Abstract
Background
Patient long-term adherence to β-blockers, HMG-CoA reductase inhibitors (statins), and angiotensin-converting-enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) after acute myocardial infarction (AMI) is alarmingly low. It is unclear how prevalent patient adherence may be across small geographic areas and whether this geographic prevalence may vary.
Methods
This is a retrospective cohort study using Medicare service claims files from 2007 to 2009 with Medicare beneficiaries ≥ 65 years who were alive 30 days after the index AMI hospitalization between 1/1/2008 to 12/31/2008 (N=85,017). The adjusted proportions of patients adherent to β-blockers, statins, and ACEIs/ARBs respectively in the 12 months after discharge across the 306 Hospital Referral Regions (HRRs) were measured and compared by control chart. The intracluster correlation coefficient (ICC) and the additional prediction power from this small-area variation on individual patient adherence were assessed.
Results
The adjusted proportion of patients adherent across HRRs ranged from 58% to 74% (median, 66%) for β-blockers, from 57% to 67% (median, 63%) for ACEIs/ARBs, and from 58% to 73% (median, 66%) for statins. The ICC was 0.053 (95% CI, 0.043–0.064) for β-blockers, 0.050 (95% CI, 0.039–0.061) for ACEIs/ARBs, and 0.041 (95% CI, 0.031–0.052) for statins. The adjusted proportion of patients adherent across HRRs increased the c-statistic by 0.01 to 0.02 (P<0.0001).
Conclusions
Non-adherence to evidence-based preventive therapies post AMI among older adults was prevalent across small geographic regions. Moderate small-area variation in patient adherence exists.
Keywords: compliance/adherence, small area variation, acute myocardial infarction, Secondary prevention, Medication Adherence, Regional Variation
INTRODUCTION
Clinical guidelines recommend long-term use of β-blockers, HMG-CoA reductase inhibitors (statins), angiotensin-converting-enzyme (ACEIs) /angiotensin receptor blockers (ARBs) as secondary preventive therapies after acute myocardial infarction (AMI).1,2 Recent studies have shown considerable improvements in the prescribing of the preventive therapies at discharge after years of implementation of the Get With The Guidelines (GWTG) program.3–5 However, evidence-based therapies may not be able to optimally reduce mortality and morbidity following AMI if patients do not take them as directed. The benefit of these preventive therapies depends on long-term adherence.6–8 Adherence to the evidence-based therapies are typically defined as more than 80% of time a patient is covered with prescription supply.7 In a recent population-based longitudinal study of 31,455 elderly AMI survivors, patients who had low adherence to the evidenced-based therapies had a 25% higher mortality risk (95% CI, 9% to 42%) as compared to patients who had high adherence.7 It was also shown that non-adherent patients had a 44% higher likelihood (95% CI, 15% to 79%) of 1-year mortality than adherent patients after AMI hospital discharge.8 Another study also showed that AMI patients who were adherent had a 53% lower risk for mortality (95% CI, 1% to 79%) and 81% lower risk for recurrent AMI (95% CI, 53% to 92%).6 However, patient medication adherence following AMI has been shown to be worryingly low in general. One year after hospital discharge, approximately half of Medicare patients have been shown to be non-adherent to statins, β-blockers, and ACEI/ARB treatments.9–11 It has also been argued that medication adherence should be a priority for health care reform.12
From a public health perspective, an efficient national intervention program may be enhanced by targeting high risk populations, and also areas with a high prevalence of non-adherence. Previous studies have shown that there were considerable regional variations in prescribing β-blockers, statins, and ACEIs/ARBs at discharge after AMI, which indicated difference in the quality of care across regions.13–18 However, it is unclear whether small-area variation exists in patient longer-term adherence to the prescribed preventive therapies following AMI discharge, and the extent of this variation. If such variation in patient adherence to the evidence-based preventive therapies exists, identifying these regions with high prevalence of patient non-adherence may facilitate future research to design more efficient and targeted public health programs and policies to address patient non-adherence to evidence-based preventive therapies.
Therefore, this study aimed to 1) assess patient adherence to the 3 evidence-based therapies post AMI among a national cohort of older adults who survived AMI in the 12 months after their discharge across Dartmouth Atlas Hospital Referral Regions (HRRs) after adjustment of non-modifiable patient characteristics; 2) to compare and identify the HRRs with high prevalence of non-adherence; and 3) to assess the extent of the variation in patient adherence across HRRs and whether the variation added explanation or prediction power of individual patient adherence status in addition to individual patient risk factors.
METHODS
Setting and Population
The cohort for this study included all Medicare beneficiaries who were 1) ≥ 65 years old; 2) continuously enrolled in the Medicare fee-for-service and prescription Part D programs at least 12 months before and until the end of the study period (or death) after an index AMI hospitalization; 3) hospitalized for the index AMI between January 1, 2008 to December 31, 2008 and survived at least 30 days after discharge; 4) were discharged to home or to skilled-nursing and long-term care facilities and had any prescription claims within 30 days after discharge. Hospitalization with AMI was defined as having an international classification of diseases (ICD) 9 code of 410.×1 as the primary or secondary discharge diagnosis in Medicare inpatient claims.19–21 The first AMI hospitalization in the study period was defined as the index AMI hospitalization for each subject.
Primary data used for this study were Medicare service claims and files from the Center for the Medicare and Medicaid Services Medicare Chronic Condition Data Warehouse (CCW) from 2007 to 2009.22 The CCW files includes inpatient, outpatient, skilled nursing facility, carrier (physician office visits), and prescription Part D event service claims files. All CCW files are linked by an encrypted and unique CCW identifier number for each beneficiary. Other data included the HRRs boundary files, which were linked to CCW files by ZIP codes of patient residence.
Treatment Measurement
The use of β-blockers, ACEIs/ARBs, or statins after AMI was defined as 1) filled a prescription within the drug class within 30 days after hospital discharge or 2) had a prescription supply greater than 30 days from the last prescription filled prior to the AMI admission and filled a prescription for the same class of the drug with 60 days after discharge. The specific drug was identified through national drug codes in the Medicare Part D prescription event files in the Medicare CCW.
Adherence Measurement
Adherence was measured as the proportion of days covered (PDC) by the prescription supply calculated from the prescription refill records in the prescription Part D event files in the 12 months (or until death if death occurred within 12 months) post AMI discharge among patients who had the preventive therapies within 30 days after AMI discharge.23 The adherence measure was also adjusted for over-stock of prescription supply from refills and hospital stays during the study period after AMI discharge. Conforming to current literature, a patient is defined as adherent if he/she had ≥ 80% of days covered with prescription supply in the study period.7
Measurement of Baseline Characteristics/Covariates
Age, gender, and race were determined by using CCW enrollment summary files from the index year. Median income for 65 years and older was measured at Census Block Groups residence level using 2010 US Census data. Comorbidities were measured by the Charlson comorbidity index in the 12 months prior to the index AMI admission using CCW claims files.24 Other variables included whether a subject was in the Medicare Part D benefit gap (“doughnut-hole”) prior to the index AMI admission, diagnosis of cardiovascular disease and other related risk factors in the 12-month baseline [including AMI, coronary artery bypass surgery (CABG), stent/percutaneous transluminal coronary angioplasty (PTCA), stroke/transient ischemic attack, unstable angina, ischemic heart disease, heart failure, atrial fibrillation, peripheral vascular disease, hypertension, diabetes, and hyperlipidemia], baseline potential contraindication or intolerant conditions (chronic kidney disease, chronic obstructive pulmonary disease, asthma, liver disease, angioedema, hyperkalemia, hypotension, sinus bradycardia, heart block, and rhabdomyoloysis/other myopathy), prescriptions of β-blockers, ACEIs/ARBs, or statins in the 6 months prior to the index AMI, AMI type (subendocardial or transmural infarction), procedures (CABG, stent/PTCA, cardiac catheterization, infusion of thrombolytic, infusion of platelet inhibitors) or complications (congestive heart failure, cardiogenic shock, acute renal failure, hypotension, cardiac dysrhythmias) during the index AMI hospitalization, and total Intensive Care Unit (ICU) and inpatient length of stay for the index AMI. Other factor measured include discharge destination (home or skilled nursing facilities) and risk factors that may be associated with nonadherence such as the total number of different prescription medications used (polypharmacy) and diagnosis of dementia in the 12-month baseline were also measured. Those characteristics were measured using files based on the standardized algorithms in the CCW and other algorithms applied in the literature.22,24,25
Measurement of Small-area Variation in Adherence
To assess small-area variation in patient adherence to the 3 evidence-based therapies post AMI, we measured the adjusted proportion of patients who were adherent to each of the therapies across Dartmouth HRRs. The Dartmouth HRRs represent regional health care markets for tertiary medical care that generally require the services of a major referral center.15 The regions were defined by determining where patients were referred for major cardiovascular surgical procedures and for neurosurgery. Each HRR has at least one city where both major cardiovascular surgical procedures and neurosurgery are performed. We assigned each patient to one of the HRRs on the basis of his/her ZIP code of residence. The adjusted proportion of patients adherent to a therapy across HRRs was calculated as the predicted number of patients adherent to a therapy divided by the total number of the patients in a HRR. The predicted number of patients adherent to a therapy was computed by summing all patients’ probabilities of being adherent in each HRR. The probability of being adherent for each individual patient was estimated using a mixed-effect hierarchical logistic regression model with HRRs as random effects and random intercept (SAS glimmix procedure).26–28 The model was adjusted for all measured baseline patient characteristics and also the clustering of patients and small and unequal sample sizes across HRRs.26–28 Sensitivity analysis was also performed for testing and adjusting for spatial autocorrelation.
Analysis
The characteristics of patients who initiated each therapy and the proportion of patients who were adherent to each therapy were described. The distribution of mean proportion of days covered by a therapy post AMI discharge to a therapy across HRRs was assessed using a box plot. The HRRs were ranked by the adjusted proportion of patients adherent to each of the 3 therapies. To compare adherence rate across HRRs and identify HRRs with higher prevalence of non-adherence, we used control chart to plot the adjusted proportion of patients adherent to a therapy across HRRs to identify the HRRs with the proportion below the 5th percentile.29
To assess the extent of the variation in patient adherence across HRR, we calculated the intraclass (intracluster) correlation coefficient (ICC).30 The ICC is a ratio between the between-cluster variance and the total variance of between- and within-cluster variance. The mean and the 95% confidence intervals of the ICC were generated by the bootstrap (re-sampling) of 1000 iterations with replacement from all patients in the cohort.31
To assess whether small-area variation in adherence adds prediction of individual patient adherence beyond individual patient risk factors, we compared the c-statistics (Area Under the Curve, AUC) from logistic regression models with measured patient characteristics and with both patient characteristics and the adjusted proportion of adherent patients across HRRs.
All analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC), and the maps were created using ArcGIS version 10 (ESRI Inc., Redlands, CA).
RESULTS
A total of 85,017 patients were included in the final analysis with 64,939 (76%) using β-blockers, 52,185 (61%) using statins, and 47,127 (55%) using ACEIs/ARBs within 30 days after discharge. About 66% users of β-blockers, 66% users of statins and 63% users of ACEIs/ARBs were adherent during the 12 months following discharge. Table 1 presents the characteristics of patients who initiated the various preventive therapies and the proportion of patients adherent for those respective characteristics. For example, approximately 58%, 59% and 56% of patients who initiated β-blockers, ACEIs/ARBs were female, respectively. The proportion of patients adherent to β-blockers increased from 65% in those 65–74 years old to 68% in those 85 years and older, from 62% to 64% for ACEIs/ARBs and from 65% to 68% for statins. The proportion of patients adherent to β-blockers decreased from 67% among patients with a Charlson comorbidity index of 0 to 65% among patients with a Charlson comorbidity index of 9 or higher. Patients who had potential contraindicative or intolerant conditions to the preventive therapies had lower adherence rates.
Table 1.
Patient characteristics and 12-month adherence for each preventive therapy post AMI
| Baseline characteristics | β-blockers (n=64,939) | ACEIs/ARBs (n=47,124) | Statins (n= 52,185) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| % of the characteristic | proportion (%) of the adherent | % of the characteristic | proportion (%) of the adherent | % of the characteristic | proportion (%) of the adherent | |
| Total | 100.0 | 66.2 | 100.0 | 62.7 | 100.0 | 66.3 |
|
| ||||||
| Demographics | ||||||
| Gender | ||||||
| Male | 42.5 | 64.5 | 41.0 | 60.9 | 44.1 | 66.6 |
| Female | 57.5 | 67.5 | 59.0 | 64.0 | 55.9 | 66.1 |
| Age | ||||||
| 65–74 | 36.1 | 64.8 | 36.3 | 61.7 | 38.5 | 65.3 |
| 75–84 | 38.1 | 66.4 | 38.6 | 63.1 | 39.0 | 66.5 |
| 85+ | 25.8 | 67.7 | 25.1 | 63.6 | 22.6 | 67.8 |
| Race | ||||||
| White | 85.4 | 67.1 | 84.5 | 63.1 | 85.0 | 67.2 |
| Black | 8.5 | 59.2 | 8.9 | 58.5 | 8.5 | 58.6 |
| Hispanic | 2.6 | 60.7 | 2.9 | 62.1 | 2.7 | 60.5 |
| Asian | 1.7 | 65.8 | 1.9 | 64.2 | 1.9 | 69.5 |
| Other | 1.7 | 63.4 | 1.8 | 64.0 | 1.9 | 65.8 |
| Median income among the age of 65+ at Census block groups of residence | ||||||
| $30,000 and below | 47.1 | 65.9 | 47.4 | 62.6 | 47.0 | 65.5 |
| $30,001–$60,000 | 41.9 | 66.8 | 41.5 | 62.4 | 41.5 | 66.8 |
| $60,001–$100,000 | 9.1 | 65.1 | 9.1 | 64.1 | 9.4 | 68.0 |
| $100,001–$150,000 | 1.5 | 63.9 | 1.6 | 64.4 | 1.6 | 67.6 |
| $150,001 and above | 0.5 | 66.8 | 0.5 | 66.8 | 0.5 | 71.4 |
|
| ||||||
| Prescription Benefit Gap | ||||||
| In Part D “doughnut-hole” prior to AMI admission | 11.2 | 69.1 | 11.7 | 65.5 | 11.4 | 71.8 |
|
| ||||||
| Baseline (12 months prior to index) co-morbidities and polypharmacy | ||||||
| AMI | 4.3 | 67.1 | 4.2 | 62.6 | 4.2 | 67.8 |
| CABG | 0.6 | 69.0 | 0.6 | 61.8 | 0.7 | 72.2 |
| Stent/PTCA | 3.2 | 64.8 | 3.3 | 61.6 | 3.4 | 66.8 |
| Stroke/TIA | 18.1 | 66.5 | 18.3 | 60.9 | 17.0 | 66.7 |
| Unstable Angina | 3.3 | 64.8 | 3.3 | 61.2 | 3.3 | 67.3 |
| IHD | 3.8 | 65.7 | 3.8 | 61.5 | 4.0 | 67.5 |
| HF | 15.0 | 67.1 | 14.9 | 60.5 | 13.7 | 67.0 |
| Atrial Fibrillation | 2.8 | 66.7 | 2.7 | 61.4 | 2.5 | 66.6 |
| PVD | 5.9 | 64.5 | 5.4 | 59.3 | 5.8 | 66.4 |
| Hypertension | 29.7 | 66.0 | 29.9 | 61.4 | 28.2 | 65.9 |
| Diabetes | 15.9 | 65.0 | 16.3 | 61.5 | 15.5 | 66.9 |
| Hyperlipidemia | 15.6 | 65.8 | 15.6 | 61.6 | 16.2 | 67.9 |
| Dementia | 15.9 | 65.0 | 16.3 | 61.5 | 15.5 | 66.9 |
| Charlson Comorbidity Index | ||||||
| 0 | 63.2 | 66.8 | 63.6 | 63.9 | 64.7 | 66.6 |
| 1–2 | 9.3 | 64.5 | 9.4 | 61.3 | 9.2 | 65.1 |
| 3–5 | 12.6 | 66.1 | 12.8 | 61.5 | 11.5 | 66.3 |
| 6–8 | 7.0 | 65.5 | 6.6 | 59.1 | 6.7 | 66.1 |
| 9+ | 3.6 | 64.7 | 3.3 | 58.5 | 3.3 | 67.8 |
| No. of Medications | ||||||
| 0–2 | 10.2 | 64.9 | 9.5 | 62.4 | 10.5 | 65.1 |
| 3–5 | 26.7 | 66.1 | 26.6 | 62.8 | 26.8 | 65.6 |
| 6–10 | 25.9 | 66.5 | 26.5 | 63.1 | 25.5 | 67.0 |
| 11–15 | 31.2 | 67.7 | 32.0 | 63.4 | 30.7 | 68.1 |
| 15+ | 9.9 | 65.9 | 8.4 | 57.5 | 9.3 | 67.0 |
|
| ||||||
| Baseline (12 months prior to index) potential contraindicative or intolerant conditions to the therapies | ||||||
| CKD | 9.8 | 65.9 | 8.4 | 57.5 | 9.3 | 66.0 |
| COPD | 2.1 | 65.0 | 2.0 | 60.4 | 1.8 | 63.0 |
| Asthma | 2.5 | 63.6 | 2.7 | 60.5 | 2.6 | 64.7 |
| Liver Disease | 6.4 | 64.2 | 6.0 | 59.3 | 6.1 | 65.9 |
| Angioedema or Hyperkalemia | 4.2 | 66.8 | 3.6 | 58.3 | 4.0 | 66.5 |
| Hypotension | 5.0 | 64.9 | 4.6 | 58.2 | 4.8 | 66.8 |
| Sinus Bradycardia or Heart Block | 2.5 | 63.6 | 14.2 | 62.6 | 13.6 | 66.7 |
| Rhabdomyolysis or other myopathy | 6.4 | 64.2 | 0.4 | 57.7 | 0.4 | 65.0 |
|
| ||||||
| Baseline (6 months prior to index) prescriptions | ||||||
| β-blockers | 55.1 | 70.7 | 52.8 | 65.0 | 51.4 | 68.8 |
| ACEIs or ARBs | 53.8 | 68.8 | 67.2 | 66.8 | 54.5 | 68.3 |
| Statins | 46.5 | 68.9 | 48.5 | 65.2 | 56.7 | 71.9 |
|
| ||||||
| AMI type, complications & procedures during AMI admission | ||||||
| Subendocardial infarction (NSTEMI) | 73.3 | 65.8 | 72.5 | 62.7 | 72.1 | 66.3 |
| Complications | ||||||
| HF | 35.1 | 67.5 | 36.0 | 61.7 | 32.6 | 66.9 |
| Cardiogenic Shock | 2.4 | 69.8 | 2.5 | 59.2 | 2.5 | 69.7 |
| Acute Renal Failure | 12.7 | 33.3 | 10.8 | 58.5 | 12.0 | 66.3 |
| Hypotension | 4.9 | 63.9 | 4.7 | 58.8 | 5.1 | 66.9 |
| Cardiac dysrhythmias | 30.4 | 67.1 | 30.5 | 62.9 | 29.7 | 67.1 |
| Procedures | ||||||
| CABG | 7.2 | 65.2 | 6.0 | 61.1 | 7.9 | 68.1 |
| Stent/PTCA | 35.7 | 67.0 | 37.0 | 63.5 | 40.0 | 66.8 |
| Cardiac catheterization | 52.6 | 66.4 | 53.9 | 62.8 | 57.3 | 66.3 |
| Infusion of thrombolytic | 0.6 | 66.6 | 0.6 | 64.8 | 0.6 | 64.9 |
| Infusion of platelet inhibitors | 4.5 | 68.1 | 4.5 | 62.9 | 5.0 | 65.8 |
|
| ||||||
| Days of hospital stays for AMI admission | ||||||
| Days of ICU stays | ||||||
| 0 | 48.5 | 66.8 | 48.4 | 63.4 | 48.1 | 66.5 |
| 1–3 | 29.1 | 65.9 | 29.5 | 63.1 | 29.9 | 66.0 |
| 4–10 | 19.5 | 64.9 | 19.4 | 60.8 | 19.1 | 66.1 |
| 11+ | 3.0 | 67.4 | 2.8 | 59.8 | 3.0 | 68.6 |
| Days of total inpatient stays | ||||||
| 1 | 4.4 | 65.6 | 4.4 | 64.4 | 4.6 | 65.1 |
| 2–5 | 56.3 | 66.2 | 57.6 | 64.1 | 57.4 | 66.3 |
| 6–10 | 38.1 | 66.3 | 37.9 | 61.1 | 36.8 | 66.3 |
| 11+ | 12.0 | 66.0 | 11.0 | 59.7 | 11.8 | 67.1 |
|
| ||||||
| Discharge destination | ||||||
| Home | 81.0 | 65.7 | 81.8 | 62.7 | 83.1 | 66.1 |
| Skilled Nursing facilities | 19.0 | 68.4 | 18.2 | 62.7 | 16.9 | 67.7 |
|
| ||||||
| Concurrent treatments | ||||||
| β-blockers | – | – | 84.2 | 63.1 | 84.9 | 66.9 |
| ACEIs/ARBs | 61.1 | 68.0 | – | – | 63.4 | 67.8 |
| Statins | 68.8 | 67.8 | 70.2 | 64.1 | – | – |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor. ARBs, angiotensin-receptor blockers. Statins, hydroxymethylglutaryl coenzyme A reductase inhibitors. AMI, acute myocardial infarction. CABG, coronary artery bypass surgery. PTCA, percutaneous transluminal coronary angioplasty. TIA, transient ischemic attack. IHD, ischemic heart disease. HF, congestive heart failure. PVD, peripheral vascular disease. CKD, chronic kidney disease. COPD, chronic obstructive pulmonary disease. NSTEMI, non-ST elevated myocardial infarction. ICU, intensive care unit.
The distribution of mean adherence (PDC) across HRR is presented in Appendix 1. The mean (standard deviation) is 0.77 (0.031), 0.80 (0.034), 0.079 (0.033) for ACEIs/ARBs, β-blockers, and statins respectively.
Figure 1 shows the mapping of the proportion of patients adherent to the 3 preventive therapies in the 12 months after discharge across HRRs after adjustment for non-modifiable factors (patient baseline sociodemographic and clinical characteristics). The proportion of patients adherent to β-blockers ranged from 58% to 74% across HRRs, from 58% to 67% for ACEIs and ARBs, and from 58% to 73% for statins. The patterns of the regional variation in adherence were similar for the 3 therapies. In general, HRRs in the south tend to have lower proportion of patients adherent to the 3 therapies than the HRRs in the north.
Figure 1.

Maps of variations in the adjusted proportion of AMI patients adherent to evidence-based preventive therapies in the 12 months after AMI discharge across hospital referral regions (HRRs)
Figure 2 A–C present the control chart plots of each HRR’s adjusted proportion of patients adherent to the 3 preventive therapies, respectively. Each circle in the plots represents a HRR. The 3 dashed reference lines represent the 95th percentile, median and 5th percentile of the adjusted proportion of patients adherent for a preventive therapy. The median of the adjusted proportion of patients adherent to β-blockers across HRRs was 66%, which means that half of the HRRs had fewer than 66% of patients adherent to β-blockers. The median of the adjusted proportion of patients adherent to statins and ACEIs/ARBs were 66% and 63%, respectively. Consistent results were found in sensitivity analysis of adjusting for spatial autocorrelation in the mixed-effect hierarchical logistic regression model.
Figure 2.
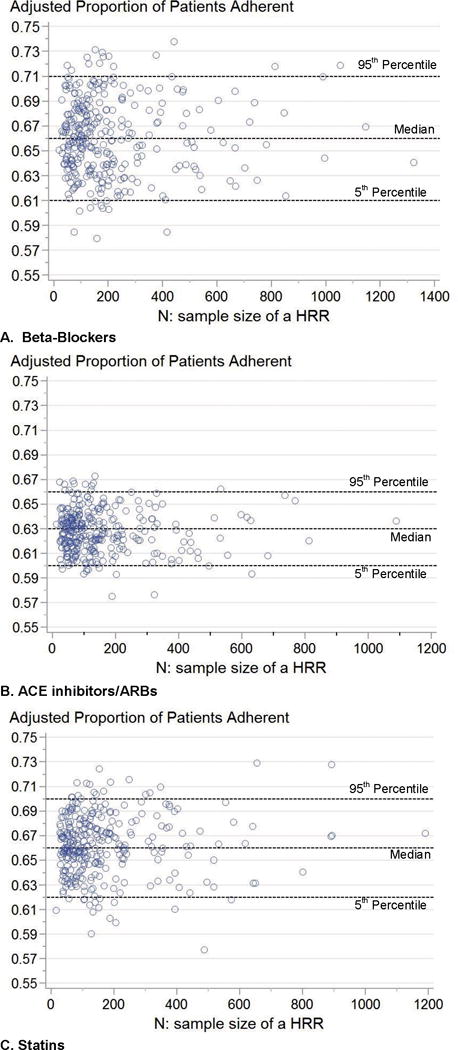
Scatter plots of the adjusted proportion of AMI patients adherent to evidence-based preventive therapies across HRRs by the adjusted proportion and sample size
The 5 percentile of adjusted proportion of patients adherent across HRRs was 61%, 60%, and 62% for beta-blockers, ACEI/ARBs and statins respectively. Appendix 2 listed the HRRs below the 5th percentile of the adjusted proportion of patients adherent for each evidence-based preventive therapy. HRR with lowest patient adherence to β-blockers was Orlando FL, Jacksonville FL for ACEIs/ARBs, and El Paso TX for Statins.
Table 2 presents the intracluster (intraclass) correlation coefficients to assess the extent of the variation in patient adherence to the 3 preventive therapies across HRRs. The ICC ranged from 0.041 for statins, to 0.050 for ACEIs/ARBs and to 0.053 for beta-blockers.
Table 2.
The intracluster (intraclass) correlation coefficients (ICC) for measuring the extent of variation in patient adherence to post AMI therapies across hospital referral regions
| Therapy | mean | 95% Confidence Intervals (CI)† |
|---|---|---|
| β-blockers | 0.053 | (0.043, 0.064) |
| ACEIs/ARBs | 0.050 | (0.039, 0.061) |
| Statins | 0.041 | (0.031, 0.052) |
Abbreviation: ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotension receptor blockers.
The 95% CI was generated by a bootstrap of 1000 iterations with replacement from all patients with respective preventive therapy in the study cohort.
Table 3 presents the difference in the AUC between the logistic model with patient characteristics and the logistic model with both patient characteristics and the adjusted proportion of patients adherent across HRRs. Adding the variation in the proportion of patients adherent across HRRs increased the AUC by 0.014 for β-blockers, 0.009 for ACEIs/ARBs, and 0.012 for statins (p < 0.0001).
Table 3.
Assessment of added prediction power by the adjusted proportion of patients adherent across Hospital Referral Regions
| Therapy | AUC of Model 1: sociodemographics + clinical characteristics* | AUC of Model 2: Model 1 + the adjusted proportion of patients adherent across HRRs | Difference in AUC | 95% CI of AUC Difference | p-value |
|---|---|---|---|---|---|
| β-blockers | 0.596 | 0.609 | 0.014 | (0.011, 0.016) | < 0.0001 |
| ACEI or ARBs | 0.597 | 0.606 | 0.009 | (0.007, 0.011) | < 0.0001 |
| statins | 0.602 | 0.614 | 0.012 | (0.010, 0.014) | < 0.0001 |
Abbreviation: ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotension receptor blockers; AUC, area under the curve, the c statistic.
all measured patient sociodemographic and clinical characteristics at baseline listed in Table 1
DISCUSSION
In our study of 85,017 elderly AMI survivors in 2008, we found that non-adherence was highly prevalent across the HRRs in the US. Half of the 306 HRRs had fewer than approximately 65% of patients adherent to the 3 evidence-based preventive therapies in the 12 months post AMI. The range in the adjusted proportion of patients adherent to the 3 evidence-based AMI therapies across HRRs were about 8 to 16 percentage points. The ICC, which measures the extent of the adherence variation across HRRs as compared variation across individuals, was 0.041 to 0.053. In a review of 31 cluster-based studies on health outcomes and clinical practice, it was shown that the median ICC was 0.005 with the interquartile ranging from 0 to 0.021 after adjusting for individual and cluster-level characteristics.32 Another study of 188 ICCs for primary care health services and binary health outcomes found that the median ICC was 0.051 with interquartile from 0.011 to 0.094.33 Therefore, the ICC value of 0.041 to 0.053 in our study suggests a moderate variation across HRRs in patient adherence relatively to typical regional variations in healthcare settings.
We ranked the HRRs based on the adjusted proportion of patients adherent and listed the HRRs below the 5th percentile. There are limitations of using quantitative comparison as performance indicators since it remains difficult to determine the meaningful extent of differences in the comparison to differentiate individual clusters in their performance.29,34,35 However, HRRs with the adjusted proportion of patients adherent below the 5th percentile may be reasonably considered as outliers.
The considerable increased hospital readmission and mortality risk associated with non-adherence to the evidence-based preventive therapies has received increasing attention from clinicians, researchers and policy makers.10,12,36–40 Given the prevalent non-adherence to evidence-based preventive therapies among patients, a public health approach to address non-adherence may be an important. In this study, the observed moderate variation in patient adherence across relatively small health service regions was unexplained by non-modifiable factors such as patient sociodemographic and clinical characteristics. This unexplained small-area variation in patient adherence may suggest that modifiable factors such as provision and quality of care may play an important role. Studies have suggested that the geographic variation in prescribing of evidence-based therapies may stem from the disparities in the provision of quality of care and processes across regions.13–18,41,42 A complexity in the processes of care potentially impacting patient long-term adherence is the involvement of patients, multiple care providers including cardiologists, family care providers, and pharmacists across institution and community settings. It has also been shown that care processes and provisions such as continuity of care (e.g., follow-up care and medication reconciliation), coordination of care (e.g., “therapeutic complexity” – having multiple prescribers, pharmacies, and care providers), and provider-patient communication affected patient adherence to prescribed preventive therapies.43–45 It is possible that differences in the quality of care process and provision and health literacy level across HRRs may be important contributors to the variation in patient adherence to evidence-based therapies unexplained by patient sociodemographic and clinical characteristics. Future studies are necessary to identify the differences in care process and provision that may lead to the small-area variation in patient adherence.
National policies and programs, for example the Health Plan Employer Data and Information Set (HEDIS) Measures and the GWTG program, are in place to enhance the prescribing of evidence-based therapies for AMI care, and improvements have been achieved in prescribing β-blockers, statins and ACEIs/ARBs at discharge.3–5 However, there is a need for more national programs and policies that specifically aim to improve the care process associated with patient adherence to the evidence-based preventive therapies. Our study suggests that it may be useful for programs to add emphasis on small health service areas with high prevalence of non-adherence. Reasons for patient non-adherence are usually multifactorial.12,38,40,46 The proportion of patients adherent across HRRs added a statistically significant but mild prediction of individual patient adherence beyond non-modifiable individual patient characteristics. The mildly added prediction for individual patients suggests that program with emphasis on small health services areas with high prevalence of non-adherence needs to be coupled with interventions on various individual risk factors to improve adherence.
Limitations of this study are common to all studies using healthcare administrative claims. To address those limitations, we applied previously validated algorithms in the literature to measure index AMI with a positive predictive value of 89% to 97%.19–21 Our data cannot elucidate whether non-adherence was due to adverse side effects. However, the 3 evidence-based therapies are generally well-tolerated, and the incidence of adverse side effects is low; it is also unlikely that adverse effects would be differential across geographic regions.7 We have also adjusted for potential intolerant and contraindicative conditions to the use of the therapies at baseline. We used prescription refill records to measure adherence without information on actual medication taking. However, prescription refill records have been shown to have good validity and correlation with pill counts.7,23 The nature of retrospective cohort analysis using claims files does not allow us to measure all potential confounding factors. It remains possible that unmeasured confounders may contribute to the observed variation in the prevalence of non-adherence across HRRs. However, it is suggested that important bias in regional variation in medical practice will be rare, and regional variation in medical practice as proxy for confounder misclassification will typically be negligible.47 Patients discharged to home may have difference adherence from that of patients discharged to skilled nursing facilities. Future study may be needed to assess whether small-area variation in patient adherence may differ by discharge location. Our study has several strengths. To our knowledge, the small-area variation in patient adherence in preventive therapies has not been assessed in other studies. We utilized a nationally representative cohort of 100% Medicare patient samples who were enrolled in the fee-for-service and Part D program and the use of recent 2007–2009 data representing contemporary settings.
In summary, moderate variation across small geographic area in patient adherence to evidence-based preventive therapies exists among older adults post AMI. This moderate small-area variation in patient adherence was unexplained by non-modifiable patient sociodemographic and clinical characteristics.
Supplementary Material
Contributor Information
Gang Fang, Email: gang_fang@unc.edu, Division of Pharmaceutical Outcomes and Policy, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, 2202 Kerr Hall, Chapel Hill, NC 27599-7517, Phone: 919-966-7573, Fax: 919-966-8486.
Jennifer Robinson, Email: jennifer-g-robinson@uiowa.edu, Department of Epidemiology, Division of Cardiology, College of Public Health, College of Medicine, University of Iowa, S455 CPHB, Iowa City, Iowa 52242, Phone: 319-384-1563, Fax: 319-384-4155.
Julie Lauffenburger, Email: jlauffen@email.unc.edu, Division of Pharmaceutical Outcomes and Policy, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, 2316 Kerr Hall, Chapel Hill, NC 27599-7573, Phone: 919-966-1169, Fax: 919-966-8486.
Mary T. Roth, Email: mroth@unc.edu, Division of Pharmaceutical Outcomes and Policy, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, 2203 Kerr Hall, Chapel Hill, NC 27599-7573, Phone: 919-843-8083, Fax: 919-966-8486.
M. Alan Brookhart, Email: mabrook@email.unc.edu, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, 2105F McGavran-Greenberg, Chapel Hill, NC 27599-7435, Phone: 919-843-2639, Fax: 919-966-2089.
References
- 1.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006 May 16;113(19):2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007 Aug 14;50(7):e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Lewis WR, Peterson ED, Cannon CP, et al. An Organized Approach to Improvement in Guideline Adherence for Acute Myocardial Infarction: Results With the Get With The Guidelines Quality Improvement Program. Arch Intern Med. 2008 Sep 8;168(16):1813–1819. doi: 10.1001/archinte.168.16.1813. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burwen DR, Galusha DH, Lewis JM, et al. National and State Trends in Quality of Care for Acute Myocardial Infarction Between 1994–1995 and 1998–1999: The Medicare Health Care Quality Improvement Program. Arch Intern Med. 2003 Jun 23;163(12):1430–1439. doi: 10.1001/archinte.163.12.1430. 2003. [DOI] [PubMed] [Google Scholar]
- 5.Jernberg T, Johanson P, Held C, Svennblad B, Lindbäck J, Wallentin L. Association Between Adoption of Evidence-Based Treatment and Survival for Patients With ST-Elevation Myocardial Infarction. JAMA: The Journal of the American Medical Association. 2011 Apr 27;305(16):1677–1684. doi: 10.1001/jama.2011.522. 2011. [DOI] [PubMed] [Google Scholar]
- 6.Wei L, Wang J, Thompson P, Wong S, Struthers AD, MacDonald TM. Adherence to statin treatment and readmission of patients after myocardial infarction: a six year follow up study. Heart. 2002 Sep 1;88(3):229–233. doi: 10.1136/heart.88.3.229. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen JN, Chong A, Alter DA. Relationship Between Adherence to Evidence-Based Pharmacotherapy and Long-term Mortality After Acute Myocardial Infarction. JAMA: The Journal of the American Medical Association. 2007 Jan 10;297(2):177–186. doi: 10.1001/jama.297.2.177. 2007. [DOI] [PubMed] [Google Scholar]
- 8.Jackevicius CA, Li P, Tu JV. Prevalence, Predictors, and Outcomes of Primary Nonadherence After Acute Myocardial Infarction. Circulation. 2008 Feb 26;117(8):1028–1036. doi: 10.1161/CIRCULATIONAHA.107.706820. 2008. [DOI] [PubMed] [Google Scholar]
- 9.Choudhry NK, Winkelmayer WC. Medication adherence after myocardial infarction: a long way left to go. Journal of general internal medicine. 2008 Feb;23(2):216–218. doi: 10.1007/s11606-007-0478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choudhry NK, Setoguchi S, Levin R, Winkelmayer WC, Shrank WH. Trends in adherence to secondary prevention medications in elderly post-myocardial infarction patients. Pharmacoepidemiology and drug safety. 2008 Dec;17(12):1189–1196. doi: 10.1002/pds.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA : the journal of the American Medical Association. 2002 Jul 24–31;288(4):462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 12.Cutler DM, Everett W. Thinking outside the pillbox–medication adherence as a priority for health care reform. The New England journal of medicine. 2010 Apr 29;362(17):1553–1555. doi: 10.1056/NEJMp1002305. [DOI] [PubMed] [Google Scholar]
- 13.Pilote L, Califf RM, Sapp S, et al. Regional variation across the United States in the management of acute myocardial infarction. GUSTO-1 Investigators. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. N Engl J Med. 1995 Aug 31;333(9):565–572. doi: 10.1056/NEJM199508313330907. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor GT, Quinton HB, Traven ND, et al. Geographic variation in the treatment of acute myocardial infarction: the Cooperative Cardiovascular Project. JAMA. 1999 Feb 17;281(7):627–633. doi: 10.1001/jama.281.7.627. [DOI] [PubMed] [Google Scholar]
- 15.Dartmouth Medical School. The Dartmouth atlas of health care in the United States. Chicago: American Hospital Publishing; 1999. [Google Scholar]
- 16.Jencks SF, Huff ED, Cuerdon T. Change in the quality of care delivered to Medicare beneficiaries, 1998–1999 to 2000–2001. JAMA. 2003 Jan 15;289(3):305–312. doi: 10.1001/jama.289.3.305. [DOI] [PubMed] [Google Scholar]
- 17.Krumholz HM, Chen J, Rathore SS, Wang Y, Radford MJ. Regional variation in the treatment and outcomes of myocardial infarction: investigating New England’s advantage. Am Heart J. 2003 Aug;146(2):242–249. doi: 10.1016/S0002-8703(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 18.Ko DT, Krumholz HM, Wang Y, et al. Regional Differences in Process of Care and Outcomes for Older Acute Myocardial Infarction Patients in the United States and Ontario, Canada. Circulation. 2007 Jan 16;115(2):196–203. doi: 10.1161/CIRCULATIONAHA.106.657601. 2007. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002 Aug;144(2):290–296. doi: 10.1067/mhj.2002.123839. [DOI] [PubMed] [Google Scholar]
- 20.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004 Jul;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Choma NN, Griffin MR, Huang RL, et al. An algorithm to identify incident myocardial infarction using Medicaid data. Pharmacoepidemiol Drug Saf. 2009 Nov;18(11):1064–1071. doi: 10.1002/pds.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chronic Condition Data Warehouse User Manual. 2009;1:5. http://www.ccwdata.org/datadoc.php. Accessed August 11th, 2009. [Google Scholar]
- 23.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Medical Care. 1988;26(8):814–823. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993 Oct;46(10):1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–1090. [DOI] [PubMed] [Google Scholar]
- 25.Andrade SE, Graham DJ, Staffa JA, et al. Health plan administrative databases can efficiently identify serious myopathy and rhabdomyolysis. J Clin Epidemiol. 2005 Feb;58(2):171–174. doi: 10.1016/j.jclinepi.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Christiansen CL, Morris CN. Improving the statistical approach to health care provider profiling. Annals of internal medicine. 1997 Oct 15;127(8 Pt 2):764–768. doi: 10.7326/0003-4819-127-8_part_2-199710151-00065. [DOI] [PubMed] [Google Scholar]
- 27.Krumholz HM, Wang Y, Mattera JA, et al. An Administrative Claims Model Suitable for Profiling Hospital Performance Based on 30-Day Mortality Rates Among Patients With an Acute Myocardial Infarction. Circulation. 2006 Apr 4;113(13):1683–1692. doi: 10.1161/CIRCULATIONAHA.105.611186. 2006. [DOI] [PubMed] [Google Scholar]
- 28.Normand S-LT, Glickman ME, Gatsonis CA. Statistical Methods for Profiling Providers of Medical Care: Issues and Applications. Journal of the American Statistical Association. 1997;92(439):803–814. [Google Scholar]
- 29.Adab P, Rouse AM, Mohammed MA, Marshall T. Performance league tables: the NHS deserves better. BMJ. 2002;324(7329):95–98. doi: 10.1136/bmj.324.7329.95. 2002-01-12 00:00:00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridout MS, Demétrio CGB, Firth D. Estimating intraclass correlation for binary data. Biometrics. 1999;55(1):137–148. doi: 10.1111/j.0006-341x.1999.00137.x. [DOI] [PubMed] [Google Scholar]
- 31.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. 1. Chapman & Hall/CRC; 1994. [Google Scholar]
- 32.Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, Campbell MJ. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. Journal of Clinical Epidemiology. 2004;57(8):785–794. doi: 10.1016/j.jclinepi.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Gulliford MC, Adams G, Ukoumunne OC, Latinovic R, Chinn S, Campbell MJ. Intraclass correlation coefficient and outcome prevalence are associated in clustered binary data. Journal of Clinical Epidemiology. 2005;58(3):246–251. doi: 10.1016/j.jclinepi.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Bird SM, Sir David C, Farewell VT, Harvey G, Tim H, Peter CS. Performance indicators: good, bad, and ugly. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2005;168(1):1–27. [Google Scholar]
- 35.Goldstein H, Spiegelhalter DJ. League Tables and Their Limitations: Statistical Issues in Comparisons of Institutional Performance. Journal of the Royal Statistical Society. Series A (Statistics in Society) 1996;159(3):385–443. [Google Scholar]
- 36.Baroletti S, Dell’Orfano H. Medication Adherence in Cardiovascular Disease. Circulation. 2010 Mar 30;121(12):1455–1458. doi: 10.1161/CIRCULATIONAHA.109.904003. 2010. [DOI] [PubMed] [Google Scholar]
- 37.Choudhry NK, Avorn J, Glynn RJ, et al. Full Coverage for Preventive Medications after Myocardial Infarction. New England Journal of Medicine. 2011;365(22):2088–2097. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- 38.Ho PM, Bryson CL, Rumsfeld JS. Medication Adherence. Circulation. 2009 Jun 16;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. 2009. [DOI] [PubMed] [Google Scholar]
- 39.Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006 Jan 17;113(2):203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 40.Osterberg L, Blaschke T. Adherence to Medication. New England Journal of Medicine. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 41.O’Connor GT, Quinton HB, Traven ND, et al. Geographic Variation in the Treatment of Acute Myocardial Infarction. JAMA: The Journal of the American Medical Association. 1999 Feb 17;281(7):627–633. doi: 10.1001/jama.281.7.627. 1999. [DOI] [PubMed] [Google Scholar]
- 42.Pilote L, Califf RM, Sapp S, et al. Regional Variation across the United States in the Management of Acute Myocardial Infarction. New England Journal of Medicine. 1995;333(9):565–572. doi: 10.1056/NEJM199508313330907. [DOI] [PubMed] [Google Scholar]
- 43.Benner JS, Tierce JC, Ballantyne CM, et al. Follow-up lipid tests and physician visits are associated with improved adherence to statin therapy. PharmacoEconomics. 2004;22(Suppl 3):13–23. doi: 10.2165/00019053-200422003-00003. [DOI] [PubMed] [Google Scholar]
- 44.Brookhart MA, Patrick AR, Schneeweiss S, et al. Physician Follow-up and Provider Continuity Are Associated With Long-term Medication Adherence: A Study of the Dynamics of Statin Use. Arch Intern Med. 2007 Apr 23;167(8):847–852. doi: 10.1001/archinte.167.8.847. 2007. [DOI] [PubMed] [Google Scholar]
- 45.Choudhry NK, Fischer MA, Avorn J, et al. The Implications of Therapeutic Complexity on Adherence to Cardiovascular Medications. Archives of internal medicine. 2011 May 9;171(9):814–822. doi: 10.1001/archinternmed.2010.495. 2011. [DOI] [PubMed] [Google Scholar]
- 46.Newby LK, Allen LaPointe NM, Chen AY, et al. Long-Term Adherence to Evidence-Based Secondary Prevention Therapies in Coronary Artery Disease. Circulation. 2006 Jan 17;113(2):203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. 2006. [DOI] [PubMed] [Google Scholar]
- 47.Huybrechts KF, Seeger JD, Rothman KJ, Glynn RJ, Avorn J, Schneeweiss S. Bias in Comparative Effectiveness Studies Due to Regional Variation in Medical Practice Intensity: A Legitimate Concern, or Much Ado About Nothing? Circulation: Cardiovascular Quality and Outcomes. 2012 Sep 1;5(5):e61–e64. doi: 10.1161/CIRCOUTCOMES.112.966093. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


