Abstract
Hyperexcitable motoneurons are likely to contribute to muscle hypertonia after a stroke injury; however, the origins of this hyperexcitability are not clear. One possibility is that the effective duration of the Ia excitatory postsynaptic potential (EPSP) is prolonged, increasing the potential for temporal summation of EPSPs, making action potential initiation easier. Accordingly, the purpose of this study was to quantify the time course of EPSPs in motoneurons of stroke survivors. The experimental protocol, which was based on parameters derived from simulation, involved sequential subthreshold electrical stimuli delivered to the median nerve of hemispheric stroke survivors. The resulting H-reflex responses were recorded in the flexor carpi radialis muscle. H-reflex response probability was then used to quantify the time course of the underlying EPSPs in the motoneuron pool. A population EPSP was estimated based on the probability of evoking an H reflex from the second electrical stimulus in the absence of a reflex response to the first stimulus. The accuracy of this time-course estimate was quantified using a computer simulation that explored a range of feasible EPSP parameters. Our experimental results showed that in all five hemispheric stroke survivors the rate of decay of the population EPSP was consistently slower in spastic compared with the contralateral motoneuron pools. We propose that one potential mechanism for hyperexcitability of motoneurons in spastic stroke survivors may be linked to this prolongation of the Ia EPSP time course. Our subthreshold double-stimulation approach also provides a noninvasive tool for quantifying the time course of EPSPs in both healthy and pathological conditions.
Keywords: EPSP time course, stroke, spasticity, reflex, double stimulation
hyperexcitability of spinal motoneurons is potentially a major contributor to muscular hypertonia after a brain injury, such as a hemispheric stroke (Katz and Rymer 1989). This hyperexcitability can be induced by a change in either the passive or active electrical properties of these neurons, which can lead to sustained voltage changes for a given synaptic current input. These abnormal voltage changes can be captured as apparent alterations in the amplitude and time course of the excitatory postsynaptic potential (EPSP), namely a larger amplitude and/or longer decay than in normal EPSPs.
The amplitude and time course of the EPSP can be measured directly in reduced animal models via intracellular recordings (Fetz and Gustafsson 1983). In contrast, such EPSP properties can only be inferred indirectly in humans, based on changes in the discharge probability of single or grouped motoneurons in response to controlled afferent stimulation (Ashby and Zilm 1982; Suresh et al. 2005; Turker and Cheng 1994). There have been a number of studies using firing probability to characterize EPSP time course in actively discharging motoneurons (Norton et al. 2008; Turker and Powers 1999); however, this approach is limited by the silencing of discharge during the afterhyperpolarization (AHP) phase that follows each action potential occurrence. Using an alternative approach in which motoneuron discharge was used only to test motoneuron excitability, we sought to noninvasively estimate the time course of the population EPSP in a motoneuron pool of stroke survivors and to assemble evidence regarding the potential origins of increased motoneuron excitability in these subjects.
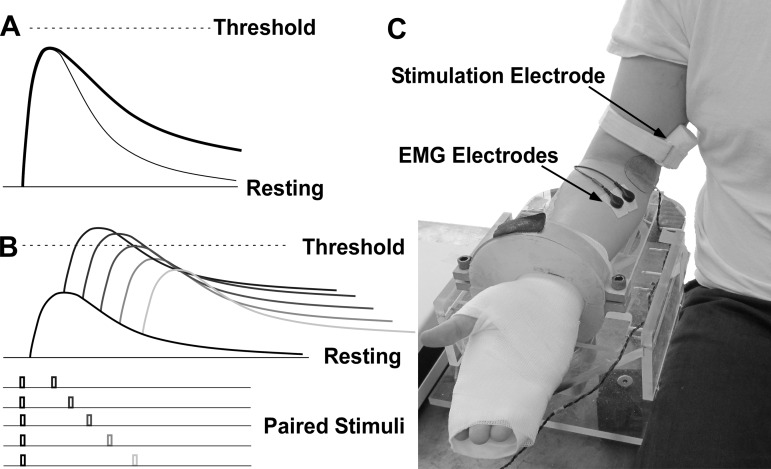
Specifically, we hypothesize that EPSPs in motoneurons innervating spastic muscle have a slower decay, compared with those motoneurons innervating the contralateral muscle (Fig. 1A). To test this hypothesis, paired electrical stimuli were delivered to the median nerve, and the resulting H reflexes of the passive flexor carpi radialis muscle were recorded. As shown in Fig. 1B, in the double-stimulation protocol, the first conditioning stimulus is of small amplitude, evoking a subthreshold EPSP (hence, AHP linked to neuron discharge is not an issue here). The second test stimulus is then delivered at a random latency. Depending on the interstimulus interval, this test stimulus could evoke an H reflex. With the use of these paired stimuli, the time course of the population EPSP can then be estimated, based on the response probability estimated using the test stimulus. The potential accuracy of this estimation was assessed through a simulation, which was also used to derive parameters for the experimental protocol.
Fig. 1.
Hypothesis, protocol, and experimental setup. A: sketch of excitatory postsynaptic potential (EPSP) time course. The EPSP (thick line) in the hyperexcitable motoneuron has a slower rate of decay than the normal motoneuron (thin line). B: subthreshold double-stimulation protocol. C: experimental setup.
This study offers a novel technique to noninvasively estimate the time course of population EPSPs and allows more systematic quantification of motoneuron and synaptic properties in intact humans and in pathological states.
METHODS
Simulation
To evaluate the accuracy of estimation, we performed a simulation with varying amplitudes (signifying different stimulus intensities) and time constants of EPSPs.
Specifically, the resting membrane potential was −70 mV and the threshold for action potential initiation was −50 mV. The time course of EPSP followed an impulse response (alpha) function (Eq. 1), with amplitude varying from 6 to 12 mV and time constant varying from 4 to 6 ms at different stimulus sequences. Paired EPSPs were summed linearly to the resting membrane potential with noise added. The amplitude of the noise followed a Gaussian distribution with a zero mean and standard deviation (σ) varying from 3 to 5 mV. When a single EPSP + noise crossed the threshold, a reflex response from the conditioning stimulus was registered; otherwise, a second EPSP with the same amplitude was added with a latency ranging from 4 to 70 ms. The response probability from the second EPSP (without a response from the first EPSP) was calculated with a 1-ms bin size. A total of 1,000 EPSP pairs were simulated at each parameter.
To estimate the time course of the EPSP decay, the impulse function (Eq. 1) was fit to the response probability distribution (Fig. 2). An exponential decay function (Eq. 2) was also used to quantify the time course. First, the exponential function was directly fit to the falling phase of the impulse function to obtain a “true” time constant. The exponential function was then fit to the response probability to estimate the time constant. The response probability from the conditioning EPSPs was also calculated, because it provides information about the effective distance-to-threshold when the second EPSPs were applied.
| (1) |
| (2) |
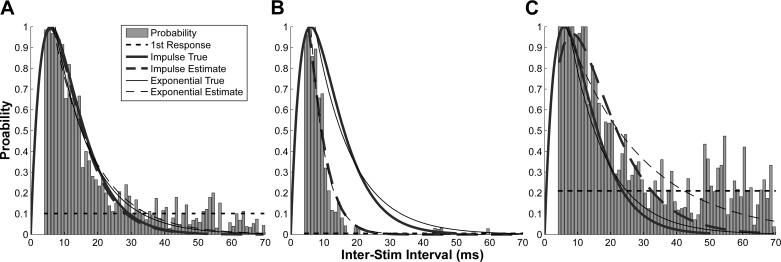
Fig. 2.
Simulated response probability at different EPSP amplitudes and synaptic noise levels. A: accurate estimate (A = 9 mV; σ = 4 mV). B: an underestimate due to low noise level (σ = 3 mV) with A = 9 mV. C: an overestimate due to high EPSP amplitude (A = 10 mV) at a given noise level (σ = 4 mV).
Here, P represents the probability of response, A represents the amplitude, ISI represents interstimulus interval, and Tc represents the time constant. For the impulse function, the time constant Tc represents the time-to-peak and is also related with the rate of decay, and the half relaxation time was Tc × 1.68. For the exponential function, Tc is related with the rate of decay, and the half relaxation time was Tc/1.44.
Experimental Data Collection and Analysis
Participants.
Five chronic hemiparetic stroke subjects (3 male and 2 female with age ranging from 58 to 67) were recruited. Inclusion criteria were as follows: spasticity present in muscles of the impaired upper extremity (modified Ashworth ≥1+ in upper extremity muscles), a single episode of stroke duration longer than 6 mo, no antispastic medication, and medically stable. All participants gave informed consent via protocols approved by the Institutional Review Board at Northwestern University.
Experimental setup.
Participants were seated upright in a Biodex chair with their shoulder placed in 45° of abduction and neutral rotation, with the elbow in 120° of extension, and the wrist in 90° of supination (Fig. 1C). The wrist and hand were cast and fixated to a ring-mount interface to standardize arm position.
The stimulating electrode (30-mm interelectrode distance) was placed in the medial bicipital groove. The electrode position was fine-tuned to elicit the maximum H reflex at a given stimulus current and was then firmly secured with medical tape and strap that applied compressive force to the electrode to minimize electrode motion and to reduce the distance to the median nerve. One surface electromyogram (EMG) electrode (two 10-mm disks, 22-mm interelectrode distance) was placed over the belly of the flexor carpi radialis (FCR). The ground electrode was placed over the cubital fossa to minimize stimulation artifact. The H reflex was evoked using a constant current stimulator (Digitimer, DS7A). Each stimulus was a 1-ms square pulse, triggered from the data acquisition interface (Power 1401, Cambridge Electronic Design). The EMG signal was amplified (Biopac) with a gain of 5,000 and band-pass filtered at 20–3,000 Hz and was sampled at 10 kHz.
Procedure.
To ensure high EMG signal quality, the skin was abraded and cleaned with alcohol pad. Ten single-pulse stimulations were applied initially, generating a consistent visible H reflex, in the absence of M wave, with the subjects instructed to relax. The stimulus current was then reduced gradually until the H reflex was visible in only about 5 out of 50 stimulations under the minimum current, which was then designated as the subthreshold current. The 10% response probability from the conditioning stimuli ensures an accurate estimation of the time course based on our simulation results. The double-stimulation procedure was then applied using the subthreshold current for both stimuli (Fig. 1B). The ISI was randomized from 4 to 70 ms in a uniform distribution. The interval between each double-stimulus sequence was set to 1 s, given that the H-reflex amplitude can fully recover within 200 ms (Rossi-Durand et al. 1999). A total of 800–1,000 double stimulations were applied. Both arms of the stroke survivors were tested in a single session.
Data analysis.
The H-reflex response was detected using a threshold-crossing approach. Specifically, the standard deviation (SD) of the baseline EMG signal from 100 to 600 ms before the conditioning stimulus was calculated first, and the H-reflex detection threshold was set at ±5 × SD. For each stimulus, a 5-ms EMG window was set to detect the H reflex. The start of the window was set manually based on the reflex latency of each stimulation series. When a reflex was detected from the conditioning stimulus, that stimulation pair was removed from later analysis. The probability of a reflex response from the test stimulus was calculated based on the number of reflex responses normalized by the number of stimulations. The probability was calculated as a function of the ISI with a 1-ms bin size. The probability of a reflex response from the conditioning stimuli was also calculated, to ensure that the estimation was accurate.
To quantify the rate of decay of the EPSP in the spastic and contralateral sides of the subjects, the impulse function (Eq. 1) and exponential function (Eq. 2) were each fitted to the distribution of response probability, and the Tc of the regression was used to quantify the EPSP time course.
RESULTS
Simulation Results
Figure 2 illustrates the response probability from the EPSP with Tc = 6 ms at different EPSP amplitude and noise levels. As shown in Fig. 2A, with an EPSP amplitude A = 9 mV and noise level σ = 4 mV, the time-course estimation was accurate. The amplitude of the actual EPSP (thick solid line) was normalized to 1 to match the probability plot. The estimated Tc was 5.84 ms from the impulse function (thick dash line). The estimated Tc was 11.90 ms from the exponential function (thin dash line), given that the “true” Tc = 11.25 ms (thin solid line). The response probability from conditioning EPSPs was 10.11% (horizontal dash line).
Examples of inaccurate estimates are shown in Fig. 2, B and C. In Fig. 2B (A = 9 mV; σ = 3 mV), the noise level was reduced, such that a smaller portion of the EPSP can be tracked. In this case, both impulse and exponential functions underestimated the time course with impulse Tc = 3.18 ms and exponential Tc = 4.42 ms. The response probability from the first EPSP was 0.5%. In Fig. 2C (A = 10 mV; σ = 4 mV), a larger EPSP (a higher stimulus current) can lead to an overestimation with impulse Tc = 8.34 ms and exponential Tc = 22.92 ms. The response probability from the first EPSPs was 21.2%.
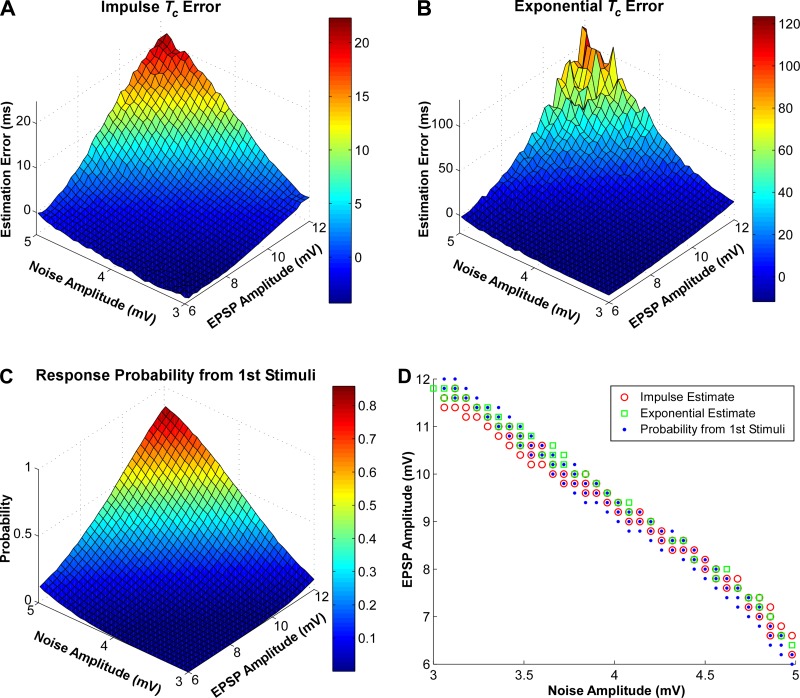
To systematically investigate the influence of EPSP and noise amplitudes on the estimation accuracy, we calculated the estimation error and the response probability from the first stimulus at different EPSP amplitude and noise levels (Fig. 3). The simulated EPSP has an impulse Tc = 6 ms. With a low noise level and low EPSP amplitude, the time constant tended to be underestimated and the response probability from the first stimuli was low and vice versa. We also found similar patterns when a shorter time constant (impulse Tc = 4 ms) was simulated.
Fig. 3.
Estimation error and response probability as a function of EPSP amplitude and noise amplitude. A: time constant (Tc) estimation error from the impulse function at different noise and EPSP amplitudes. B: Tc estimation error from the exponential function. C: response probability from the conditioning stimulus. D: EPSP amplitude and noise level combinations that lead to accurate EPSP time-course estimates.
To quantify the similarity of the different matrix landscapes, a two-dimensional correlation coefficient (Eq. 3) was calculated between the Tc error matrix (impulse or exponential) and the response probability matrix from the first stimulus. The correlation coefficients were exceptionally high (r = 0.997 between Fig. 3, A and C, and r = 0.973 between Fig. 3, B and C).
| (3) |
Here, Am × n or Bm × n represents an m × n matrix, and Ā or B̄ represents the mean of the matrix elements.
Such high correlations indicate that the response probability from the conditioning stimuli can be used to predict the estimation accuracy, given that the actual EPSP and noise amplitudes are not accessible readily in human subjects. Based on the error matrix, different EPSP and noise amplitude combinations that lead to accurate (±5% error band) estimates are shown in Fig. 3D. The combinations that lead to 7–13% response probability from the first stimuli are also plotted. Essentially, these combinations represent the numerical solutions that lead to accurate estimations. The findings were consistent with a range of EPSP time constant parameters (4–6 ms).
The primary findings of the simulation were that the combinations of EPSP and noise amplitudes that lead to accurate time constant estimates largely overlap with those that induced 7–13% response probability from the first stimulus. Namely, the double-stimulation protocol can accurately estimate the time course, if a 7–13% response probability from the conditioning stimulus is maintained.
Experimental Results
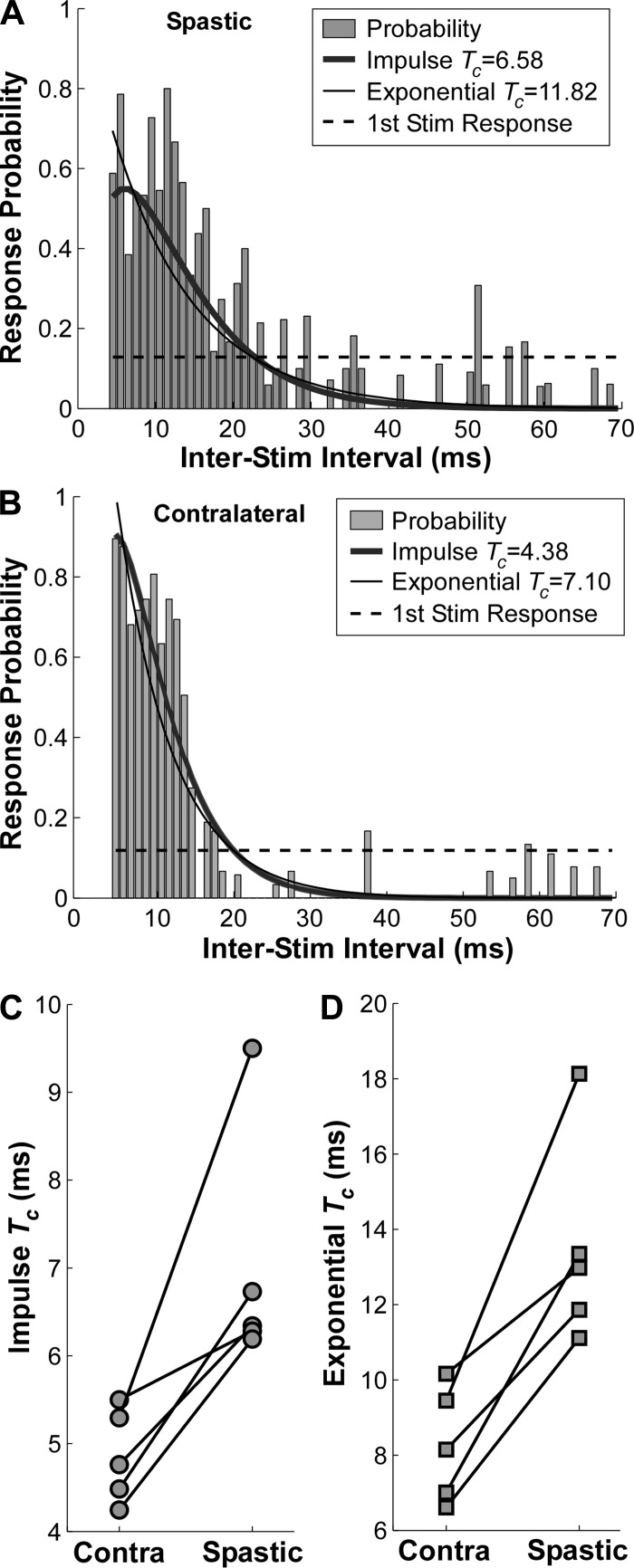
When the first stimulus elicited a subthreshold EPSP, the response probability distribution elicited from the second test stimuli can be assembled readily. A typical response for one representative stroke survivor is shown. The response probability in the spastic FCR muscle (Fig. 4A) revealed a distinctively wider distribution (i.e., a slower decay) compared with the contralateral side (Fig. 4B). The time constants (Tc) in both the impulse and exponential functions confirmed the estimates. The probability of an H-reflex response from the conditioning stimuli was 12.82% in the spastic side and 11.85% in the contralateral side, affirming that the single-pulse stimulus was essentially subthreshold and that the EPSP population estimate was accurate.
Fig. 4.
Response probability distribution from the test stimuli and Tc estimates. A and B: response probability in the spastic and contralateral muscles from a single subject, respectively. The response probability from the conditioning stimuli is shown in dashed horizontal line. C: Tc of the impulse function of 5 subjects. D: Tc of the exponential function of 5 subjects.
The time constants (Tc) of the test H-reflex occurrences in both sides of the five stroke survivors are illustrated in Fig. 4, C and D, with each symbol representing one subject. The Tc on the spastic side ranged from 6.19 to 9.50 ms in the impulse function in Fig. 4C and from 11.11 to 18.13 ms in the exponential function in Fig. 4D. These time constants were consistently longer than in the contralateral side of all the stroke survivors tested (ranging from 4.23 to 5.50 ms in the impulse function and from 6.61 to 10.16 ms in the exponential function). A paired t-test was then performed to compare the difference between the Tc in two sides of the subjects. The impulse Tc on the spastic side was significantly longer than on the contralateral side [t(4) = 3.78, P = 0.019], and similar results were found in the exponential Tc [t(4) = 4.99, P = 0.008].
DISCUSSION
This study estimated the putative time course of population EPSPs in motoneuron pools of stroke survivors, using a subthreshold double-stimulation protocol. We further sought to provide evidence regarding the origins of hyperexcitability in motoneurons innervating spastic muscles of chronic stroke survivors. Our results revealed that the rate of decay of the EPSP was measurably slower in motoneurons innervating the spastic FCR muscle compared with the contralateral muscle, a finding observed consistently in all five stroke survivors. With a slower decay of the EPSP evoked by a synaptic input from Ia afferents, the membrane potential would be in a sustained depolarization for longer time, potentially increasing the probability of more neuronal firings based on temporal summation of successive EPSPs. This effect essentially increases the net excitability of the motoneuron and could contribute to muscular hypertonia of stroke subjects. Overall, we have identified a potentially important neural mechanism of spasticity in stroke survivors, and in addition, our subthreshold double-stimulation approach may provide a novel noninvasive tool for assessing the time course of the EPSP in human motoneurons in both healthy and disease states.
Double-Stimulation Approach
One key feature of our double-stimulation approach was that the initial stimulus was subthreshold in a “passive” motoneuron. In this case, a single stimulus does not routinely evoke a reflex action potential, and the membrane potential is therefore not hyperpolarized during an AHP triggered by a previous action potential. An earlier study used a similar double-stimulation approach to perform a rough estimation of the EPSP duration (Ashby and Zilm 1982). Specifically, a subthreshold double-stimulus was applied, with intervals of 10, 15, and 20 ms with a motor unit in tonic discharge. The authors concluded that the EPSP duration was around 15–20 ms, given that the 20-ms interval did not trigger an action potential. The duration estimation was inexact due to a limited number of stimuli and potential biases from the time course of concurrent AHPs. In contrast, in our approach, by applying a larger range of inter-stimulus intervals, and a large number of stimuli in an initially quiescent neuron, we believe we are able to more accurately estimate the rate of decay of the population EPSP.
After a central nervous system injury, many changes can arise in spinal cord electrophysiology. For example, changes in descending pathways and in neuromodulatary inputs can lead to reduced presynaptic inhibition (Burke et al. 1971), to a depolarization of the resting membrane potential of spinal motoneurons (Miller et al. 2014), to an altered time course of AHP (Suresh et al. 2014), and to changes in the level of synaptic noise (Farmer et al. 1993). These various changes can bias EPSP time-course estimates based on either discharge-frequency or firing probability approaches. In contrast, given that our current double-stimulation approach begins with subthreshold excitation of an inactive neuron, our estimates are not confounded by most of these factors. For example, possible resting membrane depolarization and larger EPSPs in hyperexcitable neurons can increase the probability of neuron activation and potentially lead to biased time-course estimates; however, we are able to control for these factors in our quiescent protocol. Namely, a lower stimulus current was required to evoke smaller EPSPs, such that a fixed (∼10%) probability of reflex activation from the conditioning stimulus was maintained. Admittedly, the H reflex was recorded using surface EMG recordings, in which signals are generated by multiple active motoneurons. Future studies involving single motor unit reflex discharge are required to further explore the EPSP time course in more detail.
Mechanisms of Longer EPSP Decay Times
Multiple factors can contribute to a prolonged decay of the EPSP in motoneurons innervating hypertonic muscles. Prolonged synaptic current release or postsynaptic receptor activation can increase EPSP duration in individual motoneurons; however, this change has not been confirmed in spastic animal models. The EPSP time course can also be altered by a change in the intrinsic electrical properties of the motoneuron, including changes in input resistance of the motoneuron or changes in voltage-gated ionic conductances. Specifically, alterations in voltage-sensitive sodium and calcium membrane conductances, mediated potentially by increased neuromodulator release from different brainstem pathways can change profoundly the input-output properties of the motoneuron. Relevant neuromodulators include serotonin released from raphe neurons, norepinephrine from locus ceruleus, and potentially dopamine and even acetyl choline may all impact these conductances. These prolonged conductances can lead to sustained depolarization of the membrane. There is strong evidence that these persistent inward currents (PICs) are a dominant factor mediating spasms after spinal cord injury (Heckman et al. 2005). Our findings provide evidence for a potential increase of PIC duration in motoneurons innervating spastic muscles poststroke. This enhanced PIC appears from the outside as a prolongation in EPSP duration.
Another mechanism, potentially manifesting as a population EPSP prolongation is the addition of motoneuron excitation mediated through regional Ia excitatory interneurons. Such interneurons have been described in the cat preparation (Stecina et al. 2008), and these interneurons may also show sustained discharge after Ia afferent excitation and may also be subject to prolonged excitation via the emergence of plateau potentials that are monoamine sensitive. This prolonged excitation could lead to sustained excitation of spinal motoneurons, generating an apparent prolongation of the Ia EPSP.
Finally, it is conceivable that the onset of EPSPs in the motoneuron population is more scattered in time, so that the population response is spread out over a longer period. In other words, the motoneuron pool reflex behavior is not an accurate reflection of the individual motoneuron response. Although possible, we have no evidence that EPSP onset is differently distributed in motoneuron pools innervatings spastic muscles, as there is no slurring of the peak of the H-reflex probability distribution in spastic motoneurons.
Conclusions
Using a double-stimulation technique, we examined the time course of the population EPSP in spastic stroke survivors. The prolonged EPSP could increase the potential for temporal summation of synaptic potentials and increase the probability of sustained motoneuron discharge. These changes can contribute to the altered recruitment in spastic muscles after stroke. In addition, the double-stimulation approach opens the possibility for noninvasively assessing the time course of the EPSP in human subjects. Clearly, future studies involving a larger subject cohort and single motor unit recordings are required to understand the prevalence of the changes in EPSP time course and the reliability of the time-course estimates.
GRANTS
This study was supported by National Institutes of Health Grant R24 HD-50821-07 to W. Z. Rymer.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.H., N.L.S., and W.Z.R. conception and design of research; X.H., N.L.S., and W.Z.R. performed experiments; X.H., N.L.S., and W.Z.R. analyzed data; X.H., N.L.S., and W.Z.R. interpreted results of experiments; X.H., N.L.S., and W.Z.R. prepared figures; X.H., N.L.S., and W.Z.R. drafted manuscript; X.H., N.L.S., and W.Z.R. edited and revised manuscript; X.H., N.L.S., and W.Z.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Brian Jeon and Cindy Xue for assistance during data collection.
REFERENCES
- Ashby P, Zilm D. Characteristics of postsynaptic potentials produced in single human motoneurons by homonymous group 1 volleys. Exp Brain Res 47: 41–48, 1982. [DOI] [PubMed] [Google Scholar]
- Burke D, Gillies JD, Lance JW. Hamstrings stretch reflex in human spasticity. J Neurol Neurosurg Psychiatry 34: 231–235, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Swash M, Ingram DA, Stephens JA. Changes in motor unit synchronization following central nervous lesions in man. J Physiol 463: 83–105, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol 341: 387–410, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005. [DOI] [PubMed] [Google Scholar]
- Katz RT, Rymer WZ. Spastic hypertonia: mechanisms and measurement. Arch Phys Med Rehabil 70: 144–155, 1989. [PubMed] [Google Scholar]
- Miller DM, Klein CS, Suresh NL, Rymer WZ. Asymmetries in vestibular evoked myogenic potentials in chronic stroke survivors with spastic hypertonia: evidence for a vestibulospinal role. Clin Neurophysiol 125: 2070–2078, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA. Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain 131: 1478–1491, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-Durand C, Jones KE, Adams S, Bawa P. Comparison of the depression of H-reflexes following previous activation in upper and lower limb muscles in human subjects. Exp Brain Res 126: 117–127, 1999. [DOI] [PubMed] [Google Scholar]
- Stecina K, Jankowska E, Cabaj A, Pettersson LG, Bannatyne BA, Maxwell DJ. Premotor interneurones contributing to actions of feline pyramidal tract neurones on ipsilateral hindlimb motoneurones. J Physiol 586: 557–574, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh AK, Hu X, Powers RK, Heckman CJ, Suresh NL, Rymer WZ. Changes in motoneuron afterhyperpolarization duration in stroke survivors. J Neurophysiol 112: 1447–1456, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh NL, Ellis MD, Moore J, Heckman H, Rymer WZ. Excitatory synaptic potentials in spastic human motoneurons have a short rise-time. Muscle Nerve 32: 99–103, 2005. [DOI] [PubMed] [Google Scholar]
- Turker KS, Cheng HB. Motor-unit firing frequency can be used for the estimation of synaptic potentials in human motoneurones. J Neurosci Methods 53: 225–234, 1994. [DOI] [PubMed] [Google Scholar]
- Turker KS, Powers RK. Effects of large excitatory and inhibitory inputs on motoneuron discharge rate and probability. J Neurophysiol 82: 829–840, 1999. [DOI] [PubMed] [Google Scholar]