or SENP6 resulted in SUMO deconjugation from REV1 (Fig. 3A).
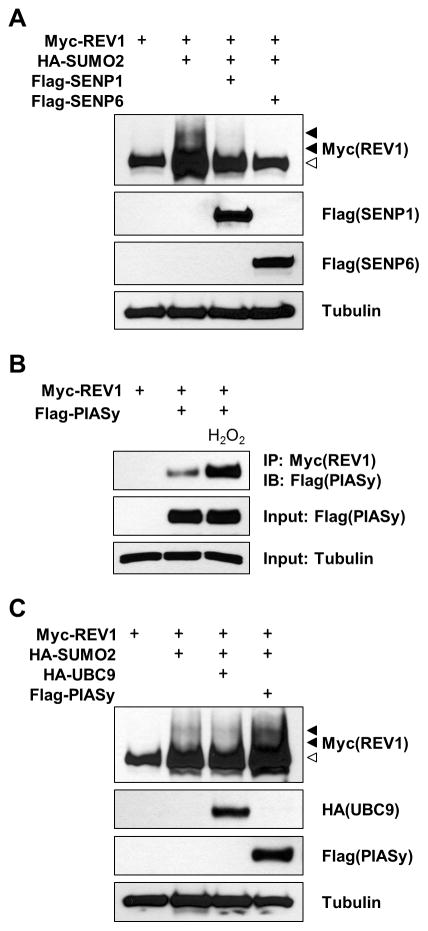
Figure 3. PIASy functions as a SUMO E3 ligase for REV1.
(A) HEK293 cells were co-transfected with Myc-REV1 and HA-SUMO2, together with either Flag-SENP1 or Flag-SENP6. Cell extracts were immunoblotted with the indicated antibodies. (B) Cells expressing Myc-REV1 and Flag-PIASy were treated with H2O2 for 15 min. Equal amounts of total cellular proteins were co-immunoprecipitated with anti-Myc antibody, and analyzed by immunoblot with anti-Flag antibody. Whole cell lysates were immunoblotted for Flag-PIASy as input control. (C) Cells were co-transfected with Myc-REV1 and HA-SUMO2 together with HA-UBC9 or Flag-PIASy, and cell extracts were immunoblotted with the indicated antibodies.
Recently, PIAS (protein inhibitor of activated STAT) proteins have been reported to be specific E3 SUMO ligases in DNA damage response (29). In an attempt to examine whether a PIAS can serve as a SUMO E3 ligase for REV1, HEK293 cells were transfected with REV1 and PIASy and cell extracts were subjected to co-immunoprecipitation (Co-IP) assay. PIASy co-precipitated with REV1 in vivo and REV1-PIASy interaction was markedly increased by ROS (Fig. 3B). Furthermore, co-expression of PIASy enhanced REV1 modification, although E2 conjugating enzyme UBC9 did not exhibit any obvious effect (Fig. 3C), suggesting that PIASy acts as an E3 SUMO ligase for REV1 SUMOylation.
SUMO modification promotes the stability of REV1 protein
SUMO is covalently bound to lysine residues in target proteins (13). Sequence analysis identified twelve putative SUMOylation sites in REV1 (Fig. 4A). Substitutions of the lysine (K) residues for arginines (R) by site-directed mutagenesis demonstrated that K119 is the major SUMOylation site of REV1 (Supplementary Fig. S2). Sequence comparison revealed that this SUMOylation site is highly conserved in vertebrates (Fig. 4B).
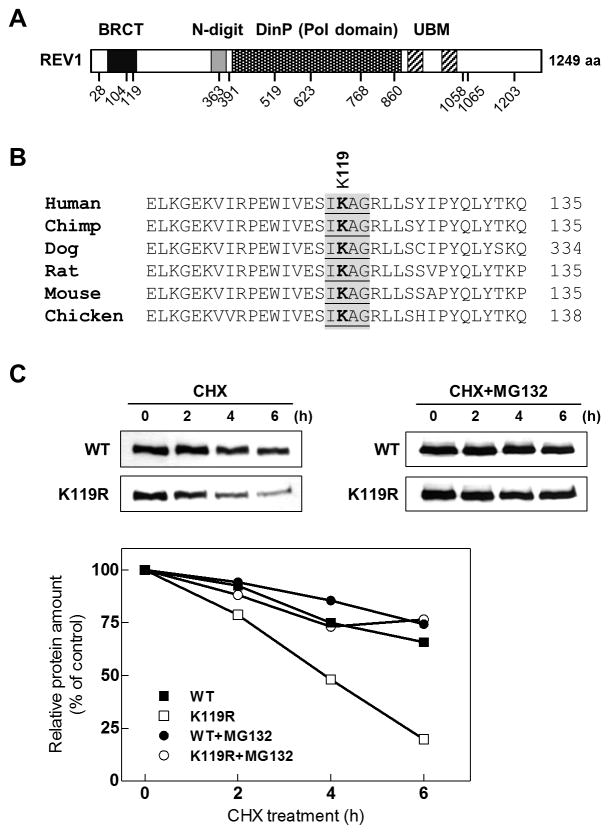
Figure 4. SUMOylation stabilizes REV1 protein.
(A) Schematic of mouse REV1 protein and its putative SUMOylation motifs predicted by Abgent SUMOplot™ (www.abgent.com/sumoplot) and SUMOsp 2.0 (50). (B) Sequence alignment of REV1 SUMOylation sites using ClustalW2 web tool indicating that SUMOylation site of murine REV1, lysine 119 (K119), is highly conserved in vertebrates. (C) Stability of wild-type REV1 and the K119R mutant. HEK293 cells expressing Myc-tagged wild-type REV1 or the K119R mutant were treated with CHX (30 μg/ml) alone or together with MG132 (50 μM) for the indicated times. REV1 protein level was analyzed by immunoblot with anti-Myc antibody and quantified using ImageJ (http://imagej.nih.gov/ij/).
A recent yeast study showed that REV1 is regulated by proteasomal degradation and that its stability may be modulated by protein modifications (30). Prior studies have also shown that SUMO contributes to regulating the stability of SUMO target proteins, including NF-κB inhibitor IκBα, transcription factor Oct4 and Huntingtin (31–34). To test whether SUMOylation would affect REV1 stability in mammalian cells, we analyzed the stability of wild-type REV1 and the SUMOylation-deficient mutant (K119R) by blocking de novo protein synthesis with cycloheximide. Wild-type REV1 was found to be stable with an estimated half-life of ~ 10 h, but mutant REV1 displayed a markedly reduced half-life of ~ 3 h. The stability of both forms was significantly increased in cells treated with MG132, a specific inhibitor of 26S proteasome (Fig. 4C). These data are consistent with the previous observations that the abundance of wild-type REV1 when co-expressed with SUMO or PIASy was higher than that of REV1 when expressed alone (Figs 2 and 3). Furthermore, the ectopic expression of SUMO also enhanced the stability of endogenous REV1 protein (Supplementary Fig. S3), indicating that SUMOylation controls REV1 stability.
REV1 is a novel interacting partner of p53 in vivo
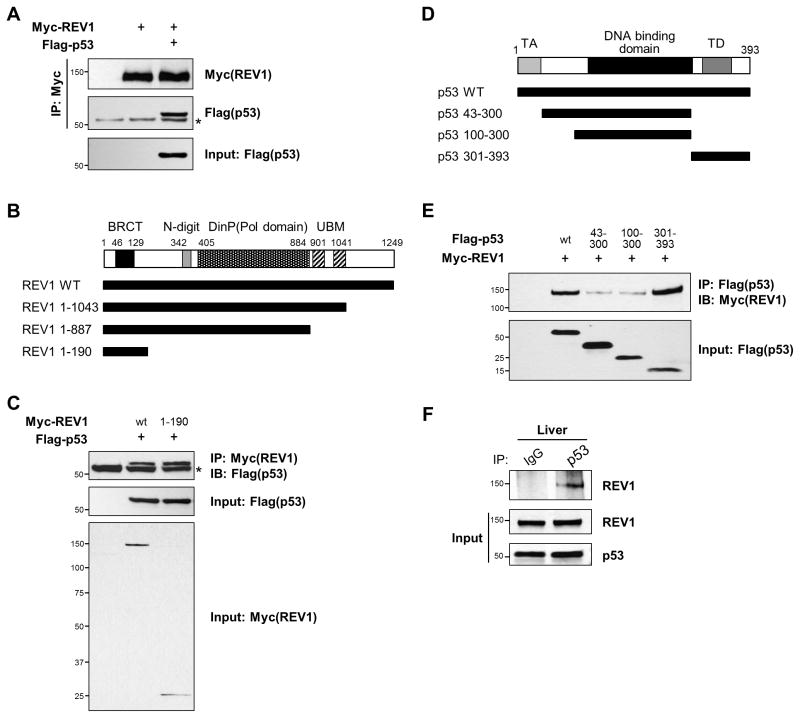
The eukaryotic REV1 protein has also been shown to function as a scaffold protein that interacts with multiple DNA metabolic and repair proteins (10,11). The p53 tumor suppressor is a key molecule in genomic maintenance and cell fate in mammals (35). Recent studies indicated that p53 and REV1 participate in overlapping cellular processes (36–38). We have sought to study whether REV1 may interact with p53. We found that full-length REV1 was able to interact with full-length p53 in vivo (Fig. 5A). To characterize the minimal p53 binding domain of REV1, we generated truncated REV1 mutants and performed co-immunoprecipitation assays with full-length p53 in HEK293 cells. Progressive deletion constructs revealed that the N-terminal domain (residues 1–190) of REV1, which contains the BRCA1 C-terminal (BRCT) domain, was sufficient for p53 binding (Figs 5B and 5C). Other truncated mutants (residues 1–887 and 1–1043) of REV1 were also immunoprecipitated with p53 (Supplementary Fig. S4).
Figure 5. REV1 interacts with p53 via its N-terminal BRCT domain.
(A) Myc-tagged full-length REV1 was transiently co-expressed with Flag-tagged full-length p53 in HEK 293 cells, and cell lysates were immunoprecipitated with anti-Myc-agarose beads. The immune complexes were analyzed by immunoblotting with anti-Myc and anti-Flag antibodies. The asterisk indicates IgG heavy chain. (B) Schematic of REV1 protein and the deletion mutants. (C) The N-terminal BRCT domain of REV1 is sufficient for its interaction with p53. Full-length or truncated (residues 1–190) Myc-REV1 was co-expressed with full-length Flag-p53 in HEK293 cells, and cell lysates were immunoprecipitated with anti-Myc conjugated beads, followed by Western blot analysis with anti-Flag antibody. Whole cell lysates were immunoblotted for Flag-p53 and Myc-REV1 as input control. (D) Schematic of Flag-tagged p53 and its fragments. (E) The C-terminal domain of p53 is sufficient for the interaction with REV1. Full-length or fragments of Flag-p53 were overexpressed with Myc-REV1. Cell lysates were immunoprecipitated with anti-Flag antibody-conjugated agarose. (F) Endogenous p53 and REV1 interact. The liver lysates from C57BL/6 mouse were immunoprecipitated with either normal rabbit IgG or anti-p53 antibody. Immunoprecipitates were then analyzed by immunoblotting with anti-REV1 antibody. Whole tissue lysates were immunoblotted for REV1 and p53 as input control.
The p53 protein is composed of several discrete functional domains, such as an N-terminal transactivation domain, a DNA binding domain, and a C-terminal region (12). To further define the REV1 interaction domain in p53, we generated p53 fragments and expressed full-length or variant p53 with wild-type REV1 in HEK293 cells. Co-IP assays revealed that REV1 can interact with the C-terminal domain as well as full-length p53 (Figs 5D and 5E), indicating that C-terminal region of p53 is sufficient for REV1 interaction. More importantly, we also examined the interaction between p53 and REV1 at the endogenous level in mouse liver (Fig. 5F), suggesting that REV1 can form a complex with p53 in vivo.
REV1 modulates p53 transactivation and stability in response to STS
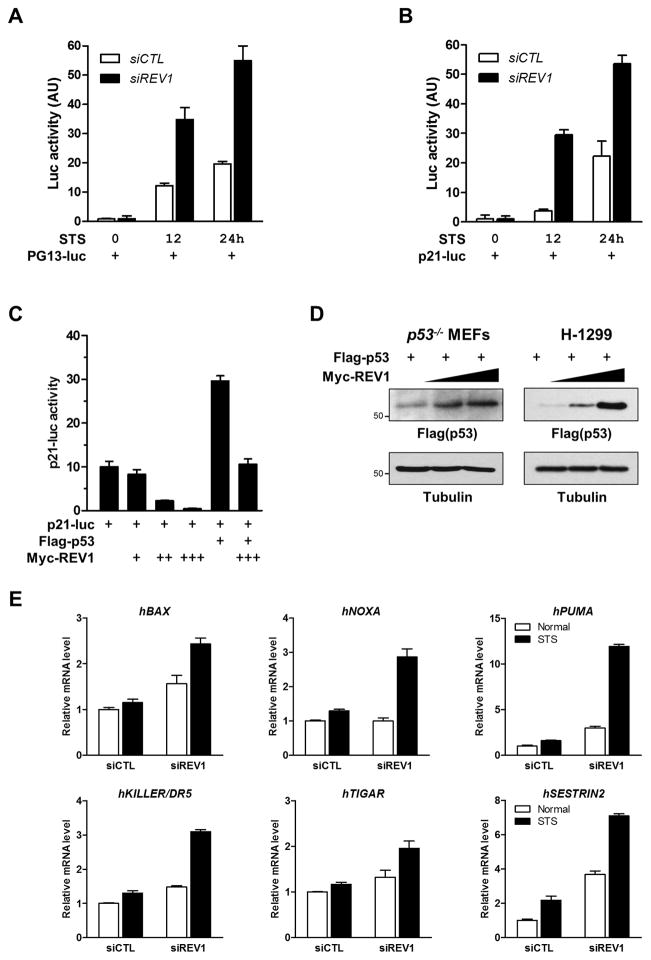
The p53 protein is a well-known transcription factor, and has been shown to mediate cellular response to metabolic stress (35). Because REV1 and p53 interact in vivo, we examined the effects of REV1 on the transcriptional activity of p53 under stress conditions. MCF7 control and REV1-knockdown cells were transfected with the p53-responsive reporter, PG13-luc, and subjected to STS. The transcriptional ability of p53 was enhanced with increasing duration of STS (Fig. 6A). Furthermore, REV1-knockdown significantly induced p53 transactivation upon starvation. Similar results were obtained with the promoter of p21, a well-known p53 target (Fig. 6B). In addition, REV1 over-expression markedly reduced the p21 promoter activity (Fig. 6C), in agreement with a negative effect of REV1 in regulating p53.
Figure 6. REV1 regulates p53 stability and transcriptional activity in response to STS.
(A, B) PG13-luc (A) or p21-luc (B) was transfected in both MCF7 control and REV1-knockdown cells. 24 h posttransfection, STS was carried out for indicated time points followed by luciferase assay. (C) p21-luc was co-transfected with Myc-REV1 and/or Flag-p53 as indicated. Luciferase assay was carried out 24 h posttransfection. (D) p53-null MEFs (left panel) or human H1299 cells (right panel) were co-transfected with Flag-p53 and increasing amounts of Myc-REV1. The level of p53 was analyzed by Western blot analysis with anti-Flag antibody. A tubulin blot was presented as a loading control (bottom panel). (E) MCF7 control and REV1-knockdown cells were subjected to STS for 48 h. Total RNA was isolated and relative mRNA levels were analyzed by qRT-PCR for the indicated genes. Results shown are reported as average ± SD.
p53 is an unstable protein and presents at low levels in normal cells, and its activity is influenced by its interaction (39). We therefore tested whether REV1 regulates p53 stability. REV1 was able to enhance the steady-state levels of p53 in both p53-null MEFs and H1299 cells (Fig. 6D).
To further assess the contribution of REV1 in p53 transactivation during starvation response, real-time PCR was carried out and the levels of p53 target genes were analyzed in the presence and absence of REV1 suppression. STS moderately increased the expression of pro-apoptotic and metabolic target genes, including BAX, NOXA, PUMA, KILLER, TIGAR, and SESTRIN2. Furthermore, REV1 suppression increased the basal levels of gene expressions, and dramatically enhanced the expression levels upon STS (Fig. 6E). These data are consistent with the reporter assays (Figs 6A and 6B), supporting the involvement of REV1 in the regulation of p53 activity in response to starvation.
REV1 modulates p53-mediated starvation response in vivo
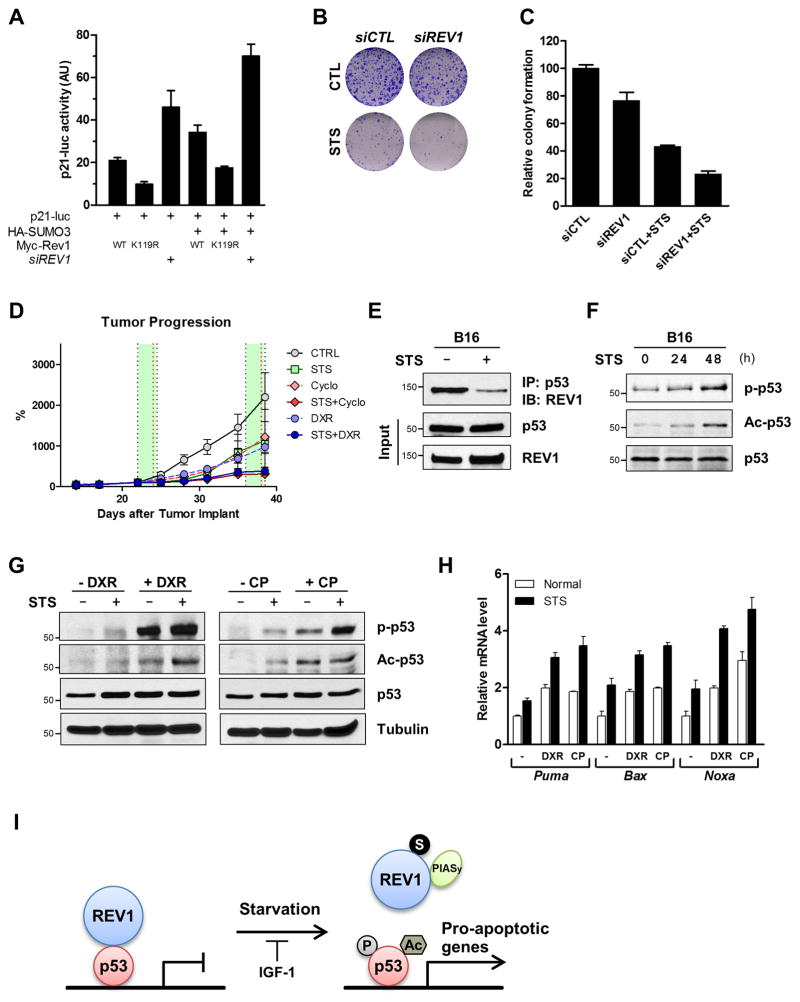
The post-translational modifications of p53 and its interacting proteins are the subject of intense research due to their profound effects on p53’s overall activity and stability (39). We therefore examined whether the modification status of REV1 could affect p53 function in in vitro and in vivo cancer models. To assess the role of REV1 SUMOylation on p53 transactivation, MCF-7 cells were transfected with p21-luc reporter in combination with wild-type or mutant REV1 and SUMO3. Co-expression of wild-type REV1 relieved its inhibitory effect on p53 when compared with SUMOylation-deficient K119R mutant (Fig. 7A). Moreover, SUMO over-expression increased overall p53 activity, suggesting that as long as REV1 is modified by SUMOylation, p53 remains highly activated under starvation conditions.
Figure 7. STS sensitizes cancer cells to chemotherapy through REV1 and p53.
(A) MCF7 cells were introduced with siREV1 for 48 h, and then transfected with p21-luc, HA-SUMO3, and wild-type Myc-REV1 or K119R mutant. After additional 24 h, cells were harvested and luciferase assay was performed as described earlier. Data are shown as average ± SD. (B, C) Colony-formation assay of MCF7 cells transfected with siCTL or siREV1 under normal or starvation conditions. MCF7 cells were transfected with control or REV1 siRNA. 24 h following siRNA transfection, cells were incubated in normal or starvation conditions for additional 48 h, and then split and subjected to colony-formation assay visualized by crystal violet staining (B) and quantification (C) of colonies formed in MCF7 cells. Values are shown as average percentage ±SD. (D) Tumor progression of allografted B16 melanoma cells treated with fasting and/or chemotherapeutic agents. C57BL/6J mice with subcutaneously implanted B16 melanoma cells were fed Ad lib or fasted with or without chemotherapy (DXR, 8 mg/kg, i.v.; CP, 100 mg/kg, i.p., as indicated by red dash line). Two cycles of fasting (48 h) and/or chemotreatment were performed. Tumor progression was presented as percentage change in tumor size. Data expressed as means ±S.E.M. (n = 5). (E) B16 cells from 48h-starved mice were harvested, and subjected to Co-IP with anti-p53 antibody, followed by immunoblot with anti-REV1 antibody. Whole cell lysates were immunoblotted for p53 and REV1 as input control. (F) Mice bearing B16 cells were starved for the indicated time, and the melanoma cells were harvested for immunoblots with anti-phospho-p53 (Ser18), anti-acetyl-p53 (Lys379) and anti-p53 antibodies. (G) Allografted B16 treated with STS and/or chemotherapeutic agents were harvested for immunoblots with anti-phospho-p53 (Ser18), anti-acetyl-p53 (Lys379) and anti-p53 antibodies. A tubulin blot was presented as a loading control. (H) Total RNA was isolated from tumors and the relative mRNA levels were analyzed by real-time PCR for the indicated genes. Results shown are reported as average ± SD. (I) A model for the regulation of p53 by REV1 SUMOylation in response to short-term starvation. Short-term starvation induces not only REV1 SUMOylation, which is catalyzed by E3 SUMO ligase PIASy, but p53 transactivation via the changes in its modification and interaction with REV1.
We further tested whether REV1 is able to affect colony formation ability of cancer cells. Knockdown of REV1 decreased the ability of MCF7 cells to form colonies (Figs 7B and 7C). Notably, colony formation was markedly suppressed by STS, and further reduced by knockdown of REV1. These results indicate that REV1 negatively influences clonogenicity and may mediate part of the effects of STS on colony formation.
To further study the role of STS in sensitization of cancer cells to chemotherapy in vivo, we employed a subcutaneous allograft model. Murine melanoma B16 cells were injected subcutaneously and allowed to form palpable tumors, and mice bearing these tumors were subjected to fasting cycles with chemotherapy. In agreement with our previous findings, two fasting cycles not only retarded tumor growth and were as effective as treatment with DXR or CP (cyclophosphamide), but also augmented the efficacy of chemotherapy drugs (Fig. 7D) (Supplementary Fig. S5)(3). Notably, the greatest therapeutic effect was observed when fasting was combined with chemotreatment.
To further investigate the molecular mechanisms underlying STS-induces sensitization of cancer cells, we examined whether the REV1-p53 pathway might be involved in sensitizing cancer cells in response to fasting. In allografted tumors, Co-IP assay revealed that STS led to the disruption of REV1-p53 interaction (Fig. 7E). Consistent with this in vivo data, ROS, which can be induced by STS (Fig. 1E), also disrupted REV1-p53 interaction in cultured cells (Supplementary Fig. S6). On the basis of these observations, we reasoned that fasting might cause disruption of an inhibitory REV1 binding, and activate p53 and its downstream effectors. We therefore examined whether fasting can activate p53 and induce the expression of the downstream genes. Phosphorylation of endogenous p53 at Ser18 (corresponding to Ser15 of human p53) as well as acetylation at Lys379 (Lys382 of human p53), which correlate with its activation (40), were increased in tumors of mice either after starvation and/or upon chemotherapy treatment (Figs 7F and 7G). Furthermore, the combination of fasting and chemotherapy exerted the strongest effects on p53 phosphorylation and acetylation (Fig. 7G). With such a dramatic increase in the levels of p53 post-translational modifications, we ascertained whether fasting and/or chemotreatment could influence p53 transactivation. Using real-time PCR, we observed that fasting up-regulated expression of p53-dependent pro-apoptotic target genes, including Puma, Bax, and Noxa, and chemotreatment also significantly induced their expressions (Fig. 7H). More importantly, the combination of fasting and chemotherapy dramatically increased the expression of these genes in implanted tumors, indicating that fasting can sensitize cancer cells to chemotherapy and induce apoptosis in a p53-dependent manner. We have previously shown that both STS and reduced IGF-1 levels retard melanoma growth in mice (3). This study indicates that these effects are mediated in part by REV1 modification and the consequent p53 activation, leading to cancer cell death.
DISCUSSION
STS or fasting has been shown to have wide and positive effects in cancer treatment by augmenting the efficacy of chemotherapy and in some cases by matching the efficacy of chemotherapy, but its mechanism of action remain poorly understood (1,3,25). This study provides evidence for a role of REV1 as a novel modulator of p53 and for REV1-p53 interaction in STS-induced enhancement of cancer cell death. According to our model, REV1 is capable of regulating p53 stability and activity via their physical interaction. STS promotes REV1 SUMOylation, which contributes to its dissociation from p53, suggesting that starvation relieves REV1-dependent repression of p53 transactivation (Fig. 7I). Starvation also promotes p53 modifications, such as phosphorylation and acetylation, which correlate well with its key function. Indeed, under DNA damage and oxidative stress, p53 and its negative regulators are post-translationally modified, leading to p53 activation by disrupting their interaction (41). Our results indicate that REV1 is acting as both a scaffold and a modulator in p53 signaling cascades in response to starvation.
Nutrient depletion causes the accumulation of ROS in cancer cells, which can lead to apoptotic cell death (3,4). ROS also function as intracellular signaling molecules regulating multiple cellular processes. Our data reveal that REV1 protein is SUMOylated in cancer cells in response to STS and ROS. DXR treatment, which can induce reactive oxygen species (ROS) through redox cycling (42), also induced REV1 SUMOylation, but the modification level was similar or less than that caused by STS (Supplementary Fig. S7). Moreover, we investigated the role of REV1 in normal cells in response to STS and chemotreatment. In contrast to its effects on cancer cells, STS had protective effects in normal cells and enhanced the resistance of primary MEFs against chemotoxicity (Supplementary Fig. S8). Cytotoxicity assays revealed that knockdown of Rev1 had no significant effects on chemotherapy-induced cytotoxicity of primary MEFs. Taken together, these data indicate that REV1 contributes to cell death selectively in cancer cells and that REV1 may be a key mediator of STS-dependent effects on cancer cells. These results are consistent with our Differential Stress Resistance (DSR) hypothesis proposing that fasting protects normal but not cancer cells from chemotherapy and the Differential Stress Sensitization (DSS) hypothesis suggesting that fasting will sensitize cancer cells by generating a complex and hostile environment to which only normal cells can adapt (3,4,43,44).
In recent years, SUMO modification has been shown to play prominent roles in controlling the function of a large number of proteins involved in signal transduction, genome maintenance and DNA repair (45). Previous studies have shown that REV1 is modified by phosphorylation in yeast and by ubiquitination in mammalian cells (28,46), but its SUMOylation had not been described. Thus, the related studies of possible cross-talk between SUMOylation and other modifications have the potential to provide important insights into the mechanism regulating REV1 function. In addition, SUMOylation at K119 contributes to REV1 stability, although other putative SUMOylation sites in REV1 may be also important. In light of the fact that K119 is in the REV1 N-terminal domain, required for p53 interaction, and that PIASy is also required for p53 modification (47), the precise interaction among REV1, PIASy, and p53 requires further investigation.
p53 has been previously proposed to regulate error-prone DNA repair process (48), but the precise mechanism by which these pathways are linked remains largely unknown. Our data indicate that the N terminus of REV1, which contains the BRCT domain, is sufficient for p53 interaction and to control p53 activity. The BRCT domain is involved in regulating DNA TLS (49). REV1 interacts with PCNA and the B family TLS polymerase Polζ via accessory subunit REV7 (Supplementary Fig. S9) (10,11). However, ROS and its induced REV1 SUMOylation did not affect REV1 interaction with PCNA or REV7 (Supplementary Fig. S9). How modification of REV1 releases p53 is unclear. Modification of the internal lysine may simply lead to masking of existing binding site or a conformational change in the REV1 structure, interfering with its interaction with p53. Indeed, Lys119 of REV1 SUMOyaltion site is located in the BRCT domain which has been identified as an important node in p53 binding (Figs 4 and 5). It is also possible that the post-translational modification either reduces p53 affinity for REV1 or disrupts p53 oligemerization, resulting in dissociation of this complex.
In conclusion, this study provides evidence for a role of REV1 and its SUMOylation in modulating p53-dependent cancer cell death in response to starvation conditions and the consequent increase in oxidative stress. The combination of starvation with other treatments has promising therapeutic potential as an intervention to promote differential REV1 and p53 regulation in normal and cancer cells, contributing to their protection and death, respectively.
Supplementary Material
Acknowledgments
We thank Dr. Errol Friedberg for Myc-REV1 construct, Dr. Shengkan Jin for p53-null MEFs, and Dr. Deborah Johnson for H1299 cells. This study was funded by NIH/NIA grants AG20642 and AG034906, The Bakewell Foundation, and a V Foundation for Cancer Research grant.
Footnotes
Conflict of Interest: Valter Longo has equity interest in L-Nutra, a company that develops and sells medical food products. Sebastian Brandhorst is a consultant for L-Nutra, a company that develops and sells medical food products.
AUTHORS’ CONTRIBUTIONS
Conception and design: H.S. Shim, V.D. Longo
Development of methodology: H.S. Shim
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): H.S. Shim, S. Brandhorst
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): H.S. Shim, M. Wei, S. Brandhorst, V.D. Longo
Writing, review, and/or revision of the manuscript: H.S. Shim, M. Wei, V.D. Longo
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): S. Brandhorst, M. Wei, V.D. Longo
Study supervision: V.D. Longo
References
- 1.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321–6. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longo VD, Lieber MR, Vijg J. Turning anti-ageing genes against cancer. Nature reviews Molecular cell biology. 2008;9(11):903–10. doi: 10.1038/nrm2526. [DOI] [PubMed] [Google Scholar]
- 3.Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Science translational medicine. 2012;4(124):124ra27. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(24):8215–20. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosarek JN, Woodruff RV, Rivera-Begeman A, Guo C, D’Souza S, Koonin EV, et al. Comparative analysis of in vivo interactions between Rev1 protein and other Y-family DNA polymerases in animals and yeasts. DNA repair. 2008;7(3):439–51. doi: 10.1016/j.dnarep.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madia F, Wei M, Yuan V, Hu J, Gattazzo C, Pham P, et al. Oncogene homologue Sch9 promotes age-dependent mutations by a superoxide and Rev1/Polzeta-dependent mechanism. The Journal of cell biology. 2009;186(4):509–23. doi: 10.1083/jcb.200906011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumstorf CA, Mukhopadhyay S, Krishnan E, Haribabu B, McGregor WG. REV1 is implicated in the development of carcinogen-induced lung cancer. Molecular cancer research: MCR. 2009;7(2):247–54. doi: 10.1158/1541-7786.MCR-08-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie K, Doles J, Hemann MT, Walker GC. Error-prone translesion synthesis mediates acquired chemoresistance. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(48):20792–7. doi: 10.1073/pnas.1011412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma S, Hicks JK, Chute CL, Brennan JR, Ahn JY, Glover TW, et al. REV1 and polymerase zeta facilitate homologous recombination repair. Nucleic acids research. 2012;40(2):682–91. doi: 10.1093/nar/gkr769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo C, Sonoda E, Tang TS, Parker JL, Bielen AB, Takeda S, et al. REV1 protein interacts with PCNA: significance of the REV1 BRCT domain in vitro and in vivo. Molecular cell. 2006;23(2):265–71. doi: 10.1016/j.molcel.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 11.Guo C, Fischhaber PL, Luk-Paszyc MJ, Masuda Y, Zhou J, Kamiya K, et al. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. The EMBO journal. 2003;22(24):6621–30. doi: 10.1093/emboj/cdg626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckerman R, Prives C. Transcriptional regulation by p53. Cold Spring Harbor perspectives in biology. 2010;2(8):a000935. doi: 10.1101/cshperspect.a000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergink S, Jentsch S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature. 2009;458(7237):461–7. doi: 10.1038/nature07963. [DOI] [PubMed] [Google Scholar]
- 14.Dou H, Huang C, Singh M, Carpenter PB, Yeh ET. Regulation of DNA repair through deSUMOylation and SUMOylation of replication protein A complex. Molecular cell. 2010;39(3):333–45. doi: 10.1016/j.molcel.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dou H, Huang C, Van Nguyen T, Lu LS, Yeh ET. SUMOylation and de-SUMOylation in response to DNA damage. FEBS letters. 2011;585(18):2891–6. doi: 10.1016/j.febslet.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Gjoerup O, Zaveri D, Roberts TM. Induction of p53-independent apoptosis by simian virus 40 small t antigen. Journal of virology. 2001;75(19):9142–55. doi: 10.1128/JVI.75.19.9142-9155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamitani T, Nguyen HP, Kito K, Fukuda-Kamitani T, Yeh ET. Covalent modification of PML by the sentrin family of ubiquitin-like proteins. The Journal of biological chemistry. 1998;273(6):3117–20. doi: 10.1074/jbc.273.6.3117. [DOI] [PubMed] [Google Scholar]
- 18.Yasugi T, Howley PM. Identification of the structural and functional human homolog of the yeast ubiquitin conjugating enzyme UBC9. Nucleic acids research. 1996;24(11):2005–10. doi: 10.1093/nar/24.11.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu B, Gross M, ten Hoeve J, Shuai K. A transcriptional corepressor of Stat1 with an essential LXXLL signature motif. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(6):3203–7. doi: 10.1073/pnas.051489598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131(3):584–95. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75(4):817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 22.Shim HS, Kim H, Lee J, Son GH, Cho S, Oh TH, et al. Rapid activation of CLOCK by Ca2+-dependent protein kinase C mediates resetting of the mammalian circadian clock. EMBO reports. 2007;8(4):366–71. doi: 10.1038/sj.embor.7400920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Zhang SS, Saito K, Williams S, Arimura Y, Ma Y, et al. PTEN regulation by Akt-EGR1-ARF-PTEN axis. The EMBO journal. 2009;28(1):21–33. doi: 10.1038/emboj.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nature protocols. 2006;1(5):2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 25.Safdie F, Brandhorst S, Wei M, Wang W, Lee C, Hwang S, et al. Fasting enhances the response of glioma to chemo- and radiotherapy. PloS one. 2012;7(9):e44603. doi: 10.1371/journal.pone.0044603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Xie K, Zhang XQ, Pridgen EM, Park GY, Cui DS, et al. Enhancing tumor cell response to chemotherapy through nanoparticle-mediated codelivery of siRNA and cisplatin prodrug. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(46):18638–43. doi: 10.1073/pnas.1303958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nature reviews Molecular cell biology. 2007;8(10):813–24. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 28.Guo C, Tang TS, Bienko M, Parker JL, Bielen AB, Sonoda E, et al. Ubiquitin-binding motifs in REV1 protein are required for its role in the tolerance of DNA damage. Molecular and cellular biology. 2006;26(23):8892–900. doi: 10.1128/MCB.01118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris JR, Boutell C, Keppler M, Densham R, Weekes D, Alamshah A, et al. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature. 2009;462(7275):886–90. doi: 10.1038/nature08593. [DOI] [PubMed] [Google Scholar]
- 30.Wiltrout ME, Walker GC. Proteasomal regulation of the mutagenic translesion DNA polymerase, Saccharomyces cerevisiae Rev1. DNA repair. 2011;10(2):169–75. doi: 10.1016/j.dnarep.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulrich HD. Mutual interactions between the SUMO and ubiquitin systems: a plea of no contest. Trends in cell biology. 2005;15(10):525–32. doi: 10.1016/j.tcb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, et al. SUMO modification of Huntingtin and Huntington’s disease pathology. Science. 2004;304(5667):100–4. doi: 10.1126/science.1092194. [DOI] [PubMed] [Google Scholar]
- 33.Hay RT. SUMO: a history of modification. Molecular cell. 2005;18(1):1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Wei F, Scholer HR, Atchison ML. Sumoylation of Oct4 enhances its stability, DNA binding, and transactivation. The Journal of biological chemistry. 2007;282(29):21551–60. doi: 10.1074/jbc.M611041200. [DOI] [PubMed] [Google Scholar]
- 35.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nature reviews Cancer. 2002;2(8):594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 36.Tsaalbi-Shtylik A, Verspuy JW, Jansen JG, Rebel H, Carlee LM, van der Valk MA, et al. Error-prone translesion replication of damaged DNA suppresses skin carcinogenesis by controlling inflammatory hyperplasia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(51):21836–41. doi: 10.1073/pnas.0909507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin X, Howell SB. DNA mismatch repair and p53 function are major determinants of the rate of development of cisplatin resistance. Molecular cancer therapeutics. 2006;5(5):1239–47. doi: 10.1158/1535-7163.MCT-05-0491. [DOI] [PubMed] [Google Scholar]
- 38.Monti P, Ciribilli Y, Russo D, Bisio A, Perfumo C, Andreotti V, et al. Rev1 and Polzeta influence toxicity and mutagenicity of Me-lex, a sequence selective N3-adenine methylating agent. DNA repair. 2008;7(3):431–8. doi: 10.1016/j.dnarep.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137(4):609–22. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y. Regulation of p53 responses by post-translational modifications. Cell death and differentiation. 2003;10(4):400–3. doi: 10.1038/sj.cdd.4401182. [DOI] [PubMed] [Google Scholar]
- 41.Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nature reviews Drug discovery. 2008;7(12):979–87. doi: 10.1038/nrd2656. [DOI] [PubMed] [Google Scholar]
- 42.Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, et al. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer research. 2007;67(18):8839–46. doi: 10.1158/0008-5472.CAN-07-1649. [DOI] [PubMed] [Google Scholar]
- 43.Lee C, Raffaghello L, Longo VD. Starvation, detoxification, and multidrug resistance in cancer therapy. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2012;15(1–2):114–22. doi: 10.1016/j.drup.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell stem cell. 2014;14(6):810–23. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes & development. 2011;25(5):409–33. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabbioneda S, Bortolomai I, Giannattasio M, Plevani P, Muzi-Falconi M. Yeast Rev1 is cell cycle regulated, phosphorylated in response to DNA damage and its binding to chromosomes is dependent upon MEC1. DNA repair. 2007;6(1):121–7. doi: 10.1016/j.dnarep.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Bischof O, Schwamborn K, Martin N, Werner A, Sustmann C, Grosschedl R, et al. The E3 SUMO ligase PIASy is a regulator of cellular senescence and apoptosis. Molecular cell. 2006;22(6):783–94. doi: 10.1016/j.molcel.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 48.Avkin S, Sevilya Z, Toube L, Geacintov N, Chaney SG, Oren M, et al. p53 and p21 regulate error-prone DNA repair to yield a lower mutation load. Molecular cell. 2006;22(3):407–13. doi: 10.1016/j.molcel.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 49.Jansen JG, Tsaalbi-Shtylik A, Langerak P, Calleja F, Meijers CM, Jacobs H, et al. The BRCT domain of mammalian Rev1 is involved in regulating DNA translesion synthesis. Nucleic acids research. 2005;33(1):356–65. doi: 10.1093/nar/gki189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren J, Gao X, Jin C, Zhu M, Wang X, Shaw A, et al. Systematic study of protein sumoylation: Development of a site-specific predictor of SUMOsp 2. 0. Proteomics. 2009;9(12):3409–12. doi: 10.1002/pmic.200800646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.