Abstract
In Parkinson's disease (PD), the dopamine (DA) neuron loss in the substantia nigra and the DA axon loss in the dorsal striatum are severe, but DA neurons in the ventral tegmental area and DA axons in middle and ventral striatal subregions are less affected. Severe DA loss leads to DA receptor supersensitivity, but it was not known whether the supersensitivity of the DA D1 receptors (D1Rs) on the striatonigral axon terminal is determined by the severe striatal or nigral DA loss. This question is important because these two possibilities affect the extent of the striatonigral terminals with supersensitive D1Rs and hence the strength of the direct pathway output. Here we have investigated this question in the transcription factor Pitx3 mutant mice that have a PD-like DA loss pattern. We found that the presynaptic D1R function was upregulated globally: the D1R-mediated facilitation was equally enhanced for the striatonigral GABA output originated in the dorsal striatum where the DA loss is severe and the somatic D1Rs are supersensitive, and for the striatonigral GABA output originated in the middle and ventral striatum where the DA loss is moderate and the somatic D1Rs are not supersensitive. These results suggest that severe nigral DA loss is sufficient to induce functional upregulation of the D1Rs on striatonigral axon terminals. Consequently, in PD, the globally enhanced D1Rs on striatonigral axon terminals originated in broad striatal subregions may strongly enhance the striatonigral GABA output upon D1R stimulation, potentially contributing to D1R agonism's profound motor-stimulating effects.
Keywords: basal ganglia; l-3,4-dihydroxyphenylalanine (l-dopa); dopamine receptor supersensitivity; Parkinson's disease; substantia nigra
the striatonigral gabaergic output promotes motor activity by inhibiting the GABAergic projection neurons in the substantia nigra pars reticulata (SNr) (Friend and Kravitz 2014; Hikosaka et al. 2000; Kravitz et al. 2010). Like their somata in the striatum, the striatonigral axon terminals express a very high level of D1 receptors (D1Rs) (Levey et al. 1993; Yung et al. 1995). The main source of dopamine (DA) in the SNr is likely the dendrites of the substantia nigra pars compacta (SNc) DA neurons (Cheramy et al. 1981; Geffen et al. 1976; Rice et al. 2011); the scattered DA neurons in the SNr may also contribute (González-Hernández and Rodríguez 2000). Activation of these presynaptic D1Rs facilitates striatonigral GABA release (Chuhma et al. 2011; Radnikow and Misgeld 1998). In Parkinson's disease (PD), nigral DA neurons, DA dendrites in the SNr, and DA axons in the dorsal striatum are severely lost, whereas ventral tegmental area (VTA) DA neurons and the DA innervation in ventral striatal subregions are less affected (Hornykiewicz 1998, 2001). As a compensatory response, in PD brains, both D1Rs and D2Rs in the striatum are upregulated or supersensitive (Aubert et al. 2005; Corvol et al. 2004; Guigoni et al. 2007; Hornykiewicz 2001). Behavioral, histochemical and biochemical studies have indicated that toxin lesion of the nigrostriatal DA system or genetic inactivation of DA synthesis also supersensitizes somatic D1Rs (Gerfen et al. 2002; Kim et al. 2000) and D1Rs at the striatonigral axon terminals (Rangel-Barajas et al. 2008).
An important question is whether the supersensitivity of the presynaptic D1Rs on the striatonigral axon terminals can be triggered by the DA loss in the SNr, independent of the functional status of the somatic D1Rs in the striatonigral neurons, or it occurs as a consequence of somatic D1R supersensitization following DA loss in the striatum, i.e., the somatic supersensitization spreads to the striatonigral axon terminals. A determination of these two possibilities is important for the following reasons. It is established that each SNr locus receives converging inputs from wide striatal subregions, including dorsal, middle and ventral areas, although some topography exists (Gerfen 1985; Hedreen and DeLong 1991; Lynd-Balta and Haber 1994). Thus, if the presynaptic D1R supersensitivity is determined by DA loss in the striatum, then in PD that has a severe nigral DA neuron loss but relatively intact VTA DA neurons, only the D1Rs on the striatonigral axon terminals originating in the dorsal striatum become supersensitive. In contrast, if the DA loss in the SNr is sufficient to sensitize the presynaptic D1Rs, then the D1Rs on all striatonigral axon terminals originating in different striatal subregions with varying degrees of DA loss may supersensitize globally, because all of these D1R-expressing axon terminals are in the same DA-deficient SNr, and such a global presynaptic D1R supersensitivity may contribute to the profound motor-stimulating effect of l-3,4-dihydroxyphenylalanine (l-dopa) and D1 agonists (Li and Zhou 2013; Nutt et al. 2010).
We hypothesize that in PD, the supersensitivity of D1Rs on the striatonigral axonal terminals may be triggered by the DA loss in the SNr, even when the somatic D1Rs are not supersensitized; this is because the DA loss in the SNr in PD is 81% and the normal DA level in the SNr is ≤1/10 of the striatal level, such that the SNr residual DA level is only 2% of the normal striatal DA level; in contrast, the DA loss in the striatum ranges 98% in the dorsal putamen and 50% in the nucleus accumbens in a dorsal-ventral gradient (Kish et al. 1988; Hornykiewicz 2001). Thus the average DA deficiency in the SNr is severer than that in the striatum, creating a fertile condition for D1R supersensitization in the SNr.
To test our hypothesis, we need an animal model with a differential DA loss in the striatal subregions, such that the DA loss is severe and the somatic D1Rs are supersensitive in one striatal subregion but not in others. The Pitx3Null mice meet these requirements because they have a PD-like DA loss pattern (Nunes et al. 2003; van den Munckhof et al. 2003). In these mice, the nigral DA neurons and SNr DA dendrites are severely lost; the DA loss in the dorsal striatum is severe (>95% DA loss), such that the somatic D1Rs in the striatonigral neurons in the dorsal striatum are supersensitive, while the middle and ventral striatum retain 10–45% DA with no detectable D1R supersensitivity, based on the l-dopa induction of phosphorylated extracellular signal-regulated kinase (pERK) (Ding et al. 2011c). These unique features make the Pitx3Null mouse an excellent animal model to determine whether the upregulation of the D1Rs on the striatonigral axon terminal is dependent on the DA loss in the striatum, or it can be triggered by DA loss in the SNr.
MATERIALS AND METHODS
Animals.
Two breeding pairs of heterozygous Pitx3-deficient mice (Nunes et al. 2003; van den Munckhof et al. 2003) were obtained from the Jackson Laboratory (Bar Harbor, ME). A small colony, including homozygous Pitx3−/− (Pitx3Null), heterozygous Pitx+/−, and wild-type Pitx+/+ (Pitx3WT) mice was generated at the University of Tennessee Health Science Center (Li et al. 2013; Li and Zhou 2013; Wei et al. 2013). The genotypes were initially determined by PCR-based genotyping to identify Pitx3WT, Pitx3Null, and heterozygotes (Li et al. 2013). Since Pitx3Null mice are fertile, survive well and are easily identified by being aphakic (Wei et al. 2013), Pitx3Null and Pitx3WT mice for this study were generated by mating Pitx3Null male mice with Pitx3Null female mice (all offspring are Pitx3Null) and mating Pitx3WT male mice with PitxWT female mice (all offspring are Pitx3WT) (Wei et al. 2013). Animal care and use were in accordance with federal guidelines and approved by Institutional Animal Care and Use Committee of University of Tennessee Health Science Center.
The nigral DA neuron loss, the gradient striatal DA axon loss and the associated pERK expression (a marker of supersensitive D1Rs) in Pitx3Null mice have been documented in previous studies (Beeler et al. 2009; Ding et al. 2007, 2011c; Luk et al. 2013; Nunes et al. 2003; van den Munckhof et al. 2003). To provide a firm foundation for our present cellular neurophysiological study, we replicated these published data on DA neuron loss and l-dopa-induced gradient pERK expression in the striatum in Pitx3Null mice.
Brain slice preparation.
The procedures to prepare parasagittal slices containing the SNr, globus pallidus, and striatum have been described in detail (Beurrier et al. 2006; Connelly et al. 2010; Ding et al. 2013). Briefly, Pitx3Null mice or wild-type mice of postnatal drug-naive days 20–30 were killed by decapitation, and brains were quickly dissected out and immediately immersed for 2 min in the following oxygenated, ice-cold cutting solution containing (in mM): 220 glycerol, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2 and 20 d-glucose. Then we cut 300- to 350-μm-thick parasagittal slices using a Leica Zero Z VT1200S vibratome (Leica Microsystems, Wetzlar, Germany) at a 15° angle to the sagittal plane. These parasagittal slices contained parts of the striatum, globus pallidus and SNr and the connections among them. These brain slices were transferred to a holding chamber filled with an artificial cerebral spinal fluid (aCSF) containing (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2.5 CaCl2, 1.3 MgCl2 and 10 d-glucose; the aCSF was continuously bubbled with 95% O2 and 5% CO2. The brain slice were first kept at 34°C for 30 min and then at room temperature (25°C) until use.
Whole cell patch-clamp recording.
We placed brain slices in a recording chamber and continuously perfused at 2 ml/min with standard aCSF saturated with 95% O2 and 5% CO2. Recordings were made at 30°C (TC 324B temperature controller, Warner Instruments) under visual guidance of a video microscope (Olympus BX51WI and Zeiss Axiocam MRm digital camera) equipped with Nomarski optics and a ×60 water immersion lens. Patch pipettes were pulled from borosilicate glass capillary tubing (KG-33, 1.1 mm inner diameter, 1.65 mm outer diameter, King Precision Glass, Claremont, CA) using a PC-10 puller (Narishige, Tokyo, Japan) and had resistances of 1–3 MΩ when filled with the following intracellular solution (in mM): 135 KCl, 0.5 EGTA, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, and 4 Na2-phosphocreatine, pH 7.25, 280–290 mOsm. The voltage-gated Na+ channel blocker lidocaine N-ethyl bromide (5 mM, QX-314, Sigma-Aldrich) was added to the intracellular solutions to block spontaneous firing when evoked inhibitory postsynaptic currents (IPSCs) were recorded. Tetrodotoxin (1 μM) was added to the extracellular solution when miniature IPSCs (mIPSCs) were recorded. All of the recordings were made in the presence of 6,7-dinitro-quinoxaline-2,3-dione (DNQX; 10 μM) and d,l-2-amino-5-phosphonovalerate (20 μM) in the aCSF to block ionotropic glutamate receptors.
We used a Multiclamp 700B amplifier, pClamp 9.2 software and Digidata 1322A interface (Molecular Devices, Sunnyvale, CA) to acquire data. For voltage clamp recording, we held cells at −70 mV. Access resistance was monitored by a −10-mV, 50-ms pulse before every evoked IPSC. Cells in which the capacitive transient was reduced by >15% were discarded. Signals were digitized at 20 kHz and filtered at 10 kHz using the built-in four-pole low-pass Bessel filter in the patch-clamp amplifier.
Electrical stimulation to evoke synaptic responses.
Focal stimulation in the SNr was delivered via a theta electrode pulled from a theta capillary. This stimulating theta electrode was filled with the extracellular solution and positioned within a 100-μm distance from the recorded cell in the SNr, laterally or medially to the recorded cell outside the cerebral peduncle. Paired (50-ms interval) pulse stimuli were delivered every 20 s. The position of the pipette and the stimulus intensity were adjusted to evoke IPSCs with a paired pulse ratio (PPR; the ratio of the second peak current over the first one) more than 1.5, presumably striatonigral IPSC, according to established criteria (Connelly et al. 2010).
Focal stimulation in the striatum was delivered through a bipolar tungsten electrode [World Precision Instruments (WPI)] placed in the dorsal, middle or ventral striatum to evoke striatonigral synaptic responses. In this study, for the convenience of description, the term “dorsal striatum” is used to describe the dorsal-most 20% of the striatum, where in Pitx3Null mice the DA loss is >95% and pERK is induced by l-dopa. The term “middle striatum” is used to describe the striatal region dorsal to the anterior commissure and ventral to the dorsal striatum defined above; the residual DA in the middle striatum is 10–30% of the WT level and has no detectable l-dopa-induced pERK. Thus the middle striatum defined here is a part of the dorsal striatum commonly defined in the literature (Voorn et al. 2004). Finally, our definition here for “ventral striatum” is identical to that commonly used in the literature, describing the striatal region ventral to the anterior commissure (Voorn et al. 2004). In Pitx3Null mice, the residual DA is gradient with no DA in the dorsal-most area to ∼45% of the normal DA level in the ventral striatum (Li and Zhou 2013; Wei et al. 2013). Anatomical studies have established that striatonigral neurons in the dorsal, middle and ventral striatum generally take the corresponding dorsal, middle and ventral route to project to the SNr (Fujiyama et al. 2011; Lévesque and Parent 2005). Therefore, stimulation in the dorsal, lower middle and ventral striatum activates the striatonigral projection neurons in the dorsal, lower middle and ventral striatum, respectively. Some fibers from the dorsal striatum may travel through the lower middle striatum; thus future studies are needed to resolve this issue by using optogenetic or single-cell stimulation. In our present study, we mitigate this issue by stimulating ventral striatum because it is unlikely that the striatonigral axons from the dorsal-most striatum travel through the ventral striatum. Stimulating pulses were generated by a Master-8 pulse generator and delivered via a WPI constant-current isolator (model A365) at 0.05 Hz to evoke IPSCs. The stimulation intensity was from 0.01 mA to 0.35 mA at a fixed duration of 0.2 ms. Low-intensity stimuli were used for local SNr stimulation, while stronger ones were for striatal stimulation.
To verifying that pallidonigral IPSCs do not interfere with striatonigral IPSCs, we evoked pallidonigral IPSCs using a minimal stimulation method to minimize the activation of the passing striatonigral axons. A saline-filled theta capillary pipet with a tip diameter of 5 μm was placed in the GPe. Paired pulses with an interpulse interval of 50 ms were delivered at 0.05 Hz. The stimulation intensities ranging from 10 to 50 μA were first adjusted to elicit IPSCs with 20–50% failure rate. Then the stimulation intensities were increased a few microamperes to decrease the failure rate to less than 10%. The resulting pallidonigral IPSCs are strikingly different from the striatonigral IPSCs, consistent with the literature (Connelly et al. 2010). Thus our striatonigral IPSCs are reasonably pure and reliable.
Puff application of GABA to SNr GABA neurons.
To study the possible postsynaptic effect of D1-like agonism on IPSCs in SNr GABA neurons, 1 mM GABA was puff-applied to evoke GABA currents in SNr GABA neurons using a Picospritzer (Parker Instrumentation) and puff pipettes with a tip diameter of ∼2 μm. The puff pipette was positioned at the same plane with and ∼50 μm away from the neuron being recorded. The pressure pulse was 3–5 psi and 25 ms. The interval between puffs was 20 s. After a stable baseline response had been obtained for 5 min, the D1-like agonist SKF81297 was bath applied for 10 min.
Analysis of electrophysiological data.
We analyzed evoked IPSCs using Clampfit 9.2 software. Averages of 10 consecutive evoked IPSCs before or after drug administration were used to evaluate baseline and drug response, respectively. Peak amplitudes after drug administration were normalized to the baseline. Latencies to the evoked IPSCs were measured from the stimulus onset to the onset of evoked IPSCs. mIPSCs were detected by MiniAnalysis. Drug effects were calculated as changes in the frequency and/or amplitude of mIPSCs. The frequency was determined from the number of mIPSCs within 5-min epochs for control, pharmacological treatment and recovery. mIPSC amplitude and frequency distributions were compared using the Kolmogoroff-Smirnoff test.
Immunostain to detect DA neurons and dendrites in the nigral area.
We used established methods to detect DA neurons and DA dendrites in the midbrain (Li and Zhou 2013; Zhou et al. 2009). The brains were fixed in 4% paraformaldehyde (PFA) dissolved in phosphate-buffered saline (PBS) at 4°C overnight and then sectioned on a vibratome. The free-floating sections (50 μm in thickness) were incubated with 2% fat-free milk, 1% bovine serum albumin (BSA), and 0.4% Triton X-100 in PBS for 1 h at room temperature to block nonspecific binding and permeabilize the cell membrane, respectively. After thorough rinsing, the free-floating sections were incubated for 48 h at 4°C with the primary antibody, a polyclonal tyrosine hydroxylase (TH) antibody raised in sheep (Millipore; diluted at 1:1,000), and then rinsed in PBS, followed by incubating with a donkey anti-sheep secondary antibody conjugated with the green Alexa Fluor 488 (diluted at 1:200; Invitrogen), for 3 h at room temperature. In a separate study, we saw no pERK in the SNc and SNr; thus double TH and pERK staining was not performed here.
Double immunostain to detect DA axons and pERK in the striatum.
We followed published double immunostaining methods to detect DA axons and pERK in the same striatal tissue sections (Ding et al. 2011c; Li and Zhou 2013). Drug-naive mice were injected intraperitoneally with 10 mg/kg l-dopa and 5 mg/kg benserazide (3 Pitx3WT mice and 3 Pitx3Null mice) or 2 mg/kg SKF81297 (3 Pitx3WT mice and 3 Pitx3Null mice). Thirty minutes later, these mice were placed under deep urethane anesthesia and intracardially perfused with 4% PFA. The brains were postfixed in the same 4% PFA at 4°C overnight and then sectioned on a vibratome. The free-floating sections (50 μm in thickness) were incubated with 2% fat-free milk, 1% BSA, and 0.4% Triton X-100 in PBS for 1 h at room temperature to block nonspecific binding and permeabilize the cell membrane, respectively. After thorough rinsing, the free-floating sections were incubated for 48 h at 4°C with the two primary antibodies (see below) and then rinsed in PBS, followed by incubating with the two secondary antibodies (see below) for 3 h at room temperature in the dark. Both the primary and secondary antigen-antibody reactions occurred in PBS containing 3% normal donkey serum, 1% BSA, and 0.2% Triton X-100. The two primary antibodies were a polyclonal TH antibody raised in sheep (Millipore; diluted at 1:1,000) and a pERK antibody raised in rabbit (Cell Signaling Technology; diluted at 1:200). The secondary antibodies were as follows: 1) donkey anti-sheep IgG antibody, conjugated with the green Alexa Fluor 488 (diluted at 1:200; Invitrogen), used for labeling TH; and 2) donkey anti-rabbit IgG antibody, conjugated with the red Alexa Fluor 568 (diluted at 1:200), used for labeling pERK.
Fluorescence images were acquired on a Zeiss 710 confocal laser scanning microscope at the Neuroscience Imaging Center of The University of Tennessee Health Science Center.
Drugs.
All drugs used in electrophysiological experiments were made into stock solutions in ddH2O or dimethyl sulfoxide. Stock solutions of drugs were diluted at least 1:1,000 to the desired concentration in aCSF immediately prior to their application. l-3,4-Dihydroxyphenylalanine methyl ester hydrochloride (l-dopa) and benserazide hydrochloride were dissolved in sterile saline. l-Dopa, benserazide, SKF38393, SKF81297, SKF83566, DNQX and dl-2-amino-5-phosphonopentanoic acid were purchased from either Tocris or Sigma-Aldrich.
Statistics.
The paired Student's t-test was used to determine significant changes before and during drug administration within the same group. One-way and two-way ANOVA with post hoc Bonferroni or least significant difference test and unpaired Student's t-test were used to compare the changes among different groups.
RESULTS
Severe DA denervation and supersensitive D1Rs in the dorsal striatum in Pitx3Null mice.
To confirm that our locally bred Pitx3Null mice have a DA loss pattern that is identical to those reported in the literature, we immunostained brain sections from Pitx3WT mice (n = 3) and Pitx3Null mice (n = 3) for TH, a marker for DA neurons in the midbrain. As shown in Fig. 1, A and B, we found that most DA neurons in the SNc in Pitx3Null mice were lost, whereas the VTA retained a considerable number of residual DA neurons. We also found that in these Pitx3Null mice, the striatum had a gradient DA loss: the DA denervation is an almost total lack of DA axons in the dorsal striatum, whereas the DA denervation was moderate in the ventral striatum (Fig. 1, C1 and D1). These results confirm that the DA loss pattern in our locally bred Pitx3Null mice is identical to that reported in the literature (Nunes et al. 2003; van den Munckhof et al. 2003). Furthermore, we detected a clear pERK expression, upon the first dose of l-dopa (10 mg/kg ip) or D1 agonist SKF81297 (2 mg/kg ip), in the dorsal striatum in Pitx3Null mice (3 Pitx3Null mice for each drug) where the DA denervation was severe, while pERK was not detectable in the middle or ventral striatum where the DA denervation was moderate; l-dopa or SKF81297 did not induce pERK in Pitx3WT mice (Fig. 1, C and D). In Pitx3Null mice, pERK was induced only in the somata of striatonigral neurons (Fig. 1D2), not in the axon terminals in the SNr (not shown), probably because pERK induction needs biochemical components that are absent in the striatonigral axon terminals, even when the D1Rs are supersensitive. These results are consistent with the literature that severe DA loss supersensitizes D1Rs in the striatonigral neurons, manifested by l-dopa-induced expression of pERK in the somata of these neurons (Ding et al. 2011c; Gerfen et al. 2002; Kim et al. 2000). Taken together, these results confirm that our locally bred Pitx3Null mice have the expected PD-like DA loss pattern and are uniquely suitable for the experimental questions in this study.
Fig. 1.
The Parkinson's disease (PD)-like dopamine (DA) neuron loss pattern and the l-3,4-dihydroxyphenylalanine (l-dopa)-induced phosphorylated extracellular signal-regulated kinase (pERK) in the striatum in Pitx3Null mice. A: tyrosine hydroxylase (TH)-immunostained DA neurons in the substantia nigra pars compacta (SNc) and the ventral tegmental area (VTA). Note the dense DA dendritic network in the substantia nigra pars reticulate (SNr) in Pitx3WT (wild-type) mice (n = 3). B: TH-immunostained DA neurons in the SNc and VTA in Pitx3Null mice (n = 3). Note that the vast majority of the SNc DA neurons and DA dendrites are lost in Pitx3Null mice. A and B are 5-μm confocal optical sections obtained with a ×20 objective under an identical scanning condition. C, C1–C3: extremely dense DA axons in the entire striatum in Pitx3WT mice, but there was no detectable pERK signal. C1: TH. C2: pERK. C3: overlay. AC, anterior commissure; OT, olfactory tubercle. D, D1–D3: extremely severe (>95%) DA axon loss and a robust pERK signal in the dorsal striatum in Pitx3Null mice. D1: TH. D2: pERK. D3: overlay. The pERK signal was exclusively in striatonigral neurons (dual-labeling data not shown). The middle striatum has a significant amount of residual DA axons but no detectable pERK signal. The boxed areas are displayed at a higher magnification at the bottom to show DA axons and pERK-positive cells in Pitx3Null mice. For C and D, drug-naive mice were injected intraperitoneally with 10 mg/kg l-dopa and 5 mg/kg benserazide (3 Pitx3WT mice and 3 Pitx3Null mice). Thirty minutes later, these mice were perfused under deep urethane anesthesia, and brain sections were processed. Each image is a 3-μm confocal optical section obtained with a ×20 objective under an identical scanning condition.
Basic properties of dorsal striatum stimulation-evoked striatonigral IPSCs in normal and DA-deficient mice.
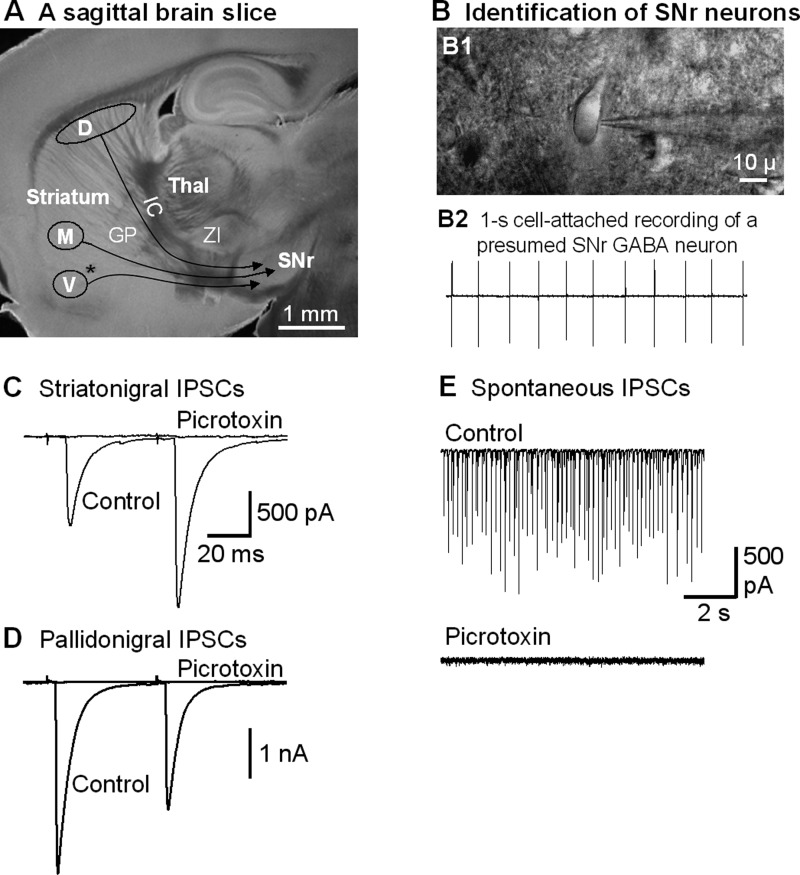
Since in Pitx3Null mice, the dorsal striatum is almost devoid of DA (>95% DA is lost) and the D1Rs there are supersensitive (Fig. 1D), we first focused on the dorsal striatum-evoked striatonigral IPSCs. The SNr and the subregions of the striatum are easily recognized in sagittal brain slices (Fig. 2A). The SNr GABA neurons were identified by their well-established electrophysiological properties: they fire spontaneously around 10 Hz under our in vitro conditions, while the sparse DA neurons fire spontaneously around 1.5 Hz (Atherton and Bevan 2005; Ding et al. 2011a, 2011b, 2013; Zhou et al. 2006; Zhou and Lee 2011). We first briefly recorded action potentials in a cell-attached mode. Neurons in the SNr with a spontaneous firing rate ≥5 Hz were presumed to be GABA neurons (Fig. 2B). After cell identification, we proceeded to whole cell recording mode and started striatal stimulation to activate striatonigral projections. As shown in Fig. 2C, focal stimulation in the dorsal striatum with two paired pulses with an interpulse interval of 50 ms evoked IPSCs in SNr GABA neurons with a latency of 7.8 ± 0.2 ms and a PPR of 2.1 ± 0.2, similar to a previous study in mice (Connelly et al. 2010). These evoked IPSCs and the spontaneous IPSCs in SNr GABA neurons were blocked by bath application of picrotoxin (100 μM) (Fig. 2, C and E), indicating that these IPSCs were mediated by GABAA receptors as expected.
Fig. 2.
Focal stimulation in the striatum evokes striatonigral inhibitory postsynaptic currents (IPSCs) in SNr GABA neurons. A: picture of a live, 15° angular sagittal brain slice taken with a ×1 objective. The SNr and the striatal subregions are clearly identifiable. D, the stimulating site in the dorsal striatum; M, the stimulating site in the lower middle striatum; V, the stimulating site in the ventral striatum. GP, globus pallidus; IC, internal capsule; Thal, thalamus; ZI, zona incerta. *AC. B, B1: picture of a live brain slice, taken under a ×60 objective, shows a typical SNr neuron being patched. B2: typical spontaneous spikes (action potentials, ∼10 Hz) in a presumed SNr GABA neuron recorded in cell-attached mode. C: two pulses with a 50-ms interval in the dorsal striatum stimulation evoked striatonigral IPSCs in an SNr neuron that were blocked by 100 μM picrotoxin. Note the typical paired pulse facilitation in this example. D: an example of typical, depressing pallidonigral IPSCs evoked by 2 paired pulses. E: spontaneous IPSCs recorded in the absence of tetrodotoxin in an SNr GABA neuron were blocked by 100 μM picrotoxin.
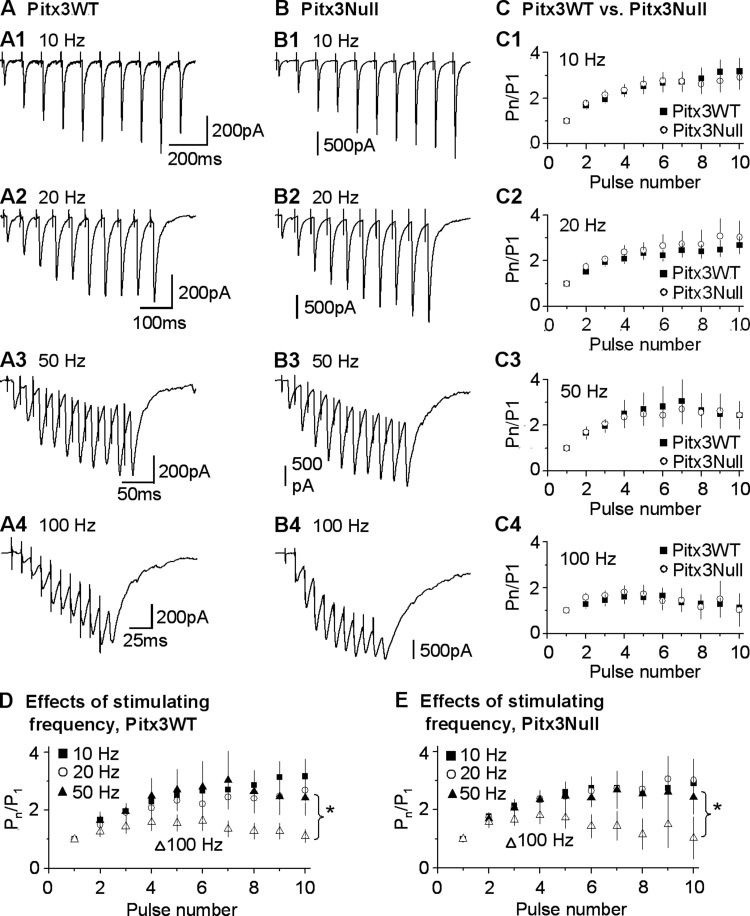
Since Pitx3Null mice have a perinatal onset DA neuron loss in the SNc, DA dendrite loss in the SNr and severe DA depletion in the dorsal striatum, we first asked the following question: does the perinatal onset nigral DA neuron loss affect the functional development of striatonigral GABAergic synapse? To answer this question, we compared the 10 stimuli-evoked striatonigral IPSC trains in Pitx3WT mice and Pitx3Null mice. We stimulated the dorsal striatum where the DA loss is severe, to evoke the striatonigral IPSCs using 10 pulses with intrapulse frequency of 10, 20, 50 and 100 Hz delivered every 30 s. As shown in Fig. 3, in both PitxWT mice and Pitx3Null mice, striatonigral IPSCs underwent significant synaptic facilitation at all these frequencies we studied [two-way ANOVA, F(9, 1100) = 9.88, P = 0.000], similar to a previous report (Connelly et al. 2010). In both types of mice, the synaptic facilitation of striatonigral IPSCs evoked at 100 Hz is significantly different from those evoked at the other frequencies (P = 0.000, 100 Hz vs. 10, 20 or 50 Hz, two-way ANOVA, post hoc Bonferroni test; Fig. 3, D and E), while there was no difference in the synaptic facilitation of striatonigral IPSCs evoked at 10, 20 and 50 Hz (P = 0.28, 10 Hz vs. 20 Hz; P = 0.42, 10 Hz vs. 50 Hz; P = 0.80, 20 Hz vs. 50 Hz, two-way ANOVA, post hoc Bonferroni test; Fig. 3, A–C), fully consistent with a previous report (Connelly et al. 2010). More important to our question, the synaptic facilitation of the striatonigral IPSCs was similar in Pitx3WT mice and Pitx3Null mice [two-way ANOVA, F(1,1100)=0.372, P = 0.542] (Fig. 3, C1–C4). These results indicate that the baseline properties of the dorsal striatum-evoked striatonigral GABAergic synapse in Pitx3Null mice are not affected by the perinatal onset DA neuron loss, consistent with Zhou and Palmiter (1995), that even a complete and perinatal onset DA loss does not affect the development of the basal ganglia. Consequently, the potential functional differences during dopaminergic stimulation are the results of different DA receptor functionalities in the DA-deficient Pitx3Null mice.
Fig. 3.
Similar baseline properties of the striatonigral IPSCs in Pitx3WT and Pitx3Null mice. A1–A4: example traces of striatonigral IPSCs evoked by dorsal striatum stimulation by a train of 10 pulses at 10 Hz, 20 Hz, 50 Hz and 100 Hz, respectively, in an SNr GABA neuron in a Pitx3WT mouse. B1–B4: example traces of striatonigral IPSCs evoked by focal dorsal striatum stimulation by a train of 10 pulses at 10 Hz, 20 Hz, 50 Hz and 100 Hz, respectively, in an SNr GABA neuron in a Pitx3Null mouse. C1–C4: pooled data showing similar ratios of peak currents to the first peak current (Pn/P1) evoked by focal dorsal striatum stimulation by a train of 10 pulses at 10 Hz, 20 Hz, 50 Hz and 100 Hz in Pitx3WT and Pitx3Null mice. D: pooled data showing activity-dependent facilitation of striatonigral IPSCs in Pitx3WT mice. E: pooled data showing activity-dependent facilitation of striatonigral IPSCs in Pitx3Null mice. *P < 0.01.
Enhanced D1R agonism facilitation of dorsal striatum-evoked striatonigral IPSCs in DA-deficient Pitx3Null mice.
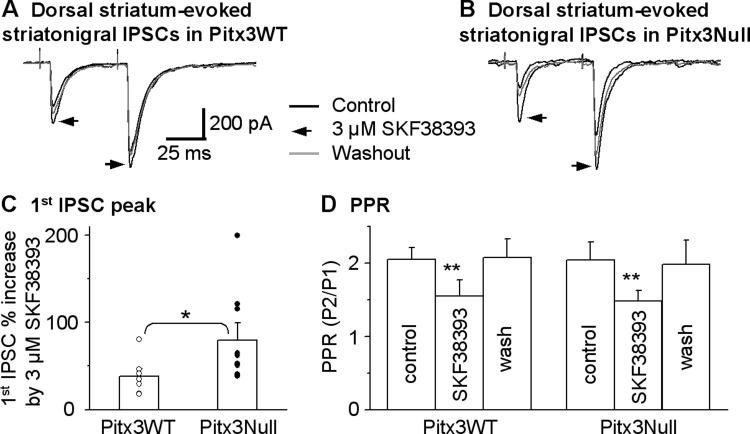
In DA-intact normal animals, previous electrophysiological studies have shown that D1R agonism facilitated the striatonigral IPSCs (Chuhma et al. 2011; Radnikow and Misgeld 1998). Based on the fact that DA receptors commonly become supersensitive following a severe DA loss, we hypothesized that D1Rs at the striatonigral terminal become functionally upregulated in DA-deficient Pitx3Null mice. To test this hypothesis, we initially examined the effects of 3 μM SKF38393, a partial D1R agonist, on the dorsal striatum-evoked striatonigral IPSCs in Pitx3WT and Pitx3Null mice. We used SKF38393 because 3 μM SKF38393 was used in a previous study on the striatonigral IPSCs in normal animals (Radnikow and Misgeld 1998). As shown in Fig. 4, A and C, 3 μM SKF38393 significantly facilitated the dorsal striatum-evoked striatonigral IPSC peak amplitude by 38.5 ± 8.0% (from 434.6 ± 53.3 pA under control to 606.5 ± 88.6 pA under SKF38393) in Pitx3WT mice (paired t-test, P = 0.0143, n = 7 cells). In DA-deficient Pitx3Null mice, 3 μM SKF38393 facilitated the dorsal striatum-evoked striatonigral IPSC peak amplitude by 79.9 ± 16.1% (from 331.5 ± 62.3 pA under control to 639.2 ± 171.8 pA under SKF83393, n = 10 cells, paired t-test, P = 0.02). When normalized to control IPSC amplitude, this SKF83393-induced facilitation was significantly larger in Pitx3Null mice than in Pitx3WT mice (unpaired t-test, P < 0.05). These results indicate that D1R-mediated enhancement of the striatonigral IPSCs became stronger after DA loss.
Fig. 4.
D1 receptor (D1R) agonist SKF38393 facilitates dorsal striatum-evoked striatonigral IPSCs more strongly in DA-deficient Pitx3Null mice than in DA-intact Pitx3WT mice. A and B: averaged traces shown before, during and after bath application of 3 μM SKF38393 in Pitx3WT mice and Pitx3Null mice, respectively. C: pooled data showing 3 μM SKF38393 had a stronger facilitatory effect on striatonigral IPSCs in Pitx3Null mice than in Pitx3WT mice. *P < 0.05, unpaired t-test. D: pooled data showing 3 μM SKF38393 decreased paired pulse ratio [PPR; ratio of the second peak current over the first one (P2/P1)] in Pitx3WT and Pitx3Null mice. **P < 0.01, paired t-test. For the PPR during baseline and SKF38393 in Pitx3WT and Pitx3Null mice, P > 0.05, unpaired t-test.
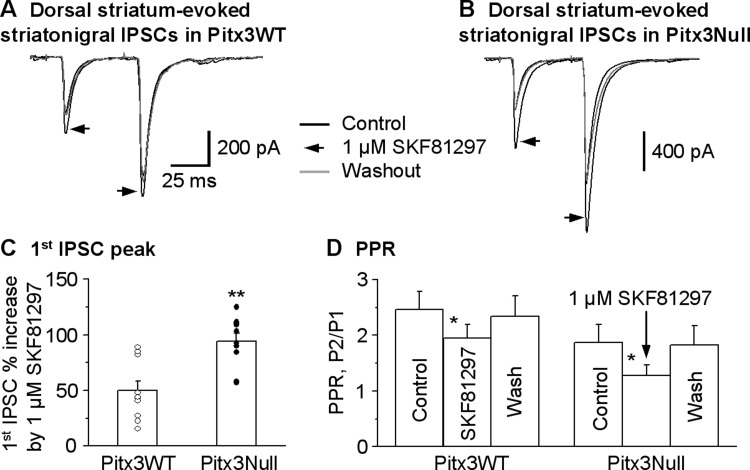
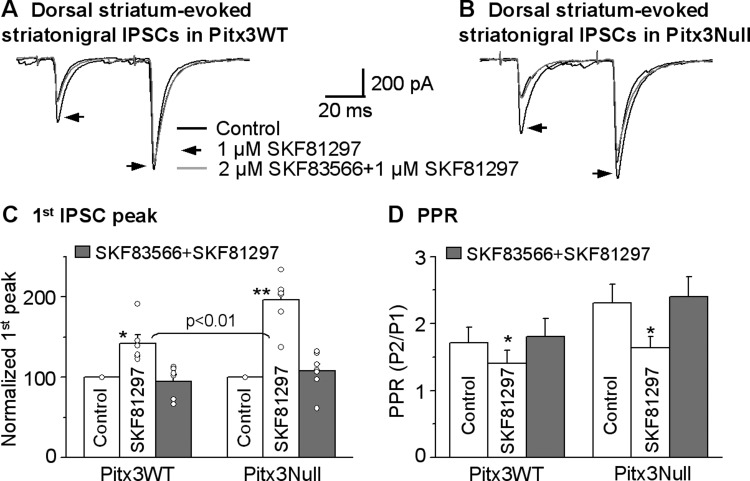
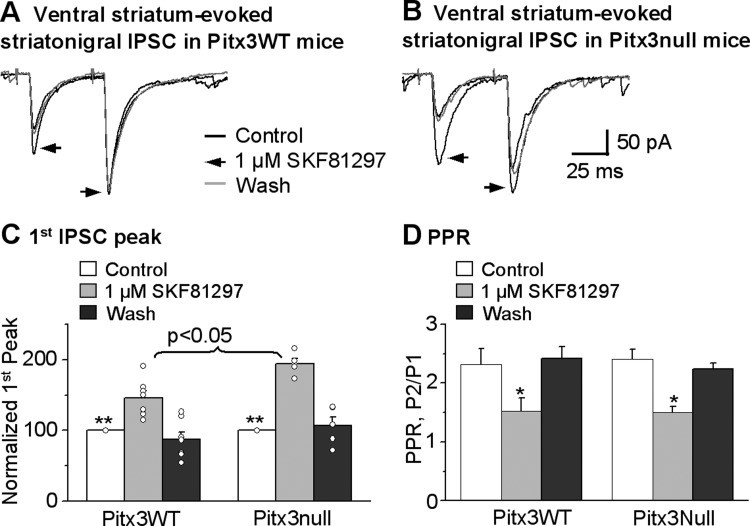
We then confirmed the results of SKF38393 by examining the effect of 1 μM SKF81297, a D1R full agonist, on the striatonigral IPSCs. As shown in Fig. 5, A–C, 1 μM SKF81297 facilitated the dorsal striatum-evoked striatonigral IPSC peak amplitude by 49.9 ± 8.4% (from 264.7 ± 37.0 pA under control to 381.2 ± 45.9 pA under SKF81297, paired t-test, P = 0.0001, n = 10 cells) in Pitx3WT mice; in Pitx3Null mice, this facilitation was 94.1 ± 7.2% (from 294.7 ± 43.3 pA under control to 574.8 ± 92.5 pA under SKF81297, paired t-test, P = 0.0002, n = 10 cells). When normalized to control IPSC amplitude, the SKF81297-induced facilitation was larger in Pitx3Null mice than in Pitx3WT mice (unpaired t-test, P < 0.01 for Pitx3WT vs. Pitx3Null, n = 10 cells for each genotype). The facilitatory effect of 1 μM SKF81297 recovered upon wash in both Pitx3WT and Pitx3Null mice. Furthermore, the facilitatory effect of 1 μM SKF81297 on the striatonigral IPSCs was blocked by 2 μM SKF83566, a D1R antagonist, in both Pitx3WT mice and Pitx3Null mice (Fig. 6).
Fig. 5.
D1R agonist SKF81297 facilitates dorsal striatum-evoked striatonigral IPSCs more strongly in DA-deficient Pitx3Null mice than in DA-intact Pitx3 WT mice. A and B: averaged traces shown before, during and after bath application of 1 μM SKF81297 in Pitx3WT mice and Pitx3Null mice, respectively. C: pooled data showing 1 μM SKF81297 had a stronger facilitatory effect on striatonigral IPSCs in Pitx3Null mice than in WT mice. **P < 0.01, unpaired t-test. D: pooled data showing 1 μM SKF81297 decreased PPR in Pitx3WT and Pitx3Null mice. *P < 0.05, paired t-test. For the PPR during baseline and SKF81297 in Pitx3WT and Pitx3Null mice, P > 0.05, unpaired t-test.
Fig. 6.
D1R antagonist SKF93566 blocks SKF81297’s effect on striatonigral IPSCs. A and B: averaged traces shown baseline, bath application of 1 μM SKF81297 and bath application of 1 μM SKF81297 + 2 μM SKF83566 in Pitx3WT mice and Pitx3Null mice, respectively. C: pooled data showing bath application of 2 μM SKF83566 blocked the facilitatory effect of 1 μM SKF81297 on striatonigral IPSCs in Pitx3WT and Pitx3Null mice. *P < 0.05, **P < 0.01, paired t-test. D: pooled data showing 1 μM SKF81297 decreased PPR in Pitx3WT and Pitx3Null mice, which were reversed by bath application of 1 μM SKF81297 + 2 μM SKF83566. *P < 0.05, unpaired t-test. For the PPR during baseline and drug application in Pitx3WT and Pitx3Null mice, P > 0.05, unpaired t-test.
Taken together, these results indicate that D1R agonism more strongly facilitates the dorsal striatum-evoked striatonigral IPSCs via D1Rs in the DA-deficient Pitx3Null mice than in normal DA-intact WT mice. This is fully consistent with the idea that DA loss in Pitx3Null mice leads to D1R supersensitization that in turn causes a stronger facilitation of striatonigral IPSCs. Our next question is: is this D1R-mediated facilitation a presynaptic effect on striatonigral axon terminals?
Evidence for a presynaptic origin of enhanced D1R facilitation of dorsal striatum-evoked striatonigral IPSCs in DA-deficient Pitx3Null mice.
Anatomical studies have established that D1Rs are strongly expressed in striatonigral axon terminals in the SNr (Yung et al. 1995). Previous studies have indicated that D1R agonism facilitated striatonigral IPSCs through a presynaptic mechanism (Chuhma et al. 2011; Radnikow and Misgeld 1998). Thus we reasoned that the activation of D1Rs at the striatonigral axon terminal mediates the D1 agonist facilitation of the striatonigral IPSCs in both Pitx3WT and Pitx3Null mice. Since a decrease or increase in the PPR indicates a presynaptic mechanism (Fioravante and Regehr 2011; Thomson 2000), we used a paired pulse stimulation protocol to answer our question. The interval between the two paired pulses was 50 ms. As shown in Figs. 4D, 5D and Fig. 6D, both 3 μM SKF38393 and 1 μM SKF81297 significantly decreased the PPR by enhancing the first IPSC more strongly than the second one in the IPSC pair (paired t-test, P < 0.05). These effects recovered upon washing out SKF38393 or SKF81297 and were also blocked by D1R antagonist SKF83566. These results indicate that D1R agonism facilitates the dorsal striatum-evoked striatonigral IPSCs through a presynaptic mechanism, consistent with the established mechanism that cAMP, a key signaling molecule produced by D1R activation, increases neurotransmitter release probability (Missale et al. 1998; Yao and Sakaba 2010).
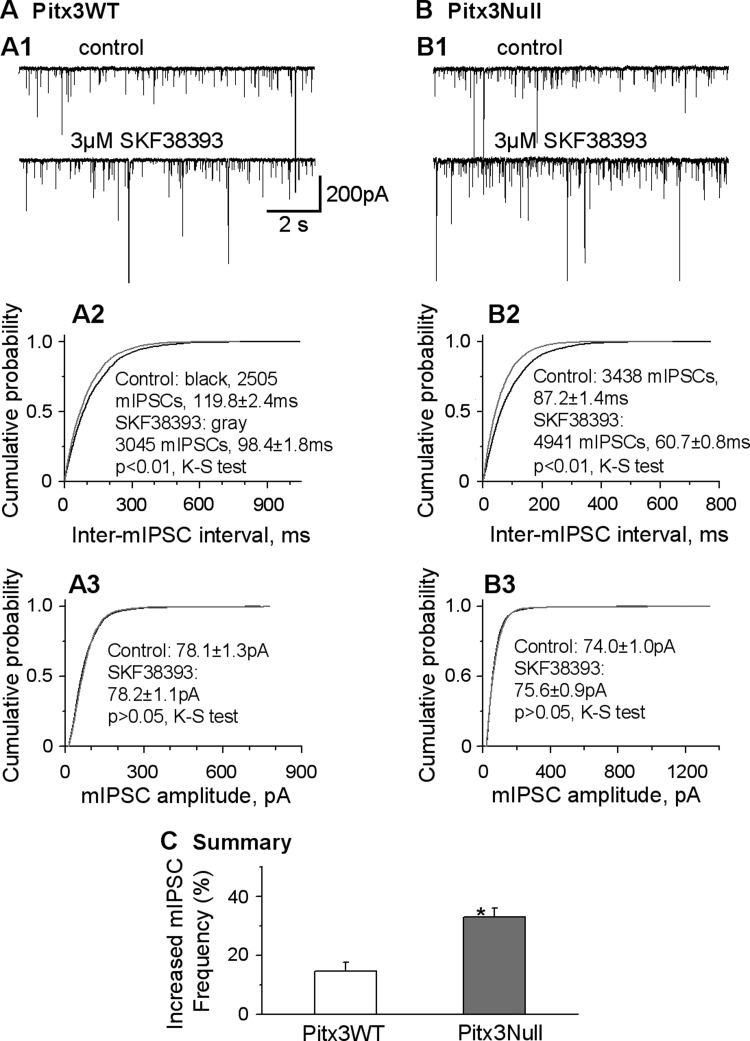
Next, we obtained further support for our conclusion by examining the effect of D1R agonist SKF38393 on the mIPSCs recorded in SNr GABA neurons in the presence of 1 μM tetrodotoxin, since changes of mIPSCs frequency reflect changes in neurotransmitter vesicle release probability at axon terminals. Although mIPSCs have striatonigral and other sources, D1R agonism predominantly affects striatonigral axons-originated mIPSCs because of the predominant, if not exclusive, D1R expression on these axons in the SNr. We found that the baseline frequency and amplitude of mIPSCs were similar in WT and Pitx3Null mice (13.2 ± 2.0 Hz and 12.1 ± 2.1 Hz, 72.5 ± 7.3 pA and 86.8 ± 8.9 pA in PitxWT and Pitx3Null mice, respectively, P > 0.05, n = 8 for both types of mice, unpaired t-test, Fig. 7). These data also indicate that perinatal onset nigral DA neuron loss does not affect the baseline GABA release properties at striatonigral axon terminals in Pitx3Null mice. In both types of mice, D1R agonist SKF38393 increased the frequency of mIPSCs in SNr GABA neurons without affecting the amplitudes (Fig. 7, A2, A3, B2, and B3). Specifically, in Pitx3WT mice, 3 μM SKF38393 significantly increased the mIPSC frequency in SNr GABA neurons by 14.5 ± 3.0% (from 13.2 ± 2.0 Hz during control to 15.1 ± 2.3 Hz during SKF38393, n = 8, paired t-test, P < 0.05), without affecting the averaged amplitudes of mIPSCs (from 72.5 ± 7.3 pA during control to 73.9 ± 8.8 pA during SKF38393, paired t-test, P > 0.05) (Fig. 7, A2, A3, and C). In Pitx3Null mice, 3 μM SKF38393 significantly increased the frequency of mIPSCs in SNr GABA neurons by 32.9 ± 3.1% in Pitx3Null mice (from 12.1 ± 2.1 Hz during control to 16.6 ± 2.8 Hz during SKF38393, n = 8, paired t-test, P < 0.05), without affecting the amplitudes of mIPSCs (from 86.8 ± 8.9 pA during control to 88.1 ± 9.3 pA during SKF38393, paired t-test, P > 0.05) (Fig. 7, B2, B3, and C). The 3 μM SKF38393-induced increase in mIPSC frequency was larger in Pitx3Null mice than in Pitx3WT mice (P = 0.0014, unpaired t-test) (Fig. 7C). These results clearly indicate that the D1R agonism facilitates GABA vesicular release from the striatonigral axon terminals, and this facilitation was stronger in the DA-deficient Pitx3Null mice.
Fig. 7.
D1R agonist SKF38393 increases the frequency of miniature IPSC (IPSC) to a larger extent in Pitx3Null mice than in Pitx3WT mice. A1 and B1: example traces showing mIPSCs in an SNr GABA neuron in Pitx3WT (A1) and Pitx3Null mice (B1) before (top) and during (bottom) bath application of 3 μM SKF38393. A2 and B2: cumulative probability plots of inter-mIPSC intervals in the SNr GABA neuron shown in A1 and B1. A3 and B3: cumulative probability plots of mIPSC amplitudes in the SNr GABA neuron shown in A1 and B1. Kolmogoroff-Smirnoff (K-S) tests were used to compare the frequency and amplitude of mIPSCs in 8 individual cells in both types of mice. C: pooled data showing 3 μM SKF38393 increased the frequency of mIPSC to a larger extent in Pitx3Null mice than in Pitx3WT mice. *P < 0.05.
To further rule out a possible postsynaptic mechanism, we puffed 1 mM GABA to SNr GABA neuron to induce GABA currents and examined the effect of 1 μM SKF81297. As shown in Fig. 8, bath application of 1 μM SKF81297 had no effect on the puffed GABA-induced currents in SNr GABA neurons in Pitx3WT mice and in Pixt3Null mice (paired t-test, control vs. SKF81297, P = 0.72 and P = 0.96, n = 5 cells each for Pitx3WT mice and Pitx3Null mice, respectively). This result excludes a postsynaptic mechanism for D1 agonism-induced facilitation of striatonigral IPSCs. Although we cannot rule out the possibility that whole cell patch-clamp recording may wash out intracellular signaling molecules and cause an apparent lack of postsynaptic effect, the likelihood of such a wash-out effect here is small, because we routinely record postsynaptic 5-HT2C receptor-mediated excitation and metabotropic glutamate responses in these neurons.
Fig. 8.
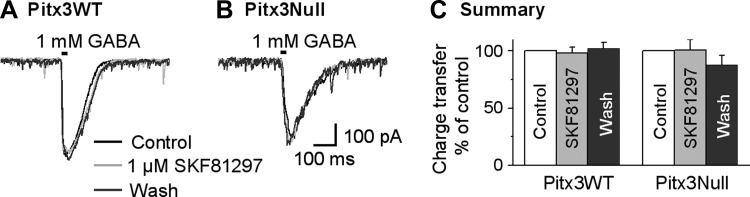
D1R agonist SKF81297 does not affect puffed GABA-induced GABA currents induced by locally puffed 1 mM GABA on SNr GABA neurons both in Pitx3WT and Pitx3Null mice. A and B: example averaged traces before, during and after bath application of 1 μM SKF81297 in Pitx3WT mice (A) and Pitx3Null mice (B). C: pooled data showing bath application of 1 μM SKF81297 had no effect on charge transfer induced by locally puffed 1 mM GABA on SNr GABA neurons both in Pitx3WT and Pitx3Null mice.
Taken together, experiments so far demonstrate that, in DA-deficient Pitx3Null mice, the function of presynaptic D1Rs is enhanced for facilitating GABA release from striatonigral axon terminals originated in the dorsal striatum where the D1Rs in striatonigral neuron somata are supersensitive, as indicated by l-dopa-induced pERK (Fig. 1D). We now turn to this question: are the D1Rs on the striatonigral axon terminals originated in the middle and ventral striatum also enhanced, although their somatic D1Rs are not supersensitive? The following experiments will answer this question.
Enhanced D1R facilitation of local SNr stimulation-evoked striatonigral IPSCs in DA-deficient Pitx3Null mice.
In this experiment, we will compare the D1R agonism's effect on the local SNr stimulation-evoked IPSC with the effect on the dorsal striatum-evoked striatonigral IPSC, because local SNr stimulation activates converging striatonigral axons originating from wide striatal subregions, including dorsal, middle and ventral striatum (Fujiyama et al. 2011; Gerfen 1985; Hedreen and DeLong 1991). Additionally, since D1R agonism may affect D1-medium spiny neuron (MSN) somata, we need to rule out the small possibility that the observed D1R agonism-induced facilitation of striatonigral IPSCs arises from potential somatic effects when the striatum was stimulated. To achieve these two goals, we cut off the striatum and then locally stimulated the SNr to evoke IPSCs in SNr GABA neurons. SNr GABA neurons receive both striatonigral projections and pallidonigral projections (Bolam et al. 1993; Smith and Bolam 1991; von Krosigk et al. 1992). Even though the striatonigral and pallidonigral axon terminals are impossible to separate from each other in the brain slice, their GABA release properties are distinct: the striatonigral IPSCs are facilitatory during repetitive stimulation (Fig. 3) and display a robust paired pulse facilitation, whereas the pallidonigral IPSCs are depressing during repetitive stimulation and display a paired pulse depression (Connelly et al. 2010). Thus striatonigral IPSCs and pallidonigral IPSCs can be distinguished based on the PPR.
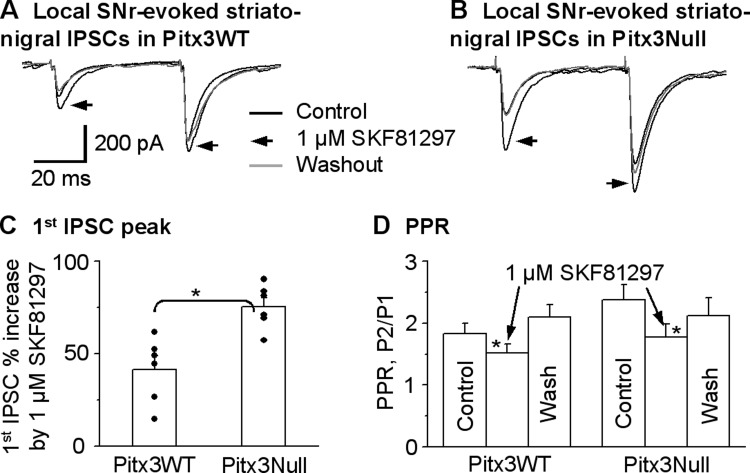
We stimulated the SNr using a saline-filled theta glass pipette with two paired pulses with an interpulse interval of 50 ms to evoke IPSCs in the SNr. By adjusting stimulating intensity and/or stimulating electrode position, we obtained locally evoked IPSCs with a PPR larger than 1.5 that were presumed to be from striatonigral axon terminals and were tested for the effects of D1R agonism. [Although not our focus, this result indicates that the distribution and/or excitability of striatonigral and pallidonigral axon terminals are not uniform in the SNr.] As shown in Fig. 9, 1 μM SKF81297 facilitated the locally evoked striatonigral IPSCs by 41.4 ± 7.2% (from 224.9 ± 22.7 pA under control to 314 ± 36.8 pA under SKF81297) in Pitx3WT mice (paired t-test, P < 0.005, n = 6 cells), accompanied by a decreased PPR from 1.8 ± 0.2 to 1.5 ± 0.1 (paired t-test, P < 0.05, n = 6 cells). These effects recovered upon washing out SKF81297. In Pitx3Null mice, 1 μM SKF81297 facilitated the locally evoked striatonigral IPSCs by 75.4 ± 4.8% (from 220.4 ± 22.8 pA under control to 385.5 ± 38.8 pA under SKF81297) in Pitx3Null mice (paired t-test, P < 0.01, n = 6 cells), accompanied by a decreased PPR from 2.4 ± 0.2 to 1.8 ± 0.2 (paired t-test, P < 0.05, n = 6 cells, Fig. 9). These effects also recovered upon washing out SKF81297. As shown in Fig. 9C, pooled data clearly indicate that 1 μM SKF81297 had a stronger facilitatory effect on the locally evoked striatonigral IPSCs in Pitx3Null mice than in Pitx3WT mice (P < 0.01, unpaired t-test). Furthermore, the D1R agonism-induced enhancement of local SNr stimulation-evoked IPSCs is similar to the D1R-induced enhancement of dorsal striatum-evoked IPSCs in both Pitx3WT (P = 0.56, one-way ANOVA) and Pitx3Null mice (P = 0.15, one-way ANOVA) (Table 1); the tendency for the enhancement to be weaker for the local SNr stimulation-evoked striatonigral IPSC than the dorsal striatum-evoked striatonigral IPSC may be due to contamination of nonstriatonigral components when local SNr stimulation was used. These data suggest that, in Pitx3Null mice, the enhancement of presynaptic D1R function is not dependent on the striatonigral axons' striatal origins that have different degrees of DA loss. Instead, the DA loss in the SNr may be the key factor triggering the functional upregulation of the D1Rs on these striatonigral axon terminals.
Fig. 9.
D1R agonist SKF81297 facilitates the striatonigral IPSCs evoked by local SNr stimulation to a larger extent in Pitx3Null mice, compared with WT mice. A and B: averaged traces shown before, during and after bath application of 1 μM SKF81297 in Pitx3WT mice and Pitx3Null mice, respectively. C: pooled data showing 1 μM SKF81297 had a stronger facilitatory effect on striatonigral IPSCs evoked by locally stimulating SNr in Pitx3Null mice than in Pitx3WT mice. *P < 0.05, unpaired t-test. D: pooled data showing 1 μM SKF81297 decreased PPR in Pitx3WT and Pitx3Null mice. *P < 0.05, paired t-test. For the PPR during baseline and SKF81297 in Pitx3WT and Pitx3Null mice, P > 0.05, unpaired t-test.
Table 1.
Similar D1 agonism-induced enhancement of dorsal striatum-evoked, middle striatum-evoked, and local SNr-evoked striatonigral IPSCs
| Mice | Bath-applied D1R Agonist | Dorsal Striatum-evoked Striatonigral IPSC (C), %increase | Middle Striatum-evoked Striatonigral IPSC (D), % increase | Ventral Striatum-evoked Striatonigral IPSC (E), % increase | Local SNr-evoked Striatonigral IPSC (F), % increase |
|---|---|---|---|---|---|
| Pitx3WT (A) | SKF81297 (1 μM) | 49.9 ± 8.4* | 43.3 ± 5.3 | 46.5 ± 10.0 | 41.4 ± 7.2 |
| Pitx3Null (B) | SKF81297 (1 μM) | 94.1 ± 7.2†‡ | 95.3 ± 14.2‡ | 94.7 ± 6.8‡ | 75.4 ± 4.8‡ |
WT, wild type; D1R, D1 receptor; IPSC, inhibitory postsynaptic current; SNr, substantia nigra pars reticulata.
C vs. D vs. E vs. F: P > 0.5;
C vs. D vs. E vs. F: P > 0.1;
A vs. B: P < 0.01: one-way ANOVA followed by post hoc least significant difference test.
Enhanced presynaptic D1R facilitation of striatonigral IPSCs originated in the middle striatum with non-supersensitive somatic D1Rs.
In the striatonigral neurons in the dorsal striatum, the supersensitization of somatic D1Rs may trigger the supersensitization of the D1Rs in the striatonigral axon terminals. However, since the vast majority of the DA dendrites, the main source of SNr DA, is lost in the SNr in Pitx3Null mice (Fig. 1), it is also possible that the DA loss in the SNr is sufficient to supersensitize the D1Rs on striatonigral axon terminals. The selective loss of nigral DA neurons and the gradient DA loss in the striatum in Pitx3Null mice offer an excellent opportunity to examine these two possibilities. Furthermore, as shown in Fig. 1, it is established that D1Rs in the dorsal striatum in Pitx3Null mice are supersensitive as indicated by l-dopa-induced pERK expression; in contrast, l-dopa also did not induce detectable pERK in middle or ventral striatum in Pitx3Null mice or in any striatal subregion in Pitx3WT mice, consistent with a previous study in Pitx3Null mice (Ding et al. 2011c). Thus we reasoned that, in Pitx3Null mice, if the increased functionality or supersensitivity of D1Rs on dorsal striatum-originated striatonigral axon terminals is dependent on the supersensitization of somatic D1Rs, then those D1Rs on middle striatum-originated striatonigral axon terminals should be non-supersensitive because the somatic D1Rs in the middle striatum are non-supersensitive. On the other hand, if the upregulation of presynaptic D1Rs is not dependent on the supersensitization of somatic D1Rs, the D1Rs on middle striatum-originated striatonigral axon terminals should be supersensitive just like the D1Rs on dorsal striatum-originated striatonigral axon terminals, because these axon terminals are in the same DA-depleted SNr.
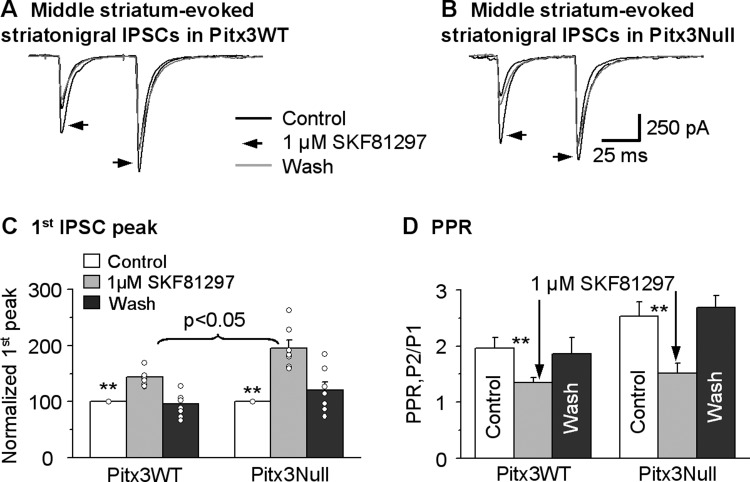
To test these possibilities, we placed the stimulating electrode in the lower middle striatum (Fig. 2A) to activate the striatonigral neurons with non-supersensitive somatic D1Rs. The lower middle striatum was chosen to minimize the activation of passing axons from the dorsal striatum. As shown in Fig. 10, we found that the middle striatum-evoked striatonigral IPSCs were facilitating in the paired pulse protocol, identical to those of dorsal striatum-evoked striatonigral IPSCs. Furthermore, bath application of 1 μM SKF81297 significantly increased the peak current of striatonigral IPSC by 43.3 ± 5.3% (from 195.1 ± 28.3 pA under control to 286.7 ± 47.8 pA under SKF81297, P < 0.01, n = 9 cells, paired t-test) in Pitx3WT mice and 95.3 ± 14.2% (from 140.9 ± 26.5 pA under control to 267.9 ± 45.4 pA under SKF81297, P < 0.01, n = 7 cells, paired t-test) in Pitx3Null mice (Fig. 10, A–C). This facilitatory effect was stronger in Pitx3Null mice than in Pitx3WT mice (P < 0.05, unpaired t-test). In these same cells, 1 μM SKF81297 also significantly decreased the PPR from 1.9 ± 0.1 during control to 1.3 ± 0.1 during SKF81297 in Pitx3WT mice and from 2.5 ± 0.3 during control to 1.5 ± 0.2 during SKF81297 in Pitx3Null mice (Fig. 10D), indicating a presynaptic mechanism. These results indicated that D1Rs on middle striatum-originated striatonigral axon terminals also had upregulated function in Pitx3Null mice, compared with WT mice. More important, the D1R agonism-induced enhancement of the middle striatum-evoked IPSCs was similar to the D1R-induced enhancement of the dorsal striatum-evoked IPSCs in both Pitx3WT mice (P = 0.61, one-way ANOVA) and Pitx3Null mice (P = 0.64, one-way ANOVA) (Table 1), indicating that the shared severe DA loss in the SNr, not the varying DA loss in striatal subregions, is the key factor that upregulates or supersensitizes the D1Rs on striatonigral axon terminals.
Fig. 10.
D1R agonist SKF81297 facilitates the middle striatum-evoked striatonigral IPSCs more strongly in Pitx3Null mice than in Pitx3WT mice. A and B: averaged traces of the middle striatum-evoked striatonigral IPSCs before, during and after bath application of 1 μM SKF81297 in Pitx3WT mice and Pitx3Null mice, respectively. C: pooled data showing 1 μM SKF81297 had a stronger facilitatory effect on the middle striatum-evoked striatonigral IPSCs in Pitx3Null mice (n = 9 cells) than in Pitx3WT mice (n = 7 cells). **P < 0.01, paired t-test. D: pooled data showing 1 μM SKF81297 decreased PPR in Pitx3WT and Pitx3Null mice. **P < 0.01, paired t-test. For the PPR during baseline and SKF81297 in Pitx3WT and Pitx3Null mice, P > 0.05, unpaired t-test.
Enhanced presynaptic D1R facilitation of striatonigral IPSCs originated in the ventral striatum with non-supersensitive somatic D1Rs.
Finally, we tested presynaptic D1R facilitation of striatonigral IPSCs originated in the ventral striatum where there was no supersensitive somatic D1Rs by placing the stimulating electrode in the ventral striatum, as illustrated in Fig. 2A. We used the anterior commissure as the landmark. We found that the ventral striatum-evoked striatonigral IPSCs were facilitating in the paired pulse protocol, identical to those of dorsal and middle striatum-evoked striatonigral IPSCs (Fig. 11). Furthermore, bath application of 1 μM SKF81297 significantly increased the peak current of striatonigral IPSC by 46.5 ± 10.0% (from 105.8 ± 33.2 pA under control to 145.1 ± 41.3 pA under SKF81297, P < 0.005, n = 7 cells, paired t-test) in Pitx3WT mice and 94.7 ± 6.8% (from 102.9 ± 44.5 pA under control to 205.7 ± 87.6 pA under SKF81297, P < 0.005, n = 5 cells, paired t-test) in Pitx3Null mice (Fig. 11, A–C). This facilitatory effect was stronger in Pitx3Null mice than in Pitx3WT mice (P < 0.005, unpaired t-test). In these same cells, 1 μM SKF81297 also significantly decreased the PPR from 2.3 ± 0.3 during control to 1.5 ± 0.2 during SKF81297 in Pitx3WT mice (n = 7 cells, P < 0.05, paired t-test) and from 2.4 ± 0.2 during control to 1.5 ± 0.1 during SKF81297 in Pitx3Null mice (n = 5 cells, P < 0.05, paired t-test) (Fig. 11D), indicating a presynaptic mechanism. These results indicated that D1Rs on ventral striatum-originated striatonigral axon terminals also had upregulated function in Pitx3Null mice, compared with WT mice. More important, the D1R agonism-induced enhancement of the ventral striatum-evoked IPSCs is similar to the D1R-induced enhancement of the dorsal striatum-evoked IPSCs in both Pitx3WT mice (P = 0.61, one-way ANOVA) and Pitx3Null mice (P = 0.64, one-way ANOVA) (Table 1), further supporting our hypothesis that the shared severe DA loss in the SNr, not the varying DA loss in striatal subregions, is the key factor that upregulates or supersensitizes the D1Rs on striatonigral axon terminals. We also need to note here that the amplitude of ventral striatum-evoked striatonigral IPSCs is smaller than these of dorsal and middle striatum-evoked striatonigral IPSCs. Although the absolute amplitudes of the IPSCs originated in different striatal areas difficult to compare in brain slices because of the potential variable preservation of the projecting fibers, one intrinsic factor for this difference may be that the projection from the ventral striatum to the SNr is less strong than the projection from the dorsal and middle striatum (Lynd-Balta and Haber 1994).
Fig. 11.
D1R agonist SKF81297 facilitates the ventral striatum-evoked striatonigral IPSCs more strongly in Pitx3Null mice than in Pitx3WT mice. A and B: averaged traces of the ventral striatum-evoked striatonigral IPSCs before, during and after bath application of 1 μM SKF81297 in Pitx3WT mice and Pitx3Null mice, respectively. C: pooled data showing 1 μM SKF81297 had a stronger facilitatory effect on the middle striatum-evoked striatonigral IPSCs in Pitx3Null mice (n = 7 cells) than in Pitx3WT mice (n = 5 cells). **P < 0.005, paired t-test. D: pooled data showing 1 μM SKF81297 decreased PPR in the same 7 cells in Pitx3WT and 5 cells in Pitx3Null mice. *P < 0.05, paired t-test. For the PPR during baseline and SKF81297 in Pitx3WT and Pitx3Null mice, P > 0.05, unpaired t-test.
DISCUSSION
Our main findings are as follows. 1) The presynaptic D1R facilitation of the motor-promoting striatonigral GABA output is upregulated following the loss of the nigrostriatal DA projection. 2) This upregulation is global: it occurs equally in both the dorsal striatum-originated striatonigral projection with supersensitive somatic D1Rs and the middle and ventral striatum-originated striatonigral projection with non-supersensitive somatic D1Rs. These findings indicate that loss of DA in the SNr is sufficient to upregulate the function of these presynaptic D1Rs intensely expressed in the striatonigral axon terminals.
We need to note here that, despite their perinatal onset of DA loss, the cellular mechanisms of DA supersensitivity in Pitx3Null mice are relevant to the pathophysiology of PD that occurs commonly in old people (Hornykiewicz 1998, 2001). This is because ample evidence shows unambiguously that DA loss leads to DA receptor supersensitivity, regardless of the induction method (toxin or genetic inactivation, progressive or nonprogressive) of DA loss and its timing (perinatal or adult onset) (Corvol et al. 2004; Ekstrand et al. 2007; Gerfen et al. 2002; Kim et al. 2000; Kostrzewa 1995). In fact, Pitx3Null mice are advantageous for studying the consequences of DA loss because their DA loss is premade with no lesion surgery required and consistent among different mice. Also the DA loss pattern in Pitx3Null is PD-like with the nigral DA neuron loss and the DA loss in the dorsal striatum being severe, while a significant number of VTA DA neurons and a considerable residual DA innervation in the ventral striatum remain (Li et al. 2013; van den Munckhof et al. 2003). Since some DA axons from VTA DA neurons also innervate the middle striatum (Haber et al. 2000), the middle striatum retains residual DA axons at 10–30% of the normal level.
Enhanced presynaptic D1R-mediated facilitation of striatonigral IPSCs in DA-deficient Pitx3Null mice.
Previous electrophysiological studies have shown that activation of D1Rs on striatonigral axon terminals facilitates the striatonigral GABAergic transmission in normal animals (Chuhma et al. 2011; de Jesús Aceves et al. 2011; Radnikow and Misgeld 1998). Neurochemical studies have indicated that D1Rs on the striatonigral axon terminals facilitated KCl-evoked GABA release in SNr tissue slices from unilateral 6-hydroxydopamine (6-OHDA)-lesioned rats more strongly than in normal rats (Rangel-Barajas et al. 2008, 2011), although KCl also evoked GABA release from all GABA processes in the SNr, including those of nonstriatal origin. Thus electrophysiological experiments in DA-deficient animals that can identify the striatonigral input were required. Our present study performed these electrophysiological experiments.
In this study, we found that D1R agonists facilitated striatonigral IPSCs in both Pitx3WT and Pitx3Null mice, and this effect was blocked by D1 antagonist SKF83566, indicating a D1R-mediated mechanism, in agreement with previous studies (Chuhma et al. 2011; de Jesús Aceves et al. 2011; Radnikow and Misgeld 1998). Furthermore, D1 agonism decreased the PPR in a paired pulse protocol, increased the frequency of mIPSCs without affecting their amplitude and did not affect puffed-GABA-induced GABA currents in SNr GABA neurons, strongly indicating a presynaptic mechanism that is consistent with the fact that D1Rs are intensely expressed on the striatonigral axon terminals (Levey et al. 1992; Yung et al. 1995).
More importantly, D1R agonist SKF38393 and SKF81297 facilitated striatonigral IPSCs to a substantially larger extent in DA-deficient Pitx3Null mice than in DA-intact Pitx3WT mice. The facilitatory effects of D1R agonist were blocked by a D1R antagonist. These results demonstrated that D1R-mediated presynaptic facilitation became stronger after severe DA depletion, consistent with the established fact of D1R supersensitization after DA loss (Aubert et al. 2005; Cai et al. 2002; Corvol et al. 2004; Guigoni et al. 2007). Our present electrophysiological data obtained in Pitx3Null mice are also consistent with previous neurochemical studies in unilaterally 6-OHDA-lesioned rats (Rangel-Barajas et al. 2008, 2011).
DA loss in the SNr is sufficient to upregulate the presynaptic D1Rs on striatonigral axon terminals.
The main goal of our present study is to answer this question: is the upregulation of the presynaptic D1Rs in the striatonigral axon terminals primarily induced by the DA loss in the SNr or in the striatum? Previous studies have not addressed this question, at least partly because unilateral DA lesion often destroys both the DA innervation in the entire striatum and DA dendrites in the SNr, such that no comparative study can be performed.
Taking advantage of the consistent PD-like DA loss pattern in Pitx3Null mice, our present study has investigated this question. In Pitx3Null mice, most nigral DA neurons are lost such that the vast majority of the DA dendrites in the SNr and the vast majority of DA axons in the dorsal striatum are lost; in contrast, a significant number of VTA DA neurons and thus a significant residual DA innervation in the ventral striatum remains (Nunes et al. 2003; van den Munckhof et al. 2003). Since some DA axons from VTA DA neurons also innervate the middle striatum (Haber et al. 2000), the middle striatum in Pitx3Null mice also retain a significant number of residual DA axons (Li and Zhou 2013; Wei et al. 2013). Consequently, the D1Rs in striatonigral neurons in the dorsal striatum are supersensitive, whereas the D1Rs in striatonigral neurons in the middle and ventral striatum are not, as indicated by the presence or absence of l-dopa-induced pERK (Fig. 1D), a hallmark of supersensitive D1Rs in the striatum (Ding et al. 2011c; Gerfen et al. 2002; Santini et al. 2009). Thus Pitx3Null mice are an excellent animal model for our question.
We found that the presynaptic D1R-mediated facilitation was similarly enhanced or supersensitive for the striatonigral GABA output originated in the dorsal striatum where DA loss is >95% and the somatic D1Rs in D1-MSNs are supersensitive, for the striatonigral GABA output originated in the middle striatum where DA loss is <90% and the somatic D1Rs in D1-MSNs are not supersensitive, and for the striatonigral GABA output originated in the ventral striatum where DA loss is <70% and the somatic D1Rs in D1-MSNs are also not supersensitive. As diagramed in Fig. 2A, anatomical studies have established that D1-MSNs in the dorsal striatum generally take the dorsal route to project to the SNr, while the D1-MSNs in middle and ventral striatum generally take the middle and ventral route to project to the SNr (Fujiyama et al. 2011; Lévesque and Parent 2005). Therefore, stimulating in the dorsal, middle and ventral striatum activates the MSNs in the dorsal, middle and ventral striatum, respectively. Thus the upregulation of these presynaptic D1Rs in striatonigral axon terminals is not determined by the varying degrees of DA loss in different striatal subregions.
These results are further supported by another line of evidence: local SNr stimulation-evoked, physiologically identified striatonigral IPSCs (based on their facilitatory PPR) were enhanced to an extent similar to dorsal striatum-evoked striatonigral IPSCs. Local SNr-stimulation likely activated striatonigral axons originated in the dorsal striatum with supersensitive somatic D1Rs, the middle striatum with non-supersensitive somatic D1Rs and even ventral striatum also with non-supersensitive D1Rs, because each SNr locus receives converging inputs from wide striatal subregions, including dorsal, middle and ventral areas (Gerfen 1985; Hedreen and DeLong 1991). Taken together, our results strongly indicate that, in Pitx3Null mice with a PD-like DA loss pattern, the increased function of D1Rs on striatonigral axon terminals is determined not by the DA loss in the striatal subregions, but by the DA loss in the SNr, the common target area of the afferents from dorsal and middle striatal subregions. This conclusion may apply to PD because the normal SNr DA content is only about 10% of that in the striatum, and 81% of the DA in the SNr is lost in late stages of PD, such that the residual SNr DA level is only about 2% of the normal striatal DA level (Hornykiewicz 2001). Thus the DA deficiency in the SNr in PD is likely to be severe, creating a fertile condition to supersensitize the D1Rs on the striatonigral axon terminals, regardless of the varying degrees of DA loss and the functional status of the somatic D1Rs in the striatum.
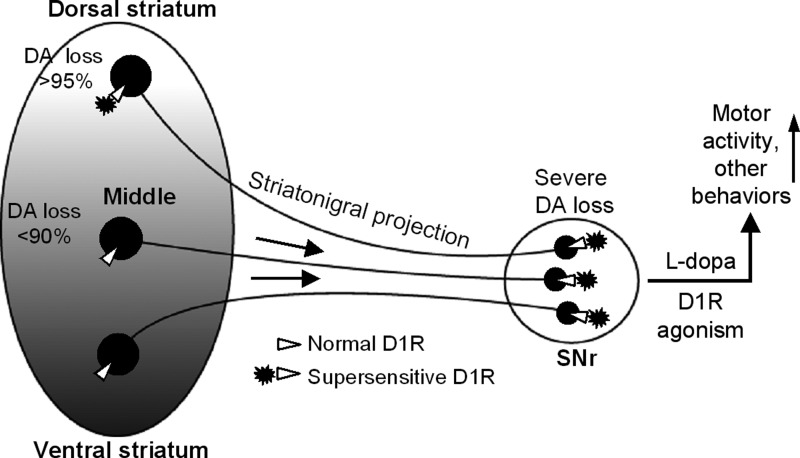
The relative independence of the striatonigral axon terminal from its soma is entirely possible, because published paired recording data (Tecuapetla et al. 2007) and our unpublished paired recording data show that the striatonigral neuron axon collateral-originated intrastriatal IPSCs are strongly depressing, while the striatonigral IPSCs are strongly facilitatory, i.e., the axon collateral behaves differently from its projection axon. Consequently, the target where these axon terminals reside may determine the function of the axon terminal. Furthermore, the dense DA dendrites in normal animals release DA, providing a key source of DA for the striatonigral axon terminals (Patel et al. 2009; Rice et al. 2011). Thus a severe loss of DA dendrites in the SNr may lead to a severe DA loss that in turn may upregulate or supersensitize the D1Rs on the striatonigral axon terminals, even when the striatal source area of these axon terminals still retains a residual DA innervation and have normal D1Rs, leading to a global enhancement of striatonigral output (Fig. 12).
Fig. 12.
Diagram summarizing our main finding that nigral DA depletion leads to a global upregulation of D1Rs at striatonigral axon terminals, regardless of the varying degrees of DA loss and the functional status of somatic D1Rs in the striatum.
Functional implications.
Because the striatonigral GABA output is motor-promoting, our finding that nigral DA loss can cause a global upregulation of D1Rs intensely expressed at the striatonigral axon terminals (Fig. 12) has important implications for PD pathophysiology, treatment and drug side effects (Fig. 12). First, the globally upregulated presynaptic D1R facilitation of GABA release may compensate for the lost nigral DA and lower striatonigral activity in PD, contributing to the maintenance of the residual motor function. Second, it can boost the motor-promoting striatonigral GABA output during the treatment of l-dopa or D1R agonists, contributing to their profound therapeutic motor-stimulating effect in DA-deficient animals and PD patients (Li and Zhou 2013; Mailman et al. 2001; Nutt et al. 2010; Robertson and Robertson 1989). Third, the global upregulation of the presynaptic D1Rs and hence the striatonigral GABA output may induce a too broad and strong inhibition of the SNr GABA projection neurons, thus degrading the normal selectivity of striatal inhibition of these basal ganglia output neurons and consequently contributing to dyskinesias and other behavioral side effects of l-dopa and D1 agonists (Li and Zhou 2013; Nutt et al. 2010; Voon et al. 2009).
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01-NS-058850 (F.-M. Zhou).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.D. and F.-M.Z. conception and design of research; S.D., L.L., and F.-M.Z. performed experiments; S.D., L.L., and F.-M.Z. analyzed data; S.D. and F.-M.Z. interpreted results of experiments; S.D. and F.-M.Z. prepared figures; S.D. and F.-M.Z. drafted manuscript; S.D. and F.-M.Z. edited and revised manuscript; S.D., L.L., and F.-M.Z. approved final version of manuscript.
REFERENCES
- Atherton JF, Bevan MD. Ionic mechanisms underlying autonomous action potential generation in the somata and dendrites of GABAergic substantia nigra pars reticulata neurons in vitro. J Neurosci 25: 8272–8281, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert I, Guigoni C, Håkansson K, Li Q, Dovero S, Barthe N, Bioulac BH, Gross CE, Fisone G, Bloch B, Bezard E. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol 57: 17–26, 2005. [DOI] [PubMed] [Google Scholar]
- Beeler JA, Cao ZF, Kheirbek MA, Zhuang X. Loss of cocaine locomotor response in Pitx3-deficient mice lacking a nigrostriatal pathway. Neuropsychopharmacology 34: 1149–1161, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurrier C, Ben-Ari Y, Hammond C. Preservation of the direct and indirect pathways in an in vitro preparation of the mouse basal ganglia. Neuroscience 40: 77–86, 2006. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Smith Y, Ingham CA, von Krosigk M, Smith AD. Convergence of synaptic terminals from the striatum and the globus pallidus onto single neurones in the substantia nigra and the entopeduncular nucleus. Prog Brain Res 99: 73–88, 1993. [DOI] [PubMed] [Google Scholar]
- Cai G, Wang HY, Friedman E. Increased dopamine receptor signaling and dopamine receptor-G protein coupling in denervated striatum. J Pharmacol Exp Ther 302: 1105–1112, 2002. [DOI] [PubMed] [Google Scholar]
- Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature 289: 537–542, 1981. [DOI] [PubMed] [Google Scholar]
- Chuhma N, Tanaka KF, Hen R, Rayport S. Functional connectome of the striatal medium spiny neuron. J Neurosci 31: 1183–1192, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly WM, Schulz JM, Lees G, Reynolds JN. Differential short-term plasticity at convergent inhibitory synapses to the substantia nigra pars reticulata. J Neurosci 30: 14854–14861, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol JC, Muriel MP, Valjent E, Féger J, Hanoun N, Girault JA, Hirsch EC, Hervé D. Persistent increase in olfactory type G-protein alpha subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson disease. J Neurosci 24: 7007–7014, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesús Aceves J, Rueda-Orozco PE, Hernández R, Plata V, Ibañez-Sandoval O, Galarraga E, Bargas J. Dopaminergic presynaptic modulation of nigral afferents: its role in the generation of recurrent bursting in substantia nigra pars reticulata neurons. Front Syst Neurosci 5: 6, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Li L, Zhou FM. Presynaptic serotonergic gating of the subthalamonigral glutamatergic projection. J Neurosci 33: 4875–4885, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Matta SG, Zhou FM. Kv3-like potassium channels are required for sustained high-frequency firing in basal ganglia output neurons. J Neurophysiol 105: 554–570, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Wei W, Zhou FM. Molecular and functional differences in voltage-activated sodium currents between GABA projection neurons and dopamine neurons in the substantia nigra. J Neurophysiol 106: 3019–3034, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Restrepo J, Won L, Hwang DY, Kim KS, Kang UJ. Chronic 3,4-dihydroxyphenylalanine treatment induces dyskinesia in aphakia mice, a novel genetic model of Parkinson's disease. Neurobiol Dis 27: 11–23, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Won L, Britt JP, Lim SA, McGehee DS, Kang UJ. Enhanced striatal cholinergic neuronal activity mediates l-DOPA-induced dyskinesia in parkinsonian mice. Proc Natl Acad Sci U S A 108: 840–845, 2011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, Cullheim S, Mohammed AH, Olson L, Larsson NG. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A 104: 1325–1330, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol 21: 269–274, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DM, Kravitz AV. Working together: basal ganglia pathways in action selection. Trends Neurosci 37: 301–303, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, Kaneko T. Exclusive and common targets of neostriatofugal projections of rat striosomes neurons: a single neuron-tracing study using a viral vector. Eur J Neurosci 33: 668–677, 2011. [DOI] [PubMed] [Google Scholar]
- Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature 260: 258–260, 1976. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol 236: 454–476, 1985. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci 22: 5042–5054, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Hernández T, Rodríguez M. Compartmental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. J Comp Neurol 421: 107–135, 2000. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Doudnikoff E, Li Q, Bloch B, Bezard E. Altered D(1) dopamine receptor trafficking in parkinsonian and dyskinetic non-human primates. Neurobiol Dis 26: 452–463, 2007. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 20: 2369–2382, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedreen JC, DeLong MR. Organization of striatopallidal, striatonigral, and nigrostriatal projections in the macaque. J Comp Neurol 304: 569–595, 1991. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80: 953–978, 2000. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Biochemical aspects of Parkinson's disease. Neurology 51: S2–S9, 1998. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Chemical neuroanatomy of the basal ganglia-normal and in Parkinson's disease. J Chem Neuroanat 22: 3–12, 2001. [DOI] [PubMed] [Google Scholar]
- Kim DS, Szczypka MS, Palmiter RD. Dopamine-deficient mice are hypersensitive to dopamine receptor agonists. J Neurosci 20: 4405–4413, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med 318: 876–880, 1988. [DOI] [PubMed] [Google Scholar]
- Kostrzewa RM. Dopamine receptor supersensitivity. Neurosci Biobehav Rev 19: 1–17, 1995. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Parent A. The striatofugal fiber system in primates: a reevaluation of its organization based on single-axon tracing studies. Proc Natl Acad Sci U S A 102: 11888–11893, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci U S A 90: 8861–8865, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Qiu G, Ding S, Zhou FM. Serotonin hyperinnervation and upregulated 5-HT2A receptor expression and motor-stimulating function in nigrostriatal dopamine-deficient Pitx3 mutant mice. Brain Res 1491: 236–250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhou FM. Parallel dopamine D1 receptor activity dependence of l-Dopa-induced normal movement and dyskinesia in mice. Neuroscience 236: 66–76, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Rymar VV, van den Munckhof P, Nicolau S, Steriade C, Bifsha P, Drouin J, Sadikot AF. The transcription factor Pitx3 is expressed selectively in midbrain dopaminergic neurons susceptible to neurodegenerative stress. J Neurochem 125: 932–943, 2013. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. Primate striatonigral projections: a comparison of the sensorimotor-related striatum and the ventral striatum. J Comp Neurol 345: 562–578, 1994. [DOI] [PubMed] [Google Scholar]
- Mailman R, Huang X, Nichols DE. Parkinson's disease and D1 dopamine receptors. Curr Opin Investig Drugs 2: 1582–1591, 2001. [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 78: 189–225, 1998. [DOI] [PubMed] [Google Scholar]
- Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci U S A 100: 4245–4250, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt JG, Chung KA, Holford NH. Dyskinesia and the antiparkinsonian response always temporally coincide: a retrospective study. Neurology 74: 1191–1197, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JC, Witkovsky P, Avshalumov MV, Rice ME. Mobilization of calcium from intracellular stores facilitates somatodendritic dopamine release. J Neurosci 29: 6568–6579, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radnikow G, Misgeld U. Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J Neurosci 18: 2009–2016, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Barajas C, Silva I, García-Ramírez M, Sánchez-Lemus E, Floran L, Aceves J, Erlij D, Florán B. 6-OHDA-induced hemiparkinsonism and chronic l-DOPA treatment increase dopamine D1-stimulated [(3)H]-GABA release and [(3)H]-cAMP production in substantia nigra pars reticulata of the rat. Neuropharmacology 55: 704–711, 2008. [DOI] [PubMed] [Google Scholar]
- Rangel-Barajas C, Silva I, Lopéz-Santiago LM, Aceves J, Erlij D, Florán B. l-DOPA-induced dyskinesia in hemiparkinsonian rats is associated with up-regulation of adenylyl cyclase type V/VI and increased GABA release in the substantia nigra reticulata. Neurobiol Dis 41: 51–61, 2011. [DOI] [PubMed] [Google Scholar]
- Rice ME, Patel JC, Cragg SJ. Dopamine release in the basal ganglia. Neuroscience 198: 112–137, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GS, Robertson HA. Evidence that l-dopa-induced rotational behavior is dependent on both striatal and nigral mechanisms. J Neurosci 9: 3326–3331, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Alcacer C, Cacciatore S, Heiman M, Hervé D, Greengard P, Girault JA, Valjent E, Fisone G. l-DOPA activates ERK signaling and phosphorylates histone H3 in the striatonigral medium spiny neurons of hemiparkinsonian mice. J Neurochem 108: 621–633, 2009. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. Convergence of synaptic inputs from the striatum and the globus pallidus onto identified nigrocollicular cells in the rat: a double anterograde labelling study. Neuroscience 44: 45–73, 1991. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Carrillo-Reid L, Bargas J, Galarraga E. Dopaminergic modulation of short-term synaptic plasticity at striatal inhibitory synapses. Proc Natl Acad Sci U S A 104: 10258–10263, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM. Molecular frequency filters at central synapses. Prog Neurobiol 62: 159–196, 2009. [DOI] [PubMed] [Google Scholar]
- van den Munckhof P, Luk KC, Ste-Marie L, Montgomery J, Blanchet PJ, Sadikot AF, Drouin J. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development 130: 2535–2542, 2003. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Smith Y, Bolam JP, Smith AD. Synaptic organization of GABAergic inputs from the striatum and the globus pallidus onto neurons in the substantia nigra and retrorubral field which project to the medullary reticular formation. Neuroscience 50: 531–549, 1992. [DOI] [PubMed] [Google Scholar]
- Voon V, Fernagut PO, Wickens J, Baunez C, Rodriguez M, Pavon N, Juncos JL, Obeso JA, Bezard E. Chronic dopaminergic stimulation in Parkinson's disease: from dyskinesias to impulse control disorders. Lancet Neurol 8: 1140–1149, 2009. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27: 468–474, 2004. [DOI] [PubMed] [Google Scholar]
- Wei W, Li L, Yu G, Ding S, Li C, Zhou FM. Supersensitive presynaptic dopamine D2 receptor inhibition of the striatopallidal projection in nigrostriatal dopamine-deficient mice. J Neurophysiol 110: 2203–2216, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Sakaba T. cAMP modulates intracellular Ca2+ sensitivity of fast-releasing synaptic vesicles at the calyx of Held synapse. J Neurophysiol 104: 3250–3260, 2010. [DOI] [PubMed] [Google Scholar]
- Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience 65: 709–730, 1995. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Lee CR. Intrinsic and integrative properties of substantia nigra pars reticulata neurons. Neuroscience 198: 69–94, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Xu JJ, Zhao Y, LeDoux MS, Zhou FM. Opposite functions of histamine H1 and H2 receptors and H3 receptor in substantia nigra pars reticulata. J Neurophysiol 96: 1581–1591, 2006. [DOI] [PubMed] [Google Scholar]
- Zhou FW, Jin Y, Matta SG, Xu M, Zhou FM. An ultra-short dopamine pathway regulates basal ganglia output. J Neurosci 29: 10424–10435, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83: 1197–1209, 1995. [DOI] [PubMed] [Google Scholar]