Abstract
In rodent cortex GABAA receptor (GABAAR)-mediated synapses are a significant source of input onto GABA neurons, and the properties of these inputs vary among GABA neuron subtypes that differ in molecular markers and firing patterns. Some features of cortical interneurons are different between rodents and primates, but it is not known whether inhibition of GABA neurons is prominent in the primate cortex and, if so, whether these inputs show heterogeneity across GABA neuron subtypes. We thus studied GABAAR-mediated miniature synaptic events in GABAergic interneurons in layer 3 of monkey dorsolateral prefrontal cortex (DLPFC). Interneurons were identified on the basis of their firing pattern as fast spiking (FS), regular spiking (RS), burst spiking (BS), or irregular spiking (IS). Miniature synaptic events were common in all of the recorded interneurons, and the frequency of these events was highest in FS neurons. The amplitude and kinetics of miniature inhibitory postsynaptic potentials (mIPSPs) also differed between DLPFC interneuron subtypes in a manner correlated with their input resistance and membrane time constant. FS neurons had the fastest mIPSP decay times and the strongest effects of the GABAAR modulator zolpidem, suggesting that the distinctive properties of inhibitory synaptic inputs onto FS cells are in part conferred by GABAARs containing α1 subunits. Moreover, mIPSCs differed between FS and RS interneurons in a manner consistent with the mIPSP findings. These results show that in the monkey DLPFC GABAAR-mediated synaptic inputs are prominent in layer 3 interneurons and may differentially regulate the activity of different interneuron subtypes.
Keywords: dorsolateral prefrontal cortex, primate, interneuron, inhibitory postsynaptic potential, disinhibition, GABAA receptor
in cortical networks, GABA neurons are driven by glutamatergic excitatory inputs and critically regulate pyramidal cell activity via synaptic inhibition (Isaacson and Scanziani 2011). Recent studies have highlighted that in the rodent neocortex and hippocampus GABA neuron activity is also controlled by GABAA receptor (GABAAR)-mediated synaptic inhibition, which may play diverse functional roles. For example, reciprocal inhibition between GABA neurons can produce oscillatory synchrony in interneuron networks (Bartos et al. 2007; Traub et al. 1996; Wang and Buzsaki 1996). Moreover, inhibiting specific GABA neuron subtypes may disinhibit pyramidal cells selectively at perisomatic locations or distal dendrites (Lovett-Barron and Losonczy 2014; Pfeffer 2014). Thus, adding to the diversity of GABAergic interneuron classes and their differential recruitment by specific patterns of excitatory input, inhibition of inhibitory GABA neurons markedly expands the range of mechanisms by which they regulate cortical network function (Kepecs and Fishell 2014).
The presence of functional inhibitory inputs onto GABA neurons was found in early studies of the cat visual neocortex (see Tamas et al. 1998 and references there), suggesting that inhibition of GABA neurons is not unique to rodent neocortex and hippocampus but might be a ubiquitous feature of cortical circuits across mammalian species and cortical areas. However, the microcircuit structure and function of the mammalian neocortex display significant area- and species-related levels of specialization (de Sousa and Proulx 2014; Elston 2003; Elston and Fujita 2014; Fuster 2008; Jacobs and Scheibel 2002; Miller and Cohen 2001; Spruston 2008). Furthermore, previous studies showed that monkey dorsolateral prefrontal cortex (DLPFC) pyramidal cells (Amatrudo et al. 2012; Elston et al. 2001, 2006, 2011) and interneurons (Povysheva et al. 2007, 2008, 2013) have some region- and species-specific features. Specifically, some electrophysiological properties of various interneuron subtypes differ significantly between rodent medial PFC and monkey DLPFC (Povysheva et al. 2007, 2008, 2013). Thus it seems plausible that inhibition of GABA neurons also has cortical region- and species-specific features and that to define the functional role of GABA inputs onto GABA neurons, these inhibitory inputs must be studied precisely in each specific microcircuit.
The macaque monkey DLPFC was identified by Brodmann in 1913 (Elston and Garey 2004) as a cortical region that lacks a clear analog or homolog in rodents or in many other groups of mammals (Preuss 1995). We previously identified at least eight different GABA neuron subtypes in layer 3 microcircuits of the monkey DLPFC (Gonzalez-Burgos et al. 2004, 2005; Krimer et al. 2005; Povysheva et al. 2007, 2008, 2013; Zaitsev et al. 2009). Monkey DLPFC layer 3 GABA neurons are divided into fast-spiking (FS) and non-FS groups (Krimer et al. 2005; Zaitsev et al. 2005, 2009). As in rodent cortex, in response to sustained excitatory currents FS interneurons of monkey DLPFC layer 3 fire at a nearly constant frequency, whereas non-FS neurons exhibit spike frequency adaptation. The FS and non-FS groups of DLPFC interneurons are subdivided into subgroups on the basis of their axonal morphology and expression of molecular markers (Zaitsev et al. 2005, 2009).
Interestingly, in rodents, the properties of inhibitory synaptic inputs onto cortical GABA neurons differ between interneuron classes distinguished by their firing patterns (Bacci et al. 2003; Pfeffer et al. 2013; Savanthrapadian et al. 2014). The relation between interneuron firing pattern and properties of inhibitory inputs, if conserved at least in part from rodents to primate neocortex, may be important for the roles that distinct GABA neuron subtypes are thought to play in shaping network activity in monkey DLPFC (Konstantoudaki et al. 2014; Wang et al. 2004). However, it is not known whether inhibition of GABA neurons is as prominent in the monkey DLPFC as in the cortex of other mammals and, if so, whether the features of inhibitory inputs vary between GABA neuron classes. Here we demonstrated that inhibitory inputs are prominent in interneurons from layer 3 of monkey DLPFC and characterized the properties of GABAAR-mediated synaptic responses in interneuron subtypes classified via their intrinsic membrane properties.
METHODS
Brain slice preparation.
Electrophysiological recordings were performed in brain slice tissue obtained from three adult macaque monkeys: one female (84 mo old) rhesus monkey (Macaca mulatta) and two male (42 and 60 mo old) long-tailed macaque monkeys (Macaca fascicularis) supplied by the University of Pittsburgh Primate Research Center. Housing and experimental procedures were conducted in accordance with US Department of Agriculture and National Institutes of Health guidelines and with approval of the University of Pittsburgh's Institutional Animal Care and Use Committee. All animals were experimentally naive at the time of entry into this study.
Tissue blocks containing portions of areas 9 and 46 were obtained from one or both hemispheres of each animal. An initial tissue block was removed from one hemisphere with a previously described surgical procedure (Gonzalez-Burgos et al. 2009), and a second tissue block was removed 1–2 wk later from the contralateral hemisphere. The locations of the blocks removed in the separate surgical procedures were offset in the rostral-caudal axis, so that nonhomotopic portions of the DLPFC were studied from each hemisphere. For removal of the second block, the animals were deeply anesthetized and perfused transcardially with a cold artificial cerebrospinal fluid (ACSF) solution of the following composition (in mM): 210.0 sucrose, 10.0 NaCl, 1.9 KCl, 1.2 Na2HPO4, 33.0 NaHCO3, 6.0 MgCl2, 1.0 CaCl2, 10.0 glucose, and 2.0 kynurenic acid, pH 7.3–7.4 when bubbled with 95% O2-5% CO2. Slices (300–350 μm thick) containing areas 9 and 46 of the DLPFC were cut in the coronal plane with a vibrating microtome (VT1000S, Leica Microsystems, Nussloch, Germany) in ice-cold ACSF. Immediately after cutting, slices were transferred to an incubation chamber maintained at room temperature and filled with a solution containing, in mM, 126.0 NaCl, 2.0 KCl, 1.2 Na2HPO4, 10.0 glucose, 25.0 Na2HCO3, 6.0 MgCl2, and 1.0 CaCl2, pH 7.3–7.4 when bubbled with 95% O2-5% CO2.
Electrophysiological recordings.
For recording, slices were submerged in a chamber superfused at 2–3 ml/min with a solution containing, in mM, 126.0 NaCl, 2.5 KCl, 1.2 Na2HPO4, 25.0 Na2HCO3, 10.0 glucose, 2.0 CaCl2, 1.0 MgCl2, 0.02 CNQX, and 0.1 d,l-AP5, bubbled with 95% O2-5% CO2, and maintained at 30–32°C. Whole cell recordings were obtained from layer 3 nonpyramidal neurons visually identified with infrared differential interference contrast video microscopy (Olympus BX51 and BX61 or Zeiss FS Axioskop microscopes). Pipettes pulled from borosilicate glass had a resistance of 3–5 MΩ when filled with a solution containing, in mM, 120.0 KCl, 10.0 NaCl, 0.2 EGTA, 10.0 HEPES, 4.0 Mg2ATP, 0.3 Na3GTP, and 14.0 Na2 phosphocreatine, with 0.5% biocytin (pH adjusted to 7.2–7.3). Assuming an intracellular HCO3− concentration of 15 mM (Farrant and Kaila 2007) and a permeability ratio PHCO3−/PCl− of 0.3 for GABAAR channels (Farrant and Kaila 2007) and using the Goldman-Hodgkin-Katz equation, we estimated a GABAAR reversal potential (EGABA) near zero (−0.66 mV). Miniature inhibitory postsynaptic potentials (mIPSPs) or currents (mIPSCs) were recorded at a potential of −70 mV in the presence of tetrodotoxin (TTX, 1 μM), CNQX (20 μM), and d,l-AP5 (100 μM) applied to the bath solution. Recordings were performed with Multiclamp 200A or Multiclamp 200B amplifiers (Axon Instruments, Union City, CA) operating in current-clamp (bridge) or voltage-clamp mode. Signals were low-pass filtered at 4 kHz, digitized at 10 or 20 kHz, and stored on disk for off-line analysis. Data acquisition was performed with Power 1401 data acquisition interface boards and Signal 3 software (Cambridge Electronic Design, Cambridge, UK). Throughout the experiments, the series resistance was monitored; if it exceeded 30 MΩ, recordings were excluded from data analysis.
To characterize the intrinsic membrane properties of neurons, rectangular hyper- and depolarizing current pulses of 500-ms duration were applied in 10-pA increments at 0.2 Hz with two repetitions. The membrane time constant was determined by fitting a single exponential to the average voltage response to hyperpolarizing current steps of −10 to −30 pA. The input resistance was estimated from the slope of the linear portion of voltage-current plots (usually between −50 and −10 pA), built measuring the amplitude of the voltage deflection near the end of the current step. Single action potential properties, including threshold, duration at half-maximal amplitude, and amplitude and size of the afterhyperpolarization (AHP), were measured from the response to current steps close to the threshold of firing for each individual cell, which usually elicited either one or a few action potentials. Spike frequency adaptation ratio was measured as the ratio between the last and the first interspike interval, measured with depolarizing current steps of 50–100 pA above the threshold of firing.
Analysis of miniature IPSPs and IPSCs.
For each neuron, we first assessed membrane properties in current clamp and then added TTX (1 μM) to study miniature synaptic events. In most neurons sampled [24 FS, 12 regular-spiking (RS), 6 burst-spiking (BS), and 6 irregular-spiking (IS) cells], we recorded mIPSPs. The effects of zolpidem on mIPSPs were tested in a subsample of these cells (14 FS, 7 RS, and 3 BS cells), as reported in Fig. 6. In a second neuron sample (10 FS and 12 RS cells), after addition of TTX the recording mode was switched to voltage clamp, and mIPSCs were studied. Table 1 reports the membrane properties of all neurons for which either mIPSPs or mIPSCs were recorded. We used Mini Analysis (Synaptosoft, Decatur, GA) to detect mIPSPs and mIPSCs. At least 200 nonoverlapping events were included to automatically generate an average event for each cell. The decay time was estimated by the weighted time constant obtained from fitting a double-exponential function to the 10–90% decay phase. The effects of zolpidem were analyzed during the last 5 min of a 15-min zolpidem application. For analysis of miniature event frequency, each data file was reanalyzed to include both nonoverlapping and overlapping miniature events.
Fig. 6.
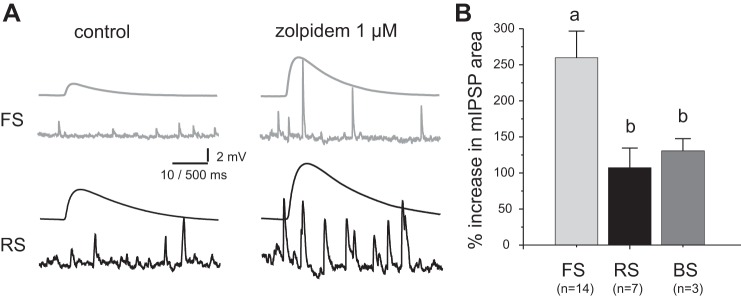
Effects of the positive modulator zolpidem on mIPSPs in FS and RS interneurons. A: mIPSPs recorded in control conditions (left) and after zolpidem application (right) from FS neurons (top) and RS neurons (bottom). Shown are representative individual mIPSPs and average mIPSP waveforms. B: bar graph summarizing the % change in mIPSP area produced by 1 μM zolpidem application. The difference between group means was significant (P < 0.05, single-factor ANOVA).
Table 1.
Summary of electrophysiological intrinsic membrane properties of subclasses of interneurons in layer 3 of monkey DLPFC
| Cell Class | Time Constant, ms | Input Resistance, MΩ | Spike Duration, ms | Adaptation Ratio (last ISI/first ISI) | Spike Threshold, mV | AHP Amplitude, mV |
|---|---|---|---|---|---|---|
| FS (n = 34) | 9.21 ± 0.62a | 155 ± 16a | 0.45 ± 0.01a | 1.07 ± 0.02a | −50.5 ± 1.0a | 18.9 ± 1.1a |
| RS (n = 24) | 22.40 ± 1.48b | 318 ± 32b | 0.84 ± 0.05b | 2.03 ± 0.13b | −51.8 ± 1.9a | 12.0 ± 0.9b |
| BS (n = 6) | 22.51 ± 5.49b | 292 ± 26b | 1.09 ± 0.11c | 4.17 ± 0.87b | −55.6 ± 1.4a | 5.7 ± 1.5c |
| IS (n = 6) | 14.3 ± 1.6a,b | 288 ± 72b | 0.66 ± 0.11b | 3.23 ± 0.69b | −53.5 ± 1.6a | 12.9 ± 2.4b |
Except for spike threshold, all other electrophysiological properties showed significant differences between group means, as determined by single-factor ANOVA. In each column, groups labeled with a different letter are significantly different, as determined by Fisher least significant difference (LSD) comparisons (P < 0.05).
DLPFC, dorsolateral prefrontal cortex; FS, fast spiking; RS, regular spiking; BS, burst spiking; IS, irregular spiking; ISI, interspike interval; AHP, afterhyperpolarization.
Morphological analysis.
Interneurons were filled with 0.5% biocytin during recording, and after recording the slices were fixed in 4% paraformaldehyde and stored in an antifreeze solution (1:1, glycerol-ethylene glycol in 0.1 M phosphate buffer) at −80°C until being processed for visualization of the biocytin label. Slices were resectioned at 50 μm, incubated with 1% H2O2, and immersed in blocking serum containing 0.5% Triton X-100 for 2 h at room temperature. Slices were then incubated with avidin biotinylated enzyme complex-peroxidase and developed using the nickel-enhanced diaminobenzidine chromogen to visualize the biocytin label. Some of the biocytin-filled and stained neurons were reconstructed with the Neurolucida tracing system from MicroBrightField.
Statistics.
Data are expressed as means ± SE. Differences between group means were tested with Student's t-test or one-way or two-way ANOVA followed by post hoc contrasts. Differences between distributions were tested with Kolmogorov-Smirnov tests.
RESULTS
Electrophysiological properties of recorded interneurons.
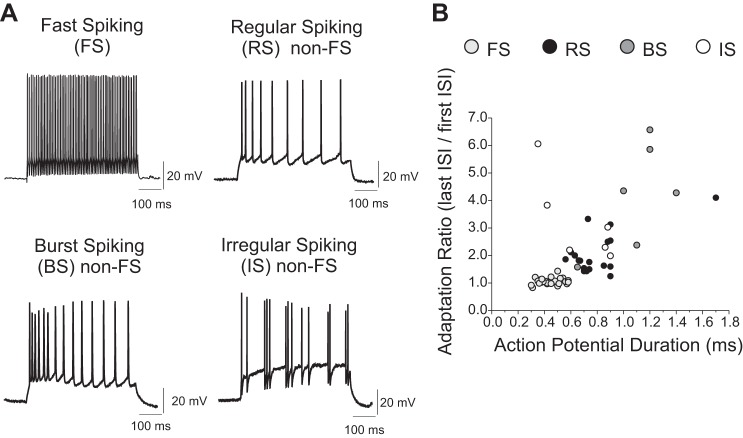
To study GABAAR-mediated synaptic inputs onto layer 3 interneurons, the recorded cells were grouped on the basis of their intrinsic electrophysiological properties (Fig. 1A). We measured action potential duration and spike frequency adaptation via an adaptation ratio (Fig. 1B). Using cluster analysis of electrophysiological parameters, we previously divided the monkey DLPFC layer 3 interneurons into two main groups: FS and non-FS cells (Krimer et al. 2005; Zaitsev et al. 2005). Here, cells with spike duration < 0.6 ms and adaptation ratio < 1.25 (Fig. 1B) were classified as FS interneurons (n = 34). All other interneurons (n = 36) were classified as non-FS cells.
Fig. 1.
Firing properties of interneurons in layer 3 of monkey dorsolateral prefrontal cortex (DLPFC). A: interneurons were divided into fast-spiking (FS) and non-FS groups, the latter containing 3 different subclasses, described as regular spiking (RS, which had simple spike frequency adaptation), burst spiking (BS, which showed an initial high-frequency burst of spikes), and irregular spiking (IS, which fired at irregular frequency but had spike frequency adaptation overall). B: the distributions of adaptation ratio vs. spike duration partially overlapped between RS, BS, and IS non-FS neurons, which were distinguished by the firing patterns shown in A and by other properties reported in Table 1.
Whereas FS neurons had homogeneous firing properties (Fig. 1), non-FS cells had heterogeneous electrophysiological properties and were divided into three subtypes (Fig. 1): regular spiking (RS), with progressive spike frequency adaptation; burst spiking (BS), with a burst of high-frequency spikes at the onset of stimulation followed by spike frequency adaptation; and irregular spiking cells (IS), with variable firing frequency and overall spike frequency adaptation. Most non-FS interneurons were RS (24/36, 66.6%), whereas BS cells (6/36; 16.7%) and IS cells (6/36; 16.7%) constituted smaller subpopulations. Table 1 summarizes some intrinsic electrophysiological properties of FS, RS, BS, and IS interneurons.
Consistent with the findings from our previous studies, FS interneurons of monkey DLPFC were either basket cells or chandelier neurons (Fig. 2) and non-FS cells had heterogeneous morphology (Fig. 2). Staining of the biocytin-filled recorded neurons confirmed that all layer 3 neurons included in this study had nonpyramidal morphology, including small rounded or ovoid-shaped cell body and lack of apical dendrite or high dendritic spine density. However, for most cells the morphological properties recovered were incomplete, precluding their classification into particular morphological subtypes. Importantly, in mouse neocortex inhibitory synaptic inputs showed different properties between interneurons classified by their firing properties into FS, RS, IS, and BS groups very similar to those studied here and independent of morphological subclass (Bacci et al. 2003; Dumitriu et al. 2007; Pfeffer et al. 2013).
Fig. 2.
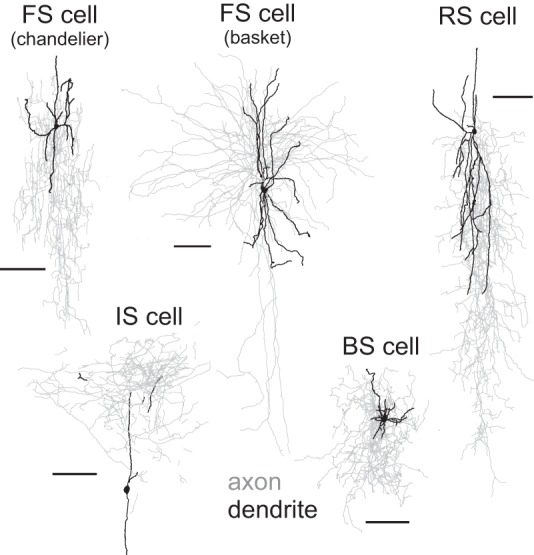
Examples of morphological properties of FS and non-FS neurons filled with biocytin during recordings and reconstructed with Neurolucida. As in our previous studies of monkey DLPFC interneurons, the FS cells with identified morphology were either chandelier or basket neurons. The non-FS cells had heterogeneous morphological properties, including cells with descending vertical axons, vertically ascending axons, or axonal arbors distributed approximately evenly around the soma. Although all non-FS interneurons recorded and included in the data analysis had nonpyramidal cell morphology, for many of the non-FS cells the recovered morphology was incomplete and could not be classified into morphological subclasses. Calibration bars, 100 μm.
Properties of miniature synaptic responses in FS and non-FS interneurons.
In each interneuron characterized by firing properties, GABAAR-mediated mIPSPs were recorded in the presence of TTX (1 μM), CNQX (20 μM), and d,l-AP5 (100 μM). To increase the driving force for the synaptic currents and improve event detection, the pipette solution contained a high Cl− concentration, yielding largely depolarizing GABAAR-mediated mIPSPs (Fig. 3, A and B). We found that the average mIPSP waveforms differed significantly between FS and non-FS interneurons. Specifically, mIPSPs had smaller amplitude and a faster exponential decay time course in FS than in non-FS neurons as a group (P < 0.0001). Moreover, mIPSPs had smaller amplitude and faster decay in FS neurons than in all non-FS subtypes (Fig. 3, C–F), with the IS subtype having mIPSP properties closest to those in FS cells. In addition, the mIPSP frequency, while showing high variability between interneurons of a given subclass (Fig. 4A), was significantly higher in FS than in RS, IS, or BS interneurons (Fig. 4, B and C).
Fig. 3.
Properties of miniature inhibitory postsynaptic potentials (mIPSPs) recorded from interneurons of different subtypes. A and B: representative examples of mIPSPs recorded from FS and RS non-FS neurons. C, top: examples of average mIPSP waveforms from FS (gray trace) and RS non-FS (black trace) neurons showing the characteristic difference in amplitude. Bottom: bar graphs summarizing mIPSP amplitude. The difference between group means was significant (P < 0.05, single-factor ANOVA). In this and all other figures, groups with a different letter are significantly different [P < 0.05, Fisher least significant difference (LSD) test]. D, top: the average waveforms in C normalized to the same amplitude illustrate the differences in decay time course. Bottom: bar graph summarizing mIPSP decay time constant. The difference between group means was significant (P < 0.05, single-factor ANOVA). E: cumulative probability distribution histograms of mIPSP amplitude. In this and all other comparisons of cumulative distribution, differences between groups were assessed by Kolmogorov-Smirnov tests with Bonferroni correction. The distributions differed significantly between all groups (P < 0.001). F: cumulative probability distribution histograms of mIPSP decay time constant. The distributions differed significantly between all groups (Kolmogorov-Smirnov test, P < 0.001).
Fig. 4.
Analysis of mIPSP frequency. A: cumulative probability distributions of interevent intervals in FS (A1), RS (A2), IS (A3), and BS (A4) interneurons. Gray curves are the distributions obtained for each neuron. Black curve is the distribution obtained for the total mIPSP sample in each cell type. B: interevent interval distributions differ significantly between all groups (Kolmogorov-Smirnov test). C: bar graph summarizing mean mIPSP frequency. The difference between group means was significant (P < 0.05, single-factor ANOVA).
The larger and longer-lasting mIPSPs in RS, IS, and BS cells (Fig. 3) may be determined, at least in part, by the longer membrane time constant and larger input resistance of non-FS neurons (Table 1). Consistent with this possibility, the mIPSP decay time and the mIPSP amplitude were significantly correlated, respectively, with the cells' membrane time constant and input resistance (Fig. 5). However, many of the correlations were weak in each interneuron class (time constant vs. mIPSP decay, FS: R = 0.55, P < 0.01; RS: R = 0.21, P = 0.51; BS: R = 0.89, P < 0.05; IS: R = 0.24, P = 0.64; input resistance vs. mIPSP amplitude, FS: R = 0.53, P < 0.01; RS: R = 0.44, P = 0.14; BS: R = 0.50, P = 0.31; IS: R = 0.41, P = 0.42). Therefore, the differences in mIPSP properties between interneuron subtypes may not be fully explained by the effects of the cells' input resistance and membrane time constant. One possibility is that the differences in mIPSPs between cell types are at least in part due to cell type-specific properties of the dendritic trees, such as their passive/active membrane properties and morphology, or the somatodendritic location of the GABA synapses involved in production of the mIPSPs. Different interneuron dendritic tree properties or different synapse locations produce different electrotonic filtering of the mIPSPs studied with somatic recording electrodes (Chitwood et al. 1999; Jaffe and Carnevale 1999; Norenberg et al. 2010). In addition, the differences in mIPSPs between interneuron subtypes could be attributed to different properties of the synapses underlying the mIPSPs.
Fig. 5.
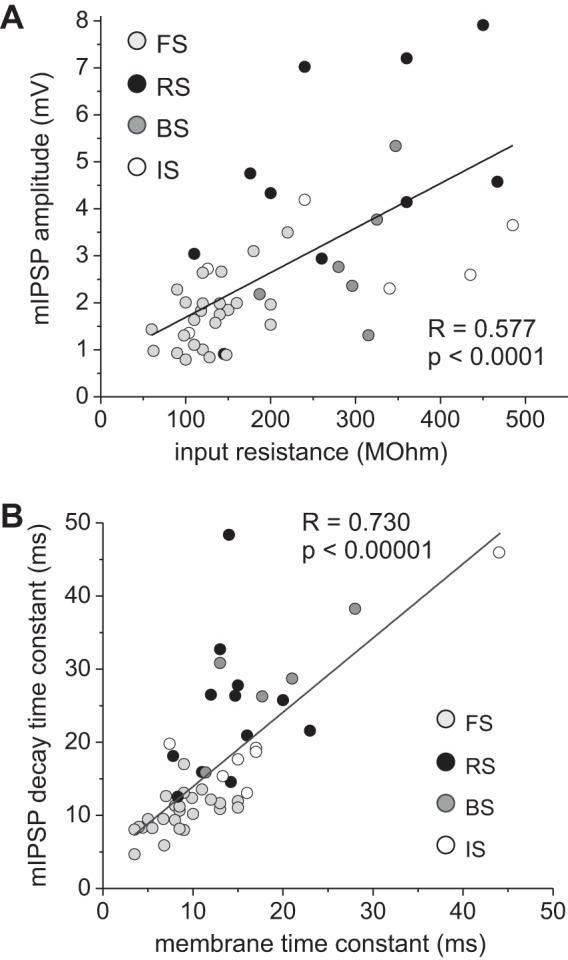
Interneuron membrane properties partially shape mIPSP amplitude and time course. A: a significant correlation was observed between mIPSP amplitude and the cells' input resistance. B: a significant correlation was observed between mIPSP decay time constant and the cells' membrane time constant.
Synaptic properties may contribute to the faster mIPSP decay kinetics in FS neurons via the subunit composition of the GABAARs, since α1 subunit-containing GABAARs (α1-GABAARs) produce currents with faster decay than other GABAAR subtypes (Farrant and Kaila 2007; Lavoie et al. 1997). To determine whether GABAAR subunit composition is an important determinant of the faster mIPSP decay kinetics in FS cells, we tested the effects of zolpidem, an allosteric modulator that binds preferentially to α1-GABAARs (Mohler 2011; Olsen and Sieghart 2009) and increases the GABA binding affinity, potentiating the response to synaptically released GABA and enhancing the IPSPs (Thomson et al. 2000). If the faster mIPSP decay in FS interneurons reflects a greater contribution of α1-GABAARs, then zolpidem should more strongly potentiate mIPSPs in FS cells. As shown previously (Thomson et al. 2000), zolpidem enhanced both the IPSP amplitude and decay (Fig. 6A). Thus we measured the changes in mIPSP area, which combine the changes in mIPSP amplitude and decay time constant. As shown in Fig. 6B, zolpidem had stronger effects in FS than in RS or BS interneurons [single-factor ANOVA F(2,21) = 4.31, P < 0.05; Fisher least significant difference (LSD) comparisons, FS vs. RS: P < 0.05, FS vs. BS: P < 0.05, RS vs. BS: P = 0.76]. These results suggest that the different properties of mIPSPs between FS and non-FS neurons are at least in part due to a greater contribution of α1-GABAARs at FS cell synapses.
To additionally determine whether synaptic properties contribute to the distinct mIPSP properties in FS cells, we recorded mIPSCs (Fig. 7A) from FS (n = 10) and RS (n = 12) interneurons. The mIPSC decay was significantly faster (P < 0.0001) in FS cells (Fig. 7, B and C), consistent with the stronger zolpidem effect in FS neurons. The mIPSC amplitude did not differ significantly between FS and RS neurons (Fig. 7, B and D), suggesting that the smaller mIPSP amplitudes in FS neurons are associated with their lower input resistance. Finally, the mIPSC frequency was significantly higher in FS neurons (Fig. 8), consistent with the findings on mIPSPs.
Fig. 7.
Properties of miniature inhibitory postsynaptic currents (mIPSCs) recorded from FS and RS interneurons. A: representative examples of mIPSCs recorded from FS and RS neurons. B, top: examples of average mIPSC waveforms recorded from FS (gray trace) and RS (black trace) neurons. Bottom: the average waveforms normalized to the same amplitude illustrate the differences in decay time course. C, left: cumulative probability distribution histograms of mIPSC decay time constant. The distributions differed significantly between groups. Right: bar graph summarizing mIPSC decay time constant. The group means were significantly different (Student's t-test). D, left: cumulative probability distribution histograms of mIPSC amplitude. The distributions did not differ significantly between groups. Right: bar graph summarizing mIPSC amplitude. The group means did not differ significantly (Student's t-test).
Fig. 8.
Analysis of mIPSC frequency. A: cumulative probability distributions of interevent intervals in FS and RS interneurons. Gray curves are the distributions obtained for each neuron. Black curve is the distribution obtained for the total mIPSP sample in each cell type. B: interevent interval distributions differ significantly between FS and RS cells. The difference between distributions was assessed by Kolmogorov-Smirnov test. C: bar graph summarizing mean mIPSC frequency. The difference between group means was assessed with Student's t-test.
The properties of mIPSCs recorded with electrodes at the soma may be altered compared with mIPSCs at the synaptic sites, because the somatic voltage clamp does not uniformly control voltage throughout the somatodendritic membrane (Spruston et al. 1993; Williams and Mitchell 2008). The magnitude of such distortion is dependent on dendritic tree properties and synapse location, and thus if those features differ between FS and RS cells then differences in mIPSC properties may be influenced by a different magnitude of space-clamp error in FS versus RS neurons. IPSCs produced by more distal GABA synapses are subject to larger space-clamp errors and have slower rise time and smaller amplitude at the soma (Spruston et al. 1993; Williams and Mitchell 2008). We found that mIPSCs recorded from individual FS or RS neurons had highly variable amplitude and 10–90% rise time (ranging from ∼0.2 to ∼3 ms; Fig. 9A), consistent with different degrees of voltage-clamp error. To obtain an estimate of mIPSC properties relatively independent of space-clamp errors, we selected from each FS or RS neuron the mIPSCs with faster rise time (10–90% rise time < 1 ms), presumably originated from proximal synapses and thus recorded with smaller space-clamp error. Consistent with more proximal synapse locations, as shown in Fig. 9B, fast-rising mIPSCs had significantly larger mean amplitude than slow-rising mIPSCs in each cell type. Moreover, the mean amplitude of fast-rising mIPSCs did not differ between FS and RS interneurons (P = 0.636), whereas the fast-rising mIPSCs had faster decay in FS neurons (Fig. 9C). Therefore, the differences in mIPSC properties between cell types appear to be relatively independent of differences in space-clamp error and to at least partly reflect different synaptic properties, in accordance with the effects of zolpidem on mIPSPs (Fig. 6).
Fig. 9.
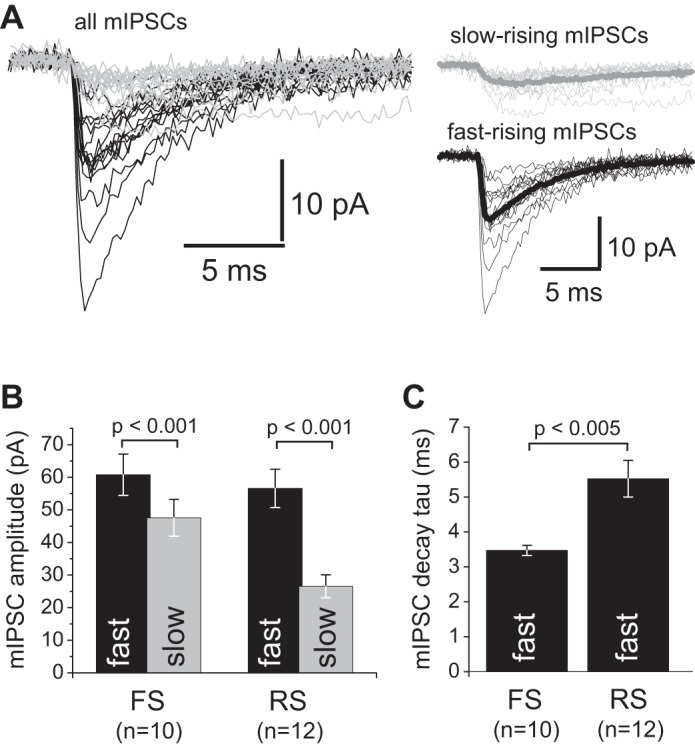
Analysis of mIPSCs with fast and slow rising phase. A, left: mIPSCs recorded from a FS interneuron aligned and superimposed. mIPSCs with 10–90% rise time ≥1 ms are shown in gray, and those with 10–90% rise time <1 ms are shown in black. Right: the slow- and fast-rising mIPSCs were separated and averaged, showing that most slow-rising mIPSCs had smaller amplitude. The average traces are shown by thick lines. B: bar graphs showing the mIPSC amplitude for fast- and slow-rising mIPSCs in FS and RS neurons. Slow-rising mIPSCs had significantly lower amplitude in both FS and RS neurons. C: bar graph summarizing the mIPSC decay time constant for fast-rising mIPSCs. Group means were significantly different (Student's test).
We previously found that the first surgical procedure did not alter the physiological or anatomical properties of the pyramidal neurons and local circuits studied in slices from the nonhomotopic tissue blocks obtained from the contralateral hemisphere during a subsequent surgical procedure (Gonzalez-Burgos et al. 2000; Henze et al. 2000). To determine the effect of prior surgical procedure on inhibitory synaptic inputs onto interneurons, we analyzed the mIPSP and mIPSC data (Figs. 3, 4, 7, and 8) by two-way ANOVA using cell type and surgical procedure (first vs. second block) as the main factors. We found that the mIPSP frequency differed between cell types [F(3,40) = 4.26, P = 0.010] but was not affected by surgical procedure [F(1,40) = 0.003, P = 0.954], nor was there a significant interaction between factors [F(3,40) = 1.99, P = 0.131]. Similar findings were obtained for mIPSP amplitude [cell type: F(3,40) = 10.9, P = 0.00002; surgical procedure: F(1,40) = 0.663, P = 0.420; interaction: F(3,40) = 0.294, P = 0.829] and mIPSP decay time constant [cell type: F(3,40) = 17.121, P = 0.000001; surgical procedure: F(1,40) = 0.896, P = 0.349; interaction: F(3,40) = 0.691, P = 0.562]. Importantly, whereas the mean mIPSP amplitude and decay time constant values were essentially identical in FS, RS, BS, or IS neurons recorded from slices of the first versus second tissue blocks (data not shown), the mean mIPSP frequency showed a tendency to differ between the first and second blocks, the difference being significant only in FS cells [FS: 5.9 ± 1.1 Hz (n = 11) vs. 3.6 ± 0.4 Hz (n = 13), P = 0.016; RS: 3.0 ± 0.7 Hz (n = 4) vs. 2.0 ± 0.3 Hz (n = 8), P = 0.468; BS: 1.4 ± 0.8 Hz (n = 2) vs. 2.0 ± 0.9 Hz (n = 4), P = 0.754; IS: 2.9 ± 0.2 Hz (n = 4) vs. 5.4 ± 1.4 Hz (n = 2), P = 0.202; Fisher LSD comparisons]. Two-way ANOVA analysis of the mIPSC data (Figs. 7, 8) similarly showed a significant effect of cell type without significant effect of surgical procedure or interaction on mIPSC amplitude [cell type: F(1,18) = 5.988, P = 0.024; surgical procedure: F(1,18) = 0.387, P = 0.542; interaction: F(1,18) = 1.401, P = 0.252] or mIPSC decay time constant [cell type: F(1,18) = 23.240, P = 0.00014; surgical procedure: F(1,18) = 0.00312, P = 0.957; interaction: F(1,18) = 1.653, P = 0.215]. However, consistent with the tendency observed for mIPSP frequency in FS and RS neurons, the mIPSC frequency was significantly affected by the prior surgical procedure [F(1,18) = 10.6, P = 0.0044] but without interaction [F(1,18) = 0.284, P = 0.600] with the significant effect of cell type [F(1,18) = 4.771, P = 0.042]. The surgical procedure indeed decreased mIPSC frequency in both FS and RS neurons, such decrease being significant only in FS cells [FS: 4.9 ± 0.58 Hz (n = 6) vs. 2.4 ± 0.53 Hz (n = 4), P = 0.020; RS: 3.11 ± 0.90 Hz (n = 6) vs. 1.32 ± 0.33 Hz (n = 6), P = 0.055]. Overall, the two-way ANOVA analysis indicated that most mIPSP and mIPSC parameters were not affected by the first surgical procedure. Whereas the prior surgery seemed to decrease the mIPSP and mIPSC frequency in FS neurons, such effect does not, however, change the conclusions of our study, as the significant differences between cell type were independent of the effects of prior surgery.
DISCUSSION
In this study we found that, as in rodent neocortex, in layer 3 of monkey DLPFC GABAAR-mediated synapses are a prominent source of synaptic input onto GABAergic interneurons, since mIPSPs and mIPSCs of significant amplitude and frequency were detected in each recorded interneuron, irrespective of subtype. We also observed that some properties of the GABAAR-mediated synaptic inputs differ between GABA neuron subtypes, suggesting that in monkey DLPFC regulation of GABA neuron activity by inhibition is GABA neuron subtype specific.
GABAAR-mediated synaptic inputs differ between DLPFC interneuron subtypes.
We divided the recorded interneurons into FS and non-FS groups that had many subthreshold and firing properties similar to those of the FS and non-FS classes described in our previous studies (Gonzalez-Burgos et al. 2004; Krimer et al. 2005; Zaitsev et al. 2005, 2009). The non-FS interneurons studied here comprised RS, BS, and IS subtypes showing differences in their inhibitory inputs, as found previously for similar electrophysiological classes of cortical interneurons in rodents (Bacci et al. 2003; Dumitriu et al. 2007; Pfeffer et al. 2013; Savanthrapadian et al. 2014). We found a significantly faster IPSP and IPSC decay in layer 3 FS cells, in a manner consistent with data from other cortical regions and layers in rodents (Bacci et al. 2003; Cossart et al. 2006; Dumitriu et al. 2007), suggesting that faster synaptic inhibition onto FS neurons is highly conserved across layers, areas, and species. The faster decay of inhibition onto FS neurons may be important for generation of synchronized oscillatory activity of FS neurons at high-frequency bands (Bartos et al. 2007).
A faster decay in FS neurons could be due to synapse morphology, which shapes the cleft GABA concentration transient (Farrant and Kaila 2007; Nusser et al. 2001; Szabadics et al. 2007), GABAAR phosphorylation (Kittler and Moss 2003; Nusser et al. 1999), and GABAAR clustering (Christie et al. 2002). In addition, GABAARs with α1 subunits have faster IPSC current decay (Farrant and Kaila 2007; Lavoie et al. 1997), and we found stronger effects of the α1 subunit-preferring positive modulator zolpidem on mIPSPs in FS neurons, consistent with a greater contribution of α1-GABAARs in FS cells. In rodents, GABA synapses onto FS cells have the highest density of α1-GABAARs (Klausberger et al. 2002) and IPSCs in FS cells have high sensitivity to zolpidem (Bacci et al. 2003). A greater contribution of α1-GABAARs in FS cells suggests that α1-GABAAR-preferring pharmacological modulators may more strongly or selectively enhance inhibition onto FS cells. In monkey DLPFC, pyramidal neurons are significantly innervated by FS cells (Gonzalez-Burgos et al. 2005) and have GABA synapses with a substantial zolpidem sensitivity (Gonzalez-Burgos et al. 2009). Thus future studies are needed to test the relative effects of α1-GABAAR subunit-preferring modulators on pyramidal cell inhibition versus FS cell-mediated disinhibition in monkey DLPFC microcircuits.
Relation to interneuron diversity.
Although here we classified the interneurons by their intrinsic membrane properties, our findings may be interpreted in the context of the interneuron diversity described in monkey DLPFC. In previous studies, we combined the analysis of electrophysiology, morphology, and molecular markers to characterize the interneurons of monkey DLPFC layer 3 (Zaitsev et al. 2005, 2009). We found that, as in rodents (Hu et al. 2014), monkey DLPFC interneurons with FS firing pattern uniquely express the calcium-binding protein parvalbumin (PV) (Zaitsev et al. 2005, 2009). PV-negative interneurons of monkey neocortex express one of two other calcium-binding proteins, calretinin or calbindin, and show non-FS firing patterns (Conde et al. 1994; DeFelipe 1997; DeFelipe et al. 1999; Jakab et al. 1997; Meskenaite 1997; Zaitsev et al. 2005, 2009). Comparison of our present data with results of our previous studies suggests that the RS interneurons belong to the calretinin-positive subclass, whereas BS cells may be calbindin-positive interneurons that also express somatostatin (Zaitsev et al. 2005, 2009). IS interneurons, which were relatively rare in our previous studies, may correspond to the cholecystokinin-positive interneurons that also express cannabinoid receptors (Eggan et al. 2010; Galarreta et al. 2004).
In the DLPFC and other areas of monkey neocortex, PV is found in symmetric GABA synapses (Blumcke et al. 1991; Williams et al. 1992), originated from chandelier and basket cells (Blumcke et al. 1991; Conde et al. 1994; DeFelipe et al. 1999; Melchitzky et al. 1999; Williams et al. 1992; Zaitsev et al. 2005, 2009). Only PV-positive basket cells, however, may provide significant inhibition onto other GABA neurons, since in monkey DLPFC, as in other cortical areas of monkey and rodent neocortex, chandelier cells mostly innervate the pyramidal cell axon initial segment (Melchitzky et al. 1999; Williams et al. 1992). The interneuron targets of PV basket cell synapses in monkey DLPFC are not fully characterized, but experiments in rodents suggest that, among interneuron targets, PV basket neurons mainly innervate other PV-positive cells (Pfeffer et al. 2013). PV-negative calretinin-positive interneurons are a main source of inhibitory inputs onto GABA neurons in monkey DLPFC, since the axon terminals from these cells target preferentially, although not exclusively, other GABA neurons via symmetric synapses (Melchitzky et al. 2005; Melchitzky and Lewis 2008). In contrast, inputs from calbindin-positive, somatostatin-containing interneurons in monkey DLPFC are much less likely to target other GABA neurons than calretinin-containing inputs (Melchitzky and Lewis 2008). Integration of the present data with the results of previous studies of monkey neocortical circuits thus suggests that inhibitory inputs are present in most, if not all, GABA neuron subtypes, and therefore that interneuron activity in primate neocortex is highly regulated by GABAAR-mediated inhibition. Since our experiments did not directly identify the sources of inhibitory input onto each interneuron subclass, future studies are necessary to understand how inhibition of GABA neurons contributes to the functional architecture of microcircuits in the monkey DLPFC.
Functional relevance.
In slices of monkey DLPFC, some IPSCs are produced via spontaneous interneuron firing (Gonzalez-Burgos et al. 2014). However, mIPSPs and mIPSCs are studied with firing blocked by TTX; thus the higher frequency of mIPSPs and mIPSCs in FS neurons is unrelated to firing activity in inputs onto these cells. If the probability of action potential-independent GABA release were similar at inputs onto FS and non-FS neurons, then the higher mIPSC frequency might indicate that FS cells have a higher density of inhibitory inputs. However, interneurons receive GABAergic input originating from multiple sources (Pfeffer et al. 2013), and mIPSCs originating from different sources may display different release probability (Goswami et al. 2012), suggesting that mIPSC frequency may not directly indicate input density.
Action potential-independent miniature synaptic events are observed in vivo (Destexhe and Pare 1999; Pare et al. 1997) and not exclusively in brain slice conditions. Miniature synaptic events may mediate maintenance of synaptic function, by regulating synaptic receptor density and clustering or acting as a trophic factor that prevents synapse loss or elimination (Kaeser and Regehr 2014). If so, then the higher miniature event frequency would make GABA synapses in FS neurons more stable and less susceptible to factors that enhance GABA synapse elimination or turnover. Importantly, action potential-dependent and -independent forms of GABA release originate from the same vesicle pool (Hua et al. 2010; Wilhelm et al. 2010), suggesting that the synaptic properties conferred by a high mIPSC frequency similarly affect action potential-evoked IPSCs.
Importantly, since we used whole cell recording conditions that modify the intracellular Cl− concentration, we could not determine the physiological value of EGABA, the reversal potential of the GABAAR current, which is mainly a Cl− current. In rodent interneurons, during early development EGABA is depolarized above the neurons' spike threshold and thus GABAAR-mediated inputs are excitatory (Sambandan et al. 2010). In mature FS and non-FS interneurons, EGABA remains depolarized (Banke and McBain 2006; Hollrigel et al. 1998; Sambandan et al. 2010; Vida et al. 2006), but the depolarizing IPSCs in mature interneurons produce shunting inhibition (Banke and McBain 2006; Hollrigel et al. 1998; Sambandan et al. 2010; Vida et al. 2006). Inhibitory mIPSCs and miniature excitatory synaptic currents (mEPSCs) could regulate interneuron activity in vivo, when firing activity in the monkey DLPFC network is low. In FS neurons of monkey DLPFC, the mEPSC frequency is ∼20 Hz (Povysheva et al. 2006), thus significantly higher than mIPSP and mIPSC frequency (∼4 Hz; see Figs. 4 and 8). A high mEPSC-to-mIPSC ratio could make monkey DLPFC FS neurons readily responsive to external inputs arriving when the local network is in a low-firing activity state. Interestingly, FS chandelier neurons are thought to have an excitatory effect on pyramidal cells under quiescent network conditions (Woodruff et al. 2011), whereas FS basket cells are generally inhibitory (Szabadics et al. 2006; Woodruff et al. 2009). Thus external inputs arriving onto chandelier versus basket cells could have opposite effects on the transition of the cortical network from quiescent to active states. Whether chandelier and basket FS neurons in monkey DLPFC have a similar mEPSC-to-mIPSC ratio remains to be determined.
A common active network state in monkey DLPFC is the persistent firing during the delay period of working memory tasks (Funahashi et al. 1989; Fuster 1973; Miller et al. 1996), thought to be mediated by recurrent excitation and inhibitory feedback control (Wang et al. 2004). Such inhibitory feedback may be provided by monkey DLPFC interneurons displaying delay-related firing (Constantinidis et al. 2002; Constantinidis and Goldman-Rakic 2002; Rao et al. 1999; Wilson et al. 1994), possibly driven by sustained synaptic input from nearby pyramidal cells (Gonzalez-Burgos et al. 2004). Pronounced spike frequency adaptation such as that displayed by monkey DLPFC layer 3 pyramidal neurons (Amatrudo et al. 2012; Henze et al. 2000; Zaitsev et al. 2012) decreases pyramidal cell excitability during persistent excitation (Carter and Wang 2007). Delay-related firing in pyramidal cells with spike frequency adaptation is thus optimized by some level of disinhibition such as that produced by endocannabinoid-mediated suppression of inhibition (Carter and Wang 2007), which is prominent in monkey DLPFC (Gonzalez-Burgos et al. 2014). Disinhibition could also be mediated by the inhibitory inputs onto GABA neurons described here. Understanding the role of pyramidal cell disinhibition in shaping delay-related firing in the monkey DLPFC requires additional studies to characterize the patterns of inhibitory connections between monkey DLPFC interneurons and to identify the interneuron subtypes that exhibit delay-related activity during working memory tasks (Constantinidis et al. 2002; Constantinidis and Goldman-Rakic 2002; Rao et al. 1999; Wilson et al. 1994).
GRANTS
This work was funded by National Institute of Mental Health Grant R01 MH-051234.
DISCLOSURES
D. A. Lewis currently receives investigator-initiated research support from Bristol-Myers Squibb and Pfizer and in 2012–2014 served as a consultant in the areas of target identification and validation and new compound development to Autifony, Bristol-Myers Squibb, Concert Pharmaceuticals, and Sunovion.
AUTHOR CONTRIBUTIONS
Author contributions: D.C.R., D.A.L., and G.G.-B. conception and design of research; D.C.R., N.V.P., A.V.Z., D.A.L., and G.G.-B. performed experiments; D.C.R., C.O., T.M., and G.G.-B. analyzed data; D.C.R., C.O., T.M., N.V.P., A.V.Z., D.A.L., and G.G.-B. interpreted results of experiments; D.C.R., T.M., and G.G.-B. prepared figures; D.C.R. and G.G.-B. drafted manuscript; D.C.R., C.O., T.M., N.V.P., A.V.Z., D.A.L., and G.G.-B. edited and revised manuscript; D.C.R., C.O., T.M., N.V.P., A.V.Z., D.A.L., and G.G.-B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Olga Krimer for her excellent assistance with histochemical methods and neuronal reconstruction.
REFERENCES
- Amatrudo JM, Weaver CM, Crimins JL, Hof PR, Rosene DL, Luebke JI. Influence of highly distinctive structural properties on the excitability of pyramidal neurons in monkey visual and prefrontal cortices. J Neurosci 32: 13644–13660, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Rudolph U, Huguenard JR, Prince DA. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J Neurosci 23: 9664–9674, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke TG, McBain CJ. GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development. J Neurosci 26: 11720–11725, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8: 45–56, 2007. [DOI] [PubMed] [Google Scholar]
- Blumcke I, Hof PR, Morrison JH, Celio MR. Parvalbumin in the monkey striate cortex: a quantitative immunoelectron-microscopy study. Brain Res 554: 237–243, 1991. [DOI] [PubMed] [Google Scholar]
- Carter E, Wang XJ. Cannabinoid-mediated disinhibition and working memory: dynamical interplay of multiple feedback mechanisms in a continuous attractor model of prefrontal cortex. Cereb Cortex 17, Suppl 1: i16–i26, 2007. [DOI] [PubMed] [Google Scholar]
- Chitwood RA, Hubbard A, Jaffe DB. Passive electrotonic properties of rat hippocampal CA3 interneurones. J Physiol 515: 743–756, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, Riquelme R, Yang BY, Charych E, Wendou Y, Daniels SB, Cantino ME, de Blas AL. Synaptic and extrasynaptic GABAA receptor and gephyrin clusters. Prog Brain Res 136: 157–180, 2002. [DOI] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol 341: 95–116, 1994. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Goldman-Rakic PS. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J Neurophysiol 88: 3487–3497, 2002. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Williams GV, Goldman-Rakic PS. A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nat Neurosci 5: 175–180, 2002. [DOI] [PubMed] [Google Scholar]
- Cossart R, Petanjek Z, Dumitriu D, Hirsch JC, Ben Ari Y, Esclapez M, Bernard C. Interneurons targeting similar layers receive synaptic inputs with similar kinetics. Hippocampus 16: 408–420, 2006. [DOI] [PubMed] [Google Scholar]
- de Sousa AA, Proulx MJ. What can volumes reveal about human brain evolution? A framework for bridging behavioral, histometric, and volumetric perspectives. Front Neuroanat 8: 51, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat 14: 1–19, 1997. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Gonzalez-Albo MC, Del Rio MR, Elston GN. Distribution and patterns of connectivity of interneurons containing calbindin, calretinin, and parvalbumin in visual areas of the occipital and temporal lobes of the macaque monkey. J Comp Neurol 412: 515–526, 1999. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Pare D. Impact of network activity on the integrative properties of neocortical pyramidal neurons in vivo. J Neurophysiol 81: 1531–1547, 1999. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Cossart R, Huang J, Yuste R. Correlation between axonal morphologies and synaptic input kinetics of interneurons from mouse visual cortex. Cereb Cortex 17: 81–91, 2007. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience 169: 1651–1661, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN. Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex 13: 1124–1138, 2003. [DOI] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, DeFelipe J. The pyramidal cell in cognition: a comparative study in human and monkey. J Neurosci 21: RC163, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, Elston A, Manger PR, DeFelipe J. Pyramidal cells in prefrontal cortex of primates: marked differences in neuronal structure among species. Front Neuroanat 5: 2, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, Elston A, Zietsch B, DeFelipe J, Manger P, Casagrande V, Kaas JH. Specializations of the granular prefrontal cortex of primates: implications for cognitive processing. Anat Rec A Discov Mol Cell Evol Biol 288: 26–35, 2006. [DOI] [PubMed] [Google Scholar]
- Elston GN, Fujita I. Pyramidal cell development: postnatal spinogenesis, dendritic growth, axon growth, and electrophysiology. Front Neuroanat 8: 78, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN, Garey LJ. New Research Findings on the Anatomy of the Cerebral Cortex of Special Relevance to Anthropological Questions. Brisbane, Australia: Univ. of Queensland Printery, 2004. [Google Scholar]
- Farrant M, Kaila K. The cellular, molecular and ionic basis of GABAA receptor signalling. Prog Brain Res 160: 59–87, 2007. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349, 1989. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J Neurophysiol 36: 61–78, 1973. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. London: Academic, 2008. [Google Scholar]
- Galarreta M, Erdelyi F, Szabo G, Hestrin S. Electrical coupling among irregular-spiking GABAergic interneurons expressing cannabinoid receptors. J Neurosci 24: 9770–9778, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Barrionuevo G, Lewis DA. Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cereb Cortex 10: 82–92, 2000. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA. Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J Neurophysiol 93: 942–953, 2005. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Krimer LS, Urban NN, Barrionuevo G, Lewis DA. Synaptic efficacy during repetitive activation of excitatory inputs in primate dorsolateral prefrontal cortex. Cereb Cortex 14: 530–542, 2004. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Miyamae T, Pafundo DE, Yoshino H, Rotaru DC, Hoftman G, Datta D, Zhang Y, Hammond M, Sampson AR, Fish KN, Bard EG, Lewis DA. Functional maturation of GABA synapses during postnatal development of the monkey dorsolateral prefrontal cortex. Cereb Cortex (June5, 2014). doi: 10.1093/cercor/bhu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Rotaru DC, Zaitsev AV, Povysheva NV, Lewis DA. GABA transporter GAT1 prevents spillover at proximal and distal GABA synapses onto primate prefrontal cortex neurons. J Neurophysiol 101: 533–547, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami SP, Bucurenciu I, Jonas P. Miniature IPSCs in hippocampal granule cells are triggered by voltage-gated Ca2+ channels via microdomain coupling. J Neurosci 32: 14294–14304, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Gonzalez-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. J Neurophysiol 84: 2799–2809, 2000. [DOI] [PubMed] [Google Scholar]
- Hollrigel GS, Ross ST, Soltesz I. Temporal patterns and depolarizing actions of spontaneous GABAA receptor activation in granule cells of the early postnatal dentate gyrus. J Neurophysiol 80: 2340–2351, 1998. [DOI] [PubMed] [Google Scholar]
- Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345: 1255263, 2014. [DOI] [PubMed] [Google Scholar]
- Hua Y, Sinha R, Martineau M, Kahms M, Klingauf J. A common origin of synaptic vesicles undergoing evoked and spontaneous fusion. Nat Neurosci 13: 1451–1453, 2010. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron 72: 231–243, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B, Scheibel AB. Regional dendritic variation in primate cortical pyramidal cells. In: Cortical Areas: Unity and Diversity, edited by Schuz A, Miller R. London: Taylor and Francis, 2002. [Google Scholar]
- Jaffe DB, Carnevale NT. Passive normalization of synaptic integration influenced by dendritic architecture. J Neurophysiol 82: 3268–3285, 1999. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic P, Leranth C. Dual role of substance P/GABA axons in cortical neurotransmission: synaptic triads on pyramidal cell spines and basket-like innervation of layer II–III calbindin interneurons in primate prefrontal cortex. Cereb Cortex 7: 359–373, 1997. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Regehr WG. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu Rev Physiol 76: 333–363, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature 505: 318–326, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol 13: 341–347, 2003. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABAA receptors in the hippocampus. J Neurosci 22: 2513–2521, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantoudaki X, Papoutsi A, Chalkiadaki K, Poirazi P, Sidiropoulou K. Modulatory effects of inhibition on persistent activity in a cortical microcircuit model. Front Neural Circuits 8: 7, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer LS, Zaitsev AV, Czanner G, Kroner S, Gonzalez-Burgos G, Povysheva NV, Iyengar S, Barrionuevo G, Lewis DA. Cluster analysis-based physiological classification and morphological properties of inhibitory neurons in layers 2–3 of monkey dorsolateral prefrontal cortex. J Neurophysiol 94: 3009–3022, 2005. [DOI] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on alpha-subunit isoform. Biophys J 73: 2518–2526, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barron M, Losonczy A. Behavioral consequences of GABAergic neuronal diversity. Curr Opin Neurobiol 26: 27–33, 2014. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Eggan SM, Lewis DA. Synaptic targets of calretinin-containing axon terminals in macaque monkey prefrontal cortex. Neuroscience 130: 185–195, 2005. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Lewis DA. Dendritic-targeting GABA neurons in monkey prefrontal cortex: comparison of somatostatin- and calretinin-immunoreactive axon terminals. Synapse 62: 456–465, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Lewis DA. Parvalbumin-immunoreactive axon terminals in macaque monkey and human prefrontal cortex: laminar, regional, and target specificity of type I and type II synapses. J Comp Neurol 408: 11–22, 1999. [PubMed] [Google Scholar]
- Meskenaite V. Calretinin-immunoreactive local circuit neurons in area 17 of the cynomolgus monkey, Macaca fascicularis. J Comp Neurol 379: 113–132, 1997. [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202, 2001. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci 16: 5154–5167, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H. The rise of a new GABA pharmacology. Neuropharmacology 60: 1042–1049, 2011. [DOI] [PubMed] [Google Scholar]
- Norenberg A, Hu H, Vida I, Bartos M, Jonas P. Distinct nonuniform cable properties optimize rapid and efficient activation of fast-spiking GABAergic interneurons. Proc Natl Acad Sci USA 107: 894–899, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Naylor D, Mody I. Synapse-specific contribution of the variation of transmitter concentration to the decay of inhibitory postsynaptic currents. Biophys J 80: 1251–1261, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Mody I. Differential regulation of synaptic GABAA receptors by cAMP-dependent protein kinase in mouse cerebellar and olfactory bulb neurones. J Physiol 521: 421–435, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56: 141–148, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Lebel E, Lang EJ. Differential impact of miniature synaptic potentials on the soma and dendrites of pyramidal neurons in vivo. J Neurophysiol 78: 1735–1739, 1997. [DOI] [PubMed] [Google Scholar]
- Pfeffer CK. Inhibitory neurons: vip cells hit the brake on inhibition. Curr Biol 24: R18–R20, 2014. [DOI] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci 16: 1068–1076, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povysheva NV, Gonzalez-Burgos G, Zaitsev AV, Kroner S, Barrionuevo G, Lewis DA, Krimer LS. Properties of excitatory synaptic responses in fast-spiking interneurons and pyramidal cells from monkey and rat prefrontal cortex. Cereb Cortex 16: 541–552, 2006. [DOI] [PubMed] [Google Scholar]
- Povysheva NV, Zaitsev AV, Gonzalez-Burgos G, Lewis DA. Electrophysiological heterogeneity of fast-spiking interneurons: chandelier versus basket cells. PLoS One 8: e70553, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povysheva NV, Zaitsev AV, Kroener S, Krimer OA, Rotaru DC, Gonzalez-Burgos G, Lewis DA, Krimer LS. Electrophysiological differences between neurogliaform cells from monkey and rat prefrontal cortex. J Neurophysiol 97: 1030–1090, 2007. [DOI] [PubMed] [Google Scholar]
- Povysheva NV, Zaitsev AV, Rotaru DC, Gonzalez-Burgos G, Lewis DA, Krimer LS. Parvalbumin-positive basket interneurons in monkey and rat prefrontal cortex. J Neurophysiol 100: 2348–2360, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci 7: 1–24, 1995. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol 81: 1903–1916, 1999. [DOI] [PubMed] [Google Scholar]
- Sambandan S, Sauer JF, Vida I, Bartos M. Associative plasticity at excitatory synapses facilitates recruitment of fast-spiking interneurons in the dentate gyrus. J Neurosci 30: 11826–11837, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savanthrapadian S, Meyer T, Elgueta C, Booker SA, Vida I, Bartos M. Synaptic properties of SOM- and CCK-expressing cells in dentate gyrus interneuron networks. J Neurosci 34: 8197–8209, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci 9: 206–221, 2008. [DOI] [PubMed] [Google Scholar]
- Spruston N, Jaffe DB, Williams SH, Johnston D. Voltage- and space-clamp errors associated with the measurement of electrotonically remote synaptic events. J Neurophysiol 70: 781–802, 1993. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Tamas G, Soltesz I. Different transmitter transients underlie presynaptic cell type specificity of GABAA,slow and GABAA,fast. Proc Natl Acad Sci USA 104: 14831–14836, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311: 233–235, 2006. [DOI] [PubMed] [Google Scholar]
- Tamas G, Somogyi P, Buhl EH. Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J Neurosci 18: 4255–4270, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP, Hughes DI, Pawelzik H. Differential sensitivity to Zolpidem of IPSPs activated by morphologically identified CA1 interneurons in slices of rat hippocampus. Eur J Neurosci 12: 425–436, 2000. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol 493: 471–484, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida I, Bartos M, Jonas P. Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron 49: 107–117, 2006. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Buzsaki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci 16: 6402–6413, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci USA 101: 1368–1373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm BG, Groemer TW, Rizzoli SO. The same synaptic vesicles drive active and spontaneous release. Nat Neurosci 13: 1454–1456, 2010. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS, Leranth C. The synaptology of parvalbumin-immunoreactive neurons in the primate prefrontal cortex. J Comp Neurol 320: 353–369, 1992. [DOI] [PubMed] [Google Scholar]
- Williams SR, Mitchell SJ. Direct measurement of somatic voltage clamp errors in central neurons. Nat Neurosci 11: 790–798, 2008. [DOI] [PubMed] [Google Scholar]
- Wilson FA, O'Scalaidhe SP, Goldman-Rakic PS. Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proc Natl Acad Sci USA 91: 4009–4013, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits 3: 15, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, McGarry LM, Vogels TP, Inan M, Anderson SA, Yuste R. State-dependent function of neocortical chandelier cells. J Neurosci 31: 17872–17886, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kroner S, Lewis DA, Krimer LS. Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cereb Cortex 15: 1178–1186, 2005. [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Povysheva NV, Gonzalez-Burgos G, Lewis DA. Electrophysiological classes of layer 2/3 pyramidal cells in monkey prefrontal cortex. J Neurophysiol 108: 595–609, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev AV, Povysheva NV, Gonzalez-Burgos G, Rotaru D, Fish KN, Krimer LS, Lewis DA. Interneuron diversity in layers 2–3 of monkey prefrontal cortex. Cereb Cortex 19: 1597–1615, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]