Abstract
Substance P (SP) and its receptor, neurokinin-1 (NK1R), have been shown to be excitatory modulators of respiratory frequency and to stabilize breathing regularity. Studies in anesthetized mice suggest that tonic activation of NK1Rs is particularly important when other excitatory inputs to the pre-Bötzinger complex in the ventral respiratory column (VRC) are attenuated. Consistent with these findings, muscarinic receptor blockade in the VRC of intact goats elicits an increase in breathing frequency associated with increases in SP and serotonin concentrations, suggesting an involvement of these substances in neuromodulator compensation. To gain insight on the contribution to breathing of endogenous SP and NK1R activation, and how NK1R modulates the release of other neurochemicals, we individually dialyzed antagonists to NK1R (133, 267, 500 μM Spantide; 3 mM RP67580) throughout the VRC of awake and sleeping goats. We found that NK1R blockade with either Spantide at any dose or RP67580 had no effect on breathing or regularity. Both antagonists significantly (P < 0.001) increased SP, while RP67580 also increased serotonin and glycine and decreased thyrotropin-releasing hormone concentrations in the dialysate. Taken together, these data support the concept of neuromodulator interdependence, and we believe that the loss of excitatory input from NK1Rs was locally compensated by changes in other neurochemicals.
Keywords: neurokinin-1 receptor, substance P, neuromodulator interdependence, control of breathing
the neuropeptide, substance p (sp), and its receptor, the neurokinin-1 receptor (NK1R), have been implicated in mediating diverse physiological functions, such as the central control of breathing, intestinal contraction, inflammation, and nociception (11, 41). NK1Rs are G protein-coupled receptors, the activation of which leads to mobilization of intracellular Ca2+ stores, activation of protein kinase C and protein kinase A, and the ERK1/2 pathway (8, 41). NK1Rs are expressed throughout the central nervous system, including nuclei involved in respiratory control (12, 27). The pre-Bötzinger complex (preBötC), the hypothesized kernel of inspiratory rhythm generation in the ventral respiratory group (VRG) of the medulla (38), has been shown to express NK1Rs (or SP immunoreactivity) in mammalian and nonmammalian animal models, including the rat (12, 14), goat (19), human (35), and lamprey (7).
Various lines of evidence in reduced preparations and intact animals demonstrate that SP is an excitatory neuromodulator in the control of breathing. Application of SP onto brainstem slices containing the preBötC increases “fictive” respiratory frequency (12), whereas blocking NK1Rs reduces respiratory frequency (10, 16, 33). In vivo studies in goats and rats in which NK1R-expressing neurons are destroyed results in abnormal breathing patterns and altered ventilatory responses to inspired gases (11, 31, 45). Furthermore, studies in anesthetized mice suggest that tonic activation of NK1Rs in the preBötC becomes particularly important when α1 noradrenergic receptors and serotonin (5-HT) type 2 receptors are blocked (10). Finally, our group has previously found that reverse dialysis of a muscarinic cholinergic receptor antagonist (atropine) in the ventral respiratory column (VRC) of intact goats, during wakefulness and sleep, elicits a significant increase in breathing frequency (28). This increase in frequency may have been mediated by concurrent increases in 5-HT and SP (28), perhaps due to corelease of these neurochemicals from a subset of raphé serotonergic neurons that colocalize SP and project to the preBötC (33). If such were the case, these findings would be consistent with the concept of neuromodulator interdependence, whereby attenuation of one neuromodulator is locally compensated for by changes in other neuromodulators (e.g., an increase in excitatory neurochemicals or a decrease in inhibitory neurotransmitters).
We sought to gain insight into the contribution to eupneic breathing of NK1Rs in the VRC of intact goats during wakefulness and natural non-rapid eye movement (NREM) sleep. Since past studies in intact animals involved destruction of NK1R-expressing neurons with neurotoxins (11, 31, 45), which would eliminate other neurochemicals coexpressed by these neurons, we utilized dialysis of receptor antagonists to meet our objectives. An additional advantage of dialysis is that it allows for the collection of effluent fluid containing endogenous neurochemicals and associating changes in neurochemical content with simultaneous changes in breathing. We hypothesize that dialysis of a NK1R antagonist would either 1) depress breathing in awake and sleeping goats without altering the effluent concentrations of excitatory and inhibitory neurochemicals or 2) result in no change in breathing, but would elicit changes in the concentrations of excitatory and/or inhibitory neurochemicals.
METHODS
Animals.
Seventeen nonpregnant, adult female goats weighing 44.9 ± 2.1 kg were used in this study. Animals were permanently housed in an environmental chamber with a fixed 12-h light/dark cycle (lights on 7:00 AM). Goats were given ad libitum access to food and water, except during a 24-h fast prior to all surgeries and while under study. All aspects of this project have been reviewed and approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin before beginning studies.
Surgical procedures.
All surgeries were performed under sterile condition. Prior to all surgeries, goats were anesthetized with 5.0 ml ketamine (100 mg/ml, IV), intubated, and then mechanically ventilated (2% isoflurane, 100% oxygen); banamine (flunixin meglumine, 1 mg/kg, IM) was given perioperatively for pain. Rectal temperature, blood oxygen saturation, and heart rate were monitored throughout and for 24 h after surgery. Naxcel (ceftiofur sodium, 4 mg/kg, IM) was given daily, and triple antibiotic was applied to all surgical sites for 7 d. To alleviate pain, buprenex (buprenorphine hydrochloride, 0.005 mg/kg, IM) was administered twice daily for 48 h after all surgeries.
Following a ≥3-d acclimatization period, an instrumentation surgery was performed, in which electroencephalogram (EEG) and electrooculogram (EOG) electrodes were permanently implanted in the midline cranium and superior orbital ridge, respectively, to monitor and score sleep state. Goats were allowed to recover for ≥1 wk before a craniotomy was performed for chronic implantation of stainless steel microtubules (MTs) (70.0-mm length, 1.27-mm outer diameter, 0.84-mm inner diameter) targeting a site just dorsal to the preBötC. The goat preBötC is ∼2.5–3.5 mm rostral from obex, 4.0–5.0 mm lateral from the midline, and 4.0–6.0 mm from the dorsal surface of the medulla, ventral to nucleus ambiguus (19, 45). For some animals, coordinates for implantation were adjusted to avoid blood vessels on the dorsal surface. MTs were secured to the skull with dental acrylic, the skin sutured closed, and stylets matching the length of the MTs were inserted, such that there was no penetration of the tissue. Animals were given ≥2 wk to recover from MT implantation.
Physiological variables.
Goats were acclimated to study equipment and procedures. Respiratory parameters were measured with a custom-made mask secured to the goat's muzzle. A one-way breathing valve was attached to the mask and connected to inspiratory and expiratory tubing. A pneumotachograph was attached to the inspiratory tubing to allow measurement of inspiratory flow (minute ventilation, VI, liter/min), breathing frequency (br/min), and tidal volume (VT, liter/br), all of which were continuously recorded on a Windaq data acquisition system. Rectal temperature was monitored with a thermocouple inserted into the rectum. Data were collected in a similar manner during night studies, except EEG and EOG activities were additionally recorded to monitor and score sleep state.
In vivo dialysis.
Dialysis probes (Harvard Apparatus, Holliston, MA) were 72.0 mm long, composed of a 70.0-mm shaft and a 2.0-mm membrane that was 0.5 mm in diameter (20 kDa cut-off, 3 μl internal volume). The dimensions of the probe thus allowed for insertion into the MT, such that only the membrane penetrated the tissue. The perfusate was either 1) mock cerebrospinal fluid (mCSF) (124 mM NaCl, 2.0 mM KCl, 2.0 mM MgCl2, 1.3 mM K2PO4, 2.0 mM CaCl2, 11 mM glucose, 26 mM NaHCO3−, and pH 7.32 in sterile distilled water) alone; 2) the peptide-based NK1R antagonist, Spantide I (SPA, 133, 267, or 500 μM; US Biological), dissolved in mCSF; or 3) the nonpeptide NK1R antagonist, RP67580 (RP, 1, 2, or 3 mM; Tocris), dissolved in mCSF and 1, 2, or 3% dimethyl sulfoxide (DMSO) (vol/vol), respectively. Because of limited sample sizes and large variability for the lower two doses of RP (1 mM, n = 4; 2 mM, n = 3), respiratory data from these studies were not reported. SPA reportedly has weak agonist effects on phospholipase C activity and is less potent than RP (4); for these reasons, we decided to use two different antagonists for this study and compare their effects. Prior to dialysis, all perfusate solutions were warmed to 39.0°C and equilibrated with 6.4% carbon dioxide and 21% oxygen (nitrogen balanced) in a tonometer. A 180-cm length of polypropylene tubing was required to connect the syringe pump to the dialysis probe.
A probe was inserted into one MT (as in previous studies) (28) and allowed to equilibrate with the tissue for 30 min, after which baseline (predialysis) data were collected for another 30 min. Three 60-min periods (Hours 1–3) of unilateral dialysis (25 μl/min) then followed in the order: 1) mCSF, 2) NK1R antagonist, 3) mCSF. In the case of RP, vehicle solutions dialyzed in Hours 1 and 3 consisted of mCSF with the appropriate concentration of DMSO. The effluent fluid (dialysate) was continuously collected in cryotubes for each hour, aliquoted, and then frozen at −80°C for later analysis of neurochemical content. Day studies were completed between 9 AM and 2 PM; night studies were completed between 8 PM and 2 AM. Any consecutive studies within an individual animal were separated by ≥36 h.
mCSF and glutamate receptor agonist injections.
Since glutamate receptor agonists at the preBötC typically elicit a tachypneic respiratory response (26, 39), we injected the glutamate receptor agonist, N-methyl-D-aspartic acid (NMDA) as a functional indicator of MT placement within the region of the preBötC. In these studies, breathing was continuously monitored, while either mCSF (500 nl) or NMDA (100 mM, 500 nl) was injected into a MT, separated by 30-min intervals. NMDA injection studies were performed on separate days at least 24 h prior to dialysis studies.
Neurochemical analysis.
All effluent dialysate samples were analyzed by a core assay lab, as previously described (28). Briefly, glutamine (GLN), glycine (GLY), GABA, and 5-HT were measured via reverse-phase high-performance liquid chromatography with a Waters Resolve C18 column. Thyrotropin-releasing hormone (TRH) and SP were measured with commercial assays from MyBioSource (MBS044339, range 0.625–20 μIU/ml) and Assay Designs (900–018, range 9.76–10,000 pg/ml), respectively, and a microplate reader at 405 nm.
Data and statistical analysis.
All ventilatory data were analyzed on a breath-by-breath basis using custom-made programs. The inspiratory flow signal at the end of each study was calibrated according to known air flow rates. For night studies, EEG and EOG activity was used to visually score each breath as being in the awake or NREM sleep states. Scoring was performed by the same investigator for all studies.
As in past reports (29), we compared daytime ventilatory data from the present study with established time control studies (28) we previously conducted, in which mCSF alone was dialyzed for Hours 1–3. The present studies were carried out by identical methodologies and under the same conditions as time control studies (28). We have decided to reuse and reanalyze these control data for two important reasons: 1) to minimize the number of goats used, since repetition of control experiments may violate policies regarding using the smallest number of animals to address scientific questions, and 2) we believe that direct comparisons between time control data and data from the present study are necessary for scientific interpretation and to frame our present results in context with previous findings.
Data were binned in 15-min bins, as per past studies (28), and 1-min bins to detect potentially small and/or rapid changes in breathing. For each bin size, a two-way repeated-measures (RM) ANOVA (one factor repetition, group and time as factors, Holm-Sidak post hoc, when appropriate) was performed for each respiratory parameter. We used the P value of the interaction term from the results of two-way RM ANOVAs to determine if the effect on breathing over time of antagonist dialysis was significantly different from time control studies. To reduce noise, we limited the period of analysis to the last 15 min of Hour 1 to the end of Hour 3. Results of two-way RM ANOVAs using 1- and 15-min bins were largely consistent; thus we report only data using the 15-min bin to allow for direct comparisons with past data. For night studies, data were binned in 15-min bins and analyzed via two-way RM ANOVA (two factor repetition, sleep state and time as factors, Holm-Sidak post hoc, when appropriate).
Similarly, two-way RM ANOVAs (one factor repetition, group and hour as factors, Holm-Sidak post hoc, when appropriate) were used to determine if NK1R antagonist dialysis had significant effects on the effluent concentrations of 5-HT, GLN, GLY, GABA, and SP relative to time control studies. The P values of the interaction term were used to determine if the effect on neurochemical concentration over time of antagonist dialysis was significantly different from time control. Since time control studies were not performed at night, and since we are unable to separate neurochemical values according to sleep state, the nighttime neurochemical values were analyzed with a one-way RM ANOVA (Holm-Sidak post hoc, when appropriate). For TRH, only daytime values from Hours 1 and 2 were available for each antagonist at the highest dose. A paired t-test was used to determine if there was a significant effect of antagonist dialysis on effluent TRH concentration (two-tailed P values reported). Simple linear regressions were performed to determine if antagonist concentration was a significant predictor of the absolute change in effluent neurochemical concentration between Hours 1 and 2 (SP) or between Hours 1 and 3 (5-HT, GLN, GLY, GABA).
Data from replicate studies within a single animal were averaged. All statistical analyses were performed with SigmaPlot (12.0 or 12.5), and all results are presented as means ± SE; values of P < 0.05 were considered statistically significant.
Histology.
Upon completion of the protocol, animals were sedated with 2.3 ml ketamine (100 mg/ml, IV). Cerebral circulation was isolated, and the goat was killed with 10.0 ml B-euthanasia (IV). The circulation of the head was perfused with phosphate buffered saline (PBS) via an arterial (carotid) catheter, followed by 4% paraformaldehyde in PBS. The brainstem was extracted and processed for sectioning and subsequent Nissl staining. Nissl-stained 4000 DPI-scanned images were captured (Nikon Super Coolscan 9000), and Metamorph software was used to determine MT placement (at the approximate middle of the rostral-caudal damage range).
RESULTS
Histology and microtubule placement.
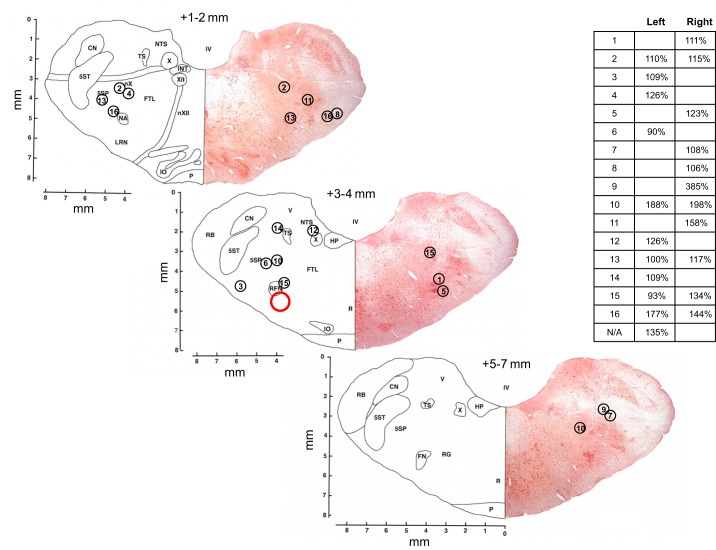
The goat preBötC is located ∼2.5–3.5 mm rostral from obex, 4.0–5.0 mm lateral from the midline, and 4.0–6.0 mm from the dorsal surface (45). Shown in Fig. 1 are the MT placements and corresponding NMDA responses for the animals studied herein. As in past studies (28, 29), there is variation in both MT placement, as determined by histology, and NMDA responses. Thus neither index provides absolute confirmation of the exact site of dialysis, as previously discussed in greater detail (28, 29). Despite this limitation, we have historically found changes in SP with antagonist dialysis to be the most highly consistent between animals for a given treatment. Since changes in SP with antagonist dialysis in these studies were largely consistent, we decided to include all animals shown in Fig. 1 for analysis.
Fig. 1.
Microtubule (MT) placements and corresponding N-methyl-D-aspartic acid injection (100 mM, 500 nl) responses expressed as percent of control (inset) for animals used in this study. MT placements ranged throughout the rostral-caudal distances indicated here, but for simplicity, placements are illustrated on only three representative sections. Tissue from one animal was unusable for Nissl staining. Numbers above each section indicate approximate rostral distance from obex. Red circle indicates approximate location of the goat pre-Bötzinger complex.
Effect on breathing of dialysis of NK1R antagonists.
The effect on breathing of dialysis of SPA or RP over time was not significantly different from time control studies. Our results also indicate that the effect on breathing over time was not significantly different between the three doses of SPA. Comparison of the effects on VI of time control studies (n = 7) and daytime dialysis of SPA at three different doses (133 μM, n = 7; 267 μM, n = 5; 500 μM, n = 6) found no significant interaction effects (P = 0.757, Fig. 2A). Likewise, there were no significant interaction effects in terms of frequency (P = 0.377, Fig. 2B) and VT (P = 0.115, Fig. 2C). Comparison of variability (coefficient of variation, CV) in VI, frequency, and VT with SPA dialysis found no significant interaction effects (P ≥ 0.061, data not shown). Comparison of the effects on breathing of time control studies and dialysis of 3 mM RP (n = 7) in the day found no significant interaction effects for any respiratory parameter (P ≥ 0.313, Fig. 3, A–C). Comparison of variability in VI, frequency, and VT with RP dialysis found no significant interaction effects (P ≥ 0.288, data not shown). There were no significant interaction effects (P ≥ 0.072, data not shown) between sleep state and time for all respiratory parameters (VI, frequency, VT) with dialysis of 500 μM SPA at night (n = 4).
Fig. 2.
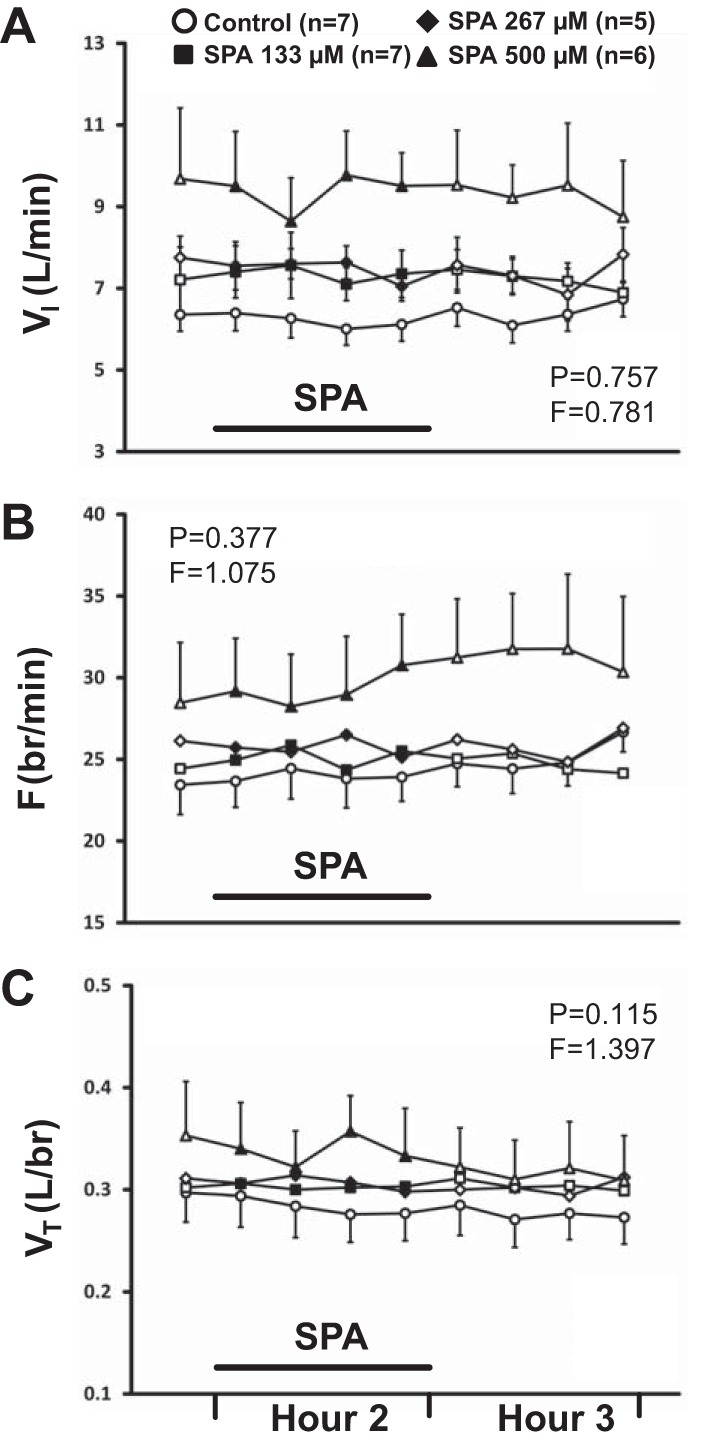
The effect on breathing over time of dialysis of Spantide (SPA) in the day at three different doses (133 μM, n = 7; 267 μM, n = 5; 500 μM, n = 6) was not significantly (P ≥ 0.115) different from the effect of time control studies (n = 7). A: minute ventilation (VI, liter/min); B: frequency (F, br/min); C: tidal volume (VT, liter/br). First data point represents last bin of Hour 1. Closed symbols and bar indicate period of SPA dialysis (Hour 2); open symbols indicate mCSF dialysis. F and P indicate values for interaction term from two-way repeated-measures (RM) ANOVA (see methods for further details on statistical analysis).
Fig. 3.
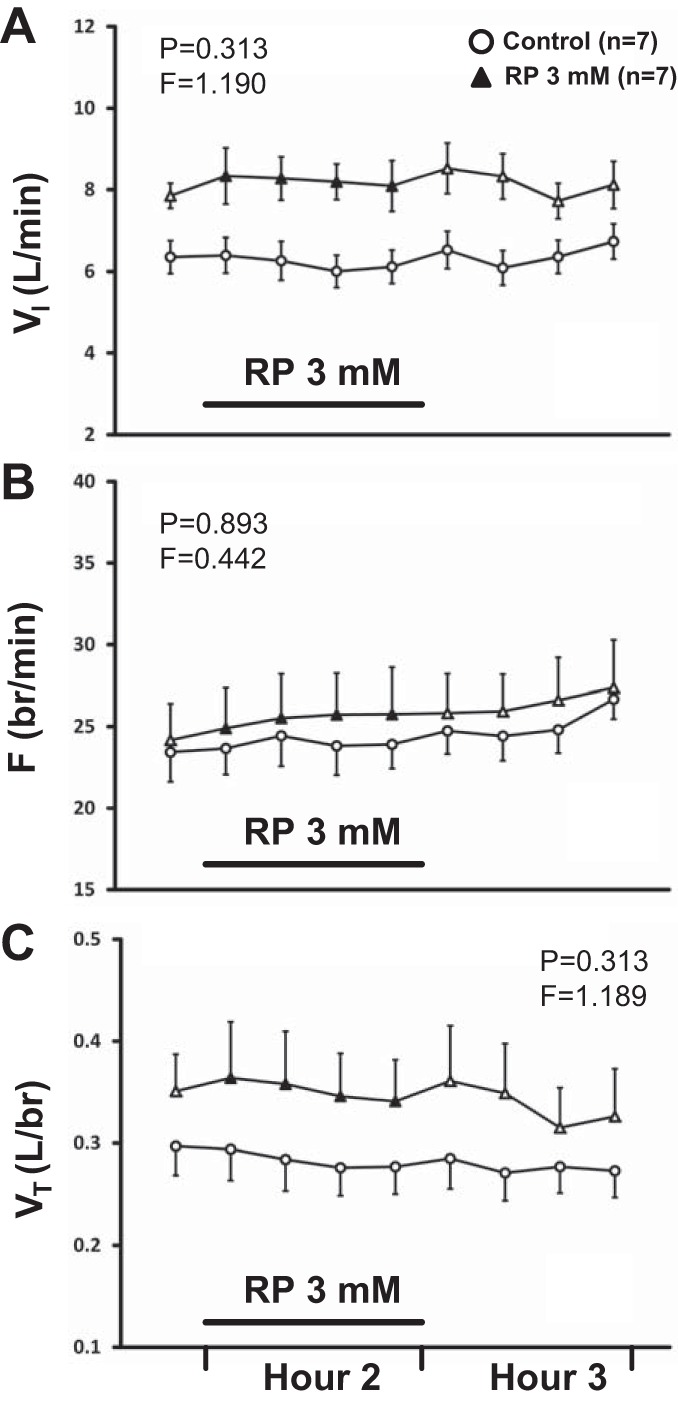
The effect on breathing over time of dialysis of 3 mM RP67580 (RP) (n = 7) in the day was not significantly different (P ≥ 0.313) from the effect of time control studies (n = 7). A: VI (liter/min); B: F (br/min); C: VT (liter/br). First data point represents last bin of Hour 1. Closed symbols and bar indicate period of RP dialysis (Hour 2); open symbols indicate mCSF dialysis. F and P indicate values for interaction term from two-way RM ANOVA (see methods for further details on statistical analysis).
Effect on neurochemicals of dialysis of NK1R antagonists.
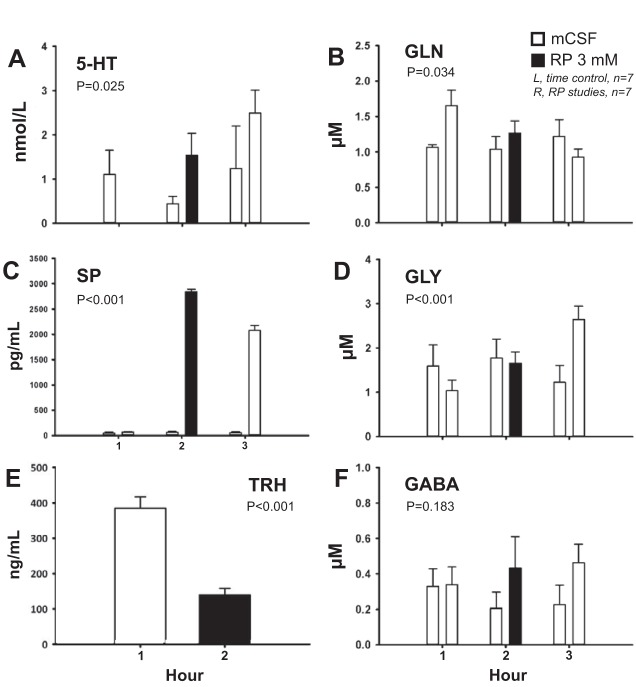
In daytime studies, comparison of the effects on neurochemicals of time control studies and the three doses of SPA (data not shown) found significant interaction effects indicating a SPA-induced increase in SP (P < 0.001) and decrease in GLN (P = 0.031). There were no significant interaction effects for 5-HT, GLY, or GABA (P ≥ 0.221, data not shown). TRH concentration was not significantly affected by 500 μM SPA (P = 0.644, data not shown). Dialysis of 3 mM RP in the day (Fig. 4, right hand bars) significantly increased the effluent concentrations of 5-HT, SP, and GLY (P ≤ 0.025, Fig. 4, A, C, and D, respectively) and significantly decreased GLN and TRH levels (P ≤ 0.034, Fig. 4, B and E, respectively) relative to time control (Fig. 4, left hand bars). RP had no significant effect on effluent GABA concentration (P = 0.183, Fig. 4F). Dialysis of 500 μM SPA at night (n = 4) significantly increased effluent SP concentration (P < 0.001), but had no effect on any other neurochemical (P ≥ 0.159, data not shown).
Fig. 4.
Dialysis of 3 mM RP in the day (n = 7) significantly increased the effluent concentrations of 5-HT (A), Substance P (SP) (C), and glycine (GLY) (D) (P ≤ 0.025). RP significantly decreased effluent concentrations of glutamine (GLN) (B) and thyrotropin-releasing hormone (TRH) (E) (P ≤ 0.034), but had no effect on GABA (F) (P = 0.183). x Axis indicates hour of dialysis. Closed bars indicate RP dialysis; open bars indicate mock cerebrospinal fluid (mCSF) dialysis. Bars on the left represent time control studies (n = 7); bars on the right represent RP studies. P values are from two-way RM ANOVA (A–D, F) or paired t-test (E).
NK1R antagonist concentration and changes in neurochemicals.
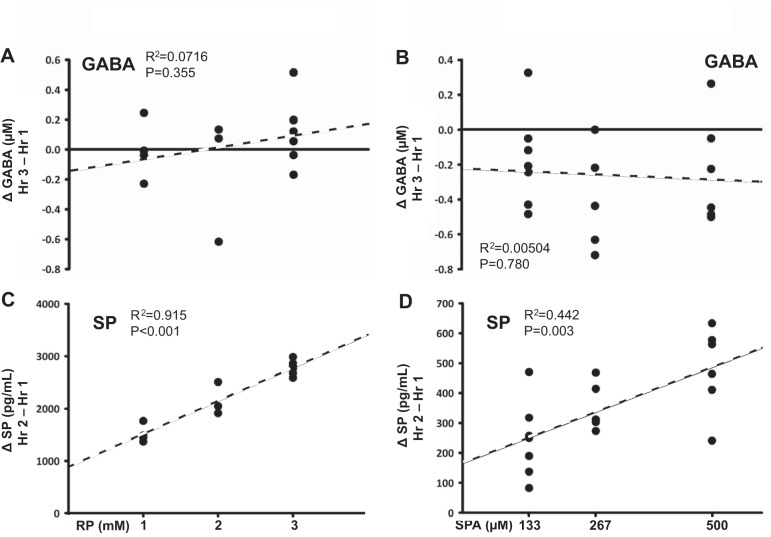
For daytime studies, simple linear regression analysis found that neither RP nor SPA concentration were significant predictors of the change in the effluent concentrations of 5-HT, GLN, GLY, or GABA (P ≥ 0.070, data shown only for GABA, Fig. 5, A and B). However, RP concentration was a significant predictor of the change in SP (P < 0.001, R2=0.915, Fig. 5C); likewise, SPA concentration was also a significant predictor of the change in SP (P = 0.003, R2=0.442, Fig. 5D). The increase in effluent SP concentration is consistently larger with dialysis of RP at all doses compared with SPA (Fig. 5C vs. 5D, note scales of y axes).
Fig. 5.
Dose-dependent effect of dialysis of three doses of RP (1 mM, n = 4; 2 mM, n = 3; 3 mM, n = 7) and three doses of SPA (133 μM, n = 7; 267 μM, n = 5; 500 μM, n = 6) on absolute change in effluent GABA and SP concentration. Neither RP nor SPA concentration were significant predictors of the change in GABA levels (A and B) (P ≥ 0.355). RP concentration was a significant predictor of the change in SP (C) (P < 0.001, R2=0.915); SPA concentration was also a significant predictor of the change in SP (D) (P = 0.003, R2=0.442). x Axis indicates antagonist concentration; y axis indicates change in effluent neurochemical concentration. P and R2 values are results from simple linear regression analyses.
DISCUSSION
Summary of main findings.
We found that, irrespective of the compound dialyzed, the effects on breathing over time of dialysis of a NK1R antagonist were not significantly different from those of time control studies, per lack of interaction effects in two-way comparisons. SPA largely had no significant effect on breathing when dialyzed at night and did not have state-dependent effects. Both antagonists significantly increased effluent SP levels in a concentration-dependent manner, but had contrasting effects on the levels of other neurochemicals, particularly with regard to 5-HT, TRH, and GLY. These findings support our second hypothesis, wherein we state that dialysis of a NK1R antagonist would not change breathing, but would elicit changes in the concentrations of excitatory and/or inhibitory neurochemicals. We believe that the lack of change in breathing with NK1R blockade, accompanied by simultaneous changes in multiple neurochemicals, provides evidence supporting the concept of neuromodulator interdependence.
Lack of effect of NK1R blockade on ventilation.
NK1R blockade had no effect on breathing or on the variability (CV) of the ventilatory parameters we measured. Others have previously found that SP has excitatory effects on respiratory activity in reduced preparations and that blockade with SPA reversibly decreases respiratory activity (43). Application of SPA in vitro also reduces regularity of respiratory activity (43), while destruction of NK1R-expressing neurons in rats (11) and goats (45) results in ataxic, irregular, and abnormal breathing patterns. Our present results are thus unexpected and suggest that the lack of effect on breathing and regularity may be due to the indirect effects of receptor blockade on other neurochemicals.
While potential excitatory effects of SP on NK2Rs and NK3Rs may also be important factors (discussed below), it is clear that in freely behaving animals, breathing can remain stable (unchanged) with the application of an antagonist, given that it elicits simultaneous significant changes in local neurochemicals. We believe that the data presented here provide evidence for the concept of neuromodulator interdependence, whereby attenuation of a single receptor subtype is compensated for by simultaneous changes in other neurochemicals, such that breathing remains unchanged. Furthermore, we postulate that in studies demonstrating irregular breathing with elimination of NK1R-expressing neurons (11, 45) compensatory changes to stabilize breathing may not have occurred or may not have been possible.
It is important to emphasize that our studies were not designed to determine the specific mechanisms of neurochemical release or the involvement of NK1Rs in such release mechanisms. Thus while our conclusions may be speculative, we believe our findings warrant further discussion and future investigation.
Effects of NK1R blockade on SP concentration.
As in past studies, in which we blocked cholinergic receptors (28, 29), the most striking and consistent finding with dialysis of NK1R antagonists was an increase in SP concentration at the site of dialysis. While we previously speculated that atropine-induced increases in SP and/or 5-HT may have been due to presynaptic disinhibition of neurochemical release (28), the increase in extracellular SP with NK1R blockade may be due to accumulation of the peptide in the synaptic space, rather than enhanced release per se.
SP has been shown to induce internalization of its receptor in vivo and in vitro in both central nervous and peripheral tissues (6, 24, 40). In cultured cells, incubation with SP caused internalization of both NK1R and its ligand (6). In the CNS, injection of SP into the rat striatum stimulates internalization of NK1Rs, which was blocked by the NK1R antagonist, RP67580 (24). Thus we postulate that dialysis of SPA or RP in the present studies blocked internalization of NK1Rs and SP, and the mechanisms for metabolizing or clearing of SP may have been unable to keep up with the blockade, leading to accumulation of the peptide in the extracellular space. Alternatively, there may also be negative feedback mechanisms that result in an increase in SP release with decreased NK1R activation due to antagonist blockade.
Another potential mechanism that may account for the increase in SP release is positive feedback modulation via NMDA receptors (NMDARs). In rat spinal cord slices, pharmacological studies provide evidence that NMDAR activation can stimulate SP release, while NMDAR blockade can inhibit evoked release of SP (20a, 23a). It has thus been suggested that stimulation of presynaptic NMDARs can modulate the release of glutamate and/or SP from primary afferent terminals in the spinal cord (20a), leading to prolongation of postsynaptic transmission via glutamate and/or NK1 receptors. In our studies, it is possible that an increase in glutamate release and subsequent enhancement of NMDAR activation may have potentiated SP release. Such an increase in glutamate release in the VRC could presumably lead to an increase in ventilation, which we did not observe. Accordingly, it seems unlikely that the increase in SP was due solely to enhanced NMDAR stimulation. A sufficiently adequate increase in glutamate release may have contributed to stabilization of breathing during NK1R blockade by compensating for the loss of excitatory transmission via NK1Rs. However, as discussed in following sections, it is unlikely that there were significant increases in glutamate with NK1R blockade.
While dialysis of both SPA and RP each increased effluent SP concentration, the increase was markedly larger with RP. One possible explanation for this difference is the use of much higher concentrations of RP than SPA, potentially allowing for greater diffusion of the drug to affect a larger area and a greater number of receptors. A second contributing factor is the greater potency of RP compared with SPA (4). Thirdly, SPA has been reported to have a weak agonist action on phospholipase C activity (4), which may have counteracted its antagonistic effects. Despite differences in the absolute magnitude of their effect on SP, both antagonists increased SP in a linear, concentration-dependent manner (Fig. 5).
Potential contribution of ATP and purinergic receptors.
There is a growing body of evidence implicating a role for ATP and signaling via P2X and P2Y receptors in the control of breathing, which has been reviewed by others (10a). ATP release has been shown to be involved in the hypercapnic ventilatory response (10b) and the hypoxic ventilatory response (10a), in which ATP is hypothesized to counter secondary hypoxic ventilatory depression. Importantly, the preBötC has been shown to be sensitive to ATP and P2 receptor agonists (22a), whereby these agents elicit an increase in fictive breathing frequency. Specifically, the increase in frequency in response to ATP appears to be mediated by P2Y1, rather than P2X2, receptors (22a). Neurons in the preBötC coexpress NK1 and P2X2 receptors, as well as NK1 and P2Y1 receptors (22a). Additionally, receptor interactions have been reported between P2 receptors and other neurochemical systems, including GABAA, nicotinic, noradrenergic, and SP receptors (10a). Thus ATP might be a source of excitation to preBötC/VRC neurons coexpressing NK1 and P2 receptors during NK1R blockade. This ATP-mediated excitation may contribute to stabilization of breathing during RP/SPA dialysis and, furthermore, may be potentiated by changes in the neuromodulatory milieu.
In anesthetized adult rats, exposure to CO2 stimulates release of ATP from known chemosensitive areas on the ventral medullary surface (10b), whereby it was suggested that ATP may act upon receptors on dendrites of neurons of the VRC that extend to the ventral surface. While we did not measure blood gases in our studies, all of our studies were conducted in room air, and we did not observe a change in breathing with NK1R blockade. These findings suggest that there was likely no or minimal PaCO2-driven release of ATP in the ventral medulla (10b). Thus if ATP is involved in compensatory mechanisms during NK1R blockade, it is unlikely to be coming from chemosensitive areas on the ventral medullary surface, such as the retrotrapezoid nucleus (10b).
Potential non-NK1R effects of SP.
SP and other neurokinins can all act on each of the three neurokinin receptors, NK1, NK2, and NK3 (8, 15). Indeed, bilateral microinjections into the presumed preBötC of rabbits of either SP, neurokinin A (NKA, the preferred ligand of NK2R), or the NK3R agonist, senktide, each increased respiratory frequency (5). Interestingly, bilateral microinjections of two different NK1R agonists did not affect respiratory frequency, whereas the NK1R antagonist, CP 99994, increased frequency and did not block the excitatory effects of SP (5). The respiratory effects of SP and NKA were either abolished or reduced by a NK2R antagonist (5). On the basis of these results, the authors concluded that NK2Rs and NK3Rs, but not NK1Rs, were involved in excitatory modulation of respiration in the rabbit and that SP may act on NK2 and NK3 receptors in the preBötC to affect breathing (5).
These findings in the rabbit indicate that we cannot exclude the possibility that SP, at high enough levels, may be exerting effects on NK2 or NK3 receptors to maintain breathing during NK1R blockade (34). Species differences in the affinity of NK1R antagonists for NK1R (8, 41) is another point to consider when comparing our results with other studies.
Distribution of NK1R and effect of NK1R blockade on effluent concentrations of other neurochemicals.
While NK1R is expressed in the goat medulla (27, 45), comprehensive double-labeling studies at the electron microscope level have not been performed in this model to determine pre- and postsynaptic distribution of NK1R and other receptors. Most studies investigating tissue expression of NK1R consistently find labeling of cell bodies and processes at synaptic and nonsynaptic sites (3, 18, 20, 22, 24, 30, 44). It should be noted, however, that presynaptic NK1R expression has also been reported in the rat and primate caudate nucleus and putamen (18).
In the VRG of the rat, at the level corresponding to the preBötC, NK1R-expressing neurons are neither cholinergic nor catecholaminergic, and rarely are they GABAergic or glycinergic (44); these neurons are also typically glutamatergic (13). Thus in our present studies, blocking NK1Rs on glutamatergic neurons would presumably decrease their activity and thus glutamate levels at the synapse. Because of technical limitations, we could not measure glutamate in our dialysate samples. However, since glutamate at the synapse is normally taken up by astrocytes and converted into GLN to maintain neurotransmitter homeostasis (2), the decrease in GLN with RP dialysis (Fig. 4B) perhaps reflects a decrease in glutamate release.
If NK1R-expressing neurons in the corresponding preBötC region of the goat are non-GABAergic and nonglycinergic like the rat (44), then the increase in GLY with RP dialysis (Fig. 4D) suggests that glycinergic neurons in the VRC were indirectly affected by changes in other neurochemicals. Indeed, the increases in 5-HT, SP, and GLY with RP dialysis (Fig. 4, A, C, and D) are reminiscent of the changes seen with dialysis of 50 mM atropine in the VRC (28). We previously proposed that the increase in inhibitory neurotransmitters with 50 mM atropine was a secondary compensatory response to increases in 5-HT and/or SP (28). Another possibility is that the increase in GLY may have been the result of enhanced activity of inhibitory inputs to the preBötC, such as the expiratory neurons of the Bötzinger complex (37, 42). These Bötzinger neurons are proposed to be part of a ring of mutual inhibitory interactions with the preBötC (37), critical for coordinated inspiratory and expiratory activity. Functional (25, 32, 36) and histological studies (21, 22, 25) demonstrate that there is a considerable number of glycinergic and GLY receptor-expressing neurons in the VRG and preBötC. A study by Manzke et al. (25) found that glycinergic neurons comprised more than 50% of the total number of neurons in the mouse preBötC and that these neurons expressed the GLY receptor α3 subtype (GlyRα3), 5-HT1A receptors, NK1R, and the μ-opioid receptor. Intriguingly, their data further suggest that interactions between 5-HT1A and GlyRα3 receptors counteracted opioid-induced respiratory depression (25). Taken together, our data and those of others indicate that glycinergic inputs within and between respiratory control centers are modulated by a complex array of neuromodulators and possibly by interactions between receptors.
The effects of NK1R blockade on 5-HT outflow (Fig. 4A) are difficult to explain. Blocking or activating NK1Rs has produced controversial results regarding 5-HT neuronal firing activity and/or 5-HT release (23). Data reviewed by Maejima (23) suggest that there is “at least a functional coupling between NK1 and 5-HT1A receptors.” More puzzling is the decrease in the excitatory peptide, TRH, with NK1R blockade with RP (Fig. 4E). Double- and triple-labeling studies of raphé neurons have shown colocalization of SP and/or TRH with 5-HT (1, 17, 46) in certain subsets of neurons. Thus one could expect 5-HT and TRH to change at least in the same direction if neurochemical release is perturbed. The fact that colocalized neurochemicals change in opposite directions in our present study indicates that release mechanisms are independent or differentially affected by receptor inactivation.
Lastly, changes in modulators that we did not measure may also account for our data and should be considered when interpreting our results. For example, we have previously shown that nonselective blockade of muscarinic receptors with 50 mM atropine in the VRC of intact goats can dramatically increase breathing frequency and 5-HT and SP concentrations (28). Conversely, a smaller dose of atropine (5 mM) does not affect breathing, but significantly increases SP concentration, albeit, to a much smaller degree (29). We were unable to measure acetylcholine/choline in these studies. However, our past studies suggest that perturbations in cholinergic level or muscarinic receptor activation may be involved in the changes in neuromodulation we report here, particularly with respect to SP, 5-HT, and GLY (Fig. 4), as these neurochemicals significantly increased with dialysis of 50 mM atropine (28).
Caveats and limitations.
Caveats and limitations of our animal model and use of dialysis have been discussed in greater detail elsewhere (28, 29) and will only be briefly summarized here. Firstly, the precise boundaries of the preBötC in the goat are unclear (19), making MT placement difficult in some animals. Methods for quantifying the diffusion area of dialyzed substances are also not exact, which is another limitation to our study. Secondly, because of the length of tubing needed for delivery of dialyzed solutions, there is always a delay (of ∼20 min) between initiation of dialysis and when the solution actually reaches the probe. Thirdly, we are unable to separate effluent fluid collected during different sleep states. Sleep state–associated changes in fractional interstitial space (47), and thus diffusion and tissue concentration of drugs and neurochemicals, are another caveat in our nighttime neurochemical data. Lastly, repeated probe insertions may have potentially caused tissue damage around the dialysis site.
Significance of findings.
It is not surprising that there exists a “safety net,” such as neuromodulator interdependence, for a function as vital as breathing. The multiplicity, redundancy, and convergence of signaling pathways in the respiratory network (9) suggest that a combination of inputs need to be blocked in an intact animal to sufficiently impact breathing. At the very least, the interaction of these inputs must be considered. Indeed, in whole animal studies where breathing was altered by blocking/enhancing the effects of a single modulator, our data suggest that the respiratory changes may have been mediated by modulator systems other than the one that was targeted. In health and disease, the concept of neuromodulator interdependence offers alternative targets for treating conditions of respiratory insufficiency, as well as ways to expand existing therapies by targeting multiple receptor systems. It would also be of great clinical and fundamental interest to investigate if and how compensatory mechanisms change under stress conditions, such as acute and chronic hypoxia or hypercapnia.
Concluding remarks.
The present studies demonstrate that perturbation of a single receptor subtype can elicit changes in multiple neurochemicals known to affect respiration without altering breathing, supporting the concept of neuromodulator interdependence. These data illustrate the complexity of interactions within and between respiratory networks, attest to the robustness of mechanisms that preserve breathing, offer alternative insights in interpretation of results from a large body of past work, and may potentially broaden therapeutic targets for a variety of respiratory conditions.
GRANTS
This project was funded by National Heart, Lung, and Blood Institute Grants HL-25739, HL-112996, and HL-007852 and by the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.M., M.R.H., and H.V.F. conception and design of research; C.M., S.E.N., S.J.O., J.R.M., L.G.P., and H.V.F. performed experiments; C.M. and H.V.F. analyzed data; C.M., M.R.H., and H.V.F. interpreted results of experiments; C.M. and S.E.N. prepared figures; C.M. drafted manuscript; C.M., S.E.N., M.R.H., and H.V.F. edited and revised manuscript; C.M., S.E.N., S.J.O., J.R.M., M.R.H., L.G.P., and H.V.F. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of J. Miller: Zablocki Veterans Affairs Medical Center, Milwaukee, WI 53295.
REFERENCES
- 1.Arvidsson U, Cullheim S, Ulfhake B, Luppi PH, Kitahama K, Jouvet M, Hokfelt T. Quantitative and qualitative aspects on the distribution of 5-HT and its coexistence with substance P and TRH in cat ventral medullary neurons. J Chem Neuroanat 7: 3–12, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem 98: 641–653, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Baude A, Shigemoto R. Cellular and subcellular distribution of substance P receptor immunoreactivity in the dorsal vagal complex of the rat and cat: a light and electron microscope study. J Comp Neurol 402: 181–196, 1998. [PubMed] [Google Scholar]
- 4.Beaujouan JC, Heuillet E, Petitet F, Saffroy M, Torrens Y, Glowinski J. Higher potency of RP 67580, in the mouse and the rat compared with other nonpeptide and peptide tachykinin NK1 antagonists. Br J Pharmacol 108: 793–800, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bongianni F, Mutolo D, Cinelli E, Pantaleo T. Neurokinin receptor modulation of respiratory activity in the rabbit. Eur J Neurosci 27: 3233–3243, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Bowden JJ, Garland AM, Baluk P, Lefevre P, Grady EF, Vigna SR, Bunnett NW, McDonald DM. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci U S A 91: 8964–8968, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cinelli E, Robertson B, Mutolo D, Grillner S, Pantaleo T, Bongianni F. Neuronal mechanisms of respiratory pattern generation are evolutionary conserved. J Neurosci 33: 9104–9112, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datar P, Srivastava S, Coutinho E, Govil G. Substance P: structure, function, therapeutics. Curr Top Med Chem 4: 75–103, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol 164: 96–104, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci 30: 8251–8262, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Funk GD, Huxtable AG, Lorier AR. ATP in central respiratory control: a three-part signaling system. Respir Physiol Neurobiol 164: 131–142, 2008. [DOI] [PubMed] [Google Scholar]
- 10b.Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436: 108–111, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science 286: 1566–1568, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci 22: 3806–3816, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyenet PG, Wang H. Pre-Botzinger neurons with preinspiratory discharges “in vivo” express NK1 receptors in the rat. J Neurophysiol 86: 438–446, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Harrison S, Geppetti P. Substance P. Int J Biochem Cell Biol 33: 555–576, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci 29: 10341–10349, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hokfelt T, Arvidsson U, Cullheim S, Millhorn D, Nicholas AP, Pieribone V, Seroogy K, Ulfhake B. Multiple messengers in descending serotonin neurons: localization and functional implications. J Chem Neuroanat 18: 75–86, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Jakab RL, Goldman-Rakic P. Presynaptic and postsynaptic subcellular localization of substance P receptor immunoreactivity in the neostriatum of the rat and rhesus monkey (Macaca mulatta). J Comp Neurol 369: 125–136, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Krause KL, Forster HV, Kiner T, Davis SE, Bonis JM, Qian B, Pan LG. Normal breathing pattern and arterial blood gases in awake and sleeping goats after near total destruction of the presumed pre-Botzinger complex and the surrounding region. J Appl Physiol 106: 605–619, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li JL, Wang D, Kaneko T, Shigemoto R, Nomura S, Mizuno N. The relationship between neurokinin-1 receptor and substance P in the medullary dorsal horn: a light and electron microscopic immunohistochemical study in the rat. Neurosci Res 36: 327–334, 2000. [DOI] [PubMed] [Google Scholar]
- 20a.Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature 386: 721–724, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Liu YY, Ju G, Wong-Riley MT. Distribution and colocalization of neurotransmitters and receptors in the pre-Botzinger complex of rats. J Appl Physiol 91: 1387–1395, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Liu YY, Wong-Riley MT, Liu JP, Jia Y, Liu HL, Jiao XY, Ju G. GABAergic and glycinergic synapses onto neurokinin-1 receptor-immunoreactive neurons in the pre-Botzinger complex of rats: light and electron microscopic studies. Eur J Neurosci 16: 1058–1066, 2002. [DOI] [PubMed] [Google Scholar]
- 22a.Lorier AR, Huxtable AG, Robinson DM, Lipski J, Housley GD, Funk GD. P2Y1 receptor modulation of the pre-Bötzinger Complex inspiratory rhythm generating network in vitro. J Neurosci 27: 993–1005, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maejima T, Masseck OA, Mark MD, Herlitze S. Modulation of firing and synaptic transmission of serotonergic neurons by intrinsic G protein-coupled receptors and ion channels. Front Integr Neurosci 7: 40, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Malcangio M, Fernandes K, Tomlinson DR. NMDA receptor activation modulates evoked release of substance P from rat spinal cord. Br J Pharmacol 125: 1625–1626, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci U S A 92: 2622–2626, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manzke T, Niebert M, Koch UR, Caley A, Vogelgesang S, Hulsmann S, Ponimaskin E, Muller U, Smart TG, Harvey RJ, Richter DW. Serotonin receptor 1A-modulated phosphorylation of glycine receptor alpha3 controls breathing in mice. J Clin Invest 120: 4118–4128, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCrimmon DR, Monnier A, Hayashi F, Zuperku EJ. Pattern formation and rhythm generation in the ventral respiratory group. Clin Exp Pharmacol Physiol 27: 126–131, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Miller JR, Neumueller S, Muere C, Olesiak S, Pan L, Bukowy JD, Daghistany AO, Hodges MR, Forster HV. Changes in glutamate receptor subunits within the medulla in goats after section of the carotid sinus nerves. J Appl Physiol 116: 1531–1542, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muere C, Neumueller S, Miller J, Olesiak S, Hodges MR, Pan L, Forster HV. Atropine microdialysis within or near the pre-Botzinger Complex increases breathing frequency more during wakefulness than during NREM sleep. J Appl Physiol 114: 694–704, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muere C, Neumueller S, Miller J, Olesiak S, Hodges MR, Pan L, Forster HV. Evidence for respiratory neuromodulator interdependence after cholinergic disruption in the ventral respiratory column. Respir Physiol Neurobiol 205: 7–15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol 347: 249–274, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Nattie E, Li A. Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol 101: 1596–1606, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Pierrefiche O, Schwarzacher SW, Bischoff AM, Richter DW. Blockade of synaptic inhibition within the pre-Botzinger complex in the cat suppresses respiratory rhythm generation in vivo. J Physiol 509: 245–254, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quartara L, Maggi CA. The tachykinin NK1 receptor. Part II: Distribution and pathophysiological roles. Neuropeptides 32: 1–49, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Schwarzacher SW, Rub U, Deller T. Neuroanatomical characteristics of the human pre-Botzinger complex and its involvement in neurodegenerative brainstem diseases. Brain 134: 24–35, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Botzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol 77: 1853–1860, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci 36: 152–162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solomon IC, Edelman NH, Neubauer JA. Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the pre-Botzinger complex in vivo. J Neurophysiol 81: 1150–1161, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Southwell BR, Seybold VS, Woodman HL, Jenkinson KM, Furness JB. Quantitation of neurokinin 1 receptor internalization and recycling in guinea-pig myenteric neurons. Neuroscience 87: 925–931, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev 94: 265–301, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stornetta RL. Identification of neurotransmitters and co-localization of transmitters in brainstem respiratory neurons. Respir Physiol Neurobiol 164: 18–27, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Telgkamp P, Cao YQ, Basbaum AI, Ramirez JM. Long-term deprivation of substance P in PPT-A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol 88: 206–213, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J Comp Neurol 434: 128–146, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah T, Davis S, Forster HV. Small reduction of neurokinin-1 receptor-expressing neurons in the pre-Botzinger complex area induces abnormal breathing periods in awake goats. J Appl Physiol 97: 1620–1628, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Wessendorf MW, Appel NM, Molitor TW, Elde RP. A method for immunofluorescent demonstration of three coexisting neurotransmitters in rat brain and spinal cord, using the fluorophores fluorescein, lissamine rhodamine, and 7-amino-4-methylcoumarin-3-acetic acid. J Histochem Cytochem 38: 1859–1877, 1990. [DOI] [PubMed] [Google Scholar]
- 47.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science 342: 373–377, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]