Abstract
Gaze is strongly attracted to visual objects that have been associated with rewards. Key to this function is a basal ganglia circuit originating from the caudate nucleus (CD), mediated by the substantia nigra pars reticulata (SNr), and aiming at the superior colliculus (SC). Notably, subregions of CD encode values of visual objects differently: stably by CD tail [CD(T)] vs. flexibly by CD head [CD(H)]. Are the stable and flexible value signals processed separately throughout the CD-SNr-SC circuit? To answer this question, we identified SNr neurons by their inputs from CD and outputs to SC and examined their sensitivity to object values. The direct input from CD was identified by SNr neuron's inhibitory response to electrical stimulation of CD. We found that SNr neurons were separated into two groups: 1) neurons inhibited by CD(T) stimulation, located in the caudal-dorsal-lateral SNr (cdlSNr), and 2) neurons inhibited by CD(H) stimulation, located in the rostral-ventral-medial SNr (rvmSNr). Most of CD(T)-recipient SNr neurons encoded stable values, whereas CD(H)-recipient SNr neurons tended to encode flexible values. The output to SC was identified by SNr neuron's antidromic response to SC stimulation. Among the antidromically activated neurons, many encoded only stable values, while some encoded only flexible values. These results suggest that CD(T)-cdlSNr-SC circuit and CD(H)-rvmSNr-SC circuit transmit stable and flexible value signals, largely separately, to SC. The speed of signal transmission was faster through CD(T)-cdlSNr-SC circuit than through CD(H)-rvmSNr-SC circuit, which may reflect automatic and controlled gaze orienting guided by these circuits.
Keywords: saccade, reward, caudate nucleus, superior colliculus, monkey
a good decision must be followed by a valuable outcome, and the basal ganglia contribute to this process (Schultz 1998). Various sensorimotor and cognitive signals are processed by many neurons in the basal ganglia (Basso and Wurtz 2002; Caan et al. 1984; DeLong 1971; Handel and Glimcher 1999; Kermadi and Joseph 1995; Kimura 1990; Matsumura et al. 1992; Rolls et al. 1983; Turner and Anderson 2005). These sensorimotor/cognitive signals are strongly modified by the following reward outcome (Apicella et al. 1992; Hikosaka et al. 1989; Samejima et al. 2005; Schultz et al. 1997). Since the basal ganglia have a powerful access to motor structures (Grillner et al. 2005), the reward-predictive signals would cause selective facilitation and inhibition among actions (Mink 1996), thereby leading to a better reward outcome (Hong and Hikosaka 2011).
However, the chosen motor action (e.g., reach and grasp) must be aimed at a valuable object (e.g., ripe apple). To obtain a reward, an animal must find the valuable object before executing the action (Hikosaka et al. 2013). For primates, the finding process heavily depends on visual information. Among many objects, one is chosen at a time based on visual information, and gaze is oriented to it (with a saccadic eye movement) (Henderson 2003; Land 2006; Yarbus 1967). This is a demanding task because many objects need to be evaluated before gaze is settled on an important object. This process may be time-consuming because individual objects may attract gaze (Treisman and Gelade 1980).
Studies on behaving monkeys suggest that the basal ganglia contribute to the visual selection of high-valued objects (Hikosaka et al. 2006). Many neurons in the monkey basal ganglia nuclei respond to visual stimuli (Hikosaka et al. 2000 for review), often much more strongly if a large reward is expected following the visual stimuli. Such reward-sensitive visual responses were found predominantly in the head of the caudate nucleus [CD(H)] (Kawagoe et al. 1998) and the substantia nigra pars reticulata (SNr) (Sato and Hikosaka 2002). Importantly, their responses changed flexibly as the reward contingency changed. Unexpectedly, however, we found that a group of SNr neurons behaved completely differently (Yasuda et al. 2012). The SNr neurons were insensitive to frequent changes in the expected reward outcome, and yet differentiated visual objects by their stable values based on long-term object-reward association learning. We also found that neurons in the tail of the caudate nucleus [CD(T)] were sensitive to stable (but not flexible) object values (Yamamoto et al. 2013). These results suggested that the object-value association memory is processed in two different manners in the basal ganglia. Indeed, another study from our laboratory found that neurons in the monkey caudate nucleus (CD) encoded object values, but in a regionally selective manner: flexible value coding in CD(H) vs. stable value coding in CD(T) (Kim and Hikosaka 2013).
It is known that both CD(H) and CD(T) project to the SNr (Saint-Cyr et al. 1990; Smith and Parent 1986), suggesting that both stable and flexible value signals are sent to the SNr. Why then did we find that SNr neurons encode stable values, but not flexible values? To answer this question, we applied a combination of electrophysiological methods (i.e., orthodromic and antidromic stimulation) and behavioral methods (i.e., flexible and stable value coding procedures) for individual neurons in the entire SNr. We found that the SNr is composed of two functional subregions: 1) the caudal-dorsal-lateral region which receives inputs from the CD(T) and encodes stable values (as we showed previously); 2) the rostral-ventral-medial region which receives inputs from the CD(H) and encodes flexible values. These results suggest parallel processing of stable and flexible object values in the basal ganglia.
MATERIALS AND METHODS
Subjects.
Three rhesus monkeys (Macaca mulatta), D (male, 13 yr old, 9 kg), G (male, 15 yr old, 11 kg), and A (male, 7 yr old, 9 kg), were used as subjects in this study. All animal care and experimental procedures were approved by the National Eye Institute and Institutional Animal Care and Use Committee and complied with the Public Health Service Policy on the humane care and use of laboratory animals. A plastic head holder, scleral search coils, and plastic recording chambers were implanted under general anesthesia and sterile surgical conditions. Anesthesia was induced with ketamine and diazepam, after which the monkey was intubated and then maintained with isoflurane.
Behavioral task.
Behavioral tasks were controlled by a QNX-based real-time experimentation data acquisition system [REX, Laboratory of Sensorimotor Research, National Eye Institute, National Institutes of Health (LSR/NEI/NIH), Bethesda, MD]. The monkey sat in a primate chair, facing a frontoparallel screen 33 cm from the monkey's eyes in a sound-attenuated and electrically shielded room. Stimuli generated by an active matrix liquid crystal display projector (PJ550, ViewSonic) were rear-projected on the screen. We created visual stimuli using fractal geometry (Yamamoto et al. 2012). One fractal was composed of four point-symmetrical polygons which were overlaid around a common center such that smaller polygons were positioned more front. Its size was ∼8° × 8°.
Flexible object-value association procedure.
The goal of this procedure was to create and test flexible (short-term) memories of object-value association. An essential feature of short-term memory is that it can be updated frequently. Therefore, learning and testing were performed simultaneously in one task procedure (object-directed saccade task, Fig. 1A).
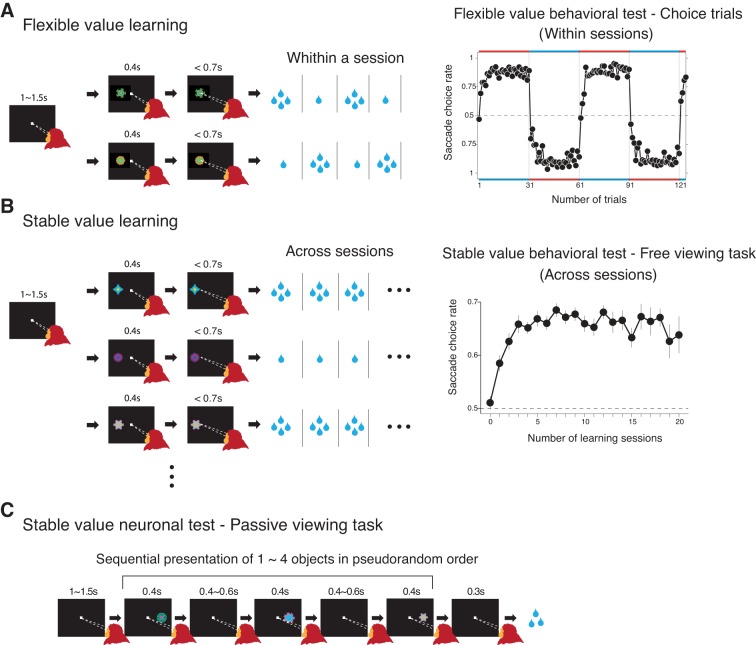
Fig. 1.
Flexible and stable value procedures. A, left: flexible value learning procedure. Two fractal objects were associated with large and small rewards in a reversible manner in blocks of trials (1 block: 30 trials). On most trials (4/5 trials), one of the two objects was presented at the neuron's preferred position, and the monkey made a saccade to it to obtain a reward (forced trials). Occasionally (1/5 trials) two objects were presented, and the monkey had to choose one of the objects and make a saccade to it (choice trials, not shown). A, right: behavioral test of flexible values (choice trials). Changes in the monkey's choice across four blocks (1 session: at least 4 blocks) (data based on monkeys A, G, and D). The data points close to the red horizontal bars indicate choices preferring the high-valued object (i.e., whichever object was recently associated with the large reward). B, left: stable value learning procedure. Among a set of eight fractal objects, four were assigned as “high-valued” objects, and the other four were assigned as “low-valued” objects. On each trial, one of the eight objects was presented at one of four positions, and the monkey made a saccade to it. If the object was high-valued, a large reward was delivered. If the object was low-valued, a small reward was delivered. For each set of visual objects, the learning procedure was done repeatedly across many daily sessions, throughout which each object remained either high-valued or low-valued. B, right: behavioral test of stable values (free viewing). The monkey's gaze preference tested during the free viewing task (see materials and methods) is plotted against the number of daily learning sessions (data based on learning of 7, 54, and 44 sets of objects in monkeys A, G, and D, respectively). The testing was done at least 1 day after the last learning session. Error bars indicate ± 1 SE. C: neuronal test of stable values (passive viewing task). While the monkey was fixating a central spot of light, one to four fractal objects (pseudorandomly chosen from a set of 8 objects) were presented sequentially at the neuron's preferred position. The monkey was rewarded 300 ms after the final object disappeared. The reward was thus not associated with particular objects.
For each monkey, a fixed set of two fractal objects (say, A and B) was used as the saccade target. Each trial started with the appearance of a central white spot, which the monkey had to fixate on. After 1,000–1,500 ms while the monkey was fixating on the central spot, one of the two fractal objects was chosen pseudorandomly and was presented at the neuron's preferred location. The fixation spot disappeared 400 ms later, and then the monkey was required to make a saccade to the object within 700 ms. If the gaze was held on the object for 400 ms, a liquid reward was delivered. The monkey received a large reward (0.20 ml) after making a saccade to one object (e.g., A) and received a small reward (0.016 ml) to the other object (e.g., B). During a block of 30 trials, the object-reward contingency was fixed (e.g., A-large/B-small), but it was reversed in a following block (e.g., B-large/A-small) without any external cue. While an SNr neuron was being recorded, these two conditions (A-large/B-small and B-large/A-small) were alternated in blocks (their order counterbalanced across neurons). Most trials (24 out of 30 trials) were forced trials: one of the two objects was presented and the monkey had to make a saccade to it. The rest of trials (6 out of 30 trials) were choice trials: two objects were presented at the same time. Their locations were chosen randomly from four possible locations (right, up, left and bottom). The monkey had to choose one of the objects by making a saccade to it to obtain the reward associated with the chosen object. If the monkey failed to make a saccade correctly on either forced or choice trials, the same trial was repeated. In each recording session, these two types of block were repeated at least twice.
Stable object-value association procedure.
The goal of this procedure was to create and test stable (long-term) memories of object-value association. To achieve this goal, each object was associated with a fixed-reward value during learning. The testing procedure was performed separately with no reward contingency to exclude possible influences of flexible (short-term) memories (see Yasuda et al. 2012 for details).
To create a fixed bias among fractal objects in their reward values (i.e., high-valued objects and low-valued objects), we used an object-directed saccade task (Fig. 1B). In each session, a set of eight fractal objects was used as the target. On each trial, one of the fractal objects was chosen pseudorandomly as the target and was presented at one of four possible locations (right, up, left and bottom). The monkey was required to make a saccade to the target to obtain a liquid reward. Importantly, half of the fractal objects were associated with a large reward (0.11 ml) (i.e., high-valued objects), whereas the other half were associated with a small reward (0.02 ml) (i.e., low-valued objects). One training session consisted of 64 trials (8 trials for each object). For each set of objects, the reward bias was maintained throughout the present study. On each training day, monkeys D, A, and G learned 1–22, 1–18, and 1–25 sets of objects, respectively. For each object set, no more than one training session was conducted in 1 day.
To test the neuronal representation of stable object-value memories, we used a passive viewing task (Fig. 1C). While the monkey was fixating a central spot of light, one to four fractal objects (pseudorandomly chosen from a set of 8 objects) were presented in the neuron's preferred location in sequence (presentation time: 400 ms, interobject time interval: 400–600 ms). The monkey was rewarded 300 ms after the final object disappeared. Thus no particular objects were associated with the reward. Each object was presented at least six times in one session.
To test the behavioral representation of stable object-value memories, we used a free viewing task (Yasuda et al. 2012). On each trial, four of a set of eight fractal objects were chosen randomly, regardless of their values, and were presented simultaneously for 2 s. The monkey was free to look at these objects (or something else) by making saccade between them, but no reward was given. After a blank period (0.5 s), another four objects were presented. Occasionally, a white small dot, instead, was presented at one of four positions. If the monkey made a saccade to it and held the gaze on it for 300–600 ms, a reward was delivered. This dot rewarded trial was used to maintain the monkey's arousal and motivation level. Each object was presented at least 25 times in one session. For data analysis, we excluded trials in which all objects were high-valued or low-valued. Therefore, the analyzed data included three groups of trials: three high-valued vs. one low-valued, two high-valued vs. two low-valued, and one high-valued vs. three low-valued.
Electrophysiology.
Based on a stereotaxic atlas (Saleem and Logothetis 2007), we placed two rectangular chambers in each monkey. For monkeys A and G, we aimed at CD(T) and CD(H) from the lateral chamber, and SNr from the posterior chamber. For monkey D, we aimed at SNr from the lateral chamber and SC from the posterior chamber (Fig. 2A). The lateral chamber was placed over the frontoparietal cortex, tilted laterally by 25° for monkey A and 35° for monkeys G and D. The posterior chamber was placed over the midline of the parietal cortex, tilted posteriorly by 40°. Magnetic resonance images (4.7T, Bruker) were then obtained along the direction of the recording chamber which was visualized with gadolinium that filled grid holes and inside the chamber. Single-unit recordings and electrical stimulations were performed using tungsten electrodes (Frederick Haer) that were advanced by an oil-driven micro-manipulator (MO-97A, Narishige). The recording and stimulation sites were determined by using a grid system, which allowed recordings at every 1 mm between penetrations. In each daily experiment, we introduced these electrodes into the brain, each through a stainless steel guide tube, which was inserted into one of the grid holes and then to the brain via the dura. For finer mapping of neurons, we also used a complementary grid, which allowed electrode penetrations between the holes of the original grid. The electrical signal from the electrode was amplified with a band-pass filter (200 Hz to 5 kHz; BAK, Mount Airy, MD) and collected at 1 kHz. Spike potentials of single neurons were isolated online using a custom voltage-time window discrimination software (MEX, LSR/NEI/NIH).
Fig. 2.
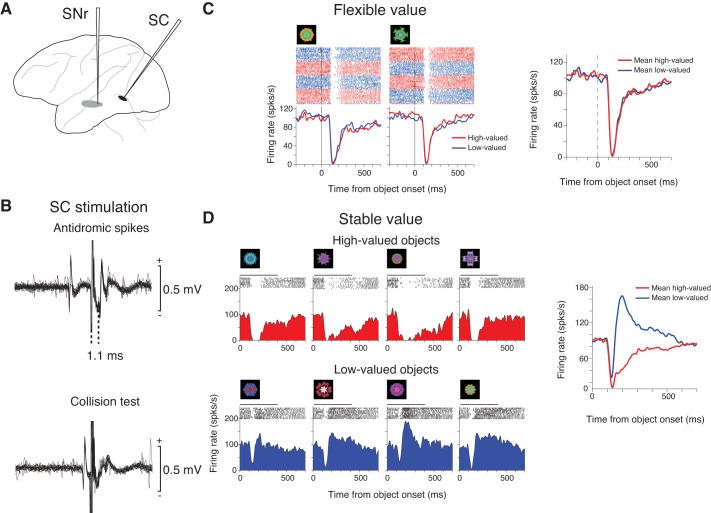
An example of superior colliculus (SC)-projecting substantia nigra pars reticulata (SNr) neuron encoding stable values (monkey D). A: stimulation-recording procedure. One electrode was inserted to SC (black) for stimulation; another electrode was inserted to SNr for recording (gray). B: antidromic activation of the SNr neuron by electrical stimulation of the ipsilateral SC (top). The antidromic nature of the spikes was confirmed by a collision test (bottom). C and D: spike activity of the SNr neuron during the flexible value procedure (C) and stable value procedure (passive viewing task) (D), shown as dot rasters and spike density functions (SDFs) aligned on the onset of the object (time 0). Red and blue SDFs indicate the responses to high-valued and low-valued objects, respectively. The raster plots in C are shown in red (large-reward trials) and blue (small-reward trials); green tick in each raster line indicates the onset of the saccade toward the presented object. The right panel shows the responses to the high-valued object (red) and the low-valued object (blue) averaged for the two objects. In D, the horizontal bar above each raster plot indicates the duration of object presentation (0–400 ms). D: before the stable value testing (pre), the monkey had learned the stable values of the eight objects for seven sessions across days; the last learning session was 6 days before the SNr neuron was recorded (recent). In the following figures, these numbers are indicated as follows: pre 7, recent 6. The neuron was also tested with three other object sets (pre 17, recent 1; pre 28, recent 1; pre 36, recent 1). The averaged responses to all tested objects are shown in the right panel.
Identification of CD-SNr and SNr-SC connections.
To test if an SNr neuron receives the direct input from CD, we electrically stimulated CD(T) and CD(H) on the same side. We adjusted the positions of the CD electrodes, first by recording neuronal activity through them until neurons responding to visual fractals were found. This was particularly critical for the CD(T) electrode, because it could be placed outside CD(T) due to a small mislocation. The stereotaxic coordinates of the stimulation sites were as follows: CD(T) = 8–10 mm anterior, 8–9.4 mm dorsal, 15–16 mm lateral, and CD(H) = 20 mm anterior, 18.9 mm dorsal, 6.5 mm lateral for monkey G; CD(T) = 14 mm anterior, 6.1–6.8 mm dorsal, 12.9–14.1 mm lateral, and CD(H) = 21–26 mm anterior, 14.1–16 mm dorsal, 5.2–6.4 mm lateral for monkey A (Saleem and Logothetis 2007). After switching the CD electrodes from recording to stimulation, we lowered another electrode into SNr. For stimulation, we used a biphasic pulse with cathodal and anodal components. The currents for the cathodal pulse ranged from 10 to 180 μA; the currents for the anodal pulse were made lower than those for the cathodal pulse. The duration of each pulse was 200 μs.
To test if an SNr neuron projects its axon to SC, we used the antidromic activation method by electrically stimulating SC on the same side (Hikosaka and Wurtz 1983b; Yasuda et al. 2012). To position the SC electrode, we lowered the SC electrode until presaccadic activity was recorded. After switching the SC electrode from recording to stimulation, we lowered another electrode into SNr. To find a SC-projecting SNr neuron, we stimulated the SC until spikes with a fixed latency were detected. The antidromic nature of the spikes was confirmed using a collision test (Hikosaka and Wurtz 1983b). For stimulation, we used a biphasic pulse with cathodal and anodal components (as for CD stimulation), but the duration of each pulse was 100 μs. The currents for the cathodal pulse ranged from 10 to 180 μA.
Data analysis.
We analyzed the effects of object-value association learning on neuronal and behavioral discriminations of high- and low-valued objects. To assess the neuronal discrimination, we first measured the magnitude of SNr neuron's response to each fractal object by counting the numbers of spikes within a test window in individual trials. For both stable and flexible object value testing, we put the test window from 100–400 ms after the onset of the object. The neuronal discrimination was defined as the area under the receiver operating characteristic (ROC) based on the response magnitudes of SNr neurons to high-valued objects vs. low-valued objects (see Figs. 4 and 9). The statistical significance of the neuronal discrimination was tested using Wilcoxon rank-sum test.
Fig. 4.

Segregation of neurons in SNr by value coding (monkey D). A: the distribution of SC-projecting SNr neurons and their value codings. Their locations are projected onto a horizontal plane, together with the contours of SNr (solid line) and substantia nigra pars compacta (SNc) (dotted line) based on a brain atlas (Saleem and Logothetis 2007). R-C, rostral-caudal; M-L, medial-lateral. For each neuron, the diameter of the circle indicates the magnitude of stable value coding (red) or flexible value coding (green). The magnitude was calculated using absolute receiver operating characteristic (ROC) area, which ranged from 0.5 (diameter: 0) to 1.0 (diameter, as shown) (Bromberg-Martin et al. 2010). The recording sites of the neurons shown in Figs. 2 and 3 are indicated by red and green stars, respectively. B: the relationship between stable (ordinate) and flexible (abscissa) value codings of neurons in rostral SNr (left) and caudal SNr (right). Green circle: significant coding of flexible value (P < 0.05, rank sum test) (rostral: 28/71, caudal: 2/64). Red circle: significant coding of stable value (rostral: 4/71, caudal: 29/64). Black circle: significant coding of both stable and flexible values (rostral: 14/71, caudal: 22/64). Small dot: no significant value coding (rostral: 25/71, caudal: 11/64). Data include neurons whose projections to SC were not identified.
Fig. 9.

The relationship between stable and flexible value coding of CD(H)- and CD(T)-recipient SNr neurons. The format is the same as in Fig. 4B.
To assess the behavioral discrimination, we used the monkey's saccade to high-valued or low-valued objects as the behavioral choice. We computed the choice rate by dividing the number of saccades to high-valued objects by the number of saccades to all object during the behavioral test. For flexible values, the computation was based on choice trials (Fig. 1A, right) which were included in the flexible value learning task (Fig. 1A, left). For stable values, the computation was based on the free viewing task (Fig. 1B, right) which was performed separately from the stable value learning task (Fig. 1B, left).
To examine possible contributions of SNr neuronal activity to object-value learning, we first focused on the periods in which the monkey showed clear saccade biases (well-learned periods): five or more daily training sessions in stable object-value learning; five or more trials after the reversal of the object-reward contingency in flexible object-value learning.
The latency of the effect by CD stimulation was calculated by using a bootstrap analysis. We set the time window in 20-ms period before stimulation (pre-period) and another sliding test window (duration: 5 ms) starting from 5 ms after stimulation (post-period). Each time we slid the test window by 1 ms, we compared the mean firing rate in the pre-period with the mean firing rate in the post-period. Trials were randomly resampled with replacements to form a new bootstrap data set, which is composed of 50 trials. Such random resampling and comparison were repeated 1,000 times. If the averaged discharge rate was smaller (larger) during the post-period than during the pre-period in 975 repetitions, the time point was regarded as the time of a significant decrease (increase). The beginning of four consecutive significant decreases (increase) was defined as the latency of stimulation effect. When the bootstrap analysis could not detect the latency of the stimulation effect, but the stimulation effect was observed by qualitative assessment, we applied t-test between pre-period and the period when the effect was detected to test the significance of the stimulation effect (significance level: P = 0.05).
For quantitative assessment of the spatial distribution of the recorded SNr neurons, we drew a three-dimensional (3D) plane (discriminative plane) by performing linear discriminant analysis. In this analysis (see Fig. 6A), first we plotted the location of recorded neurons in a 3D space, then we set a plane in the same 3D space so that the separation between two neuronal groups is maximized when all neuronal plots were projected onto the plane (linear classifier plane), then the discriminative plane which was perpendicular to the linear classifier plane was set on the same 3D space where it best separates two neuronal groups.
Fig. 6.
Separate distributions of CD(T)- and CD(H)-recipient SNr neurons. A: discriminative analysis. A discriminative plane (green) largely separated CD(T)-recipient neurons (red) and CD(H)-recipient neurons (black) in SNr in both monkeys. V, ventral; D, dorsal. B: distributions of CD(T)-recipient neurons (red) and CD(H)-recipient neurons (black) projected to the three-dimensional coordinates. Data are from monkey A (top) and monkey G (bottom). Between CD(T)- and CD(H)-recipient neurons, the means of the distributions (indicated by triangles) were significantly different in all dimensions in both monkeys (P < 0.01). AC, anterior commissure; rvmSNr, rostral-ventral-medial SNr; cdlSNr, caudal-dorsal-lateral SNr.
RESULTS
To test the neuronal coding of flexible and stable object values, we had the monkeys experience fractal objects associated with a large or small reward, but in two different ways (Fig. 1): 1) each object frequently changing its associated value (flexible values) (Fig. 1A); and 2) each object consistently associated with either a large or small reward (stable values) (Fig. 1B) (see Yasuda et al. 2012 for details).
In the flexible value procedure (Fig. 1A), one of two objects was presented on each trial, and the monkey made a saccade to it. The saccade was followed by a large or small reward, depending on the object. The object-reward contingency was reversed across blocks of 30 trials. On some trials, the two objects were presented simultaneously, and the monkey chose one by making a saccade to it. The monkey's choice changed quickly across blocks, largely aiming at the recently high-valued object (Fig. 1A, right), reflecting the quick updating of the flexible object values. For testing the flexible value coding of SNr neurons, neuronal activity was recorded during the learning session (Fig. 1A, left), because the value of each object was updated frequently.
In the stable value learning (Fig. 1B), the monkey performed a similar saccade task with eight objects, but the object-reward contingency remained stable throughout learning sessions across days. Behavioral (Fig. 1B, right) or neuronal (Fig. 1C) coding of stable value was tested in a separate session with a long delay (≥1 day) after the last learning session with no contingent reward. By doing so, we could test if the object value effect was stably retained for a long time. In the behavioral testing (free viewing, see materials and methods), four of the eight objects were presented simultaneously, and the monkey looked at them freely. To eliminate any short-term (i.e., flexible) reward effect, we gave no reward feedback. The monkey's choice of the high-valued objects increased gradually, depending on the number of daily learning sessions (Fig. 1B, right), reflecting slow acquisition of the stable object values. For testing the stable value coding of SNr neurons, we used the passive viewing task in which learned objects were presented in the neuron's response field (mostly contralateral). To eliminate any short-term (i.e., flexible) reward effect, a reward at the end of a trial was not contingently associated with any object.
Another difference between the two procedures was the number of objects employed. For the flexible value procedure, we used a small number of objects (two sets of objects, two objects in each set) so that the monkey could flexibly kept track of the changes in the values of individual objects based on short-term memory. For the stable value procedure, the monkeys experienced many objects with high or low values (87, 7, and 85 sets for monkey D, A and G, respectively, 8 objects in each set) so that the monkeys could not rely on short-term memory, which is characterized by a small capacity (Cowan 2001).
Separate processing of stable and flexible object values in SNr.
Consistent with our previous report (Yasuda et al. 2012), we found many neurons in SNr that encoded stable values of object, but not flexible values. An example is shown in Fig. 2. In the stable value testing procedure (passive viewing task) (Fig. 2D), the neuron was strongly inhibited by high-valued objects, but was mostly excited by low-valued objects (ROC = 1, P ≈ 0, Wilcoxon rank-sum test). In the flexible value procedure (Fig. 2C), the neuron was inhibited by the two objects tested, but the responses were not influenced by their changing values (ROC = 0.52, P = 0.79, Wilcoxon rank-sum test).
However, assuming that both stable value-coding CD(T) neurons and flexible value-coding CD(H) neurons project to SNr, we hypothesized that SNr contains neurons that encode flexible values. We found that this was true after exploring SNr widely, particularly in the rostral direction. An example is shown in Fig. 3. In the flexible value procedure (Fig. 3C), the neuron showed differential responses, depending on the object value (ROC = 0.96, P ≈ 0, Wilcoxon rank-sum test): when either of the two objects was high-valued, the neuron was strongly inhibited; when the object was low-valued, the neuron was first inhibited, but was then excited. In the stable value procedure (Fig. 3D), the neuron showed no value-dependent differential response (ROC = 0.48, P = 0.78, Wilcoxon rank-sum test).
Fig. 3.
An example of SC-projecting rostral SNr neuron encoding flexible values (monkey D). The format is the same as in Fig. 2. For D, left, pre 31, recent 1 (see Fig. 2). The neuron was also tested with the other two object sets (pre 29, recent 1; pre 20, recent 1).
Common oculomotor goal of stable and flexible object value signals.
The monkey's eye movements were strongly influenced by both stable and flexible values of visual objects (Fig. 1, A, right, and B, right). This suggested that the stable and flexible value signals in SNr converge before reaching the saccadic mechanism. Consistent with this hypothesis, the two example neurons were activated antidromically by the electrical stimulation of the superior colliculus (SC) (Figs. 2B and 3B). We placed the SC electrode in the intermediate layer where neurons showed presaccadic activity (Yasuda et al. 2012). The SC stimulation evoked an action potential (i.e., spike) at a fixed latency (1.1 ms for the stable value-coding neuron, Fig. 2B, top; 1.76 ms for the flexible value-coding neuron, Fig. 3B, top). When the stimulation was immediately followed by a spontaneous spike, no spike was evoked (collision test), indicating that the SC-evoked spike was an antidromic response (i.e., the SNr neuron projected its axon to SC). These results suggest that stable and flexible value signals which were separately encoded by the two SNr neurons converged in SC. In monkey D, we found 43 neurons that were antidromically activated from SC. Among them, 16 neurons encoded stable values, and 5 encoded flexible values selectively.
Notably, the two types of value-coding neurons were distributed differentially in SNr, particularly along its rostrocaudal dimension (Fig. 4A). Flexible value-coding neurons tended to be located more rostrally than stable value-coding neurons [average location: 8.1 mm vs. 10.8 mm posterior to the anterior commissure (AC); P = 8.39 × 10−6, t-test]. The flexible value-coding neuron shown in Fig. 3 was located 3 mm more rostrally than the stable value-coding neuron shown in Fig. 2.
However, antidromic spikes were not readily detectable, because a neuron may project its axon in a limited portion of the target area (Hikosaka and Wurtz 1983b). Therefore, we decided to add more data about the distribution of stable and flexible value signals in SNr without antidromic activation in the same monkey (D). We found that their distributions were similar to the antidromically activated neurons: among 90 SNr neurons that were not antidromically activated, flexible value-coding neurons tended to be located more rostrally than stable value-coding neurons (average location: 8.2 mm vs. 10.6 mm posterior to AC, P = 2.3 × 10−9, t-test). We then divided SNr into the rostral and caudal parts by applying linear discriminant analysis (see materials and methods), and plotted the magnitudes of stable and flexible codings for all SNr neurons tested in monkey D (including antidromically activated and nonactivated neurons) (Fig. 4B). In caudal SNr, most data points were located in the upper quadrant (the area above two diagonal lines), indicating that most value-coding neurons (42/53) encoded stable values more strongly than flexible values and did so by showing stronger inhibitions to stably high-valued objects than to stably low-valued objects. In rostral SNr, most data points were located in the right or left quadrant, indicating that most neurons (33/46) encoded flexible values more strongly than stable values; some of them (20/46) were more inhibited by flexibly high-valued objects (right quadrant), while the others (13/46) were more inhibited by flexibly low-valued objects (left quadrant).
To summarize, stable and flexible value signals tended to be processed in different regions in SNr, but both signals may influence the initiation of saccades through their common destination, SC.
Processing of stable-flexible values in CD-SNr connection.
Where do the stable and flexible value signals in SNr neurons come from? Previous studies from our laboratory showed that stable and flexible values are represented topographically within CD: stable values by CD(T) neurons and flexible values by CD(H) neurons (Kim and Hikosaka 2013; Yamamoto et al. 2013). We thus hypothesized that CD(T)-recipient SNr neurons encode stable values, and CD(H)-recipient SNr neurons encode flexible values. To test this hypothesis, we inserted three electrodes in each experiment to CD(T), CD(H), and SNr in monkeys A and G (Fig. 5). We placed the CD(T) and CD(H) electrodes where neurons responded to fractal objects; thereafter, the electrodes were used for electrical stimulation.
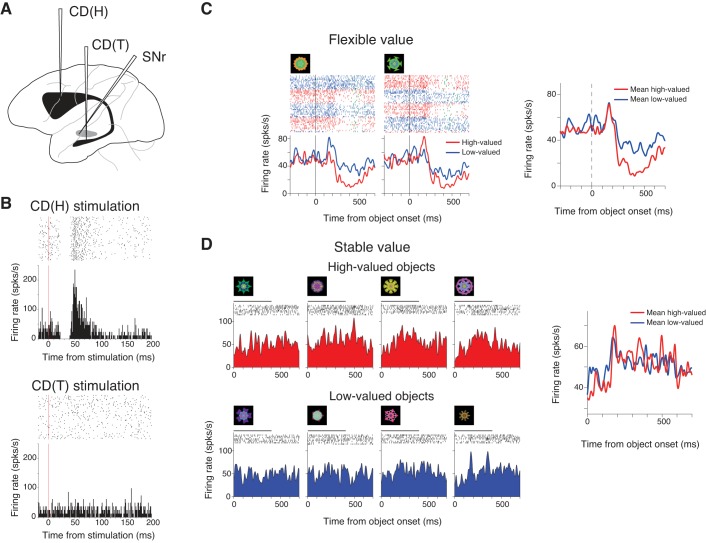
Fig. 5.

Responses of two SNr neurons (left and right) to the electrical stimulation of head of the caudate nucleus [CD(H); top] and tail of the caudate nucleus [CD(T); bottom; monkey G]. For each panel, the neuron's spike activity is shown as dot rasters (top) and a peristimulus time histogram (PSTH; bottom), which are aligned on the onset of CD stimulation (time 0). The drawing (above) illustrates two stimulating electrodes in CD(H) and CD(T), and one recording electrode in SNr.
We found that many SNr neurons responded to the stimulation at CD(T) or CD(H). A common response was an inhibition. Two examples are shown in Fig. 5. The neuron recorded from caudal SNr was inhibited by CD(T) stimulation (latency: 6 ms) (Fig. 5A, bottom), but showed no response to CD(H) stimulation (Fig. 5A, middle). In contrast, the neuron recorded from rostral SNr was inhibited by CD(H) stimulation (latency: 19 ms) (Fig. 5B, middle), but not by CD(T) stimulation (Fig. 5B, bottom). These inhibitions are likely to reflect direct inhibitory connections from CD to SNr (Graybiel 1990; Hikosaka et al. 1993; Yoshida and Precht 1971). In SNr of the two monkeys, 183 neurons (monkey A: 78, monkey G: 106) were inhibited by CD stimulation. Among them, 57 neurons (monkey A: 25, monkey G: 32) were selectively inhibited by CD(T) stimulation, whereas 94 (monkey A: 23, monkey G: 71) neurons were selectively inhibited by CD(H). Only one neuron was inhibited by both CD(T) and CD(H) stimulation. These results suggest that SNr neurons are segregated into two groups by the source of their direct inhibitory inputs from CD: CD(T)-recipient neurons and CD(H)-recipient neurons.
According to our hypothesis, CD(T)-recipient SNr neurons would encode stable values, and CD(H)-recipient SNr neurons would encode flexible values. This hypothesis, together with data shown in Fig. 4, predicted that CD(T)-recipient neurons would be located in caudal SNr, and CD(H)-recipient neurons would be located in rostral SNr. We confirmed this prediction in both monkeys A and G [monkey A: average location of CD(H)- vs. CD(T)-recipient SNr neurons: 8.3 mm vs. 9.9 mm posterior to AC, P = 1.5 × 10−10, t-test, monkey G: average location: 10.2 mm vs. 12.8 mm posterior to AC, P = 5.4 × 10−24, t-test] (Fig. 6B, left). The spatial segregation was also present in the other two dimensions: ventral-dorsal (monkey A: average location: 6.4 mm vs. 5.6 mm ventral to AC, P = 9 × 10−4, t-test, monkey G: average location: 8 mm vs. 7.5 mm posterior to AC, P = 2.5 × 10−4, t-test) and medial-lateral (monkey A: average location: 4.4 mm vs. 5.9 mm lateral to AC, P = 2.4 × 10−7, t-test, monkey G: average location: 5.7 mm vs. 7.3 mm posterior to AC, P = 7.1 × 10−25, t-test) (Fig. 6B, center and right). In 3D space, these two types of SNr neurons could be largely separated by a single linear plane (Fig. 6A): 96% (23/25 for monkey A, and 32/32 for monkey G) of CD(T)-recipient neurons and 94% (20/23 for monkey A, and 68/71 for monkey G) of CD(H)-recipient neurons were located in each of the space separated by the discriminating plane. In summary, CD(T)-recipient neurons tended to be distributed in the caudal-dorsal-lateral part of SNr (hereafter called cdlSNr), whereas CD(H)-recipient neurons tended to be distributed in the rostral-ventral-medial part of SNr (hereafter called rmvSNr).
We then tested our hypothesis directly: do CD(T)-recipient neurons encode stable values and CD(H)-recipient neurons encode flexible values? Data from two example neurons (Figs. 7 and 8) followed the hypothesis. The neuron shown in Fig. 7 was inhibited by CD(T) stimulation (latency: 8 ms), but not by CD(H) stimulation (Fig. 7B). In the stable value testing procedure, the neuron was strongly inhibited by high-valued objects, but was only weakly inhibited by low-valued objects (Fig. 7D) (ROC = 0.95, P = 4.64 × 10−9, Wilcoxon rank-sum test). In the flexible value procedure, the neuron was inhibited by the two objects, but showed no activity bias based on object values (Fig. 7C) (ROC = 0.42, P = 0.22, Wilcoxon rank-sum test). The neuron shown in Fig. 8 was inhibited by CD(H) stimulation (latency: 22 ms) (followed by an excitation), but not by CD(T) stimulation (Fig. 8B). In the flexible value procedure, the neuron was more strongly inhibited when either of the high-valued objects was presented (Fig. 8C) (ROC = 0.63, P = 0.025, Wilcoxon rank-sum test). In the stable value testing procedure, the neuron's activity showed no significant difference between high- and low-valued objects (Fig. 8D) (ROC = 0.47, P = 0.75, Wilcoxon rank-sum test).
Fig. 7.
An example of CD(T)-recipient SNr neuron encoding stable values (monkey G). A and B: the neuron's responses to CD(H) stimulation (A) and CD(T) stimulation (B). The format is the same as in Fig. 5. The neuron was recorded from cdlSNr. The stimulation current was 150 μA in both CD(T) and CD(H) stimulation. C and D: spike activity of the SNr neuron during the flexible (C) and stable (D) value procedures. The format is the same as in Fig. 2, C and D. For D, left, pre 54, recent 1. The neuron was also tested with another object set (pre 51, recent 4).
Fig. 8.
An example of CD(H)-recipient SNr neuron encoding flexile values (monkey G). The format is the same as in Fig. 7. The neuron was recorded from rvmSNr. The stimulation current was 150 μA in both CD(T) and CD(H) stimulation. For D, left, pre 14, recent 1. The neuron was also tested with another object set (pre 12, recent 8).
We found that CD(T)-recipient SNr neurons mostly followed the hypothesis. Most of the CD(T)-recipient neurons (37/45, 85%) encoded object values (Table 1). Among them, significantly more neurons encoded only stable values than chance level (26/37, 70%, P = 4.85 × 10−6, binomial test). Figure 9 (right) shows how individual CD(T)-recipient neurons encoded the two types of values. Most data points were located in the upper quadrant (Fig. 9, right, the area above the two diagonal lines), indicating that most neurons encoded stable values more strongly than flexible values and did so by showing stronger inhibitions to stably high-valued objects than to stably low-valued objects.
Table 1.
Inhibition effect of caudate stimulation on SNr neurons of different type of value coding
| Stable | Stable and Flexible | Flexible | No Value | Total | |
|---|---|---|---|---|---|
| CD(T) | 26 | 8 | 3 | 8 | 45 |
| CD(T and H) | 0 | 0 | 1 | 0 | 1 |
| CD(H) | 5 | 3 | 9 | 35 | 52 |
| Total | 31 | 11 | 13 | 43 |
Values are no. of neurons.
SNr, substantia nigra pars reticulate; CD, caudate nucleus; T, tail; H, head.
These data, together with data in Fig. 4, suggest that the CD(T)-cdlSNr-SC circuit primarily processes stable values of visual objects. This was supported by another experiment (Fig. 10) in which we inserted one electrode to SNr for recording and two electrodes to CD(T) and SC for stimulation in monkey A. We found an SNr neuron that was inhibited by CD(T) stimulation (Fig. 10B, top, latency: 6 ms) and was activated antidromically by SC stimulation (Fig. 10B, bottom, latency: 1.24 ms). The neuron encoded stable value (Fig. 10D), but not flexible value (Fig. 10C).
Fig. 10.
An example of SNr neuron that transmits stable value signals from CD(T) to SC (monkey A). A: stimulation-recording procedure. The electrodes in SC and CD(T) were used for stimulation, and the electrode in SNr was used for recording. The neuron was recorded from cdlSNr. B: the SNr neuron's response to CD(T) stimulation (80 μA; top) and antidromic activation of the SNr neuron by electrical stimulation of the ipsilateral SC. C and D: spike activity of the SNr neuron during the flexible (C) and stable (D) value procedures. For D, pre 16, recent 15. The neuron was also tested with the other 3 object sets (pre 16, recent 1, pre 21, recent 1, pre 27, recent 14).
In contrast, CD(H)-recipient SNr neurons were more heterogeneous. Less than half of the CD(H)-recipient neurons (17/52, 33%) encoded object values (Table 1). Dominant among them were neurons selectively encoding flexible values (9/17, 53%), compared with those selectively encoding stable values (5/17, 29%), but the bias was not statistically significant (P = 0.076, binomial test). In the scatterplot (Fig. 9, left), more data points were located in the right quadrant, showing a tendency for preferential coding of flexible values with stronger inhibitions by high-valued objects.
To summarize, CD(T)-recipient SNr neurons preferentially processed stable values, whereas CD(H)-recipient SNr neurons processed flexible and/or stable values. The encoding of stable values by CD(H)-recipient neurons might be explained by the contribution of the signals transmitted through the indirect pathway originating from CD(T), as exemplified in Fig. 11. The neuron was inhibited by CD(H) stimulation (Fig. 11B, top) and was thus classified as a CD(H)-recipient neuron. As expected from this response, the neuron was more inhibited when high-valued object was presented in flexible value procedure (ROC = 0.82, P = 7.70 × 10−6, Wilcoxon rank-sum test) (Fig. 11C). Interestingly, the neuron was excited by CD(T) stimulation (Fig. 11B, bottom). Correspondingly, the neuron was excited in the stable value procedure (Fig. 11D), more strongly in response to low-valued objects than high-valued objects (ROC = 0.80, P = 7.70 × 10−7, Wilcoxon rank-sum test).
Fig. 11.
An example of CD(H)-recipient SNr neuron encoding both flexible and stable values (monkey A). The neuron was recorded from the rostral part of cdlSNr close to the junction to rvmSNr. The stimulation current was 150 μA in both CD(T) and CD(H) stimulation. The format is the same as in Fig. 7. For D, left, pre 12, recent 2. The neuron was also tested with another object set (pre 15, recent 3).
Since the output neurons in CD are GABAergic and inhibitory (Graybiel 1990; Precht and Yoshida 1971; Yoshida and Precht 1971), the CD(T)-induced excitatory response was likely to be caused by a disinhibition through the indirect pathway mediated by the globus pallidus external segment (GPe) and possibly the subthalamic nucleus (STN) (Hikosaka et al. 2000). Among the 123 SNr neurons that were tested with the flexible and stable value procedures, 32 neurons responded to CD(T) or CD(H) stimulation first with an excitatory (or disinhibitory) response (Table 2). However, the origin of the excitatory responses [CD(T) or CD(H)] showed no clear correlation with the type of value-coding. These results suggest that the value signals mediated by the indirect pathway may not follow the topographic projections of the direct pathway. In other words, the indirect pathway may act as a mediator of the interaction between the stable and flexible values signals (see Fig. 13).
Table 2.
Excitation effect of caudate stimulation on SNr neurons of different type of value coding
| Stable | Stable and Flexible | Flexible | No Value | Total | |
|---|---|---|---|---|---|
| CD(T) | 2 | 4 | 2 | 3 | 11 |
| CD(T and H) | 1 | 1 | 2 | 3 | 7 |
| CD(H) | 3 | 1 | 2 | 8 | 14 |
| Total | 6 | 6 | 6 | 14 |
Values are no. of neurons.
Fig. 13.
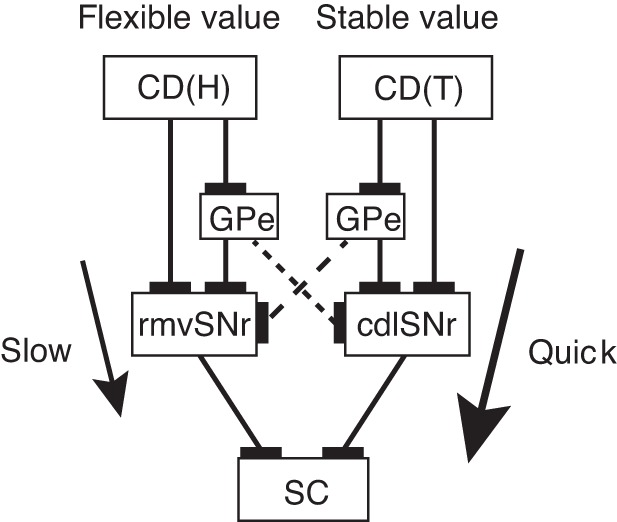
Parallel processing of visual object values in basal ganglia. Stable values are learned slowly but executed quickly in CD(T)-cdlSNr-SC pathway (thick arrow), whereas flexible values are learned quickly but executed slowly in CD(H)-rvmSNr-SC pathway (thin arrow). The two value signals may interact with each other by passing through globus pallidus external segment (GPe) (dashed lines).
Transmission speeds of stable and flexible value signals.
We found another difference between the stable and flexible value circuits: speed of signal transmission. The difference was revealed in the signal transmission from CD to SNr (Fig. 12A) and the signal conduction from SNr to SC (Fig. 12B). The latencies of the inhibitory responses of SNr neurons (Fig. 12A) were shorter by CD(T) stimulation (mean: 8.8 ms, median: 8 ms) than by CD(H) stimulation (mean: 22.5 ms, median: 20.5 ms) (P = 3.12 × 10−6, t-test). Examples were shown in Figs. 5, 7, and 8. The latencies of the SC-evoked antidromic responses (Fig. 12B) were shorter for stable value-coding SNr neurons (mean: 1.33 ms) than for the flexible value-coding SNr neurons (mean: 2.33 ms) (P = 6.57 × 10−4, t-test). Examples were shown in Figs. 2, 3, and 10. These results suggest that the stable value signal primarily mediated by CD(T)-cdlSNr-SC circuit influences the oculomotor output more quickly than the flexible value signal primarily mediated by CD(H)-rvmSNr-SC pathway (Fig. 13).
Fig. 12.

Stable and flexible value signals are transmitted at different speeds in CD-SNr-SC circuit. A: latencies of inhibitions in SNr neurons caused by stimulation of CD(T) (white) and CD(H) (gray). Number of neurons in each bin is indicated. B: latencies of SC-induced antidromic responses in stable value-coding SNr neurons (white) and flexible value-coding SNr neurons (gray). The means of the latencies (indicated by arrows) were significantly different for the othodromic responses (P = 3.12 × 10−6, t-test; A) and the antidromic responses (P = 6.57 × 10−4, t-test; B).
DISCUSSION
Parallel CD-SNr-SC circuits transmit stable and flexible object values.
Our data suggest that stable and flexible values of visual objects are processed in mostly separate circuits in the basal ganglia, both of which may be used for oculomotor control (Fig. 13). The two circuits are confined in the CD-SNr-SC connections, but are mediated by different portions of CD and SNr. Specifically, stable value signals are processed mainly in the connection from the CD(T) to the cdlSNr, whereas flexible value signals are processed mainly in the connection from the CD(H) to the rvmSNr. Both of the stable and flexible value signals are then, at least partially, sent to SC, thereby biasing gaze to high-valued objects.
This conclusion was supported by a basic electrophysiological experiment: electrical stimulation of CD(T) inhibited neurons mostly in cdlSNr [CD(T)-recipient neurons], while stimulation of CD(H) inhibited neurons mostly in rmvSNr [CD(H)-recipient neurons] (Fig. 6). These inhibitory effects are likely caused by the direct connections of CD to SNr, because all projection neurons in the striatum (including CD) are thought to be GABAergic and inhibitory (Graybiel 1990; Precht and Yoshida 1971). Our finding is consistent with anatomical studies, each of which focused on either CD(H) (Smith and Parent 1986; Szabo 1970) or CD(T) (Francois et al. 1994; Saint-Cyr et al. 1990; Szabo 1972). Previous electrophysiological studies identified inhibitory connections from CD to SNr by stimulating CD(H) and caudate body (Hikosaka et al. 1993; Yoshida and Precht 1971). Notably, Hikosaka et al. (1993) found that neurons in the caudal-dorsal part of SNr were not affected by CD(H) stimulation, consistent with the anatomical study by Smith and Parent (1986). This CD(H)-unaffected region seems to correspond to the region that was inhibited by CD(T) stimulation in this study (i.e., cdlSNr).
An important question remained after these anatomical or electrophysiological experiments: the functional significance of these CD-SNr circuits. Our behavioral experiment provided an answer to this question: CD(T)-recipient neurons in cdlSNr tended to encode stable object values, whereas CD(H)-recipient neurons in rvmSNr tended to encode flexible object values (Table 1). The functional dichotomy of SNr is likely related to, or caused by, the functional dichotomy of CD: CD(T) neurons encode stable values, whereas CD(H) neurons encode flexible values (Kim and Hikosaka 2013; Yamamoto et al. 2013). However, some SNr neurons encoded both stable and flexible values (Table 1). This may be explained by inputs from caudate body (Hikosaka et al. 1993) which contains a mixture of stable and flexible value-coding neurons (Kim and Hikosaka 2013).
We showed that the stable and flexible value signals in SNr are both transmitted to SC, at least partially. This was shown by antidromic activation of both stable value-coding SNr neurons and flexible value-coding SNr neurons by electrical stimulation of SC (Figs. 2 and 3). These results suggest that stable and flexible value signals in the basal ganglia converge to SC (Fig. 13), so that the monkey can make saccades to visual objects with high reward values, whether the values remain unchanged for a long time (stable values) or change quickly (flexible values). However, SC may not be the sole destination of the basal ganglia-derived value signals; they may also be sent to other SNr-target areas, including the thalamo-cortical circuits (Beckstead and Frankfurter 1982; Parent et al. 1983; Tanibuchi et al. 2009).
Involvement of indirect pathway for object value processing.
Another factor that may affect the functional dichotomy is the indirect pathway. CD can influence SNr through the direct pathway (i.e., monosynaptic inhibitory connection) and the indirect pathway (i.e., disynaptic or multisynaptic connection mediated by GPe and possibly STN as well) (Hikosaka et al. 2000). Since GPe neurons are GABAergic inhibitory and STN neurons are glutamatergic excitatory, the net effect through the indirect pathway should be excitatory. This is opposite to the inhibitory effect of the direct pathway.
Our data suggested that CD(T) and CD(H) have separate goals in SNr (cdlSNr and rvmSNr) using their separate direct pathways. Do they also have separate indirect pathways? Although not conclusive, one way to address this question is to investigate excitatory responses of SNr neurons, which are likely mediated through the indirect pathway. In response to CD(T) stimulation, cdlSNr neurons were often inhibited and then excited (Figs. 7 and 10). Similarly, in response to CD(H) stimulation, rvmSNr neurons were often inhibited and then excited (Fig. 8). These results suggest that the direct and indirect pathways have the same goal (Fig. 13). This appears to occur also in natural viewing conditions, especially in the CD(T)-cdlSNr connection: cdlSNr neurons were typically inhibited by stably high-valued objects and excited by stably low-valued objects (Figs. 2 and 10). This combination of responses would lead to a purposive behavior through the SNr-SC inhibitory connection: facilitation of saccades to stably high-valued objects and suppression of saccades to stably low-valued objects (as shown in Fig. 1B).
It is known anatomically that CD(T) and CD(H) project to different parts of GPe: CD(T) to the caudal-ventral part of GPe (Saint-Cyr et al. 1990), and CD(H) to the rostral-dorsal part of GPe (Cowan and Powell 1966; Parent et al. 1984). This separate circuit arrangement is necessary for the stable value signal to be processed without being interrupted by the flexible value signal, and vice versa. The caudal-ventral part of GPe would receive inputs from CD(T) and send outputs to cdlSNr, whereas the rostral-dorsal part of GPe would receive inputs from CD(H) and send outputs to rvmSNr (Fig. 13).
However, SNr neurons sometimes showed excitatory responses with no preceding inhibitory responses in response to CD stimulation (Fig. 11B) or visual stimuli (Fig. 11D). Such initial excitatory responses occurred without clearly following the stable-flexible value dichotomy (Table 2). This raises the possibility that each part of GPe does have access to the incongruent part of SNr. The stable value signal could then be integrated into the flexible value signal, and vice versa (Fig. 13). The responses of the SNr neuron shown in Fig. 11 may be explained by this hypothetical integration mechanism. Alternatively, the integration (or interaction) of the two value signals may occur in STN, which may receive converging inputs from the two parts of GPe and send outputs to cdlSNr or rvmSNr, or both. These circuit schemes remain to be investigated.
Functional subdivisions of SNr.
Our circuit and information analysis suggests a topographical dichotomy of SNr. This may have an anatomical correlate: pars lateralis vs. pars reticulata (Francois et al. 1985). The dorsolateral part of SNr is sometimes called “pars lateralis,” which contains a cluster of neurons projecting to SC (Beckstead and Frankfurter 1982; Francois et al. 1984). Indeed, visual-saccadic neurons are mostly clustered in the dorsolateral SNr (or pars lateralis) (Hikosaka and Wurtz 1983a), which roughly corresponds to cdlSNr encoding stable values. In contrast, pars reticulata (i.e., more ventromedial part of SNr) contains neurons that project to different areas, such as the thalamus, pedunculopoine nucleus, or SC (Beckstead and Frankfurter 1982). This may be related to the fact that rvmSNr receives direct inputs from CD(H), which contains a variety of neuron types (Hikosaka et al. 1989).
Notably, neurons in the pars lateralis in monkeys and humans have larger cell bodies and longer dendrites than neurons in the pars reticulata (Francois et al. 1984; Yelnik et al. 1987). Consistent with this observation, the latency of the SC-evoked antidromic response was shorter for stable value-coding cdlSNr neurons than for flexible value-coding rvmSNr neurons (Fig. 12B). The clustering of large SC-projecting neurons in the pars lateralis seems unique to primates (Beckstead and Frankfurter 1982; Francois et al. 1985). Our data suggest that the pars lateralis receives inputs from CD(T) and sends outputs mainly to SC, whereas the pars reticulata receives inputs from CD(H) and sends outputs to SC and other areas. Following Francois et al. (1985), we speculate that the pars lateralis is a phylogenetically new division of the substantia nigra, which is devoted to the control of saccadic eye movements.
Functional significance of the parallel value processing in the CD-SNr-SC connection.
What is the functional significance of mostly separate processing of flexible and stable value? In a familiar environment, many objects have their stable values based on our long-term experiences (e.g., I like cucumber, I don't like carrot), and we can easily choose our favorite objects (i.e., objects with high stable values) (Seitz et al. 2009; Shiffrin and Schneider 1977). However, performing this choice requires high-capacity memories because we are surrounded by so many objects with different values. The memories must be retained for a long time because we may not encounter some of the familiar objects for a long time. cdlSNr neurons satisfy these requirements (Yasuda et al. 2012): they are inhibited by stably high-valued objects, even when they are no longer associated with rewards (Figs. 2, 7, and 10). Therefore, cdlSNr-SC mechanism would guide our gaze automatically to stably high-valued objects. Without this mechanism, we would need to let our gaze explore many objects until it happens to land on a high-valued object. Thus cdlSNr-SC mechanism would work well if the environment is stable.
However, the environment may change. We may experience one of our favorite objects, but with a bad outcome (e.g., favorite cucumber tastes bitter). In this case, cdlSNr-SC mechanism would be useless, because it would carry long-term memories (e.g., favorite cucumber) stubbornly and acquire newly experienced values too slowly (e.g., bitter cucumber). On the other hand, rvmSNr-SC mechanism is flexible because it relies on short-term memories of object values. It would guide our gaze based on recently acquired values. The rvmSNr-SC mechanism would thus be ideal in a flexible environment. On the contrary, this mechanism would be much less useful in a stable environment because it does not encode long-term values of individual objects.
In summary, the stable cdlSNr-SC mechanism and the flexible rvmSNr-SC mechanism, together, would guide gaze to high-valued objects, regardless of whether their values remain unchanged (i.e., stable) or change frequently (i.e., flexible). Such value-based gaze orienting is crucial for survival, because it guides motor actions (Hayhoe and Ballard 2005; Johansson et al. 2001; Land and Hayhoe 2001; Miyashita et al. 1996) or initiates cognitive mnemonic processes (Just and Carpenter 1976; Yarbus 1967). This stable-flexible scheme is consistent with Bayesian decision theory (Kording and Wolpert 2006; McNamara et al. 2006) which postulates two statistical distributions: 1) “prior” based on long-term experiences; and 2) “likelihood” based on short-term information. The stable cdlSNr-SC mechanism would process “prior” information, whereas the flexible rvmSNr-SC mechanism would process “likelihood” information. The final selection (“posterior”) would occur in SC (Kim and Basso 2010).
Speed of signal transmission through the parallel CD-SNr-SC circuits.
SNr-SC connection is directed to the intermediate and deep layers of SC (Hikosaka and Wurtz 1983b; Karabelas and Moschovakis 1985), which sends burst signals to gaze (or saccade) centers in the brain stem and upper spinal cord (Sparks 2002). This suggests that both stable and flexible value signals originating from the basal ganglia are capable of influencing gaze orienting. Notably, the speed of signal transmission was different between the two value signals. First, the inhibition of cdlSNr neurons by CD(T) stimulation occurred much earlier than the inhibition of rvmSNr neurons by CD(H) stimulation (Fig. 12A). Second, the signal conduction to SC was faster for stable value-coding cdlSNr neurons than for flexible value-coding rvmSNr neurons (Fig. 12B).
These results suggest that the stable value signal reaches SC earlier than the flexible value signal, and therefore the gaze tends to be attracted first by stably high-valued objects rather than flexibly high-valued objects. Moreover, the gaze orienting to stably high-valued objects would occur even when there is no rewarding outcome, both in monkeys (Bichot and Schall 1999; Peck et al. 2009; Yamamoto et al. 2013; Yasuda et al. 2012) and humans (Anderson and Yantis 2012; Della Libera and Chelazzi 2009; Theeuwes and Belopolsky 2012). The automatic nature of the cdlSNr-guided gaze orienting may be characterized as a habit. Indeed, many studies have suggested the basal ganglia contribute to habit formation (Fernandez-Ruiz et al. 2001; Graybiel 2008; Yin and Knowlton 2006). From a different perspective, however, this can be viewed as a skill (Hikosaka et al. 2013), because it allows monkeys and humans to choose high-valued objects among many low-valued objects accurately and quickly in a familiar environment, a behavior critical for survival.
GRANTS
This research was supported by the Intramural Research Program at the National Institutes of Health, National Eye Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.Y. and O.H. conception and design of research; M.Y. performed experiments; M.Y. analyzed data; M.Y. and O.H. interpreted results of experiments; M.Y. prepared figures; M.Y. and O.H. drafted manuscript; M.Y. and O.H. edited and revised manuscript; M.Y. and O.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank H. F. Kim, A. Ghazizadeh, I. Monosov, D. McMahon, and H. Amita for discussions; and A. M. Nichols, M. K. Smith, T. W. Ruffner, D. Parker, G. Tansey, I. Bunea, J. W. McClurkin, and L. P. A. V. Hays for technical assistance.
REFERENCES
- Anderson BA, Yantis S. Value-driven attentional and oculomotor capture during goal-directed, unconstrained viewing. Atten Percept Psychophys 74: 1644–1653, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol 68: 945–960, 1992. [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Neuronal activity in substantia nigra pars reticulata during target selection. J Neurosci 22: 1883–1894, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM, Frankfurter A. The distribution and some morphological features of substantia nigra neurons that project to the thalamus, superior colliculus and pedunculopontine nucleus in the monkey. Neuroscience 7: 2377–2388, 1982. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Effects of similarity and history on neural mechanisms of visual selection. Nat Neurosci 2: 549–554, 1999. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O, Nakamura K. Coding of task reward value in the dorsal raphe nucleus. J Neurosci 30: 6262–6272, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caan W, Perrett DI, Rolls ET. Responses of striatal neurons in the behaving monkey. 2. Visual processing in the caudal neostriatum. Brain Res 290: 53–65, 1984. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci 24: 87–114; discussion 114–185, 2001. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Powell TP. Strio-pallidal projection in the monkey. J Neurol Neurosurg Psychiatry 29: 426–439, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Libera C, Chelazzi L. Learning to attend and to ignore is a matter of gains and losses. Psychol Sci 20: 778–784, 2009. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol 34: 414–427, 1971. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Wang J, Aigner TG, Mishkin M. Visual habit formation in monkeys with neurotoxic lesions of the ventrocaudal neostriatum. Proc Natl Acad Sci U S A 98: 4196–4201, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois C, Percheron G, Yelnik J. Localization of nigrostriatal, nigrothalamic and nigrotectal neurons in ventricular coordinates in macaques. Neuroscience 13: 61–76, 1984. [DOI] [PubMed] [Google Scholar]
- Francois C, Percheron G, Yelnik J, Heyner S. A histological atlas of the macaque (Macaca mulatta) substantia nigra in ventricular coordinates. Brain Res Bull 14: 349–367, 1985. [DOI] [PubMed] [Google Scholar]
- Francois C, Yelnik J, Percheron G, Fenelon G. Topographic distribution of the axonal endings from the sensorimotor and associative striatum in the macaque pallidum and substantia nigra. Exp Brain Res 102: 305–318, 1994. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci 31: 359–387, 2008. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci 13: 244–254, 1990. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA. Mechanisms for selection of basic motor programs–roles for the striatum and pallidum. Trends Neurosci 28: 364–370, 2005. [DOI] [PubMed] [Google Scholar]
- Handel A, Glimcher PW. Quantitative analysis of substantia nigra pars reticulata activity during a visually guided saccade task. J Neurophysiol 82: 3458–3475, 1999. [DOI] [PubMed] [Google Scholar]
- Hayhoe M, Ballard D. Eye movements in natural behavior. Trends Cogn Sci 9: 188–194, 2005. [DOI] [PubMed] [Google Scholar]
- Henderson JM. Human gaze control during real-world scene perception. Trends Cogn Sci 7: 498–504, 2003. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol 95: 567–584, 2006. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Miyashita N. Effects of caudate nucleus stimulation on substantia nigra cell activity in monkey. Exp Brain Res 95: 457–472, 1993. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. J Neurophysiol 61: 814–832, 1989. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80: 953–978, 2000. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol 49: 1230–1253, 1983a. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol 49: 1285–1301, 1983b. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Yamamoto S, Yasuda M, Kim HF. Why skill matters. Trends Cogn Sci 17: 434–441, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. Dopamine-mediated learning and switching in cortico-striatal circuit explain behavioral changes in reinforcement learning. Front Behav Neurosci 5: 15, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Westling G, Bäckström A, Flanagan JR. Eye-hand coordination in object manipulation. J Neurosci 21: 6917–6932, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. Eye fixations and cognitive processes. Cogn Psychol 8: 441–480, 1976. [Google Scholar]
- Karabelas AB, Moschovakis AK. Nigral inhibitory termination on efferent neurons of the superior colliculus: an intracellular horseradish peroxidase study in the cat. J Comp Neurol 239: 309–329, 1985. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci 1: 411–416, 1998. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Joseph JP. Activity in the caudate nucleus of monkey during spatial sequencing. J Neurophysiol 74: 911–933, 1995. [DOI] [PubMed] [Google Scholar]
- Kim B, Basso MA. A probabilistic strategy for understanding action selection. J Neurosci 30: 2340–2355, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HF, Hikosaka O. Distinct basal ganglia circuits controlling behaviors guided by flexible and stable values. Neuron 79: 1001–1010, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. Behaviorally contingent property of movement-related activity of the primate putamen. J Neurophysiol 63: 1277–1296, 1990. [DOI] [PubMed] [Google Scholar]
- Kording KP, Wolpert DM. Bayesian decision theory in sensorimotor control. Trends Cogn Sci 10: 319–326, 2006. [DOI] [PubMed] [Google Scholar]
- Land MF. Eye movements and the control of actions in everyday life. Prog Retin Eye Res 25: 296–324, 2006. [DOI] [PubMed] [Google Scholar]
- Land MF, Hayhoe M. In what ways do eye movements contribute to everyday activities? Vision Res 41: 3559–3565, 2001. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Kojima J, Gardiner TW, Hikosaka O. Visual and oculomotor functions of monkey subthalamic nucleus. J Neurophysiol 67: 1615–1632, 1992. [DOI] [PubMed] [Google Scholar]
- McNamara JM, Green RF, Olsson O. Bayes' theorem and its applications in animal behaviour. Oikos 112: 243–251, 2006. [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425, 1996. [DOI] [PubMed] [Google Scholar]
- Miyashita K, Rand MK, Miyachi S, Hikosaka O. Anticipatory saccades in sequential procedural learning in monkeys. J Neurophysiol 76: 1361–1366, 1996. [DOI] [PubMed] [Google Scholar]
- Parent A, Bouchard C, Smith Y. The striatopallidal and striatonigral projections: two distinct fiber systems in primate. Brain Res 303: 385–390, 1984. [DOI] [PubMed] [Google Scholar]
- Parent A, Mackey A, Smith Y, Boucher R. The output organization of the substantia nigra in primate as revealed by a retrograde double labeling method. Brain Res Bull 10: 529–537, 1983. [DOI] [PubMed] [Google Scholar]
- Peck CJ, Jangraw DC, Suzuki M, Efem R, Gottlieb J. Reward modulates attention independently of action value in posterior parietal cortex. J Neurosci 29: 11182–11191, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precht W, Yoshida M. Blockage of caudate-evoked inhibition of neurons in the substantia nigra by picrotoxin. Brain Res 32: 229–233, 1971. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Thorpe SJ, Maddison SP. Responses of striatal neurons in the behaving monkey. 1. Head of the caudate nucleus. Behav Brain Res 7: 179–210, 1983. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Ungerleider LG, Desimone R. Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey. J Comp Neurol 298: 129–156, 1990. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates. London: Academic, 2007. [Google Scholar]
- Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science 310: 1337–1340, 2005. [DOI] [PubMed] [Google Scholar]
- Sato M, Hikosaka O. Role of primate substantia nigra pars reticulata in reward-oriented saccadic eye movement. J Neurosci 22: 2363–2373, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1–27, 1998. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 275: 1593–1599, 1997. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Kim D, Watanabe T. Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron 61: 700–707, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic human information processing. II. Perceptual learning, automatic attending, and a general theory. Psychol Rev 84: 127–190, 1977. [Google Scholar]
- Smith Y, Parent A. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus). Neuroscience 18: 347–371, 1986. [DOI] [PubMed] [Google Scholar]
- Sparks DL. The brainstem control of saccadic eye movements. Nat Rev Neurosci 3: 952–964, 2002. [DOI] [PubMed] [Google Scholar]
- Szabo J. The course and distribution of efferents from the tail of the caudate nucleus in the monkey. Exp Neurol 37: 562–572, 1972. [DOI] [PubMed] [Google Scholar]
- Szabo J. Projections from the body of the caudate nucleus in the rhesus monkey. Exp Neurol 27: 1–15, 1970. [DOI] [PubMed] [Google Scholar]
- Tanibuchi I, Kitano H, Jinnai K. Substantia nigra output to prefrontal cortex via thalamus in monkeys. I. Electrophysiological identification of thalamic relay neurons. J Neurophysiol 102: 2933–2945, 2009. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Belopolsky AV. Reward grabs the eye: oculomotor capture by rewarding stimuli. Vision Res 74: 80–85, 2012. [DOI] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cogn Psychol 12: 97–136, 1980. [DOI] [PubMed] [Google Scholar]
- Turner RS, Anderson ME. Context-dependent modulation of movement-related discharge in the primate globus pallidus. J Neurosci 25: 2965–2976, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Kim HF, Hikosaka O. Reward value-contingent changes of visual responses in the primate caudate tail associated with a visuomotor skill. J Neurosci 33: 11227–11238, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Monosov IE, Yasuda M, Hikosaka O. What and where information in the caudate tail guides saccades to visual objects. J Neurosci 32: 11005–11016, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbus AL. Eye Movements and Vision. New York: Plenum, 1967. [Google Scholar]
- Yasuda M, Yamamoto S, Hikosaka O. Robust representation of stable object values in the oculomotor basal ganglia. J Neurosci 32: 16917–16932, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelnik J, Francois C, Percheron G, Heyner S. Golgi study of the primate substantia nigra. I. Quantitative morphology and typology of nigral neurons. J Comp Neurol 265: 455–472, 1987. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci 7: 464–476, 2006. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Precht W. Monosynaptic inhibition of neurons of the substantia nigra by caudato-nigral fibers. Brain Res 32: 225–228, 1971. [DOI] [PubMed] [Google Scholar]