Highlights
-
•
Krüppel-like factor 4 (KLF4) was downregulated in breast cancer tissues.
-
•
KLF4 was upregulated in TNF-α–stimulated SK-BR-3 breast cancer cells.
-
•
KLF4 inhibited proliferation and promoted apoptosis in breast cancer cells.
-
•
Overexpression of KLF4 suppressed SK-BR-3 cell tumorigenicity in vivo.
Abbreviations: KLF4, Krüppel-like factor 4; iPSC, induced pluripotent stem cell; EMT, epithelial–mesenchymal transition; FBS, fetal bovine serum; SNK, Student–Newman–Keuls; TNF-α, tumor necrosis factor-α; FACS, fluorescence-activated cell sorting
Keywords: Apoptosis, Breast cancer, Krüppel-like factor 4, Proliferation, Tumorigenicity
Abstract
Krüppel-like factor 4 (KLF4) functions as either a tumor suppressor or an oncogene in different tissues by regulating the expression of various genes. The aim of this study was to reveal the functions of KLF4 in regulating breast cancer apoptosis, proliferation, and tumorigenic progression. KLF4 expression levels in breast cancer tissues and breast cancer cell lines were found to be much lower than those in nontumorous tissues and a nontransformed mammary epithelial cell line. KLF4 was upregulated in the tumor necrosis factor-α–induced SK-BR-3 breast cancer cell apoptotic process. Overexpression of KLF4 promoted SK-BR-3 breast cancer cell apoptosis and suppressed SK-BR-3 cell tumorigenicity in vivo.
1. Introduction
Breast cancer remains a major health problem with respect to malignancy and is composed of distinct biological subtypes with diverse clinical, pathological, molecular, and genetic features as well as different therapeutic responsiveness and outcomes [1,2]. Despite this clinical importance, the exact pathological processes of tumor proliferation and metastasis remain poorly understood, and deciphering the exact molecular mechanisms of these processes is of paramount importance to identifying specific therapeutic targets for this devastating disease.
Krüppel-like factor 4 (KLF4), a zinc finger-containing transcription factor, is expressed in a variety of tissues [3,4]. KLF4 regulates numerous biological processes, including proliferation, differentiation, apoptosis, migration, invasion, and the blood-tumor barrier [4–7]. In addition, KLF4 plays an important role in reprogramming differentiated somatic cells into induced pluripotent stem cells (iPSCs) [8–10]. The role of KLF4 in cancers remains controversial [11–14]. In renal cell cancer, KLF4 is a putative tumor suppressor gene and is epigenetically silenced by promoter CpG methylation [5]. In hepatocellular carcinoma, KLF4 suppresses SLUG transcription and acts as a tumor suppressor. KLF4 is downregulated in colon cancer tissues and may serve as a prognostic biomarker [15]. KLF4 also acts as a tumor suppressor in prostate cancer, cervical carcinoma, and pancreatic ductal carcinoma [16–18]. Several studies have revealed that KLF4 participates in the maintenance of breast cancer stem cells as well as metastasis and invasion in breast cancer [19–21]. However, KLF4 was also shown to inhibit tumorigenic progression and metastasis in a mouse model of HER2/NEU/ERBB2-positive breast cancer [22] and to suppress estrogen-dependent breast cancer growth by inhibiting the transcriptional activity of estrogen receptor alpha [23]. Moreover, KLF4 is transcriptionally activated by p53 following DNA damage and is considered to participate in apoptosis in breast cancer cells [23]. KLF4 is significantly downregulated during epithelial–mesenchymal transition (EMT) in mammary epithelial cells and breast cancer cells and plays a central role in the maintenance of epithelial cell differentiation and the prevention of EMT and metastasis [24,25].
In this study, the authors examined KLF4 expression levels in breast cancer tissues and several breast cell lines, detected the potential role of KLF4 in SK-BR-3 breast cancer cell apoptosis, and investigated the role of KLF4 in tumorigenic progression by transient adenoviral expression of KLF4 in a xenograft model.
2. Materials and methods
2.1. Cell culture, breast cancer cell lines, and clinical samples
The mammary epithelial cell line HBL-100 and breast cancer cell lines MCF-7, SK-BR-3, and MDA-MB-231 were obtained from Cell Resource Center of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, China. HBL-100 cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA). MCF-7 and SK-BR-3 cells were maintained in Dulbecco’s Modified Eagle’s Medium with 10% FBS, and MDA-MB-231 cells were maintained in L-15 Medium with 10% FBS. All cell lines were cultured in a humidified atmosphere with 5% CO2 at 37 °C.
Human breast cancer samples and their nontumorous counterparts were collected from patients with breast cancer who underwent surgical resection at Hebei University Affiliated Hospital between June 2013 and December 2013. Tumor stage was classified according to National Comprehensive Cancer Network guidelines, and all cases were found to be infiltrating ductal carcinoma stage II. The resected tissues were immediately snap-frozen in liquid nitrogen and stored at −80 °C or fixed in 10% neutral-buffered formalin and embedded in paraffin. Written informed consent was obtained from patients, and approval of the use of human specimens in this study was obtained from the Institutional Review Board of Hebei University Affiliated Hospital.
2.2. Immunohistochemical staining
A manual immunohistochemical procedure was applied using tissue sections with a thickness of 4 μm. After deparaffinization in xylene and rehydration in graded ethanol, endogenous peroxidase was blocked with 3% aqueous H2O2 for 25 min. Then heat-induced antigen retrieval was accomplished by immersing the slides in 10 mM citrate buffer, pH 6, at 120 °C for 10 min. The slides were incubated with the anti-KLF4 antibody (sc-20691, dilution: 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4 °C. Then the slides were washed four times, processed with ready-to-use peroxidase-conjugated antibodies for 30 min at room temperature, developed with 3,3′-diaminobenzidine as a chromogen, and counterstained with hematoxylin. The results were reviewed independently by two pathologists. As negative controls, slides were treated by replacing the primary antibody with nonimmune serum.
The expression of KLF4 was determined using a semiquantitative visual approach. Immunostaining scoring was performed while blinded to patients’ information and outcomes. The percentage of positive tumor cells was determined semiquantitatively by assessing the entire slide. Each sample was assigned to one of the following categories: 0 (0–4%), 1 (5–24%), 2 (25–49%), 3 (50–74%), or 4 (75–100%). The intensity of immunostaining was determined as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). Immunostaining score formula = score of positive cell percentage × score of staining intensity.
2.3. Adenovirus expression vector and plasmid construction
The human KLF4 cDNA (accession NM_004235.4) was cloned into the pEGFP-C2 plasmid to create the pEGFP-KLF4 expression vector, and the KLF4 cDNA sequence was confirmed by sequencing. For the adenovirus expression vector, the GFP-KLF4 or green fluorescent protein (GFP) cDNA was cloned into the pAD/CMV/V5-DEST vector (Invitrogen, Carlsbad, CA, USA) to create the GFP-KLF4 and GFP adenovirus pAd-GFP-KLF4 and pAd-GFP. The resulting constructs were packaged in A293 cells by transfection with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions to generate an adenoviral stock. Then the adenovirus was amplified by infecting with A293 cells, and culture supernatant from A293 cells with a titer of 3 × 108 pfu/mL was used to infect breast cancer cells. After adenovirus delivery, GFP and GFP-KLF4 were detected using Western blot analysis.
2.4. siRNA transfection
Nonspecific siRNA (si-NS) and the siRNAs specific for human KLF4 were purchased from Santa Cruz Biotechnology. Transfection was performed using Lipofectamine (Invitrogen) following the manufacturer’s instructions. After the indicated times of transfection, HBL-100 cells were harvested and subjected to Western blotting and MTS proliferation analysis.
2.5. RNA preparation and quantitative RT-PCR
Total RNA was isolated with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Complementary DNA was synthesized from the total RNA (0.5 μg) using the Moloney Murine Leukemia Virus Reverse Transcriptase (Promega, Madison, WI, USA) following the manufacturer’s instructions. Subsequently, quantitative reverse transcription polymerase chain reaction (RT-PCR) of KLF4 was performed using the Platinum SYBR Green quantitative PCR SuperMix UDG Kit (Invitrogen). Each RT-PCR consisted of 2 μL diluted RT product, 10 μL SYBR Green PCR Master Mix, and 250 nM forward and reverse primers in a total volume of 20 μL. Reactions were carried out on a 7300 RT-PCR System (Applied Biosystems, Foster City, CA, USA) for 40 cycles (95 °C for 5 s, 60 °C for 40 s) after an initial 2-min incubation at 95 °C. As an internal control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene primers were used for RNA template normalization. The relative expression level was calculated using the following equation: relative gene expression = 2 – (ΔCtSample – ΔCtControl) [4]. The following primers were used: KLF4, 5′-ATCTTTCTCCACGTTCGCGTCTG-3′ (sense) and 5′-AAGCACTGGGGGAAGTCGCTTC-3′ (antisense); GAPDH, 5′-AAGGTCGGAGTCAACGGATTT-3′ (sense) and 5′-ACCAGAGTTAAAAGCAGCCCTG-3′ (antisense). Experiments were performed at least three times with samples analyzed in triplicate.
2.6. Western blotting
Western blotting was carried out as described previously [26]. Briefly, crude proteins were extracted, resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). Membranes were blocked with 5% milk in Tris-buffered saline with Tween 20 for 2 h at 37 °C and then incubated overnight at 4 °C with the following primary antibodies: rabbit anti-KLF4 (1:400, Santa Cruz Biotechnology), rabbit anti-cleaved caspase-3 (1:500, Proteintech, Chicago, IL, USA), rabbit anti-bax (1:500, Proteintech), or mouse anti-β-actin (1:1000, Santa Cruz Biotechnology). After incubation with the appropriate secondary antibody, the immunoreactive signal of antibody–antigen pairs was visualized using the Chemiluminescence Plus Western Blot analysis kit (Santa Cruz Biotechnology). Experiments were performed at least three times.
2.7. MTS cell proliferation assay
The MTS cell proliferation assay was performed using the commercially available CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega). About 1 × 104 cells were seeded in 96-well plates and grown for the indicated times. Then, 20 μl MTS reagent was added to each well, and the plate was incubated in the dark for 2 h before detection. The absorbance was measured in a Thermo Fluroscan Ascent spectrometer at 490 nm. Experiments were performed at least three times.
2.8. Assessment of apoptosis by flow cytometry
Cells were stained with phycoerythrin (PE) annexin V and 7-amino-actinomycin (PE Annexin V Apoptosis Detection Kit I; BD Biosciences, Franklin Lakes, NJ, USA) as recommended by the manufacturer. A total of 10,000 fluorescent events were acquired using a fluorescence-activated cell sorting (FACS)-Calibur equipped with CellQuest Pro software (BD Biosciences).
2.9. Animal model of breast cancer xenograft
Nude mice were subcutaneously injected as described previously [27]. Female BALB/c-nu mice, 4–6 weeks old, were purchased from the Beijing HFK Bioscience Co., Ltd (Beijing, China). All of the mice were bred and housed in a specific pathogen-free environment at Hebei University Laboratory Animal Research Center. All experiments were approved by the Animal Research Ethics Committee of the authors’ institution. SK-BR-3 breast cancer cells were added with pAd-GFP, pAd-GFP-KLF4, or PBS for 24 h, and GFP- or GFP-KLF4-overexpressing SK-BR-3 cells and null-overexpressing SK-BR-3 cells were obtained. The cells were suspended in their regular medium separately. Then, 100 μL of suspensions containing 5 × 106 cells was injected subcutaneously into the right mammary fat pad of a nude mouse. Each experimental group was tested in a group of 5 mice. The size of the tumor formed was monitored closely and measured every 2 days using a caliper. The volume of the tumors was estimated according the formula: Volume = 1/2 × a × b2, where a and b represent the largest and smallest diameters, respectively [28]. The mice were sacrificed 14 days after injection. Tumors were harvested, measured, weighed, and photographed.
2.10. Statistical analyses
Data are presented as histograms of means ± standard error of the mean (SEM) values for data from three or more independent experiments. Statistical analyses were performed using the Student’s t-test or one-way analysis of variance (ANOVA) according to the number of groups compared. Student–Newman–Keuls (SNK) test was used to test specific differences after the ANOVA for post hoc analysis. Differences were considered significant at P < 0.05.
3. Results
3.1. KLF4 expression was significantly decreased in breast cancer tissues and breast cells
To explore whether KLF4 is associated with breast cancer, KLF4 messenger RNA (mRNA) expression levels were detected in breast infiltrating ductal carcinoma tissues and adjacent normal breast tissues. Breast cancer samples and their nontumorous counterparts from 16 patients were collected, total RNA was isolated, and quantitative RT-PCR was performed to evaluate KLF4 expression levels. As shown in Fig. 1A, KLF4 mRNA expression levels in breast cancer tissues were much lower than those in their nontumorous counterpart tissues. KLF4 protein levels were detected by immunohistochemical analysis. The results revealed that KLF4 was expressed in normal breast tissues. However, the expression of KLF4 can hardly be detected in breast cancer tissues (Fig. 1B). Furthermore, KLF4 expression was low in breast cancer cell lines (SK-BR-3, MCF-7, and MDA-MB-231) compared with the expression in a nontransformed mammary epithelial cell line (HBL-100) (Fig. 1C and D).
Fig. 1.
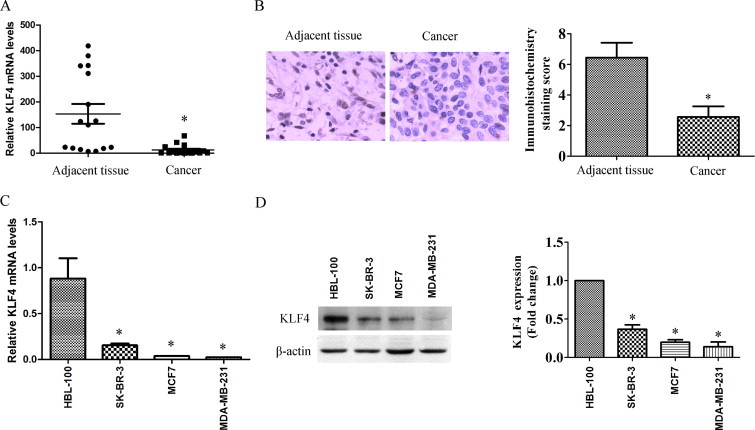
KLF4 expression was significantly decreased in breast cancer tissues and breast cells. (A) Samples and adjacent tissues were obtained from 16 patients with breast cancer, total RNA was isolated, and quantitative RT-PCR was performed to evaluate KLF4 mRNA levels. ∗P < 0.05 vs adjacent tissue group. (B) KLF4 protein levels in breast cancer tissues and adjacent tissues were detected by immunohistochemical staining. Magnification 200×. Left, representative photographs; right, immunohistochemical staining scores. ∗P < 0.05 vs control group. (C) KLF4 mRNA levels in breast cancer cell lines and a nontransformed mammary epithelial cell line were detected by quantitative RT-PCR. Experiments were performed at least three times. ∗P < 0.05 vs HBL-100 group. (D) Crude proteins were extracted from the cells and subjected to Western blotting with anti-KLF4 antibody. β-Actin was used as a control for equal protein loading. Left, Blots from a representative experiment. Right, Densitometry; results were normalized to β-actin. Bars represent the mean ± SEM from 3 independent experiments. ∗P < 0.05 vs HBL-100 group.
3.2. KLF4 was upregulated in TNF-α–stimulated SK-BR-3 breast cancer cells
To explore the actual roles of KLF4 in breast cell proliferation and apoptosis, we first examined the expression of KLF4 in TNF-α–stimulated SK-BR-3, MCF-7, and MDA-MB-231 breast cancer cell lines. KLF4 mRNA and protein levels were increased significantly in TNF-α–stimulated SK-BR-3 cells (Fig. 2A and B). However, unlike the change in SK-BR-3 cells, only a slight change in KLF4 mRNA levels was observed in TNF-α–treated MCF-7 and MDA-MB-231 breast cancer cells (Fig. 2A and B). Moreover, TNF-α increased KLF4 protein expression in SK-BR-3 cells in a time-dependent manner. As shown in Fig. 2C, 6 h after treatment with 10 ng/mL TNF-α, KLF4 protein expression increased and that increase was maintained until 34 h after treatment in SK-BR-3 cells.
Fig. 2.
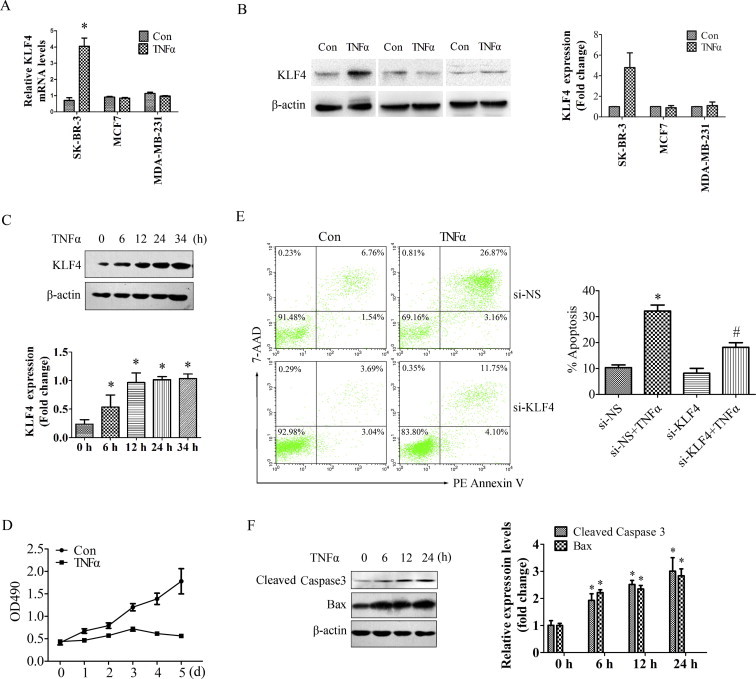
KLF4 was upregulated in TNF-α–stimulated SK-BR-3 breast cancer cells. (A) SK-BR-3, MCF7, and MDA-MB-231 breast cancer cells were treated with 10 ng/mL TNF-α for 24 h. Total RNA was isolated and subjected to qRT-PCR. Bars represent the mean ± SEM from 3 independent experiments. ∗P < 0.05 vs control group. (B) SK-BR-3, MCF7, and MDA-MB-231 breast cancer cells were treated with 10 ng/mL TNF-α for 24 h. Crude proteins were extracted from the treated cells and subjected to Western blotting with anti-KLF4 antibody. β-Actin was used as a control for equal protein loading. Left, Blots from a representative experiment. Right, Densitometry; results were normalized to β-actin. Bars represent the mean ± SEM from 3 independent experiments. ∗P < 0.05 vs control group. (C) SK-BR-3 breast cancer cells were treated with 10 ng/mL TNF-α for the indicated times (0, 6, 12, 24, and 34 h). Crude proteins were extracted from the treated cells and then subjected to Western blotting with anti-KLF4 antibody. β-Actin was used as a control for equal protein loading. Upper, Blots from a representative experiment. Lower, Densitometry; results were normalized to β-actin. Bars represent the mean ± SEM from 3 independent experiments. ∗P < 0.05 vs control group. (D) SK-BR-3 cells were treated with 10 ng/mL TNF-α for 0, 1, 2, 3, 4 or 5 days, and cell proliferation was tested using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay Kit. The absorbance was measured at 490 nm. ∗P < 0.05 vs control group. (E) SK-BR-3 cells were transfected with si-KLF4 or si-NS for 24 h and then treated with 10 ng/mL TNF-α for 48 h and analyzed for Annexin-V/7-AAD by FACS using PE Annexin V Apoptosis Detection Kit. One representative experiment is shown (upper); the results from 3 independent experiments were summarized (lower). ∗P < 0.05 vs control group. (E) SK-BR-3 breast cancer cells were treated with 10 ng/mL TNF-α for 0, 6, 12, and 24 h. Crude proteins were extracted from the treated cells and subjected to Western blotting with antibodies against cleaved caspase-3 and Bax. β-Actin was used as a control for equal protein loading. Bars represent the mean ± SEM from 3 independent experiments. ∗P < 0.05 vs control group.
Cell proliferation and apoptosis were also examined in TNF-α–treated SK-BR-3 breast cancer cells. The MTS cell proliferation assay was performed, and the results revealed that treatment with 10 ng/mL TNF-α significantly decreased SK-BR-3 cell proliferation (Fig. 2D). Then, apoptosis was examined in TNF-α–stimulated SK-BR-3 cells. As shown in Fig. 2E, the number of apoptotic cells was significantly greater after treatment with 10 ng/mL TNF-α for 48 h, and knocking down endogenous KLF4 by transfection with si-KLF4 in TNF-α–treated cells significantly attenuated the pro-apoptotic role of TNF-α in SK-BR-3 breast cancer cells. The apoptosis-related genes cleaved caspase-3 and Bax were detected by Western blotting. The results revealed that the levels of cleaved caspase-3 and Bax proteins in SK-BR-3 cells increased after TNF-α treatment in a time-dependent manner (Fig. 2F). These results revealed that KLF4 expression levels were significantly increased during TNF-α–induced apoptosis, which suggests the important role of KLF4 in SK-BR-3 breast cancer cell proliferation and apoptosis.
3.3. KLF4 inhibited proliferation and promoted apoptosis in breast cancer cells
To validate the roles of KLF4 in breast cancer cell proliferation and apoptosis, GFP or GFP-KLF4 overexpression was introduced by infection of the adenovirus vector pAd-GFP or pAd-GFP-KLF4. Forty-eight hours after infection, the exogenous GFP or GFP-KLF4 expression levels in SK-BR-3 cells were observed by fluorescence microscopy (Fig. 3A). The MTS proliferation assay was performed to examine the effects of KLF4 on SK-BR-3 cell proliferation. The results revealed that GFP-KLF4 overexpression significantly decreased cell proliferation (Fig. 3B). To further identify the role of KLF4 in breast cancer cell proliferation, we performed MTS proliferation assays in the highly KLF4-expressing breast cell line HBL-100 in which KLF4 was knocked down by RNAi (Fig. 3C). As shown in Fig. 3D, proliferative activity was significantly higher in KLF4-knockdown HBL-100 cells, which suggested an anti-proliferative role of KLF4 in breast cells. Next, we examined apoptosis in KLF4-overexpressing SK-BR-3 cells by FACS. As shown in Fig. 3C, the number of apoptotic cells was significantly increased after pAd-GFP-KLF4 overexpression for 72 h. Then, the cells were stained with PE-conjugated Annexin V. As shown in Fig. 3D, Annexin V expression on the cell membrane was increased significantly in GFP-KLF4-overexpressing SK-BR-3 cells. Moreover, the apoptosis-related genes, cleaved caspase-3 and Bax, were detected by Western blotting. The results revealed that the levels of cleaved caspase-3 and Bax proteins in KLF4-overexpressing SK-BR-3 cells were significantly higher than those in the control group (Fig. 2E). These results suggest that KLF4 suppressed proliferation and promoted apoptosis by upregulating the expression of the apoptosis-related genes caspase-3 and Bax in SK-BR-3 breast cancer cells.
Fig. 3.
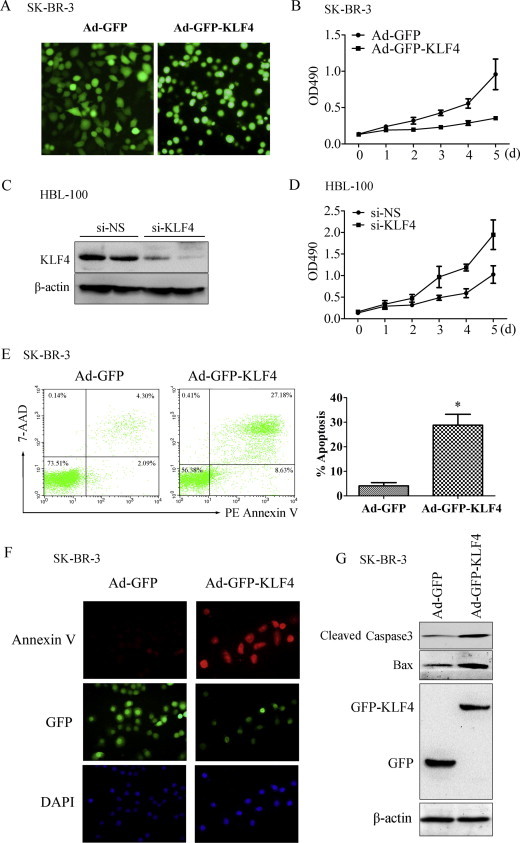
KLF4 promoted SK-BR-3 breast cancer cell apoptosis. (A) SK-BR-3 cells were infected with Ad-GFP and Ad-GFP-KLF4 for 48 h. The exogenous GFP or GFP-KLF4 expression was observed by fluorescence microscopy. Magnification, 200×. (B) SK-BR-3 cells were infected with Ad-GFP and Ad-GFP-KLF4 for 0, 1, 2, 3, 4, or 5 days. Cell proliferation was tested using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay Kit, and the absorbance was measured at 490 nm. ∗P < 0.05 vs Ad-GFP group. (C) HBL-100 breast cells were transfected with si-KLF4 or si-NS for 24 h, and expression of KLF4 was assessed by Western blot analysis. β-Actin was used as a control for equal protein loading. (D) HBL-100 cells were transfected with si-KLF4 or si-NS, and cell proliferation was tested using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay Kit at 0, 1, 2, 3, 4, or 5 days after transfection. (E) SK-BR-3 cells were infected with Ad-GFP and Ad-GFP-KLF4 for 72 h and analyzed for Annexin-V/7-AAD by FACS using the PE Annexin V Apoptosis Detection Kit. One representative experiment is shown (left); results from 3 independent experiments were summarized (right). ∗P < 0.05 vs Ad-GFP group. (F) SK-BR-3 cells were infected with Ad-GFP and Ad-GFP-KLF4 for 72 h and then stained with PE-conjugated Annexin V. Pictures show the GFP-tag (green), annexin V (red), and 4′,6-diamidino-2-phenylindole (purple). Magnification, 400×. (G) SK-BR-3 cells were infected with Ad-GFP and Ad-GFP-KLF4 for 48 h. The cell lysates were analyzed by Western blotting with the indicated antibodies, and β-actin was the loading control. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Overexpression of KLF4 suppressed SK-BR-3 cell tumorigenicity in vivo
SK-BR-3 cells were infected with Ad-GFP or Ad-GFP-KLF4 for 48 h to obtain GFP- or GFP-KLF4-overexpressing cells. Then, 5 × 106 naive, GFP- or GFP-KLF4-overexpressing SK-BR-3 breast cancer cells were injected into nude mice. After the cells were injected subcutaneously into the right mammary fat pad of the nude mice, tumor growth was monitored carefully and the size of the tumor formed was measured every 2 days. As shown in Fig. 1A, GFP-KLF4-overexpressing SK-BR-3 cells resulted in significantly slower growing and smaller tumors than both naive and GFP-overexpressing cells. At day 14, the mice were sacrificed and the tumors were harvested, photographed, measured, and weighed. Tumor volumes in the GFP-KLF4-overexpressing group were significantly smaller than those in the naive or GFP-overexpressing groups (Fig. 4B and C). There was no significant difference in tumor volume between the control group and GFP group (Fig. 4C). This trend was also reflected in the weights of the tumors, with the average weight of tumors in the GFP-KLF4-overexpressing group being significantly less (Fig. 4D). These results suggest that overexpression of KLF4 in SK-BR-3 breast cancer cells attenuated the ability of SK-BR-3 cells to form tumors in nude mice.
Fig. 4.
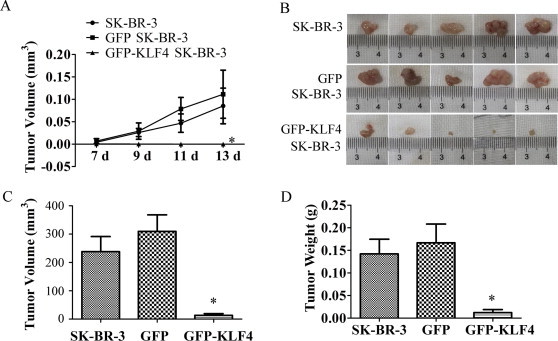
KLF4 overexpression suppressed in vivo tumorigenicity of SK-BR-3 cells. (A) Naive or GFP- or GFP-KLF4-overexpressing SK-BR-3 breast cancer cells were injected subcutaneously into the right mammary fat pad of nude mice at 5 × 106 cells per site. Tumor volume was monitored every 2 days after subcutaneous injection and tumor growth curve is shown. Tumor volume was calculated by the formula: V = 1/2 × a (length) × b2 (width). ∗P < 0.05 vs SK-BR-3 group. (B) Tumors were harvested from the mice 14 days after subcutaneous injection and photographed. (C) The volume of the harvested tumors was calculated by the formula: V = 1/2 × a (length) × b2 (width). ∗P < 0.05 vs SK-BR-3 group. (D) The weight of the tumors was measured. ∗P < 0.05 vs SK-BR-3 group.
4. Discussion
KLF4 has been recognized as a tumor suppressor in many types of cancers [11–13]. However, conflicting data exist regarding the function of KLF4 in breast cancer. Initial studies using immunohistochemical and in situ analyses of human breast tumors reported increased KLF4 expression [29]. KLF4 can also induce cancer stem cell (CSC)-like properties that may promote cell migration and invasion [21], whereas it can also inhibit EMT and block metastasis [22,25]. Subsequent reports have shown that KLF4 mRNA levels are decreased in breast cancer tissues, relative to normal tissue, and inversely correlated with increasing tumor grade [23]. KLF4 was also reported to participate estrogen receptor signaling by upregulating the estrogen level in breast cancer cells, and ablation of KLF4 inhibits estrogen-induced growth of tumor cells [30]. Despite these findings, the exact roles of KLF4 in breast cancer cell proliferation and apoptosis remain unclear. This study demonstrated that KLF4 expression is lost in breast infiltrating ductal carcinoma tissues and several breast cancer cell lines, and KLF4 exhibits an anti-proliferative and pro-apoptotic role in SK-BR-3 breast cancer cells, which suggested a role for KLF4 as a tumor suppressor.
TNF-α is a cytokine that acts as an important mediator of the apoptotic process that also demonstrates selective cytotoxicity against malignant breast tumor cells [31,32]. It can also increase expression of KLF4 in vascular smooth muscle cells [33]. Therefore, we examined whether KLF4 participates TNF-α–induced breast cancer cell apoptosis. In this study, treatment of SK-BR-3 breast cancer cells with 10 ng/mL TNF-α significantly suppressed proliferation and induced apoptosis, as well as induced the expression of apoptosis-related genes, including cleaved caspase-3 and Bax. Recently, KLF4 has been shown to be transcriptionally activated by p53 following DNA damage [23] and to be involved in breast cancer cell apoptosis [22,24,34], suggesting its potential role as a tumorigenic suppressor. Moreover, KLF4 suppresses histone deacetylase inhibitor-induced caspase activation and the stress-activated protein kinase pathway by targeting p57(Kip2) [35]. In this study, KLF4 protein and mRNA expression levels were significantly increased in the TNF-α–induced apoptotic process in SK-BR-3 cells, which suggests the participation of KLF4 in SK-BR-3 breast cancer cell apoptosis.
KLF4 is an important modulator of cell proliferation, differentiation, and transformation and has recently been considered a possible prognostic factor in breast cancer [36]. The mechanisms of KLF4 in SK-BR-3 cell proliferation and apoptosis remain unknown. In this study, KLF4 overexpression was introduced by infection of the adenovirus vector pAd-GFP-KLF4 to validate the roles of KLF4 in SK-BR-3 breast cancer cell, and the results revealed induction of apoptosis and apoptosis-related gene expression and significantly decreased cell proliferation. Tumorigenic progression is a multistep process. Our results show that KLF4 overexpression progressively suppressed SK-BR-3 mammary tumorigenicity in a mouse model.
In summary, we focused on the functions of KLF4 in breast cancer in the present study and identified the anti-proliferative and pro-apoptotic roles of KLF4 by targeting caspase-3 and Bax. While these findings shed some light on the function of KLF4 in breast cancer apoptosis and mammary tumorigenic progression using a mouse model, it is important that additional models, both in vitro and in vivo, representing various breast cancer subtypes, be examined. As KLF4 plays a role in the induction and maintenance of iPSCs [8], it is also important to determine whether similar regulation occurs in mammary and tumor stem cells. Further elucidating the crucial functions of KLF4 in breast cancer formation and processing will better define the utility of KLF4 as a target for diagnostic and therapeutic interventions.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31301143), the Natural Science Foundation of Hebei Province (No. C2013201271), and the Hebei Youth Top-notch Talent Support Program. Nai-peng Cui and Bao-ping Chen conceived and designed the project. Bing Wang, Ming-zhi Zhao, Dan-dan Lin, An-yi Zhang, Yan Qin, Cai-yun Liu, Nai-peng Cui and Wei-tao Yan acquired the data. Bing Wang, Ming-zhi Zhao, Wei-tao Yan and Jian-hong Shi analyzed and interpreted the data. Jian-hong Shi and Bao-ping Chen wrote the paper.
Contributor Information
Jian-hong Shi, Email: shijianhong1980@126.com.
Bao-ping Chen, Email: cbp1962@126.com.
References
- 1.Perou C.M., Sorlie T., Eisen M.B. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T., Perou C.M., Tibshirani R. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghaleb A.M., Aggarwal G., Bialkowska A.B. Notch inhibits expression of the Kruppel-like factor 4 tumor suppressor in the intestinal epithelium. Mol. Cancer Res. 2008;6:1920–1927. doi: 10.1158/1541-7786.MCR-08-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi J.H., Zheng B., Chen S. Retinoic acid receptor alpha mediates all-trans-retinoic acid-induced Klf4 gene expression by regulating Klf4 promoter activity in vascular smooth muscle cells. J. Biol. Chem. 2012;287:10799–10811. doi: 10.1074/jbc.M111.321836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H., Wang J., Xiao W. Epigenetic alterations of Kruppel-like factor 4 and its tumor suppressor function in renal cell carcinoma. Carcinogenesis. 2013;34:2262–2270. doi: 10.1093/carcin/bgt189. [DOI] [PubMed] [Google Scholar]
- 6.Lin Z.S., Chu H.C., Yen Y.C. Kruppel-like factor 4, a tumor suppressor in hepatocellular carcinoma cells reverts epithelial mesenchymal transition by suppressing slug expression. PLoS One. 2012;7:e43593. doi: 10.1371/journal.pone.0043593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma J., Wang P., Liu Y. Kruppel-like factor 4 regulates blood-tumor barrier permeability via ZO-1, occludin and claudin-5. J. Cell. Physiol. 2014;229:916–926. doi: 10.1002/jcp.24523. [DOI] [PubMed] [Google Scholar]
- 8.Ho P.J., Yen M.L., Lin J.D. Endogenous KLF4 expression in human fetal endothelial cells allows for reprogramming to pluripotency with just OCT3/4 and SOX2–brief report. Arterioscler. Thromb. Vasc. Biol. 2010;30:1905–1907. doi: 10.1161/ATVBAHA.110.206540. [DOI] [PubMed] [Google Scholar]
- 9.D’Anselmi F., Masiello M.G., Cucina A. Microenvironment promotes tumor cell reprogramming in human breast cancer cell lines. PLoS One. 2013;8:e83770. doi: 10.1371/journal.pone.0083770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi S., Marumoto T., Nii T. Characterization of common marmoset dysgerminoma-like tumor induced by the lentiviral expression of reprogramming factors. Cancer Sci. 2014;105:402–408. doi: 10.1111/cas.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan H., Xie L., Leithauser F. KLF4 is a tumor suppressor in B-cell non-Hodgkin lymphoma and in classic Hodgkin lymphoma. Blood. 2010;116:1469–1478. doi: 10.1182/blood-2009-12-256446. [DOI] [PubMed] [Google Scholar]
- 12.Choi W.J., Youn S.H., Back J.H. The role of KLF4 in UVB-induced murine skin tumor development and its correlation with cyclin D1, p53, and p21(Waf1/Cip1) in epithelial tumors of the human skin. Arch. Dermatol. Res. 2011;303:191–200. doi: 10.1007/s00403-010-1101-0. [DOI] [PubMed] [Google Scholar]
- 13.Hu W., Hofstetter W.L., Li H. Putative tumor-suppressive function of Kruppel-like factor 4 in primary lung carcinoma. Clin. Cancer Res. 2009;15:5688–5695. doi: 10.1158/1078-0432.CCR-09-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C.C., Liu Z., Li X. KLF4 and PCNA identify stages of tumor initiation in a conditional model of cutaneous squamous epithelial neoplasia. Cancer Biol Ther. 2005;4:1401–1408. doi: 10.4161/cbt.4.12.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel N.V., Ghaleb A.M., Nandan M.O. Expression of the tumor suppressor Kruppel-like factor 4 as a prognostic predictor for colon cancer. Cancer Epidemiol. Biomarkers Prev. 2010;19:2631–2638. doi: 10.1158/1055-9965.EPI-10-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J., Place R.F., Huang V. Prognostic value and function of KLF4 in prostate cancer: RNAa and vector-mediated overexpression identify KLF4 as an inhibitor of tumor cell growth and migration. Cancer Res. 2010;70:10182–10191. doi: 10.1158/0008-5472.CAN-10-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang W.T., Zheng P.S. Kruppel-like factor 4 functions as a tumor suppressor in cervical carcinoma. Cancer. 2012;118:3691–3702. doi: 10.1002/cncr.26698. [DOI] [PubMed] [Google Scholar]
- 18.Zammarchi F., Morelli M., Menicagli M. KLF4 is a novel candidate tumor suppressor gene in pancreatic ductal carcinoma. Am. J. Pathol. 2011;178:361–372. doi: 10.1016/j.ajpath.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu F., Shi Y., Wang J. Deficiency of Kruppel-like factor KLF4 in mammary tumor cells inhibits tumor growth and pulmonary metastasis and is accompanied by compromised recruitment of myeloid-derived suppressor cells. Int. J. Cancer. 2013;133:2872–2883. doi: 10.1002/ijc.28302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuda H., Xing F., Pandey P.R. MiR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res. 2013;73:1434–1444. doi: 10.1158/0008-5472.CAN-12-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu F., Li J., Chen H. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011;30:2161–2172. doi: 10.1038/onc.2010.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yori J.L., Seachrist D.D., Johnson E. Kruppel-like factor 4 inhibits tumorigenic progression and metastasis in a mouse model of breast cancer. Neoplasia. 2011;13:601–610. doi: 10.1593/neo.11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akaogi K., Nakajima Y., Ito I. KLF4 suppresses estrogen-dependent breast cancer growth by inhibiting the transcriptional activity of ERalpha. Oncogene. 2009;28:2894–2902. doi: 10.1038/onc.2009.151. [DOI] [PubMed] [Google Scholar]
- 24.Tiwari N., Meyer-Schaller N., Arnold P. Klf4 is a transcriptional regulator of genes critical for EMT, including Jnk1 (Mapk8) PLoS One. 2013;8:e57329. doi: 10.1371/journal.pone.0057329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yori J.L., Johnson E., Zhou G. Kruppel-like factor 4 inhibits epithelial-to-mesenchymal transition through regulation of E-cadherin gene expression. J. Biol. Chem. 2010;285:16854–16863. doi: 10.1074/jbc.M110.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi J.H., Zheng B., Li Y.H. Novel insight into Y-box binding protein 1 in the regulation of vascular smooth muscle cell proliferation through targeting GC box-dependent genes. FEBS Lett. 2013;587:1326–1332. doi: 10.1016/j.febslet.2013.02.047. [DOI] [PubMed] [Google Scholar]
- 27.Arpino G., Gutierrez C., Weiss H. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J. Natl. Cancer Inst. 2007;99:694–705. doi: 10.1093/jnci/djk151. [DOI] [PubMed] [Google Scholar]
- 28.Chi C.W., Chen C.C., Chen Y.J. Therapeutic and radiosensitizing effects of armillaridin on human esophageal cancer cells. Evidence-based complementary and alternative medicine: eCAM. 2013;2013:459271. doi: 10.1155/2013/459271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster K.W., Frost A.R., McKie-Bell P. Increase of GKLF messenger RNA and protein expression during progression of breast cancer. Cancer Res. 2000;60:6488–6495. [PubMed] [Google Scholar]
- 30.Hu D., Zhou Z., Davidson N.E. Novel insight into KLF4 proteolytic regulation in estrogen receptor signaling and breast carcinogenesis. J. Biol. Chem. 2012;287:13584–13597. doi: 10.1074/jbc.M112.343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y., Wang L., Lin X.Y. Anti-apoptotic effect of claudin-1 on TNF-alpha-induced apoptosis in human breast cancer MCF-7 cells. Tumour Biol. 2012;33:2307–2315. doi: 10.1007/s13277-012-0493-1. [DOI] [PubMed] [Google Scholar]
- 32.Estevam F.R., Augusto S.F., Rodrigues S.A. Apoptosis and production of TNF-alpha by tumor-associated inflammatory cells in histological grade III breast cancer. Cancer Immunol. Immunother. 2005;54:671–676. doi: 10.1007/s00262-004-0639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali M.S., Starke R.M., Jabbour P.M. TNF-alpha induces phenotypic modulation in cerebral vascular smooth muscle cells: implications for cerebral aneurysm pathology. J. Cereb. Blood Flow Metab. 2013;33:1564–1573. doi: 10.1038/jcbfm.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin B., Ma Z.Y., Zhou Z.W. The TrkB+ cancer stem cells contribute to post-chemotherapy recurrence of triple-negative breast cancers in an orthotopic mouse model. Oncogene. 2014;34:761–770. doi: 10.1038/onc.2014.8. [DOI] [PubMed] [Google Scholar]
- 35.Ky N., Lim C.B., Li J. KLF4 suppresses HDACi induced caspase activation and the SAPK pathway by targeting p57(Kip2) Apoptosis. 2009;14:1095–1107. doi: 10.1007/s10495-009-0368-0. [DOI] [PubMed] [Google Scholar]
- 36.Tetreault M.P., Yang Y., Katz J.P. Kruppel-like factors in cancer. Nat. Rev. Cancer. 2013;13:701–713. doi: 10.1038/nrc3582. [DOI] [PubMed] [Google Scholar]


