Abstract
Orexigenic mediators can impact the hypothalamic feeding circuitry via the activation of AMP-dependent protein kinase (AMPK). Given that testosterone is an orexigenic hormone, we hypothesized that androgenic changes in energy balance are due to enhanced cannabinoid-induced inhibition of anorexigenic proopiomelanocortin (POMC) neurons via activation of AMPK. To this end, whole animal experiments were carried out in gonadectomized male guinea pigs treated subcutaneously with either testosterone propionate (TP; 400 μg) or its sesame oil vehicle (0.1 ml). TP-treated animals displayed increases in energy intake associated with increases in meal size. TP also increased several indices of energy expenditure as well as the p-AMPK/AMPK ratio in the arcuate nucleus (ARC) measured 2 and 24 h posttreatment. Subcutaneous administration of the CB1 receptor antagonist AM251 (3 mg/kg) rapidly blocked the hyperphagic effect of TP. This was mimicked largely upon third ventricular administration of AM251 (10 μg). Electrophysiological studies revealed that TP potentiated the ability of the cannabinoid receptor agonist WIN 55,212-2 to decrease the frequency of miniature excitatory postsynaptic currents in ARC neurons. TP also increased the basal frequency of miniature inhibitory postsynaptic currents. In addition, depolarization-induced suppression (DSE) is potentiated in cells from TP-treated animals and blocked by AM251. The AMPK inhibitor compound C attenuated DSE from TP-treated animals, whereas the AMPK activator metformin enhanced DSE from vehicle-treated animals. These effects occurred in a sizable number of identified POMC neurons. Collectively, these results indicate that the androgen-induced increases in energy intake are mediated via an AMPK-dependent augmentation in endocannabinoid tone onto POMC neurons.
Keywords: testosterone, AMP-activated protein kinase, proopiomelanocortin, cannabinoid, energy balance
cannabinoids stimulate appetite in both humans (1, 10, 25) and rodent animal models (5, 16, 37) and do so via complex interactions between the gut, liver, pancreas, brainstem, hypothalamus, and limbic forebrain (9). Two well-known endogenous ligands for cannabinoid receptors are anandamide (13) and 2-arachidonoylglycerol (2-AG) (49), which are produced and released from neurons in a Ca2+-dependent manner (59). Anorexigenic proopiomelanocortin (POMC) neurons in the hypothalamic arcuate nucleus (ARC) serve as one such source of endocannabinoids (29). After endocannabinoid ligands activate their respective CB1 receptors, pleiotropic actions will ensue, including inhibition of voltage-gated Ca2+ channels, activation of inwardly rectifying K+ channels, and suppression of neurotransmitter release (46). As a result, the endocannabinoid system plays a crucial role in controlling neuronal excitability and synaptic transmission. In fact, hypothalamic levels of endocannabinoids are sensitive to fluctuations in energy status and inversely correlated with circulating leptin concentrations (29). Short-term synaptic plasticity mediated by endocannabinoids is referred to as depolarized-induced suppression of inhibition (DSI) (58, 79, 81) and excitation (DSE) (43, 47). DSI and DSE are initiated postsynaptically by an elevation of cytoplasmic Ca2+ concentration and are expressed presynaptically as a suppression of the transmitter release. For example, postsynaptic depolarizing stimuli provoked by the influx of extracellular calcium elicited DSI, in which somatodendritic release of endogenous cannabinoids presynaptically inhibited GABAergic synaptic currents, increasing the excitability of hippocampal pyramidal (58, 79) and cerebellar Purkinje (81) neurons. Likewise, DSE was also found to occur at excitatory synapses in the cerebellum, which was also mediated by endocannabinoids (43, 47). This retrograde signaling has also been reported in the hypothalamic paraventricular nucleus (PVN), where glucocorticoids stimulate the somatodendritic release of endogenous cannabinoids, which presynaptically decrease glutamatergic synaptic currents, proposing a cellular mechanism for negative feedback in parvocellular neurons (15). Given that cannabinoids presynaptically inhibit glutamatergic input onto POMC neurons in both mice (31) and guinea pigs (16, 34), these findings suggest that cannabinoids may inhibit POMC neurons in part by retrograde signaling that involves DSE.
It is well known that androgens play an integral role in controlling energy balance. For example, testosterone increases energy intake in rodents (54, 62) and rams (4). Testosterone has also been found to increase muscle mass, protein synthesis, and glycogen accumulation in muscle (6). In addition, it has also been reported that there is a higher energy obligation for muscle mass than fat and therefore less fat deposition in intact vs. castrated males (8). Furthermore, testosterone levels in men positively correlate with levels of orexigenic ghrelin (23). Moreover, the use of synthetic drugs derived from the naturally occurring compound Δ-9-tetrahydrocannabinol, coadministered with anabolic androgens like nandrolone, has been implemented in cachexia patient therapy to stimulate appetite by promoting lean body mass (7, 12).
AMPK is an energy sensor that restores energy balance by activating processes that produce energy (e.g., lipid oxidation and glucose uptake) while inhibiting those that consume energy (e.g., protein synthesis) (28). It is a heterotrimeric complex consisting of an α-, β-, and γ-subunit and is activated by the upstream kinases such as calmodulin-dependent protein kinase kinase-β and liver kinase B1 under conditions of high intracellular calcium levels or stressful cellular conditions, such as low ATP levels that cause a rise in the cellular AMP/ATP ratio (30). After activation, AMPK turns on catabolic pathways to generate ATP and turns off biosynthetic pathways requiring ATP consumption (26). AMPK signaling is regulated in certain regions of the hypothalamus by nutrient status indicators such as fasting/refeeding, leptin, insulin, glucose, fatty acids, and ghrelin and has been shown to control whole body energy metabolism (2, 40, 51). In addition to fasting conditions activating AMPK, it was found that anorexigenic signals stimulated by hormones like leptin and insulin reduce the expression of AMPK in orexigenic neuropeptide Y (NPY)/agouti-related peptide (AgRP) neurons as well as excite POMC neurons via signal transducer and activator of transcription 3 (STAT3) and phosphatidylinositol 3-kinase (PI3K), which causes the release of anorexigenic α-MSH and subsequent activation of MC4 receptors in the PVN (51, 61).
Ghrelin is an orexigenic gut peptide that increases the overall activity of AMPK (2, 51). There is also an important interaction between ghrelin and cannabinoids, as subanorectic doses of the CB1 receptor antagonist rimonabant inhibited the orexigenic effect of ghrelin upon central administration into the PVN (73). Furthermore, ghrelin and cannabinoids increase hypothalamic AMPK activity, and an intact CB1 receptor is crucial for these effects (41). Ghrelin increased, whereas leptin decreased, 2-AG and anandamide concentrations in the hypothalamus (14, 41). Tetrahydrocannabinol, 2-AG, and ghrelin increased AMPK activity in the hypothalamus, and ghrelin's ability to augment hypothalamic activity of AMPK is blocked by rimonabant (41, 42).
Based on these interactions between cannabinoids, orexigenic mediators like ghrelin and testosterone, and cellular substrates like AMPK that regulate energy homeostasis, it is apparent that androgens are poised to play an important role in modulating the hypothalamic feeding circuitry as well. Indeed, there are high levels of androgen receptors found in hypothalamic nuclei like the ARC (32, 65, 67, 68). Therefore, we wanted to test the hypothesis that testosterone's effects on energy intake are mediated through augmented endocannabinoid and AMPK signaling. To this end, we conducted whole animal experiments in orchidectomized male guinea pigs to determine testosterone-induced changes in energy balance and AMPK activation in the ARC microdissected from hypothalamic slices. We also examined whether CB1 receptor blockade could dampen the hyperphagia caused by the steroid. In vitro electrophysiological recordings were performed to explore whether AMPK inhibition could block the androgenic potentiation of the cannabinoid-induced presynaptic inhibition of excitatory input onto ARC POMC neurons and whether AMPK activation could act in lieu of testosterone to augment this presynaptic inhibition of transmitter release in slices from vehicle-treated animals.
MATERIALS AND METHODS
Animals.
All animal procedures described in this study are in accordance with institutional guidelines based on National Institutes of Health standards and were approved by the Institutional Animal Care and Use Committee at Western University of Health Sciences. Male Topeka guinea pigs (500–900 g, 50–75 days old) were acquired from Elm Hill Breeding Laboratories (Chelmsford, MA) or bred in our animal care facility, maintained under controlled temperature (69–73°F) and a coordinated 12:12-light-dark cycle (12 h lights on, 12 h lights off), and provided with food and water ad libitum.
Drugs.
Unless otherwise indicated, all drugs were purchased through Tocris Cookson (Bioscience, Minneapolis, MN). For the behavioral experiments, testosterone propionate (TP; Sigma-Aldrich, St. Louis, MO) was initially prepared as a 1 mg/ml stock solution in punctilious ethanol. A known quantity of this stock solution was added to a volume of sesame oil that was sufficient to produce a final concentration of 4 mg/ml following evaporation of the ethanol. N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251; 3 mg/ml) was dissolved in cremephor-ethanol-0.9% saline (CES; 1:1:18, vol/vol/vol). For the studies that called for the administration of AM251 into the third ventricle (I3V), the compound was dissolved in CES at a concentration of 5 μg/μl and delivered in a total volume of 2 μl.
For the electrophysiological experiments, the voltage-gated Na+ channel blocker tetrodotoxin (TTX) with citrate (Alomone Laboratories, Jerusalem, Israel) was dissolved in ultrapure H2O to a stock concentration of 1 mM and diluted further with artificial cerebrospinal fluid (aCSF) to a working concentration of 500 nM. The GABAA receptor antagonist 6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (SR 95531) was dissolved in ultrapure H2O to stock concentrations of 10 mM, and the stock concentrations were diluted further with aCSF to the working concentration of 10 μM. The cannabinoid receptor agonist (R)-(+)-{2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl}-1-naphthalenylmethanone mesylate (WIN 55,212-2) was dissolved in dimethylsulfoxide to stock concentrations of 1 mM, and the stock concentrations were diluted further with aCSF to working concentrations ranging from 10 nM to 3 μM. AM251 was dissolved in dimethylsulfoxide to stock concentrations of 1 mM, and the stock concentrations were diluted further with aCSF to the working concentration of 1 μM. The AMPK activator N,N-dimethylimidodicarbonimidic diamide hydrochloride (metformin) was dissolved in ultrapure H2O to stock concentrations of 50 mM, and the stock concentrations were diluted further with aCSF to the working concentration of 500 μM. The AMPK inhibitor 6-{4-[2-(1-piperidinyl)ethoxyphenyl]-3-(4-pyridinyl)-pyrazolo[1,5-a]} pyrimidine dihydrochloride (compound C) was dissolved in ultrapure H2O to stock concentrations of 30 mM, and the stock concentrations were diluted further with aCSF to the working concentration of 30 μM. The NMDA receptor antagonist cis-4-[phosphomethyl]-piperidine-2-carboxylic acid (CGS 19755) was dissolved in ultrapure H2O to stock concentrations of 10 mM, and the stock concentrations were diluted further with aCSF to the working concentration of 10 μM. The AMPA receptor antagonist 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX) was dissolved in ultrapure H2O to stock concentrations of 10 mM, and the stock concentrations were diluted further with aCSF to the working concentration of 3 μM. Aliquots of the stock solutions were stored at −20°C until needed.
Feeding and metabolic studies.
The analyses for energy balance were performed in a Comprehensive Lab Animal Monitoring System (Columbus Instruments, Columbus, OH) as described previously and validated (19, 61, 77). After a 3-day acclimation period, energy intake, expenditure, and meal pattern were monitored around the clock for 5–7 days. A meal was defined as an event in which an animal consumed ≥10 mg of food. Once the animal had eaten at least this threshold amount, the computer logged this event as a meal in the experimental data file the instant the animal withdrew its head from the food dish. We calculated meal frequency as the number of meals consumed per unit of time and meal size as the amount of food eaten in a given hour divided by the number of meals in the same hour. We also measured O2 consumption, CO2 production, and the metabolic heat production as indices of energy expenditure. These whole animal experiments were conducted with male guinea pigs that were orchidectomized 6 days prior to experimentation, some of whom had a temperature data logger (SubCue, Calgary, AL, Canada) implanted into the abdominal cavity at the time of the orchidectomy to monitor temporal fluctuations in core body temperature, as described previously (16). The initial experiment examined the effects of TP and the CB1 receptor antagonist AM251 administered systemically over a 7-day monitoring period. Thus, each morning at 8 AM, animals were given either the CB1 receptor antagonist AM251 (3 mg/kg sc) or its CES vehicle (1 ml/kg sc) Every other day they were injected with TP (400 μg sc) or its sesame oil vehicle (0.1 ml sc). In the second experiment, we wanted to better resolve how testosterone influences cannabinoid sensitivity within the hypothalamic feeding circuitry. As a result, two survival surgeries were conducted. Stereotaxic guide cannulas implanted into the third ventricle, as well as orchidectomies performed 7 days later, were carried out as described previously (16, 61). During the 5-day monitoring phase, animals were treated every day with AM251 (10 μg I3V) or its CES vehicle (2 μl I3V) and every other day with TP (400 μg sc) or its sesame oil vehicle (0.1 ml sc). These treatment schedules produce physiological steroid levels and do not result in tolerance of cannabinoid effects (34, 39).
Western blot analysis.
Unless otherwise stated, all primary antibody solutions were prepared in TBS containing 0.1% Tween-20 and Odyssey blocking buffer at a 1:1,000 dilution. Primary antibodies directed against the following antigens were used as follows: glyceride-3-phosphate dehydrogenase (GAPDH; 1:10,000; Millipore, Billerica, MA), total AMPK (Abcam, Cambridge, MA), and AMPK phosphorylated at the threonine residue located in the 172nd position of the α-subunit (p-AMPK; Cell Signaling Technology, Danvers, MA). At the end of the 5-day experimental period, we set out to determine whether the hyperphagic effect of TP (400 μg sc) involved activation of the AMPK pathway in the ARC. Thus, animals were treated once again with TP (400 μg sc) or its sesame oil vehicle (0.1 ml sc), anesthetized either 2 or 24 h later with 32% isoflurane, and rapidly decapitated. Trunk blood was collected for subsequent determination of serum testosterone concentrations conducted at the Oregon Health and Science University, Endocrine Technology and Support Core Laboratory (OHSU ETSC, Beaverton, OR). Following brain removal, two to three coronal slices (1 mm in thickness) spanning the rostral-caudal extent of the ARC were prepared using a guinea pig brain matrix (Ted Pella, Redding, CA) and stored in RNAlater (Ambion, Austin, TX) for 2–3 h. The ARC was then microdissected from the slices. ARC microdissections were homogenized in cold lysis buffer (50 mM Tris·HCl, pH 7.4, 0.5 M EDTA, and 0.5 M EGTA) containing a protease inhibitor cocktail (Sigma-Aldrich). Protein levels were quantified using a Bradford assay (Bio-Rad Laboratories, Hercules, CA) to establish equal loading into the gel. Proteins were separated by electrophoresis on a 10% sodium dodecyl sulfate polyacrylamide gel and transferred to a nitrocellulose membrane. Membranes were blocked for 1 h with odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) and incubated overnight with primary antibodies at 4°C. They were then washed four times with Tris-buffered saline with Tween (TBST) for 10 min, followed by incubation with Odyssey infrared-conjugated secondary antibodies diluted 1:10,000 in Odyssey blocking buffer for 2 h at room temperature. After 4 × 10-min washes with TBST followed by 4 × 10-min washes with Tris-buffered saline (TBS), membranes were scanned using an Odyssey infrared imager (LI-COR Biosciences). All membranes were probed with GAPDH as a loading control. Levels of p-AMPK and AMPK expression were determined by calculating the ratio of phosphoprotein density to total protein density for each experimental group and then normalizing the ratio to the values observed in vehicle-treated animals.
Electrophysiology.
Electrophysiological recordings from ARC neurons using biocytin-filled electrodes were performed in hypothalamic slices prepared from orchidectomized guinea pigs treated 24 h prior with either TP (400 μg sc) or its sesame oil vehicle (0.1 ml sc), as described previously (16). Briefly, electrode resistances varied from 3 to 8 MΩ. Membrane currents were recorded in voltage clamp with access resistances ranging from 8 to 20 MΩ and underwent analog-digital conversion via a Digidata 1322A interface coupled to pClamp 8.2 software (Axon Instruments). The access resistance, as well as the resting membrane potential (RMP) and the input resistance (Rin), were monitored throughout the course of the recording. If the access resistance deviated greater than 20% of its original value, the recording was ended.
To evaluate whether TP modulates cannabinoid-induced presynaptic inhibition of excitatory input onto ARC neurons via an AMPK pathway, we first monitored miniature excitatory postsynaptic current (mEPSC) frequency and amplitude from a holding potential of −75 mV, using an internal solution in which Cs+ was substituted for K+. We perfused slices with aCSF containing TTX (500 nM) and SR 95531 (10 μM) to block GABAA receptor-mediated synaptic input. These agents were bath applied for 3–4 min prior to the attainment of 3–4 min worth of baseline mEPSC frequency and amplitude. Slices were then perfused with the cannabinoid receptor agonist WIN 55,212-2 for 3–4 min, and an additional 3–4 min of data were collected. To pharmacologically characterize the effects of CB1 receptor activation on mEPSC frequency, ARC neurons from vehicle- and TP-treated animals were tested with various concentrations of WIN 55,212-2 (0.01–3 μM). Composite dose-response curves were generated from the following modification of the Hill equation
where Δfmax is the maximal inhibition of mEPSC frequency, IC50 represents the agonist potency to inhibit this parameter, and n is the Hill slope. Currents measured in the presence of varying concentrations of WIN 55,212-2 were normalized with respect to their baseline frequency.
For comparison, we also evaluated the effect of TP and cannabinoids on miniature inhibitory postsynaptic current (mIPSC) frequency and amplitude. Briefly, aCSF was perfused over cells in hypothalamic slices from TP- or vehicle-treated animals in the presence of TTX (500 nM) along with the CGS 19755 (10 μM) and NBQX (3 μM) to block excitatory synaptic input elicited by the activation of ionotropic glutamate receptors. Baseline recordings were performed from a holding potential of −30 mV for 3–4 min. WIN 55,212-2 (1 μM) was then applied for an additional 3–4 min, and 3–4 more minutes of data were collected.
The threshold for event detection was set ≥3 pA below (for mEPSCs) or above (for mIPSCs) the baseline holding current, as assessed from the headstage output, and monitored continuously throughout each 3- to 4-min recording period. This was done to ensure that the smaller amplitude events were not inadvertently omitted from the analysis. Synaptic events were detected and analyzed using Clampfit 8.2 (Axon Instruments) in combination with the SigmaPlot (IBM/SPSS, New York, NY) and StatGraphics (StatPoint, Warrenton, VA) programs. When we analyzed the data to determine mEPSC/mIPSC frequency and amplitude for the ≥100 contiguous synaptic events per condition, we poured over each 250-ms sweep in the entire range to ensure that each event that we included in the analysis bore the classic kinetic profile of a fast EPSC or IPSC. We used this information to evaluate cannabinoid-induced alterations in mEPSC/mIPSC frequency and amplitude as assessed from cumulative probability plots.
Next, we set out to determine whether endocannabinoids retrogradely inhibit excitatory input via DSE. To elicit DSE, cells from TP- or vehicle-treated animals were given a 60-mV depolarization (0.75–3 s in duration) from a holding potential of −75 mV. These pulses were delivered every 60 s for up to 15 consecutive trials. The pulses were delivered in the presence of SR 95531 (10 μM) to block GABAA receptor-mediated synaptic input. Prior to executing the DSE protocol, we monitored spontaneous EPSCs (sEPSCs) for 3–4 min to establish baseline frequency and amplitude. In some experiments, we perfused the AM-251 (1 μM) along with SR 95531 to assess the role of the CB1 receptor in the expression of DSE occurring at ARC synapses. In other experiments, either metformin (500 μM) or compound C (30 μM) was used to determine the role of AMPK signaling. The doses of the drugs used in these electrophysiological experiments were chosen based on our prior work as well as the published work of others (16, 38, 63, 64). Data were analyzed by looking at the average poststimulation amplitude and frequency acquired from at least three separate trials over 5-s bins ≤20 s normalized to that observed under basal conditions.
Immunohistochemistry.
Following electrophysiological recording, slices were fixed with 4% paraformaldehyde in Sorensen's phosphate buffer (pH 7.4) for 90–180 min (38). They then were immersed overnight in 20% sucrose dissolved in Sorensen's buffer and frozen in Tissue-Tek embedding medium (Miles, Elkhart, IN) the next day. Coronal sections (20 μm) were cut on a cryostat and mounted on slides. These sections were washed with 0.1 M sodium phosphate buffer (pH 7.4) and then processed with streptavidin-AF488 (Molecular Probes, Eugene, OR) at a 1:300 dilution. After the biocytin-filled neuron was localized via fluorescence microscopy, the slides containing the appropriate sections were processed with polyclonal antibodies directed against either β-endorphin (1:400 dilution; Immunostar, Hudson, WI), α-MSH (1:200 dilution; Immunostar), or cocaine-amphetamine-regulated transcript (1:2,000 dilution; Phoenix Pharmaceuticals, Burlingame, CA) using fluorescence immunohistochemistry (38).
Statistical analyses.
Comparisons between two groups were made with either the Student's t-test or the Mann-Whitney U-test. Comparisons between more than two groups were performed using either the one-way, multifactorial, or rank-transformed two-way analysis of variance (ANOVA), followed by the least significant difference (LSD) test, or alternatively via the Kruskal-Wallis test, followed by analysis of the median-notched box-and-whisker plot. If the multifactorial ANOVA uncovered a significant interaction between the variables, then we proceeded with the one-way ANOVA, followed by the LSD test. Comparisons of the mEPSC interval distributions were evaluated via the Kolmogorov-Smirnov test. Differences were considered statistically significant if the probability of error was <5%.
RESULTS
Experiment no. 1: the effects of TP on energy intake and expenditure.
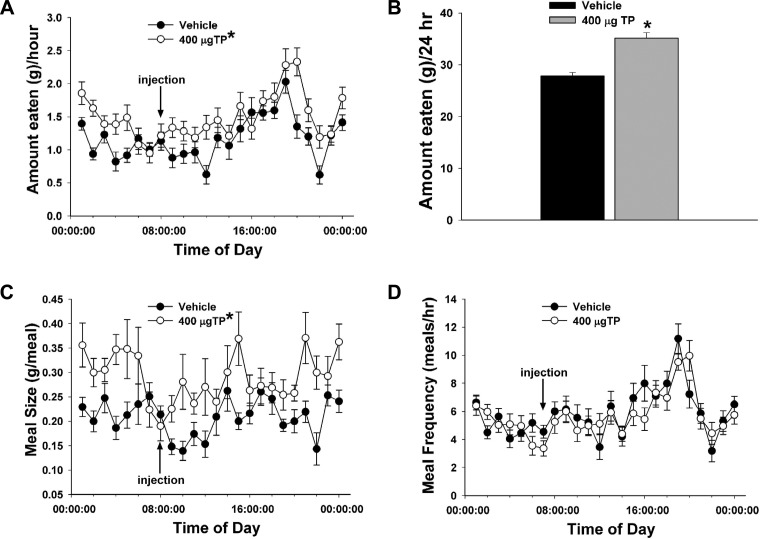
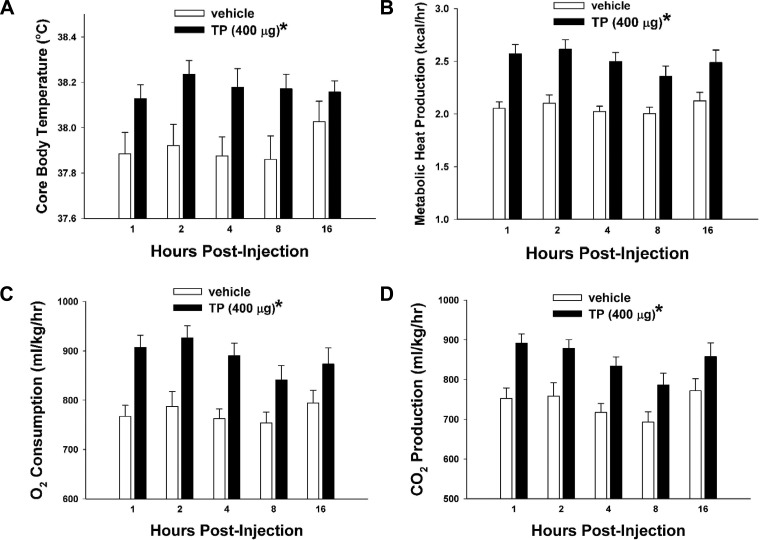
We first used in vivo behavioral studies in orchidectomized male guinea pigs to examine the role of testosterone on energy intake and expenditure. To validate our model of TP replacement in orchidectomized male guinea pigs, we measured serum testosterone levels taken from gonadally intact as well as orchidectomized animals 2 and 24 h post-TP treatment (400 μg sc) and from orchidectomized animals 2 and 24 h post-vehicle treatment. As shown in Table 1, TP (400 μg sc) produced a marked rise in circulating testosterone concentrations 2 h after administration that was comparable with those observed in gonadally intact males. Serum testosterone concentrations were still elevated at 24 h after delivery (1-way ANOVA/LSD, F = 16.35, df = 4, P < 0.0001). TP increased incremental (multifactorial ANOVA/LSD, Fsteroid = 26.04, df = 1, P < 0.0001, Ftime = 6.20, df = 23, P < 0.0001, Finteraction = 1.54, df = 23, P < 0.05; Fig. 1A) and cumulative food intake (Student's t-test, t = −5.55181, P < 0.0001; Fig. 1B). Increases in hourly intake appeared biphasic, with an acute increase observed 1–4 h after injection and a latent increase seen throughout much of nocturnal period. These changes were associated with elevations in meal size (multifactorial ANOVA/LSD, Fsteroid = 49.32, df = 1, P < 0.0001, Ftime = 1.90, df = 23, P < 0.008, Finteraction = 1.58, df = 23, P < 0.05; Fig. 1C) but not frequency (multifactorial ANOVA/LSD, Fsteroid = 1.26, df = 1, P < 0.27, Ftime = 7.20, df = 23, P < 0.0001, Finteraction = 1.10, df = 23, P < 0.34; Fig. 1D). Likewise, TP increased core body temperature (multifactorial ANOVA/LSD, Fsteroid = 21.32, df = 1, P < 0.0001, Ftime = 0.36, df = 4, P < 0.84, Finteraction = 0.79, df = 4, P < 0.53; Fig. 2A), metabolic heat production (multifactorial ANOVA/LSD, Fsteroid = 57.00, df = 1, P < 0.0001, Ftime = 1.03, df = 4, P < 0.39, Finteraction = 0.37, df = 4, P < 0.83; Fig. 2B), oxygen consumption (multifactorial ANOVA/LSD, Fsteroid = 43.02, df = 1, P < 0.0001, Ftime = 1.21, df = 4, P < 0.31, Finteraction = 0.57, df = 4, P < 0.69; Fig. 2C), and carbon dioxide production (multifactorial ANOVA/LSD, Fsteroid = 38.24, df = 1, P < 0.0001, Ftime = 3.17, df = 4, P < 0.02, Finteraction = 0.28, df = 4, P < 0.89; Fig. 2D).
Table 1.
Serum testosterone levels in gonadally intact and castrated male guinea pigs measured 2 or 24 h following injection with either TP (400 μg sc) or its sesame oil vehicle (0.1 ml sc)
| Experimental Group | Testosterone, pg/ml |
|---|---|
| Gonadally intact | 3,858.0 ± 724.6 |
| Orch/vehicle (2 h) | 365.0 ± 27.6 |
| Orch/TP (2 h) | 3,067.5 ± 329.0* |
| Orch/vehicle (24 h) | 387.7 ± 81.4 |
| Orch/TP (24 h) | 1,085.3 ± 179.1* |
Values are means ± SE of serum testosterone concentrations; n = 4–25. Orch, castrated; TP, testosterone propionate.
Values from TP-treated Orch animals that are significantly different (1-way ANOVA/least significant difference, P < 0.05) from those from vehicle-treated controls.
Fig. 1.
Testosterone propionate (TP) increases hourly and cumulative (24-h) intake that is associated with increased meal size but not meal frequency. A, C, and D: symbols represent means and vertical lines 2 SE of the hourly intake (A), meal size (C), or meal frequency (D), which were monitored around the clock. Multifactorial ANOVA/least significant difference (LSD); n = 4–6. B: bars represent means and vertical lines 1 SE of the cumulative 24-h intake measured over the monitoring period. Students t-test; n = 4–6. *P < 0.05.
Fig. 2.
TP increases core body temperature, metabolic heat production, O2 consumption, and CO2 production. A–D: bars represent means and vertical lines 1 SE of core body temperature, metabolic heat production, O2 consumption, and CO2 production, which were monitored at 1, 2, 4, 8, and 16 h after steroid administration. Multifactorial ANOVA/LSD; n = 4–6. *P < 0.05.
Experiment no. 2: the effects of CB1 receptor blockade on TP-induced changes in energy intake.
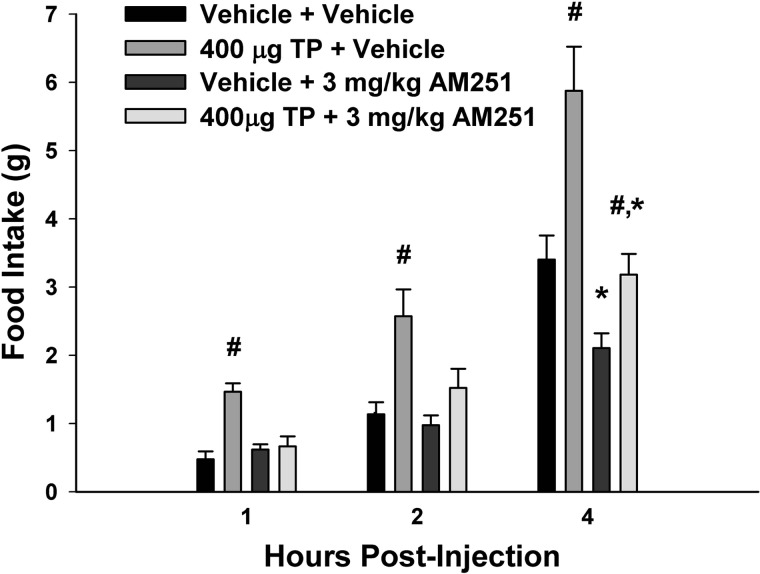
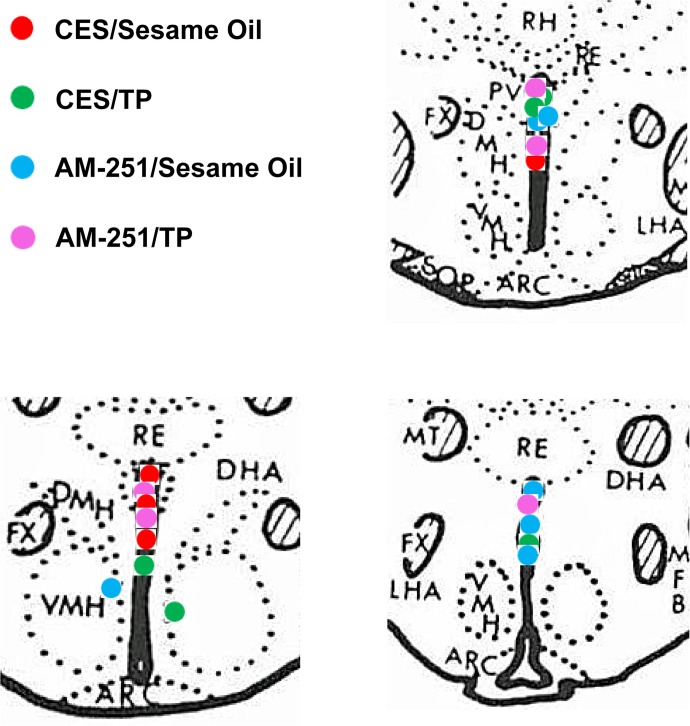
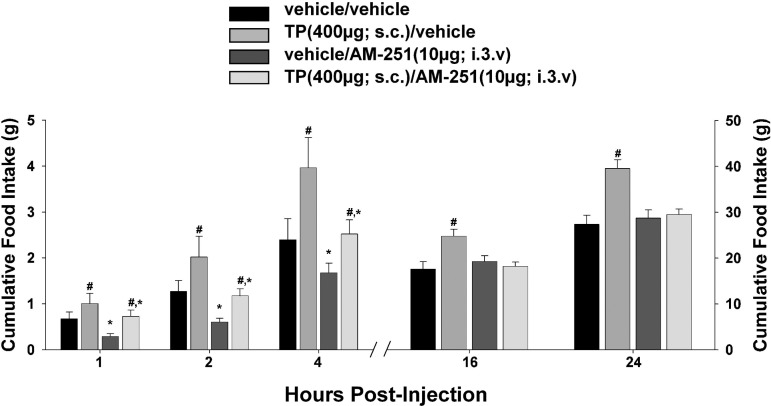
To examine the possible role of the CB1 receptor in mediating the hyperphagic effect of TP, the CB1 receptor antagonist AM251 was administered peripherally (3 mg/kg sc) along with the steroid. As shown in Fig. 3, AM251 rapidly and completely blocked the TP-induced increase in cumulative intake as early as 1 h postadministration (multifactorial ANOVA/LSD, Fsteroid = 13.21, df = 1, P < 0.0006, FAM251 = 5.37, df = 1, P < 0.03, Finteraction = 11.02, df = 1, P < 0.002). To more precisely determine the location of these CB1 receptors that contribute to the TP-induced hyperphagia, we examined the ability of AM251 administered directly into third ventricle to abrogate the increase in intake caused by the steroid. The locations of the guide cannula placements can be seen in Fig. 4. As shown in Fig. 5, centrally administered AM251 (10 μg I3V) per se produced a rapid and marked decrease in energy intake that was clearly evident from 1–4 h postadministration and attenuated the TP-induced hyperphagia over this same period (multifactorial ANOVA/LSD, Fsteroid = 6.69, df = 1, P < 0.02, FAM251 = 6.50, df = 1, P < 0.02, Finteraction = 0.47, df = 1, P < 0.50). Moreover, it completely blocked the increase in intake caused by TP observed at 16 and 24 h postadministration (multifactorial ANOVA/LSD, Fsteroid = 8.37, df = 1, P < 0.005, FAM251 = 7.20, df = 1, P < 0.009, Finteraction = 6.15, df = 1, P < 0.02). This suggests that activation of hypothalamic CB1 receptors by endogenous cannabinoids contributes to the hyperphagic effect of TP.
Fig. 3.
Systemic administration of the CB1 receptor antagonist N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) rapidly blocks the hyperphagic effect of TP. Bars represent means and vertical lines 1 SE of the energy intake measured in animals at 1, 2, and 4 h after treatment with TP (400 μg sc) and/or AM251 (3 mg/kg sc) or their respective vehicles. #Values from TP-treated animals that are significantly different (P < 0.05, multifactorial ANOVA/LSD; n = 4–6) from vehicle-treated controls; *Values from AM251-treated animals that are significantly different (P < 0.05, multifactorial ANOVA/LSD; n = 4–6) from those from their vehicle-treated counterparts.
Fig. 4.
Coronal sections of guinea pig brain at the level of the hypothalamus that illustrate the placement of the guide cannulas used to deliver AM251 into the 3rd ventricle. Only those animals with guide cannulas placed directly into the 3rd ventricle were used in this study. RH, rhomboid thalamic nucleus; RE, reuniens thalamic nucleus; PV, paraventricular hypothalamic nucleus; FX, fornix; DMH, dorsomedial hypothalamic nucleus; VMH, ventromedial hypothalamic nucleus; LHA, lateral hypothalamic area; MT, mammillothalamic tract; MFB, medial forebrain bundle; CES, cremephor-ethanol-0.9% saline; ARC, arcuate nucleus.
Fig. 5.
Third ventricular administration of AM251 appreciably attenuates the hyperphagic effect of TP. Bars represent means and vertical lines 1 SE of energy intake monitored at 1, 2, 4, 16, and 24 h after treatment with TP (400 μg sc) and/or AM251 [10 μg into the 3rd ventricle (I3V)] or their respective vehicles. #Values from TP-treated animals that are significantly different (P < 0.05, multifactorial ANOVA/LSD; n = 4–6) from those from their vehicle-treated counterparts; *values from AM251-treated animals that are significantly different (P < 0.05, multifactorial ANOVA/LSD; n = 4–6) from vehicle-treated controls.
Experiment no. 3: the effects of TP on activation of AMPK in the ARC.
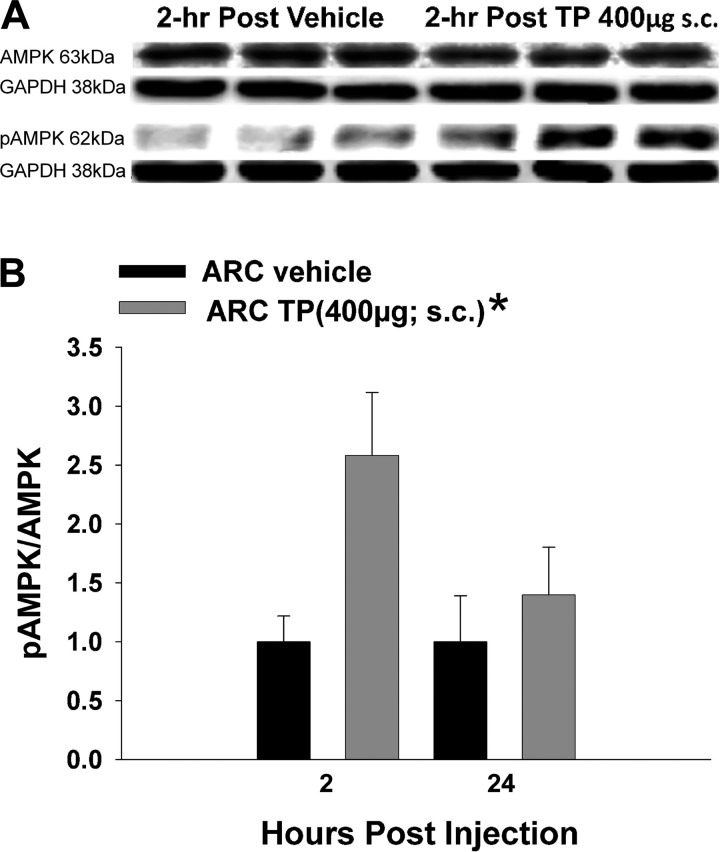
AMPK is a vital energy sensor that becomes activated in various hypothalamic nuclei under conditions of negative energy balance, such as fasting (27). Therefore, we tested whether TP treatment could alter phosphorylated levels of this signaling molecule in hypothalamic nuclei microdissected from animals at the end of the behavioral studies. Western blotting showed clear p-AMPK and total AMPK bands registering at 62 kDa in the ARC. TP elevated the p-AMPK/AMPK ratio significantly in the ARC, an effect that appeared to peak at 2 h after treatment (rank-transformed 2-way ANOVA/LSD, Fsteroid = 5.54, df = 1, P < 0.03, Ftime = 1.99, df = 1, P < 0.17, Finteraction = 1.99, df = 1, P < 0.17; Fig. 6).
Fig. 6.
TP activates AMP-activated protein kinase (AMPK) in the ARC. A: representative Western blots illustrating the levels of AMPK, phosphorylated AMPK (p-AMPK), and the loading control GAPDH in the ARC microdissected from TP- and vehicle-treated animals. B: composite bar graph illustrating the p-AMPK/AMPK ratio determined in ARC microdissections from animals treated 2 or 24 h prior with either TP or its sesame oil vehicle. Bars represent means and vertical lines 1 SE. *Values from TP-treated animals that are significantly different (P < 0.05; rank-transformed 2-way ANOVA/LSD; n = 5–8) from vehicle-treated controls.
Experiment no. 4: the effects of TP on CB1 receptor-mediated changes in excitatory and inhibitory synaptic input in POMC neurons.
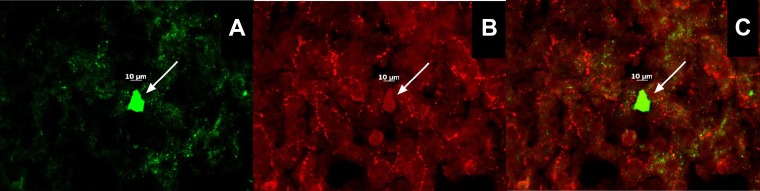
For experiment nos. 4 and 5 (see below), we made recordings from a total of 581 ARC neurons. The cells from vehicle-treated animals had a RMP of −51.7 ± 1.4 mV and a Rin of 648.3 ± 52.5 MΩ, whereas those from TP-treated animals had a RMP of −52.9 ± 1.4 mV and a Rin of 755.6 ± 63.3 MΩ. One hundred sixty-two of these neurons exhibited conductances like the hyperpolarization-activated cation current and A-type K+ current, which are characteristic of POMC neurons (36, 39). Of these, 113 were immunopositive for POMC neurons like the one shown in Fig. 7.
Fig. 7.
The double-labeling of an ARC neuron that is immunopositive for a phenotypic marker characteristic of proopiomelanocortin (POMC) neurons. A: biocytin visualized with AF488. B: α-MSH immunoreactivity visualized with AF546. C: composite overlay. Calibration bar, 10 μm.
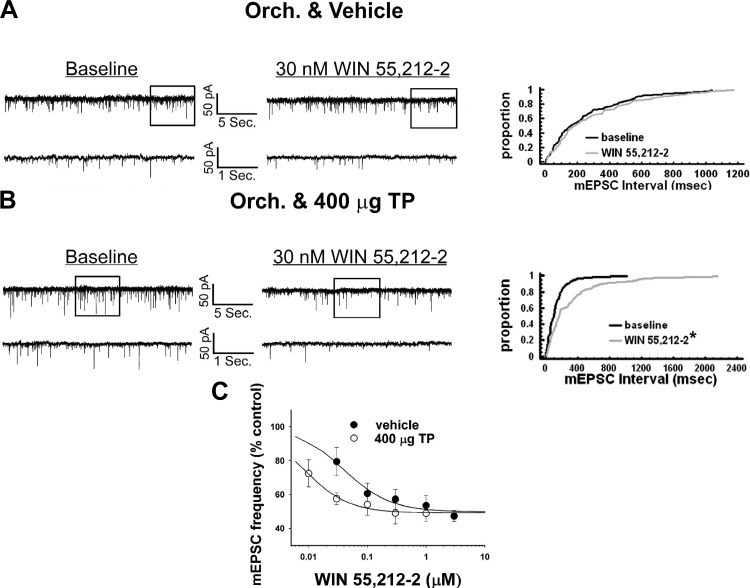
In a parallel series of experiments, we endeavored to gain a better understanding of how these TP-induced changes in energy intake are occurring on a cellular level. We first tested whether TP could modulate the ability of the CB1 receptor agonist WIN 55,212-2 to decrease mEPSC frequency in POMC neurons. As shown in Fig. 8A, a 30-nM dose of WIN 55,212-2 modestly increased the interval between contiguous mEPSCs in recordings from orchidectomized, vehicle-treated animals. However, this did not result in a significant change in the mEPSC interval distribution following treatment with WIN 55,212-2 (Kolmogorov-Smirnov, K-S statistic = 0.777817, P < 0.59). By contrast, this same dose of WIN 55,212-2 produced a robust increase in the interval between contiguous synaptic events in POMC neurons from TP-treated animals that significantly altered the mEPSC interval distribution following agonist treatment (Kolmogorov-Smirnov, K-S statistic = 2.05061, P < 0.0005; Fig. 8B). This is reflected in a nearly fivefold increase in agonist potency to decrease mEPSC frequency (vehicle: IC50 = 39.1 nM, Δfmax = 50.2%; TP: IC50 = 8.4 nM, Δfmax = 50.9%; Fig. 8C). TP did not affect basal mEPSC frequency (vehicle: 7.0 ± 1.7 Hz; TP: 4.4 ± 0.9 Hz; Student's t-test, t = 1.27342, P < 0.21), and neither TP nor WIN 55,212-2 affected mEPSC amplitude (data not shown). This suggests that TP enhances the cannabinoid-induced presynaptic inhibition of glutamatergic input because of an increase in CB1 receptor sensitivity.
Fig. 8.
TP potentiates CB1 receptor agonist-induced decreases in miniature excitatory postsynaptic current (mEPSC) frequency. A and B: membrane current traces under baseline (left) and in the presence of 30 nM (R)-(+)-{2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl}-1-naphthalenylmethanone mesylate (WIN 55,212-2; middle) and their corresponding cumulative distributions (right) in recordings from vehicle- (A) and TP-treated animals (B). C: composite dose-response curves for the decrease in mEPSC frequency produced by WIN 55,212-2 in POMC neurons from orchidectomized, vehicle-treated (●), and TP-treated (○) animals. The curves were fit via logistic equation to the data points. Symbols represent means and vertical lines 2 SE (n = 3–12) of the mEPSC frequency seen with varying concentrations of WIN 55,212-2 that were normalized to their respective control values. Note the prominent TP-induced leftward shift in the agonist dose-response curve. *P < 0.05, Kolmogorov-Smirnov test.
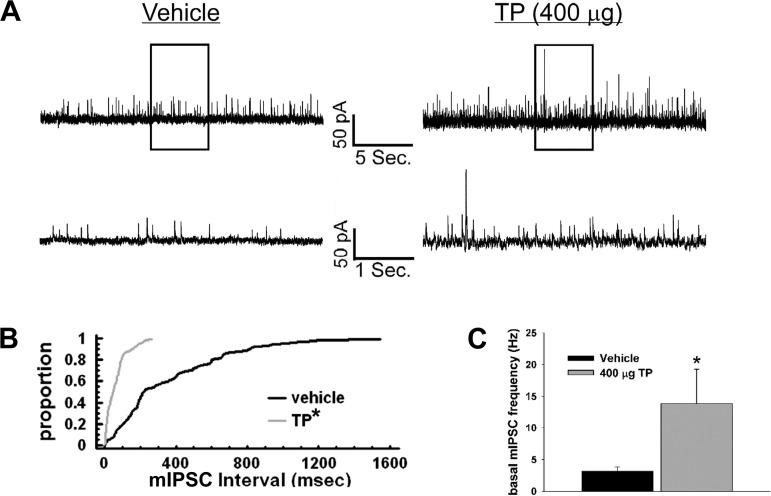
We then wanted to look at whether TP could impact basal and cannabinoid-induced changes in mIPSC frequency. Membrane current traces (Fig. 9A) demonstrate that TP increases the number of inhibitory synaptic events per unit of time in ARC neurons, which is illustrated further by the cumulative probability distributions (Kolmogorov-Smirnov, K-S statistic = 3.46482, P < 0.0001; Fig. 9B) and composite bar graph (Student's t-test, t = −2.10677, P < 0.05; Fig. 9C). Consistent with what we have shown previously (16), WIN 55,212-2 was comparatively ineffective in decreasing mIPSC frequency. A relatively high dose of WIN 55,212-2 (1 μM) was required to see any appreciable effect, and TP did not alter the reduction in mIPSC frequency caused by the agonist (vehicle: 64.5 ± 7.8% of baseline; TP: 67.8 ± 9.3% of baseline; Mann-Whitney U-test, W = 8.0, P < 0.51, n = 8–10). Overall, there was no effect of TP or WIN 55,212-2 on mIPSC amplitude (data not shown).
Fig. 9.
TP increases basal miniature inhibitory postsynaptic current (mIPSC) frequency in POMC neurons. Membrane current traces (A), cumulative probability distributions (B), and a composite bar graph (C) showing the increase in basal frequency after TP (400 μg sc) administration. *P < 0.05, Kolmogorov-Smirnov (B) and Student's t-tests (C); n = 13.
Experiment no. 5: the effects of TP on retrograde endocannabinoid signaling at POMC synapses.
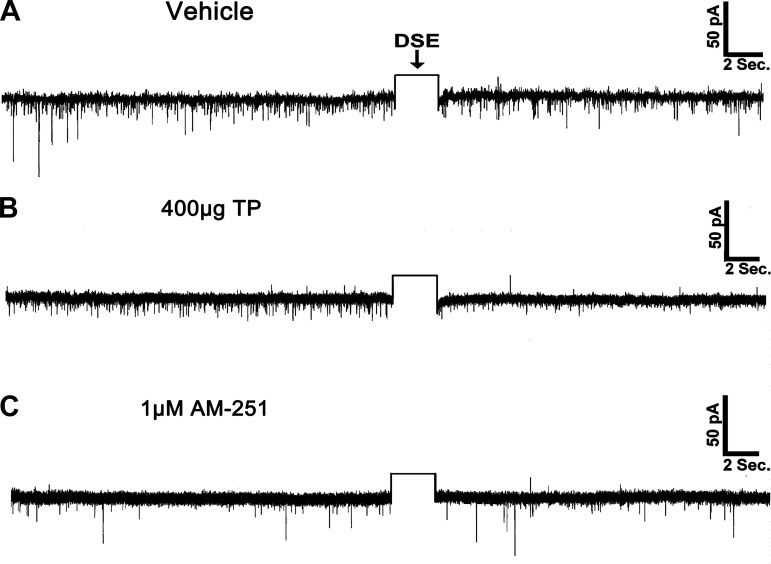
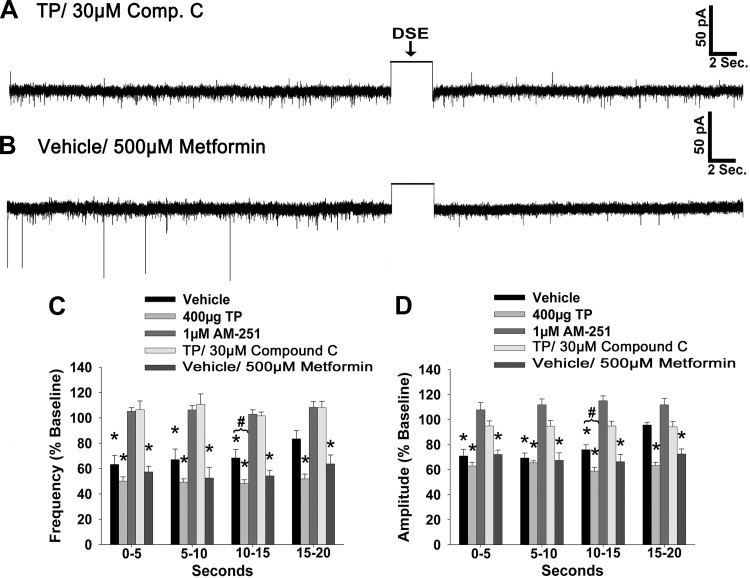
Thus far, we have demonstrated that TP elicits a prominent increase in energy intake that is, at least in part, sensitive to blockade of hypothalamic CB1 receptors and that these changes are somehow associated with increased activation of AMPK and augmented inhibitory tone onto POMC neurons. The latter occurs through enhanced inhibitory synaptic input, as well as a potentiation of the CB1 receptor-mediated presynaptic inhibition of excitatory input, onto these cells. To provide additional evidence concerning the role of endogenous cannabinoids in mediating the androgenic increase in energy intake, we set out to examine whether they were involved in eliciting DSE at ARC POMC synapses in the male guinea pig. We found that in recordings from orchidectomized, vehicle-treated animals, a 3-s, 60-mV depolarization of the postsynaptic cell triggers a significant reduction in both the frequency and amplitude of sEPSCs. These changes were first evident within 5 s of the depolarizing stimulus (Kruskal-Wallis/median-notched box-and-whisker analysis; frequency: test statistic = 20.4731, P < 0.0005; amplitude: test statistic = 21.0242, P < 0.0004), sustained for ≥15 s (frequency: test statistic = 22.0649, P < 0.0002; amplitude: test statistic = 23.5851, P < 0.0001), and appeared to be returning to baseline levels by 20 s poststimulation (frequency: test statistic = 19.9478, P < 0.0006; amplitude: test statistic = 21.3483, P < 0.0003; Figs. 10A and 11, C and D). TP administered 24 h prior to experimentation potentiated the extent of the DSE, as the reductions in sEPSC frequency and amplitude persisted for ≥20 s following the stimulus (Figs. 10B and 11, C and D). The stimulus-induced decreases in sEPSC frequency and amplitude were completely reversed by pretreatment of the slice with the CB1 receptor antagonist AM251 (1 μM; Figs. 10C and 11, C and D). In addition, pretreatment with the AMPK inhibitor compound C (30 μM) attenuated the robust DSE observed in recordings from TP-treated animals (Fig. 11, A, C, and D). On the other hand, bath application of the AMPK activator metformin (500 μM) in slices from vehicle-treated animals prolonged the stimulus-induced reductions in sEPSC frequency and amplitude to a level that is nearly identical to that observed in recordings from TP-treated animals (Fig. 11, B–D). Collectively, this suggests that the ability of TP to further reduce excitatory synaptic input onto anorexigenic POMC neurons requires, at least in part, an AMPK-dependent increase in endocannabinoid tone that leads to a more extensive activation of presynaptic CB1 receptors, as summarized in Fig. 12.
Fig. 10.
Depolarized-induced suppression of excitation (DSE) is potentiated in POMC neurons from TP-treated animals and blocked by the CB1 receptor antagonist AM251. The representative membrane current traces illustrate the changes in spontaneous excitatory postsynaptic current (sEPSC) frequency and amplitude elicited by DSE during recordings in slices from vehicle- (A) and TP-treated animals (B) as well as in slices pretreated with AM251 (C). The rectangular wave under the arrow labeled DSE represents the truncated change in membrane current caused by the 3-s, 60-mV depolarizing voltage command.
Fig. 11.
The AMPK inhibitor compound C (Comp. C) attenuates DSE in POMC neurons from TP-treated animals, whereas the AMPK activator metformin enhances DSE in POMC neurons from vehicle-treated animals. A and B: representative membrane current traces illustrating the changes in sEPSC frequency and amplitude elicited by DSE during recordings in slices from TP-treated animals that were pretreated with 30 μM of Comp. C (A) and in slices from vehicle-treated animals that were pretreated with 500 μM of metformin (B). The rectangular wave under the arrow labeled DSE represents the truncated change in membrane current caused by the 3-s, 60-mV depolarizing voltage command. C and D: composite bar graphs illustrate the DSE-induced changes in sEPSC frequency and amplitude, respectively, observed during the various treatment conditions. Bars and vertical lines represent means and 1 SE, respectively. *Values of poststimulus sEPSC frequency and amplitude that are significantly different (P < 0.05, Kruskal-Wallis/median-notched box-and-whisker analysis; n = 4–8) from those observed under basal conditions; #values of poststimulus sEPSC frequency and amplitude observed during recordings from TP-treated slices that were significantly different (P < 0.05, Kruskal-Wallis/median-notched Box-and-Whisker analysis; n = 4–8) from those from vehicle-treated slices.
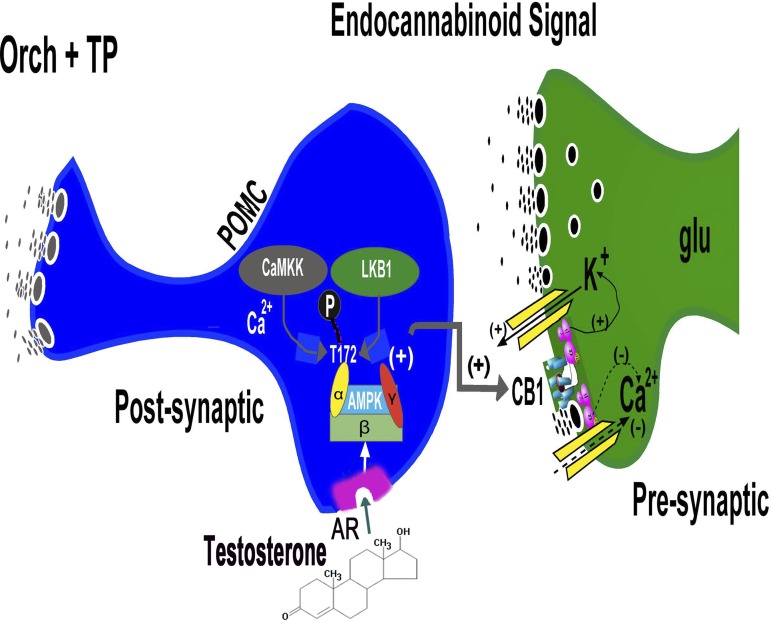
Fig. 12.
Schematic for how testosterone enhances CB1 receptor-mediated signaling at glutamatergic inputs impinging upon POMC neurons. Testosterone induces hyperphagia and potentiates cannabinoid tone at the CB1 receptors in the glutamatergic nerve terminals by activating cellular energy sensor AMPK. The rapidity of the testosterone-induced changes in energy balance suggests that the steroid may be acting at a membrane androgen receptor like that shown in striated muscle (17, 74, 78). The activation of this heterotrimeric AMPK complex may be due to upstream kinases like calmodulin-dependent protein kinase kinase (CaMKK) and liver kinase B1 (LKB1) via phosphorylation of threonine residue 172 (T172) of the α-subunit that occurs in response to increases in intracellular calcium and the AMP/ATP ratio. This in turn may lead to increased endocannabinoid tone by either enhancing synthesis, inhibiting breakdown, or attenuating reuptake and removal from the synaptic cleft. The endocannabinoids could then act transynaptically in a retrograde fashion at the CB1 receptor to augment the cannabinoid-induced presynaptic inhibition of glutamate release.
DISCUSSION
Taken together, these data demonstrate that androgen-induced increases in energy intake are mediated via an increase in cannabinoid sensitivity by a mechanism that involves the AMPK pathway in the ARC of the hypothalamus. These conclusions are based on the following observations: 1) TP increases energy intake and expenditure, 2) the hyperphagic effect of TP is rapidly reduced by antagonism of the hypothalamic CB1 receptors, 3) TP activates AMPK in the ARC, 4) TP potentiates the ability of cannabinoids to decrease mEPSC frequency and elicit DSE at POMC synapses, 5) TP enhances inhibitory GABAergic tone onto POMC neurons, 6) the AMPK inhibitor compound C attenuates DSE in POMC neurons from TP-treated animals, and 7) the AMPK activator metformin enhances DSE in POMC neurons from vehicle-treated animals.
Presently, we show that testosterone stimulates energy intake in orchidectomized male guinea pigs, which is consistent with what has been shown previously in other rodents (54, 62) as well as rams (4). This increase in consumption was associated with an increase in meal size but not meal frequency. We have previously validated our meal pattern criteria by performing logarithmic transformations of our intermeal interval and meal size data, which yielded unimodal frequency distributions and probability densities (19). Thus, we feel that our method is valid and that we can draw appropriate conclusions from the data obtained. The meal pattern data shown in the present study may reflect the more constant grazing pattern of consumption seen in guinea pigs, which eat smaller, more frequent meals and also ingest a greater proportion of their energy during the daylight (lights on) period than do rats (33, 35). Given these inherent differences in meal pattern and the diurnal fluctuations in energy intake, it should not be surprising that in orchidectomized rats testosterone increases meal frequency concomitant with decreased meal size (11). We also found that testosterone increased energy expenditure. This is congruent with the reduced energy expenditure observed in androgen receptor null mice that has been associated with decreased lipolysis and uncoupling protein 1 expression (18), decreased voluntary activity (62), or leptin and insulin resistance (45). In addition, testosterone dosing has been found to ameliorate the metabolic profile and reduce visceral adipose tissue in a high-fat diet-induced rabbit model of the metabolic syndrome (48).
Given that the testosterone-induced hyperphagia observed in the present study occurred as early as 1 h after administration, this rapid action could presumably be mediated by a plasma membrane receptor (17, 74) and carried out in a fashion similar to estrogens activating Gq-coupled membrane estrogen receptors (44, 60). Indeed, testosterone activates a plasma membrane G protein-coupled receptor, which results in the hydrolysis of phosphatidylinositol 4,5-bisphosphate by phospholipase C, which generates diacylglycerol and inositol 1,4,5-trisphosphate, the latter of which causes rapid Ca2+ release (74). Other membrane androgen receptors include the Gi-coupled GPRC6A that evokes extracellular signal-regulated protein kinase signaling and promotes the development of the male reproductive system, the Gs-coupled ZIP9 protein that promotes cellular apoptosis in cloaker ovarian follicular cells, and the one activated by testosterone albumin conjugates that suppresses c-Src stimulation and β-catenin expression to facilitate apoptosis in colonic tumor cells (24, 76). Although cardiac myocytes are clearly excitable cells, they reside in the periphery. Coupled with the observation that androgens can exert a rapid, positive allosteric modulation of GABAA receptor function in medial preoptic area (POA) neurons (56), our findings represent one of the first reports of rapid androgen actions in the central nervous system. However, the androgenic orexigenesis is also sustained, as can be seen by the increases in hourly intake during the nocturnal period (≤22 h after steroid administration). Cumulative intake was still elevated 24 h after administration, and this provided the rationale to examine the androgenic modulation of cannabinoid-induced changes in synaptic transmission to POMC neurons at this time point. However, an in-depth evaluation of the mechanisms underlying rapid, membrane-initiated androgenic signaling is certainly warranted and will provide the basis for future experimentation along these lines.
Arguably the most seminal finding of the present study is that TP activates of AMPK in the ARC. This is in agreement with the work of Wilson et al. (78), who showed that testosterone stimulates glucose uptake through glucose transporter type 4 (GLUT4) and activation of the CaMK II/AMPK signaling pathway in cultured cardiomyocytes. AMPK is a dynamic enzyme that plays an integral role in energy homeostasis. It has recently been shown that anorexigenic signals like leptin, insulin, and glucose reduce AMPKα2 activity, whereas orexigenic signals like ghrelin and hypoglycemia stimulate AMPKα2 activity in mediobasal hypothalamic lysates (2, 40, 42, 51). We propose that testosterone-induced activation of AMPK in the ARC is at least partly responsible for increased food intake caused by the steroid, which is in line with reports that virally mediated expression of activated or dominant-negative mutants of AMPK in the hypothalamus results in increased or decreased food intake, respectively (40, 51). A proposed mouse model for the role of AMPK activity in anorexigenic signaling in the hypothalamus suggest mediations by the MC4 receptor that occur in conjunction with changes in activity of ARC NPY/AGRP neurons (51). The signaling cascade is initiated by anorexigenic signals such as leptin, insulin, glucose or refeeding, which activates POMC neurons by STAT3 and PI3K producing a second anorexigenic signal facilitated by α-MSH in the PVN. These same anorexigenic signals inhibit AMPK activity and thus NPY/AgRP neuronal excitability, ultimately facilitating the activation of the MC4 receptor in PVN neurons. MC4 activation then decreases AMPK activity in the PVN, which may be necessary as a final common pathway in the regulation of energy balance (51). Our data, along with the work of others (4, 54, 62), clearly indicate that TP is serving as an orexigenic signal working opposite that of hormones like leptin and insulin to inhibit POMC cells. This notion provides further saliency for the increased AMPK activation in the ARC after TP treatment. Given that AMPK activity is enhanced during fuel deficiency (28), coupled with the anabolic and metabolic actions of testosterone (6, 8, 18, 45, 62), it is certainly conceivable that the orexigenesis elicited by the steroid is due to the sensation of a negative energy balance triggered within the hypothalamic feeding circuitry.
Presently, we found that systemic administration of the CB1 receptor antagonist AM251 completely blocked the androgenic hyperphagia, which occurred prior to the onset of its own anorexigenic effect. On the other hand, third ventricular administration of AM251 per se exerted a powerful anorexigenic effect seen 1–4 h after administration. Although AM251 did not fully reduce TP-induced changes in energy intake during this time to levels seen in the presence of AM251 alone, it did bring consumption down to levels observed in vehicle-treated controls. This is reflected in the statistical readout, where both AM251 and TP exerted significant, diametrically opposed main effects on energy intake, with no interaction between the two. Therefore, the most parsimonious explanation is that AM251 is dampening the hyperphagic effect of TP at the early time points. The cannabinoid regulation of energy homeostasis occurs at many levels, including, but not limited to, the gut and the mesolimbic dopamine pathways (9). We realize that the hypothalamic feeding circuitry is just one of the components involved. However, we believe that antagonism of hypothalamic CB1 receptors, although only partially effective early on, effectively disrupts the mechanism through which testosterone elicits its orexigenic effect. The fact that AM251 completely blocks the TP-induced increase in cumulative energy intake at time points when the anorexigenic effect of the antagonist has long since vanished lends particular credence to the idea that hypothalamic CB1 receptors play an instrumental role in this process.
Our results demonstrating that testosterone stimulates energy intake that is blocked by hypothalamic CB1 receptor antagonism and promotes ARC AMPK activity that heightens endocannabinoid tone onto POMC neurons are consistent with what has been shown for other orexigenic hormones. For example, ghrelin increases hypothalamic AMPK activity, and an intact CB1 receptor is crucial for this effect (41). Ghrelin was also found to inhibit the excitatory inputs onto parvocellular neurons of the PVN, and this effect was eliminated by introducing either the CB1 receptor antagonist rimonabant or the diacylglycerol lipase inhibitor tetrahydrolipstatin that blocks 2-AG synthesis (41). Thus, testosterone, like ghrelin, increases hypothalamic AMPK activity, reduces excitatory input onto critical elements of the hypothalamic feeding circuitry, and stimulates appetite via a CB1 receptor-dependent mechanism.
The dynamic actions of cannabinoids at POMC synapses are sexually differentiated. For example, the potency of WIN 55,212-2 to presynaptically inhibit GABAA receptor-mediated input is reduced approximately six times in males compared with females (16). Cannabinoids also enhance a postsynaptic A-type K+ current (IA) in the POMC neurons of females, whereas in males CB1 receptor activation leads to the activation of G protein-gated, inwardly-rectifying K+ channels (34, 72). This sexually differentiated cannabinoid regulation of appetite can be additionally modified by gonadal steroid hormones. For example, estradiol substantially reduces the ability of CB1 receptor agonists to presynaptically inhibit ionotropic glutamate receptor-mediated excitation of POMC cells and enhance the IA in these cells (39, 53). This steroid is able to elicit its negative modulatory effect within minutes after bath application to hypothalamic slices and lasts for at least 24 h after systemic treatment (39, 53). Estradiol's ability to disrupt cannabinoid signaling is due to the activation of estrogen receptor-α and the Gq-coupled membrane estrogen receptor, which triggers a signaling cascade involving the activation of PI3K, protein kinase C-δ, and, to some extent, protein kinase A (38, 77). Therefore, CB1 receptors are less effective in decreasing glutamatergic neurotransmission, leading to increased excitation of POMC neurons and enhanced anorexigenic tone within the hypothalamic feeding circuitry that accounts for the ability of estradiol to reduce cannabinoid-induced hyperphagia in females (39, 77). Presently, we found the exact opposite to be true for testosterone in males. Testosterone enhanced the cannabinoid-induced presynaptic inhibition of glutamate release onto POMC neurons and augmented endocannabinoid-mediated DSE in these cells via activation of AMPK. The fact that we clearly observed DSE in our guinea pig animal model contrasts with what was reported in transgenic mice (31, 69). Guinea pigs have proven to be more sensitive than rats or mice during the development of appetite-suppressing drugs like fenfluramine and fluoxetine, and like humans, they lack the capacity to synthesize their own vitamin C (3, 50, 57). Thus, our guinea pig animal model has clear utility and translational relevance in the study of the synaptic and hormonal determinants underlying the hypothalamic control of energy homeostasis. Finally, although testosterone was without effect on the rather modest, cannabinoid-induced, presynaptic inhibition of GABA release onto POMC neurons, it markedly elevated basal inhibitory input received by these cells. This is in keeping with the fact that testosterone increases GABA turnover in the POA and median eminence of male rodents (20–22). This is consistent with the observation that anabolic steroids increase firing in GABAergic neurons in the medial POA as well as spontaneous IPSC frequency in downstream gonadotropin-releasing hormone (GnRH) neurons, the latter of which suppresses firing in these cells (55). The androgenic potentiation of GABAergic tone in the medial POA is reportedly dependent on the protein kinase C-mediated phosphorylation state of the β3-subunit of the GABAA receptor (56). Similar findings reported by Sullivan and Moenter (71) were seen in prenatally androgenized female rats, which exhibited augmented GABAA receptor-mediated input onto GnRH neurons in the medial POA. We have observed previously that cannabinoid agonists are approximately six times less potent in decreasing mIPSC frequency in orchidectomized males than in ovariectomized females (16). However, presently, we found that testosterone replacement does not appreciably alter cannabinoid agonist potency in this regard. Thus, the androgenic enhancement of GABAA receptor function appears to be independent of the enhancement of endocannabinoid tone. These collective modulatory actions of testosterone serve to enhance inhibitory tone onto POMC neurons and are congruent with the fact that the steroid increases and decreases the number of NPY/AgRP and POMC neurons, respectively, in the ARC (54, 68). This provides a regulatory framework that helps explain the mechanisms underlying testosterone-induced hyperphagia in males.
In conclusion, the results of the present study demonstrate that testosterone increases energy intake in male guinea pigs via activation of AMPK in the ARC and augmentation of endocannabinoid tone in anorexigenic POMC neurons. Most of the clinical studies touting the ability of cannabinoids to stimulate appetite in cachexic patients used experimental subjects that were almost exclusively male (25, 52, 66, 75, 80). However, in one of the few double-blind, placebo-controlled studies where the sex ratio was more evenly split, the results were far less impressive (10a). This reinforces the need to develop rational, sex difference-based therapeutic adjuncts for use in ameliorating the symptoms associated with HIV/AIDS and cancer.
GRANTS
This study was supported by US Public Health Service Grants DA-024314 and HD-058638.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.B. and E.J.W. conception and design of research; A.B., C.M., and E.J.W. performed experiments; A.B., C.M., and E.J.W. analyzed data; A.B., C.M., and E.J.W. interpreted results of experiments; A.B. and E.J.W. prepared figures; A.B. and E.J.W. drafted manuscript; A.B., C.M., and E.J.W. edited and revised manuscript; A.B., C.M., and E.J.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Steve Do for technical assistance.
REFERENCES
- 1.Abel EL. Effects of marihuana on the solution of anagrams, memory and appetite. Nature 231: 260–261, 1971. [DOI] [PubMed] [Google Scholar]
- 2.Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279: 12005–12008, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Anelli M, Bizzi A, Caccia S, Codegoni AM, Fracasso C, Garattini S. Anorectic activity of fluoxetine and norfluoxetine in mice, rats and guinea-pigs. J Pharm Pharmacol 44: 696–698, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Anukulkitch C, Rao A, Dunshea FR, Blache D, Lincoln GA, Clarke IJ. Influence of photoperiod and gonadal status on food intake, adiposity, and gene expression of hypothalamic appetite regulators in a seasonal mammal. Am J Physiol Regul Integr Comp Physiol 292: R242–R252, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Avraham Y, Menachem AB, Okun A, Zlotarav O, Abel N, Mechoulam R, Berry EM. Effects of the endocannabinoid noladin ether on body weight, food consumption, locomotor activity, and cognitive index in mice. Brain Res Bull 65: 117–123, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bardin CW. The anabolic action of testosterone. N Engl J Med 335: 52–53, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Batterham MJ, Garsia R. A comparison of megestrol acetate, nandrolone decanoate and dietary counselling for HIV associated weight loss. Int J Androl 24: 232–240, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab 82: 407–413, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Borgquist A, Wagner EJ. On the cannabinoid regulation of energy homeostasis: past, present and future. In: Endocannabinoids: Molecular, Pharmacological, Behavioral and Clinical Features, edited by Murillo-Rodŕiguez E, Onaivi ES, Darmani NA, and Wagner EJ. Oak Park, IL: Bentham Science, 2013, p. 60–91. [Google Scholar]
- 10.Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry 58: 917–924, 2001. [DOI] [PubMed] [Google Scholar]
- 10a.Cannabis-In-Cachexia-Study-Group, Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, Ko YD, Schnelle M, Reif M, Cerny T. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol 24: 3394–3400, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Chai JK, Blaha V, Meguid MM, Laviano A, Yang ZJ, Varma M. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am J Physiol Regul Integr Comp Physiol 276: R1366–R1373, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Corcoran C, Grinspoon S. Treatments for wasting in patients with the acquired immunodeficiency syndrome. N Engl J Med 340: 1740–1750, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258: 1946–1949, 1992. [DOI] [PubMed] [Google Scholar]
- 14.Di Marzo V, Goparaju SK, Wang L, Liu J, Bátkai S, Járai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410: 822–825, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 23: 4850–4857, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz S, Farhang B, Hoien J, Stahlman M, Adatia N, Cox JM, Wagner EJ. Sex differences in the cannabinoid modulation of appetite, body temperature and neurotransmission at POMC synapses. Neuroendocrinology 89: 424–440, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estrada M, Espinosa A, Müller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology 144: 3586–3597, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S, Nawata H. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes 54: 1000–1008, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Farhang B, Pietruszewski L, Lutfy K, Wagner EJ. The role of the NOP receptor in regulating food intake, meal pattern, and the excitability of proopiomelanocortin neurons. Neuropharmacology 59: 190–200, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grattan DR, Rocca MS, Sagrillo CA, McCarthy MM, Selmanoff M. Antiandrogen microimplants into the rostral medial preoptic area decrease gamma-aminobutyric acidergic neuronal activity and increase luteinizing hormone secretion in the intact male rat. Endocrinology 137: 4167–4173, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Grattan DR, Selmanoff M. Castration-induced decrease in the activity of medial preoptic and tuberoinfundibular GABAergic neurons is prevented by testosterone. Neuroendocrinology 60: 141–149, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Grattan DR, Selmanoff M. Prolactin- and testosterone-induced inhibition of LH secretion after orchidectomy: role of preoptic and tuberoinfundibular gamma-aminobutyric acidergic neurones. J Endocrinol 143: 165–174, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Greenman Y, Rouach V, Limor R, Gilad S, Stern N. Testosterone is a strong correlate of ghrelin levels in men and postmenopausal women. Neuroendocrinology 89: 79–85, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Gu S, Honisch S, Kounenidakis M, Alkahtani S, Alarifi S, Alevizopoulos K, Stournaras C, Lang F. Membrane androgen receptor down-regulates c-Src-activity and beta-catenin transcription and triggers GSK-3beta-phosphorylation in colon tumor cells. Cell Physiol Biochem 34: 1402–1412, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV+ marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology (Berl) 181: 170–178, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Hardie DG. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci 117: 5479–5487, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546: 113–120, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Harrold JA, Williams G. The cannabinoid system: a role in both the homeostatic and hedonic control of eating? Br J Nutr 90: 729–734, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2: 9–19, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci 25: 9746–9751, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herbison AE, Skinner DC, Robinson JE, King IS. Androgen receptor-immunoreactive cells in ram hypothalamus: distribution and co-localization patterns with gonadotropin-releasing hormone, somatostatin and tyrosine hydroxylase. Neuroendocrinology 63: 120–131, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch E, Collier G. The ecological determinants of reinforcement in the guinea pig. Physiol Behav 12: 239–249, 1974. [DOI] [PubMed] [Google Scholar]
- 34.Ho J, Cox JM, Wagner EJ. Cannabinoid-induced hyperphagia: correlation with inhibition of proopiomelanocortin neurons? Physiol Behav 92: 507–519, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horton BJ, West CE, Turley SD. Diurnal variation in the feeding pattern of guinea pigs. Nutr Metab 18: 294–301, 1975. [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology 144: 1331–1340, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol 134: 1151–1154, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeffery GS, Peng KC, Wagner EJ. The role of phosphatidylinositol-3-kinase and AMP-activated kinase in the rapid estrogenic attenuation of cannabinoid-induced changes in energy homeostasis. Pharmaceuticals 4: 630–651, 2011. [Google Scholar]
- 39.Kellert BA, Nguyen MC, Nguyen C, Nguyen QH, Wagner EJ. Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis. Eur J Pharmacol 622: 15–24, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med 10: 727–733, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, Harvey-White J, Liposits Z, Kunos G, Grossman AB, Fekete C, Korbonits M. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One 3: e1797, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem 280: 25196–25201, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29: 717–727, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Levin ER. Cellular functions of the plasma membrane estrogen receptor. Trends Endocrinol Metab 10: 374–377, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Lin HY, Xu Q, Yeh S, Wang RS, Sparks JD, Chang C. Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes 54: 1717–1725, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci 15: 6552–6561, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron 31: 463–475, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Maneschi E, Morelli A, Filippi S, Cellai I, Comeglio P, Mazzanti B, Mello T, Calcagno A, Sarchielli E, Vignozzi L, Saad F, Vettor R, Vannelli GB, Maggi M. Testosterone treatment improves metabolic syndrome-induced adipose tissue derangements. J Endocrinol 215: 347–362, 2012. [DOI] [PubMed] [Google Scholar]
- 49.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50: 83–90, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Mennini T, Bizzi A, Caccia S, Codegoni A, Fracasso C, Frittoli E, Guiso G, Padura IM, Taddei C, Uslenghi A, Garattini S. Comparative studies on the anorectic activity of d-fenfluramine in mice, rats, and guinea pigs. Naunyn Schmiedebergs Arch Pharmacol 343: 483–490, 1991. [DOI] [PubMed] [Google Scholar]
- 51.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428: 569–574, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Nelson K, Walsh D, Deeter P, Sheehan F. A phase II study of delta-9-tetrahydrocannabinol for appetite stimulation in cancer-associated anorexia. J Palliat Care 10: 14–18, 1994. [PubMed] [Google Scholar]
- 53.Nguyen QH, Wagner EJ. Estrogen differentially modulates the cannabinoid-induced presynaptic inhibition of amino acid neurotransmission in proopiomelanocortin neurons of the arcuate nucleus. Neuroendocrinology 84: 123–137, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Nohara K, Zhang Y, Waraich RS, Laque A, Tiano JP, Tong J, Münzberg H, Mauvais-Jarvis F. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology 152: 1661–1669, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oberlander JG, Penatti CAA, Porter DM, Henderson LP. The buzz about anabolic androgenic steroids: electrophysiological effects in excitable tissues. Neuroendocrinology 96: 141–151, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oberlander JG, Porter DM, Onakomaiya MM, Penatti CAA, Vithlani M, Moss SJ, Clark AS, Henderson LP. Estrous cycle variations in GABAA receptor phosphorylation enable rapid modulation by anabolic androgenic steroids in the medial preoptic area. Neuroscience 226: 397–410, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Odumosu A. Vitamin C and weight reducing drugs on brain ascorbic acid in guinea pigs. Acta Vitaminol Enzymol 3: 96–102, 1981. [PubMed] [Google Scholar]
- 58.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 29: 729–738, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Piomelli D, Giuffrida A, Calignano A, Rodríguez de Fonseca F. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol Sci 21: 218–224, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Qiu J, Rønnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids 73: 985–991, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, Ku S, Wagner EJ, Rønnekleiv OK, Kelly MJ. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab 19: 682–693, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rana K, Fam BC, Clarke MV, Pang TP, Zajac JD, MacLean HE. Increased adiposity in DNA binding-dependent androgen receptor knockout male mice associated with decreased voluntary activity and not insulin resistance. Am J Physiol Endocrinol Metab 301: E767–E778, 2011. [DOI] [PubMed] [Google Scholar]
- 63.Roland AV, Moenter SM. Glucosensing by GnRH neurons: inhibition by androgens and involvement of AMP-activated protein kinase. Mol Endocrinol 25: 847–858, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roland AV, Moenter SM. Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (GnRH) neurons that is reversed by metformin treatment in adulthood. Endocrinology 152: 618–628, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roselli CE, Horton LE, Resko JA. Time-course and steroid specificity of aromatase induction in rat hypothalamus-preoptic area. Biol Reprod 37: 628–633, 1987. [DOI] [PubMed] [Google Scholar]
- 66.Sacks N, Hutcheson JR, Watts JM, Webb RE. Case report: the effect of tetrahydrocannabinol on food intake during chemotherapy. J Am Coll Nutr 9: 630–632, 1990. [DOI] [PubMed] [Google Scholar]
- 67.Scott CJ, Clarke IJ, Rao A, Tilbrook AJ. Sex differences in the distribution and abundance of androgen receptor mRNA-containing cells in the preoptic area and hypothalamus of the ram and ewe. J Neuroendocrinol 16: 956–963, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Sheppard KM, Padmanabhan V, Coolen LM, Lehman MN. Prenatal programming by testosterone of hypothalamic metabolic control neurones in the ewe. J Neuroendocrinol 23: 401–411, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sinnayah P, Jobst EE, Rathner JA, Caldera-Siu AD, Tonelli-Lemos L, Eusterbrock AJ, Enriori PJ, Pothos EN, Grove KL, Cowley MA. Feeding induced by cannabinoids is mediated independently of the melanocortin system. PLoS One 3: e2202, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA 101: 7129–7134, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang SL, Tran V, Wagner EJ. Sex differences in the cannabinoid modulation of an A-type K+ current in neurons of the mammalian hypothalamus. J Neurophysiol 94: 2983–2986, 2005. [DOI] [PubMed] [Google Scholar]
- 73.Tucci SA, Rogers EK, Korbonits M, Kirkham TC. The cannabinoid CB1 receptor antagonist SR141716 blocks the orexigenic effects of intrahypothalamic ghrelin. Br J Pharmacol 143: 520–523, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vicencio JM, Ibarra C, Estrada M, Chiong M, Soto D, Parra V, Diaz-Araya G, Jaimovich E, Lavandero S. Testosterone induces an intracellular calcium increase by a nongenomic mechanism in cultured rat cardiac myocytes. Endocrinology 147: 1386–1395, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Walsh D, Kirkova J, Davis MP. The efficacy and tolerability of long-term use of dronabinol in cancer-related anorexia: a case series. J Pain Symptom Manage 30: 493–495, 2005. [DOI] [PubMed] [Google Scholar]
- 76.Wang C, Liu Y, Cao JM. G protein-coupled receptors: extranuclear mediators for the non-genomic actions of steroids. Int J Mol Sci 15: 15412–15425, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Washburn N, Borgquist A, Wang K, Jeffery GS, Kelly MJ, Wagner EJ. Receptor subtypes and signal transduction mechanisms contributing to the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis. Neuroendocrinology 97: 160–175, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson C, Contreras-Ferrat A, Venegas N, Osorio-Fuentealba C, Pávez M, Montoya K, Durán J, Maass R, Lavandero S, Estrada M. Testosterone increases GLUT4-dependent glucose uptake in cardiomyocytes. J Cell Physiol 228: 2399–2407, 2013. [DOI] [PubMed] [Google Scholar]
- 79.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410: 588–592, 2001. [DOI] [PubMed] [Google Scholar]
- 80.Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manage 29: 358–367, 2005. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida T, Hashimoto K, Zimmer A, Maejima T, Araishi K, Kano M. The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar Purkinje cells. J Neurosci 22: 1690–1697, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]